Abstract
Phthalates are environmental contaminants mostly used as plasticizers and additives in different products. Having endocrine-disrupting properties, phthalates are known as potential reproductive toxicants. The present study was conducted to evaluate the reproductive toxicity of di-n-butyl phthalate (DBP) in pregnant rats and their offspring and also to assess the ability of vitamin E in the elimination or reducing reproductive toxicity of DBP. Sixty-six pregnant Wistar rats were exposed to 100, 500 or 1,000 mg kg-1 per day DBP or 500 mg kg-1 per day DBP along with 100 mg kg-1 per day vitamin E during gestation. After delivery, they were divided into two groups. In one group gavage was finished after litter while in the other DBP administration was continued till weaning. The results showed that DBP affected many aspects of reproductive performance in pregnant rats and their offspring. It could be suggested that vitamin E could ameliorate the adverse effects of DBP, especially in male pups.
Key Words: Di-n-butyl phthalate, Pregnancy, Reproductive toxicology, Vitamin E
Introduction
Phthalates are a category of synthetic chemicals.1,2 They are mainly used as plasticizers to increase softness and flexibility of polyvinyl chloride products like wires and packaging equipment and as solvents in consumer and cosmetic products like enteric-coated oral medications and dietary supplements.1,2
Phthalates are not covalently bound to the polymer matrix; hence, they can leach into the environment.3 Food, water and air which are in contact with phthalate-containing materials can be contaminated and act as the source of exposure.4
Several studies have reported association linked phthalate exposure with reproductive system malf-ormations.5 Di-n-butyl phthalate (DBP) is one of the most commonly used phthalates.3,6 Adverse effects of DBP on pregnancy have been reported at doses of 500 mg kg-1 and higher.7,8 These studies either reported side effects as mild as changes in maternal food consumption and weight gain or more intense adverse effects like increase in pregnancy loss or elevation in the number of dead fetuses.2,8 Several studies have reported association of DBP to the oxidative stress.9 Farombi et al. reported that malondialdehyde, as an indicator of oxidative stress, was increased in rats exposed to DBP.9
There are growing studies indicating the role of oxidative stress (OS) in the etiology of female reproductive issues like birth defects and abortions.10 It has been reported that OS can influence early embryo development and may also play a major role both in the implantation and fertilization of eggs.10
Previous studies have demonstrated that exposure to phthalates resulted in male reproductive complications.1,11 DBP exposure- whether in utero or postnatal- has been reported to cause adverse effects on the male reproductive system, like increased incidence of undescended testes, delayed puberty and abnormal spermatogenesis.6 Oxidative stress can act both at the level of testis (disrupting the steroidogenic capacity of Leydig cells and the capacity of the germinal epithelium to differentiate normal spermatozoa) and spermatozoa (inducing lipid peroxidation and DNA damage).12 It has been reported that DBP alters the testicular structure and function, at least partly, by inducing oxidative stress in testes of adult rats.13
There are studies demonstrating that nutritional supplements and antioxidants like vitamin E can protect against OS.14 Vitamin E is an essential nutrient for mammalian reproduction and plays a critical role in preventing different pathological complications through its antioxidant properties. Vitamin E is essential for the protection of the fetus against oxidative damage.15
The goals of the present study were to explore the effects of three different doses of DBP on reproductive outcomes of pregnant rats and their offspring during gestation only or gestation and lactation period, and to figure out if vitamin E can alleviate or prevent the adverse effects of DBP on dams or pups.
Materials and Methods
Animals. Adult Wistar strain albino rats (200 – 250 g) obtained from the Laboratory Animal House, Urmia University, Urmia, Iran. Animals were housed under standard laboratory conditions (27.00 ± 2.00 ˚C and 12-hr dark-light cycle) and had access to standard pellet diet and tap water ad libitum. Animals were treated humanely and with regard for alleviation of suffering in accordance with the guidelines of the Urmia University Ethics Committee (IR-UU-ACE-103/3AD/13/05/2020).
Chemicals. Di-n-butyl phthalate were supplied from Merck (Darmstadt, Germany). Vitamin E was purchased from Sigma-Aldrich (St. Louis, USA). Corn oil (Ladan Inc., Tehran, Iran) was used as the vehicle.
Treatments. After one week of acclimatization period to laboratory conditions, 72 male and 72 female adult Wistar rats were maintained in cages at a rate of 1:1 (monogamous breeding system) for 24 hr. After observation of vaginal plug, male rats were separated, female rats were considered as pregnant dams and the day was considered as gestation day 1 (GD 1). Pregnant dams were randomly assigned to six groups, 12 per each, and were caged alone until parturition. Treatment groups included: (1) 100 mg kg-1 DBP; (2) 500 mg kg-1 DBP; (3) 1,000 mg kg-1 DBP; (4) 500 mg kg-1 DBP + 100 mg kg-1 vitamin E; (5) control group (corn oil) and (6) vitamin E group. Pregnant rats were weighed once daily and then gavaged with DBP in corn oil (as carrier).
In each treatment, six dams were gavaged every day from GD 1 up to parturition (pups were exposed prenatally only) while the other six were gavaged every day from GD 1 to postnatal day 21 (PND 21) when the pups were weaned from dams (pups exposed both prenatally and postnatally). In all groups, dams were allowed to deliver naturally and nurse their pups until PND 21.
