Abstract
Neuroendocrine tumors (NETs) occur more commonly in lungs, gastrointestinal tract, or pancreas. NETs in locations such as ovaries are rare, and they have been described mainly in case reports. Here we describe a patient with primary NET of ovary presenting with distant metastases to peritoneum, liver, lung, and mediastinal lymph nodes.
Keywords: metastatic, neuroendocrine, ovary
Introduction
Neuroendocrine tumors (NETs) are a group of heterogeneous tumors arising from neuroendocrine cells. They were earlier known as carcinoid tumors, but this term has been replaced by the term “NET” in the last World Health Organization and European NET Society classifications of tumors of digestive system. 1 NETs are uncommon, with an incidence ranging between 1 and 5/100,000 patients. 2 They are seen more frequently in lungs, rectum, small bowel, stomach, and pancreas. 3 However, they may also be seen in colon, cecum, or appendix, and are very rare in other locations. In a study of 350,000 patients by Yao et al in 2008, only 15% of all NETs were located in sites other than gastrointestinal tract, pancreas, or lungs. 2
Ovarian NETs account for 0.5% of all carcinoid tumors. 4 One-third of patients with ovarian NETs show clinical neuroendocrine symptoms such as facial flushing, diarrhea, and abdominal cramping. 5 The differential diagnosis of NET in the ovary includes germ cell tumors, sex-cord and granulosa cell cancers, other gynecologic cancers, and metastatic neoplasms. 6
Pathological diagnosis is critical to guide management. They may range from indolent well-differentiated NETs to high-grade aggressive neuroendocrine cancers. Neuroendocrine carcinomas in the ovary include small cell carcinomas, large cell variants of high-grade NETs, and well-differentiated NETs. 6
They often need a multidisciplinary therapeutic approach after determination of the extent of disease and the primary organ of involvement.
Case Report
A 32-year-old nulliparous woman presented with lower abdominal and back pain for 3 months but no other gastrointestinal symptoms. She was initially evaluated outside with ultrasound that revealed bilateral adnexal masses with hepatic space occupying lesions. She was referred to our hospital for further evaluation and management. Physical examination demonstrated a nodular mass at POD on PV examination. However, cervix was healthy. Her initial cancer antigen (CA) 125, CA 19–6, and carcinoembryogenic antigen (CEA) levels were 29.6 U/mL, 153.5U/mL, and 7.85ng/mL, respectively. Liver function test was deranged with raised aspartate aminotransferase, alkaline phosphatase, and gamma GT levels. Upper gastrointestinal endoscopy/colonoscopy showed no remarkable findings.
Contrast enhanced computed tomographic (CECT) scanning of thorax and abdomen revealed large bilateral heterogeneous appearing solid adnexal masses, showing moderate contrast enhancement ( Fig. 1A, B ). Large irregular hypoattenuating lesions were seen in right lobe of liver ( Fig. 2 ). CECT showed enhancing nodules in infracolic omentum, one abutting the ascending colon on right side ( Fig. 3 ). CECT revealed compression over distal ureter by the left adnexal mass causing upstream hydroureteronephrosis on left side ( Fig. 4 ). No free fluid in peritoneal cavity. Thoracic scan revealed large mediastinal nodes ( Fig. 5A, B ) along with some lung parenchymal nodules seen bilaterally ( Fig. 6A, B ).
Fig. 1.
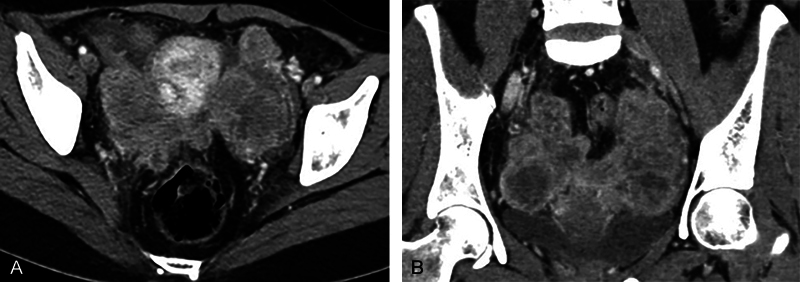
( A and B ) Axial and coronal contrast-enhanced computed tomography images show bilateral solid heterogeneously enhancing adnexal masses. Ovaries are not seen separately. Masses are closely abutting the uterus on either side.
