Abstract
Postpartum depression (PPD) is the most common psychological health issue among women, which often comorbids with anxiety (PPD-A). PPD and PPD-A showed highly overlapping clinical symptoms. Identifying disorder-specific neurophysiological markers of PDD and PPD-A is important for better clinical diagnosis and treatments. Here, we performed functional connectivity density (FCD) and resting-state functional connectivity (rsFC) analyses in 138 participants (45 unmedicated patients with first-episode PPD, 31 PDD-A patients and 62 healthy postnatal women, respectively). FCD mapping revealed specifically weaker long-range FCD in right lingual gyrus (LG.R) for PPD patients and significantly stronger long-range FCD in left ventral striatum (VS.L) for PPD-A patients. The follow-up rsFC analyses further revealed reduced functional connectivity between dorsomedial prefrontal cortex (dmPFC) and VS.L in both PPD and PPD-A. PPD showed specific changes of rsFC between LG.R and dmPFC, right angular gyrus and left precentral gyrus, while PPD-A represented specifically abnormal rsFC between VS.L and left ventrolateral prefrontal cortex. Moreover, the altered FCD and rsFC were closely associated with depression and anxiety symptoms load. Taken together, our study is the first to identify common and disorder-specific neural circuit disruptions in PPD and PPD-A, which may facilitate more effective diagnosis and treatments.
Keywords: postpartum depression, postpartum depression with anxiety, resting-state fMRI, functional connectivity density, functional connectivity
Introduction
Childbirth is an exciting, greatly anticipated and desired life event. Yet, it also brings physical and psychological challenges to the parents, especially to the mother. Early motherhood could be vulnerable with a high prevalence and incidence of postpartum depression (PPD) and postpartum anxiety (Lee and Chung, 2007; Marcus, 2009; Smith et al., 2011; Tebeka et al., 2016) due to the immense alternations of biological, financial, social conditions of the mother, as well as the infant’s vulnerability and safety concerns during this period (Leckman et al., 1999), PPD and postpartum anxiety pose tremendous negative impacts on the well-being of the mother. Further, maternal depression also influences infants’ physical, cognitive and mental health development (Glasheen et al., 2010; Drury et al., 2016).
PPD and postpartum anxiety share numerous common symptoms with generalized depression and anxiety that occurs at other times in a woman’s life. Yet, emerging evidence indicates that PPD is distinct from major depressive disorder (MDD) with respect to symptom severity, hormone contributions, heritability, epigenetic mechanisms and response to standard and novel treatment interventions (Batt et al., 2020). Moreover, unique neural circuits underlying PPD are also identified when compared with generalized depression (Pawluski et al., 2017). In a resting-state functional magnetic resonance imaging (rsfMRI) study, PPD patients showed hypoactivities in both cortical and limbic regions, while non-parturient patients showed hypoactivity in lateral cognitive regions and hyperactivity in medial affective and subcortical limbic regions (Alcaro et al., 2010; Northoff et al., 2011; Pawluski et al., 2017). In addition, the neurobiological distinctions between postpartum anxiety and non-postpartum anxiety are also reported in previous fMRI studies (Moses-Kolko et al., 2010; Gingnell et al., 2015). Moreover, PPD is often comorbid with anxiety (PPD-A), with overlapping symptomatology, genetic and risk factors (Ohara and Mccabe, 2013). Thus, it is important to reveal the unique neural profiles for PPD and PPD-A, so as to delineate their neurophysiological basis for effective treatments.
rsfMRI is extensively adopted to evaluate functional couplings between spatially distinct cortical areas by capturing low-frequency fluctuations of spontaneous brain activity (Fox and Raichle, 2007; Wang et al., 2020b). Cortical and subcortical hubs play an important role in energy-efficient neuronal communication and information integration (Laughlin and Sejnowski, 2003; Barabasi, 2009). Alterations to the functional hubs are closely linked to cognitive disturbances among brain systems (i.e. neuropsychiatric disorders) (Buckner et al., 2009; Wu et al., 2016). In the current study, we used voxel-level functional connectivity density (FCD) mapping, an ultrafast, novel data-driven approach, which has recently, developed to identify functional hubs with high sensitivity and reproducibility (Tomasi and Volkow, 2011). A large number of studies have illustrated the effectiveness of the FCD mapping approach in investigating the abnormal dynamics of functional hubs in patients with neuropsychological diseases (Tomasi and Volkow, 2012a; Liu et al., 2015; Zhang et al., 2015; Wang et al., 2017).
In this study, we used the FCD approach to examine the shared and disorder-specific abnormal functional integration and communications in first-episode, treatment-naive PDD and PPD-A patients and healthy individuals. This method provides an objective, unbiased perspective in observing the common and disorder-specific neuropathology of PPD and PPD-A and also facilitates future developments of effective diagnosis and treatment strategies.
Materials and methods
Participants
The data were collected from a longitudinal project that aimed to investigate the determinants of health, well-being of patients with PPD in Chengdu, China. From 1 June 2018 to 1 January 2020, postpartum women were screened and recruited at the Maternity clinic (within 1 year after birth), West China Second University Hospital of Sichuan University (Table 1 for details). One hundred thirty-eight right-handed, age- and education-matched postnatal women, who have normal puerperium and healthy infant, were enrolled in this study. Finally, 45 drug-naive patients with PPD, 31 drug-naive PPD-A and 62 healthy postnatal women (HPW) were included. All enrolled patients were experiencing their first episode of depressive symptoms and were treatment-naive. Written informed consents were obtained from all participants. This study was approved by the local ethics committee of West China Second University Hospital of Sichuan University followed by the Helsinki Declaration. All participants conducted psychiatry assessments, sex hormone test (prolactin, estradiol and progesterone) and MRI scanning. The diagnosis of PPD or PPD-A was made according to the criteria of the Structured Clinical Interview for DSM-IV Axis I Disorders by two experienced psychiatrists at the psychiatry department, West China Hospital of Sichuan University. All participants were free from medical diseases such as cardiovascular diseases and diabetes, depression or anxiety and any other Axis I mental disorders such as schizophrenia, bipolar disorder and substance dependence (other than nicotine). None had a history of suicide, alcohol or drug abuse, hormonal contraception, and none were undergoing psychotropic medications, vasoactive medications, cognitive behavior therapy or MRI contradiction.
Table 1.
