Abstract
Telomeres are located at the end of chromosomes. They are known to protect chromosomes and prevent cellular senescence. Telomere length shortening has been considered an important marker of aging. Many studies have reported this concept in connection with neurodegenerative disorders. Considering the role of telomeres, it seems that longer telomeres are beneficial while shorter telomeres are detrimental in preventing neurodegenerative disorders. However, several studies have shown that people with longer telomeres might also be vulnerable to neurodegenerative disorders. Before these conflicting results can be explained through large-scale longitudinal clinical studies on the role of telomere length in neurodegenerative disorders, it would be beneficial to simultaneously review these opposing results. Understanding these conflicting results might help us plan future studies to reveal the role of telomere length in neurodegenerative disorders. In this review, these contradictory findings are thoroughly discussed, with the aim to better understand the role of telomere length in neurodegenerative disorders.
Keywords: Telomere, Neurodegenerative Disorders, Shortening, Alzheimer’s Disease, Parkinson’s Disease, Frontotemporal Dementia
INTRTODUCTION
The prevalence of age-related neurodegenerative disorders including Alzheimer’s disease (AD), Parkinson’s disease (PD), and frontotemporal dementia (FTD) continue to increase worldwide. Although many studies have attempted to reveal the exact etiologies and mechanisms of each disease, they remain unclear. Understanding the role of aging in neurodegenerative disorders could be helpful because aging is a robust nonmodifiable risk factor.1 Aging of all organisms is eventually associated with progressive physical deterioration and increased vulnerability to death.2 Aging can result in genomic instability, telomere shortening, epigenetic changes, mitochondrial dysfunction, cellular senescence, altered intercellular communications, and so on.2 Among these alterations, telomere attribution is considered one of the most remarkable markers for aging and cellular senescence.3
Telomeres are repetitive nucleotide sequences of TTAGGG at the end of chromosomes. The length of double-stranded telomeric DNA ranges from 5 to 15 bp in humans. Telomeres form special cap structures with t-loop.4,5,6 It is well known that telomeres contribute to the maintenance of chromosomal stability by preventing fusion and degradation of chromosomes and regulating the ability of cells to replicate via mitosis.4,5,6 However, telomere length decreases during aging and under various stressful conditions. For example, our previous study has shown that approximately 48 bp per year is naturally shortened in healthy Korean males and approximately 56 bp per year in healthy Korean females.7 Shortening of telomere length can be faster under stressful and pathological conditions. Oxidative stress and other harmful stressors can shorten telomere length. Furthermore, telomere length shortening can also happen in various neurodegenerative disorders.8 These findings suggest that people with shorter telomere lengths are more vulnerable to neurodegenerative disorders, whereas people with longer telomere lengths might be more resistant to such disorders. Results of many studies support this hypothesis. However, several studies have reported contradictory results, showing that patients with neurodegenerative disorders have longer telomeres.9,10 These conflicting results should be discussed and explained to better understand the role of telomere length in neurodegenerative disorders.
Thus, the purpose of this review was to summarize papers that have examined the relationship between telomere length and neurodegenerative disorders and to suggest future studies for answering any remaining questions.
AGING AND NEURODEGENERATIVE DISORDERS
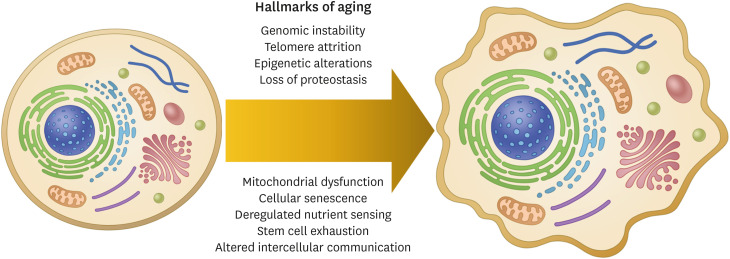
Aging is an inevitable event in the lifecycle and an undeniable risk factor for neurodegenerative disorders.1 Aging leads to many changes such as genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, mitochondrial dysfunction, cellular senescence, deregulated nutrient sensing, stem cell exhaustion, and altered intercellular communication (Fig. 1).1 These hallmarks of aging will be briefly discussed here.