Fig. 2.
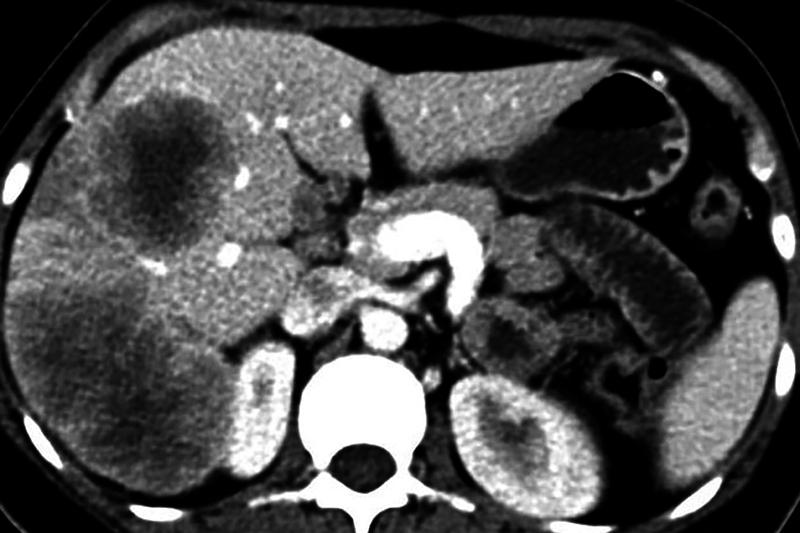
Axial contrast-enhanced computed tomography image shows large hypoattenuating lesions in right lobe of liver—metastases.
Fig. 3.
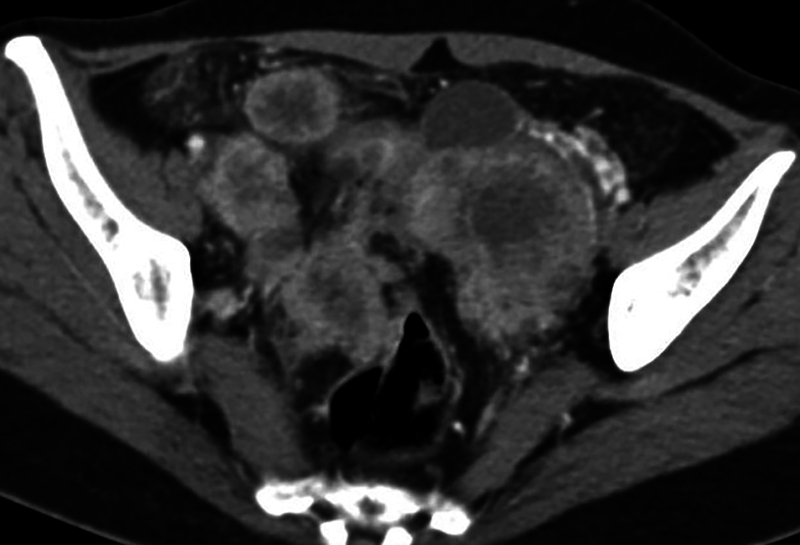
Axial contrast-enhanced computed tomography image shows enhancing infracolic omental nodules with some surrounding omental fat stranding.
Fig. 4.
