Abstract
Introduction
Varicella zoster virus (VZV) reactivation has been reported following vaccination for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), but the real extent remains unknown.
Methods
We conducted a systematic review to summarize evidence of VZV reactivation or infection following SARS-CoV-2 vaccination. Episodes after coronavirus disease-2019 (COVID-19) were also identified. Related articles were identified in PubMed and EMBASE databases till December 31, 2021 using the terms “varicella zoster” and “COVID-19″. PROSPERO Register Number: CRD42021289399.
Results
The search revealed 314 articles, of which 55 met the inclusion criteria. VZV manifestations were documented in 179 (82.1%) subjects following SARS-CoV-2 vaccination and in 39 (17.9%) patients with COVID-19. Among the vaccinated, median (IQR) age was 56.5 (42–70) years, and 56.8% were female. Twenty-one (16.8%) were immunosuppressed. The median (IQR) latency time after vaccination was 6 (3–10) days, and 84.4% received mRNA vaccines. VZV reactivation occurred following a first dose (68.2%), a second dose (12.8%) or a booster (0.6%). The most important VZV manifestation was dermatome herpes zoster rash, which accounted for 86.4% of events in vaccinated subjects. Twenty patients (11.3%) presented serious VZV events after vaccination, with Herpes Zoster ophthalmicus (5.6%) and post-herpetic neuralgia (3.4%) predominating. No VZV pneumonia or deaths were recorded. Antiviral prescriptions were made in 96.2% of vaccinated subjects. No significant differences between vaccinated and infected subjects were found.
Conclusion
This study indicates that the occurrence of VZV reactivation is clinically relevant. However, our findings suggest that COVID-19 vaccination is safe, and remains strongly recommended.
Keywords: Covid-19, Herpes virus, Herpes zoster rash, Bnt162b2, Mrna-1273, Adverse events
1. Introduction
Coronavirus disease-2019 (COVID-19) is caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The systemic manifestations of COVID-19 comprise respiratory, gastrointestinal, neurological and endocrine symptoms, cardiovascular and hematological manifestations, and septic shock [2]. This disease has been associated with numerous cutaneous manifestations [3], ranging from vesicular and chilblain-like lesions to urticaria and maculopapular eruptions. Recent case reports have documented cutaneous lesions attributable to varicella zoster virus (VZV) in patients with COVID-19, or shortly after SARS-CoV-2 vaccination [4]. It would be interesting to establish whether there is a causal relationship between these events or whether it is just a coincidence.
The main objective of this systematic literature review was to summarize the evidence concerning clinical VZV reactivation following SARS-CoV-2 vaccination. A secondary objective was to record the timing and distribution of VZV reactivation, and to identify serious events among SARS-CoV-2 vaccinated subjects compared with those with COVID-19 acute infection.
2. Material and methods
2.1. Registration and protocol
This study was performed following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [5]. The protocol was pre-registered on PROSPERO (CRD42021289399). PRISMA checklist is reported in Supplemental Table 1.
2.2. Data sources
The search was performed in the PubMed and EMBASE databases using the terms “varicella zoster” and “COVID-19″. Articles published from January 1 2020, to December 31 2021, (including Ahead of Print publication studies) were reviewed. Only studies related to humans were considered for inclusion. No language restrictions were applied. Preprint servers such as medRxiv were not scanned.
Reasons for exclusion were the following: (a) reviews and conference abstracts that did not present original data; (b) unpublished studies; (c) surveillances or registers; (d) evidence of poor quality or low relevance; (e) reports associated with Epstein-Barr virus, cytomegalovirus or other herpes viruses.
2.3. Selection criteria
The articles included were observational studies, case series, case reports, and letters. Subjects (any age) who reported VZV manifestations after (i) SARS-CoV-2 vaccination or (ii) COVID-19 disease were eligible for inclusion. Outcomes assessed were: latency time (days), VZV manifestations (frequency and related complications), and antiviral treatment.
2.3.1. Definitions
VZV reactivation was defined as the appearance of herpes zoster symptoms (a painful, unilateral vesicular eruption, which usually occurs in a restricted dermatome distribution) after a confirmed diagnosis of COVID-19 or after the administration of the SARS-CoV-2 vaccine. Status of the COVID-19 infection was considered as active when the subjects had symptoms confirmed by laboratory confirmation, and recovered when the symptoms abated. Severity of illness was defined as mild when the patient could remain at home and severe when hospital admission was required due to the infection. Immune status was considered as immunocompetent when subjects neither had an autoimmune disease nor had received immunosuppressive treatment during the past year.
2.4. Data extraction and study selection process
Two authors (GARA, FRP) independently searched and analysed all the articles. All articles identified were retrieved by reading and assessing their titles, abstracts and full-texts. A third reviewer (CC) was consulted in order to resolve any differences.
The articles were classified according to whether the subjects enrolled were infected or vaccinated. For the SARS-CoV-2 vaccinated subjects, the data extracted consisted of: subject's number, sex and age, type of vaccination, latency, VZV manifestations, and antiviral treatment administered. For the COVID-19 infected subjects, the data extracted consisted of: subject's number, sex and age, SARS-CoV-2 infection status, severity of illness, latency, VZV manifestations, and antiviral treatment administered. When the diagnosis of herpes zoster was not confirmed by laboratory tests, the cases were evaluated by an expert author (MCM).