Demographics and clinical characteristics of the subjects used in present study
| HC (n = 62) | PPD (n = 45) | PPD-A (n = 31) | F value | HC vs PPD | HC vs PPD-A | PPD vs PPD-A | |
|---|---|---|---|---|---|---|---|
| Age (years) | 32.42 ± 3.92 | 31.11 ± 3.19 | 31.03 ± 3.83 |
F
2,135 = 2.29 (P = 0.11) |
P = 0.069 | P = 0.11 | P = 0.92 |
| Education (years) | 16.5 ± 1.61 | 16.69 ± 1.92 | 16.52 ± 1.57 |
F
2,135 = 0.18 (P = 0.84) |
P = 0.58 | P = 0.96 | P = 0.68 |
| Postpartum time (days) | 96.27 ± 58.86 | 94.29 ± 56.29 | 100.35 ± 53.16 |
F
2,135 = 0.11 (P = 0.9) |
P = 0.86 | P = 0.75 | P = 0.64 |
| Estradiol (pg/ml) | 81.23 ± 372.8 | 67.2 ± 241.09 | 32.01 ± 19.5 |
F
2,135 = 0.31 (P = 0.74) |
P = 0.83 | P = 0.47 | P = 0.42 |
| Progesterone (ng/ml) | 0.79 ± 1.41 | 0.85 ± 2.75 | 0.92 ± 1.67 |
F
2,135 = 0.04 (P = 0.96) |
P = 0.89 | P = 0.71 | P = 0.9 |
| Prolactin (ng/ml) | 77.66 ± 67.39 | 91.44 ± 70.23 | 65.95 ± 53.37 |
F
2,135 = 1.43 (P = 0.24) |
P = 0.31 | P = 0.4 | P = 0.09 |
| EPDS scores | 7.06 ± 4.14 | 16.2 ± 3.22 | 18.87 ± 5.46 |
F
2,135 = 104.07 (P < 10–27) |
P < 10–21 | P < 10–18 | P = 0.0092 |
| BAI scores | 30.73 ± 7.23 | 37.18 ± 5.94 | 53.71 ± 10.23 |
F
2,135 = 93.83 (P < 10–25) |
P < 10–5 | P < 10–20 | P < 10–12 |
| PSQI | 6.85 ± 3.41 | 9.29 ± 3.46 | 10.71 ± 3.85 |
F
2,135 = 13.94 (P < 10–5) |
P < 0.001 | P < 10–5 | P = 0.097 |
| PSQ: emotion importance | 52.79 ± 10.16 | 54.04 ± 8.36 | 53.68 ± 10.23 |
F
2,135 = 0.24 (P = 0.79) |
P = 0.5 | P = 0.69 | P = 0.86 |
| emotion support | 46.08 ± 11.39 | 38.49 ± 10.83 | 34.42 ± 9.06 |
F
2,135 = 14.02 (P < 10–5) |
P < 10–3 | P < 10–5 | P = 0.09 |
| material importance | 48.81 ± 7.85 | 49.82 ± 7.98 | 47.61 ± 8.59 |
F
2,135 = 0.69 (P = 0.50) |
P = 0.51 | P = 0.50 | P = 0.25 |
| material support | 45.66 ± 8.85 | 40.67 ± 11.1 | 36.84 ± 9.35 |
F
2,135 = 9.35 (P < 10–3) |
P < 0.011 | P < 10–4 | P = 0.12 |
| information importance | 57.73 ± 7.7 | 58 ± 9.58 | 55 ± 10.12 |
F
2,135 = 1.23 (P = 0.29) |
P = 0.87 | P = 0.15 | P = 0.19 |
| information support | 50.39 ± 9.33 | 46.6 ± 10.18 | 42.35 ± 9.7 |
F
2,135 = 7.29 (P < 10–3) |
P < 0.049 | P < 10–3 | P = 0.073 |
| comparison importance | 25.13 ± 4.82 | 24.91 ± 5.84 | 24.32 ± 7.04 |
F
2,135 = 0.21 (P = 0.81) |
P = 0.83 | P = 0.52 | P = 0.69 |
| comparison support | 23.94 ±5.39 | 21.51 ± 6.14 | 20.48 ± 6.08 |
F
2,135 = 4.39 (P < 0.014) |
P < 0.033 | P < 0.0064 | P = 0.47 |
One-way ANOVA was first used to identify differences in demographics and clinical characteristics. Post hoc two-sample t-tests were further used to determine the between-group differences in all the indices. A Pearson chi-squared test was used for gender comparison. Two-sample t-tests were used for age and education comparisons. HC: healthy control.
Clinical assessments
The depression and anxiety loads were assessed with Edinburgh Postnatal Depression Scale (EPDS) (Cox et al., 1987) and Beck’s Anxiety Inventory (BAI) (Beck et al., 1988). The quality of sleep was evaluated using Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989). Moreover, we evaluated the postpartum social support level with the Postpartum Support Questionnaire (PSQ) (Logsdon and Usui, 2006). PSQ is used to measure the expected support and the received support of postpartum women (Logsdon et al., 1996), which includes four sub-scales: (i) material, (ii) emotional, (iii) informational and (iv) comparison.
MRI data acquisition
The rsfMRI data were acquired on a clinical Siemens 3.0 T MRI scanner using a standard echo planar imaging (EPI) sequence at the West China Second University Hospital of Sichuan University. The foam padding and earplugs were used to reduce head motion and to muffle scanner noise. Before scanning, participants were instructed to relax, to remain awake with their eyes closed and to avoid thinking during the MRI acquisition. Experimenters confirmed that none of them fell asleep during the scan. Resting-state functional images were collected with repetition time = 3.05 s, echo time = 22.5 ms, flip angle = 30°, 36 slices, thickness/gap = 4.0/1 mm, voxel size = 2.45 × 2.45 × 4 mm3, matrix size = 94 × 94 and field of view = 230 × 230 mm2.
rsfMRI data preprocessing
The rsfMRI data were preprocessed with the following steps: discarding the first six volumes to allow for magnetization equilibrium; realigning all the images to the first volume to reduce head motion; normalizing all the images to the Montreal Neurological Institute EPI template and resampled at 3 × 3 × 3mm3; regressing out Friston 24-parameter model of head motion, white matter, cerebrospinal fluid and global mean signals; filtering with a temporal band pass of 0.01–0.1 Hz. For FCD calculation, no spatial smoothing was adopted to control the smoothing effect on local correlations (Tomasi and Volkow, 2012d). For the resting-state functional connectivity (rsFC) analysis, the normalized fMRI images were smoothed using a Gaussian kernel of 6 mm full width at the half maximum (FWHM), and then regression and filtering were completed. To minimize the head motion effects, participants with a displacement of more than 2.5 mm and an angular motion of more than 2.5° were excluded. No participants were excluded under this criterion. Moreover, scrubbing method with linear interpolation was further used to eliminate the bad images exceeding the pre-set criteria (frame displacement: FD; FD < 0.5) for excessive motion.
FCD mapping
To identify the abnormalities of brain hubs in PPD and PPD-A, FCD mapping was used to identify the functional hubs in the brain and to characterize the differences in functional integration in this study (Tomasi and Volkow, 2010, 2012b). FCD maps mainly include local/short-range (lFCD) and long-range FCD (lrFCD) and are calculated within a gray matter (GM) mask by counting the number of voxels with rsFC strength above 0.6. Previous studies have demonstrated that 0.6 is the most stable and optimal threshold in revealing the functional modules in the brain (Tomasi and Volkow, 2010, 2012b, 2012d; Tomasi et al., 2016; Zhang et al., 2016; Zou et al., 2016). Connectivity strength less than 0.4 increases false-positive rates, while connectivity strength greater than 0.7 leads to FCD maps with reduced dynamic range and lower sensitivity (Tomasi and Volkow, 2010). Thus, we also adopted this threshold when calculating the FCD maps in this study. The lFCD at a given voxel x0 was calculated as the local number of connections, n(x0), between x0 and its neighboring voxels using a ‘growing’ algorithm. First, the FC between x0 and each voxel (xi) that is directly neighboring with x0 was calculated, and the FC greater than 0.6 was considered functionally connected to x0. Next, the FC between x0 and xj that is directly neighboring with xi not with x0 was computed, and the FC greater than 0.6 was considered functionally connected to x0 as a neighbor. This search strategy was continued until no further voxels could be included. This procedure was repeated for all GM voxels to obtain a whole-brain lFCD map for each subject. To calculate lrFCD, a voxel-wise whole-brain global FCD (gFCD) map was first calculated in each subject. The gFCD at x0 was calculated as the total number of connections, n(x0), between x0 and all other voxels with connectivity strength above 0.6 in the GM mask. Then, the lrFCD was defined as gFCD minus lFCD to remove the number of local connections. To improve the normality, the lFCD and lrFCD maps were normalized using z-transformation for each subject and were spatial smoothing with FWHM = 6 mm for statistical analyses.
