Abstract
Background
Lifelong pulmonary consequences of being born extremely preterm or with extremely low birth weight remain unknown. We aimed to describe lung function trajectories from 10 to 35 years of age for individuals born extremely preterm, and address potential cohort effects over a period that encompassed major changes in perinatal care.
Methods
We performed repeated spirometry in three population-based cohorts born at gestational age ≤28 weeks or with birth weight ≤1000 g during 1982–85, 1991–92 and 1999–2000, referred to as extremely preterm-born, and in term-born controls matched for age and gender. Examinations were performed at 10, 18, 25 and 35 years. Longitudinal data were analysed using mixed models regression, with the extremely preterm-born stratified by bronchopulmonary dysplasia (BPD).
Results
We recruited 148/174 (85%) eligible extremely preterm-born and 138 term-born. Compared with term-born, the extremely preterm-born had lower z-scores for forced expiratory volume in 1 s (FEV1) at most assessments, the main exceptions were in the groups without BPD in the two youngest cohorts. FEV1 trajectories were largely parallel for the extremely preterm- and term-born, also during the period 25–35 years that includes the onset of the age-related decline in lung function. Extremely preterm-born had lower peak lung function than term-born, but z-FEV1 values improved for each consecutive decade of birth (p=0.009). More extremely preterm—than term-born fulfilled the spirometry criteria for chronic obstructive pulmonary disease, 44/148 (30%) vs 7/138 (5%), p<0.001.
Conclusions
Lung function after extremely preterm birth tracked in parallel, but significantly below the trajectories of term-born from 10 to 35 years, including the incipient age-related decline from 25 to 35 years. The deficits versus term-born decreased with each decade of birth from 1980 to 2000.
Keywords: lung physiology, COPD epidemiology, clinical epidemiology, health economist, paediatric lung disaese
Key messages.
What is already known on this topic?
Extremely premature birth is associated with airway obstruction during childhood, adolescence and young adulthood. Survival was uncommon in these infants before the 1980s and their lifetime health prospects are therefore unknown.
What this study adds?
This is the first population-based longitudinal study to describe lung function trajectories well into adulthood in extremely preterm-born survivors, describing persistent airway obstruction beyond peak function in early adult life and throughout the onset of the age-related decline from 25 to 35 years.
How this study might affect research, practice and/or policy?
Chronic obstructive pulmonary disease roots in early life, and extremely premature birth represents an important precursor that needs to be acknowledged by chest physicians and targeted by the research community.
Introduction
Rarely in the history of medicine were the ‘frontiers of the possible’ pushed further and faster than in our neonatal intensive care units (NICU) during the 1980s and 1990s.1 By the turn of the millennium, survival approached 80%–90% for 28 weeks’ gestation, the cut-off for being labelled extremely premature.2 In high-income countries, extremely preterm-born infants currently account for one in 200 children growing up.3 They are born at a time when the lungs normally undergo complex developmental processes in a protected intrauterine environment. In contrast, the lungs of infants born at this early stage are exposed to lifesaving, but also harmful, intensive care. Survivors are at increased risk of chronic lung disease as well as premature death from a range of causes.4
Lung function normally increases through childhood and adolescence, reaches a peak in the mid-20s, and then gradually declines with age.5 The trajectory is modulated by genetic mechanisms, antenatal factors, and multiple early events and exposures.6 For most people, the adult decline proceeds unrecognised as symptoms only appear if lung function reaches very low levels.7 For infants born extremely preterm, this lifespan perspective represents uncharted territory, as their high survival rates are recent achievements.
Most extremely preterm-born do not appear to reach their expected peak adult lung function.8 A few longitudinal studies have traced their lung function up to the mid-20s, finding both deterioration or tracking in parallel although at lower levels, when compared with subjects born at term.9–13 To date, no longitudinal studies have traced lung function of extremely preterm-born past 25 years of age, and thus we lack information regarding the nature of their age-related decline. Given their common early morbidities, the decline might start at a younger age or be steeper than in the general population, and early chronic obstructive pulmonary disease (COPD) is a feared scenario.14 Bronchopulmonary dysplasia (BPD) is a respiratory complication affecting approximately 50% of extremely preterm-born neonates, primarily characterised by prolonged requirement for supplemental oxygen in the neonatal period.2 BPD is associated with subsequent airway obstruction and bronchial hyper-responsiveness, and there are concerns for additional negative impact on adult pulmonary health.5 14
We have previously reported lung function data from three population-based cohorts born extremely preterm or with extremely low birth weight (BW) (hereafter referred to as extremely preterm-born) during the early 1980s, 1990s and 2000s.12 15 16 With the present extension of these studies, we aimed to describe their lung function trajectories well into adulthood, and to address potential cohort effects over almost twenty years of inclusion, a period that encompassed major changes in perinatal care. We hypothesised that lung function in subjects born extremely preterm tracked in parallel, but below the level of matched term-born controls, and that differences decreased with succeeding decade of birth.
Material and methods
Subjects and study design
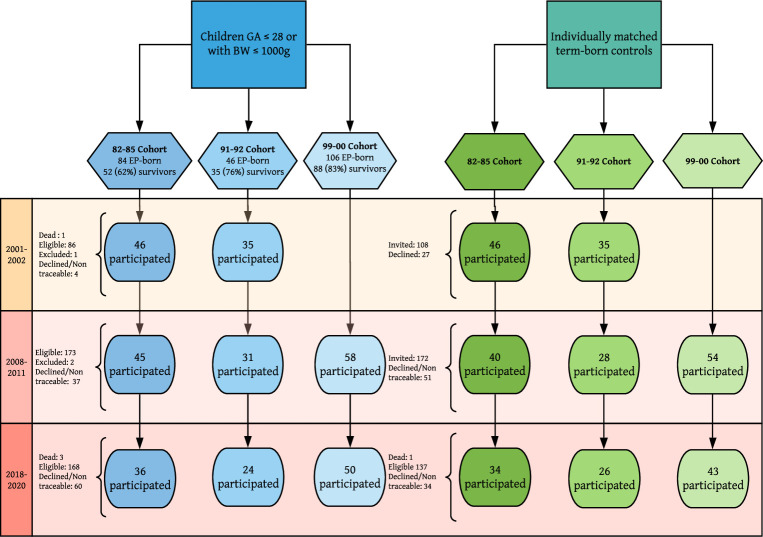
Three regional population-based cohorts born during 1982–1985 (82–85 cohort), 1991–1992 (91–92 cohort) and 1999–2000 (99–00 cohort) at gestational age (GA) ≤28 (82–85 and 91–92 cohort) or ≤27 weeks (99–00 cohort), or with BW ≤1000 g were included. For each extremely preterm-born, the temporally closest individual born at term at the same hospital and of the same sex with BW 3–4 kg (Norwegian 10–90 percentiles)17 was recruited as a control. The cohorts were assessed at three (two for the 99–00 cohort) study-visits performed in 2001–2002, 2008–2011 and 2018–2020 at, respectively, 18, 25 and 35 years; 10, 18 and 25 years; and 10 and 18 years (figure 1). Inclusion and study design has been described previously.12 15 16 The subjects completed the International Study of Asthma and Allergy in Childhood18 questionnaire at each visit.
