Abstract
Gout is characterized by monosodium urate (MSU) crystal deposits in and within joints. These deposits result from persistent hyperuricaemia and most typically lead to recurrent acute inflammatory episodes (gout flares). Even though some aspects of gout are well characterized, uncertainties remain; this upcoming decade should provide further insights into many of these uncertainties. Synovial fluid analysis allows for the identification of MSU crystals and unequivocal diagnosis. Non-invasive methods for diagnosis are being explored, such as Raman spectroscopy and imaging modalities. Both ultrasound and dual-energy computed tomography (DECT) allow the detection of MSU crystals; this not only provides a mean of diagnosis, but also has furthered gout knowledge defining the presence of a preclinical deposition in asymptomatic hyperuricaemia. Scientific consensus establishes the beginning of gout as the beginning of symptoms (usually the first flare), but the concept is currently under review. For effective long-term gout management, the main goal is to promote crystal dissolution treatment by reducing serum urate below 6 mg/dL (or 5 mg/dL if faster crystal dissolution is required). Current urate-lowering therapies’ (ULTs) options are limited, with allopurinol and febuxostat being widely available, and probenecid, benzbromarone, and pegloticase available in some regions. New xanthine oxidase inhibitors and, especially, uricosurics inhibiting urate transporter URAT1 are under development; it is probable that the new decade will see a welcomed increase in the gout therapeutic armamentarium. Cardiovascular and renal comorbidities are common in gout patients. Studies determining whether optimal treatment of gout will positively impact these comorbidities are currently lacking, but will hopefully be forthcoming. Overall, the single change that will most impact gout management is greater uptake of international rheumatology society recommendations. Innovative strategies, such as nurse-led interventions based on these recommendations have recently demonstrated treatment success for people with gout.
Keywords: diagnosis, gout, pathogenesis, treatment, urate
Where do we stand now?
Gout is the most common inflammatory arthritis world-wide. 1 Gout in its most typical form is characterized by recurring, self-limiting acute inflammatory episodes known as gout flares. 2 However, gout can also cause a variety of different symptoms. Tophi, accumulations of urate crystals surrounded by an inflammatory corona 3 are common in patients with insufficiently treated and advanced disease, but can occasionally be the presenting symptom in the absence of flares; this seems to be especially common in elderly women under diuretic treatment. 4 Tophi can put pressure on nearby structures, especially in space-constrained areas, such as the spine 5 or the carpal tunnel. Advanced disease can lead to persistent joint inflammation, frequently polyarticular. 6 Gout can also cause foot pain and disability in-between flares, 7 perhaps even before the first inflammatory flare occurs. 8 Gout also happens more frequently in patients with some rheumatological diseases, such as psoriatic arthritis or osteoarthritis. An association of gout with several comorbidities, such as chronic kidney disease (CKD), hypertension and cardiovascular events, has also been clearly established; whether this association is causal or an epiphenomenon, is currently still under debate. Furthermore, the potential effect of urate-lowering therapy (ULT) on these comorbidities remains uncertain.
Gout is the result of monosodium urate (MSU) crystals’ deposition in and around joints and in other tissues. Osteoarthritis can predispose to MSU crystals’ deposition, especially in weight-bearing joints of the lower extremities; thus, both can coexist. Deposited MSU crystals can trigger gout flares through activation of the NLRP3 inflammasome; in the absence of therapy, flares tend to be recurrent. 6 MSU crystals can be found in joints prior to the first gout attack for an undefined, but likely extended, time period; 9 whether gout starts with the first crystal deposition or with the first gout attack is still under debate. What is clear is that once crystals are found within a joint, and in the absence of urate-lowering management, the crystals will remain. 10
Gout prevalence has been estimated at 2–3% of adult population. Wide variations occur between world regions, with very high prevalence throughout the Pacific area. 1 Data also suggest that there has been an increase in gout incidence in the past decades. Different studies in North America and Scandinavia have reported a 1.5–2-fold increase in gout incidence in recent decades. 1 The Global Burden of Disease study has also shown a doubling of gout incidence from 1990 to 2017, with similar increases across all world regions. 11 In parallel, gout hospitalizations and inpatient medical costs have doubled in Canada from 2000 to 2011. 12 Similar increases have been reported in Aotearoa New Zealand, UK 13 and Spain. 14 In addition, the number of gout patients may be even higher as a notable underreporting of gout diagnosis in records has been detected.15,16
To treat gout, the aim is to reduce serum urate levels, thereby allowing deposited crystals to dissolve and, with time, disappear. Current guidelines recommend achieving serum urate levels under 6 mg/dL.17,18 Given that the serum urate level will determine the rate of crystal dissolution, a lower serum urate target (i.e. <5 mg/dL) may be indicated in many patients. Even though gout is one of the few rheumatic diseases, which can be ‘cured’ and that effective treatment is readily available, successive audits and studies have demonstrated a suboptimal treatment of gout. 19 Studies report that less than half of patients diagnosed with gout receive ULT; in the UK database Clinical Practice Research Datalink UK, only 40% of eligible patients were on ULT 1 year after diagnosis. 20 Even when prescribed, ULT is often not titrated to achieve urate target. 21 The use of ULT without achieving urate target implies that crystal deposits will continue to increase and the patient is at risk for adverse events from the medication. With regional variations, rheumatologists provide care for severe or complex patients, while most of the care for gout patients relies on primary care. Unfortunately, poor management is seen in both settings. Improving gout patient management to comply with current recommendations is the single change that, if achieved within the next decade, could most positively impact gout patients.
When does gout start?
The central pathology of gout is MSU crystal deposition. 22 MSU crystals form in the presence of elevated urate above saturation concentration, which is dependent on sodium ion concentration, temperature and pH. 23 The clinical symptoms of gout result from the individual’s immune response to deposited crystals. 24 Hyperuricaemia is the most important risk factor for development of gout, with a concentration dependent relationship between serum urate and incident gout.25,26 However, most people with hyperuricaemia do not develop gout, even when observed over many years.
Numerous synovial fluid and advanced imaging studies have demonstrated that many people with asymptomatic hyperuricaemia have evidence of MSU crystal deposition; most studies estimate this as 25–33% depending on the serum urate threshold and method of detection of MSU crystal deposition.9,27–29 It is likely that asymptomatic MSU crystal deposition is a precursor to development of symptomatic disease. However, it is unknown why some, but not all, people with hyperuricaemia form MSU crystals, and why some, but not all, people with hyperuricaemia and asymptomatic MSU crystal deposition develop symptomatic disease. Analysis of dual-energy computed tomography (DECT) MSU crystal volume has shown that MSU crystal deposits occur more frequently and at higher volumes in those with symptomatic gout than in asymptomatic hyperuricaemia, suggesting that a threshold of urate crystal volume may be required before symptomatic disease occurs. 28
Genetic studies comparing people with symptomatic gout to asymptomatic hyperuricaemia have identified some genetic variants associated with symptomatic gout. A Japanese genome-wide association study (GWAS) identified three gout-associated loci; CNTN5, MIR302F and ZNF724. 30 In a study of Han Chinese participants, three gout-associated loci were identified; at 17q23.2, 9p24.2 and 11p15.5. 31 In an analysis of UK Biobank, 13 gout-associated loci were identified (ABCG2, SLC2A9, SLC22A11, GCKR, MEPE, PPM1 K-DT, LOC105377323 and ADH1B); all variants in urate transporters and metabolic genes, but none in inflammatory genes. 32 Variants in ABCG2 have been consistently reported as associated with gout compared with hyperuricaemic controls.32,33 It is noteworthy that many of the genetic variants identified as risk variants associated with gout when compared with asymptomatic hyperuricaemia are also associated with elevated serum urate concentrations. While it is possible that some variants may have pleiotropic effects, potentially on the inflammatory response to deposited MSU crystals, it is also possible that these variants increase the lifelong exposure to elevated serum urate levels, and are thus associated with development of MSU crystal deposition and symptomatic gout.
Traditionally, gout has been diagnosed when clinical symptoms develop. This is most often at the time of a gout flare, although tophaceous gout or chronic gouty arthritis can occasionally be the initial presentation. An important conceptual question, particularly given that MSU crystals can be observed in those without clinical symptoms, is when does gout start? Gout is a disorder of MSU crystal deposition, and some have argued that evidence of MSU crystal deposition is sufficient for a definition of gout.34,35 This is even more uncertain when imaging evidence of tophus is demonstrated on ultrasound or DECT. In established gout, MSU crystal burden, as measured by tophus burden or DECT volume, predicts cardiovascular disease and mortality.36–38 In asymptomatic hyperuricaemia, synovial fluid or imaging evidence of MSU crystals has been associated with increased joint inflammation and cardiovascular disease,27,39 suggesting that asymptomatic deposition might contribute to adverse outcomes, even when symptomatic disease is not evident.