Seed-based functional connectivity analyses
Whole-brain rsFC analysis was performed to further identify whether the brain areas with changes in local or long-range FCD showed altered distal functional couplings, The Fisher’s z-transformation was used to convert functional connectivity (FC) value to z value for statistical analyses.
Statistical analyses
The analysis of variance (ANOVA) was performed with age and head motion parameter of mean FD values added as covariates in order to identify the differences in local and short-range FCD and rsFC. Significance was determined using a cluster-level Monte Carlo simulation-corrected threshold of P < 0.05 (cluster-forming threshold at voxel-level P < 0.001).
Functional characterization with BrainMap database
We also examined which behavioral domains were significantly associated with the observed changes in FCD and functional connectivities using the behavioral domain analysis on the BrainMap database (www.brainmap.org). The behavioral domain analysis included 5 behavioral domains and 51 behavioral sub-domains, and the functional characterization of the regions was determined using forward inferences (Bzdok et al., 2013). The significance level was established using a binomial test [P < 0.05 corrected for multiple comparisons using false discovery rate (FDR)]. The detailed procedures for functional characterization have been described in our previous studies (Wang et al., 2017, 2019, 2020b).
Correlation analysis
To explore whether the altered FCD or rsFC was associated with the clinical performances, the correlation analyses were performed and the significant level was set at P < 0.05 corrected with FDR-BH method.
Results
Demographic and clinical characteristics
Participants in HPW, PPD and PPD-A groups were well matched in age (P = 0.11), education level (P = 0.84) and postpartum time (P = 0.9). No significant differences in estradiol (P = 0.74), progesterone (P = 0.96) and prolactin (P = 0.24) among the three groups were found. ANOVA analyses revealed significant main effects of groups for depressive symptom load (EPDS, F2,135 = 104.07, P ≪ 0.001), anxiety symptom load (BAI, F2,135 = 93.83, P ≪ 0.001), sleep quality (PSQI, F2,135 = 13.94, P ≪ 0.001) and received social support (PSQ, F2,135 = 14.02, P ≪ 0.001). Post hoc analyses indicated that depressive and anxiety symptom loads were higher in both PPD and PPD-A patients compared to healthy individuals and in PDD-A compared to PPD patients. The sleep quality and received social support were lower in both PPD and PPD-A relative to HPW, but no significant differences were observed between the PPD and PPD-A groups (Table 1 for details).
FCD mapping results
Significant differences in long-range FCD in right lingual gyrus (LG.R) and left ventral striatum (VS.L) were found (Table 2), while the difference of the local FCD in these regions were not significant (Figure 1). Post hoc analysis identified significantly weaker lrFCD in LG.R in PPD patients as compared to PPD-A and HPW, and no significant difference was found between PPD-A and HPW (Figure 1). VS.L showed significantly stronger lrFCD in PPD-A as compared to PPD and HPW, but the difference between PPD and HPW (Figure 1) was not significant. FCD mapping revealed special circuit disruptions in PPD and PPD-A patients.
Table 2.
Regions with changed long-range FCD and FC in HPW, PPD and PPD-A patients
| Peak MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Indices | Brain regions | L/R | X | Y | Z | F values |
| FCD: | Lingual gyrus | R | 18 | −90 | −6 | 12.03 |
| Ventral striatum | L | −27 | −6 | 9 | 11.69 | |
| FC: LG.R | Angular gyrus | L | 42 | −75 | 45 | 10.56 |
| Dorsomedial prefrontal cortex | L | −9 | 33 | 48 | 9.39 | |
| Precentral/postcentral gyrus | L | −33 | −24 | 60 | 12.94 | |
| FC: VS.L | Dorsomedial prefrontal cortex | R | −6 | 42 | 39 | 9.8 |
| Ventrolateral prefrontal cortex | L | −42 | 33 | 0 | 11.46 | |
One-way ANOVA revealed changed long-range FCD and rsFC among HPW, PPD and PPD-A. MNI: Montreal Neurological Institute.
Fig. 1.
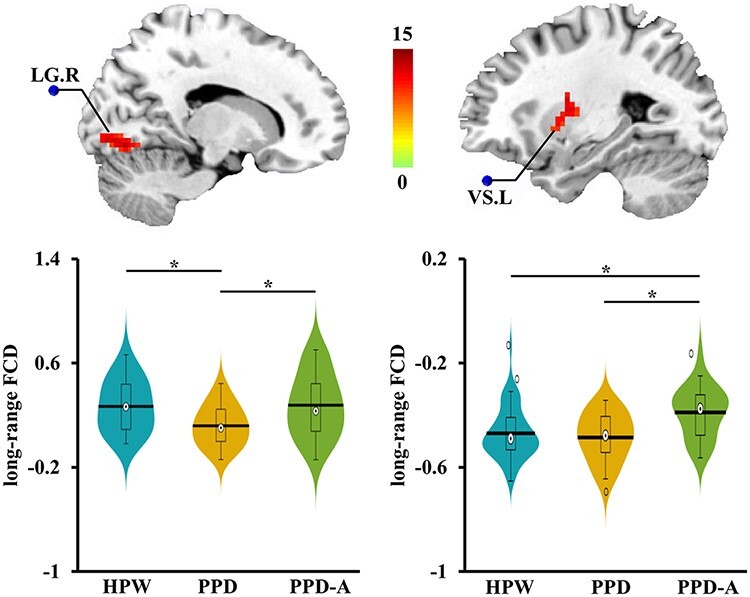
Altered long-range FCD maps. One-way ANOVA for FCD maps was performed and only found altered long-range FCD among HPW, PPD and PPD-A participants. Posthoc two-sample t-tests analyses identified decreased long-range FCD of LG.R in PPD compared to both HPW and PPD-A. No difference in long-range FCD of LG.R between PPD-A and HPW was found. In addition, increased long-range FCD of VS.L was found in PPD-A compared to both PPD and HPW. No difference in long-range FCD of VS.L between PPD and HPW was found.
Changed seed-based functional connectivities
Seed-based, whole-brain rsFC analyses were performed to identify the abnormal, distal functional couplings between LG.R and VS.L with other cortical regions. Significant differences in rsFC between LG.R and right angular gyrus (AG.R), bilateral dorsomedial prefrontal cortex (dmPFC), left precentral gyrus (PreCG.L) and between VS.L and left ventrolateral prefrontal cortex (vlPFC.L) and dmPFC were found (Figure 2A, B, Table 2). Post hoc analyses identified significantly increased rsFC between LG.R and AG.R, and significantly decreased rsFC between LG.R and dmPFC, PreCG.L in PPD patients as compared to PPD-A patients and healthy individuals (Figure 2A). No significant differences in rsFCs between LG.R and AG.R, dmPFC and PreCG were found between HPW and PPD-A (Figure 2A). In addition, the rsFC between VS.L and dmPFC was stronger in HPW than that in both patient groups, but no significant difference was found between PPD and PPD-A. The rsFC strength between VS.L and vlPFC.L was stronger in PPD-A than HPW and PPD patients, and no significant difference between HPW and PPD (Figure 2B). The rsFC analyses further revealed specific functional couplings for both PPD and PPD-A patients.
Fig. 2.