Figure 1.
Patient inclusion. The inclusion procedure for the extremely preterm (EP)-born (born at gestational age ≤28 weeks or birth weight ≤1000 g) and their matched term-born controls. BW, Birth weight; GA, gestational age.
BPD was defined as need for supplemental oxygen or respiratory support at postmenstrual age ≥36 weeks. The mode of determination of GA, and the definitions of small for GA (SGA), smoking status, atopy and asthma have been described previously,12 15 16 and is also provided in the online supplemental file.
thoraxjnl-2021-218400supp001.pdf (168.7KB, pdf)
Lung function measurements
Spirometry without prior administration of bronchodilator was performed and reviewed according to standard criteria19 in a lung physiology laboratory with experienced test-personnel, using a Vmax spirometer (SensorMedics, Anaheim, California, USA) for the first two study visits, and a Vyntus pneumo spirometer (Vyaire, Hoechberg, Germany) for the last. Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), the ratio FEV1/FVC and forced expiratory flow at 25%–75% of FVC (FEF25–75%) were recorded. Raw data were normalised for age, sex, ethnicity and height.20 The lower limit of normal (LLN) was defined as z-score ≤ −1.645. The Global lung function initiative (GLI) equations were used to estimate peak expected lung function at 20 and 22 years for females and males, respectively, defining participants’ final height as measured at 18 or 25 years, whichever was highest. The spirometry criteria for COPD was defined as FEV1/FVC z-score ≤LLN.
Statistical methods
Descriptive data are reported as means, SD, ranges, counts and/or percentages, as appropriate. Questionnaire data are presented as at least one positive response at any visit. Welch’s one-way analysis of variance (ANOVA)/t-tests, Kruskall-Wallis, Wilcoxon-Mann-Whitney and Pearson’s χ2 tests, were performed to compare exposure variables between cohorts and between extremely preterm and term-born subjects within cohorts, as appropriate. We fitted linear mixed-effects longitudinal models to estimate, for each time point, mean z-scores for FEV1, FVC, FEV1/FVC and FEF25–75%, and FEV1 as percentage of expected adult peak. The explanatory variables were cohort, age group and subject category (term-born vs extremely preterm-born without BPD vs extremely preterm-born with BPD). For maximal flexibility, all possible interactions of these explanatory variables were included, and subjects were included as a random intercept. The use of mixed-effects models allows inclusion of subjects with incomplete follow-up data, reduces bias caused by missing data, and increases the precision of the estimates.21
We fitted simplified versions of the full model to test for cohort effects and parallel longitudinal patterns, and we compared the full and simplified models using likelihood ratio tests. To compare cohorts of extremely preterm-born subjects, we omitted the age variable, as FEV1 z-scores were stable over time. To assess the effects of neonatal variables, we fitted a multiple regression model for z-FEV1 at 18 years, the only age where all cohorts had measurements. Explanatory variables were cohort, BPD status, GA, BW z-score, maternal smoking and treatment with surfactant and antenatal steroids. We evaluated the effect of current smoking at 18 years on z-FEV1 for the 82–85 and 91–92 cohorts by Welch’s t-test (data missing for the 99–00 cohort).
All models were fitted using R V.4.0.2, and the mixed-effects models by using the R package ‘lme4’ V.1.1–25. Values of p≤0.05 were characterised as statistically significant.
The study was originally designed in 2001 to have 90% power to detect differences exceeding 7.5 percentage points in FEV1 between the extremely preterm-born and term-born groups, given normally distributed data, a within-group SD of 10 percentage points, and a two-sided significance level of 0.05. This required a sample-size of 39 in each group.
Results
Subjects
In total, 217 extremely preterm-born were admitted to the two NICUs during the three inclusion periods; 174 (80%) were alive at first follow-up. Survival rates at hospital discharge were 62%, 76% and 83% in the 82–85, 91–92 and 99–00 cohorts, respectively. During the follow-up period, three died and three were excluded due to inability to perform spirometry. In total, 148/174 (85%) participated at least once, 122/174 (70%) at two or more visits, and 99/171 (58%) participated at all possible visits (three for the 82–85 and 91–92 cohort, two for the 99–00 cohort) (figure 1). Sixteen subjects were included solely on the BW criterion, six in the 82–85 cohort (mean GA 31 weeks), two in the 91–92 cohort (GA 30 and 31 weeks), and eight in the 99–00 cohort (mean GA 29.6 weeks).
To recruit a full 1:1 control group, we approached 1.3 term-born subjects per extremely preterm-born in the 82–85 and 91–92 cohorts, and 1.6 in the 99–00 cohort. Altogether 138 controls were included, one died during the study period. In total, 93/137 (68%) participated at all possible visits and 115/138 (83%) at two or more visits.
Perinatal characteristics
For term-born participants, mean BW increased with birth-decade. For extremely preterm-born, mean BW and GA decreased, and the proportion born SGA increased with birth-decade. Days on oxygen supplementation and the proportion with BPD increased with birth-decade, as did the use of CPAP, surfactant and antenatal as well as postnatal steroids, whereas the number of ventilator days decreased. Exogenous surfactant was unavailable for the 82–85 cohort, available as synthetic surfactant (Exosurf) provided according to the OSIRIS study protocol for the 91–92-cohort,22 and as porcine-derived surfactant (Curosurf) provided according to existing guidelines to the 99–00 cohort. Details on neonatal management have been described previously.15 16 Maternal smoking decreased with birth-decade. Perinatal characteristics did not differ between participants and non-participants in the 99–00 cohort, except that more non-participants were exposed to maternal smoking during pregnancy (56% vs 25%; online supplemental table E1). Non-participation rates for the two oldest cohorts were negligible (table 1).
Table 1.