2.5. Quality assessment
Two investigators (RMR and MCM) independently assessed the risk of bias in the studies included. Any disagreement regarding quality assessment was resolved by a third author (ST).
The risk of bias was evaluated using the Newcastle Ottawa scale [6] for cohort and case-control studies, and an adapted form of the Newcastle Ottawa cohort scale for cross-sectional studies [7].
The tool proposed by Murad et al. [8] was used to assess the quality of case reports and case series. The scale combines criteria previously described by Pierson [9], Bradford Hills [10], and the modified Newcastle-Ottawa scale [6].
2.6. Statistical analysis
Continuous variables were described as medians and their interquartile ranges (IQR), while categorical variables were presented as counts and percentages. Heterogeneity was investigated by ordering tables, displaying SARS-CoV-2 vaccination and COVID-19 disease characteristics and outcomes separately. When relevant information was not reported, the authors were contacted and the missing data requested.
2.7. Statement of ethics compliance
Ethics committee approval was not required since the article was based on previously conducted studies and did not contain any new studies with human participants or animals.
3. Results
3.1. Study selection
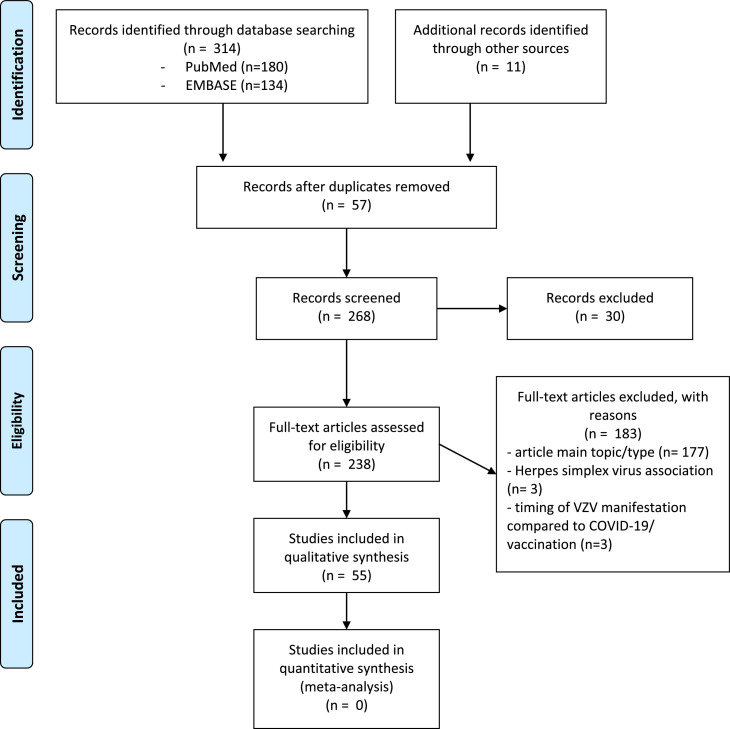
A total of 314 studies were identified. After applying the inclusion and exclusion criteria, 55 studies [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65] of VZV manifestations in subjects with SARS-CoV-2 vaccinations or COVID-19 disease were deemed eligible for inclusion. A flow diagram of the selection of the studies is presented in Fig. 1 .
Fig. 1.
PRISMA flow diagram of the selection of studies.
3.2. Study and population characteristics
Twenty-three case reports, 22 letters, seven case series, and three observational studies met the eligibility criteria. Thirty-three studies were conducted in subjects vaccinated against SARS-CoV-2 and 22 in subjects with COVID-19 disease.
A total of 218 subjects were included. The median (IQR) age was 58 (42–70) years. Sex was reported in 203 participants, 114 (56.1%) of whom were female. Study and population characteristics are detailed in Table 1 and Supplemental Table 2.
Table 1.
Characteristics of subjects with varicella zoster reactivation after SARS-CoV-2 vaccine (33 studies) or COVID-19 disease (22 studies).
| SARS-CoV-2 vaccine | COVID-19 disease | |
|---|---|---|
| Number of patients | 179* | 39 |
| Age, median (interquartile range) | 56.5 (42–70) | 62 (43–70) |
| Sex, n (%) | 169 | 34 |
| Women | 96 (56.8) | 18 (52.9) |
| Men | 73 (43.2) | 16 (47.1) |
| Immune status, n (%) | 125 | 29 |
| Immunocompetent | 104 (83.2) | 26 (89.6) |
| Immunocompromised | 21 (16.8) | 3 (10.4) |
| Vaccination type, n (%) | 179 | 0 |
| mRNA | 151 (84.4) | – |
| Viral vector | 23 (12.8) | – |
| Inactivated virus vaccine | 5 (2.8) | – |
| Doses, n (%) | 173 | 0 |
| 1st dose | 118 (68.2) | – |
| 2nd dose | 54 (31.2) | – |
| booster | 1 (0.6) | – |
| COVID-19 status & severity, n (%) | 0 | 38 |
| Active | – | 30 (78.9) |
| mild | – | 13 (34.2) |
| severe | – | 17 (44.7) |
| Recovered | – | 8 (21.1) |
| mild | – | 5 (13.1) |
| severe | – | 3 (7.9) |
| COVID-19 treatment prescriptionsa, n (%) | 0 | 32 |
| Hydroxycloroquine | – | 10 (31.3) |
| Azithromycin | – | 4 (12.5) |
| Oseltamivir | – | 2 (6.2) |
| Other | – | 16 (50) |
Six vaccinated subjects also had previous COVID-19 disease.