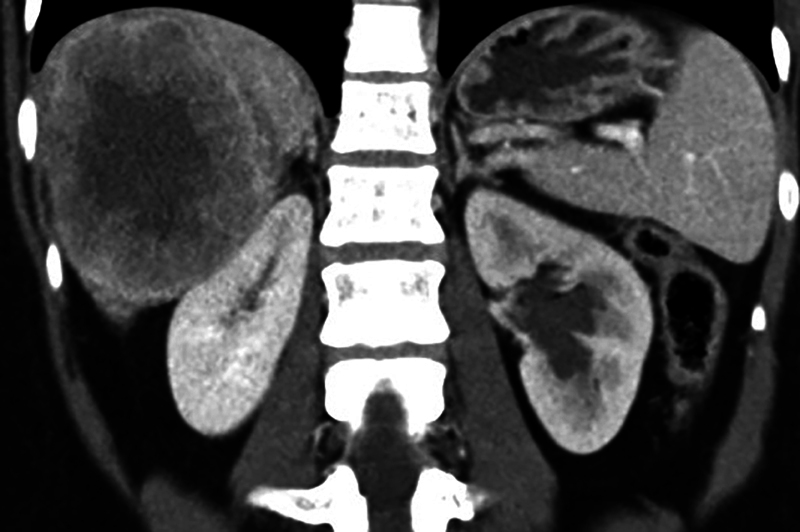
Coronal contrast-enhanced computed tomography image shows left hydronephrotic kidney due to compression over left ureter by left adnexal mass. Large right lobe of liver metastases is also seen.
Fig. 5.
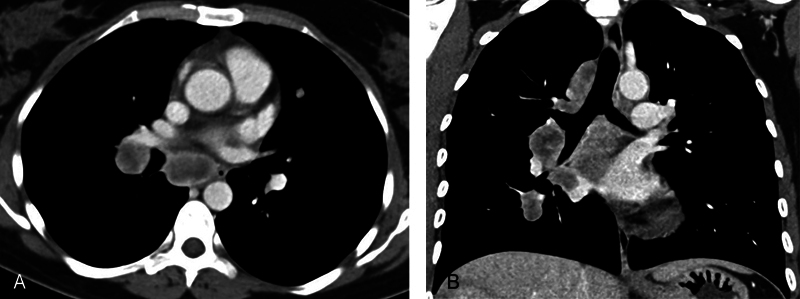
( A and B ) Axial and coronal contrast-enhanced computed tomography images show large heterogeneously enhancing, necrotic appearing mediastinal nodes.
Fig. 6.
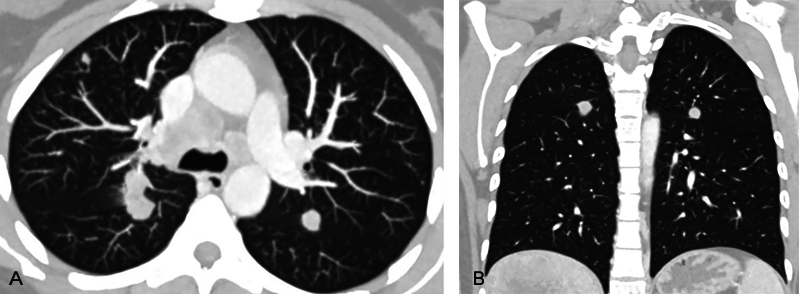
( A and B ) Axial and coronal computed tomographic images with lung window settings show bilateral solid lung parenchymal nodules; some in perifissural location.
Ultrasound-guided biopsy of right-sided pelvic nodule was done under aseptic/antiseptic precautions with local anesthetic (2% lignocaine) cover using 18 G biopsy gun (Bard's).
The sections showed cores of fibrocollagenous tissue infiltrated by solid nests of pleomorphic tumor cells with variably prominent nucleoli and areas of necrosis ( Fig. 7 ). Immunohistochemistry (IHC) showed the tumor cells to be positive for synaptophysin ( Fig. 8 ), chromogranin ( Fig. 9 ), CK and SATB2 and focally expressing CDX2, CK7, and CK20 while negative for CD56, PAX8, ER, WT1, TTF1, GATA3, p40, desmin, calretinin, and CD34. Proliferation index as measured by Ki67 index is 80% ( Fig. 10 ). Final impression was a high-grade metastatic neuroendocrine carcinoma.