Perinatal data
| 82–85 cohort | 91–92 cohort | 99–00 cohort | P value | ||||
| Term-born, participated/eligible | 46/46 | 35/35 | 57/57 | − | |||
| EP-born, participated/eligible | 49/51 | 35/35 | 64/88 | − | |||
| Perinatal data of term-born and EP-born subjects | |||||||
| Birth weight in grams, mean (range) | |||||||
| Term-born | 3441 | (3000–4000) | 3564 | (3010–4000) | 3696 | (2990–4500) | 0.003 |
| EP-born | 1005 | (580–1480) | 933 | (570–1400) | 844 | (450–1250) | <0.001 |
| EP no BPD | 1047 | (580–1480) | 976 | (620–1400) | 862 | (450–1250) | 0.001 |
| EP BPD | 888 | (670–1080) | 851 | (570–1200) | 827 | (520–1100) | 0.39 |
| Maternal smoking during pregnancy, n (%) | |||||||
| Term-born | 10 | (22) | 9 | (26) | − | (26)* | 0.72 |
| EP-born | 22 | (48) | 13 | (38) | 15 | (25) | 0.06 |
| Perinatal data of the EP-born subjects | |||||||
| Gestational age in weeks, mean (range) | 27.5 | (23–34) | 26.7 | (23–31) | 26.7 | (24–31) | 0.03 |
| SGA, n (%) | 9 | (18) | 5 | (14) | 23 | (36) | 0.03 |
| Birth weight Z score, mean (SD) | −0.5 | (1.2) | −0.3 | (0.9) | −0.8 | (1.3) | 0.13 |
| Postnatal days with oxygen treatment, median (quartiles)† | 42 | (26–56) | 49 | (26–71) | 61 | (40–78) | 0.05 |
| Days on CPAP, median (quartiles) | 3 | (0–14) | 8 | (1–28) | 29 | (19–42) | <0.001 |
| Ventilator days, median (quartiles) | 8 | (1–16) | 4 | (1–12) | 5 | (2–9) | 0.04 |
| BPD n (%) | 13 | (27) | 12 | (34) | 33 | (52) | 0.02 |
| Antenatal steroids, n (%) | 17 | (35) | 15 | (44) | 52 | (81) | <0.001 |
| Surfactant, n (%) | 0 | (0) | 17 | (49) | 54 | (84) | <0.001 |
| Postnatal steroids, n (%) | 4 | (8) | 10 | (29) | 20 | (31) | 0.01 |
| PDA closure, medical or surgical, n (%) | 13 | (27) | 17 | (49) | 14 | (22) | 0.02 |
Cohort differences were tested using Welch’s one-way ANOVA for continuous variables and Pearson’s χ2 test for categorical variables.
*Data not recorded for term-born from this cohort; the reported value represents percentage of smoking mothers of all births in the region, from the medical birth registry of Norway. The corresponding p value is based on comparison of the two earliest cohorts.
†Cohort differences were tested using Kruskall-Wallis test due to skewed data.
ANOVA, analysis of variance; BPD, bronchopulmonary dysplasia (supplemental oxygen or respiratory support at postmenstrual age ≥36 weeks); CPAP, continuous positive airway pressure; EP, subjects born extremely preterm or with extremely low birth weight (gestational age ≤28 weeks or birth weight ≤1000 g); PDA, persistent ductus arteriosus; SGA, small for gestational age (BW <10th percentile for GA according to Norwegian growth curves).
Anthropometric measurements and information from questionnaires
Adult extremely preterm-born attained a lower height than term-born. Questionnaires revealed no significant differences between the extremely preterm-born and term-born participants regarding wheeze, use of asthma medication or current asthma. Significantly more extremely preterm-born responded ‘yes’ to the question ‘Have you ever had asthma’, although atopy tended to be more common in term-born. Smoking became less common from the 82–85 to the 91–92 cohort for extremely preterm-born but not term-born (p<0.001 and p=0.24, respectively). Complete questionnaire data from each visit are available in online supplemental table E2 (table 2).
Table 2.
Background variables (n=286)
| Cohort | 82–85 cohort | 91–92 cohort | 99–00 cohort | |||||||
| Participated/eligible* | Term-born | 46/46 | (100) | − | 35/35 | (100) | − | 57/57 | (100) | − |
| EP-born | 49/51 | (96) | 35/35 | (100) | 64/88 | (73) | ||||
| Female sex, n (%), p value | Term-born | 21 | (46) | − | 22 | (63) | − | 27 | (47) | − |
| EP-born | 21 | (43) | 22 | (63) | 31 | (48) | ||||
| Final Height in cm, mean (SD), p value | Term-born | 174 | (8) | 0.04 | 171 | (9) | 0.09 | 177 | (10) | <0.001 |
| EP-born | 170 | (8) | 167 | (9) | 168 | (10) | ||||
| Adult Weight in kg, mean (SD), p value | Term-born | 73 | (14) | 0.73 | 73 | (14) | 0.34 | 73 | (14) | 0.02 |
| EP-born | 74 | (18) | 69 | (14) | 66 | (14) | ||||
| Self-reported smoking, n (%), p value | Term-born | 16 | (35) | 0.42 | 8 | (23) | 0.04 | −† | − | − |
| EP-born | 21 | (43) | 2 | (6) | 2 | (6) | ||||
| Atopy, n (%), p value | Term-born | 20 | (43) | 0.13 | 8 | (23) | 0.78 | 20 | (40) | 0.04 |
| EP-born | 13 | (28) | 9 | (26) | 12 | (22) | ||||
| Wheeze last 12 months, n (%), p value | Term-born | 17 | (38) | 0.27 | 15 | (43) | 0.34 | 7 | (13) | 0.10 |
| EP-born | 24 | (49) | 19 | (54) | 15 | (24) | ||||
| Asthma med. last 12 months, n (%), p value | Term-born | 6 | (13) | 0.34 | 4 | (13) | 0.17 | 3 | (5) | 0.25 |
| EP-born | 10 | (20) | 9 | (26) | 7 | (11) | ||||
| Asthma ever, n (%), p value | Term-born | 9 | (20) | 0.06 | 10 | (29) | 0.14 | 5 | (9) | <0.001 |
| EP-born | 18 | (37) | 16 | (46) | 22 | (35) | ||||
| Current asthma, n (%), p value | Term-born | 6 | (13) | 0.10 | 8 | (23) | 0.29 | 4 | (7) | 0.31 |
| EP-born | 13 | (27) | 12 | (34) | 8 | (13) | ||||
The listed variables ‘Allergy’, ‘self-reported smoking’, ‘wheeze last 12 months’ and ‘asthma med. last 12 months’ refer to the number (fraction) with a least one positive response or finding at any visit. ‘Current asthma’ refers to a combination of wheeze in the last 12 months plus a positive response to the questions ‘asthma ever’ or ‘asthma medication last 12 months’ on minimum one visit. P value refers to comparisons between EP and term-born (Welch’s t-test for continuous data and Pearson’s χ2 test for categorical data).
*At least one visit (all percentage and p values are based only on visits where the subject participated).
†Data not presented due to large amount of missing data.
Adult weight, weight at 25 years (or 18 years if not applicable); Asthma med, asthma medication; EP, subjects born extremely preterm or with extremely low birth weight (gestational age ≤28 weeks or birth weight ≤1000 g); Final height, the height achieved at 18 or 25 years, whichever was highest.