Pregnant rats were examined daily for any signs of toxicity. Possible abortions or deaths were recorded. On the day of delivery (PND 1), gestation length, litter size and live birth index, defined as the proportion of live litter to total litter, were recorded for each dam. All pups were weighed and genders were determined. Sex ratio, defined as the proportion of male pups to female pups, was recorded for each dam. At PND 3, the anogenital distance (AGD) of each pup was measured and recorded. For measuring AGD, under a magnifying glass, the length of the perineum from the base of the sex papilla to the proximal end of the anal opening was measured using a caliper. On PND 21, dams were euthanized by overdose of xylazine hydrochloride (Alfasan, Woerden, Netherlands) and ketamine hydrochloride (Alfasan) intraperitoneally.16 After necropsy and systematic examination, uterus and ovary were dissected out and the number of implantation sites was counted. From 21 days of age in females and 30 days of age in males, all offspring were examined daily for detection of puberty. Puberty onset in females was defined as the time of vaginal opening, and in males as the capacity for preputial separation, determined by manual retraction of the prepuce.17,18 After puberty, female pups were euthanized by xylazine-ketamine overdose.16 In the male pups, following sacrifice, the testes and epididymises of each rat were removed and weighed. For sperm analysis, one cauda epididymis was excised on a petri dish containing 1.00 mL human tubal fluid (HTF) medium at 37.00 ˚C.16 The sperm were allowed to diffuse into the medium for 30 min. For sperm count, a sample of sperm suspension was placed on a pre-warmed Neubauer hemocytometer and an optical microscope was used to count the sperm. For analyzing sperm motility one drop of sperm suspension was placed on a pre-warmed Neubauer chamber for light microscope observation at a magnification of 100×, a total number of 200 sperm per sample were evaluated.16 To evaluate sperm morphology, an aliquot of sperm suspension was smeared on a clean glass, stained with H&E and a total number of 200 sperm per animal were screened under a light microscope (Olympus, Tokyo, Japan) at a magnification of 400×, with classification of sperm as normal and different abnormal types.19
Statistical analysis. Statistical analyses were carried out with SPSS Statistics for Windows (version 22.0; IBM Corp., Armonk, USA). The results were presented as mean ± SD whenever possible. The litter was used as the basis for analysis of fetal variables. Analysis of variance and Bonferroni test were used where appropriate. A value of p < 0.05 was considered significant.
Results
In the present study, vaginal bleeding was observed in two dams receiving DBP at 1,000 mg kg-1 and one dam at 500 mg kg-1, on gestation days 10, 12 and 15, respectively. This observation was recorded as mid- pregnancy abortion. Since no sign of parturition was seen after 25 days, dams were euthanized. The uteruses contained no fetuses but implantation sites were observed. These rats were excluded from the study either.
The results of the implantation number, litter size, sex ratio and viability index are shown in Table 1 and the data of maternal body weight gain changes from GD 6-21 and fetal body weight at birth are represented in Table 2. The gestation length showed no significant change in any of the treatment groups (Table 2). While the AGD of female pups in any of DBP treated groups had no significant change, the male pups showed a significant reduction in AGD at 500 mg kg-1 and 1,000 mg kg-1, in both lactation and non-lactation treatments compared to the control and vitamin E groups. When vitamin E was administered along with DBP, the AGD of the male pups was increased significantly (p < 0.05) compared to the DBP at 500 mg kg-1 (Table 2).
Table 1.
Implantation site number, litter size, viability index and sex ratio of rats given a dose range of DBP or DBP along with vitamin E during pregnancy. Values are means ± standard deviation
| Groups | Implantation site number | Litter size | Viability index (%) | Sex ratio (%) |
|---|---|---|---|---|
| Control | 12.83 ± 0.75 | 12.17 ± 0.85 | 96.83 ± 1.17 | 51.17 ± 4.60 |
| Vitamin E | 13.10 ± 0.87 | 12.33 ± 0.61 | 95.83 ± 1.47 | 49.86 ± 5.10 |
| 100 mg kg -1 DBP | 12.50 ± 0.83 | 12.04 ± 0.63 | 96.33 ± 1.21 | 49.51 ± 5.80 |
| 500 mg kg -1 DBP | 9.00 ± 0.63a | 7.33 ± 0.83a | 77.33 ± 3.50a | 49.37 ± 4.20 |
| 1,000 mg kg -1 DBP | 7.32 ± 0.52b | 4.30 ± 0.54b | 64.50 ± 3.94b | 36.83 ± 6.10a |
| 500 mg kg -1 DBP + 100 mg kg -1 vitamin E | 10.83 ± 0.75c | 9.33 ± 0.82c | 85.01 ± 2.83c | 49.16 ± 5.50 |
abc Different superscripts denote statistical significance at p < 0.05.
Table 2.