However, at present, there is no conclusive evidence that treating MSU crystal deposition without symptoms is beneficial, and treatment of asymptomatic MSU crystal deposition is not recommended by major rheumatology society gout management guidelines.17,40 The 2015 American College of Rheumatology and European League Against Rheumatism Gout Classification Criteria require ‘at least one episode of swelling, pain, or tenderness in a peripheral joint or bursa’ as entry criterion for classification of gout.41,42 Similarly, in a recent consensus statement, the Gout and Crystal Arthritis Network (G-CAN) has recently defined gout as ‘a disease caused by monosodium urate crystal deposition with any of the following clinical presentations (current or prior): gout flare, chronic gouty arthritis or subcutaneous tophus’. Bursill et al., 2 indicating that most experts currently believe that both classification and diagnosis of gout requires clinical features of joint inflammation or tophus. Longitudinal studies are essential to understand whether imaging evidence of MSU crystal deposition in asymptomatic hyperuricaemia has prognostic implications for development of future gout flares, or other clinical features of gout, or development of associated comorbid conditions, such as cardiovascular disease or CKD. Furthermore, clinical trials are required to determine the benefits and harms of ULT or anti-inflammatory medications for those with asymptomatic MSU crystal deposition.
Will we still use crystal analysis for diagnosis?
Synovial fluid analysis
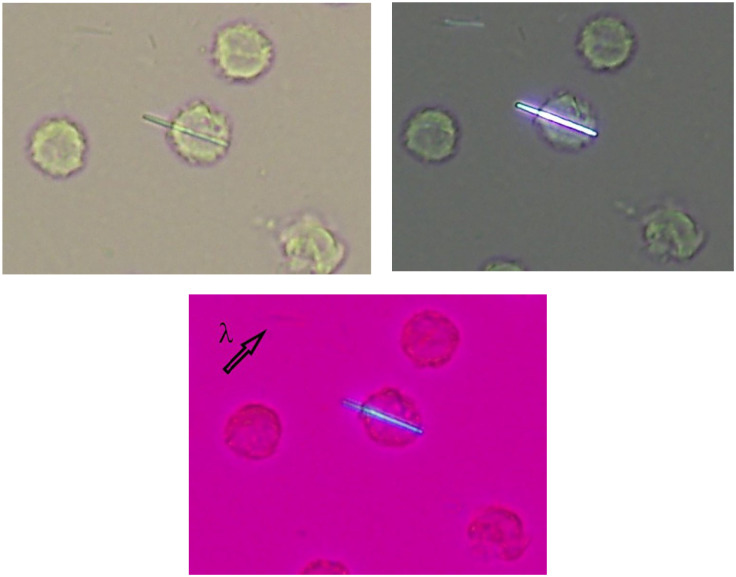
Since discovering MSU and calcium pyrophosphate (CPP) crystals in the synovial fluid from patients suffering from gout 22 or CPP crystal arthritis, 43 their identification by compensated polarized microscopy became the gold standard diagnosis for crystal arthritis (Figure 1).44,45 The rationale behind this is that all the clinical manifestations come from the presence of crystals, so that, these must be demonstrated. This approach of proving the pathogenic factor is used in other deposition diseases (i.e. amyloidosis) or infectious diseases, and is highly specific, especially for MSU crystals. When identified, MSU crystals are considered responsible for the arthritis, 46 however, CPP crystals are commonly found in advanced osteoarthritic joints, 47 are doubtfully pathogenic in some settings and are likely part of the process of joint damage and repair.
Figure 1.
MSU crystals in a synovial fluid sample seen under light microscopy (a) using ordinary light, (b) polarized light, and (c) first-order red compensator. λ shows the axis of the first-order red compensator.
Synovial fluid analysis for crystals under polarized light microscopy is reliable, 48 can be learnt after a short period, 49 provides an immediate diagnosis (even in-between flares 10 ), prompts the therapeutic plan and avoids subsequent investigations. However, in the literature, concerns have been raised about the procedure; 50 methodological issues in the studies, such as the source of crystals, the medium of storage, or the experience and usual dedication of the observers, may explain the suboptimal results that have been published. Although the coexistence of MSU and calcium pyrophosphate (CPP) crystals has received scant attention, identification of both crystal types has been reported in 1–8% of patient with confirmed gout. 51 Given that both can result in acute self-limiting arthritis, symptom attribution can be problematic. In addition, patients with psoriatic arthritis have a higher risk of presenting with gout 52 potentially mediated by an increased risk of hyperuricaemia. Interestingly, hyperuricaemic subjects with psoriatic arthritis depicted more erosions, higher disease activity and lesser response to treatments. 53 The presence of MSU crystals should be ruled out in cases with poor evolution. These caveats highlight the need for accurate diagnosis as an integral part of the management strategy.
However, before considering whether the synovial fluid analysis will remain the principal diagnostic method for gout and other crystal arthritis in the future, it is worth reviewing its current use in clinical practice. Available data from some European countries can be deemed poor. For example, in the United Kingdom and Spain, crystal-proven diagnoses in gout are limited to 18% 54 and 32% 55 of patients in rheumatology clinics, respectively. These rates are presumably lower in cases managed solely in a primary care setting. Not having access to a polarized microscope or the inconvenience of performing a joint aspiration are common justifications, despite the risk of misclassification when relying solely on clinical data 56 or the good performance and tolerability of thinner needles, such as 29G. 57 Moreover, efforts in building diagnostic clinical approaches have not achieved criteria accurate enough,58,59 though they may have a place in settings with no access to microscopy.
A potential option might be identifying MSU crystals without polarized microscopy; some efforts have been made in this regard.60,61 Raman spectroscopy, a chemical technique used mainly by geologists to detect the presence of crystals and provide a specific identification based on their chemical constituents. Large and expensive machines limited the application in clinical practice, but a smaller device more suitable for hospitals showed good agreement with the microscopic identification of MSU crystals (kappa = 0.84), but moderate for CPP crystals (kappa = 0.61). 60 A limitation is that joint sampling for synovial fluid remains indispensable. A pilot study showed good sensitivity in detecting MSU crystal deposits in vivo, 62 although only studied in a superficial joint (the first metatarsophalangeal joint). Thus, these devices should not replace the microscope yet. Synovial fluid analysis helps, not only in screening for microcrystals, but also permits an immediate leukocyte count to differentiate mechanical and inflammatory fluids, prompting further investigations.
Imaging for diagnosing crystal arthritis
Conventional radiography is not useful for gout diagnosis, 63 although typical gout erosions are a feature of 2015 ACR/EULAR classification criteria. 41 However, in the last decade, imaging techniques have experienced significant advances in the field of crystal arthritis, especially ultrasound (Figure 2) and DECT (Figure 3), contributing to a better understanding of gout: the existence of a significant crystal load at the time of first flares;28,64 the presence of a preclinical deposition in asymptomatic hyperuricaemia;28,65 deposition of crystals following a symmetrical, polyarticular fashion; the involvement of periarticular structures not often clinically apparent66,67 or how tophi formation and growth lead to bone erosions. 68 Also, as will be discussed later in this article, imaging can aid following the reduction of crystal deposits during ULT69,70 and may help demonstrate the clearance of the disease.
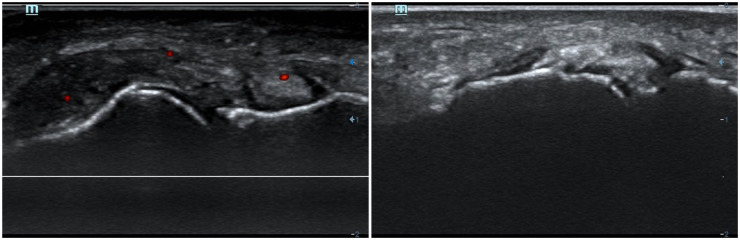
Figure 2.
Ultrasound tophi in the first metatarsophalangeal (MTP) joint (left image, dorsal aspect; right image, medial aspect) from two patients with gout. Identification of sonographic deposits in the medial scans of the first MTP is quite common in gout. Note: in the left image, a positive power-Doppler signal inside the tophus, despite the patient being in-between flares, indicating subclinical inflammation.
Figure 3.
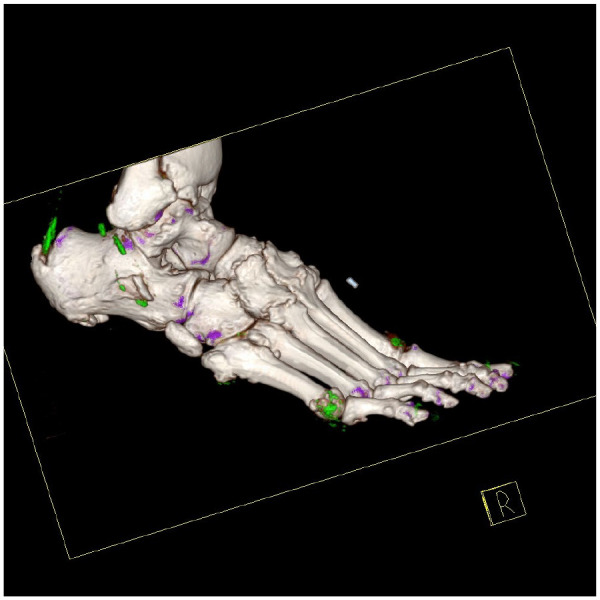
Dual-energy CT of the right foot in a patient with tophaceous gout. Three-dimensional volume rendered images demonstrating MSU crystal deposition (colour coded green), including at first metatarsophalangeal joint, fifth metatarsophalangeal joint and the Achilles tendon.