COVID-19 treatment prescriptions reported in COVID-19 disease group (32 prescriptions in 16 subjects).
3.2.1. Severe acute respiratory syndrome coronavirus 2 vaccine
A total of 179 subjects were identified (Table 1). The median (IQR) age was 56.5 (42–70) years and 96 (56.8%) were female. Twenty-one (16.8%) subjects were immunocompromised. Six subjects [20,29,47,55] reported a history of COVID-19 disease prior to SARS-CoV-2 vaccination. Regarding the type of vaccines associated with VZV reactivation, the manifestations occurred after mRNA vaccines in 151 (84.4%) subjects, after adenovirus vector vaccines in 23 (12.8%), and after inactivated virus vaccine in five (2.8%). VZV reactivation occurred following the first dose in 68.2% of cases, the second dose in 31.2%, and the booster in 0.6%.
A total of 157 uncomplicated VZV manifestations were reported, with dermatome herpes zoster rash (86.4%) the most common. Disseminated rash was observed in fewer than 5% of subjects. Among the 20 severe VZV manifestations, Herpes Zoster ophthalmicus was the most frequent, representing 5.6% of events. Post-herpetic neuralgia was described in six vaccinated subjects, accounting for 3.4% of manifestations. The median (IQR) latency time associated with VZV reactivation was 6 days (3–10). Antiviral prescriptions were made in 151 subjects, in most cases valacyclovir or acyclovir. Outcomes are detailed in Table 2 .
Table 2.
Outcomes of subjects with varicella zoster reactivation after SARS-CoV-2 vaccine (33 studies) or COVID-19 disease (22 studies).
| SARS-CoV-2 vaccine (n = 179*) | COVID-19 disease (n = 39) | |
|---|---|---|
| Latency, median (interquartile range) | 6 (3–10) | 7 (5–20) |
| Varicella zoster virus manifestationsa, n (%) | 177 | 41 |
| Uncomplicated infections, events | 157 (88.7) | 31 (75.6) |
| Dermatomal herpes zoster rash | 153 (86.4) | 28 (68.3) |
| Disseminated rash | 4 (2.3) | 3 (7.3) |
| Serious infections, events | 20 (11.3) | 10 (24.4) |
| Herpes Zoster ophthalmicus | 10 (5.6) | 7 (17.1) |
| Post-herpetic neuralgia | 6 (3.4) | 1 (2.4) |
| Encephalitis-meningitis | 1 (0.5) | 1 (2.4) |
| Vasculitis | 1 (0.5) | 1 (2.4) |
| Acute retinal necrosis | 1 (0.5) | 0 |
| Ramsay-Hunt Syndrome | 1 (0.5) | 0 |
| Pneumonia | 0 | 0 |
| VZV treatment, n (%) | ||
| Pharmacological treatment, patients | 157 | 34 |
| Received treatment | 151 (96.2) | 30 (88.2) |
| No treatment | 6 (3.8) | 4 (11.8) |
| Antiviral treatment, prescriptionsb | 160 | 31 |
| Acyclovir | 20 (12.5) | 19 (61.3) |
| Valacyclovir | 51 (31.9) | 11 (35.5) |
| Famciclovir | 1 (0.6) | 1 (3.2) |
| Ganciclovir | 1 (0.6) | 0 |
| Brivudine | 1 (0.6) | 0 |
| NR antiviral | 86 (53.8) | 0 |
Six vaccinated subjects also had previous COVID-19 disease.
Manifestations reported in SARS-CoV-2 vaccination group (177 events in 175 subjects) and COVID-19 disease group (41 events in 39 subjects).
Antiviral prescriptions reported in SARS-CoV-2 vaccination group (160 prescriptions in 151 subjects) and COVID-19 disease group (31 prescriptions in 30 subjects).
3.2.2. Coronavirus disease 2019
Thirty-nine subjects were identified (Table 1). The median (IQR) age was 62 (43–70) years, and 18 (52.9%) subjects were female. Three (10.4%) subjects were immunocompromised. Thirty (78.9%) had active SARS-CoV-2 infection, while eight (21.1%) had already recovered. Eighteen (47.3%) outpatients had a mild disease course and 20 (52.6%) subjects were hospitalized due to severe COVID-19 symptomatology. Hydroxychloroquine was the most frequent pharmacological prescription (31.3%). One patient was treated with tocilizumab and one with dexamethasone.
Thirty-one uncomplicated VZV manifestations were reported, the most common being dermatome herpes zoster rash (68.3%). Among the 10 severe VZV infections, Herpes Zoster ophthalmicus was the most frequently reported complication (17.1%). Post-herpetic neuralgia, encephalitis-meningitis and vasculitis manifestations were also described in one subject each. The median (IQR) latency time associated with VZV reactivation was 7 days (5–20). Antiviral prescriptions were made in 30 subjects, the most frequently used being acyclovir. Outcomes are detailed in Table 2.
3.3. Quality assessment
Two observational studies were assessed by the Newcastle Ottawa Scale (Supplemental Table 3A), with a moderate quality of evidence. This scale was adapted from the Newcastle-Ottawa Quality Assessment Scale for cohort studies to assess the quality of cross-sectional studies for the systematic review. One cross-sectional study presented satisfactory quality (Supplemental Table 3B).