Duration of pregnancy, maternal and fetal body weight and AGD of female and male pups in rats given a dose range of DBP or DBP along with vitamin E during pregnancy. Values are means ± standard deviation
| Groups | Duration of Pregnancy | Maternal body weight gain during pregnancy (g) | Fetal body weight (mm) | AGD of female pups (mm) | AGD of male pups (mm) |
|---|---|---|---|---|---|
| Control | 19.51 ± 0.55 | 147.33 ± 9.54 | 4.72 ± 0.09 | 2.22 ± 0.12 | 3.85 ± 0.12 |
| Vitamin E | 19.52 ± 0.44 | 147.66 ± 7.89 | 4.66 ± 0.08 | 2.18 ± 0.10 | 3.88 ± 0.13 |
| 100 mg kg -1 DBP | 19.67 ± 0.81 | 142.43 ± 12.20 | 4.63 ± 0.07 | 2.29 ± 0.17 | 3.90 ± 0.11 |
| 500 mg kg -1 DBP | 19.83 ± 0.83 | 117.50 ± 7.47a | 4.23 ± 0.09a | 2.33 ± 0.15 | 3.13 ± 0.09a |
| 1,000 mg kg -1 DBP | 20.0 ± 0.89 | 78.16 ± 7.85b | 5.21 ± 0.09b | 2.23 ± 0.14 | 2.87 ± 0.12b |
| 500 mg kg -1 DBP + 100 mg kg -1 vitamin E | 19.67 ± 0.81 | 138.67 ± 6.28c | 4.49 ± 0.08c | 2.25 ± 0.10 | 3.51 ± 0.14c |
abc Different superscripts denote statistical significance at p < 0.05.
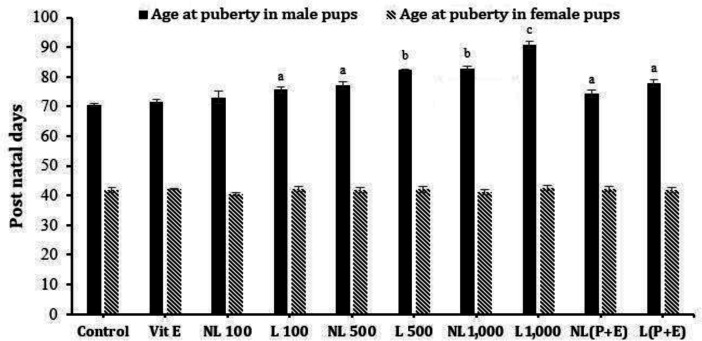
In the present study, DBP had no effect on female pups’ puberty both in the lactation and non-lactation groups at any of the applied doses. Also, DBP exposure at 500 mg kg-1 and 1,000 mg kg-1 in both lactation and non-lactation groups resulted in a significant (p < 0.05) delay at the onset of puberty in the male pups. Also, DBP at 100 mg kg-1 during lactation led to a significant delay at the onset of puberty in male offspring. However, preputial separation was occurred later in the lactation treatments compared to the non-lactation groups. Vitamin E administration along with 500 mg kg-1 DBP did not affect age at puberty in male or female offspring compared to the DBP at 500 mg kg-1 (Table 3, Fig. 1). In the present study, DBP exposure led to nipple retention in male offspring at all doses. Also, cryptorchidism was observed in DBP at 500 mg kg-1 and 1,000 mg kg-1 in both lactation and non-lactation groups.
Table 3.
Effect of a dose range of DBP or DBP along with vitamin E on sperm parameters of F1 male rats at puberty. Values are means ± standard deviation
| Groups | Sperm count (10 6 mL -1 ) | Sperm motility (%) | Abnormal sperm (%) |
|---|---|---|---|
| Control | 35.25 ± 0.92 | 72.78 ± 0.36 | 1.05 ± 0.05 |
| Vitamin E | 36.56 ± 0.44 | 73.31 ± 0.39 | 0.98 ± 0.08 |
| Non-lactation treatment (100 mg kg -1 DBP) | 35.07 ± 0.22 | 71.85 ± 0.14 | 1.11 ± 0.27 |
| Lactation treatment (100 mg kg -1 DBP) | 36.49 ± 0.36 | 71.73 ± 0.15 | 1.07 ± 0.19 |
| Non-lactation treatment (500 mg kg -1 DBP) | 24.26 ± 0.31a | 54.68 ± 0.26a | 41.94 ± 0.12a |
| Lactation treatment (500 mg kg -1 DBP) | 21.13 ± 0.23b | 50.73 ± 0.18b | 46.56 ± 0.33b |
| Non-lactation treatment (1,000 mg kg -1 DBP) | 7.87 ± 0.23c | 24.10 ± 0.22c | 63.62 ± 0.95c |
| Lactation treatment (1,000 mg kg -1 DBP) | 5.02 ± 0.26d | 20.28 ± 0.32d | 69.59 ± 1.89d |
| Non-lactation treatment (500 mg kg -1 DBP + 100 mg kg -1 vitamin E) | 32.82 ± 0.31e | 72.00 ± 0.72e | 6.52 ± 1.03e |
| Lactation treatment (500 mg kg -1 DBP + 100 mg kg -1 vitamin E) | 33.2 ± 0.44e | 71.78 ± 0.39e | 6.39 ± 0.83e |
abcde Different superscripts denote statistical significance at p < 0.05.
Fig. 1.