Lung function
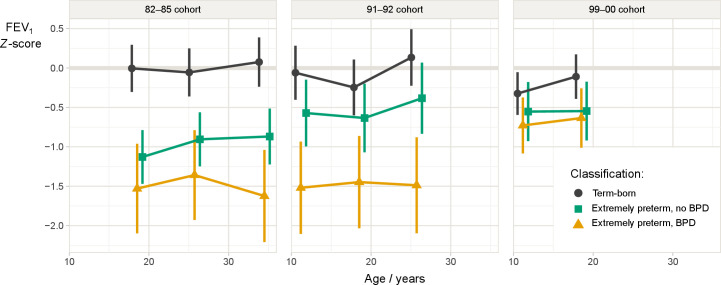
For term-born controls, mean z-scores for spirometry variables were close to zero at most assessments (table 3). Notably, for the next-to last visit of the 99–00 cohort, mean z-FEV1 was –0.3 and the 95% CI did not include zero (table 3), thus, development was not completely stable over time (likelihood ratio test comparing a full model to a model without age effect for term-born, p=0.005, figure 2).
Table 3.
Spirometric lung function data comparing term-born with extremely preterm subjects (n=286 subjects)
| 82–85 cohort* | 91–92 cohort | 99–00 cohort | ||||||||||||||||||||||
| Age (mean, range) | 17.7 (15.3–19.9) | 24.9 (22.5–27.0) | 34.5 (32.1–37.6) | 10.6 (9.7–11.5) | 17.8 (17.0–19.2) | 27.1 (26.1–28.4) | 11.6 (10.1–13.8) | 19.8 (18.2–21.4) | ||||||||||||||||
| n/eligble | ||||||||||||||||||||||||
| Term-born | 46/46 | 40/46 | 34/46 | 35/35 | 28/35 | 26/34 | 54/57 | 43/57 | ||||||||||||||||
| EP no BPD | 34/38 | 32–33/38 | 25–26/36 | 23/23 | 19/23 | 15/23 | 26/43 | 27/43 | ||||||||||||||||
| EP BPD | 12/13 | 12/13 | 9–10/12 | 12/12 | 12/12 | 9/12 | 32/45 | 23/45 | ||||||||||||||||
| Mean | 95% CI | P value | Mean | 95% CI | P value | Mean | 95% CI | P value | Mean | 95% CI | P value | Mean | 95% CI | P value | Mean | 95% CI | P value | Mean | 95% CI | P value | Mean | 95% CI | P value | |
| FEV1 z-score | ||||||||||||||||||||||||
| Term-born (ref) | 0.0 | −0.3 to 0.3 | − | −0.1 | −0.4 to 0.2 | − | 0.1 | −0.2 to 0.4 | − | −0.1 | −0.4 to 0.3 | − | −0.2 | −0.6 to 0.1 | − | 0.1 | −0.2 to 0.5 | − | −0.3 | −0.6 to −0.1 | − | −0.1 | −0.4 to 0.2 | − |
| EP no BPD | −1.1 | −1.5 to −0.8 | <0.001 | −0.9 | −1.2 to −0.6 | <0.001 | −0.9 | −1.2 to −0.5 | <0.001 | −0.6 | −1.0 to −0.1 | 0.07 | −0.6 | −1.1 to −0.2 | 0.17 | −0.4 | −0.8 to 0.1 | 0.08 | −0.6 | −0.9 to −0.2 | 0.33 | −0.5 | −0.9 to −0.2 | 0.07 |
| EP BPD | −1.5 | −2.1 to −1.0 | <0.001 | −1.4 | −1.9 to −0.8 | <0.001 | −1.6 | −2.2 to −1.0 | <0.001 | −1.5 | −2.1 to −0.9 | <0.001 | −1.4 | −2.0 to −0.9 | <0.001 | −1.5 | −2.1 to −0.9 | <0.001 | −0.7 | −1.1 to −0.4 | 0.08 | −0.6 | −1.0 to −0.3 | 0.03 |
| FVC z-score | ||||||||||||||||||||||||
| Term-born (ref) | −0.2 | −0.5 to 0.1 | − | 0.0 | −0.3 to 0.3 | − | 0.2 | −0.2 to 0.5 | − | −0.1 | −0.5 to 0.2 | − | −0.2 | −0.6 to 0.2 | − | 0.4 | 0.1 to 0.8 | − | −0.2 | −0.4 to 0.1 | − | 0.0 | −0.3 to 0.3 | − |
| EP no BPD | −0.9 | −1.3 to −0.6 | 0.003 | −0.4 | −0.7 to 0.0 | 0.11 | −0.3 | −0.7 to 0.0 | 0.05 | −0.5 | −1.0 to −0.1 | 0.17 | −0.4 | −0.9 to 0.0 | 0.41 | −0.1 | −0.5 to 0.4 | 0.07 | −0.1 | −0.5 to 0.3 | 0.67 | −0.1 | −0.5 to 0.3 | 0.66 |
| EP BPD | −1.1 | −1.6 to −0.5 | 0.01 | −0.6 | −1.2 to 0.0 | 0.07 | −0.9 | −1.5 to −0.3 | 0.002 | −0.7 | −1.2 to −0.1 | 0.15 | −0.2 | −0.8 to 0.4 | 0.95 | −0.1 | −0.7 to 0.5 | 0.12 | −0.2 | −0.5 to 0.2 | 0.96 | −0.3 | −0.7 to 0.1 | 0.20 |
| FEV1/FVC z-score | ||||||||||||||||||||||||
| Term-born (ref) | 0.4 | 0.1 to 0.6 | − | −0.2 | −0.4 to 0.1 | − | −0.2 | −0.5 to 0.1 | − | 0.1 | −0.2 to 0.4 | − | −0.1 | −0.5 to 0.2 | − | −0.5 | −0.9 to −0.2 | − | −0.3 | −0.6 to 0.0 | − | −0.2 | −0.5 to 0.0 | − |
| EP no BPD | −0.3 | −0.7 to 0.0 | 0.002 | −0.9 | −1.3 to −0.6 | <0.001 | −0.9 | −1.3 to −0.5 | 0.004 | 0.0 | −0.4 to 0.4 | 0.73 | −0.3 | −0.7 to 0.2 | 0.55 | −0.5 | −1.0 to −0.1 | 0.96 | −0.8 | −1.2 to −0.4 | 0.03 | −0.7 | −1.1 to −0.3 | 0.06 |
| EP BPD | −0.7 | −1.2 to −0.1 | 0.001 | −1.0 | −1.6 to −0.5 | 0.007 | −1.2 | −1.8 to −0.6 | 0.003 | −1.4 | −2.0 to −0.8 | <0.001 | −1.7 | −2.3 to −1.2 | <0.001 | −1.9 | −2.5 to −1.3 | <0.001 | −0.9 | −1.2 to −0.6 | 0.008 | −0.5 | −0.9 to −0.1 | 0.30 |
| FEF 25–75% z-score | ||||||||||||||||||||||||
| Term-born (ref) | 0.2 | −0.1 to 0.5 | − | −0.1 | −0.4 to 0.2 | − | −0.1 | −0.4 to 0.2 | − | −0.2 | −0.6 to 0.1 | − | −0.3 | −0.7 to 0.0 | − | −0.4 | −0.8 to −0.1 | − | −0.6 | −0.8 to −0.3 | − | −0.3 | −0.5 to 0.0 | − |
| EP no BPD | −1.0 | −1.3 to −0.7 | <0.001 | −1.2 | −1.6 to −0.9 | <0.001 | −1.1 | −1.5 to −0.8 | <0.001 | −0.8 | −1.2 to −0.4 | 0.05 | −0.6 | −1.0 to −0.2 | 0.32 | −0.7 | −1.1 to −0.2 | 0.35 | −1.0 | −1.3 to −0.6 | 0.06 | −0.8 | −1.2 to −0.4 | 0.02 |
| EP BPD | −1.5 | −2.0 to −0.9 | <0.001 | −1.5 | −2.0 to −0.9 | <0.001 | −1.6 | −2.2 to −1.1 | <0.001 | −1.9 | −2.5 to −1.4 | <0.001 | −1.9 | −2.5 to −1.4 | <0.001 | −2.1 | −2.7 to −1.5 | <0.001 | −1.2 | −1.6 to −0.9 | 0.003 | −0.9 | −1.3 to −0.5 | 0.007 |
All results are based on longitudinal mixed-effects models. The p values compare EP subjects with controls.