Several studies have assessed imaging in gout diagnosis compared with synovial fluid analysis or other reference standards, with better diagnostic performances shown by ultrasound and DECT. Studies assessing the sonographic double-contour sign yielded a pooled sensitivity and specificity of 74–83% and 76–88%, respectively; 71 sonographic tophi are less accurate (sensitivity of 65%, specificity of 80%), other features, such as aggregates or hyperechoic clouds show a poorer performance. Pooled results for DECT showed a sensitivity of 74–90% and a specificity of 80–88%. 71 Ultrasound is cheaper, widespread in rheumatology clinics, but operator-dependent, while DECT is radiant, vastly more expensive but highly reliable. 72 Some experts support that ultrasound is more useful to discriminate between crystal and non-crystal arthritides, rather than just for gout, as CPP crystal deposition disease may sometimes form tophaceous-like accumulations and show a double contour-like sign. 73 However, DECT can discriminate between urate and calcium deposits. Thus, the choice will vary according to the setting. In addition, DECT has also demonstrated MSU deposition in the context of other diseases when persistent hyperuricaemia is present. A fifth of patients with established rheumatoid arthritis and persistent hyperuricaemia showed MSU deposits on DECT, 74 especially in patients diagnosed with seronegative rheumatoid arthritis. In these instances, symptom attribution might be complex and a ULT trial might be warranted.
A major issue in using imaging for gout diagnosis is the variable sensitivity regarding the disease duration. 75 Poor diagnostic performance at early stages is a significant limitation for the technique, as it is at this stage that excluding other forms of arthritis and initiating proper gout management to avoid progression to advanced tophaceous forms is crucial. In fact, the detection of subcutaneous tophi by physical exam has high diagnostic utility in itself, 63 and the diagnosis is generally straightforward, but late. Thus, improving the early detection of crystal deposits is a crucial condition for a technique to be useful for diagnosis.
What will we know about gout outside the ‘typical’ presentation?
The prototypical gout patient is a middle-aged White male, obese, with comorbidities and, according to popular depictions, indulging in dietary excesses. 76 However, this depiction is far from accurate. Beer, liquor, fructose-rich soft drinks and meat have been shown to associate with increased urate levels. However, in the general population, diet patterns explain less than 1% of urate level variation, while genome-wide single-nucleotide variations explain almost a quarter of this variation. 77 Public perception of gout as a result of self-indulgence should be challenged to increase the understanding of the disease and the uptake of ULT. 76
Non-White populations experience, not only a higher incidence and prevalence, but also a higher disease burden and a greater risk of inappropriate management. 78 In the United States, gout is estimated to affect 4% of non-Hispanic Whites, but 5% of African Americans. 79 In addition, African Americans are at a three- to six-fold higher risk of developing severe cutaneous adverse reactions to allopurinol, 80 probably reflecting the difference in allele frequency of HLA-B*5801. In Aotearoa New Zealand, disparities are even more pronounced: gout is present in 4.3% of people of NZ European ethnicity, 8.3% in Māori and 13.6% in Pacific peoples. 81 Māori and Pacific peoples living in Aotearoa have early age of onset, high flare frequency, pain and activity limitation and high prevalence of associated comorbidities. 82 Even in the face of the greater disease burden, Māori and Pacific peoples are less likely to receive regular dispensing of ULT, compared with people of NZ European ethnicity. 83 Culturally, safe approaches to gout management that focus on providing better care to those with the greatest disease burden should be a priority.
Overall, gout is more common in men, but little specific data are available to characterize gout in women. Under 65 years, gout prevalence is approximately four times higher in men. In later decades, the prevalence of gout acquires a more equal sex distribution due to the increase in incidence in older women. 1 This is usually attributed to the increase in serum urate levels after menopause. However, most studies are performed in predominantly male populations with only a few studies examining gender differences. When other predominantly male diseases, such as myocardial infarction or spondyloarthritis, have been assessed from a gender perspective, findings have been striking. Women with spondyloarthritis have a longer diagnostic delay despite seeking medical attention, resulting not only from a difference in symptoms presentation at onset, but also to the preconceived concepts of the physicians. 84 In short, if you do not expect to find gout in women, then you will never suspect it, nor diagnose it.
Women tend to have later-onset gout, and more commonly a polyarticular distribution. Women also tend to have lower alcohol intake, a higher body mass index, 85 a higher intake of diuretics and common gout-related comorbidities (such as hypertension or CKD).86,87 Given that many of the studies analyse gout patients in a rheumatology setting, these may not be generalizable to the general population. A greater identification and physician awareness of any differences in disease manifestations between genders could increase the diagnostic suspicion and therefore the number of gout diagnosis in women.
Gender and ethnic inequalities in enrolment in studies of ULTs have also recently become apparent. Men and White participants have been over-represented (when compared with estimated prevalence) in trials testing most mainstream drugs, such as xanthine oxidase inhibitors (XOIs) or uricosurics. 82 Enrolment of non-White participants has always been limited, but has substantially decreased in the past decade. Studies that explore ethnic and gender differences in gout’s natural history or treatment response are currently unavailable. More data in diverse ethnicity and gender groups will hopefully be forthcoming on the next decade. Even more importantly, efforts to address these inequities are urgently needed.
Will our treatment options develop/change?
Given that gout results from inflammation triggered by MSU crystal deposition, the main aim in gout treatment is to achieve the dissolution of urate crystals. Saturation point for serum urate levels is approximately 6.8 mg/dL in physiological conditions. When urate is lowered below this saturation point, crystals will slowly, but steadily, dissolve. Achieving serum urate target is associated with improvement in patient-reported outcomes, such as gout flares. 88 To ensure serum urate is below saturation point even with mild differences in other crystal facilitators (temperature, pH, etc.), recommendations suggest reducing serum urate level in gout patients below 6 mg/dL. Given that crystals dissolve quicker at lower serum urate levels, urate levels below 5 mg/dL are recommended in patients with erosions, a high urate burden, tophi and comorbidities. Some of the patient profiles that would benefit from lower urate targets are still to be defined; in essence, lower targets should be considered in any patient in which a quicker resolution is deemed beneficial. Once all urate crystals have dissolved, gout can be considered cured; continuing treatment with ULT then aims at preventing the recurrence of crystal deposition. 89
The key to adequate gout management is therefore the use of ULT. Available treatment options are limited. Current ULT options work by decreasing urate synthesis (allopurinol and febuxostat), increasing urate kidney excretion (benzbromarone and probenecid) or through urolytic enzymes (pegloticase), which cleave urate into more soluble, easily disposed of products. Allopurinol and febuxostat are widely accessible, but not all of uricosuric or urolytic therapies are available in all countries or regions. 90
Lesinurad, another uricosuric drug, has recently been removed from all markets following a poor market penetration. This has probably resulted from a combination of a need for use in conjunction with an XOI and concerns over renal adverse effects. It is expected – and hoped – that the next 10 years will bring an increase in the number of treatment options available to gout patients. When used appropriately, current ULTs achieve target serum urate levels in most patients. 91 However, a small percentage of gout patients are unable to achieve their target due to refractoriness or, more commonly, intolerance or contraindication to current treatments.
Allopurinol and febuxostat inhibit xanthine oxidase enzyme, key in urate synthesis. Advances in research on the three-dimensional structure of this enzyme could lead to the development of new drugs. 92 Topiroxostat is a non-purine XOI currently licenced only in Japan. It was reported to be non-inferior to allopurinol in hyperuricaemic patients with or without gout, even if the dose of allopurinol to which it was compared was low (200 mg daily). 93 The addition of topiroxostat to the gout armamentarium could prove a useful alternative. Tigulixostat, a non-purine selective XOI, reduced serum urate levels by around 50% and was well tolerated in a phase II study. 94 Another XOI (LC350189), is currently finalizing a phase II, dose-ranging, randomized controlled trial (RCT); if the results are positive, it could be developed within the next 10 years. Even though some positive results have been reported, alternative ways of decreasing purine synthesis production, such as the inhibition of purine nucleoside phosphorylase by ulodesine, 95 do not seem to be under current development.
Given that hyperuricaemia in most gout patients is mediated by a decreased urate kidney excretion, uricosuric drugs have always been pathogenically appealing. Enhanced understanding of the localization and mechanisms of the different urate transporters, could lead to the development of drugs targeting these transporters, especially URAT1. 92 Dotinurad, a selective URAT1 inhibitor, has recently been approved for gout and hyperuricaemia in Japan; this upcoming decade might see its approval in other markets. In two RCTs, dotinurad has been reported as non-inferior to both febuxostat (up to 40 mg daily) 96 and benzbromarone (50 mg daily); 97 as with topiroxostat, the ULT doses used in the control group were low. Verinurad, another URAT1 inhibitor, has been shown to be effective in lowering serum urate both alone and when combined with febuxostat in a phase II trial. 98 As with lesinurad, an increase in renal-related adverse events when used in monotherapy was noted and will have to be monitored. 99 Arhalofenate, initially developed as an insulin sensitizer for type 2 diabetes mellitus, has showed a moderate urate-lowering ability through inhibition of URAT1. 100 SHR4640 is currently being assessed in a phase III RCT in Chinese gout patients, which could lead at least to regional market authorization in the future. Other uricosuric drugs, mostly centred in inhibiting URAT1, are at earlier stages of development. 101 However, overall, only a few phase III clinical trials are currently planned or registered and some drugs with successful phase II trials have not been developed further. Results from well-conducted phase III trials are awaited to see how new drugs fit into the, relatively small, existing gout therapy armamentarium.