Fig. 1. Hallmarks of aging. Hallmarks of aging include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, mitochondrial dysfunction, cellular senescence, deregulated nutrient sensing, stem cell exhaustion, and altered intercellular communication.
Genomic instability, including nucleic acid sequences, chromosomal rearrangements, and aneuploidy, is known to play a vital role in the biology of aging.11 Genomic instability means mutations within the genome, including nucleic acid sequences, chromosomal rearrangements, and aneuploidy. External stress-caused or endogenous DNA damage can be an important and major source of genomic instability.12 DNA damage can be repaired in healthy situations through the following 5 DNA repair pathways: base excision repair, nucleotide excision repair, mismatch repair, DNA double-strand break repair, and direct reversal.13,14 In pathological conditions, these repair pathways do not work properly. Damaged DNAs cause genomic instability and lead to pathological signaling cascades that promote cellular senescence and inflammation known to be the main pathologies of neurodegenerative disorders.1
Telomere shortening has been emphasized in aging biology. The role of telomere shortening in aging and senescence will be thoroughly and separately discussed later. Epigenetic alterations will be covered here. Epigenetic alterations refer to changes in the chemical structure of DNA without changing DNA coding sequence. These alterations include methylation, PARylation, and acetylation of DNA. Histones can collectively influence the chromatin tertiary structure. Epigenetic alterations are known to affect chromatin activity and function, including transcription and replication, thus initiating the pathology of neurodegenerative disorders.15
Loss of proteostasis means an imbalance between protein synthesis and degradation due to improper regulation of the proteasome, autophagy, ubiquitination machinery, and lysosome.16 It is well established that loss of proteostasis can increase protein misfolding, aggregation, and deposition, which are strongly associated with neurodegenerative disorders.
Mitochondria are the main source of intracellular energy. Therefore, they play critical roles in neuronal activity. However, mitochondria damaged by aging can produce a glut of reactive oxygen species, one of the most important triggers of the pathogenic mechanisms of neurodegenerative disorders.17 Furthermore, damaged mitochondria can impair lipid biosynthesis, calcium signaling, and cell apoptosis, which are known to have vital roles in the development of neurodegenerative disorders.18
Cellular senescence is the cessation of cell division triggered by a DNA damage response to diverse stresses.19 It contributes to the survival of healthy cells and the removal of damaged cells by stress-induced stable cell cycle arrest.20 Several pathways are known to be related to cellular senescence, including stress-induced premature senescence, replicative senescence, oncogenic-induced senescence, and mitochondrial dysfunction-associated senescence.20 Recently, autophagy has been emphasized as another key process associated with cellular senescence.21 However, it remains unclear whether autophagy promotes or inhibits senescence.
Deregulated nutrient sensing refers to impaired ability of cells to adjust their metabolism to the amount of available nutrients. Cells must sense nutrient levels to maintain a stable status. Nutrient sensing is essential for cell survival. However, it is deregulated with aging, resulting in altered metabolism and cell death. Caloric restriction can downregulate the nutrient signaling pathway, expand lifespan, and exert neuroprotective effects through various nutrient sensing pathways associated with insulin, insulin-like growth factor 1, mechanistic target of rapamycin, AMP-activated protein kinase, and sirtuins.16
Stem cell exhaustion is an important hallmark of aging. If enough stem cells exist in the brain and contribute to the regeneration of damaged neurons, humans could live healthier and longer. However, in the real world, the number and function of stem cells decline with age. The loss of stem cells can be due to DNA damage, telomere attrition, epigenetic alterations, loss of proteostasis, mitochondrial dysfunction, and cellular senescence.22
Cells in our body do not survive in isolation from one another. They give and receive external signals termed intercellular communication. Aging alters intercellular communication through deregulated neurohormonal signaling. Altered intercellular communication is known to cause inflammation and neurodegenerative disorders.23 Several mechanisms have been suggested to be involved in inflammation. Therapeutic strategies targeting these mechanisms have been studied with the aim to treat neurodegenerative disorders.
