Summary
Objective
Lifestyle interventions can be efficacious in reducing cardiovascular disease risk factors and are recommended as first-line interventions in England. However, recent information on the use of these interventions in primary care is lacking. We investigated for how many patients with newly diagnosed hypertension, hyperlipidaemia or obesity, lifestyle interventions were recorded in their primary care electronic health record.
Design
A retrospective cohort study.
Setting
English primary care, using UK Clinical Practice Research Datalink.
Participants
A total of 770,711 patients who were aged 18 years or older and received a new diagnosis of hypertension, hyperlipidaemia or obesity between 2010 and 2019.
Main outcome measures
Record of lifestyle intervention and/or medication in 12 months before to 12 months after initial diagnosis (2-year timeframe).
Results
Analyses show varying results across conditions: While 55.6% (95% CI 54.9–56.4) of individuals with an initial diagnosis of hypertension were recorded as having lifestyle support (lifestyle intervention or signposting) within the 2-year timeframe, this number was reduced to 45.2% (95% CI 43.8–46.6) for hyperlipidaemia and 52.6% (95% CI 51.1–54.1) for obesity. For substantial proportions of individuals neither lifestyle support nor medication (hypertension: 12.2%, 95% CI 11.9–12.5; hyperlipidaemia: 32.2%, 95% CI 31.2–33.3; obesity: 43.9%, 95% CI 42.3–45.4) were recorded. Sensitivity analyses confirm that limited proportions of patients had lifestyle support recorded in their electronic health record before they were first prescribed medication (diagnosed and undiagnosed), ranging from 12.1% for hypertension to 19.7% for hyperlipidaemia, and 19.5% for obesity (23.4% if restricted to Orlistat).
Conclusions
Limited evidence of lifestyle support for individuals with cardiovascular risk factors (hypertension, hyperlipidaemia, obesity) recommended by national guidelines in England may stem from poor recording in electronic health records but may also represent missed opportunities. Given the link between progression to cardiovascular disease and modifiable lifestyle factors, early support for patients to manage their conditions through non-pharmaceutical interventions by establishing lifestyle modification as first-line treatment is crucial.
Keywords: Disease prevention, cardiovascular disease, primary care, lifestyle intervention
Introduction
Despite the decrease of cardiovascular disease seen in many Western countries in recent decades, it continues to be a major health problem. 1 The most recent Global Burden of Disease study estimated that, in 2019, cardiovascular disease accounted for 2.4 million disability-adjusted life years and over 150,000 deaths in England, representing over 14% of total disability-adjusted life years and 30% of all deaths in England, with a large proportion of this mortality being attributable to high systolic blood pressure (42.9%), high low-density lipoprotein cholesterol (25.3%) and high body mass index (16.4%). 2
Several well-established risk factors predispose individuals to developing cardiovascular disease including age, gender, high systolic blood pressure, high total cholesterol, elevated low-density lipoprotein cholesterol, smoking behaviour and diabetes status. 3 Several of these risk factors are directly linked to lifestyle-related behaviours which can be modified through interventions that support individuals to, for example, maintain a healthy diet and body weight, 4 engage in physical activity, 5 reduce sodium intake 6 and alcohol consumption, 7 and abstain from tobacco smoking. 8 General practitioners can play a particularly important role on this by recommending and monitoring lifestyle-related changes for reduction in cardiovascular disease risk. 9
Lifestyle interventions are generally seen to be successful in controlled clinical trials.4,5,10–12 While there are concerns about the long-term effectiveness 13 and the large variability in responses to these interventions, with some individuals being highly successful while others achieve very little lifestyle change,14,15 in the UK, the National Institute for Health and Care Excellence have recommended addressing lifestyle behaviours as a first-line intervention for individuals diagnosed with conditions such as type 2 diabetes, hypertension, hyperlipidaemia and obesity.16–18 In line with their recommendations, several lifestyle intervention programmes are used through England’s National Health Service to support individuals in changing their lifestyle, and they include more formal programmes like DESMOND for individuals with type 2 diabetes 19 as well as programmes that target behaviours related to diet and/or physical activity. While more formal programmes like DESMOND have specific referral pathways, the pathways for hypertension, hyperlipidaemia and obesity are not as well established despite these conditions having a high prevalence in England. Hypertension is estimated to affect 12.5 million adults, over 20% of the population, in the UK 20 and was associated with about 30% of deaths in the UK Biobank study. 21 Dyslipidaemias come in various forms with the most common being raised cholesterol. In 2017, it was reported that the prevalence of raised cholesterol in UK adults was 48%. 22 Obesity, another common risk factor for cardiovascular disease, has a prevalence of 28% in the UK. 23
To better understand how cardiovascular disease risk is managed in primary care in England, this study aimed to quantify use of lifestyle interventions for individuals with an initial diagnosis of hypertension, hyperlipidaemia or obesity. We use a well-established primary care dataset from England, and the results have important implications for policy and practice to ensure that individuals with modifiable risk factors linked to cardiovascular disease can benefit from interventions that can help to improve their health, prevent disease and, most importantly, put their health back in their hands.