*Two of the eligible non-participants could not be classified according to BPD status, but are presented in the non-BPD group.
BPD, bronchopulmonary dysplasia (supplemental oxygen or respiratory support at postmenstrual age ≥36 weeks); EP, subjects born extremely preterm or with extremely low birth weight (gestational age ≤28 weeks or birth weight ≤1000 g); FEF25–75%, forced expiratory flow at 25%–75% of FVC; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ref, reference level.
Figure 2.
Estimated mean FEV1 z-scores with 95% CIs in three cohorts of extremely preterm-born (born at gestational age ≤28 weeks or birth weight ≤ 1000 g) with or without BPD, and individually matched controls. BPD, bronchopulmonary dysplasia; FEV1, forced expiratory volume in 1 s.
For extremely preterm-born, mean z-scores for FEV1, FEV1/FVC and FEF 25–75% were significantly below zero and below those of term-born at most assessments, with a few exceptions for the non-BPD group in the 91–92 and 99–00 cohorts (table 3). The deficits were largest for the BPD groups in the 82–85 and 91–92 cohorts. Within each of the extremely preterm-born cohorts, z-FEV1 remained stable from childhood to adulthood (likelihood ratio test comparing a full model to a model without age effect for extremely preterm-born, p=0.27, figure 2).
Significantly more extremely preterm-born than term-born had z-scores for FEV1, FEV1/FVC and FEF25-75% below LLN at minimum one visit (table 4). Notably, 44 (30%) extremely preterm-born compared with 7 (5%) term-born fulfilled the COPD spirometry criteria (p<0.001), with respectively 20 and 5 also reporting wheeze. The fraction below LLN for extremely preterm-born with vs without BPD were 24 (41%) vs 20 (22%), respectively.
Table 4.
Spirometry variables below the lower limit of normal at any time
| Cohort | 82–85 cohort | 91–92 cohort | 99–00 cohort | |||||||
| Z-FEV1 below LLN at any time, n (%), p value | Term-born (ref.) | 3 | (7) | – | 3 | (9) | – | 6 | (11) | – |
| EP no BPD | 10 | (28) | 0.01 | 4 | (17) | 0.31 | 7 | (23) | 0.13 | |
| EP BPD | 7 | (54) | <0.001 | 6 | (50) | 0.002 | 8 | (24) | 0.08 | |
| Z-FVC below LLN at any time, n (%), p value | Term-born (ref.) | 3 | (7) | – | 0 | (0) | – | 4 | (7) | – |
| EP no BPD | 8 | (22) | 0.04 | 2 | (9) | 0.08 | 1 | (3) | 0.46 | |
| EP BPD | 2 | (15) | 0.31 | 1 | (8) | 0.08 | 4 | (12) | 0.41 | |
| Z-FEV1/FVC below LLN at any time, n (%), p value | Term-born (ref.) | 1 | (2) | – | 5 | (14) | – | 1 | (2) | – |
| EP no BPD | 8 | (22) | 0.004 | 3 | (13) | 0.89 | 9 | (29) | <0.001 | |
| EP BPD | 5 | (38) | <0.001 | 9 | (75) | <0.001 | 10 | (30) | <0.001 | |
| Z-FEF25-75% below LLN at any time, n (%), p value | Term-born (ref.) | 2 | (4) | – | 6 | (17) | – | 6 | (11) | – |
| EP no BPD | 11 | (31) | 0.001 | 3 | (13) | 0.67 | 9 | (29) | 0.03 | |
| EP BPD | 7 | (54) | <0.001 | 9 | (75) | <0.001 | 12 | (36) | 0.003 | |
| Any variable below LLN at any time, n (%), p value | Term-born (ref.) | 5 | (11) | – | 7 | (20) | – | 8 | (14) | – |
| EP no BPD | 17 | (47) | <0.001 | 6 | (26) | 0.59 | 12 | (39) | 0.008 | |
| EP BPD | 9 | (69) | <0.001 | 10 | (83) | <0.001 | 15 | (45) | <0.001 | |
The presented data are the number (fraction) of subjects with at least one value ≤LLN at least one visit.
P value refers to comparisons between term-born and EP-born groups using Pearson’s χ2 test.
EP, subjects born extremely preterm or with extremely low birth weight (gestational age ≤28 weeks or birth weight ≤1000 g); FEF25–75%, forced expiratory flow at 25–75% of FVC; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LLN, Lower limit of normal (z-score ≤ −1.645); ref., reference level.
Lung function differences across three decades
Across the three birth cohorts, z-FEV1 for extremely preterm-born increased (likelihood ratio test comparing a model with a cohort effect for extremely preterm-born to one without a cohort effect, p=0.009), and approached the term-born in the last cohort. The improvements occurred in the subgroup without BPD from the 1980s and in the subgroup with BPD from the 1990s (figure 2). When exploring effects from neonatal variables, only birth cohort and BW z-score had a significant impact on z-FEV1 at 18 years (p=0.03 and 0.006, respectively), not maternal smoking, GA, BPD status, or the use of surfactant or antenatal steroids (online supplemental table E3). Current smoking was not associated with z-FEV1 at 18 years (p=0.15 and 0.92 for term and extremely preterm-born, respectively).
