Abstract
Observational findings achieved that gut microbes mediate human metabolic health and disease risk. The types of intestinal microorganisms depend on the intake of food and drugs and are also related to their metabolic level and genetic factors. Recent studies have shown that chronic inflammatory pain is closely related to intestinal microbial homeostasis. Compared with the normal intestinal flora, the composition of intestinal flora in patients with chronic inflammatory pain had significant changes in Actinomycetes, Firmicutes, Bacteroidetes, etc. At the same time, short-chain fatty acids and amino acids, the metabolites of intestinal microorganisms, can regulate neural signal molecules and signaling pathways, thus affecting the development trend of chronic inflammatory pain. Glucocorticoids and non-steroidal anti-inflammatory drugs in the treatment of chronic inflammatory pain, the main mechanism is to affect the secretion of inflammatory factors and the abundance of intestinal bacteria. This article reviews the relationship between intestinal microorganisms and their metabolites on chronic inflammatory pain and the possible mechanism.
Keywords: arthritis, chronic inflammatory pain, glucocorticoids, intestinal microflora, nonsteroidal anti-inflammatory drugs
Introduction
Chronic pain (CP) has always been a public medical problem in the world. Usually, the duration of pain lasting more than 3 months is defined as CP. It is not only a high incidence rate but also a relapse after recovery, which brings great obstacles to the physiology and psychology of patients and seriously affects the quality of life of patients. CP is divided into seven categories in the international classification of diseases. This article is divided into one kind of chronic primary pain and six kinds of secondary chronic cancer-related pain, chronic neuropathic pain, chronic secondary visceral pain, chronic posttraumatic and postoperative pain, chronic secondary headache, and orofacial pain, and chronic secondary musculoskeletal pain. 1 The above factors lead to the occurrence of CP, resulting in the pathogenesis of CP is not clear, more difficult to cure, higher treatment costs and most of the vulnerable groups are middle-aged and elderly, and the economic situation is not optimistic.
Chronic inflammatory pain refers to local or systemic pain caused by long-term infiltration of an organ or joint by inflammatory factors, which is an important part of CP. Here are a few examples of chronic inflammatory pain. Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a long-term urinary system pain or discomfort in the pelvic region, accompanied by reproductive system disorders. Inflammatory CP/CPPS (NIH category III a) is the cause of abnormal leukocytes in the prostate. 2 Inflammation and autoimmunity are thought to be major contributors to CP/CPPS. TNF-α, IL-6, COX-2, and other factors were abnormally increased in the blood and semen of patients. 3 The incidence of rheumatoid arthritis (RA) in Europe and North America ranges from 0.5% to 1% but is likely to decline in the coming years. 4 The pathogenesis of RA is related to immune protein G and citrulline. 5 It is characterized by inflammatory and destructive reactions in synovial tissue, and pain is a key problem in RA. Peripheral sensitization of nociceptors is caused by cytokines (IL-1β, IL-6, and TNF), chemokines, and growth factors in synovial membrane and synovial fluid. 6 The prevalence of gout ranged from < 1% to 6.8% and the incidence ranged from 0.58 to 2.89 per thousand people per year. 7 The pathogenesis of gout is the activation of the NLRP3 receptor and inflammasome IL-1 and IL-18. Urate crystals also induce microglia to secrete inflammatory factors. It occurs when high levels of uric acid (supersaturation) in the body cause sodium urate to deposit in joints and cause pain. 8 The pathogenesis of knee osteoarthritis (OA) is the production and release of inflammatory factors, such as IL-6, IL-1β, TNF-α, and IL-8 in serum, synovial fluid, and synovial tissue. In addition, some pain-related neurotransmitters, such as substance P and nerve growth factor, are released from osteoarthritic joints. 9
There are many kinds and inducements of chronic inflammatory pain. And more and more evidence shows the role of intestinal microorganisms in chronic inflammatory pain. In this article, we explain the pathogenesis mechanisms of CP based on the relationship between gut microbes and their metabolites and CP. 10 In addition, the progress of the regulation and mechanism of intestinal microorganisms of chronic inflammatory pain drugs were discussed to provide a scientific basis for the pathogenesis of CP and its effective drug research and development.