Effects of a dose range of DBP or DBP along with vitamin E on the age at puberty in F1 male and female rats. Vit E, vitamin E group; NL 100, Non-lactation treatment of 100 mg kg-1 DBP; L 100, Lactation treatment of 100 mg kg-1 DBP; NL 500, Non-lactation treatment of 500 mg kg-1 DBP; L 500, Lactation treatment of 500 mg kg-1 DBP; NL 1,000, Non-lactation treatment of 1,000 mg kg-1 DBP; L 1,000, Lactation treatment of 1,000 mg kg-1 DBP; NL(P+E), Non-lactation treatment of 500 mg kg-1 DBP +100 mg kg-1 vitamin E; L(P+E), Lactation treatment of 500 mg kg-1 DBP +100 mg kg-1 vitamin E. Each column and vertical bar represent the mean ± SD
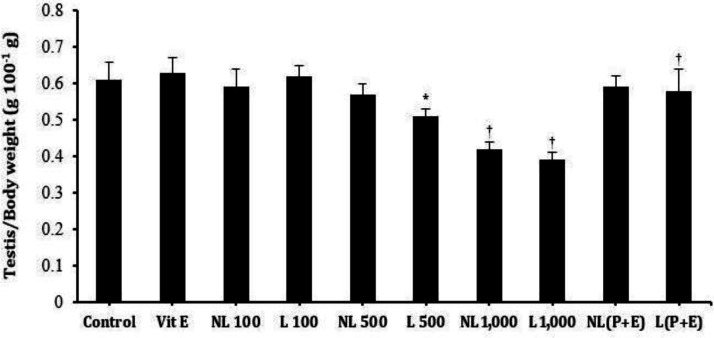
In the lactation groups, testis weight was significantly decreased at 500 mg kg-1 and 1,000 mg kg-1 DBP compared to the control and vitamin E groups. Also, in non-lactation groups, DBP at 1,000 mg kg-1 decreased the testis weight significantly (p < 0.05) compared to the control and vitamin E groups. Administration of vitamin E improved the testis weight significantly (p < 0.05) in lactation treatments of DBP at 500 mg kg-1 compared to 500 mg kg-1 DBP group (Table 3, Fig. 2).
Fig. 2.
Effects of a dose range of DBP or DBP along with vitamin E on relative testis weight. Vit E, vitamin E group; NL 100, Non-lactation treatment of 100 mg kg-1 DBP; L 100, Lactation treatment of 100 mg kg-1 DBP; NL 500, Non-lactation treatment of 500 mg kg-1 DBP; L 500, Lactation treatment of 500 mg kg-1 DBP; NL 1,000, Non-lactation treatment of 1,000 mg kg-1 DBP; L 1,000, Lactation treatment of 1,000 mg kg-1 DBP; NL(P+E), Non-lactation treatment of 500 mg kg-1 DBP +100 mg kg-1 vitamin E; L(P+E), Lactation treatment of 500 mg kg-1 DBP +100 mg kg-1 vitamin E. Each column and vertical bar represent the mean ± SD
*, † Significantly different from control group, p < 0.05 and ‡ Significantly different from Lactation treatment of 500 mg kg-1 DBP, p < 0.05.
Discussion
In the present study, DBP had no effect on the duration of pregnancy that was consistent with the previous studies.6 However, in a conflicting study, Ahmad et al. found that DBP at 2, 10 and 50 mg kg-1 elevated gestational length.20 This inconsistency might be due to some unique effects of phthalates that might occur at one dose that was not observed in other doses.21 In two dams at 1,000 mg kg-1 and one dam at 500 mg kg-1 DBP, vaginal bleeding were observed on gestation days 10, 12 and 15, respectively. Since it was reported that mid-pregnancy was sensitive to disruption by phthalates these findings could be considered as DBP-mediated mid-pregnancy abortion which was in agreement with the previous studies.8,22 It was reported that phthalates had adverse effects on ROS scavenging system either by increasing the concentration of ROS or by decreasing the activity of the antioxidant enzymes.3 Oxidative stress affects implantation and early development of embryos and also leads to luteal regression.14,23 Likewise, it was suggested that the inhibition of DNA transcription in the rapidly divided fetal cells, which might be accelerated under the condition of oxidative stress, might also play a role in fetal death.15 Vitamin E had cytoprotective and antioxidant properties in embryo cultured cells, maternal and fetal tissues and pregnant rats that could explain lack of mid-pregnancy loss in co-administration of vitamin E and DBP in the current study.15
The DBP at 100 mg kg-1 had no significant effect on litter size or the number of implantations which was in agreement with the previous studies.24 Although, in a controversial study, a significant reduction in the litter size was observed in the pregnant rats treated with 100 mg kg-1 DBP.25 In the current study, litter size and implantation number were decreased at 500 mg kg-1 and 1,000 mg kg-1 DBP. It has been shown that phthalate exposure could cause implantation loss and consequently reduction of litter size.21 In the present study, implantation sites were found with no associated embryos. It was reported that following phthalate administration to pregnant rats, there was an improper spacing of some implanted embryos which could more easily lead to pregnancy loss.26 Co-administration of vitamin E and DBP in the present study increased the litter size and the number of implantations. As discussed earlier, the increased level of oxidative stress following administration of DBP could explain decreased litter size and implantation number in the present study.3,14,15 Administration of vitamin E in the present study decreased the disrupting effects of DBP probably by its potent antioxidant properties.15