Twenty-three case reports, 22 letters, and seven case series were assessed by the Murad et al. study (Supplemental Table 3C). The quality of evidence was high in seven studies, moderate in 40 and low in only five, mainly due to selection and reporting issues. Causality Q5 was not applicable in this systematic review, due to the lack of a dose–response effect to assess.
4. Discussion
This study summarizes the available evidence on VZV reactivation following the administration of SARS-CoV-2 vaccines or after COVID-19 infection. Supplemental Table 4 summarizes the differences between our current study and the five previous literature reviews [66], [67], [68] assessing the potential association between SARS-CoV-2 and VZV reactivation. In our study, nearly 90% of reactivations were non-serious, among both SARS-CoV-2-vaccinated and infected subjects. The majority of the VZV manifestations were VZV skin rashes with dermatome distributions. Most manifestations appeared within ten days of the first dose inoculation or infection onset. The majority of subjects received antiviral therapy. Herpes Zoster ophthalmicus was described as the most common serious adverse event, followed by post-herpetic neuralgia, which accounted for two-thirds of the serious events among vaccinated subjects. Pneumonia and meningoencephalitis were extremely rare, and all reported patients survived. Nevertheless, the authors agreed that the benefits of vaccination against SARS-CoV-2 far outweigh the chance of developing these symptoms, which are not life-threatening and can be successfully addressed with the proper antiviral regimen.
The exact mechanism that causes VZV reactivation is not clear [69]. It appears that a defective cellular immunity may be involved [70]. Interestingly, tissue-resident memory T cells and satellite glial cells can secrete interferon and regulate VZV reactivation. Emerging evidence shows that it may be induced by the upregulation of the phosphatidylinositol-3 kinase-Akt pathway and the mitogen-activated protein kinase pathway [71]. The immune response induced by respiratory viruses is complex and it may predispose to bacterial or viral superinfections [72]. Moreover, COVID-19 depletes the immune system [73,74] and makes it susceptible to attack by other viruses, which may explain the reactivation of VZV. Yu et al. [75] suggested that patients with COVID-19 may have an increased risk of developing shingles due to their high levels of Th17 cells and interleukin-17. Moreover, the association between the infection by the novel coronavirus and VZV reactivation can be attributed to the impaired cellular immunity that characterizes COVID-19. Indeed, the age-related and immunosuppressant-related declines in cell-mediated defenses are established risk factors for herpes zoster [76]. Interestingly, Levinson et al. [77] proposed that mitochondrial haplogroups, which coordinate T-cell functionality, are linked to the risk of VZV reactivation. Haplogroup clade IWX is associated with a high risk of VZV reactivation in individuals of European ancestry. The mechanism that underpins VZV reactivation after SARS-CoV-2 vaccination remains obscure, since vaccination is supposed to strengthen the immune response rather than weaken it. The study by Rodríguez-Jiménez et al. [53] hypothesized that SARS-CoV-2 vaccines may induce some type of immunomodulatory process that leads to the reactivation of VZV, because this phenomenon has not been reported after other vaccinations. A retrospective single-center study showed that SARS-CoV-2 vaccination might indeed trigger VZV manifestations in rare instances [78]. Further studies investigating the mechanism underlying the presumptive causative relationship between COVID-19/SARS-CoV-2 vaccination and VZV reactivation are now warranted.
The current study does not examine the incidence of VZV reactivation after SARS-CoV-2 vaccination or infection in the real world. Several surveillance and register studies have been conducted to aid decision-making regarding risks and benefits and to estimate the prevalence of this adverse phenomenon [79], [80], [81], [82]. A post-surveillance study [79] conducted during research into the Pfizer BNT162b2 vaccine, reported no evidence of a significant increase in herpes zoster risk (relative risk 1.07; 95%CI 0.85–1.35). In contrast, analysing observational data, Barda et al. [80] recorded an elevated risk (risk ratio 1.43, 95%CI 1.20–1.73) of VZV reactivation among BNT162b2 mRNA vaccine recipients. The analyses of the Vaccine Adverse Event Reporting System (VAERS) database [81] investigated the occurrence of herpes virus reactivation in the US after COVID-19 vaccines using a nested case-control study. In this database 5934 cases of VZV reactivation were recorded among adverse events, and a slightly higher risk of VZV (Reporting Odds Ratio: 1.49) infection following the Pfizer BioNTech vaccine was identified, with estimated incidence of 0.7/100,000 doses. In the US, the VAERS database [81] reported the most common adverse event alongside VZV reactivation to be “rash” (26.1%), and only 4.1% of cases were serious. Wan et al. [82] conducted a self-controlled search of case series for herpes zoster-related hospitalization after mRNA (BNT162b2, BioNTech/Fosun Pharma) and inactivated virus (CoronaVac, Sinovac) SARS-CoV-2 vaccines, reporting an incidence of hospitalization herpes zoster for 7.9 per 1000,000 doses of BNT162b2 administered and 7.1 per 1000,000 doses of CoronaVac.