Materials and methods
Data source and ethics
This retrospective observational study used data from Clinical Practice Research Datalink Aurum, an ongoing database of pseudonymised routine primary care records from general practitioners in the UK. The data are representative of the population in England with respect to geographical spread, deprivation, age and gender. 24 Data were extracted from the Clinical Practice Research Datalink Aurum database in July 2020, containing electronic health records from 35.9 million patients and 1296 currently contributing practices in England. In this release, over 93% of permanent registrations were deemed to have research quality data based on Clinical Practice Research Datalink metrics derived from internal consistency of key variables including date of birth, practice registration date and transfer out date. 24 The Clinical Practice Research Datalink Independent Scientific Advisory Committee approved study protocols for each condition (20_000180, 20_000181 and 20_000182) in accordance with the Declaration of Helsinki.
Study period
The study period spans from 2010 to 2019. For hypertension, it spans from 2011 to 2019, as we were only interested in periods during which clinical guidelines by the National Institute for Health and Care Excellence specifically recommended lifestyle interventions as a first-line treatment option for the condition.
Study population
The eligible population had an initial diagnosis of hypertension, hyperlipidaemia or obesity at adult age during the study period, at least 12 months of continuous registration after said initial diagnosis and was deemed as research quality based on Clinical Practice Research Datalink metrics. The diagnosis had to be clearly indicated by the presence of an electronic health record code entered by the general practitioner, whereas a physical measurement or laboratory test result exceeding commonly applied disease thresholds (e.g. a systolic blood pressure measurement above 140 mmHg) did not qualify as a diagnosis (Supplementary Table 1). In addition, patients that received any condition-specific medication (e.g. statins for hyperlipidaemia) during the 15 years before their initial electronic health record recorded diagnosis or had any record indicating genetic causes for the condition (e.g. familial hypercholesterolemia) were excluded. Code lists for medication and exclusion diagnoses are provided in the Supplementary Data (Supplementary Tables 2–3). Follow-up ended at the last data collection from the general practitioner practice, practice deregistration or death.
Outcomes
We were interested in the course of treatment of patients who had an initial diagnosis of hypertension, hyperlipidaemia or obesity. The primary outcome was thus the presence of any record of signposting to, performance of or referral to lifestyle intervention appropriate for each condition before and after a patient’s initial diagnosis. We identified relevant electronic health record codes based on a string-based term search which was performed independently by two members of our research team (Supplementary Box 1, Supplementary Table 4). For lifestyle support, we distinguished between codes that indicated signposting (i.e. signposting such as ‘Physical activity opportunity signposted’, brief advice such as ‘Diet leaflet given’ or ‘Advice about exercise’) and codes that indicated an offer, referral or performance of a lifestyle intervention on-site or off-site (i.e. counselling such as ‘Dietary management education, guidance, and counselling’ or referrals such as ‘Referral to community dietician’ or ‘Exercise on prescription’). When presenting what proportion of patients received lifestyle support, we distinguished by signposting (including brief advice) and lifestyle intervention, with the latter being less susceptible to underreporting as general practitioners often act as gatekeepers for referrals to lifestyle intervention services and programmes. In addition, we determined whether patients received any prescription of condition-specific medication. We identified medication prescriptions using British National Formulary terms (Supplementary Table 3). Presence and absence of relevant codes were determined for different time periods in relation to the initial diagnosis – within 12 months or 6 months before the initial diagnosis; at time of diagnosis, for which we allowed the records to be entered 1 week before to 1 week after the initial diagnosis; and within 1 month, 2 months, 3 months, 4 months, and 12 months after the initial diagnosis.
Statistical analysis
R Version 4.0.3 was used to conduct the analyses. Baseline characteristics were described for all patients with an initial diagnosis of hypertension, hyperlipidaemia or obesity that fulfilled our inclusion criteria. We calculated the proportion and number of patients among those with an initial diagnosis that had an electronically recorded lifestyle intervention and/or medication for each condition. Confidence intervals were computed with standard errors using a variance-stabilising transformation for the binomial distribution and are clustered at the general practitioner practice level. We compared the proportion of patients that had these interventions recorded in their electronic health record before and after their initial diagnosis as well as the cumulative proportion of patients that had these interventions recorded in their electronic health record at any point in time from 12 months before to 12 months after their initial diagnosis.
Sensitivity analysis
While there is evidence that correctness of diagnosis codes in Clinical Practice Research Datalink Aurum is high, 25 Persson et al. report that half of patients who had either high cholesterol values or medication had no hyperlipidaemia diagnosis codes. The authors concluded that there may be a substantial number of patients with hyperlipidaemia treated by their general practitioner but with no diagnosis code in their electronic health record. Thus, as sensitivity analysis, we created an alternative cohort, where we used the first recorded condition-specific medication as the index date instead of the initial recorded diagnosis and determined what proportion of patients (diagnosed and undiagnosed) had a lifestyle intervention recorded in their electronic health record in the 12 months before said prescription.