ROLE OF TELOMERES IN AGING AND SENESCENCE
Telomeres with unique DNA-protein structures can be found at the end of each chromosome (Fig. 2). They defend the genome by preventing nucleolytic degradation, unnecessary recombination, repair, and interchromosomal fusion. Telomere length is shortened with cell division and aging (Fig. 2). When the shortening is over a critical limit, the cell will go through senescence and induce chronic inflammation and tissue dysfunction.24 To protect chromosomes from various conditions, telomeres have specific loop structures with telomeric DNA and telomere-binding proteins mediated by TRF2.25 These loop structures are considered to prime telomeric DNA synthesis by telomerase. Telomerase activity can be observed in most proliferating cells. However, somatic cells have low or undetectable levels of telomerase activity. Therefore, telomeres in somatic cells cannot be repaired when shortened.
Fig. 2. Telomere shortening and aging. Telomere (TTAGGG) length is shortened with cell division. Telomere shortening is an important marker of aging. (A) The structure of telomere and its shortening with cell division. (B) The Brief mechanism of telomere shortening and elongation. (C) The relationship between aging, telomere shortening, and tissue dysfunction.
Telomere shortening is thought to be able to reflect the pace of aging. Telomeres are inevitably lost with aging in normal diploid cells. For example, human liver cells lose 55 bp of telomeric DNA per year and leukocyte telomere length shortens at an average rate of 30–35 bp per year.26,27
Telomere length is inversely correlated with age. Thus, a long telomere length is considered to be associated with aging. Several genetic disorders and stressful conditions can accelerate telomere shortening. It has been reported that people with shorter telomeres have significantly poor survival due to higher mortality rates caused by age-related disorders.28 In addition, shorter telomere length has been continuously reported to be associated with neurodegenerative disorders, which will be discussed later.
TELOMERE LENGTH IN NEURODEDENERATIVE DISORDERS
AD
AD is the most common neurodegenerative disorder. It is a progressive neurological disorder that causes brain atrophy due to neuronal cell death. As AD is one of the most representative age-related diseases and telomere length is strongly correlated with aging, the relationship between the 2 has been studied.1 It is impossible to obtain brain tissues of living patients with AD. Most studies have used peripheral blood leukocytes (PBLs) to measure telomere length in patients with AD. To accept these findings, it is important to confirm that telomere length of neurons in the brain is well correlated with that of PBLs. Regarding this, Lukens et al.29 have shown that telomere length in PBLs is strongly correlated with that in the cerebellum of patients with AD. This finding suggests that the telomere length of PBLs can be used to reveal the relationship between telomere length and AD.
Consistent with the hypothesis that shorter telomere length reflecting more rapid aging is associated with neurodegenerative disorders, many studies have shown that patients with AD have shorter telomeres than healthy people.29,30,31 Telomere length of patients with AD has been reported to be even shorter than that of patients with mild cognitive impairment (MCI).31 In Koreans, telomere shortening has been found to be correlated with cognitive decline and dementia conversion in MCI due to AD.32 Furthermore, patients with AD have greater telomere shortening every year than healthy individuals and patients with MCI.7,32 Another study has also confirmed that longer AD duration is associated with shorter telomere length.33 It has been reported that short telomere length could be useful for predicting the incidence of AD among non-APOE ε4 carriers.34
These results regarding the relationship between telomere length and AD suggest that individuals with shorter telomeres are vulnerable to AD. One in vitro study using human hippocampal progenitors has suggested that telomere shortening has a negative influence on human cognitive function, namely, a short telomere length can decrease the proliferative capacity and lead to cognitive function impairment in humans.35
However, results of several papers are inconsistent with these findings. One study has reported no correlation between telomere length and cognitive performance among non-demented and demented people in long-life family study participants.36 It has also been reported that telomere length is not predictive of dementia or MCI conversion in the oldest old.37 One study has shown that both longer telomere length and shorter telomere length are associated with an increased risk of AD in the Rotterdam study.10 An in vivo study has shown that telomere shortening can reduce AD amyloid pathology in mice, suggesting that shorter telomere length could be beneficial in AD progression.38 This is in contrast to findings described above.