Pegloticase treatment is restricted to patients with tophaceous gout in which XOI have been ineffective, or the patient has shown intolerance or contraindication. Given that over 40% of patients develop antibodies against pegloticase, which are linked to inefficacy and adverse events, new strategies for delivering pegloticase are being assessed. The use of concomitant mycophenolate mofetil halved the lack of efficacy and prevented infusion reactions at 6 months. 102 The use of other immunomodulatory medication, such as methotrexate is currently under study. 103 Alternatively, the co-administration of tolerogenic particles at the time of uricase administration is also being explored. A proof-of-concept study has shown that the production of anti-drug antibodies is mitigated by the concomitant administration of pegadricase with ImmTor, which consists of rapamycin encapsulated in nanoparticles. 104 ImmTor induces specific immune tolerance, instead of the general immunosuppression produced by methotrexate or mycophenolate, which is an attractive approach.
Currently, most patients with gout are on concomitant flare prophylaxis on ULT initiation, commonly with colchicine for a minimum of 6 months. However, the need for prophylaxis if ULT is initiated at a low dose with a slow upwards titration is under review. 105 In a decade, further information about whether colchicine is needed will be available.
For gout flare treatment, anti-inflammatory drugs – colchicine, non-steroidal anti-inflammatory drugs (NSAIDs) or glucocorticoids – are the mainstay treatment, with interleukin-1 (IL-1) blocking therapy used in selected patients. Canakinumab is currently licenced in Europe, while anakinra, a cheaper IL-1 blocking drug, is unlicensed; several publications report the use of anakinra in off-label contexts and a phase II trial has recently been published. 106 Given the central role of NLRP3 inflammasome for crystal inflammation, the development of NLRP3 inhibitors could prove of additional benefit to gout patients. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, has been shown to be safe in gout flares 107 and is awaiting trials against current mainstream therapies.
Will gout management entail benefits on related diseases?
Cardiovascular disease.
Cumulative data have proven that gout is an independent risk factor for the development of atherosclerotic cardiovascular events, such as cardiovascular death, 108 coronary heart disease, 109 stroke 110 or peripheral artery disease, 111 on a level similar to diabetes for certain outcomes. 112 By having gout, the increased risk of cardiovascular and coronary mortality was estimated at 30% and 40%, respectively, compared with those without gout, 113 while usual risk assessment tools, such as Framingham or SCORE underestimate the risk. 114 In patients with established cardiovascular disease, gout increased the possibility of new episodes after the first event, regardless of a management adherent to cardiology guidelines.115,116 Thus, it is clear that gout management should be included in the primary and secondary cardiovascular prevention strategies, especially as the derived cardiovascular risk is believed to derive from the hyperuricaemic state and the crystal-driven persistent inflammation. 117
However, whether gout management results in cardiovascular benefits should be clarified in the following years. We lack clinical trials on this issue, as it would be unethical to randomize gout patients to receive placebo or no therapy; therefore, we must rely on observational data. Several studies have analysed the cardiovascular impact of gout management, with contradictory findings.118–121 Differences in populations and ancestries, disease stages, serum urate levels or type, dose or duration of urate-lowering agents may account for the inconclusive data. However, the principal limitation is that gout management remains unsatisfactory at both primary care122,123 and rheumatology clinics;54,55 therefore, data extracted from these needs to be taken with caution. Larger observational studies in which patients with gout are treated according to standards are needed to prove the expected cardiovascular benefit.
It is uncertain whether the choice of the urate-lowering agent – XOI, reversible (allopurinol) or not reversible (febuxostat), uricosurics or recombinant uricases – might carry a differential cardiovascular profile. When the CARES trial was published, significant concerns arose with febuxostat, as more cardiovascular and all-cause deaths were detected compared with allopurinol. 124 However, major methodological issues, such as a 44% drop-out rate and 85% of events registered after drug withdrawal, limited the value of the data, and the similar profile between both agents reported in the recent Febuxostat versus Allopurinol Streamlined Trial (FAST) 125 and Comparative Effectiveness in Gout: Allopurinol versus Febuxostat (CSP594) 126 trials is reassuring. Some observational studies suggest better cardiovascular outcomes using uricosurics (probenecid or benzbromarone) than XOI.127,128 Preliminary data suggest effects also on endothelial function and adipokines regulation, 129 but more interventional studies are needed. No studies have assessed the cardiovascular impact of pegloticase yet.
Colchicine is an old drug, used for gout flare management or prevention when ULT is introduced. When used for prevention, colchicine is commonly recommended for at least six months, as this was the duration of the trials. 130 In our practice, patients with very recurrent gout flares, persistent arthritis or tophaceous disease, may benefit from longer colchicine schemes. 131 Moreover, from the cardiovascular perspective, patients at high risk or with an established disease might also be candidates for this strategy, considering the benefits of adding colchicine 0.5 mg once daily to the standard cardiovascular care. 132 In the cardiovascular trials, patients with gout were poorly represented (only 8% of total), but nothing indicates that benefits would not be translatable, considering some promising, observational data. 133
Renal disease
Gout and the kidney are closely and bidirectionally related.134,135 The MSU crystal deposition at the renal medulla occurs with an undetermined frequency in patients with gout, but is likely more common in severe cases with larger crystal load. 136 It is presumable that some patients with marked hyperuricaemia, despite being asymptomatic, also present renal deposits. The inflammation associated with MSU crystal deposits favours fibrosis and a progressive decline in renal function that, in association with the hyperuricaemic state, the coexistence of hypertension or diabetes, and the use of non-steroidal anti-inflammatory agents for flares, all suggest how gout could lead to CKD 137 and its progression to final stages.138,139
An unsolved question is whether ULT would reduce the incidence and progression of renal disease. 140 No clinical trials have formally assessed this endpoint in the gout population. However, studies in patients with CKD without gout, many of whom had elevated serum urate levels, have not demonstrated that ULT with allopurinol prevents progression of CKD.141,142 Allopurinol (dosed to achieve target urate levels and not limited by creatinine clearance-based formulae) and febuxostat are effective and safe in people with gout and CKD,143,144 and recent data suggest that lowering urate levels to achieve crystal dissolution may prevent renal function decline in people with gout.145,146 However, the combination of gout and renal disease is full of uncertainties in multiple areas (pathophysiology, prognosis, management, among others) and merits focussed research. 140
How can we manage gout better?
While effective urate-lowering medications, such as allopurinol and probenecid, have been available for gout management for more than half a century, and newer agents, such as febuxostat, have been available for more than a decade, major gaps remain in gout management. A recent global meta-analysis of gout management has shown that approximately half of people with gout receive ULT, half of those on ULT receive regular uninterrupted ULT, and only one-third on ULT achieve serum urate target. 21 Collectively, these gaps in treatment lead to ongoing preventable pain, disability, and poor health-related quality of life.
In addition to the structural challenges of chronic disease management, there are specific challenges to gout that impact both health care practitioner and patient behaviours. The benefits of ULT are realized over months or years, rather than days. 147 Indeed, when prescribed at high dose without anti-inflammatory prophylaxis, gout flares are common when initiating ULT, 105 and severe gout flares when starting ULT may lead to cessation of treatment. 148 The disease course, with symptom-free inter-critical periods, can reinforce the perception of gout as an intermittently flaring disease. Physicians’ knowledge of gout and adherence to relevant recommendations has been reported as poor in a multitude of settings.149,150 Patients may choose to stop treatment to test treatment (‘To see if I can do without it’, ‘To see if I really need it’) or to resist illness (‘Because I want to think of myself as a healthy person again’, ‘Because I want to lead a normal life again’). 151 The perception of gout as a humorous condition, reinforced by historical cartoons of dietary excess, can trivialize the impact of disease. 76 The persistent contemporary cultural narratives about the role of dietary and alcohol excess as the cause of disease lead to excessive focus on dietary solutions as the primary approach to manage gout and can create stigma and embarrassment about the condition.152,153
Health systems and health care professionals play an important role in enabling adherence and improving gout care. Low-cost pharmacy interventions have shown modest improvements.154,155 Most impressive has been the impact of nurse-led care, with a large clinical trial demonstrating the clinical benefits of a treat-to-target nurse-led care approach within primary care. 156 The nurse-led programme included addressing illness perceptions, building understanding, proving regular follow-up with intermittent serum urate testing and dose titration of ULT. At 2 years, 95% of the patient receiving nurse-led care achieved serum urate levels under 6 mg/dL, compared with 30% in the usual care group. Improvements in gout flares, tophi, adherence and patient-reported outcomes were also observed. 157 Nurse-led care was cost-effective in the short term and potentially cost saving in the long term.
Periodic serum urate testing provides a useful approach to monitor adherence and reinforce the role of persistent ULT once treatment is established. Imaging may also provide important opportunities to build understanding about gout as a chronic disease of MSU crystal deposition and the role of ULT, and monitor response to treatment. Viewing images during gout education improves understanding about the condition. 158 Viewing their own clinical images leads to greater patient involvement in their care, 159 and people with gout find personalized images more engaging and interesting than generic images. 160 Serial scanning with DECT or ultrasound can also allow visualization of changes in MSU crystal deposition, further reinforcing the benefits of treatment.69,70,161,162 While exposure to radiation limits frequent DECT assessments in clinical practice, point of care ultrasound allows serial assessments over time as part of the clinical visit.
Acknowledgments
None.
Footnotes
ORCID iD: Francisca Sivera  https://orcid.org/0000-0002-3414-1667
https://orcid.org/0000-0002-3414-1667
Contributor Information
Francisca Sivera, Rheumatology Unit, Hospital General Universitario Elda, Ctra Sax s/n, Elda 03600, Alicante, Spain; Department Medicine, Universidad Miguel Hernandez, Elche, Spain.