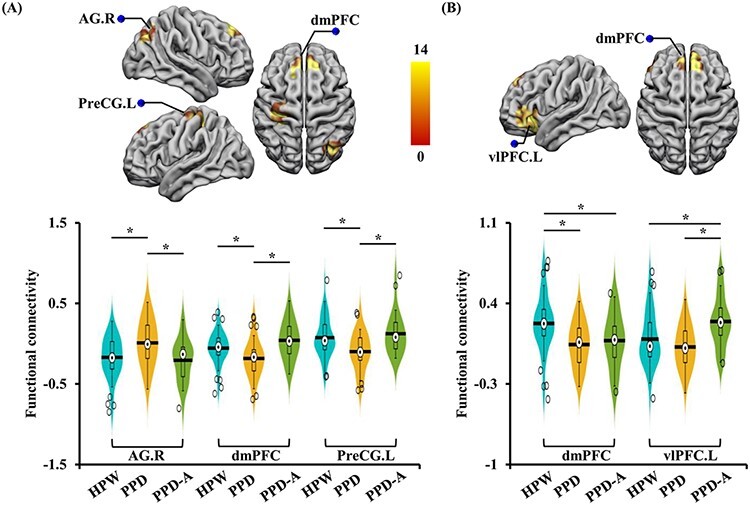
Altered rsFC of LG.R and VS.L. Seed-based FC analyses of LG.R identified increased functional couplings between LG.R and AG.R and decreased functional couplings between LG.R and dmPFC; PreCG.L in PPD compared to both PPD-A and HPW. (B) Seed-based FC analyses of VS.L identified decreased functional connectivities between VS.L and dmPFC in both PPD and PPD-A patients compared to HPW and increased functional connectivities between VS.L and vlPFC.L in PPD-A compared to both PPD and HPW.
Correlation analyses
In the PPD group, the lrFCD of LG.R was negatively correlated with postpartum time, while the rsFC between LG.R and AG.R was positively correlated with postpartum time. Positive correlations between rsFC of VS.L-vlPFC.L and the behavioral scores of Beck anxiety symptom load were found in both PPD and PPD-A patients. The rsFC between VS.L and dmPFC and lrFCD in LG.R were, respectively, positively correlated with behavioral scores of PPD symptom load and PSQ emotion support in all HPW, PPD and PPD-A participants (shown in Figure 3).
Fig. 3.
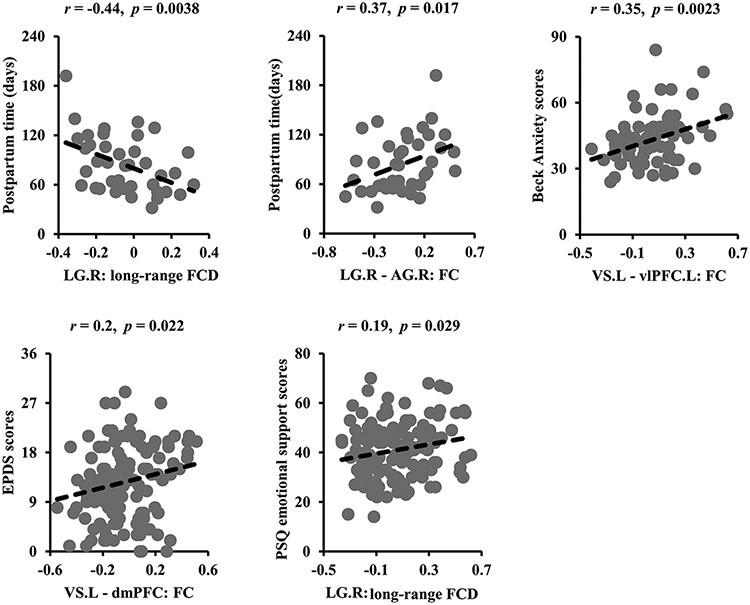
Associations between changed neural measures and clinical characteristics. Negative and positive correlations between postpartum time and long-range FCD of LG.R, functional connectivities of LG.R with AG.R in PPD patients were identified, respectively. Functional connectivities between VS.L and vlPFC.L were positively correlated with anxiety symptom load in PPD and PPD-A patients. The positive correlations between functional connectivities of VS.L with dmPFC, long-range FCD of LG.R and depression load and emotion support were, respectively, identified in all the HPW, PPD and PPD-A participants.
Meta-analysis based functional characterization
Functional characterization for each brain area with altered FCD or rsFC was performed with the BrainMap database. Functional characterization revealed significant associations between the following brain regions and brain functions: (i) LG.R with action preparation, language, orthography, visual shape and visual motion; (ii) VS.L with action execution; (iii) dmPFC with social cognition and emotion; (iv) AG.R with working memory; (v) PreCG.L with action execution, motor learning and somesthesis and (vi) vlPFC.L with language, gustation, semantics and explicit memory (Figure 4).
Fig. 4.
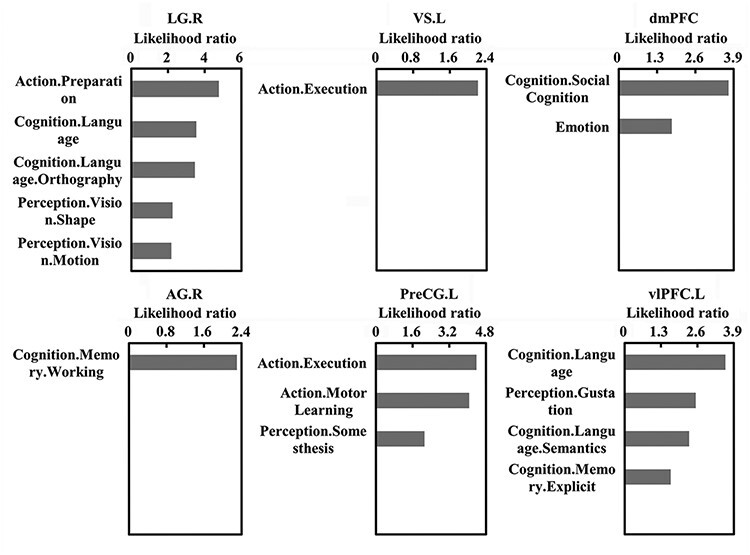
Functional characterization for the brain areas with changed long-range FCD or functional connectivities using BrainMap database. Behavioral characterization of the brain areas of LG.R, VS.L dmPFC, AG.R, PreCG.L and left inferior frontal gyrus (IFG.L) was performed. Only behavioral domains significantly associated with these brain areas at P < 0.05 (FDR corrected) are shown.
Common and specific circuits for PPD and PPD-A
In summary, the common and disorder-specific circuit disruptions were identified in PPD and PPD-A patients based on both FCD and FC results. The commonly disrupted circuit involves the medial fronto-striatal network, i.e. dmPFC-VS.L, which showed significantly weaker rsFC in both PPD and PPD-A patients as compared to HPW. Abnormalities in the lateral prefrontal-striatal circuit were observed in the PPD-A group only (indexed by a stronger rsFC in PPD-A group relative to PPD and HPW), while disruptions to the occipito-parieto-frontal circuit, including LG with AG, dmPFC and PreCG, were specifically identified in the PPD group (Figure 5).
Fig. 5.
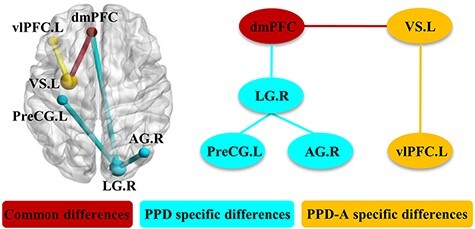
Common and specific neural circuits for PPD and PPD-A. Based on the findings from the previous long-range FCD and functional connectivities analyses, common and specific changes of PPD and PPD-A were revealed.