It remains unclear whether shorter or longer telomeres are preferable for AD. However, more studies have reported that shorter telomeres have harmful effects, whereas longer telomeres have protective effects on AD. This question should first be answered to understand the effect of telomere length on AD. Considering our finding that the annual rate of telomere length change is more important in sleep quality (including sleep duration, sleep latency, and sleep efficiency) than telomer length at a single time point and that poor sleep quality is strongly correlated with faster longitudinal shortening of telomere length,38 longitudinal or annual measurement of telomere length in healthy people and patients with MCI or dementia could provide some clues.
PD
PD is a progressive nervous system disorder that affects movement, causing tremors, stiffness, or slowing. It is the second most common neurodegenerative disease affecting approximately 2% of the population aged over 60 years.39,40 In contrast to AD, there have been few studies about the relationship between telomere length and findings about PD are much more conflicting than ones about AD.
Some studies are consistent with the hypothesis that people with shorter telomeres might be more vulnerable to PD. It has been reported that telomere length is shorter in patients with PD than in age-matched healthy controls.41 Wu et al.42 have shown that telomere length is significantly shortened in Chinese patients with PD than in controls and that the shortening of telomere length is independent of LRRK2 variants. Another study has reported that telomere length appears to be associated with the time to the onset of motor complications after levodopa treatment initiation.43 However, the significance of the association disappeared after adjusting for age at inclusion and disease duration, although relationships between telomere length and other PD-related phenotypes remained the same.43
However, some studies have reported conflicting results. For example, long telomere length at diagnosis has been reported to be a risk factor for dementia progression in idiopathic parkinsonism.44 Another study has reported that shorter telomere length is correlated with reduced PD risk.45
Other studies have shown no relationship between telomere length and PD. Wang et al.46 have reported that shorter telomeres are not related to the risk of PD. Hudson et al.47 have suggested that telomere shortening is not involved in the pathogenesis of PD by showing that longer telomere length is found in blood from patients with PD. However, a difference in telomere length is not found in the substantia nigra compared to controls. A meta-analysis has also suggested that there is no consistent evidence of shorter telomeres in patients with PD.48
FTD and amyotrophic lateral sclerosis (ALS)
In the past, FTD and ALS were thought to be completely different diseases. However, the finding that C9orf72 gene mutation can cause both FTD and ALS suggests that they might be spectrum disorders.49,50 Unfortunately, only a few studies have reported the relationship between telomere length and FTD or ALS.
Regarding ALS, it has been reported that shorter telomeres are associated with earlier ALS onset in animal models.51 De Felice et al.52 have also reported that the telomere length of 50 patients with sporadic ALS is significantly shorter than that of 50 healthy subjects. However, a case-control study including 1,241 European patients with ALS has shown that telomere length is longer in patients with ALS than in healthy controls, suggesting that longer telomere length is not favorable for ALS.53 In contrast, a Mendelian randomization study has suggested that there is no direct causal association between telomere length and ALS. However, Xia et al.54 have recently used a 2-sample Mendelian randomization approach and shown that longer telomere length is inversely associated with a lower risk of ALS. Therefore, more longitudinal studies are needed to resolve these discrepancies.
In terms of the association between telomere length and FTD, there have been no reports except our previous study,9 which shows that patients with FTD have longer telomere lengths. Nevertheless, our study had a small sample size. Thus, we could not conclude whether longer telomere length could be a cause of FTD. Therefore, more studies with a larger number of FTD patients and controls should be performed to determine the effect of telomere length on FTD.9
CONCLUSION
This review included a number of studies that focused on the relationship between telomere length and neurodegenerative disorders such as AD, PD, FTD, and ALS. Over 50% of papers reported that shorter telomere length could be a definite risk factor for neurodegenerative disorders. However, other studies reported that there were no relationship between them or that longer telomere length could be a risk factor. These conflicting results might be due to differences in study protocols and no lifestyle regulation before blood sampling as well as the use of different ethnicities and time points. Considering our finding that the amount of telomere length alteration might be more important than telomere length at a single time point, large-scale longitudinal studies are necessary in the future. These studies should help us elucidate the causal relationship between telomere length and various neurodegenerative disorders.