Results
After excluding patients who had receipt of medication recorded in their electronic health record before their initial recorded diagnosis (n = 223,414 for hypertension, n = 38,221 for hyperlipidaemia and n = 32,296 for obesity), the full sample consisted of 770,711 patients who met our inclusion criteria and received an initial diagnosis of hypertension, hyperlipidaemia or obesity during our study period. Table 1 provides the baseline characteristics for all newly diagnosed patients stratified by condition.
Table 1.
Characteristics of patients by condition.
| Hypertension | Hyperlipidaemia | Obesity | ||||
|---|---|---|---|---|---|---|
| Variable | (n = 403,129) |
(n = 105,900) |
(n = 261,682) |
|||
| Median | IQR | Median | IQR | Median | IQR | |
| Year of diagnosis | 2014 | 2012–2017 | 2015 | 2013–2017 | 2014 | 2012–2017 |
| Age a | 57 | 48–67 | 55 | 46–65 | 48 | 34–61 |
| Follow-up time (in months) | 55.6 | 32.5–82.0 | 51.8 | 31.2–80.0 | 54.4 | 31.4–84.3 |
| % |
n |
% |
n |
% |
n |
|
| Gender | ||||||
| Female | 44.3 | 178,746 | 49.8 | 52,697 | 57.2 | 149,809 |
| Male | 55.7 | 224,380 | 50.2 | 53,203 | 42.8 | 111,868 |
| Non-binary | 0.0 | 3 | 0.0 | 0 | 0.0 | 5 |
| Region | ||||||
| North East | 3.3 | 13,254 | 2.0 | 2,144 | 8,829 | 3.4 |
| North West | 16.1 | 64,794 | 20.6 | 21,827 | 43,739 | 16.7 |
| Yorkshire and the Humber | 3.2 | 12,929 | 2.0 | 2,134 | 8,317 | 3.2 |
| East Midlands | 2.5 | 9,898 | 2.0 | 2,115 | 5,468 | 2.1 |
| West Midlands | 17.2 | 69,284 | 19.2 | 20,286 | 41,838 | 16.0 |
| East of England | 4.3 | 17,237 | 3.7 | 3,894 | 8,240 | 3.1 |
| South West | 12.1 | 48,950 | 5.9 | 6,282 | 40,612 | 15.5 |
| South Central | 12.7 | 51,377 | 8.6 | 9,088 | 31,257 | 11.9 |
| London | 19.6 | 78,922 | 26.2 | 27,719 | 52,311 | 20.0 |
| South East Coast | 8.9 | 35,680 | 9.4 | 9,992 | 20,799 | 7.9 |
| Unknown | 0.2 | 804 | 0.4 | 419 | 272 | 0.1 |
Table shows characteristics of patients who met all inclusion criteria.
aAge in years at time of the initial diagnosis. IQR; interquartile range.
Hypertension
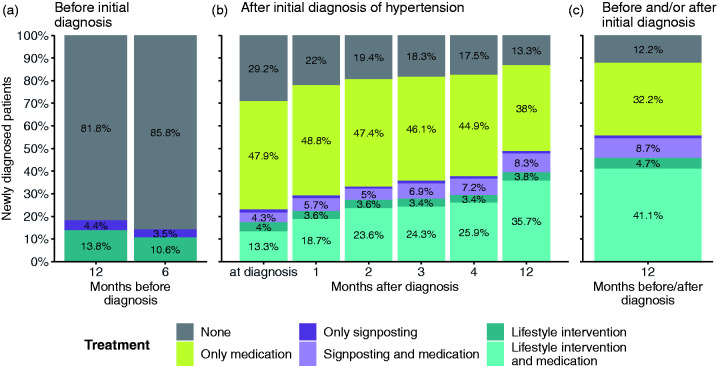
Among newly diagnosed hypertension patients between 2011 and 2019 (N = 403, 129), 86.8% (95% confidence interval [CI] 86.5–87.1) had a combination of medication, signposting for lifestyle-related support, and/or lifestyle intervention recorded in their electronic health record within 12 months after their initial diagnosis (Figure 1(b) – all coloured bars except for grey). This number increases to 87.8% (95% CI 87.5–88.1) if we also take lifestyle intervention up to 12 months before the initial diagnosis into account (Table 2: ‘All patients with medication and/or lifestyle support record’). While 82.0% (95% CI 81.6–82.5) of diagnosed patients were recorded in their electronic health record as having been prescribed medication within 12 months after their diagnosis (either alone or in combination with signposting and/or lifestyle intervention), only about half of newly diagnosed patients (55.6%, 95% CI 54.9–56.4) had any lifestyle intervention or signposting recorded in their electronic health record in a window from up to 12 months before to within 12 months after their diagnosis (Figure 1(c) – dark and light purple and dark and light teal bars; Table 2). The percentage of patients who had any lifestyle intervention or signposting recorded in their electronic health record after their initial diagnosis rose from 22.9% (95% CI 22.4–23.4) at the time of diagnosis to 48.7% (95% CI 48.0–49.4) within 12 months after their diagnosis (Figure 1(b) – dark and light purple and dark and light teal bars).
Figure 1.