Discussion
This study applied a voxel-wise, data-driven approach to identify the common and disorder-specific neuromarkers for PPD and PPD-A using FCD mapping and FC analyses. PPD patients showed specifically weaker lrFCD in LG.R, functional couplings between LG.R and dmPFC and PreCG.L and specifically stronger functional coupling between LG.R and AG.R. For PPD-A, significantly higher FCD in VS.L and significantly stronger rsFC between VS.L and vlPFG.L were found. Moreover, both PPD and PPD-A patients exhibited weaker intrinsic functional coupling between VS.L and dmPFC as compared to HPW. Correlation analyses further confirmed the neural measurements in these abnormal brain areas were significantly associated with clinical measures, including postpartum time, depression and anxiety loads and emotion support. Our findings identified common and disorder-specific neural circuit disruptions in PPD and PPD-A, consequently shedding light on the potential neurophysiological mechanisms underlying different PPD phenotypes.
A common medial frontal-striatal neural circuit for PPD and PPD-A
The core symptoms of depression are characterized by low mood and/or by a persistent loss of overall interest, pleasure (hedonism) or reward in life. Our findings showed that the medial prefrontal-striatal network, i.e. dmPFC with ventral striatum (VS), was disrupted in both PPD and PPD-A patients. The VS is the hub for hedonia and reinforcement reward by modulating dopamine and serotonin releases, and the connections of the limbic structures with the prefrontal cortices. The previous fMRI studies in HPW have confirmed that VS is activated in response to the pleasant stimulus of one’s own infant smiling (Strathearn et al., 2008), and the activity level of VS is modulated by the quality of maternal attachment security (Strathearn et al., 2009). Yet, in PPD patients, reduced activation of the VS was found in response to reward stimuli and positive words (Laurent and Ablow, 2012; Silverman et al., 2007), thus implicating the importance of the VS in maintaining positive emotion and hedonism/reward in postpartum period for the health of the mother–infant dyad.
Task-based fMRI studies have indicated stronger activity of the dmPFC when participants were instructed to deploy cognitive strategies in reducing negative emotional experience (Ochsner and Gross, 2005). As a nexus of the central executive network, the affective network and the default mode network, the dmPFC plays a key role not only in social cognition and cognitive control (Garavan et al., 2002; Seeley et al., 2007) but also in regulating both positive and negative emotions (Ochsner et al., 2004). The dysfunction of dmPFC was reported in individuals with depressive symptoms (Sheline et al., 2010), and functional recovery could predict the treatment response in MDD patients (Bai et al., 2019; Wang et al., 2020a). The dmPFC is also engaged in cognitive reappraisal through reinterpreting the meaning of affective stimuli to alter emotional impacts. Successful reappraisal involves both inhibiting activity of the dmPFC-amygdala pathway and evoking activity in the dmPFC–VS pathway. The dmPFC exerts top-down modulation of reward-related firing to influence VS activity (Kim and Hamann, 2007; Arco and Mora, 2008), while the VS sends out signals to the dmPFC for the purpose of reward-based motivation and learning (Moses-Kolko et al., 2011). In healthy individuals, task-based fMRI studies found that anticipation and reward-receiving were associated with significantly stronger activation in the VS (Delgado et al., 2003), whereas the dmPFC was activated during learning of reward-based contingencies (O’Doherty, 2004). Thus, the reduced dmPFC–VS functional connection might be related to disrupted cognitive control and reward processing in both PPD and PPD-A patients.
A specific lateral fronto-striatal neural circuit for PPD-A
Previous studies have demonstrated that anxiety symptoms primarily caused by the disturbances of specific neural circuits, in particular, the prefrontal cortex (PFC) and the amygdala loop, which are known to involve in detecting and responding to threat-related cues in the surrounding environment (Davis and Whalen, 2001; Hariri et al., 2003; Monk et al., 2008). The PFC exerts a top-down control over the limbic structures in regulating emotion categorization (Meaux and Vuilleumier, 2016) and modulating attention bias to threat (Monk et al., 2006; Bishop, 2007). Specifically, the prefrontal-limbic circuit is a neurocognitive model that mediates threat-related attention orientation (Bishop, 2008; Shechner et al., 2012; Heeren et al., 2013). Within this network, the vlPFC plays an important role in modulating amygdala responses to threat to maintain goal-directed behavior (Corbetta and Shulman, 2002; Fox and Pine, 2012). Monk et al. found that adolescents with generalized anxiety disorder exhibited greater vlPFC response to angry faces compared to healthy adolescents (Monk et al., 2006). Hypoactivity in the vlPFC was associated with the reduction of subjective distress in anxiety-prone participants (Campbell-Sills et al., 2011). In early postpartum mothers (within 3 weeks), the stronger connection between vlPFC and VS is associated with heightened intrusive/anxious responses to infants’ cries, stimulating stronger mother–infant attachment (Judd et al., 2012), Consistent with the previous findings, the elevated rsFC strength between vlPFC and VS found in the PPD-A group may serve as a protective mechanism to modulate negative emotions and to regulate/ease anxiety symptoms (Shechner et al., 2012; Guyer et al., 2013).
A specific occipto-parieto-frontal neural circuit for PPD
Despite ample studies highlighting the frontal-limbic system in the neurobiology of depression, emerging evidence implicates that the activity levels of the LG and AG are closely related to depression (Sun et al., 2016, 2018; Wu et al., 2016; Wang et al., 2020a). Through direct links to the parietal cortex, frontal cortex and limbic system, LG plays an important role in processing and synthesis of visual information, visual memory, visuospatial attention and affective identification (Fujita, 2002; Collins and Olson, 2014) (Conrad and Stumpf, 1975; Bogousslavsky et al., 1987; Hopfinger et al., 2000). Aberrant structural and functional deficits in LG in depressed patients have been identified using fMRI tools (Desseilles et al., 2009; Veer et al., 2010; Ling-Li et al., 2012; Laurent and Jennifer, 2013; Jung et al., 2014). Recent studies reported reduced long-range FCD in LG and disrupted functional connections with frontal, parietal and posterior cingulate cortices, indicating that the LG may be a critical hub for visual recognition circuit in depression (Sun et al., 2016, 2018). Besides, LG is demonstrated to be part of the ‘fear’ network to modulate fear processing (Carlson et al., 2009). In our study, we identified specifically weaker lrFCD in LG in PPD as compared to PPD-A and HPW. The weakened lrFCD is negatively correlated with postpartum time and positively correlated with emotion support. Our findings highlight that the abnormal neural profile of LG may serve as a neural marker for PPD, possibly related to negative bias for visual cognition, attention and emotion processing through mother–infant interaction.
In the current study, specifically stronger FC between right LG and AG and specifically weaker FC between the LG and bilateral dmPFC were found. A past study showed that individuals with cognitive vulnerability to depression had greater functional couplings between the LG and the AG relative to HPW (Sun et al., 2018), and the right AG is primarily involved in spatial memory and attention reorienting (Thiel et al., 2004; Corbetta et al., 2008; Wang et al., 2016). Moreover, using the grapy-theory-based brain network analysis, Zhang and colleagues revealed reduced nodal centrality in AG with other visual regions (Zhang et al., 2011). Recent findings have also demonstrated that functional recoveries of the AG and the dmPFC in depressive patients contribute to the positive responses in electroconvulsive therapy (Wang et al., 2018, 2020a; Bai et al., 2019; Gao et al., 2020). These findings emphasize the critical role of the AG in processing emotionally relevant visual stimuli (Lang et al., 1998; Adolphs, 2002) and also support the hypothesis that top-down influences from the frontal-parietal regions on the visual areas could cause abnormalities in visual cognition in depressive patients (Drevets, 2010). Additionally, PPD patients showed specifically weaker rsFC in the dmPFC–LG connection, implicating a reduced top-down attention control. The specifically stronger rsFC between the LG and the AG, which showed a positive correlation with postpartum time, suggests a compensatory mechanism in visual processing or visual memory in postpartum women.