Fig. 7.
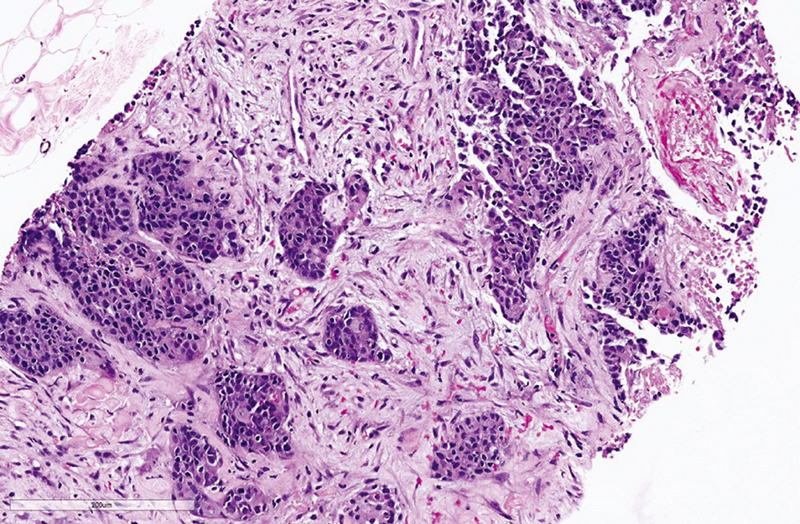
Hematoxylin and eosin stained slide of the tumor 20x.
Fig. 8.
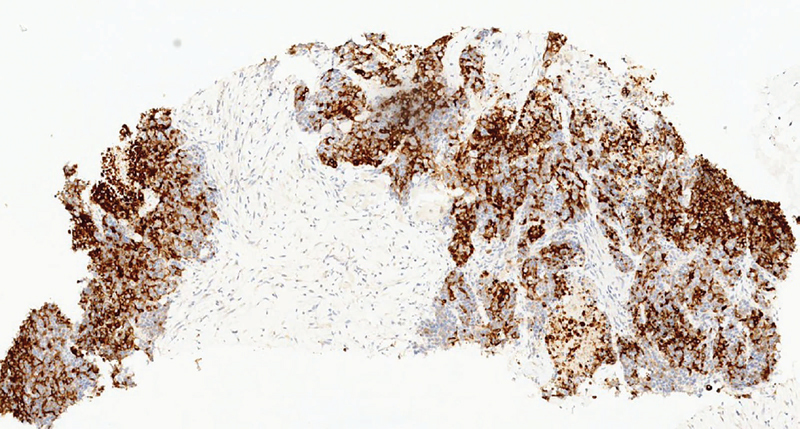
Synaptophysin stain by immunohistochemistry shows diffuse cytoplasmic staining in tumor cells.
Fig. 9.
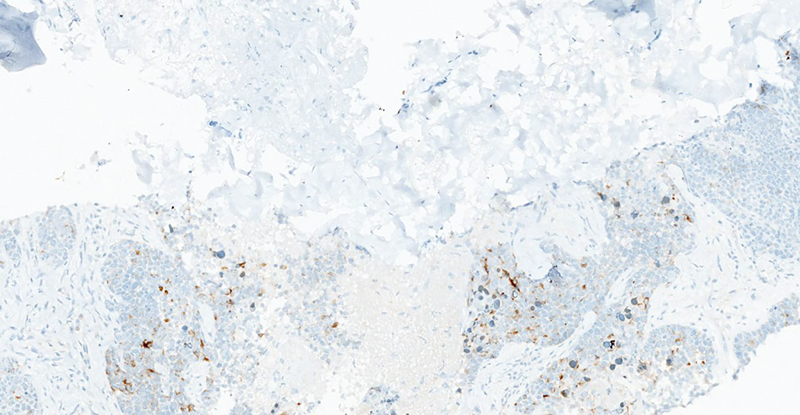
Chromogranin stain by immunohistochemistry shows patchy cytoplasmic staining in tumor cells.