Mariano Andres, Department Medicine, Universidad Miguel Hernandez, Elche, Spain; Rheumatology Unit, Hospital General Universitario Alicante, Alicante, Spain; Alicante Institute of Sanitary and Biomedical Research (ISABIAL), Alicante, Spain.
Nicola Dalbeth, The University of Auckland, Auckland, New Zealand.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Francisca Sivera: Conceptualization; Data curation; Writing – original draft; Writing – review & editing.
Mariano Andres: Conceptualization; Data curation; Writing – original draft; Writing – review & editing.
Nicola Dalbeth: Conceptualization; Data curation; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Competing Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: FS reports research grants from Novartis and speaking fees from Menarini. M. A. reports research grants from Grünenthal and speaking fees from Menarini. N.D. reports grants from AstraZeneca and Amgen, consulting fees from Dyve BioSciences, AstraZeneca JW Pharmaceuticals, Selecta, Arthrosi, Horizon, and PK Med, and speaker fees from Abbvie and Janssen.
Availability of data and materials: Not applicable.
References
- 1. Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol 2020; 16: 380–390. [DOI] [PubMed] [Google Scholar]
- 2. Bursill D, Taylor WJ, Terkeltaub R, et al. Gout, hyperuricaemia and crystal-associated disease network (G-CAN) consensus statement regarding labels and definitions of disease states of gout. Ann Rheum Dis 2019; 78: 1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dalbeth N, Pool B, Gamble GD, et al. Cellular characterization of the gouty tophus: a quantitative analysis. Arthritis Rheum 2010; 62: 1549–1556. [DOI] [PubMed] [Google Scholar]
- 4. Macfarlane DG, Dieppe PA. Diuretic induced gout in elderly women. Br J Rheumatol 1985; 24: 155–157. [DOI] [PubMed] [Google Scholar]
- 5. Jin HJ, Son ES, Kim DH. The frequency of axial deposition in Korean patients with gout at a tertiary spine center. Front Med (Lausanne) 2020; 7: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dalbeth N, Stamp L. Hyperuricaemia and gout: time for a new staging system? Ann Rheum Dis 2014; 73: 1598–1600. [DOI] [PubMed] [Google Scholar]
- 7. Petty HR, Rathod-Mistry T, Menz HB, et al. Foot structure, pain and functional ability in people with gout in primary care: cross-sectional findings from the Clinical Assessment Study of the Foot. J Foot Ankle Res 2019; 12: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alammari YM, Gheta D, Flood RM, et al. Urate-lowering therapy (ULT) reduces non-episodic foot pain in patients who fail to meet ACR/EULAR 2015 gout classification criteria: an effect predicted by ultrasound and potential rationale for reclassification. Ann Rheum Dis 2019; 78: 579–580. [DOI] [PubMed] [Google Scholar]
- 9. De Miguel E, Puig JG, Castillo C, et al. Diagnosis of gout in patients with asymptomatic hyperuricaemia: a pilot ultrasound study. Ann Rheum Dis 2012; 71: 157–158. [DOI] [PubMed] [Google Scholar]
- 10. Pascual E, Batlle-Gualda E, Martinez A, et al. Synovial fluid analysis for diagnosis of intercritical gout. Ann Intern Med 1999; 131: 756–759. [DOI] [PubMed] [Google Scholar]
- 11. Jin Z, Wang D, Zhang H, et al. Incidence trend of five common musculoskeletal disorders from 1990 to 2017 at the global, regional and national level: results from the global burden of disease study 2017. Ann Rheum Dis 2020; 79: 1014–1022. [DOI] [PubMed] [Google Scholar]
- 12. Rai SK, Avina-Zubieta JA, McCormick N, et al. Trends in gout and rheumatoid arthritis hospitalizations in Canada from 2000 to 2011. Arthritis Care Res (Hoboken) 2017; 69: 758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robinson PC, Merriman TR, Herbison P, et al. Hospital admissions associated with gout and their comorbidities in New Zealand and England 1999-2009. Rheumatology (Oxford) 2013; 52: 118–126. [DOI] [PubMed] [Google Scholar]
- 14. Benavent D, Peiteado D, Martinez-Huedo MA, et al. Healthcare-related impact of gout in hospitalized patients in Spain. Sci Rep 2021; 11: 13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calabuig I, Gomez-Garberi M, Andres M. Gout is prevalent but under-registered among patients with cardiovascular events: a field study. Front Med (Lausanne) 2020; 7: 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quilis N, Sivera F, Seoane-Mato D, et al. Prevalence of gout in the adult general population in Spain: estimating the proportion of undiagnosed cases. Joint Bone Spine 2021; 89: 105257. [DOI] [PubMed] [Google Scholar]
- 17. Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017; 76: 29–42. [DOI] [PubMed] [Google Scholar]
- 18. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken) 2020; 72: 744–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pascual E, Sivera F. Why is gout so poorly managed? Ann Rheum Dis 2007; 66: 1269–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuo C-F, Grainge MJ, Mallen C, et al. Eligibility for and prescription of urate-lowering treatment in patients with incident gout in England. JAMA 2014; 312: 2684–2686. [DOI] [PubMed] [Google Scholar]
- 21. Son CN, Stewart S, Su I, et al. Global patterns of treat-to-serum urate target care for gout: systematic review and meta-analysis. Semin Arthritis Rheum 2021; 51: 677–684. [DOI] [PubMed] [Google Scholar]
- 22. McCarty DJ, Hollander JL. Identification of urate crystals in gouty synovial fluid. Ann Intern Med 1961; 54: 452–460. [DOI] [PubMed] [Google Scholar]
- 23. Loeb JN. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum 1972; 15: 189–192. [DOI] [PubMed] [Google Scholar]
- 24. Faires J, McCarty D, Jr. Acute arthritis in man and dog after intrasynovial injection of sodium urate crystals. Lancet 1962; 280: 682–685. [DOI] [PubMed] [Google Scholar]
- 25. Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med 1987; 82: 421–426. [DOI] [PubMed] [Google Scholar]
- 26. Dalbeth N, Phipps-Green A, Frampton C, et al. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Ann Rheum Dis 2018; 77: 1048–1052. [DOI] [PubMed] [Google Scholar]
- 27. Andres M, Bernal JA, Arenas MD, et al. Synovial fluid leukocyte count in asymptomatic hyperuricaemia with crystal deposition: a proof-of-concept study. Rheumatology (Oxford) 2019; 58: 1104–1105. [DOI] [PubMed] [Google Scholar]
- 28. Dalbeth N, House ME, Aati O, et al. Urate crystal deposition in asymptomatic hyperuricaemia and symptomatic gout: a dual energy CT study. Ann Rheum Dis 2015; 74: 908–911. [DOI] [PubMed] [Google Scholar]
- 29. Howard RG, Pillinger MH, Gyftopoulos S, et al. Reproducibility of musculoskeletal ultrasound for determining monosodium urate deposition: concordance between readers. Arthritis Care Res (Hoboken) 2011; 63: 1456–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawamura Y, Nakaoka H, Nakayama A, et al. Genome-wide association study revealed novel loci which aggravate asymptomatic hyperuricaemia into gout. Ann Rheum Dis 2019; 78: 1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li C, Li Z, Liu S, et al. Genome-wide association analysis identifies three new risk loci for gout arthritis in Han Chinese. Nat Commun 2015; 6: 7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sandoval-Plata G, Morgan K, Abhishek A. Variants in urate transporters, ADH1B, GCKR and MEPE genes associate with transition from asymptomatic hyperuricaemia to gout: results of the first gout versus asymptomatic hyperuricaemia GWAS in Caucasians using data from the UK Biobank. Ann Rheum Dis 2021; 80: 1220–1226. [DOI] [PubMed] [Google Scholar]
- 33. Wrigley R, Phipps-Green AJ, Topless RK, et al. Pleiotropic effect of the ABCG2 gene in gout: involvement in serum urate levels and progression from hyperuricemia to gout. Arthritis Res Ther 2020; 22: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Puig JG, Beltrán LM, Mejía-Chew C, et al. Ultrasonography in the diagnosis of asymptomatic hyperuricemia and gout. Nucleosides Nucleotides Nucleic Acids 2016; 35: 517–523. [DOI] [PubMed] [Google Scholar]
- 35. Perez-Ruiz F, Marimon E, Chinchilla SP. Hyperuricaemia with deposition: latest evidence and therapeutic approach. Ther Adv Musculoskelet Dis 2015; 7: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vincent ZL, Gamble G, House M, et al. Predictors of mortality in people with recent-onset gout: a prospective observational study. J Rheumatol 2017; 44: 368–373. [DOI] [PubMed] [Google Scholar]
- 37. Perez-Ruiz F, Martinez-Indart L, Carmona L, et al. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann Rheum Dis 2014; 73: 177–182. [DOI] [PubMed] [Google Scholar]
- 38. Marty-Ané A, Norberciak L, Andrès M, et al. Crystal deposition measured with dual-energy computed tomography: association with mortality and cardiovascular risks in gout. Rheumatology (Oxford, England) 2021; 60: 4855–4860. [DOI] [PubMed] [Google Scholar]
- 39. Andres M, Quintanilla MA, Sivera F, et al. Silent monosodium urate crystal deposits are associated with severe coronary calcification in asymptomatic hyperuricemia: an exploratory study. Arthritis Rheumatol 2016; 68: 1531–1539. [DOI] [PubMed] [Google Scholar]