Prescribed medications and recorded lifestyle interventions for patients with an initial diagnosis of hypertension. Proportions of patients by hypertension treatment (hypertension medication, signposting to lifestyle intervention and lifestyle intervention) 12 months before to 12 months after an initial recorded diagnosis of hypertension. Patients with a prescription of hypertension medication before any initial diagnosis of hypertension are excluded. Grey bars – no intervention; yellow bars – only medication; dark purple bars – only signposting; light purple bars – signposting and medication; dark blue bars – only lifestyle intervention; light blue bars – lifestyle intervention and medication.
Table 2.
Proportion of patients with electronic health records of medication prescription and/or lifestyle support in a period of 12 months before to 12 months after initial diagnosis for each condition.
| Hypertension | Hyperlipidaemia | Obesity | ||||
|---|---|---|---|---|---|---|
| Treatment | (N = 403,129) |
(N = 105,900) |
(N = 261,682) |
|||
| Per cent (95% CI) | n | Per cent (95% CI) | n | Per cent (95% CI) | n | |
| None | 12.2 (11.9–12.5) | 49,039 | 32.2 (31.2–33.3) | 34,144 | 43.9 (42.3–45.4) | 114,789 |
| Medication | ||||||
| Medication only | 32.2 (31.5–32.9) | 129,821 | 22.5 (21.6–23.5) | 23,858 | 3.5 (3.3–3.7) | 9231 |
| Medication w/signposting | 8.7 (8.4–9.1) | 35,210 | 4.1 (3.8–4.4) | 4,355 | 0.9 (0.8–1.0) | 2364 |
| Medication w/ lifestyle intervention | 41.1 (40.3–41.8) | 165,575 | 17.6 (16.9–18.3) | 18,623 | 3.7 (3.5–3.9) | 9769 |
| Lifestyle support | ||||||
| Lifestyle Intervention only | 4.7 (4.4–5.0) | 18,917 | 17.8 (16.6–19.0) | 18,834 | 38.4 (37.0–39.7) | 100,441 |
| Signposting only | 1.1 (1.0–1.2) | 4,567 | 5.7 (5.3–6.2) | 6,086 | 9.6 (9.0–10.3) | 25,088 |
| Total | 100 | 403,129 | 100 | 105,900 | 100 | 261,682 |
| Prop. (95% CI) |
n |
Prop. (95% CI) |
n |
Prop. (95% CI) |
n |
|
| All patients with medication record | 82.0 (81.6–82.4) | 330,606 | 44.2 (42.8–45.6) | 46,836 | 8.2 (7.7–8.6) | 21,364 |
| All patients with lifestyle support record | 55.6 (54.9–56.4) | 224,269 | 45.2 (43.8–46.6) | 47,898 | 52.6 (51.1–54.1) | 137,662 |
| All patients with medication and/or lifestyle support record | 87.8 (87.5–88.1) | 354,090 | 67.8 (66.7–68.8) | 71,756 | 56.1 (54.6–57.7) | 146,893 |
CI: confidence interval. Table shows the proportions of patients by treatment group and condition. The patient must have a record of a given treatment at any point between 12 months prior to and/or 12 months after their initial diagnosis. Confidence intervals were computed with standard errors using a variance-stabilising transformation for the binomial distribution and are clustered at the practice level. Patients that have received a lifestyle intervention may have also concurrently received signposting.
Among patients who had a recorded medication prescription in their electronic health record within 12 months of their initial diagnosis (n = 330, 606; Table 2: ‘Medication total’), 60.7% (95% CI 60.0–61.5) also had any lifestyle intervention or signposting recorded in their EHR during 12 months prior to 12 months after their diagnosis (200,785 out of 330,606 patients; Table 2: ‘Medication w/signposting’ and ‘Medication w/lifestyle intervention’ divided by ‘Medication total’). 5.8% (95% CI 5.5–6.1) of patients had any lifestyle intervention or signposting recorded in their electronic health record up to 12 months before to within 12 months after their initial diagnosis without being prescribed any medication within 12 months after their diagnosis (23,484 out of 403,129 patients; Table 2: ‘Lifestyle intervention only’ and ‘Signposting only’ divided by ‘Total’).