The maternal weight gain was decreased at 500 mg kg-1 and 1,000 mg kg-1 DBP. Reportedly, different doses of DBP caused reduced maternal body weight which showed correlation with the other adverse developmental data such as increased incidence of resorptions, pre- and post-implantation embryonic loss, decreased litter size, decreased fetal weight and even fetal anomalies.8,21 In the present study, administration of DBP at 100 mg kg-1 did not affect maternal body weight which was consistent with the results of the others.27 This suggested that DBP at a dose of 100 mg kg-1 did not provoke maternal toxicity. However, Salazar et al. reported that DBP at 12 or 50 mg kg-1 decreased the maternal weight gain.6 Also, Howdeshell et al. did not observe any reduction in maternal body weight gain following administration of DBP at 33, 50, 100, 300, and 600 mg kg-1.11 These inconsistencies might be due to differences in the dose and period of DBP administration. In the present study, co-administration of vitamin E with DBP improved maternal weight gain. It has been reported that co-administration of vitamins E and C in pregnant diabetic rats improved diabetes-induced weight loss of the mother and fetuses.28
The DBP administration at 100 mg kg-1 had no effect on fetal body weight. However, DBP at 500 mg kg-1 and 1,000 mg kg-1 resulted in a reduction of fetal body weight. Several studies indicated that phthalates are fetotoxic, which is in part evidenced by the decreased fetal body weight after administration of the compounds to the dams.7,21,29 Saillenfait et al. reported that fetal weight reduction after administration of phthalate was along with different abnormalities of fetuses and a sign of developmental retardation of offspring.30 On the contrary, Giribabu et al. reported that DBP at 100 mg kg-1 and 500 mg kg-1 had no effect on body weight of male fetuses which might be due to the limited time of exposure.25 Here, co-administration of vitamin E with DBP improved fetal body weight. It has been reported that vitamin E enhanced the maternal immune status and reduced disease which might provide additional nutrients for growth of fetus and so increasing intrauterine growth.31
A significant reduction was observed in the viability index at 500 mg kg-1 and 1,000 mg kg-1 DBP. It has been reported that DBP at 500 mg kg-1 and 750 mg kg-1 caused a significant reduction in the number of live fetuses per litter.32 Besides the fetotoxic effects of DBP, increased number of dead fetuses at 500 mg kg-1 and 1,000 mg kg-1 DBP could be due to the inappropriate formation of the placenta and decidual cells degeneration.26 In the present study, viability index was improved after administration of vitamin E which could be explained by antioxidant properties of vitamin E.15
The maternal exposure to DBP at 100 mg kg-1 and 500 mg kg-1 had no effect on the sex ratio. However, DBP administration at 1,000 mg kg-1 led to a reduction of the sex ratio. It has been reported that the survival rate of male progeny before and after birth was less than female offspring in mammalian species. Also, it has been reported that males were more susceptible to the hazardous effects of phthalates.33 Hence, reduction of the sex ratio in DBP at 1,000 mg kg-1 in the present study might be due to more in utero fetal male death that was in agreement with the previous studies.24,32
The DBP had no effect on the AGD of female pups at any of the applied doses which was in agreement with the previous investigations.2,22 However, DBP exposure led to a significant decrease in the AGD of male pups at 500 mg kg-1 and 1,000 mg kg-1 that was in agreement with the previous studies.34,35 Also, co-administration of vitamin E with DBP in the present study improved the AGD of male pups. It has been reported that AGD in male rats was an androgen-dependent factor, so it was sensitive to endocrine disruptors such as DBP.36 It has been demonstrated that oxidative stress played an important role in phthalates-mediated testosterone reduction, hence, the antioxidant properties of vitamin E may reduce the effects of DBP on AGD.37
The DBP exposure had no effect on the onset of puberty in female offspring at any of the applied doses that was in agreement with the previous studies.2,8,27 However, Lee et al. reported delayed vaginal opening in rats exposed to 10,000 ppm DBP in diet.38 Also, Salazar et al. reported the same effect at DBP at 12 or 50 mg kg-1.6 The different dosing and route of administration in the mentioned studies might be the point of inconsistencies. Overall, the evidences suggest that DBP did not affect puberty in females.2 In the present study, DBP administration at 500 mg kg-1 and 1,000 mg kg-1 in male offspring resulted in delayed onset of puberty in both lactation and non-lactation groups. Also, administration of DBP at 100 mg kg-1 resulted in delayed puberty in the male offspring of the lactation treatment. In previous studies, oral doses of DBP resulted in delayed puberty in male rats.6,39,40 The delay might be due to decreased serum testosterone reported in rats treated with DBP during gestation.39 Resumed administration of DBP during the lactation period might result in more reduction of serum testosterone and later onset of puberty.