- 40. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Rheumatol (Hoboken, NJ) 2020; 72: 879–895. [DOI] [PubMed] [Google Scholar]
- 41. Neogi T, Jansen TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2015; 74: 1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neogi T, Jansen TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol 2015; 67: 2557–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kohn NN, Hughes RE, McCarty DJ, Jr, et al. The significance of calcium phosphate crystals in the synovial fluid of arthritic patients: the ‘pseudogout syndrome’. II. Identification of crystals. Ann Intern Med 1962; 56: 738–745. [DOI] [PubMed] [Google Scholar]
- 44. Sivera F, Andres M, Carmona L, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systematic literature review and expert opinion of a broad panel of rheumatologists in the 3e initiative. Ann Rheum Dis 2014; 73: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Richette P, Doherty M, Pascual E, et al. 2018 updated European League against Rheumatism evidence-based recommendations for the diagnosis of gout. Ann Rheum Dis 2020; 79: 31–38. [DOI] [PubMed] [Google Scholar]
- 46. Pascual E, Sivera F, Andres M. Synovial fluid analysis for crystals. Curr Opin Rheumatol 2011; 23: 161–169. [DOI] [PubMed] [Google Scholar]
- 47. Derfus BA, Kurian JB, Butler JJ, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol 2002; 29: 570–574. [PubMed] [Google Scholar]
- 48. Pascual E, Tovar J, Ruiz MT. The ordinary light microscope: an appropriate tool for provisional detection and identification of crystals in synovial fluid. Ann Rheum Dis 1989; 48: 983–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lumbreras B, Pascual E, Frasquet J, et al. Analysis for crystals in synovial fluid: training of the analysts results in high consistency. Ann Rheum Dis 2005; 64: 612–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Segal JB, Albert D. Diagnosis of crystal-induced arthritis by synovial fluid examination for crystals: lessons from an imperfect test. Arthritis Care Res 1999; 12: 376–380. [DOI] [PubMed] [Google Scholar]
- 51. Robier C, Neubauer M, Quehenberger F, et al. Coincidence of calcium pyrophosphate and monosodium urate crystals in the synovial fluid of patients with gout determined by the cytocentrifugation technique. Ann Rheum Dis 2011; 70: 1163–1164. [DOI] [PubMed] [Google Scholar]
- 52. Merola JF, Wu S, Han J, et al. Psoriasis, psoriatic arthritis and risk of gout in US men and women. Ann Rheum Dis 2015; 74: 1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Widawski L, Fabacher T, Spielmann L, et al. Psoriatic arthritis with hyperuricemia: more peripheral, destructive, and challenging to treat. Clin Rheumatol 2022; 41: 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roddy E, Packham J, Obrenovic K, et al. Management of gout by UK rheumatologists: a British Society for Rheumatology national audit. Rheumatology (Oxford) 2018; 57: 826–830. [DOI] [PubMed] [Google Scholar]
- 55. Perez Ruiz F, Sanchez-Piedra CA, Sanchez-Costa JT, et al. Improvement in diagnosis and treat-to-target management of hyperuricemia in gout: results from the GEMA-2 transversal study on practice. Rheumatol Ther 2018; 5: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Malik A, Schumacher HR, Dinnella JE, et al. Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid crystal analysis. J Clin Rheumatol 2009; 15: 22–24. [DOI] [PubMed] [Google Scholar]
- 57. Sivera F, Aragon R, Pascual E. First metatarsophalangeal joint aspiration using a 29-gauge needle. Ann Rheum Dis 2008; 67: 273–275. [DOI] [PubMed] [Google Scholar]
- 58. Janssens HJ, Fransen J, van de Lisdonk EH, et al. A diagnostic rule for acute gouty arthritis in primary care without joint fluid analysis. Arch Intern Med 2010; 170: 1120–1126. [DOI] [PubMed] [Google Scholar]
- 59. Kienhorst LB, Janssens HJ, Fransen J, et al. The validation of a diagnostic rule for gout without joint fluid analysis: a prospective study. Rheumatology (Oxford) 2015; 54: 609–614. [DOI] [PubMed] [Google Scholar]
- 60. Li B, Singer NG, Yeni YN, et al. A point-of-care Raman spectroscopy-based device for the diagnosis of gout and pseudogout: comparison with the clinical standard microscopy. Arthritis Rheumatol 2016; 68: 1751–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Park S, Lee LE, Kim H, et al. Detection of intracellular monosodium urate crystals in gout synovial fluid using optical diffraction tomography. Sci Rep 2021; 11: 10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Abhishek A, Curran DJ, Bilwani F, et al. In vivo detection of monosodium urate crystal deposits by Raman spectroscopy-a pilot study. Rheumatology (Oxford) 2016; 55: 379–380. [DOI] [PubMed] [Google Scholar]
- 63. Sivera F, Andres M, Falzon L, et al. Diagnostic value of clinical, laboratory, and imaging findings in patients with a clinical suspicion of gout: a systematic literature review. J Rheumatol Suppl 2014; 92: 3–8. [DOI] [PubMed] [Google Scholar]
- 64. Ottaviani S, Allard A, Bardin T, et al. An exploratory ultrasound study of early gout. Clin Exp Rheumatol 2011; 29: 816–821. [PubMed] [Google Scholar]
- 65. Pineda C, Amezcua-Guerra LM, Solano C, et al. Joint and tendon subclinical involvement suggestive of gouty arthritis in asymptomatic hyperuricemia: an ultrasound controlled study. Arthritis Res Ther 2011; 13: R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dalbeth N, Kalluru R, Aati O, et al. Tendon involvement in the feet of patients with gout: a dual-energy CT study. Ann Rheum Dis 2013; 72: 1545–1548. [DOI] [PubMed] [Google Scholar]
- 67. Ventura-Rios L, Sanchez-Bringas G, Pineda C, et al. Tendon involvement in patients with gout: an ultrasound study of prevalence. Clin Rheumatol 2016; 35: 2039–2044. [DOI] [PubMed] [Google Scholar]
- 68. Sapsford M, Gamble GD, Aati O, et al. Relationship of bone erosion with the urate and soft tissue components of the tophus in gout: a dual energy computed tomography study. Rheumatology (Oxford) 2017; 56: 129–133. [DOI] [PubMed] [Google Scholar]
- 69. Hammer HB, Karoliussen L, Terslev L, et al. Ultrasound shows rapid reduction of crystal depositions during a treat-to-target approach in gout patients: 12-month results from the NOR-Gout study. Ann Rheum Dis 2020; 79: 1500–1505. [DOI] [PubMed] [Google Scholar]
- 70. Dalbeth N, Billington K, Doyle A, et al. Effects of allopurinol dose escalation on bone erosion and urate volume in gout: a dual-energy computed tomography imaging study within a randomized, controlled trial. Arthritis Rheumatol 2019; 71: 1739–1746. [DOI] [PubMed] [Google Scholar]
- 71. Stewart S, Su I, Gamble GD, et al. Diagnostic value of different imaging features for patients with suspected gout: a network meta-analysis. Semin Arthritis Rheum 2021; 51: 1251–1257. [DOI] [PubMed] [Google Scholar]
- 72. Ramon A, Bohm-Sigrand A, Pottecher P, et al. Role of dual-energy CT in the diagnosis and follow-up of gout: systematic analysis of the literature. Clin Rheumatol 2018; 37: 587–595. [DOI] [PubMed] [Google Scholar]
- 73. Loffler C, Sattler H, Peters L, et al. Distinguishing gouty arthritis from calcium pyrophosphate disease and other arthritides. J Rheumatol 2015; 42: 513–520. [DOI] [PubMed] [Google Scholar]
- 74. Petsch C, Araujo EG, Englbrecht M, et al. Prevalence of monosodium urate deposits in a population of rheumatoid arthritis patients with hyperuricemia. Semin Arthritis Rheum 2016; 45: 663–668. [DOI] [PubMed] [Google Scholar]
- 75. Zhang B, Yang M, Wang H. Diagnostic value of ultrasound versus dual-energy computed tomography in patients with different stages of acute gouty arthritis. Clin Rheumatol 2020; 39: 1649–1653. [DOI] [PubMed] [Google Scholar]
- 76. Duyck SD, Petrie KJ, Dalbeth N. ‘You Don’t Have to Be a Drinker to Get Gout, But It Helps’: a content analysis of the depiction of gout in popular newspapers. Arthritis Care Res (Hoboken) 2016; 68: 1721–1725. [DOI] [PubMed] [Google Scholar]
- 77. Major TJ, Topless RK, Dalbeth N, et al. Evaluation of the diet wide contribution to serum urate levels: meta-analysis of population based cohorts. BMJ 2018; 363: k3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Alvarado-de la Barrera C, Lopez-Lopez CO, Alvarez-Hernandez E, et al. Are target urate and remission possible in severe gout? A five-year cohort study. J Rheumatol 2020; 47: 132–139. [DOI] [PubMed] [Google Scholar]
- 79. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum 2011; 63: 3136–3141. [DOI] [PubMed] [Google Scholar]
- 80. Keller SF, Lu N, Blumenthal KG, et al. Racial/ethnic variation and risk factors for allopurinol-associated severe cutaneous adverse reactions: a cohort study. Ann Rheum Dis 2018; 77: 1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dalbeth N, Dowell T, Gerard C, et al. Gout in aotearoa New Zealand: the equity crisis continues in plain sight. NZ Med J 2018; 131: 8–12. [PubMed] [Google Scholar]
- 82. Guillen AG, Te Karu L, Singh JA, et al. Gender and ethnic inequities in gout burden and management. Rheum Dis Clin North Am 2020; 46: 693–703. [DOI] [PubMed] [Google Scholar]