Hyperlipidaemia
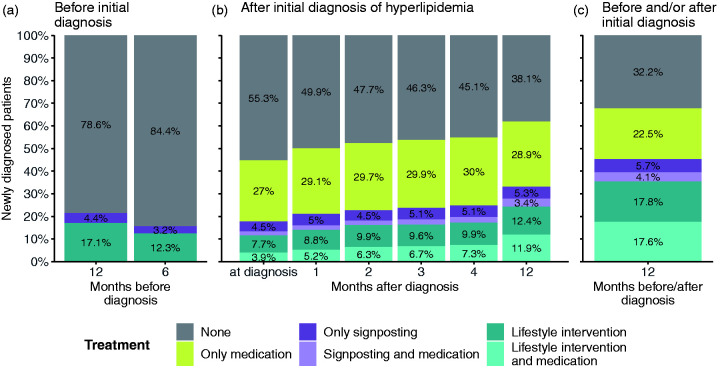
Among newly diagnosed hyperlipidaemia patients between 2010 and 2019, 61.9% (95% CI 60.8–62.9) had a combination of medication, signposting for lifestyle-related support, and/or lifestyle intervention recorded in their electronic health record within 12 months after their initial diagnosis (Figure 2(b) – all coloured bars except for grey). This number increases to 67.8% (95% CI 66.7–68.8) if we also take lifestyle intervention up to 12 months before the initial diagnosis into account (Table 2: ‘All patients with medication and/or lifestyle support record). While 44.2% (95% CI 42.8–45.6) of diagnosed patients were recorded as having received a medication prescription within 12 months after their diagnosis (either alone or in combination with signposting and/or lifestyle intervention), 45.2% (95% CI 43.8–46.6) had any record in their electronic health record of lifestyle intervention or signposting in a window from up to 12 months before, to within 12 months after their diagnosis (Figure 2(c) – dark and light purple and dark and light teal bars; Table 2). The percentage of patients who had any record of lifestyle intervention or signposting in their electronic health record after their initial diagnosis rose from 17.7 (95% CI 16.8–18.7) at the time of diagnosis to 33.0% (95% CI 31.7–34.3) within 12 months after their diagnosis (Figure 2(b) – dark and light purple and dark and light teal bars).
Figure 2.
Prescribed medications and recorded lifestyle interventions for patients with an initial diagnosis of hyperlipidaemia. Proportions of patients by hyperlipidaemia treatment (hypertension medication, signposting to lifestyle intervention and lifestyle intervention) 12 months before to 12 months after an initial recorded diagnosis of hypertension. Patients with a prescription of hyperlipidaemia medication before any initial diagnosis of hyperlipidaemia are excluded. Grey bars – no intervention; yellow bars – only medication; dark purple bars – only signposting; light purple bars – signposting and medication; dark blue bars – only lifestyle intervention; light blue bars – lifestyle intervention and medication.
Among patients who had a record of receiving a medication prescription within 12 months of their initial diagnosis (n = 46 836; Table 2: ‘Medication total’), 49.1% (95% CI 47.8–50.3) also had any lifestyle intervention or signposting recorded in their electronic health record during 12 months prior to 12 months after their diagnosis (22,978 out of 46, 836 patients; Table 2: ‘Medication w/signposting’ and ‘Medication w/lifestyle intervention’ divided by ‘Medication total’). Of patients, 23.5% (95% CI 22.2–24.9) had any lifestyle intervention or signposting recorded in their electronic health record within 12 months before to 12 months after their initial diagnosis without being prescribed any medication up to 12 months after their diagnosis (24,920 out of 105,900 patients; Table 2: ‘Lifestyle intervention only’ and ‘Signposting only’ divided by ‘Total’).
Obesity
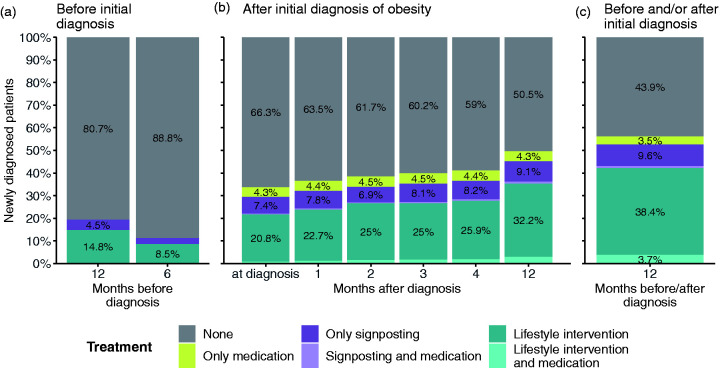
Among newly diagnosed obesity patients between 2010 and 2019, 49.5% (95% CI 47.9–51.1) had combination of medication, signposting for lifestyle-related support and/or lifestyle intervention recorded in their electronic health record within 12 months after their initial diagnosis (Figure 3(b) – all coloured bars except for grey). This number increases to 56.1% (95% CI 54.6–57.7) if we also take lifestyle intervention up to 12 months before the initial diagnosis into account (Table 2: ‘All patients receiving medication and/or lifestyle intervention’). Given the lack of approved anti-obesity medicines, only 8.2% (95% CI 7.7–8.6) had receipt of medication commonly used to target obesity recorded in their electronic health record (including gastro intestinal anti-obesity drugs such as Orlistat, appetite suppressants and bulk-forming laxatives, see Supplementary Table 3; Table 2: ‘All patients with medication record’). The percentage of patients who had any record in their electronic health record of lifestyle intervention or signposting after their initial diagnosis rose from 29.4% (95% CI 27.9–31.0) at the time of diagnosis to 45.2% (95% CI 43.7–46.7) within 12 months after their diagnosis (Figure 3(b) – dark and light purple and dark and light teal bars). If we also take lifestyle intervention up to 12 months before their diagnosis into account, this number increases by 7.4 percentage points, resulting in 52.6% (95% CI 51.1–54.1) of patients that had any record of lifestyle intervention or signposting in a window from up to 12 months before to within 12 months after their diagnosis (Figure 3(c) – dark and light purple and dark and light teal bars).
Figure 3.