Footnotes
Funding: This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health & Welfare, Republic of Korea (grant numbers: HI20C0253, HU21C0113, and HU21C0007) and the Medical Research Center (2017R1A5A2015395).
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Yu HJ, Koh SH.
- Writing - original draft: Yu HJ.
- Writing - review & editing: Koh SH.
References
- 1.Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 2.Azam S, Haque ME, Balakrishnan R, Kim IS, Choi DK. The ageing brain: molecular and cellular basis of neurodegeneration. Front Cell Dev Biol. 2021;9:683459. doi: 10.3389/fcell.2021.683459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 2016;8:3–11. doi: 10.18632/aging.100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright WE, Tesmer VM, Huffman KE, Levene SD, Shay JW. Normal human chromosomes have long G-rich telomeric overhangs at one end. Genes Dev. 1997;11:2801–2809. doi: 10.1101/gad.11.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEachern MJ, Krauskopf A, Blackburn EH. Telomeres and their control. Annu Rev Genet. 2000;34:331–358. doi: 10.1146/annurev.genet.34.1.331. [DOI] [PubMed] [Google Scholar]
- 6.Sarek G, Kotsantis P, Ruis P, Van Ly D, Margalef P, Borel V, et al. CDK phosphorylation of TRF2 controls t-loop dynamics during the cell cycle. Nature. 2019;575:523–527. doi: 10.1038/s41586-019-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koh SH, Choi SH, Jeong JH, Jang JW, Park KW, Kim EJ, et al. Telomere shortening reflecting physical aging is associated with cognitive decline and dementia conversion in mild cognitive impairment due to Alzheimer’s disease. Aging (Albany NY) 2020;12:4407–4423. doi: 10.18632/aging.102893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Liu Y, Xia Q, Xia Q, Wang B, Yang C, et al. Potential roles of telomeres and telomerase in neurodegenerative diseases. Int J Biol Macromol. 2020;163:1060–1078. doi: 10.1016/j.ijbiomac.2020.07.046. [DOI] [PubMed] [Google Scholar]
- 9.Kim EJ, Koh SH, Ha J, Na DL, Seo SW, Kim HJ, et al. Increased telomere length in patients with frontotemporal dementia syndrome. J Neurol Sci. 2021;428:117565. doi: 10.1016/j.jns.2021.117565. [DOI] [PubMed] [Google Scholar]
- 10.Fani L, Hilal S, Sedaghat S, Broer L, Licher S, Arp PP, et al. Telomere length and the risk of Alzheimer’s disease: the Rotterdam Study. J Alzheimers Dis. 2020;73:707–714. doi: 10.3233/JAD-190759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow HM, Herrup K. Genomic integrity and the ageing brain. Nat Rev Neurosci. 2015;16:672–684. doi: 10.1038/nrn4020. [DOI] [PubMed] [Google Scholar]
- 12.Møller P. Genotoxicity of environmental agents assessed by the alkaline comet assay. Basic Clin Pharmacol Toxicol. 2005;96(Suppl 1):1–42. [PubMed] [Google Scholar]
- 13.Jeppesen DK, Bohr VA, Stevnsner T. DNA repair deficiency in neurodegeneration. Prog Neurobiol. 2011;94:166–200. doi: 10.1016/j.pneurobio.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKinnon PJ. Maintaining genome stability in the nervous system. Nat Neurosci. 2013;16:1523–1529. doi: 10.1038/nn.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang JY, Aromolaran KA, Zukin RS. The emerging field of epigenetics in neurodegeneration and neuroprotection. Nat Rev Neurosci. 2017;18:347–361. doi: 10.1038/nrn.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johri A, Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther. 2012;342:619–630. doi: 10.1124/jpet.112.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keogh MJ, Chinnery PF. Mitochondrial DNA mutations in neurodegeneration. Biochim Biophys Acta. 2015;1847:1401–1411. doi: 10.1016/j.bbabio.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Kültz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- 20.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narita M, Young AR, Arakawa S, Samarajiwa SA, Nakashima T, Yoshida S, et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–970. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh J, Lee YD, Wagers AJ. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014;20:870–880. doi: 10.1038/nm.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Currais A. Ageing and inflammation - A central role for mitochondria in brain health and disease. Ageing Res Rev. 2015;21:30–42. doi: 10.1016/j.arr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Shin JS, Hong A, Solomon MJ, Lee CS. The role of telomeres and telomerase in the pathology of human cancer and aging. Pathology. 2006;38:103–113. doi: 10.1080/00313020600580468. [DOI] [PubMed] [Google Scholar]
- 25.van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 26.Takubo K, Nakamura K, Izumiyama N, Furugori E, Sawabe M, Arai T, et al. Telomere shortening with aging in human liver. J Gerontol A Biol Sci Med Sci. 2000;55:B533–B536. doi: 10.1093/gerona/55.11.b533. [DOI] [PubMed] [Google Scholar]
- 27.Herrmann M, Pusceddu I, März W, Herrmann W. Telomere biology and age-related diseases. Clin Chem Lab Med. 2018;56:1210–1222. doi: 10.1515/cclm-2017-0870. [DOI] [PubMed] [Google Scholar]
- 28.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 29.Lukens JN, Van Deerlin V, Clark CM, Xie SX, Johnson FB. Comparisons of telomere lengths in peripheral blood and cerebellum in Alzheimer’s disease. Alzheimers Dement. 2009;5:463–469. doi: 10.1016/j.jalz.2009.05.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, et al. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol Aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 31.Scarabino D, Broggio E, Gambina G, Corbo RM. Leukocyte telomere length in mild cognitive impairment and Alzheimer’s disease patients. Exp Gerontol. 2017;98:143–147. doi: 10.1016/j.exger.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Lee EH, Han MH, Ha J, Park HH, Koh SH, Choi SH, et al. Relationship between telomere shortening and age in Korean individuals with mild cognitive impairment and Alzheimer’s disease compared to that in healthy controls. Aging (Albany NY) 2020;13:2089–2100. doi: 10.18632/aging.202206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu M, Huo YR, Wang J, Wang C, Liu S, Liu S, et al. Telomere shortening in Alzheimer’s disease patients. Ann Clin Lab Sci. 2016;46:260–265. [PubMed] [Google Scholar]
- 34.Hackenhaar FS, Josefsson M, Adolfsson AN, Landfors M, Kauppi K, Hultdin M, et al. Short leukocyte telomeres predict 25-year Alzheimer’s disease incidence in non-APOE ε4-carriers. Alzheimers Res Ther. 2021;13:130. doi: 10.1186/s13195-021-00871-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmos AB, Duarte RR, Smeeth DM, Hedges EC, Nixon DF, Thuret S, et al. Telomere length and human hippocampal neurogenesis. Neuropsychopharmacology. 2020;45:2239–2247. doi: 10.1038/s41386-020-00863-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashrafi A, Cosentino S, Kang MS, Lee JH, Schupf N, Andersen SL, et al. Leukocyte telomere length is unrelated to cognitive performance among non-demented and demented persons: an examination of long life family study participants. J Int Neuropsychol Soc. 2020;26:906–917. doi: 10.1017/S1355617720000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zekry D, Herrmann FR, Irminger-Finger I, Ortolan L, Genet C, Vitale AM, et al. Telomere length is not predictive of dementia or MCI conversion in the oldest old. Neurobiol Aging. 2010;31:719–720. doi: 10.1016/j.neurobiolaging.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Rolyan H, Scheffold A, Heinrich A, Begus-Nahrmann Y, Langkopf BH, Hölter SM, et al. Telomere shortening reduces Alzheimer’s disease amyloid pathology in mice. Brain. 2011;134:2044–2056. doi: 10.1093/brain/awr133. [DOI] [PubMed] [Google Scholar]