- 83. Dalbeth N, Gow P, Jackson G, et al. Gout in aotearoa New Zealand: are we going to ignore this for another 3 years? NZ Med J 2016; 129: 10–13. [PubMed] [Google Scholar]
- 84. Jovani V, Blasco-Blasco M, Pascual E, et al. Challenges to conquer from the gender perspective in medicine: the case of spondyloarthritis. PLoS ONE 2018; 13: e0205751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Maynard JW, McAdams DeMarco MA, Baer AN, et al. Incident gout in women and association with obesity in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Med 2012; 125: 717.e9–717.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Te Kampe R, Janssen M, van Durme C, et al. Sex differences in the clinical profile among patients with gout: cross-sectional analyses of an observational study. J Rheumatol 2021; 48: 286–292. [DOI] [PubMed] [Google Scholar]
- 87. Drivelegka P, Sigurdardottir V, Svard A, et al. Comorbidity in gout at the time of first diagnosis: sex differences that may have implications for dosing of urate lowering therapy. Arthritis Res Ther 2018; 20: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stamp L, Frampton C, Morillon M, et al. Association between serum urate and flares in people with gout and evidence for surrogate status: a secondary analysis of two randomised controlled trials. Lancet 2021; 4: E53–E60. [DOI] [PubMed] [Google Scholar]
- 89. Perez-Ruiz F, Herrero-Beites AM, Carmona L. A two-stage approach to the treatment of hyperuricemia in gout: the ‘dirty dish’ hypothesis. Arthritis Rheum 2011; 63: 4002–4006. [DOI] [PubMed] [Google Scholar]
- 90. Lee MH, Graham GG, Williams KM, et al. A benefit-risk assessment of benzbromarone in the treatment of gout. Drug Saf 2008; 31: 643–665. [DOI] [PubMed] [Google Scholar]
- 91. Janssen CA, Jansen TLTA, Oude Voshaar MAH, et al. Quality of care in gout: a clinical audit on treating to the target with urate lowering therapy in real-world gout patients. Rheumatol Int 2017; 37: 1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Otani N, Ouchi M, Kudo H, et al. Recent approaches to gout drug discovery: an update. Expert Opin Drug Discov 2020; 15: 943–954. [DOI] [PubMed] [Google Scholar]
- 93. Hosoya T, Ogawa Y, Hashimoto H, et al. Comparison of topiroxostat and allopurinol in Japanese hyperuricemic patients with or without gout: a phase 3, multicentre, randomized, double-blind, double-dummy, active-controlled, parallel-group study. J Clin Pharm Ther 2016; 41: 290–297. [DOI] [PubMed] [Google Scholar]
- 94. Terkeltaub R, Mune J, Lee J, et al. Phase 2 results from a randomized, double-blind, plaebo-controlled, dose-finding study to evaluate efficacy and safety of tigulixostat, a novel non-purine selective xanthine oxidase inhibitor in gout patients with hyperuicemia. Arthritis Rheumatol 2021; 73: 5. [DOI] [PubMed] [Google Scholar]
- 95. Hollister A, Maetzel A, Becker MA, et al. Ulodesine (BCX4208) long-term safety when added to allopurinol in the chronic management of gout: a phase 2 24-week blinded safety extensin and vaccine challenge study. Arthritis Rheumatol 2012, https://acrabstracts.org/abstract/ulodesine-bcx4208-long-term-safety-when-added-to-allopurinol-in-the-chronic-management-of-gout-a-phase-2-24-week-blinded-safety-extension-and-vaccine-challenge-study/
- 96. Hosoya T, Furuno K, Kanda S. A non-inferiority study of the novel selective urate reabsorption inhibitor dotinurad versus febuxostat in hyperuricemic patients with or without gout. Clin Exp Nephrol 2020; 24: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hosoya T, Sano T, Sasaki T, et al. Dotinurad versus benzbromarone in Japanese hyperuricemic patient with or without gout: a randomized, double-blind, parallel-group, phase 3 study. Clin Exp Nephrol 2020; 24: 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shiramoto M, Liu S, Shen Z, et al. Verinurad combined with febuxostat in Japanese adults with gout or asymptomatic hyperuricaemia: a phase 2a, open-label study. Rheumatology (Oxford) 2018; 57: 1602–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fitz-Patrick D, Roberson K, Niwa K, et al. Safety and efficacy of verinurad, a selective URAT1 inhibitor, for the treatment of patients with gout and/or asymptomatic hyperuricemia in the United States and Japan: findings from two phase II trials. Mod Rheumatol 2019; 29: 1042–1052. [DOI] [PubMed] [Google Scholar]
- 100. Neogi T, Choi HK. Editorial: pursuit of a dual-benefit antigout drug: a first look at arhalofenate. Arthritis Rheumatol 2016; 68: 1793–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Shen Z, Colton C, Yan R, et al. Combination treatment of AR882, a new URAT1 inhibitor and xanthine oxidase inhibitors allopurinol or febuxostat: effect on uric acid, hypoxanthine and xanthine in plasma or serum and urine. Ann Rheum Dis 2021; 80: 843. [Google Scholar]
- 102. Khanna PP, Khanna D, Cutter G, et al. Reducing immunogenicity of pegloticase (RECIPE) with concomitant use of mycophenolate mofetil in patients with refractory gout- a phase II double blind placebo controlled randomized trial. Arthritis Rheumatol 2021; 73: 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Botson JK, Tesser JRP, Bennett R, et al. Pegloticase in combination with methotrexate in patients with uncontrolled gout: a multicenter, open-label study (MIRROR). J Rheumatol 2021; 48: 767–774. [DOI] [PubMed] [Google Scholar]
- 104. Kishimoto TK. Development of ImmTOR tolerogenic nanoparticles for the mitigation of anti-drug antibodies. Front Immunol 2020; 11: 969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yamanaka H, Tamaki S, Ide Y, et al. Stepwise dose increase of febuxostat is comparable with colchicine prophylaxis for the prevention of gout flares during the initial phase of urate-lowering therapy: results from FORTUNE-1, a prospective, multicentre randomised study. Ann Rheum Dis 2018; 77: 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Saag KG, Khanna PP, Keenan RT, et al. A randomized, phase II study evaluating the efficacy and safety of anakinra in the treatment of gout flares. Arthritis Rheumatol 2021; 73: 1533–1542. [DOI] [PubMed] [Google Scholar]
- 107. Klück V, Jansen TLTA, Janssen M, et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol 2020; 2: e270–e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 2007; 116: 894–900. [DOI] [PubMed] [Google Scholar]
- 109. Krishnan E, Baker JF, Furst DE, et al. Gout and the risk of acute myocardial infarction. Arthritis Rheum 2006; 54: 2688–2696. [DOI] [PubMed] [Google Scholar]
- 110. Seminog OO, Goldacre MJ. Gout as a risk factor for myocardial infarction and stroke in England: evidence from record linkage studies. Rheumatology (Oxford) 2013; 52: 2251–2259. [DOI] [PubMed] [Google Scholar]
- 111. Clarson LE, Hider SL, Belcher J, et al. Increased risk of vascular disease associated with gout: a retrospective, matched cohort study in the UK clinical practice research datalink. Ann Rheum Dis 2015; 74: 642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Singh JA, Ramachandaran R, Yu S, et al. Is gout a risk equivalent to diabetes for stroke and myocardial infarction? A retrospective claims database study. Arthritis Res Ther 2017; 19: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Clarson LE, Chandratre P, Hider SL, et al. Increased cardiovascular mortality associated with gout: a systematic review and meta-analysis. Eur J Prev Cardiol 2015; 22: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Andres M, Bernal JA, Sivera F, et al. Cardiovascular risk of patients with gout seen at rheumatology clinics following a structured assessment. Ann Rheum Dis 2017; 76: 1263–1268. [DOI] [PubMed] [Google Scholar]
- 115. Pagidipati NJ, Clare RM, Keenan RT, et al. Association of gout with long-term cardiovascular outcomes among patients with obstructive coronary artery disease. J Am Heart Assoc 2018; 7: e009328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Stamp LK, Frampton C, Drake J, et al. Associations of gout and baseline serum urate level with cardiovascular outcomes: analysis of the coronary disease cohort study. Arthritis Rheumatol 2019; 71: 1733–1738. [DOI] [PubMed] [Google Scholar]
- 117. Singh JA. When gout goes to the heart: does gout equal a cardiovascular disease risk factor. Ann Rheum Dis 2015; 74: 631–634. [DOI] [PubMed] [Google Scholar]
- 118. Perez Ruiz F, Richette P, Stack AG, et al. Failure to reach uric acid target of < 0.36 mmol/L in hyperuricaemia of gout is associated with elevated total and cardiovascular mortality. RMD Open 2019; 5: e001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kim SC, Schneeweiss S, Choudhry N, et al. Effects of xanthine oxidase inhibitors on cardiovascular disease in patients with gout: a cohort study. Am J Med 2015; 128: 653e7–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yen FS, Hsu CC, Li HL, et al. Urate-lowering therapy may prevent the development of coronary artery disease in patients with gout. Front Med (Lausanne) 2020; 7: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Stack AG, Hanley A, Casserly LF, et al. Independent and conjoint associations of gout and hyperuricaemia with total and cardiovascular mortality. QJM 2013; 106: 647–658. [DOI] [PubMed] [Google Scholar]