It has been reported that gestational exposure of rats to high doses of DBP impaired steroidogenesis by the fetal testis, decreased testosterone and led to post-natal disorders such as hypospadias, cryptorchidism, and reduced AGD.41,42 Androgen action during this time might also be a determinant of sperm production in adulthood.41 Phthalate are anti-androgenic compounds negatively correlated with the decreased fertility in males.5 The anti-androgenic effect of DBP exposure during gestational, lactation and pubertal age was reported in rodents.43 The infertility of males caused by DBP might be due to decreased testosterone secreted by Leydig cells or the direct damage of germ cells and Sertoli cells.37 Also, earlier studies showed that phthalates increased ROS production in the testes of rats.44,45 Excessive production of ROS have been associated with impaired sperm motility, concentration and morphology.46
The DBP exposure at all doses led to nipple retention in male offspring in the both lactation and non-lactation groups that was in agreement with the results of others.1,6,45 It has been reported that nipple retention in male was resulted from alteration of androgen levels or blockade of the androgen receptor.47
In the present study, cryptorchidism was observed in DBP at 500 mg kg-1 and 1,000 mg kg-1 in both lactation and non-lactation groups that was in agreement with the results of others.48 DBP-induced cryptorchidism could result from multiple factors including suppression of insulin-like factor 3, suppression of testosterone and decreased testes weight due to DBP exposure.49 In the present study, DBP administration at 1,000 mg kg-1 led to a significant reduction in testis weight that was in agreement with the former studies.1,39 This reduction might be associated with the loss of germ cells and atrophy of Leydig cells following administration of DBP.48 Although administration of DBP at 100 mg kg-1 in the present study had no effect on sperm characteristics, Zhang et al. reported that DBP at 50 mg kg-1 significantly decreased sperm count and motility and increased the percentage of sperm abnormalities.7 In the present study, DBP at 500 mg kg-1 and 1,000 mg kg-1 decreased the sperm count and motility and increased the morphological abnormalities significantly both in the lactation and non-lactation groups. Adverse effects of prenatal and/or neonatal phthalate exposure on cauda epididymal sperm count, sperm morphology and sperm motility have been described in rodents.1,20,37,49
It has been reported that increased ROS levels in seminal plasma resulted in oligozoospermia. Also, oxidative stress disrupts the steroidogenic capacity of Leydig cells and the ability of the germinal epithelium to differentiate into normal spermatozoa.46,50 Oxidative stress affects the motility of spermatozoa that has been suggested to be due to decreased flexibility and decreased energy availability required for sperm motility.50 In the present study, co-administration of vitamin E with DBP improved sperm count, motility and morphology that could be explained by the potent antioxidant properties of vitamin E.46
Finally, this study showed that DBP at the doses of 500 mg kg-1 and 1,000 mg kg-1 had significant embryolethality which was evidenced by the incidence of pre- and post-implantation loss, increase in the number of resorptions and elevation of dead fetuses per litter. Also, DBP at 100, 500 and 1,000 mg kg-1 had adverse effects on male reproductive system. Administration of vitamin E at 100 mg kg-1 along with DBP could protect the dam and offspring from phthalate-induced adverse effects on the reproductive system, probably due to its antioxidative effects. Accordingly, many signs of DBP-induced reproductive toxicity might be reduced or eliminated by vitamin E consumption.
Conflict of interest
The authors declare there is no conflict of interest.
Acknowledgments
The authors would like to thank Dr. Mostafa Araghi and Dr. Ali Soleimanzadeh for technical assistance.
References
- 1.Kay VR, Bloom MS, Foster WG. Reproductive and developmental effects of phthalate diesters in males. Crit Rev Toxicol. 2014;44(6):467–498. doi: 10.3109/10408444.2013.875983. [DOI] [PubMed] [Google Scholar]
- 2.Kay VR, Chambers C, Foster WG. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol. 2013;43(3):200–219. doi: 10.3109/10408444.2013.766149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasanth GK, Divya LM, Sadasivan C. Effects of mono and di(n-butyl) phthalate on superoxide dismutase. Toxicology. 2009;262(1):38–42. doi: 10.1016/j.tox.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 4.Katsikantami I, Sifakis S, Tzatzarakis MN, et al. A global assessment of phthalates burden and related links to health effects. Environ Int. 2016;97:212–236. doi: 10.1016/j.envint.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Helal MA. Celery oil modulates DEHP-induced reproductive toxicity in male rats. Reprod Biol. 2014;14(3):182–189. doi: 10.1016/j.repbio.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Salazar V, Castillo C, Ariznavarreta C, et al. Effect of oral intake of dibutyl phthalate on reproductive parameters of Long Evans rats and pre-pubertal development of their offspring. Toxicology. 2004;205(1-2):131–137. doi: 10.1016/j.tox.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Jiang X, Chen B. Reproductive and developmental toxicity in F1 Sprague–Dawley male rats exposed to di-n-butyl phthalate in utero and during lactation and determination of its NOAEL. Reprod Toxicol. 2004;18(5):669–676. doi: 10.1016/j.reprotox.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Gray LE Jr, Laskey J, Ostby J. Chronic di-n-butyl phthalate exposure in rats reduces fertility and alters ovarian function during pregnancy in female Long Evans hooded rats. Toxicol Sci. 2006;93(1):189–195. doi: 10.1093/toxsci/kfl035. [DOI] [PubMed] [Google Scholar]