Development of FEV1 as percentages of peak expected values
FEV1 expressed as percentages of expected adult peak values revealed parallel trajectories for term- and extremely preterm-born in the 82–85 and 91–92 cohorts (global test for lack of parallelism for these two cohorts, p=0.33). In the 99–00 cohort, the trajectories diverged between the term- and extremely preterm-born (global test for lack of parallelism for all three cohorts, p=0.03), with term-born developing marginally better. The two extremely preterm-born cohorts who had reached 25 years of age did not achieve their expected peak lung function, mean FEV1% of expected adult peak (95% CI) were 99% (96% to 101%) for term-born; 89% (84% to 94%) and 86% (83% to 90%) for extremely preterm-born in the 91–92 and 82–85 cohorts, respectively (figure 3).
Figure 3.
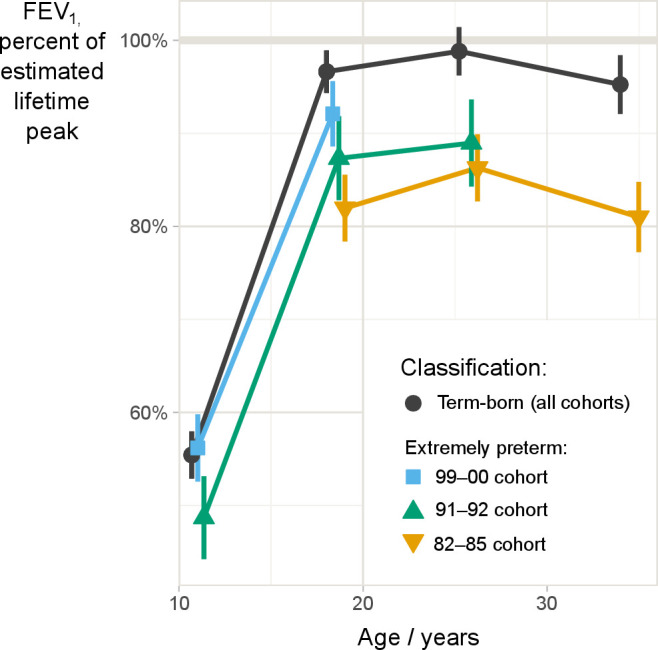
Mean measured FEV1 as fraction of expected peak FEV1 (with 95% CIs) in three population based cohorts born extremely preterm (born at gestational age ≤28 weeks or birth weight ≤1000 g) during three different decades (blue, green and yellow lines), and aggregated data for their individually matched term-born controls (black line), based on mixed-effects longitudinal model. Expected peak FEV1 was calculated for each individual using the GLI reference equation. FEV1: forced expiratory volume in 1 s; GLI, global lung function initiative.
From 25 to 35 years of age (82–85 cohort), FEV1 declined for both term-born and extremely preterm-born. Relative to their expected peak values, the decline did not differ significantly between the groups (from 98.8% to 95.2% in term-born and 86.3% to 81.0% in extremely preterm-born, mean absolute difference in decline 1.7%, 95% CI (−2.2% to 5.7%), p=0.4). FEV1% of expected peak values stratified by BPD status are presented in online supplemental figure E1.
thoraxjnl-2021-218400supp002.pdf (25.7KB, pdf)
Discussion
This is the first longitudinal population based study to report lung function trajectories from childhood to the mid-30s for extremely preterm-born cohorts, including also the incipient age-related physiological decline. FEV1 tracked in parallel, but significantly below the trajectories of term-born controls, including a parallel peak and onset of the age-related decline from 25 to 35 years. Lung function deficits were most pronounced for those born extremely preterm in the early 1980s, and successively smaller for each succeeding decade of birth. One in three extremely preterm-born participants met the spirometry criteria for COPD, although without prior administration of bronchodilator.
Development of lung function from childhood to 35 years of age
Population studies underline the importance of intrauterine and early life events for adult peak lung function,23 the starting point for physiological age-related decline. Even subtle reductions in adult FEV1 are associated with cardiovascular disease, poor respiratory health, COPD, and overall mortality.24 There is increasing awareness that COPD is a heterogeneous condition that originates early in life.25 Until now, lifelong lung function trajectories for extremely preterm-born have represented uncharted territory, as no longitudinal studies have presented data beyond 25 years of age. This study highlights that a disturbingly high proportion individuals born extremely preterm commence their journey towards old age with FEV1 levels significantly below normal, and that childhood and adolescent airway obstruction follow stable trajectories well into adulthood. This is in accordance with most longitudinal studies of younger extremely preterm-born cohorts,9 10 13 although an Australian study reported deterioration of lung function from 8 to 18 years, most prominent in the BPD subgroup.11 We observed an apparent decline in FEV1 z-score from 25 to 35 years in our 82–85 BPD subgroup (figure 2), but this was not significant, possibly due to small sample size.
Extremely preterm-born harbour numerous risk factors known to be implicated in the development of COPD, such as bronchial hyper-responsiveness and early life lung infections.23 25 As our spirometries were performed without prior bronchodilator and the questionnaires were not validated for assessing COPD symptoms, we could not classify COPD. Nevertheless, 30% of extremely preterm-born had FEV1/FVC below LLN, and 45% of these also reported wheeze in the ISAAC questionnaire. It was encouraging that the onset of the physiological lung function decline from 25 to 35 years did not differ between extremely preterm-born and term-born, suggesting that further age-related decline may not be as steep as reported for other high-risk groups.5
Cohort effects associated with improved perinatal care
Neonatal intensive care improved substantially during the recruitment period, with more extensive use of prenatal steroids, introduction of surfactant, better respiratory care, and generally improved perinatal management.1 Improvements in care may have contributed to increased survival for the most immature, but the long-term effects on morbidity are less obvious,26 27 since a greater number of immature survivors conceivably could lead to poorer outcomes. The scarce literature on these matters is inconsistent. Doyle et al assessed three cohorts at 8 years of age born extremely preterm during 1991–2005, and found the poorest airflow patterns in the most recent cohort.28 They argued this could be due to decreased use of postnatal corticosteroids or increased use of CPAP. We also observed increasing use of CPAP with birth cohort, however, no decrease in the use of postnatal steroids, which may have contributed to the differing results. Contrasting Doyle et al and in line with our findings, a meta-analysis addressing the effect of preterm birth on FEV1 % suggested improvements with year of birth from 1980 to 1995.29 One may even read a dose–response effect into our data-set, as improvements from the 1980s primarily occurred in those without BPD, presumably with the least traumatic neonatal history, whereas improvement from the 1990s also encompassed the BPD group (figure 2).