Our study has some limitations. The first was publication bias; it is reasonable to assume that some cases of VZV reactivation may have not been published because they were not exceptional. In contrast, the incidence of more severe complications like Herpes Zoster ophthalmicus may be over-rated. Therefore, accurate data on the real occurrence of reactivation cannot be derived from this type of study. The second limitation was the incompleteness of the data provided by some reports, which influenced the results of this systematic review. The third limitation was that our study did not explore any biological causes for the relationship between SARS-CoV-2 and VZV reactivation which ranges widely in terms of the interval between the two events (3–10 days [vaccine], 5–20 days [disease]). Whether differences existed between COVID-19 disease and anti-SARS-CoV-2 vaccinations remains unknown. Lastly, the lack of a simultaneous control group means that the evidence of association between SARS-CoV-2 infection/vaccination is poor and causality cannot be inferred. However, no articles related to influenza H1N1pdm2009 infection or vaccinations were documented in a literature search carried out prior to COVID-19 onset. Like other observational studies of vaccine safety, a potential selection bias may be inferred because of the differences in safety outcomes between subjects who accept vaccination and those who do not. Moreover, the risk of herpes zoster is highly dependent on comorbidities and age, factors that give priority for vaccination.
5. Conclusion
This systematic review should be viewed as a real-world example generating a signal of interest for the first step identification of rare adverse events. Our findings suggest that subjects with SARS-CoV-2 vaccine or infection are exposed to a clinically relevant risk of varicella herpes zoster reactivation. VZV reactivation often presents as a self-limited localized dermatome rash within ten days of the inoculation of the first dose or infection. Most patients received antivirals and serious events were rare, with post-herpetic neuralgia affecting fewer than 5% of cases. Further analytical post-surveillance studies should be conducted to discern the potential association with vaccination. Our findings support the notion that COVID-19 vaccination is safe, and remains strongly recommended.
Funding source
This work was funded by CIBERES, Instituto de Salud Carlos III, Madrid, Spain (Fondos FEDER) (CB06–06–036).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
Raquel Martinez-Reviejo: Methodology, Formal analysis, Writing-original draft,Writing- Review & Editing. Sofia Tejada: Methodology, Validation, Formal analysis, Writing-original draft, Writing- Review & Editing. Ganiyat AR. Adebanjo: Methodology,Writing-original draft, Writing Review & Editing. Camilla Chello: Validation, Writing - Review & Editing. Miriam C. Machado: Methodology, Writing - Review & Editing. Francesca R. Parisella: Methodology, Writing - Review & Editing. Magda Campins: Writing - Review & Editing. Antonella Tammaro: Conceptualization, Writing - Review & Editing. Jordi Rello: Conceptualization, Writing - Review & Editing, Supervision. All authors read and approved the final manuscript
Disclosures
Jordi Rello served as consultant for Pfizer and ROCHE Advisory Boards. Magda Campins served as consultant for GSK, Sanofi, Pfizer, Novartis, Sequirus and MSD advisory boards. Magda Campins also participated in vaccine related projects from GSK, Janssen, Sanofi, Pfizer and Novavax. Other authors declare that they have no conflict of interest.
Compliance with ethics guidelines
Ethics committee approval was not required, since the article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by the authors.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Prior presentation
This study was presented in part at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Lisbon 23–26 April 2022.
Acknowledgments
Thanks to Prof. Dr. Jose Nart, Chairman and Program Director, Department of Periodontology UIC-Barcelona for helping with the diagnosis of oral herpes zoster. Thanks to Michael Maudsley for assisting with the English language review.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2022.07.022.
Appendix. Supplementary materials
References
- 1.Gorkhali R., Koirala P., Rijal S., Mainali A., Baral A., Bhattarai H.K. Structure and Function of Major SARS-CoV-2 and SARS-CoV Proteins. Bioinform Biol Insights. 2021;15 doi: 10.1177/11779322211025876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahajan R.K., Paul G., Mahajan R., Gautam P.L., Paul B. Systemic manifestations of COVID-19. J Anaesthesiol Clin Pharmacol. 2020;36:435–442. doi: 10.4103/joacp.JOACP_359_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh H., Kaur H., Singh K., Sen C.K. Cutaneous Manifestations of COVID-19: a Systematic Review. Adv Wound Care (New Rochelle) 2021;10:51–80. doi: 10.1089/wound.2020.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tammaro A., Adebanjo G a.R, Parisella F.R., Pezzuto A., Rello J. Cutaneous manifestations in COVID-19: the experiences of Barcelona and Rome. J Eur Acad Dermatol Venereol. 2020;34:e306–e307. doi: 10.1111/jdv.16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Wells G., Shea B., O'Connell D., Peterson J., Welch V., Losos M., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ott Hosp Res Inst Nd n.d. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed February 2, 2022).