- 9.Farombi EO, Abarikwu SO, Adedara IA, et al. Curcumin and kolaviron ameliorate di-n-butylphthalate-induced testicular damage in rats. Basic Clin Pharmacol Toxicol. 2007;100(1):43–48. doi: 10.1111/j.1742-7843.2007.00005.x. [DOI] [PubMed] [Google Scholar]
- 10.Sultana Z, Maiti K, Aitken J, et al. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am J Reprod Immunol. 2017;77:5. doi: 10.1111/aji.12653. [DOI] [PubMed] [Google Scholar]
- 11.Howdeshell KL, Wilson VS, Furr J, et al. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008;105(1):153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- 12.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1(1):15–24. doi: 10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou D, Wang H, Zhang J, et al. Di-n-butyl phthalate (DBP) exposure induces oxidative damage in testes of adult rats. Syst Biol Reprod Med. 2010;56(6):413–419. doi: 10.3109/19396368.2010.509902. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28 . doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdou HM, Mohamed NA, El Mekkawy DA, et al. Vitamin E and/or wheat germ oil supplementation ameliorate oxidative stress induced by cadmium chloride in pregnant rats and their fetuses. Jordan J Biol Sci. 2017;10(1):39–48. [Google Scholar]
- 16.Soleimanzadeh A, Kian M, Moradi S, et al. Carob (Ceratonia siliqua L fruit hydro-alcoholic extract alleviates reproductive toxicity of lead in male mice: Evidence on sperm parameters, sex hormones, oxidative stress biomarkers and expression of Nrf2 and iNOS. Avicenna J Phytomed. 2020;10(1):35–49. [PMC free article] [PubMed] [Google Scholar]
- 17.Smith JT, Waddell BJ. Increased fetal glucocorticoid exposure delays puberty onset in postnatal life. Endocrinology. 2000;141(7):2422–2428. doi: 10.1210/endo.141.7.7541. [DOI] [PubMed] [Google Scholar]
- 18.Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17(2):298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- 19.Narayana K, D’Souza UJ, Seetharama Rao KP. Ribavirin-induced sperm shape abnormalities in Wistar rat. Mutat Res. 2002;513(1-2):193–196. doi: 10.1016/s1383-5718(01)00308-4. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad R, Gautam AK, Verma Y, et al. Effects of in utero di-butyl phthalate and butyl benzyl phthalate exposure on offspring development and male reproduction of rat. Environ Sci Pollut Res Int. 2014;21(4):3156–3165. doi: 10.1007/s11356-013-2281-x. [DOI] [PubMed] [Google Scholar]
- 21.Mahaboob Basha P, Radha MJ. Gestational di-n-butyl phthalate exposure induced developmental and teratogenic anomalies in rats: a multigenerational assessment. Environ Sci Pollut Res Int. 2017;24(5):4537–4551. doi: 10.1007/s11356-016-8196-6. [DOI] [PubMed] [Google Scholar]
- 22.Hannas BR, Howdeshell KL, Furr J, et al. In utero phthalate effects in the female rat: A model for MRKH syndrome. Toxicol Lett. 2013;223(3):315–321. doi: 10.1016/j.toxlet.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CK, Lee JT, Yu SJ, et al. Effects of cadmium on the expression of placental lactogens and Pit-1 genes in the rat placental trophoblast cells. Mol Cell Endocrinol. 2009;298(1-2):11–18. doi: 10.1016/j.mce.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 24.Shirai M, Wakui S, Wempe MF, et al. Male Sprague-Dawley rats exposed to in utero di(n-butyl) phthalate: dose dependent and age-related morphological changes in Leydig cell smooth endoplasmic reticulum. Toxicol Pathol. 2013;41(7):984–991. doi: 10.1177/0192623312474725. [DOI] [PubMed] [Google Scholar]
- 25.Giribabu N, Sainath SB, Sreenivasula Reddy P. Prenatal di‐n‐butyl phthalate exposure alters reproductive functions at adulthood in male rats. Environ toxicol. 2014;29(5):534–544. doi: 10.1002/tox.21779. [DOI] [PubMed] [Google Scholar]
- 26.Li R, Yu C, Gao R, et al. Effects of DEHP on endometrial receptivity and embryo implantation in pregnant mice. J Hazard Mater. 2012;241-242:231–240. doi: 10.1016/j.jhazmat.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 27.Guerra MT, Scarano WR, de Toledo FC, et al. Reproductive development and function of female rats exposed to di-eta-butyl-phthalate (DBP) in utero and during lactation. Reprod Toxicol. 2010;29(1):99–105. doi: 10.1016/j.reprotox.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Cederberg J, Simán CM, Eriksson UJ. Combined treatment with vitamin E and vitamin C decreases oxidative stress and improves fetal outcome in experimental diabetic pregnancy. Pediatr Res. 2001;49(6):755–762. doi: 10.1203/00006450-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Saillenfait AM, Sabaté JP, Gallissot F. Developmental toxic effects of diisobutyl phthalate, the methyl-branched analogue of di-n-butyl phthalate, administered by gavage to rats. Toxicol Lett. 2006;165(1):39–46. doi: 10.1016/j.toxlet.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Saillenfait AM, Gallissot F, Sabaté J-P. Differential developmental toxicities of di-n-hexyl phthalate and dicyclohexyl phthalate administered orally to rats. J Appl Toxicol. 2009;29(6):510–521. doi: 10.1002/jat.1436. [DOI] [PubMed] [Google Scholar]