Prescribed medications and recorded lifestyle interventions for patients with an initial diagnosis of obesity. Proportions of patients by obesity treatment (anti-obesity medication, signposting to lifestyle intervention and lifestyle intervention) 12 months before to 12 months after an initial recorded diagnosis of obesity. Patients with a prescription of anti-obesity medication before any initial diagnosis of obesity are excluded. Grey bars – no intervention; yellow bars – only medication; dark purple bars – only signposting; light purple bars – signposting and medication; dark blue bars – only lifestyle intervention; light blue bars – lifestyle intervention and medication.
Sensitivity analyses
Given that a substantial number of patients were already recorded as having been prescribed medication before their initial recorded diagnosis, we performed additional analyses where we explored the recorded use of lifestyle interventions before an initial prescription of medication rather than using the initial diagnosis as an index date (Supplementary Table 6). The proportion of individuals (diagnosed and undiagnosed) who had any record in their electronic health record of lifestyle support up to 12 months before their initial prescription ranges from 12.1% (95% CI 11.8–12.5) for antihypertensive medication to 19.7% (95% CI 19.2–20.3) for lipid-lowering medication and 19.5% (95% CI 19.0–20.0) for anti-obesity medication, respectively. If we restrict anti-obesity medication to Orlistat, which is the only medication singularly used for obesity treatment, the number slightly increases to 23.4% (95% CI 22.8–24.1). If we restrict this sample further to those that have been diagnosed at the time they had their first recorded medication prescription, the numbers increase slightly: The proportion of individuals (diagnosed) who had any record of lifestyle support up to 12 months before their initial prescription then ranges from 21.6% (95% CI 21.0–22.2) for antihypertensive medication to 26.7% (95% CI 25.7–27.8) for lipid-lowering medication and 33.1% (95% CI 32.3–34.0) for anti-obesity medication, respectively. We present figures for these analyses in the Supplementary Data (Supplementary Figures 1–4).
Discussion
Summary
Based on the primary care eletronic health records in our analysis, the main finding of our study points to a general lack of adherence to guidelines by the National Institute for Health and Care Excellence on the recommended use of lifestyle interventions for individuals with an initial diagnosis of hypertension, hyperlipidaemia or obesity. Given that these conditions are often linked to modifiable lifestyle factors, it is a missed opportunity to fail to support these individuals to manage their conditions through non-pharmaceutical routes.
Through our analyses we also find heterogeneity in the recorded use of lifestyle interventions across conditions with 45.8% of individuals with an initial diagnosis of hypertension having a electronic health record of some sort of lifestyle intervention (55.6% if signposting is included), going down to about 35.4% (45.2% if signposting is included) for those with an initial diagnosis of hyperlipidaemia. There are also substantial proportions of individuals not having any recorded support (medication, lifestyle intervention and/or signposting) at all within 12 months of diagnosis – ranging from only 12% of people with an initial diagnosis of hypertension to 44% for people with an initial diagnosis of obesity. In addition, our results suggest that only a small proportion of patients had a recorded lifestyle intervention before they were first prescribed medication for hypertension, hyperlipidaemia or obesity, raising the question why not more individuals receive a lifestyle intervention as first-line intervention.
Limitations
Our study used a large population-based primary care dataset that is representative of the adult population of England. However, this study also has several limitations. First, there are alternative explanations pertaining to the lack of recorded lifestyle interventions that could be related to our findings. Given that our analyses only capture what is recorded in primary care electronic health records, it is possible that interventions are being given but are not recorded. Brief advice, in particular, may be given to patients but not recorded by general practitioners, which could have led to an underestimation of lifestyle intervention rates. It may also be that patients are offered lifestyle interventions but decline this, or that there is a lack of services in the area that provide lifestyle interventions.
Second, our primary analysis sample only includes patients for which an initial diagnosis was recorded in their electronic health record, excluding patients who had any medication prescription prior to their initial diagnosis. Sensitivity analyses revealed low recorded lifestyle intervention rates for patients prior to their initial medication prescription (independent of their diagnosis status), substantiating a generally low recorded use of lifestyle intervention as first-line treatment.
Third, our analyses do not capture all details related to the diagnosis (e.g. exact lipid profile) and health status of the individual. However, this should not reflect a lack of general practitioners utilising lifestyle interventions. Given the importance of lifestyle-related factors in perpetuating cardiovascular disease risk, even patients who are given medications because of advanced disease at their initial diagnosis should receive lifestyle support to help them better manage their condition and, ideally, bring it into remission. 26
Fourth, in these analyses, we did not distinguish lifestyle interventions by content, duration or intensity and we also did not determine whether patients actually received or completed a given lifestyle intervention. While even brief advice by physicians can help patients change their health behaviours in the short term, 27 oftentimes more intensive lifestyle and maintenance programmes are needed to induce the long-term lifestyle modification needed for cardiovascular disease risk reduction. 28 Thus, our results reflect the proportion of patients that can potentially access lifestyle interventions through general practices rather than the proportions of patients eventually benefitting from lifestyle interventions. This information is still valuable, however, in guiding future modifications to primary care pathways to improve care and outcomes for patients with these conditions.