Fig. 10.
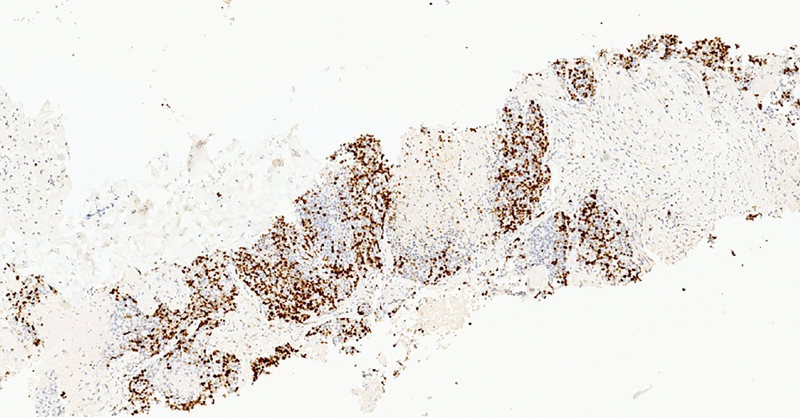
Ki67 stain by immunohistochemistry shows nuclear staining in tumor cells.
Due to metastatic disease at presentation, patient was not deemed suitable for operative management. Subsequently, systemic therapy with etoposide and cisplatin alongside symptomatic management was suggested. Patient was followed up 3 monthly and post six cycles of chemotherapy; imaging findings revealed stable disease. Patient is currently doing well clinically and due for next follow-up and imaging.
Discussion
NETs of ovary are more often detected incidentally; however, some patients may present with vague clinical symptoms like abdominal discomfort and pain as seen in our patient. Other symptoms include persistent facial flushing, episodic hypertension, bloating, nausea, vomiting, and even abdominal distension owing to ascites. 7
On cross-section imaging, the usual appearances are bilateral solid enhancing or large complex solid cystic tumor masses with implants in the pelvic peritoneum, omentum, and small bowel loops. In addition, metastases to liver and lung may be seen. 7 8 Hence, imaging features are often nonspecific and indistinguishable from other neoplasms.
NETs in the ovary may present as small cell carcinomas, large cell variants, and well-differentiated carcinoids tumors. 6 IHC is useful in the diagnosis, as synaptophysin, chromogranin, and CD56 are common markers of ovarian carcinoid tumors. 9 Carcinoid tumors are usually negative for EMA, estrogen receptors, progesterone receptors, and sex-cord stromal markers such as inhibin and calretinin. 6 Recent studies state that the immunohistochemical marker caudal-type homeobox transcription factor 2 (CDX2) may be useful for distinguishing primary ovarian carcinoid from small intestine metastasis and appendiceal NET. 10 It is imperative to differentiate a primary ovarian NET from a metastatic gastrointestinal carcinoid to choose appropriate therapy. In our patient, IHC showed the tumor cells to be positive for synaptophysin, chromogranin, CK and SATB2 and focally expressing CDX2, CK7, and CK20, while negative for CD56, PAX8, ER, WT1, TTF1, GATA3, p40, desmin, calretinin, and CD34. The primary tumor was more aggressive as she had metastases to the liver and lung. According to Kurabayashi et al, the proliferation activity of a primary ovarian carcinoid is an important prognostic factor. Our patient had a K i -67 positive rate of 80%. This highly positive K i -67 result indicates a poor prognosis. 11
The most frequent sites of metastatic carcinoids are lymph nodes (89.8%), liver (44.1%), lung (13.6%), peritoneum (13.6%), and pancreas (6.8%). 4 Ovarian carcinoids will be more often metastatic rather than primary. This may be from direct spread from adjacent appendix or small bowel or with Krukenberg tumors from hematogenous spread. However, metastases from primary gastrointestinal carcinoids must always be ruled out especially in case of bilateral ovarian tumors. 6 In our patient, though bilateral ovarian NETs were present, owing to CDX2 positivity and negative endoscopy findings, diagnosis of primary ovarian NET was favored.