Another disorder-specific neural circuit disruption in PPD involves the decreased functional interaction between the LG and the PreCG. These regions are associated with emotion recognition, somatic symptoms and inhibitory control. The PreCG is involved in cognitive processing and emotion regulation (Seo et al., 2014), and abnormalities of this region could lead to emotion-recognition deficits (Adolphs, 2010). PPD patients accompanied by gastrointestinal symptoms have decreased GM volume in the right PreCG (Liu et al., 2019), and weaker FC between the LG and the PreCG was observed in regular depressive patients, even after they receive electroconvulsive therapeutic treatments (Wang et al., 2020a). More importantly, the PreCG has a potential role in impulsivity regulation, especially in regulating impulsive suicidal behaviors. At the same time, the PreCG and the neighboring motor areas also participate in executive response inhibition and inhibitory control (Bush et al., 1998; Nachev et al., 2008; Bari and Robbins, 2013; Jha et al., 2015). Deficits in inhibitory control is associated with a greater propensity to act on aggressive feelings (Mann, 2003) and is linked to suicide vulnerability in patients with MDD (Hwang et al., 2010; Sublette et al., 2013). Thus, the specifically weaker rsFC between the LG and the PreCG in PPD patients suggests the attenuated inhibitory control, which may lead to abnormal somatosensory symptoms and incurred vulnerability for impulsive behavior or increased risk of suicide.
Limitations
There were several limitations in our study. First, our study failed to identify the frequently reported brain abnormalities in the cingulate cortex, amygdala and orbitofrontal cortex. This inconsistent finding might be due to different sample sizes and methodological differences. Second, our study did not exclude potential confounding factors such as fertility pattern (natural or assisted), natural or caesarean section, primipara or multipara. A more delicately designed study examining the specific effects is needed. Third, we only applied resting-state not task fMRI for the cross-sectional study of PPD, PPD-A and HPW. Future investigations with multimodal fMRI, including both resting and task, are warranted to better reveal the neuropathology of PPD phenotype. Fourth, this study included PPD patients with anxiety, but patients only with anxiety without depression not included, which may be better to reveal the specific neural circuits. Finally, the non-PPD patients should be included to reveal the specific neural circuits for PPD in future studies.
Conclusion
The current study revealed common and disorder-specific neural circuits for PPD and PPD-A using the FCD and resting-state fMRI approach. Both PPD and PPD-A patients exhibited disrupted medial prefronto-striatal circuit (dmPFC-VS). Deficits in the lateral prefrontal-striatal circuit (vlPFC-VS) were observed in the PPD-A group only, while abnormal occipito-parieto-frontal circuit of LG with AG, dmPFC and PreCG was specifically identified in the PPD group. These findings suggest that disrupted cognitive control and reward may be the shared characteristics for both PPD and PPD-A. Attention bias, impaired maternal–infant interaction, somatic symptoms and inhibitory control may be primary characteristics for PPD, while excessively negative emotion regulation and overreaction to threat may lead to anxiety in PPD patients. The specific neurofunctional markers for PPD and PPD-A found in this study may provide directions to more effective treatment strategies for PPD and anxiety.
Contributor Information
Bochao Cheng, Department of Radiology, West China Second University Hospital of Sichuan University, Chengdu 610041, China; Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, China.
Yushan Zhou, Department of Nuclear Medicine, West China Hospital of Sichuan University, Chengdu 610041, China; Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu 610041, China.
Veronica P Y Kwok, Center for Language and Brain, Shenzhen Institute of Neuroscience, Shenzhen 518057, China.
Yuanyuan Li, Key Laboratory for NeuroInformation of the Ministry of Education, School of life Science and technology, University of Electronic Science and Technology of China, Chengdu 625014, China.
Song Wang, Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, China.
Yajun Zhao, School of Sociality and Psychology, Southwest Minzu University, Chengdu 610225, China.
Yajing Meng, Department of Psychiatry, West China Hospital of Sichuan University, Chengdu 610041, China.
Wei Deng, Department of Psychiatry, West China Hospital of Sichuan University, Chengdu 610041, China.
Jiaojian Wang, State Key Laboratory of Primate Biomedical Research, Institute of Primate Translational Medicine, Kunming University of Science and Technology, Kunming 650500, China.
Acknowledgments
The authors also would like to thank Dr Qiang Yao and Aiyun Xing, Department of Gynecology and Obstetrics, West China Second Hospital, Chengdu, for their instruction and assistance with patients enrollment.
Funding
This study was supported by the National Natural Science Foundation of China (No.: 62176044); the Sichuan Science and Technology Program (Grant No. 2021YJ0186, 2019YJ0080; 21YYJC2988); the National Key Technology R&D Program of the Ministry of Science and Technology of China (Grant No. 2016YFC1307200) and Scientific research project of Sichuan health planning committee (Grant No.20PJ078).
Author contribution
B.C. designed the study. B.C., Yu.Z., Ya.Z., Y.M. and W.D. diagnosed the patients and collected the data; J.W., Y.L. and S.W. analysed the data. J.W., B.C. and V.K. drafted the manuscript. All authors discussed the results and commented on the manuscript.
Conflict of interest
None declare.
Data availability
All data and code used for data analysis are available upon request.