- 7.Herzog R., Álvarez-Pasquin M.J., Díaz C., Del Barrio J.L., Estrada J.M., Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid-Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierson D.J. How to read a case report (or teaching case of the month) Respir Care. 2009;54:1372–1378. [PubMed] [Google Scholar]
- 10.Hill A.B. The Environment and Disease: association or Causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aksu S.B., Öztürk G.Z. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? Clin Exp Vaccine Res. 2021;10:198–201. doi: 10.7774/cevr.2021.10.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Algaadi S.A. Herpes zoster after COVID-19 vaccine: a case report. Pak J Med Health Sci. 2021;15:1165–1166. [Google Scholar]
- 13.Alpalhão M., Filipe P. Herpes Zoster following SARS-CoV-2 vaccination - a series of four cases. J Eur Acad Dermatol Venereol. 2021;35:e750–e752. doi: 10.1111/jdv.17555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arora P., Sardana K., Mathachan S.R., Malhotra P. Herpes zoster after inactivated COVID-19 vaccine: a cutaneous adverse effect of the vaccine. J Cosmet Dermatol. 2021;20:3389–3390. doi: 10.1111/jocd.14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayaz C.M., Dizman G.T., Metan G., Alp A., Unal S. Out-patient management of patients with COVID-19 on home isolation. Infez Med. 2020;28:351–356. [PubMed] [Google Scholar]
- 16.Bhargava P., Singdia H., Mathur R., Garg R., Rani N., Nijhawan S., et al. Herpes Zoster Duplex Unilateralis as a manifestation of severe lymphopenia in COVID19. Eur J Pain. 2021;25:508–509. doi: 10.1002/ejp.1709. [DOI] [Google Scholar]
- 17.Bostan E., Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566–1567. doi: 10.1111/jocd.14035. [DOI] [PubMed] [Google Scholar]
- 18.Brambilla L., Maronese C.A., Tourlaki A., Veraldi S. Herpes zoster following COVID-19: a report of three cases. Eur J Dermatol. 2020;30:754–756. doi: 10.1684/ejd.2020.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao X., Zhang X., Meng W., Zheng H. Herpes Zoster and Postherpetic Neuralgia in an Elderly Patient with Critical COVID-19: a Case Report. J Pain Res. 2020;13:2361–2365. doi: 10.2147/JPR.S274199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Català A., Muñoz-Santos C., Galván-Casas C., Roncero Riesco M., Revilla Nebreda D., Solá-Truyols A., et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142–152. doi: 10.1111/bjd.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Channa L., Torre K., Rothe M. Herpes zoster reactivation after mRNA-1273 (Moderna) SARS-CoV-2 vaccination. JAAD Case Rep. 2021;15:60–61. doi: 10.1016/j.jdcr.2021.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu H.-.H., Wei K.-.C., Chen A., Wang W.-.H. Herpes zoster following COVID-19 vaccine: report of 3 cases. QJM. 2021;114:531–532. doi: 10.1093/qjmed/hcab208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.David E., Landriscina A. Herpes Zoster Following COVID-19 Vaccination. J Drugs Dermatol. 2021;20:898–900. doi: 10.36849/JDD.6146. [DOI] [PubMed] [Google Scholar]
- 24.Desai H.D., Sharma K., Patoliya J.V., Ahadov E., Patel N.N. A Rare Case of Varicella-Zoster Virus Reactivation Following Recovery From COVID-19. Cureus. 2021;13:e12423. doi: 10.7759/cureus.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eid E., Abdullah L., Kurban M., Abbas O. Herpes zoster emergence following mRNA COVID-19 vaccine. J Med Virol. 2021;93:5231–5232. doi: 10.1002/jmv.27036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsaie M.L., Nada H.A. Herpes zoster (shingles) complicating the course of COVID19 infection. J Dermatolog Treat. 2020;33:1123–1125. doi: 10.1080/09546634.2020.1782823. [DOI] [PubMed] [Google Scholar]
- 27.Elsaie M.L., Youssef E.A., Nada H.A. Herpes zoster might be an indicator for latent COVID 19 infection. Dermatol Ther. 2020;33:e13666. doi: 10.1111/dth.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elsaie M.L., Youssef E.A., Nada H.A. Herpes zoster may be a marker for COVID-19 infection during pregnancy. Cutis. 2020;106:318–320. doi: 10.12788/cutis.0133. [DOI] [PubMed] [Google Scholar]
- 29.Fathy R.A., McMahon D.E., Lee C., Chamberlin G.C., Rosenbach M., Lipoff J.B., et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venereol. 2022;36:e6–e9. doi: 10.1111/jdv.17646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Nieto D., Ortega-Quijano D., Suarez-Valle A., Burgos-Blasco P., Jimenez-Cauhe J., Fernandez-Guarino M. Comment on: “To consider varicella-like exanthem associated with COVID-19, virus varicella zoster and virus herpes simplex must be ruled out. Characterization of herpetic lesions in hospitalized COVID-19 patients. J Am Acad Dermatol. 2020;83:e257–e259. doi: 10.1016/j.jaad.2020.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira ACA de F., Romão T.T., Macedo Y.S., Pupe C., Nascimento O.J.M., Fellow of the American Academy of Neurology (FAAN) COVID-19 and herpes zoster co-infection presenting with trigeminal neuropathy. Eur J Neurol. 2020;27:1748–1750. doi: 10.1111/ene.14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster C.E., Penella A.D.V., Davis A.L., Arrington A.S., Campbell J., Palazzi D.L. Varicella-Zoster virus reactivation following SARS-CoV-2 immunization in two patients with leukemia. Pediatr Blood Cancer. 2021;68:e29191. doi: 10.1002/pbc.29191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furer V., Zisman D., Kibari A., Rimar D., Paran Y., Elkayam O. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology. 