Prospects for the future
Advanced NICUs have facilitated survival in high numbers of immature infants for more than 30 years, paralleled by continuously refined treatment tools.2 In our data set, these developments were paralleled by an apparent diminishing long-term significance of BPD. Instead, antenatal factors became more evident, reflected by negative effects from low BW z-scores, findings also reported in a recent Finnish study.30 Preterm birth happens for a reason, and the pathology that leads to early deliveries are likely implicated in the causal pathways that lead to subsequent pulmonary disease—irrespective of optimal postnatal care.
Huge efforts and resources are invested in acute perinatal care of extremely preterm-born compared with the resources spent on the long-term respiratory challenges that face adult NICU-graduates, with little evidence supporting that therapeutic interventions improve the pulmonary prognosis of BPD after discharge.31 Notably, premature birth is not listed as a risk factor for COPD in authoritative statements, and data suggest that few pulmonologists inquire about early life factors.32 33 Almost one in three extremely preterm-born participants met the post-bronchodilator spirometry criteria for COPD, and their upcoming age-related lung function decline will inevitably be a concern for adult pulmonologists. Thus, COPD after preterm birth appears to be a neglected condition, reflected even by the taxonomy; we still use the neonatal term BPD to label their adult lung disease—decades after their NICU discharge.
Strengths and limitations
Strengths of this study were enrolment of three population-based cohorts born in three different decades, the longitudinal approach, relatively high follow-up rates, and free access to healthcare for all children in the recruitment area, reducing the risk of socioeconomic bias. Similar growth patterns drawn from multiple visits of three cohorts born in eras with different treatment opportunities, support the notion that lung function trajectories in extremely preterm-born parallel those of term-born. The same and unselected control groups throughout the study period minimised risks of selection bias. The senior NICU staff and senior members of the research group were largely the same throughout the study periods.
The main limitation was a relatively low number of participants; however, the population-based design ensured unbiased inclusion. The initial power analysis did not account for stratifying data by BPD or comparisons across cohorts, making subgroup analyses vulnerable to type II errors. GA was assessed by the last menstrual period during 1982–85 and by early ultrasound thereafter. We have previously shown that the different methods probably did not introduce significant bias.34 Our extremely preterm-born cohorts were relatively mature at birth when compared with participants of other recent studies, such as the EPICure.10 This was probably related to our inclusion criteria, also allowing participation of individuals with BW at or below 1000 g irrespective of GA, resulting in inclusion of more mature SGA infants. Thus, our outcome data are not directly generalisable to all extremely preterm-born cohorts. Categorising the long-term lung disease of preterms by their neonatal BPD status is debated. The definitions of BPD have varied historically and between institutions, with no specific information on anatomy, mechanics, inflammation or pathology in the respiratory organs.35 Due to the longitudinal design, we opted to stick with the ISAAC questionnaire throughout the study, although it is validated for children, not adults.18 The 99–00 cohort were asked to respond to the questionnaires electronically at the last study-visit, unfortunately with lower response rates. Participation rates decreased over time and with more recent birth cohorts, but attrition did not exceed similar follow-up studies.36 A higher rate of maternal smoking during pregnancy for those lost to follow-up in the 99–00 cohort may suggest that average outcomes were somewhat better in the participants. The spirometry results of the control groups largely confirmed that the GLI equations fitted our population, except the next-to-last visit for the youngest cohort where 95% CI for mean z-FEV1 did not include zero. We have no clear explanation for this finding. This finding was most likely not attributable to the upgrading of spirometry equipment, as it did not apply to all control-cohorts. We therefore consider the risk of bias due to upgrading of equipment to be small, as laboratory standards were otherwise unchanged during the complete course of the study, and as spirometry is a robust technique.
Conclusion
This is the first longitudinal study to describe lung function trajectories from childhood and well into adulthood for population-based cohorts of extremely preterm-born survivors. Spirometry variables tracked in parallel, but significantly below the trajectories of matched term-born controls, including a parallel peak and onset of the age-related decline from 25 to 35 years. On average, lung function deficits were most pronounced for participants born extremely preterm in the early 1980s, and successively smaller for each decade of birth. Irrespective of birth decade, almost one in three had spirometry values that met the spirometry criteria for COPD.
Acknowledgments
We would like to thank all participants and their parents who patiently participated in this project over three decades, several travelling from far away. This would not have been possible without you.
Footnotes
Contributors: TB drafted the manuscript, prepared figure 1 and contributed to data collection, quality control, figure preparation and statistical analysis. ODR was responsible for quality control and contributed to data collection. KOH performed the statistical analysis, wrote the statistical methods section and prepared the figures 2 and 3. MRB, HHC, IBM and KØ contributed to data collection. TM contributed to the design of the study, data collection, and quality control. MV and TH had intellectual oversight of the project, designed the study and contributed to manuscript drafting, data collection and quality control. All authors contributed to the writing and critical review of the manuscript, and approved the final version. MV is responsible for the overall content as the guarantor.
Funding: Western Norway Regional Health Authority.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by reference number: REK nr. 2017-00628. For each study visit, approval was obtained from the Regional Committee for Research Ethics in Western Norway, and signed informed consents from all participants and/or guardians. Participants gave informed consent to participate in the study before taking part.