- 31.Capper JL, Wilkinson RG, Kasapidou E, et al. The effect of dietary vitamin E and fatty acid supplementation of pregnant and lactating ewes on placental and mammary transfer of vitamin E to the lamb. Br J Nutr. 2005;93(4):549–557. doi: 10.1079/bjn20051376. [DOI] [PubMed] [Google Scholar]
- 32.Ema M, Miyawaki E. Adverse effects on development of the reproductive system in male offspring of rats given monobutyl phthalate, a metabolite of dibutyl phthalate, during late pregnancy. Reprod Toxicol. 2001;15(2):189–194. doi: 10.1016/s0890-6238(01)00111-3. [DOI] [PubMed] [Google Scholar]
- 33.Bae J, Kim S, Kannan K, et al. Couples’ urinary bisphenol A and phthalate metabolite concentrations and the secondary sex ratio. Environ Res. 2015;137:450–457. doi: 10.1016/j.envres.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barlow NJ, Foster PM. Pathogenesis of male reproductive tract lesions from gestation through adulthood following in utero exposure to Di(n-butyl) phthalate. Toxicol Pathol. 2003;31(4):397–410. doi: 10.1080/01926230390202335. [DOI] [PubMed] [Google Scholar]
- 35.Struve MF, Gaido KW, Hensley JB, et al. Reproductive toxicity and pharmacokinetics of di-n-butyl phthalate (DBP) following dietary exposure of pregnant rats. Birth Defects Res B Dev Reprod Toxicol. 2009;86(4):345–354. doi: 10.1002/bdrb.20199. [DOI] [PubMed] [Google Scholar]
- 36.Welsh M, Saunders PT, Fisken M, et al. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest. 2008;118(4):1479–1490. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen H, Liao K, Wu HF, et al. In utero exposure of high-dose di-n-butyl phthalate resulted in opposite effects on testicular cell apoptosis in late embryonic and pubertal male rat offspring. Hum Exp Toxicol. 2017;36(12):1236–1247. doi: 10.1177/0960327116685886. [DOI] [PubMed] [Google Scholar]
- 38.Lee KY, Shibutani M, Takagi H, et al. Diverse developmental toxicity of di-n-butyl phthalate in both sexes of rat offspring after maternal exposure during the period from late gestation through lactation. Toxicology. 2004;203(1-3):221–238. doi: 10.1016/j.tox.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Kim TS, Jung KK, Kim SS, et al. Effects of in utero exposure to DI(n-Butyl) phthalate on development of male reproductive tracts in Sprague-Dawley rats. J Toxicol Environ Health A. 2010;73(21-22):1544–1559. doi: 10.1080/15287394.2010.511579. [DOI] [PubMed] [Google Scholar]
- 40.Gray LE Jr, Barlow NJ, Howdeshell KL, et al. Trans-generational effects of Di (2-ethylhexyl) phthalate in the male CRL:CD(SD) rat: added value of assessing multiple offspring per litter. Toxicol Sci. 2009;110(2):411–425. doi: 10.1093/toxsci/kfp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drake AJ, van den Driesche S, Scott HM, et al. Glucocorticoids amplify dibutyl phthalate-induced disruption of testosterone production and male reproductive development. Endocrinology. 2009;150(11):5055–5064. doi: 10.1210/en.2009-0700. [DOI] [PubMed] [Google Scholar]
- 42.Johnson KJ, Heger NE, Boekelheide K. Of Mice and men (and rats): phthalate-induced fetal testis endocrine disruption is species-dependent. Toxicol Sci. 2012;129(2):235–248. doi: 10.1093/toxsci/kfs206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foster PM, Mylchreest E, Gaido KW, et al. Effects of phthalate esters on the developing reproductive tract of male rats. Hum Reprod Update. 2001;7(3):231–235. doi: 10.1093/humupd/7.3.231. [DOI] [PubMed] [Google Scholar]
- 44.Nair N. Dose-dependent short-term study of di-n-butyl phthalate on the testicular antioxidant system of Wistar rats. Environ Sci Pollut Res Int. 2015;22(3):2196–2204. doi: 10.1007/s11356-014-3457-8. [DOI] [PubMed] [Google Scholar]
- 45.Oda SS, Waheeb RS. Ginger attenuated di (n-butyl) phthalate-induced reproductive toxicity in pubertal male rabbits. World Rabbit Sci. 2017;25(4):387–398. [Google Scholar]
- 46.Agarwal A, Virk G, Ong C, et al. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32(1):1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barlow NJ, McIntyre BS, Foster PM. Male reproductive tract lesions at 6, 12, and 18 months of age following in utero exposure to di(n-butyl) phthalate. Toxicol Pathol. 2004;32(1):79–90. doi: 10.1080/01926230490265894. [DOI] [PubMed] [Google Scholar]
- 48.Jiang XP, Tang JY, Xu Z, et al. Sulforaphane attenuates di-N-butylphthalate-induced reproductive damage in pubertal mice: Involvement of the Nrf2-antioxidant system. Environ Toxicol. 2017;32(7):1908–1917. doi: 10.1002/tox.22413. [DOI] [PubMed] [Google Scholar]
- 49.Mahood IK, Scott HM, Brown R, et al. In utero exposure to di(n-butyl) phthalate and testicular dysgenesis: comparison of fetal and adult end points and their dose sensitivity. Environ Health Perspect. 2007;115(Suppl 1):55–61. doi: 10.1289/ehp.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner H, Cheng JW, Ko EY. Role of reactive oxygen species in male infertility: An updated review of literature. Arab J Urol. 2017;16(1):35–43. doi: 10.1016/j.aju.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]