2021;60:SI90–SI95. doi: 10.1093/rheumatology/keab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh B., Gajjar R.A., Modi V.K., Jadeja D.M. A Rare Case of Herpes Zoster in an Adult Patient Recovered From Symptomatic Reinfection of COVID-19. Cureus. 2021;13:e16274. doi: 10.7759/cureus.16274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kluger N., Klimenko T., Bosonnet S. Herpes simplex, herpes zoster and periorbital erythema flares after SARS-CoV-2 vaccination: 4 cases. Ann Dermatol Venereol. 2021;149:58–60. doi: 10.1016/j.annder.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koumaki D., Krueger-Krasagakis S.-.E., Papadakis M., Katoulis A., Koumaki V., Evangelou G., et al. Herpes zoster viral infection after AZD1222 and BNT162b2 coronavirus disease 2019 mRNA vaccines: a case series. J Eur Acad Dermatol Venereol. 2022;36:e85–e86. doi: 10.1111/jdv.17720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee C., Cotter D., Basa J., Greenberg H.L. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960–1964. doi: 10.1111/jocd.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maldonado M.D., Romero-Aibar J., Pérez-San-Gregorio M.A. COVID-19 pandemic as a risk factor for the reactivation of herpes viruses. Epidemiol Infect. 2021;149:e145. doi: 10.1017/S0950268821001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maruki T., Ishikane M., Suzuki T., Ujiie M., Katano H., Ohmagari N. A case of varicella zoster virus meningitis following BNT162b2 mRNA COVID-19 vaccination in an immunocompetent patient. Int J Infect Dis. 2021;113:55–57. doi: 10.1016/j.ijid.2021.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMahon D.E., Amerson E., Rosenbach M., Lipoff J.B., Moustafa D., Tyagi A., et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishra S.B., Mahendradas P., Kawali A., Sanjay S., Shetty R. Reactivation of varicella zoster infection presenting as acute retinal necrosis post COVID 19 vaccination in an Asian Indian male. Eur J Ophthalmol. 2021 doi: 10.1177/11206721211046485. [ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Mohta A., Arora A., Srinivasa R., Mehta R.D. Recurrent herpes zoster after COVID-19 vaccination in patients with chronic urticaria being treated with cyclosporine-A report of 3 cases. J Cosmet Dermatol. 2021;20:3384–3386. doi: 10.1111/jocd.14437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nastro F., Fabbrocini G., di Vico F., Marasca C. Small vessel vasculitis related to varicella-zoster virus after Pfizer-BioNTech COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021;35:e745–e747. doi: 10.1111/jdv.17550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nofal A., Fawzy M.M., Sharaf El Deen S.M., El-Hawary E.E. Herpes zoster ophthalmicus in COVID-19 patients. Int J Dermatol. 2020;59:1545–1546. doi: 10.1111/ijd.15240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Özdemir A.K., Kayhan S., Çakmak S.K. Herpes zoster after inactivated SARS-CoV-2 vaccine in two healthy young adults. J Eur Acad Dermatol Venereol. 2021;35:e846–e847. doi: 10.1111/jdv.17577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palanivel J.A. Herpes zoster after COVID-19 vaccination-Can the vaccine reactivate latent zoster virus? J Cosmet Dermatol. 2021;20:3376–3377. doi: 10.1111/jocd.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papasavvas I., de Courten C., Herbort C.P. Varicella-zoster virus reactivation causing herpes zoster ophthalmicus (HZO) after SARS-CoV-2 vaccination - report of three cases. J Ophthalmic Inflamm Infect. 2021;11:28. doi: 10.1186/s12348-021-00260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel P., Undavia A., Choudry R., Zhang Y., Prabhu A.M. COVID-19 Associated With Concomitant Varicella Zoster Viral Encephalitis. Neurol Clin Pract. 2021;11:e219–e221. doi: 10.1212/CPJ.0000000000000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pona A., Jiwani R.A., Afriyie F., Labbe J., Cook P.P., Mao Y. Herpes zoster as a potential complication of coronavirus disease 2019. Dermatol Ther. 2020;33:e13930. doi: 10.1111/dth.13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Psichogiou M., Samarkos M., Mikos N., Hatzakis A. Reactivation of Varicella Zoster Virus after Vaccination for SARS-CoV-2. Vaccines (Basel) 2021;9:572. doi: 10.3390/vaccines9060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 52.Rehman O., Arya S.K., Jha U.P., Nayyar S., Goel I. Herpes Zoster Ophthalmicus After COVID-19 Vaccination: chance Occurrence or More? Cornea. 2022;41:254–256. doi: 10.1097/ICO.0000000000002881. [DOI] [PubMed] [Google Scholar]
- 53.Rodríguez-Jiménez P., Chicharro P., Cabrera L-M, Seguí M., Morales-Caballero Á., Llamas-Velasco M., et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58–59. doi: 10.1016/j.jdcr.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saati A., Al-Husayni F., Malibari A.A., Bogari A.A., Alharbi M. Herpes Zoster Co-Infection in an Immunocompetent Patient With COVID-19. Cureus. 2020;12:e8998. doi: 10.7759/cureus.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Said J.T., Virgen C.A., Lian C.G., Cutler C.S., Merola J.F., LeBoeuf N.R. Disseminated varicella-zoster virus infections following messenger RNA-based COVID-19 vaccination. JAAD Case Rep. 2021;17:126–129. doi: 10.1016/j.jdcr.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santovito L.S., Pinna G. A case of reactivation of varicella-zoster virus after BNT162b2 vaccine second dose? Inflamm Res. 2021;70:935–937. doi: 10.1007/s00011-021-01491-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shors A.R. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656–657. doi: 10.1016/j.jdcr.