References
- Adolphs R. (2002). Neural systems for recognizing emotion. Current Opinion in Neurobiology, 12, 169–77. [DOI] [PubMed] [Google Scholar]
- Adolphs R. (2010). Emotion. Current Biology, 20, R549. [DOI] [PubMed] [Google Scholar]
- Alcaro A., Panksepp J., Witczak J., Hayes D.J., Northoff G. (2010). Is subcortical-cortical midline activity in depression mediated by glutamate and GABA? A cross-species translational approach. Neuroscience Biobehavioral Reviews, 34, 592–605. [DOI] [PubMed] [Google Scholar]
- Arco A.D., Mora F. (2008). Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacology, Biochemistry, and Behavior, 90, 226–35. [DOI] [PubMed] [Google Scholar]
- Bai T., Wei Q., Zu M., et al. (2019). Functional plasticity of the dorsomedial prefrontal cortex in depression reorganized by electroconvulsive therapy: validation in two independent samples. Human Brain Mapping, 40, 465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabasi A.L. (2009). Scale-free networks: a decade and beyond. Science, 325, 412–3. [DOI] [PubMed] [Google Scholar]
- Bari A., Robbins T. (2013). Inhibition and impulsivity: behavioral and neural basis of response control. Progress in Neurobiology, 108, 44–79. [DOI] [PubMed] [Google Scholar]
- Batt M., Duffy K., Novick A., Metcalf C., Epperson C. (2020). Is postpartum depression different from depression occurring outside of the perinatal period? A review of the evidence. Focus, 18, 106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Epstein N., Brown G., Steer R.A. (1988). An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology, 56, 893–7. [DOI] [PubMed] [Google Scholar]
- Bishop S. (2008). Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences, 1129, 141–52. [DOI] [PubMed] [Google Scholar]
- Bishop S.J. (2007). Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences, 11, 307–16. [DOI] [PubMed] [Google Scholar]
- Bogousslavsky J., Miklossy J., Deruaz J.P., Assal G., Regli F. (1987). Lingual and fusiform gyri in visual processing: a clinico-pathologic study of superior altitudinal hemianopia. Journal of Neurology, Neurosurgery, and Psychiatry, 50, 607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Sepulcre J., Talukdar T., et al. (2009). Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29, 1860–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Whalen P.J., Rosen B.R., Jenike M.A., McInerney S.C., Rauch S.L. (1998). The counting Stroop: an interference task specialized for functional neuroimaging—validation study with functional MRI. Human Brain Mapping, 6, 270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D.J., Iii C.F.R., Monk T.H., Berman S.R., Kupfer D.J. (1989). The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Research-neuroimaging, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Bzdok D., Laird A.R., Zilles K., Fox P.T., Eickhoff S.B. (2013). An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Human Brain Mapping, 34, 3247–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L., Simmons A.N., Lovero K.L., Rochlin A.A., Paulus M.P., Stein M.B. (2011). Functioning of neural systems supporting emotion regulation in anxiety-prone individuals. NeuroImage, 54, 689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J.M., Reinke K.S., Habib R. (2009). A left amygdala mediated network for rapid orienting to masked fearful faces. Neuropsychologia, 47, 1386–9. [DOI] [PubMed] [Google Scholar]
- Collins J.A., Olson I.R. (2014). Beyond the FFA: the role of the ventral anterior temporal lobes in face processing. Neuropsychologia, 61, 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C.D., Stumpf W.E. (1975). Direct visual input to the limbic system: crossed retinal projections to the nucleus anterodorsalis thalami in the tree shrew. Experimental Brain Research, 23, 141–9. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron, 58, 306–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3, 201–15. [DOI] [PubMed] [Google Scholar]
- Cox J., Holden J.M., Sagovsky R. (1987). Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. British Journal of Psychiatry, 150, 782–6. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. (2001). The amygdala: vigilance and emotion. Molecular Psychiatry, 6, 13–34. [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Locke H.M., Stenger V.A., Fiez J.A. (2003). Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cognitive, Affective and Behavioral Neuroscience, 3, 27–38. [DOI] [PubMed] [Google Scholar]
- Desseilles M., Balteau E., Sterpenich V., et al. (2009). Abnormal neural filtering of irrelevant visual information in depression. Journal of Neuroscience the Official Journal of the Society for Neuroscience, 47, S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W.C. (2010). Orbitofrontal cortex function and structure in depression. Annals of the New York Academy of Sciences, 1121, 499–527. [DOI] [PubMed] [Google Scholar]
- Drury S.S., Scaramella L., Zeanah C.H. (2016). The neurobiological impact of postpartum maternal depression: prevention and intervention approaches. Child Adolescent Psychiatric Clinics of North America, 25, 179–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8, 700–11. [DOI] [PubMed] [Google Scholar]
- Fox N.A., Pine D.S. (2012). Temperament and the emergence of anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita I. (2002). The inferior temporal cortex: architecture, computation, and representation. Journal of Neurocytology, 31, 359–71. [DOI] [PubMed] [Google Scholar]
- Gao J., Li Y., Wei Q., et al. (2020). Habenula and left angular gyrus circuit contributes to response of electroconvulsive therapy in major depressive disorder. Brain Imaging and Behavior, 15, 2246–53. [DOI] [PubMed] [Google Scholar]
- Garavan H., Ross T.J., Murphy K., Roche R.A., Stein E.A. (2002). Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage, 17, 1820–9. [DOI] [PubMed] [Google Scholar]
- Gingnell M., Bannbers E., Moes H., et al. (2015). Emotion reactivity is increased 4-6 weeks postpartum in healthy women: a longitudinal fMRI study. PLoS One, 10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasheen C., Richardson G.A., Fabio A. (2010). A systematic review of the effects of postnatal maternal anxiety on children. Archives of Womens Mental Health, 13, 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Masten C.L., Pine D.S. (2013). Neurobiology of pediatric anxiety disorders. In: Vasa, R., Roy, A., editors. Pediatric Anxiety Disorders. Current Clinical Psychiatry, New York, NY: Humana Press. [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., Fera F., Weinberger D.R. (2003). Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry, 53, 494–501. [DOI] [PubMed] [Google Scholar]
- Heeren A., De Raedt R., Koster E.H., Philippot P. (2013). The (neuro)cognitive mechanisms behind attention bias modification in anxiety: proposals based on theoretical accounts of attentional bias. Frontiers in Human Neuroscience, 7, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger J., Buonocore M., Mangun G. (2000). The neural mechanisms of top-down attentional control. Nature Neuroscience, 3, 284–91. [DOI] [PubMed] [Google Scholar]
- Hwang J.-P., Lee T.-W., Tsai S.-J., et al. (2010). Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. Journal of Geriatric Psychiatry and Neurology, 23, 171–84. [DOI] [PubMed] [Google Scholar]
- Jha A., Nachev P., Barnes G., Husain M., Brown P., Litvak V. (2015). The frontal control of stopping. Cerebral Cortex (New York, N.Y.: 1991), 25, 4392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd L., Schettler P., Akiskal H., et al. (2012). Prevalence and clinical significance of subsyndromal manic symptoms, including irritability and psychomotor agitation, during bipolar major depressive episodes. Journal of Affective Disorders, 138, 440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J., Kang J., Won E., et al. (2014). Impact of lingual gyrus volume on antidepressant response and neurocognitive functions in Major Depressive Disorder: a voxel-based morphometry study. Journal of Affective Disorders, 169, 179–87. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Hamann S. (2007). Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroence, 19, 776–98. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Fitzsimmons J.R., et al. (1998). Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology, 35, 199–210. [PubMed] [Google Scholar]
- Laughlin S.B., Sejnowski T.J. (2003). Communication in neuronal networks. Science, 301, 1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent H.K., Ablow J.C. (2012). A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Social Cognitive and Affective Neuroscience, 7, 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent H.K.A., Jennifer C. (2013). A face a mother could love: depression-related maternal neural responses to infant emotion faces. Social Neuroscience, 8, 228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman J.F., Mayes L.C., Feldman R., Evans D.W., King R.A., Cohen D.J. (1999). Early parental preoccupations and behaviors and their possible relationship to the symptoms of obsessive-compulsive disorder. Acta Psychiatrica Scandinavica, 100, 1–26. [DOI] [PubMed] [Google Scholar]
- Lee D.T.S., Chung T.K.H. (2007). Postnatal depression: an update. Best Practice Research in Clinical Obstetrics Gynaecology, 21, 183–91. [DOI] [PubMed] [Google Scholar]
- Ling-Li Z., Hui S., Li L., et al. (2012). Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain: A Journal of Neurology, 1498, 1498–507. [DOI] [PubMed] [Google Scholar]