Fifth, comparisons across conditions cannot be made without important limitations as it is unknown whether accuracy of and type of recorded lifestyle intervention information varies systematically across conditions.
Comparison with existing literature
While several studies have evaluated the effectiveness of primary care lifestyle interventions,5,11,12 few studies have investigated how patients access lifestyle intervention in primary care. Booth et al. evaluated the use of weight management interventions among overweight and obese patients (based on body mass index) in English primary care and found that the proportion of patients that received a weight management intervention ranged from 8.7% to 28.1%, depending on their body mass index category. 29 Similarly, Sheppard et al. reported that in a cohort of patients with mild hypertension, only 12% received lifestyle advice. 30 We identified a higher proportion of patients receiving lifestyle interventions. Differences between the present study and these other studies include a more recent study period for our study, an inclusion of lifestyle interventions pertaining not only to weight management but also to general lifestyle and physical activity, and a cohort identification based on clinical diagnosis codes rather than body mass index categories.
Implications for research and/or practice
The results of this study suggest that lifestyle interventions to reduce cardiovascular disease risk are underutilised in English primary care. Given the strong link between modifiable lifestyle factors and progression to cardiovascular disease, establishing early support for lifestyle modification as a first-line intervention is crucial. It is possible that intervention rates have been underestimated through a lack of formal recording in medical records and it is important that our study outcomes are interpreted as recorded lifestyle interventions by general practitioners rather than interventions received. However, given the large burden of cardiovascular disease on primary healthcare services and lack of long-term follow-up on the effectiveness and equity of access to lifestyle interventions for conditions predisposing patients to cardiovascular disease, the use of electronic health records will be indispensable to better understand their use and impact. Thus, as a starting point, improvements in formal recording of lifestyle interventions in routine medical records are needed. Furthermore, though unpicking the factors that contribute to our results will take additional study, our results have important implications for policy and practice to ensure we can create more efficient and effective mechanisms for primary care to utilise and refer individuals with risk factors linked to cardiovascular disease to lifestyle interventions to promote health and prevent further exacerbations of cardiovascular disease.
Supplemental Material
Supplemental material, sj-pdf-1-jrs-10.1177_01410768221077381 for Use of lifestyle interventions in primary care for individuals with newly diagnosed hypertension, hyperlipidaemia or obesity: a retrospective cohort study by Julia M Lemp, Meghana Prasad Nuthanapati, Till W Bärnighausen, Sebastian Vollmer, Pascal Geldsetzer and Anant Jani in Journal of the Royal Society of Medicine
Declarations
Competing Interests
None declared.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: PG was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR003143. TB was supported by the Alexander von Humboldt Foundation through the Alexander von Humboldt Professor award, funded by the Federal Ministry of Education and Research. The research used the data storage service SDS@hd supported by the Ministry of Science, Research, and the Arts Baden-Wuerttemberg (MWK), Germany, and the German Research Foundation (DFG) through grant INST 35/1314-1 FUGG as well as by the state of Baden-Wuerttemberg, Germany, through bwHPC and the German Research Foundation (DFG) through grant INST 35/1134-1 FUGG.
Ethics approval
Permission for data usage was obtained from the Clinical Practice Research Datalink Independent Scientific Advisory Committee (protocol numbers 20_000180, 20_000181, and 20_000182). Linked pseudonymised data were provided by Clinical Practice Research Datalink. Analysis of pseudonymised data (i.e. data that could not be linked to individuals without additional information that was not available to the analysts) was considered exempt for nonhuman subjects research by the institutional review board of the Heidelberg University Medical Faculty.
Guarantor
AJ.
Contributorship
JML, PG and AJ contributed to the idea generation and protocol development. JML and MPN prepared the data for analysis, and JML performed the statistical analyses. JML, SV, PG, and AJ interpreted study results, and JML, MPN, and AJ had primary responsibility in writing the manuscript. TWB, SV and PG also contributed to manuscript writing. All authors critically reviewed the manuscript.
Acknowledgements
This study is based on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare products Regulatory Agency. The data is provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the authors alone. Due to Clinical Practice Research Datalink license restrictions, we are unable to share data.
Provenance
Not commissioned; peer-reviewed by Lee Aiyegbusi and Julie Morris.
ORCID iDs
Julia M Lemp https://orcid.org/0000-0002-1524-3641
Sebastian Vollmer https://orcid.org/0000-0002-7863-0462
Anant Jani https://orcid.org/0000-0002-7046-6768
Supplemental material
Supplemental material for this article is available online.
References
- 1.World Health Organization. Cardiovascular diseases (CVDs). See www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (last checked 2 March 2021).
- 2.Institute for Health Metrics and Evaluation (IHME). Global Health Data Exchange: Results Tool. See http://ghdx.healthdata.org/gbd-results-tool (last checked 2 March 2021).