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tammaro A., Adebanjo G a.R, Parisella F.R., Pezzuto A., Rello J. Cutaneous manifestations in COVID-19: the experiences of Barcelona and Rome. J Eur Acad Dermatol Venereol. 2020;34:e306–e307. doi: 10.1111/jdv.16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tartari F., Spadotto A., Zengarini C., Zanoni R., Guglielmo A., Adorno A., et al. Herpes zoster in COVID-19-positive patients. Int J Dermatol. 2020;59:1028–1029. doi: 10.1111/ijd.15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tessas I., Kluger N. Ipsilateral herpes zoster after the first dose of BNT162b2 mRNA COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021;35:e620–e622. doi: 10.1111/jdv.17422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thimmanagari K., Veeraballi S., Roach D., Al Omour B., Slim J. Ipsilateral Zoster Ophthalmicus Post COVID-19 Vaccine in Healthy Young Adults. Cureus. 2021;13:e16725. doi: 10.7759/cureus.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toscani I., Troiani A., Citterio C., Rocca G., Cavanna L. Herpes Zoster Following COVID-19 Vaccination in Long-Term Breast Cancer Survivors. Cureus. 2021;13:e18418. doi: 10.7759/cureus.18418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Dam C.S., Lede I., Schaar J., Al-Dulaimy M., Rösken R., Smits M. Herpes zoster after COVID vaccination. Int J Infect Dis. 2021;111:169–171. doi: 10.1016/j.ijid.2021.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Voisin O., Deluca N., Mahé A., Lelorc'h E., Hubert S., Ménage E., et al. Disseminated Herpes Zoster During COVID-19. Infect Dis Clin Pract (Baltim Md) 2021;29:e109–e110. doi: 10.1097/IPC.0000000000000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu R., Zhou Y., Cai L., Wang L., Han J., Yang X., et al. Co-reactivation of the human herpesvirus alpha subfamily (herpes simplex virus-1 and varicella zoster virus) in a critically ill patient with COVID-19. Br J Dermatol. 2020;183:1145–1147. doi: 10.1111/bjd.19484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diez-Domingo J., Parikh R., Bhavsar A.B., Cisneros E., McCormick N., Lecrenier N. Can COVID-19 Increase the Risk of Herpes Zoster? A Narrative Review. Dermatol Ther (Heidelb) 2021;11:1119–1126. doi: 10.1007/s13555-021-00549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Algaadi S.A. Herpes zoster and COVID-19 infection: a coincidence or a causal relationship? Infection. 2022;50:289–293. doi: 10.1007/s15010-021-01714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rahangdale R.R., Tender T., Balireddy S., Pasupuleti M., Hariharapura R.C. Interplay between stress and immunity triggers herpes zoster infection in COVID-19 patients: a review. Can J Microbiol. 2022;68:303–314. doi: 10.1139/cjm-2021-0242. [DOI] [PubMed] [Google Scholar]
- 69.Zerboni L., Sen N., Oliver S.L., Arvin A.M. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol. 2014;12:197–210. doi: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kennedy P.G.E., Gershon A.A. Clinical Features of Varicella-Zoster Virus Infection. Viruses. 2018;10:E609. doi: 10.3390/v10110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Depledge D.P., Sadaoka T., Ouwendijk W.J.D. Molecular Aspects of Varicella-Zoster Virus Latency. Viruses. 2018;10:E349. doi: 10.3390/v10070349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reijnders T.D.Y., Schuurman A.R., van der Poll T. The Immune Response to Respiratory Viruses: from Start to Memory. Semin Respir Crit Care Med. 2021;42:759–770. doi: 10.1055/s-0041-1736459. [DOI] [PubMed] [Google Scholar]
- 73.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 74.Darif D., Hammi I., Kihel A., El Idrissi Saik I., Guessous F., Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong? Microb Pathog. 2021;153 doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu X., Li L., Chan M.T.V., Wu W.K.K. Bioinformatic analyses suggest augmented interleukin-17 signaling as the mechanism of COVID-19-associated herpes zoster. Environ Sci Pollut Res Int. 2021;28:65769–65775. doi: 10.1007/s11356-021-15567-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marra F., Parhar K., Huang B., Vadlamudi N. Risk Factors for Herpes Zoster Infection: a Meta-Analysis. Open Forum Infect Dis. 2020;7:ofaa005. doi: 10.1093/ofid/ofaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levinson R.T., Hulgan T., Kalams S.A., Fessel J.P., Samuels D.C. Mitochondrial Haplogroups as a Risk Factor for Herpes Zoster. Open Forum Infect Dis. 2016;3:ofw184. doi: 10.1093/ofid/ofw184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abu-Rumeileh S., Mayer B., Still V., Tumani H., Otto M., Senel M. Varicella zoster virus-induced neurological disease after COVID-19 vaccination: a retrospective monocentric study. J Neurol. 2021;269:1751–1757. doi: 10.1007/s00415-021-10849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shasha D., Bareket R., Sikron F.H., Gertel O., Tsamir J., Dvir D., et al. Real-world safety data for the Pfizer BNT162b2 SARS-CoV-2 vaccine: historical cohort study. Clin Microbiol Infect. 2022;28:130–134. doi: 10.1016/j.cmi.2021.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gringeri M., Battini V., Cammarata G., Mosini G., Guarnieri G., Leoni C., et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675–684. doi: 10.1080/14760584.2022.2044799. [DOI] [PubMed] [Google Scholar]
- 82.Wan E.Y.F., Chui C.S.L., Wang Y., Ng V.W.S., Yan V.K.C., Lai F.T.T., et al. Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: a self-controlled case series and nested case-control study. Lancet Reg Health West Pac. 2022;21 doi: 10.1016/j.lanwpc.2022.100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.