Metastatic ovarian NETs are typically associated with a poor prognosis. A combination of surgical resection and etoposide/platinum therapy is used for these patients as is also seen in our patient who is undergoing systemic therapy with etoposide and cisplatin. 12
The purpose of this case report was to drive home the point that the role of radiology alone is limited in the diagnosis of primary NET of ovary unless coupled with IHC. Further studies are necessary to determine the best possible management of women with gynecologic carcinoid tumors.
Acknowledgment
None.
Conflict of Interest None declared.
Patient Consent
Written informed consent was obtained from the patient for publication of this case report and the accompanying images.
References
- 1.Klimstra D S, Modlin I R, Adsay N V et al. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol. 2010;34(03):300–313. doi: 10.1097/PAS.0b013e3181ce1447. [DOI] [PubMed] [Google Scholar]
- 2.Yao J C, Hassan M, Phan A et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Modlin I M, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79(04):813–829. doi: 10.1002/(sici)1097-0142(19970215)79:4<813::aid-cncr19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Modlin I M, Lye K D, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(04):934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 5.Robboy S J, Norris H J, Scully R E. Insular carcinoid primary in the ovary. A clinicopathologic analysis of 48 cases. Cancer. 1975;36(02):404–418. doi: 10.1002/1097-0142(197508)36:2<404::aid-cncr2820360216>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Reed N S, Gomez-Garcia E, Gallardo-Rincon Det al. Gynecologic Cancer InterGroup (GCIG) consensus review for carcinoid tumors of the ovary Int J Gynecol Cancer 201424(09, Suppl 3):S35–S41. [DOI] [PubMed] [Google Scholar]
- 7.Vora M, Lacour R A, Black D R, Turbat-Herrera E A, Gu X. Neuroendocrine tumors in the ovary: histogenesis, pathologic differentiation, and clinical presentation. Arch Gynecol Obstet. 2016;293(03):659–665. doi: 10.1007/s00404-015-3865-0. [DOI] [PubMed] [Google Scholar]
- 8.Hsu W-W, Mao T-L, Chen C-H. Primary ovarian mucinous carcinoid tumor: a case report and review of literature. Taiwan J Obstet Gynecol. 2019;58(04):570–573. doi: 10.1016/j.tjog.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 9.McCluggage W G, Young R H. Immunohistochemistry as a diagnostic aid in the evaluation of ovarian tumors. Semin Diagn Pathol. 2005;22(01):3–32. doi: 10.1053/j.semdp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Desouki M M, Lioyd J, Xu H, Cao D, Barner R, Zhao C. CDX2 may be a useful marker to distinguish primary ovarian carcinoid from gastrointestinal metastatic carcinoids to the ovary. Hum Pathol. 2013;44(11):2536–2541. doi: 10.1016/j.humpath.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Kurabayashi T, Minamikawa T, Nishijima S et al. Primary strumal carcinoid tumor of the ovary with multiple bone and breast metastases. J Obstet Gynaecol Res. 2010;36(03):567–571. doi: 10.1111/j.1447-0756.2010.01231.x. [DOI] [PubMed] [Google Scholar]
- 12.Gardner G J, Reidy-Lagunes D, Gehrig P A. Neuroendocrine tumors of the gynecologic tract: a Society of Gynecologic Oncology (SGO) clinical document. Gynecol Oncol. 2011;122(01):190–198. doi: 10.1016/j.ygyno.2011.04.011. [DOI] [PubMed] [Google Scholar]