- 122. Proudman C, Lester SE, Gonzalez-Chica DA, et al. Gout, flares, and allopurinol use: a population-based study. Arthritis Res Ther 2019; 21: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kempny A, Martin J. Management of gout and adherence to current guidelines in general practice surgery. Br J Gen Pract 2020; 70: bjgp20X711617. [DOI] [PubMed] [Google Scholar]
- 124. White WB, Saag KG, Becker MA, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 2018; 378: 1200–1210. [DOI] [PubMed] [Google Scholar]
- 125. Mackenzie IS, Ford I, Nuki G, et al. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. The Lancet 2020; 396: 1745–1757. [DOI] [PubMed] [Google Scholar]
- 126. O’Dell J, Neogi T, Pillinger M, et al. Urate lowering therapy in the treatment of gout: a multicenter randomized, double-blind comparison of allopurinol and febuxostat using a treat-to-target strategy. Arthritis Rheumatol 2021; 73, https://acrabstracts.org/abstract/urate-lowering-therapy-in-the-treatment-of-gout-a-multicenter-randomized-double-blind-comparison-of-allopurinol-and-febuxostat-using-a-treat-to-target-strategy/ [Google Scholar]
- 127. Kim SC, Neogi T, Kang EH, et al. Cardiovascular risks of probenecid versus allopurinol in older patients with gout. J Am Coll Cardiol 2018; 71: 9941004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kang EH, Park EH, Shin A, et al. Cardiovascular risk associated with allopurinol vs. benzbromarone in patients with gout. Eur Heart J 2021; 42: 4578–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Nakata T, Ikeda S, Koga S, et al. Randomized, open-label, cross-over comparison of the effects of benzbromarone and febuxostat on endothelial function in patients with hyperuricemia. Int Heart J 2020; 61: 984–992. [DOI] [PubMed] [Google Scholar]
- 130. Seth R, Kydd AS, Falzon L, et al. Preventing attacks of acute gout when introducing urate-lowering therapy: a systematic literature review. J Rheumatol Suppl 2014; 92: 42–47. [DOI] [PubMed] [Google Scholar]
- 131. Pascual E, Andres M, Vazquez-Mellado J, et al. Severe gout: strategies and innovations for effective management. Joint Bone Spine 2017; 84: 541–546. [DOI] [PubMed] [Google Scholar]
- 132. Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in patients with chronic coronary disease. N Engl J Med 2020; 383: 1838–1847. [DOI] [PubMed] [Google Scholar]
- 133. Solomon DH, Liu CC, Kuo IH, et al. Effects of colchicine on risk of cardiovascular events and mortality among patients with gout: a cohort study using electronic medical records linked with Medicare claims. Ann Rheum Dis 2016; 75: 1674–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med 2012; 125: 679–687. [DOI] [PubMed] [Google Scholar]
- 135. Jing J, Kielstein JT, Schultheiss UT, et al. Prevalence and correlates of gout in a large cohort of patients with chronic kidney disease: the German Chronic Kidney Disease (GCKD) study. Nephrol Dial Transplant 2015; 30: 613–621. [DOI] [PubMed] [Google Scholar]
- 136. Bardin T, Nguyen QD, Tran KM, et al. A cross-sectional study of 502 patients found a diffuse hyperechoic kidney medulla pattern in patients with severe gout. Kidney Int 2021; 99: 218–226. [DOI] [PubMed] [Google Scholar]
- 137. Roughley M, Sultan AA, Clarson L, et al. Risk of chronic kidney disease in patients with gout and the impact of urate lowering therapy: a population-based cohort study. Arthritis Res Ther 2018; 20: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yu KH, Kuo CF, Luo SF, et al. Risk of end-stage renal disease associated with gout: a nationwide population study. Arthritis Res Ther 2012; 14: R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Stack AG, Johnson ME, Blak B, et al. Gout and the risk of advanced chronic kidney disease in the UK health system: a national cohort study. BMJ Open 2019; 9: e031550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Stamp LK, Farquhar H, Pisaniello HL, et al. Management of gout in chronic kidney disease: a G-CAN Consensus Statement on the research priorities. Nat Rev Rheumatol 2021; 17: 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Badve SV, Pascoe EM, Tiku A, et al. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med 2020; 382: 2504–2513. [DOI] [PubMed] [Google Scholar]
- 142. Doria A, Galecki AT, Spino C, et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med 2020; 382: 2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Stamp LK, Chapman PT, Barclay ML, et al. A randomised controlled trial of the efficacy and safety of allopurinol dose escalation to achieve target serum urate in people with gout. Ann Rheum Dis 2017; 76: 1522–1528. [DOI] [PubMed] [Google Scholar]
- 144. Saag KG, Whelton A, Becker MA, et al. Impact of febuxostat on renal function in gout patients with moderate-to-severe renal impairment. Arthritis Rheumatol 2016; 68: 2035–2043. [DOI] [PubMed] [Google Scholar]
- 145. Kim WJ, Song JS, Choi ST. The role of a ‘Treat-to-Target’ approach in the long-term renal outcomes of patients with gout. J Clin Med 2019; 8: 1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Novella-Navarro M, Cabrera-Alarcon JL, Diaz-Torne C, et al. A treat-to-target approach for gout confers renoprotective effect in patients with chronic kidney disease stage 3. Rheumatol Int 2020; 40: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 147. Stamp L, Morillon MB, Taylor WJ, et al. Serum urate as surrogate endpoint for flares in people with gout: a systematic review and meta-regression analysis. Semin Arthritis Rheum 2018; 48: 293–301. [DOI] [PubMed] [Google Scholar]
- 148. Elmelegy D, Abhishek A. Reasons for discontinuing urate-lowering treatment in community-dwelling adults with gout: results of a primary care-based cross-sectional study. Rheumatol Adv Pract 2021; 5: rkab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Oderda GM, Shiozawa A, Walsh M, et al. Physician adherence to ACR gout treatment guidelines: perception versus practice. Postgrad Med 2014; 126: 257–267. [DOI] [PubMed] [Google Scholar]
- 150. Spaetgens B, Pustjens T, Scheepers LEJM, et al. Knowledge, illness perceptions and stated clinical practice behaviour in management of gout: a mixed methods study in general practice. Clin Rheumatol 2016; 35: 2053–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Weinman J, Graham S, Canfield M, et al. The Intentional Non-Adherence Scale (INAS): initial development and validation. J Psychosom Res 2018; 115: 110–116. [DOI] [PubMed] [Google Scholar]
- 152. Kleinstauber M, Wolf L, Jones ASK, et al. Internalized and anticipated stigmatization in patients with gout. ACR Open Rheumatol 2020; 2: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Petrie KJ, MacKrill K, Derksen C, et al. An illness by any other name: the effect of renaming gout on illness and treatment perceptions. Health Psychol 2018; 37: 37–41. [DOI] [PubMed] [Google Scholar]
- 154. Goldfien R, Pressman A, Jacobson A, et al. A pharmacist-staffed, virtual gout management clinic for achieving target serum uric acid levels: a randomized clinical trial. Perm J 2016; 20: 15–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Mikuls TR, Cheetham TC, Levy GD, et al. Adherence and outcomes with urate-lowering therapy: a site-randomized trial. Am J Med 2019; 132: 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Doherty M, Jenkins W, Richardson H, et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet 2018; 392: 1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Fuller A, Jenkins W, Doherty M, et al. Nurse-led care is preferred over GP-led care of gout and improves gout outcomes: results of Nottingham Gout Treatment Trial follow-up study. Rheumatology (Oxford) 2020; 59: 575–579. [DOI] [PubMed] [Google Scholar]
- 158. Krasnoryadtseva A, Dalbeth N, Petrie KJ. The effect of different styles of medical illustration on information comprehension, the perception of educational material and illness beliefs. Patient Educ Couns 2020; 103: 556–562. [DOI] [PubMed] [Google Scholar]
- 159. Bourke S, Taylor WJ, Doyle AJ, et al. The patient experience of musculoskeletal imaging tests for investigation of inflammatory arthritis: a mixed-methods study. Clin Rheumatol 2018; 37: 2261–2268. [DOI] [PubMed] [Google Scholar]
- 160. Krasnoryadtseva A, Dalbeth N, Petrie K. Does seeing personal medical images change beliefs about illness and treatment in people with gout? A randomised controlled trial. Psychol Health 2020; 35: 107–123. [DOI] [PubMed] [Google Scholar]
- 161. Uhlig T, Eskild T, Karoliussen LF, et al. Two-year reduction of dual-energy CT urate depositions during a treat-to-target strategy in gout in the NOR-Gout longitudinal study. Rheumatology (Oxford) 2022; 61: SI81–SI85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Perez-Ruiz F, Martin I, Canteli B. Ultrasonographic measurement of tophi as an outcome measure for chronic gout. J Rheumatol 2007; 34: 1888–1893. [PubMed] [Google Scholar]