References
- 1. Owen LS, Manley BJ, Davis PG, et al. The evolution of modern respiratory care for preterm infants. Lancet 2017;389:1649–59. 10.1016/S0140-6736(17)30312-4 [DOI] [PubMed] [Google Scholar]
- 2. Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 2015;314:1039–51. 10.1001/jama.2015.10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chawanpaiboon S, Vogel JP, Moller A-B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7:e37–46. 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Risnes K, Bilsteen JF, Brown P, et al. Mortality among young adults born preterm and early term in 4 Nordic nations. JAMA Netw Open 2021;4:e2032779. 10.1001/jamanetworkopen.2020.32779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med 2019;7:358–64. 10.1016/S2213-2600(18)30529-0 [DOI] [PubMed] [Google Scholar]
- 6. Bush A. Lung development and aging. Ann Am Thorac Soc 2016;13 Suppl 5:S438–46. 10.1513/AnnalsATS.201602-112AW [DOI] [PubMed] [Google Scholar]
- 7. Jakeways N, McKeever T, Lewis SA, et al. Relationship between FEV1 reduction and respiratory symptoms in the general population. Eur Respir J 2003;21:658–63. 10.1183/09031936.03.00069603 [DOI] [PubMed] [Google Scholar]
- 8. Doyle LW, Andersson S, Bush A, et al. Expiratory airflow in late adolescence and early adulthood in individuals born very preterm or with very low birthweight compared with controls born at term or with normal birthweight: a meta-analysis of individual participant data. Lancet Respir Med 2019;7:677–86. 10.1016/S2213-2600(18)30530-7 [DOI] [PubMed] [Google Scholar]
- 9. Gibson A-M, Reddington C, McBride L, et al. Lung function in adult survivors of very low birth weight, with and without bronchopulmonary dysplasia. Pediatr Pulmonol 2015;50:987–94. 10.1002/ppul.23093 [DOI] [PubMed] [Google Scholar]
- 10. Hurst JR, Beckmann J, Ni Y, et al. Respiratory and cardiovascular outcomes in survivors of extremely preterm birth at 19 years. Am J Respir Crit Care Med 2020;202:422–32. 10.1164/rccm.202001-0016OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doyle LW, Adams A-M, Robertson C, et al. Increasing airway obstruction from 8 to 18 years in extremely preterm/low-birthweight survivors born in the surfactant era. Thorax 2017;72:712–9. 10.1136/thoraxjnl-2016-208524 [DOI] [PubMed] [Google Scholar]
- 12. Vollsæter M, Røksund OD, Eide GE, et al. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax 2013;68:767–76. 10.1136/thoraxjnl-2012-202980 [DOI] [PubMed] [Google Scholar]
- 13. Moschino L, Stocchero M, Filippone M, et al. Longitudinal assessment of lung function in survivors of bronchopulmonary dysplasia from birth to adulthood. The Padova BPD study. Am J Respir Crit Care Med 2018;198:134–7. 10.1164/rccm.201712-2599LE [DOI] [PubMed] [Google Scholar]
- 14. Bolton CE, Bush A, Hurst JR, et al. Lung consequences in adults born prematurely. Thorax 2015;70:574–80. 10.1136/thoraxjnl-2014-206590 [DOI] [PubMed] [Google Scholar]
- 15. Vollsæter M, Skromme K, Satrell E, et al. Children born preterm at the turn of the millennium had better lung function than children born similarly preterm in the early 1990s. PLoS One 2015;10:e0144243. 10.1371/journal.pone.0144243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Halvorsen T, Skadberg BT, Eide GE, et al. Better care of immature infants; has it influenced long-term pulmonary outcome? Acta Paediatr 2006;95:547–54. 10.1080/08035250500477529 [DOI] [PubMed] [Google Scholar]
- 17. Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand 2000;79:440–9. [PubMed] [Google Scholar]
- 18. Asher MI, Keil U, Anderson HR, et al. International study of asthma and allergies in childhood (Isaac): rationale and methods. Eur Respir J 1995;8:483–91. 10.1183/09031936.95.08030483 [DOI] [PubMed] [Google Scholar]
- 19. Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American thoracic Society and European respiratory Society technical statement. Am J Respir Crit Care Med 2019;200:e70–88. 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-Ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324–43. 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mallinckrodt CH, Clark WS, David SR. Accounting for dropout bias using mixed-effects models. J Biopharm Stat 2001;11:9–21. 10.1081/BIP-100104194 [DOI] [PubMed] [Google Scholar]
- 22. Early versus delayed neonatal administration of a synthetic surfactant--the judgment of OSIRIS. The OSIRIS Collaborative Group (open study of infants at high risk of or with respiratory insufficiency--the role of surfactant. Lancet 1992;340:1363–9. [PubMed] [Google Scholar]
- 23. Svanes C, Sunyer J, Plana E, et al. Early life origins of chronic obstructive pulmonary disease. Thorax 2010;65:14–20. 10.1136/thx.2008.112136 [DOI] [PubMed] [Google Scholar]
- 24. Agustí A, Noell G, Brugada J, et al. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med 2017;5:935–45. 10.1016/S2213-2600(17)30434-4 [DOI] [PubMed] [Google Scholar]
- 25. Bui DS, Lodge CJ, Burgess JA, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med 2018;6:535–44. 10.1016/S2213-2600(18)30100-0 [DOI] [PubMed] [Google Scholar]
- 26. Cheong JL, Spittle AJ, Burnett AC, et al. Have outcomes following extremely preterm birth improved over time? Semin Fetal Neonatal Med 2020;25:101114. 10.1016/j.siny.2020.101114 [DOI] [PubMed] [Google Scholar]
- 27. Marlow N, Ni Y, Lancaster R, et al. No change in neurodevelopment at 11 years after extremely preterm birth. Arch Dis Child Fetal Neonatal Ed 2021;106:418–24. 10.1136/archdischild-2020-320650 [DOI] [PubMed] [Google Scholar]
- 28. Doyle LW, Carse E, Adams A-M, et al. Ventilation in extremely preterm infants and respiratory function at 8 years. N Engl J Med 2017;377:329–37. 10.1056/NEJMoa1700827 [DOI] [PubMed] [Google Scholar]
- 29. Kotecha SJ, Edwards MO, Watkins WJ, et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax 2013;68:760–6. 10.1136/thoraxjnl-2012-203079 [DOI] [PubMed] [Google Scholar]
- 30. Ronkainen E, Dunder T, Kaukola T, et al. Intrauterine growth restriction predicts lower lung function at school age in children born very preterm. Arch Dis Child Fetal Neonatal Ed 2016;101:F412–7. 10.1136/archdischild-2015-308922 [DOI] [PubMed] [Google Scholar]
- 31. Duijts L. ERS TF BPD (TF-2015-18); European respiratory Society tas force on bronchopulmonary dysplasia. Society ER, 2020. [Google Scholar]
- 32. GOLD . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2020 report published in Fontana, WI, USA, 2020. Available: wwwgoldcopdorg
- 33. Bolton CE, Bush A, Hurst JR, et al. Are early life factors considered when managing respiratory disease? A British thoracic Society survey of current practice. Thorax 2012;67:1110. 10.1136/thoraxjnl-2012-202637 [DOI] [PubMed] [Google Scholar]
- 34. Markestad T, Kaaresen PI, Rønnestad A, et al. Early death, morbidity, and need of treatment among extremely premature infants. Pediatrics 2005;115:1289–98. 10.1542/peds.2004-1482 [DOI] [PubMed] [Google Scholar]
- 35. Jobe AH, Steinhorn R. Can we define bronchopulmonary dysplasia? J Pediatr 2017;188:19–23. 10.1016/j.jpeds.2017.06.064 [DOI] [PubMed] [Google Scholar]
- 36. MacBean V, Drysdale SB, Zivanovic S, et al. Participant retention in follow-up studies of prematurely born children. BMC Public Health 2019;19:1233. 10.1186/s12889-019-7575-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2021-218400supp001.pdf (168.7KB, pdf)
thoraxjnl-2021-218400supp002.pdf (25.7KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.