- 39.Lee A, Gilbert RM. Epidemiology of Parkinson disease. Neurol Clin. 2016;34:955–965. doi: 10.1016/j.ncl.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Simon DK, Tanner CM, Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med. 2020;36:1–12. doi: 10.1016/j.cger.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin-Ruiz C, Williams-Gray CH, Yarnall AJ, Boucher JJ, Lawson RA, Wijeyekoon RS, et al. Senescence and inflammatory markers for predicting clinical progression in Parkinson’s disease: the ICICLE-PD study. J Parkinsons Dis. 2020;10:193–206. doi: 10.3233/JPD-191724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Y, Pei Y, Yang Z, Li K, Lou X, Cui W. Accelerated telomere shortening independent of LRRK2 variants in Chinese patients with Parkinson’s disease. Aging (Albany NY) 2020;12:20483–20492. doi: 10.18632/aging.103878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levstek T, Redenšek S, Trošt M, Dolžan V, Podkrajšek KT. Assessment of the telomere length and its effect on the symptomatology of Parkinson’s disease. Antioxidants. 2021;10:137. doi: 10.3390/antiox10010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Degerman S, Domellöf M, Landfors M, Linder J, Lundin M, Haraldsson S, et al. Long leukocyte telomere length at diagnosis is a risk factor for dementia progression in idiopathic parkinsonism. PLoS One. 2014;9:e113387. doi: 10.1371/journal.pone.0113387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schürks M, Buring J, Dushkes R, Gaziano JM, Zee RY, Kurth T. Telomere length and Parkinson’s disease in men: a nested case-control study. Eur J Neurol. 2014;21:93–99. doi: 10.1111/ene.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Chen H, Gao X, McGrath M, Deer D, De Vivo I, et al. Telomere length and risk of Parkinson’s disease. Mov Disord. 2008;23:302–305. doi: 10.1002/mds.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hudson G, Faini D, Stutt A, Eccles M, Robinson L, Burn DJ, et al. No evidence of substantia nigra telomere shortening in Parkinson's disease. Neurobiol Aging. 2011;32:2107.e3. :2107.e5. doi: 10.1016/j.neurobiolaging.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forero DA, González-Giraldo Y, López-Quintero C, Castro-Vega LJ, Barreto GE, Perry G. Telomere length in Parkinson’s disease: a meta-analysis. Exp Gerontol. 2016;75:53–55. doi: 10.1016/j.exger.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan BK, Yokoyama JS, Takada LT, Sha SJ, Rutherford NJ, Fong JC, et al. Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9ORF72 hexanucleotide expansion. J Neurol Neurosurg Psychiatry. 2012;83:358–364. doi: 10.1136/jnnp-2011-301883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strong MJ, Abrahams S, Goldstein LH, Woolley S, Mclaughlin P, Snowden J, et al. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:153–174. doi: 10.1080/21678421.2016.1267768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linkus B, Wiesner D, Meßner M, Karabatsiakis A, Scheffold A, Rudolph KL, et al. Telomere shortening leads to earlier age of onset in ALS mice. Aging (Albany NY) 2016;8:382–393. doi: 10.18632/aging.100904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Felice B, Annunziata A, Fiorentino G, Manfellotto F, D’Alessandro R, Marino R, et al. Telomerase expression in amyotrophic lateral sclerosis (ALS) patients. J Hum Genet. 2014;59:555–561. doi: 10.1038/jhg.2014.72. [DOI] [PubMed] [Google Scholar]
- 53.Al Khleifat A, Iacoangeli A, Shatunov A, Fang T, Sproviero W, Jones AR, et al. Telomere length is greater in ALS than in controls: a whole genome sequencing study. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:229–234. doi: 10.1080/21678421.2019.1586951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia K, Zhang L, Zhang G, Wang Y, Huang T, Fan D. Leukocyte telomere length and amyotrophic lateral sclerosis: a Mendelian randomization study. Orphanet J Rare Dis. 2021;16:508. doi: 10.1186/s13023-021-02135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]