- Liu B., Fan L., Cui Y., et al. (2015). DISC1 Ser704Cys impacts thalamic-prefrontal connectivity. Brain Structure and Function, 220, 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Li G., Zhang A., et al. (2019). The prognosis and changes of regional brain gray matter volume in MDD with gastrointestinal symptoms. Neuropsychiatric Disease and Treatment, 15, 1181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon M.C., Usui W., Birkimer J.C., McBride A.B. (1996). The postpartum support questionnaire: reliability and validity. Journal of Nursing Measurement, 4, 129–42. [PubMed] [Google Scholar]
- Logsdon M.C., Usui W.M. (2006). The postpartum support questionnaire: psychometric properties in adolescents. Journal of Child and Adolescent Psychiatric Nursing, 19, 145–56. [DOI] [PubMed] [Google Scholar]
- Mann J.J. (2003). Neurobiology of suicidal behaviour. Nature Reviews Neuroscience, 4, 819–28. [DOI] [PubMed] [Google Scholar]
- Marcus S.M. (2009). Depression during pregnancy: rates, risks and consequences—Motherisk update 2008. The Canadian Journal of Clinical Pharmacology, 16, e15. [PubMed] [Google Scholar]
- Meaux E., Vuilleumier P. (2016). Facing mixed emotions: analytic and holistic perception of facial emotion expressions engages separate brain networks. NeuroImage, 141, 154–73. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Nelson E.E., McClure E.B., et al. (2006). Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. The American Journal of Psychiatry, 163, 1091–7. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Telzer E.H., Mogg K., et al. (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry, 65, 568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko E.L., Perlman S.B., Wisner K.L., James J., Saul A.T., Phillips M.L. (2010). Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. The American Journal of Psychiatry, 167, 1373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko E.L., Fraser D., Wisner K.L., et al. (2011). Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biological Psychiatry, 70, 395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P., Kennard C., Husain M. (2008). Functional role of the supplementary and PRE-supplementary motor areas. Nature Reviews Neuroscience, 9, 856–69. [DOI] [PubMed] [Google Scholar]
- Northoff G., Wiebking C., Feinberg T.E., Panksepp J. (2011). The ‘resting-state hypothesis’ of major depressive disorder—A translational subcortical–cortical framework for a system disorder. Neuroscience Biobehavioral Reviews, 35, 1929–45. [DOI] [PubMed] [Google Scholar]
- O’Doherty J.P. (2004). Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology, 14, 769–76. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage, 23, 483–99. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–9. [DOI] [PubMed] [Google Scholar]
- Ohara M.W., Mccabe J.E. (2013). Postpartum depression: current status and future directions. Annual Review of Clinical Psychology, 9, 379–407. [DOI] [PubMed] [Google Scholar]
- Pawluski J.L., Lonstein J.S., Fleming A.S. (2017). The neurobiology of postpartum anxiety and depression. Trends in Neurosciences, 40, 106–20. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27, 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D., Olman C.A., Haut K.M., Sinha R., MacDonald A.W. 3rd, Patrick C.J. (2014). Neural correlates of preparatory and regulatory control over positive and negative emotion. Social Cognitive and Affective Neuroscience, 9, 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T., Britton J.C., Pérez-Edgar K., et al. (2012). Attention biases, anxiety, and development toward or away from threats or rewards? Depression and Anxiety, 29, 282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Yan Z., Mintun M.A. (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America, 107, 11020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M.E., Loudon H., Safier M., et al. (2007). Neural dysfunction in postpartum depression: an fMRI pilot study. CNS Spectrums, 12, 853–62. [DOI] [PubMed] [Google Scholar]
- Smith M.V., Shao L., Howell H.B., Lin H., Yonkers K.A.J.M., Journal C.H. (2011). Perinatal depression and birth outcomes in a healthy start project. Maternal and Child Health Journal, 15, 401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L., Li J., Fonagy P., Montague P.R. (2008). What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics, 122, 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L., Fonagy P., Amico J., Montague P.R. (2009). Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology Official Publication of the American College of Neuropsychopharmacology, 34, 2655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sublette M.E., Milak M., Galfalvy H., Oquendo M., Malone K., Mann J. (2013). Regional brain glucose uptake distinguishes suicide attempters from non-attempters in major depression. Archives of suicide research. Official Journal of the International Academy for Suicide Research, 17, 434–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Luo L., Yuan X., et al. (2018). Regional homogeneity and functional connectivity patterns in major depressive disorder, cognitive vulnerability to depression and healthy subjects. Journal of Affective Disorders, 235, 229–35. [DOI] [PubMed] [Google Scholar]
- Sun X., Fei G., Qing L., Zhiliang C., Huafu Z. (2016). Abnormal functional connectivity density in first-episode, drug-naive adult patients with major depressive disorder. Journal of Affective Disorders, 194, 153–8. [DOI] [PubMed] [Google Scholar]
- Tebeka S., Strat Y.L., Dubertret C. (2016). Developmental trajectories of pregnant and postpartum depression in an epidemiologic survey. Journal of Affective Disorders, 203, 62–8. [DOI] [PubMed] [Google Scholar]
- Thiel C.M., Zilles K., Fink G.R. (2004). Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: an event-related fMRI study. NeuroImage, 21, 318–28. [DOI] [PubMed] [Google Scholar]
- Tomasi D., Shokri-Kojori E., Volkow N.D. (2016). High-resolution functional connectivity density: hub locations, sensitivity, specificity, reproducibility, and reliability. Cerebral Cortex, 26, 3249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. (2010). Functional connectivity density mapping. Proceedings of the National Academy of Sciences of the United States of America, 107, 9885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. (2011). Functional connectivity hubs in the human brain. NeuroImage, 57, 908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. (2012a). Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biological Psychiatry, 71, 443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. (2012b). Aging and functional brain networks. Molecular Psychiatry, 17, 549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. (2012d). Resting functional connectivity of language networks: characterization and reproducibility. Molecular Psychiatry, 17, 841–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer I., Beckmann C., Tol M.-J., et al. (2010). Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Frontiers in Systems Neuroscience, 4, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhang J., Rong M., et al. (2016). Functional topography of the right inferior parietal lobule structured by anatomical connectivity profiles. Human Brain Mapping, 37, 4316–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wei Q., Yuan X., et al. (2017). Local functional connectivity density is closely associated with the response of electroconvulsive therapy in major depressive disorder. Journal of Affective Disorders, 225, 658–64. [DOI] [PubMed] [Google Scholar]
- Wang J., Wei Q., Wang L., et al. (2018). Functional reorganization of intra- and internetwork connectivity in major depressive disorder after electroconvulsive therapy. Human Brain Mapping, 39, 1403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Becker B., Wang L., Li H., Zhao X., Jiang T. (2019). Corresponding anatomical and coactivation architecture of the human precuneus showing similar connectivity patterns with macaques. NeuroImage, 200, 562–74. [DOI] [PubMed] [Google Scholar]
- Wang J., Ji Y., Li X., et al. (2020a). Improved and residual functional abnormalities in major depressive disorder after electroconvulsive therapy. Progress in Neuro-psychopharmacology and Biological Psychiatry, 100, 109888. [DOI] [PubMed] [Google Scholar]
- Wang J., Yang Y., Zhao X., Zuo Z., Tan L.-H. (2020b). Evolutional and developmental anatomical architecture of the left inferior frontal gyrus. NeuroImage, 117268. [DOI] [PubMed] [Google Scholar]
- Wu H., Sun H., Xu J., et al. (2016). Changed hub and corresponding functional connectivity of subgenual anterior cingulate cortex in major depressive disorder. Frontiers in Neuroanatomy, 10, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang J., Wu Q., et al. (2011). Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biological Psychiatry, 70, 334–42. [DOI] [PubMed] [Google Scholar]
- Zhang J., Bi W., Zhang Y., et al. (2015). Abnormal functional connectivity density in Parkinson’s disease. Brain Topography, 280, 113–8. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xie B., Chen H., Li M., Liu F., Chen H. (2016). Abnormal functional connectivity density in post-traumatic stress disorder. Brain Topography, 29, 405–11. [DOI] [PubMed] [Google Scholar]
- Zou K., Gao Q., Long Z., et al. (2016). Abnormal functional connectivity density in first-episode, drug-naive adult patients with major depressive disorder. Journal of Affective Disorders, 194, 153–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and code used for data analysis are available upon request.