- 3.D’Agostino RB Sr, Pencina MJ, Massaro JM, et al. Cardiovascular disease risk assessment: insights from Framingham. Glob Heart 2013; 8: 11–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeBlanc ES, Patnode CD, Webber EM, et al. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults. JAMA 2018; 320: 1172–1172. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor EA, Evans CV, Rushkin MC, et al. Behavioral counseling to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors. JAMA 2020; 324: 2076–2076. [DOI] [PubMed] [Google Scholar]
- 6.Aburto NJ, Ziolkovska A, Hooper L, et al. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 2013; 346: f1326–f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaner EF, Beyer FR, Muirhead C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2018; 2: CD004148–CD004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lancaster T and Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2017; 3: CD001292. [DOI] [PubMed]
- 9.Rippe JM and Angelopoulos TJ. Lifestyle strategies for risk factor reduction, prevention and treatment of cardiovascular disease. In: Rippe JM (ed.) Lifestyle Medicine. Third edition. Boca Raton, FL: Taylor & Francis, 2019, pp.19–36. [DOI] [PMC free article] [PubMed]
- 10.Rees K, Dyakova M, Wilson N, et al. Dietary advice for reducing cardiovascular risk. Cochrane database Syst Rev 2013; CD002128. [DOI] [PMC free article] [PubMed]
- 11.Booth HP, Prevost TA, Wright AJ, et al. Effectiveness of behavioural weight loss interventions delivered in a primary care setting: a systematic review and meta-analysis. Fam Pract 2014; 31: 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vetter ML, Wadden TA, Chittams J, et al. Effect of lifestyle intervention on cardiometabolic risk factors: results of the POWER-UP trial. Int J Obes 2013; 37: S19–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aucott L, Rothnie H, McIntyre L, et al. Long-term weight loss from lifestyle intervention benefits blood pressure? Hypertension 2009; 54: 756–762. [DOI] [PubMed] [Google Scholar]
- 14.Unick JL, Hogan PE, Neiberg RH, et al. Evaluation of early weight loss thresholds for identifying nonresponders to an intensive lifestyle intervention. Obesity 2014; 22: 1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadden TA, West DS, Neiberg RH, et al. One-year weight losses in the Look AHEAD Study: factors associated with success. Obesity 2009; 17: 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NICE (National Institute for Health and Care Excellence). Hypertension in adults: diagnosis and management (CG 127). See www.nice.org.uk/guidance/cg127 (last checked 2 March 2021).
- 17.NICE (National Institute for Health and Care Excellence). Cardiovascular disease: risk assessment and reduction, including lipid modification (CG 181). See www.nice.org.uk/guidance/cg181 (last checked 2 March 2021).
- 18.NICE (National Institute for Health and Care Excellence). Obesity: identification, assessment and management (CG 189). See www.nice.org.uk/guidance/cg189 (last checked 2 March 2021). [PubMed]
- 19.Davies MJ, Heller S, Skinner TC, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008; 336: 491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Public Health England. Health matters: combating high blood pressure. See www.gov.uk/government/publications/health-matters-combating-high-blood-pressure/health-matters-combating-high-blood-pressure (last checked 2 March 2021).
- 21.Hammami I, Lacey B, Lewington S. The burden of hypertension and associated risk for cardiovascular mortality in the UK biobank. Eur Heart J 2018; 39: ehy563.3028.
- 22.NHS Digital. Health Survey for England 2017. See https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2017 (2018, last checked 2 March 2021).
- 23.Digital N. Health Survey for England 2019. See https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2019 (2020, last checked 2 March 2021).
- 24.Wolf A, Dedman D, Campbell J, et al. Data resource profile: clinical practice research datalink (CPRD) Aurum. Int J Epidemiol 2019; 48: 1740–1740g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Persson R, Vasilakis‐Scaramozza C, Hagberg KW, et al. CPRD Aurum database: assessment of data quality and completeness of three important comorbidities. Pharmacoepidemiol Drug Saf 2020; 29: 1456–1464. [DOI] [PubMed] [Google Scholar]
- 26.Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin 2017; 35: 231–246. [DOI] [PubMed] [Google Scholar]
- 27.Whatnall MC, Patterson AJ, Ashton LM, et al. Effectiveness of brief nutrition interventions on dietary behaviours in adults: a systematic review. Appetite 2018; 120: 335–347. [DOI] [PubMed] [Google Scholar]
- 28.Webb VL, Wadden TA. Intensive lifestyle intervention for obesity: principles, practices, and results. Gastroenterology 2017; 152: 1752–1764. [DOI] [PubMed] [Google Scholar]
- 29.Booth HP, Prevost AT, Gulliford MC. Access to weight reduction interventions for overweight and obese patients in UK primary care: population-based cohort study. BMJ Open 2015; 5: e006642–e006642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheppard JP, Stevens S, Stevens RJ, et al. Association of guideline and policy changes with incidence of lifestyle advice and treatment for uncomplicated mild hypertension in primary care: a longitudinal cohort study in the Clinical Practice Research Datalink. BMJ Open 2018; 8: e021827–e021827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jrs-10.1177_01410768221077381 for Use of lifestyle interventions in primary care for individuals with newly diagnosed hypertension, hyperlipidaemia or obesity: a retrospective cohort study by Julia M Lemp, Meghana Prasad Nuthanapati, Till W Bärnighausen, Sebastian Vollmer, Pascal Geldsetzer and Anant Jani in Journal of the Royal Society of Medicine