Gut–brain axis and chronic inflammatory pain
The human gut is a large, diverse, and dynamic intestinal microbiota with more than 100 trillion microorganisms, including at least 1000 different species. 11 The microorganisms in the human intestine can be divided into bacteria, archaea, acellular phages, and eukaryotes. Bacteroides and Firmicutes accounted for more than 90% of the gastrointestinal bacteria.12,13 Different intestinal microorganisms cause functional differences. Through the current popular genomics, high-throughput sequencing technology, 16SrDNA, and polymerase chain reaction (PCR) technology, it is found that the sources of differences in intestinal microorganisms in vivo may be related to genetics, environment, diet, stress, age, infection, drugs (antibiotics), etc. 14 Intestinal microorganisms can participate in host metabolism, have digestion and absorption function, and are also an important component of the human immune system. Intestinal bacterial disturbance can lead to disorder of the immune system, leading to obesity, diabetes, cardiovascular and cerebrovascular diseases, and other diseases.15,16
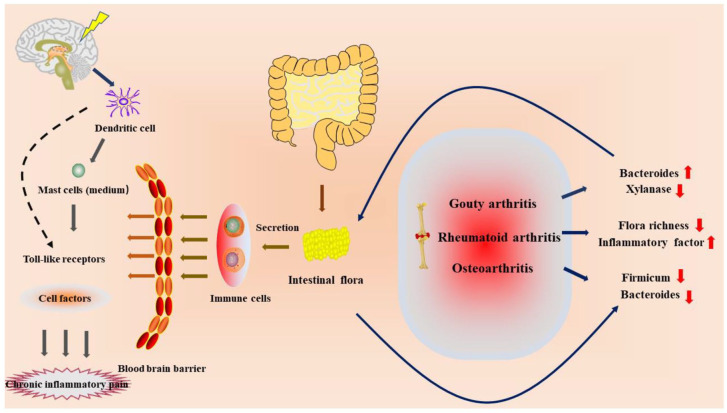
With the development of the ‘gut–brain axis’ and ‘microbe–gut–brain axis’, the research on gut and central nervous system has become a hot topic. Low-fat and low-sugar diet can reduce oxidative stress and prevent the activation of microglia cells after digestion and absorption. 17 Immune cells produced by intestinal flora can cross the blood–brain barrier and are activated by dendritic cells of the central nervous system, which can change cytokines through nerve signaling pathways, such as Toll-like receptors. 18 Mast cells may be the mediators that lead to the association between intestinal flora and the neuroimmune system, thus generating sensory signals. 19 Recent studies have shown a strong link between gut bacteria and CP. The differences in the levels of intestinal flora in patients with CP/CPPS were reflected in that the relative abundance of Actinomycetes was lower than that in normal people. 20 At the class level, the proportion of γ-Proteobacteria increased and that of β-Proteobacteria decreased. At the genus level, the relative abundance of Clostridium and Escherichia was significantly higher than that of the normal human body, while the relative abundance of Dorea, Hallella Moore and Moore, Alistipes, and Dialister was significantly lower than that of the normal human body. At the species level, the relative abundance of Bacteroides and Escherichia coli was higher than that of a normal human body, while the relative abundance of Eucobacteria nitpicks and Eucobacteria rectum was lower than that of a normal human body. 21 The intestinal bacteria of patients with RA were lower than those of normal people and showed a negative correlation with inflammatory indicators C-reactive protein (CRP), TNF-α, and IL-6, and the levels of inflammatory factors were significantly increased, and the diversity of intestinal flora in RA patients was lower than that in normal people. 22 Gout belongs to a kind of arthritis and belongs to the category of metabolic rheumatism. During the attack, the joints will be accompanied by severe pain and changes in intestinal bacteria, among which the contents of Bacteroides and xylanase are high, and the synthesis of butyric acid that protects intestinal mucosa in patients with gout is reduced. 23 OA is a degenerative disease that can lead to advanced pain and joint deformity. In patients with this disease, there is mild intestinal inflammation, 24 and the intestinal flora of the susceptible population, such as Firmicutes and Bacteroidetes, is significantly reduced compared with the normal population. 25 At the same time, vitamin D deficiency can lead to pain response and decreased microbial diversity in mice, characterized by an increase in Firmicutes and a decrease in microflora Verrucomicrobium and Bacteroidetes. 26
These studies suggest that chronic inflammatory pain may alter the intestinal microbiota, thereby negatively stimulating the chronic inflammatory response and generating metabolic resistance to promote CP, suggesting that intestinal bacteria are involved in many chronic inflammatory diseases. 27 It is believed that the changes of intestinal bacteria are related to the brain, affecting nerve signal transduction and inducing pain (Figure 1).
Figure 1.
Relationship between intestinal flora and chronic inflammatory pain.
The changes of intestinal flora in patients with arthritis lead to the disorder of immune cells. Through the blood–brain barrier, immune cells use dendritic cells located in the central nerve to conduct signal transduction and produce pain.
Relationship between intestinal microorganisms and their metabolites and chronic inflammatory pain
Short-chain fatty acid
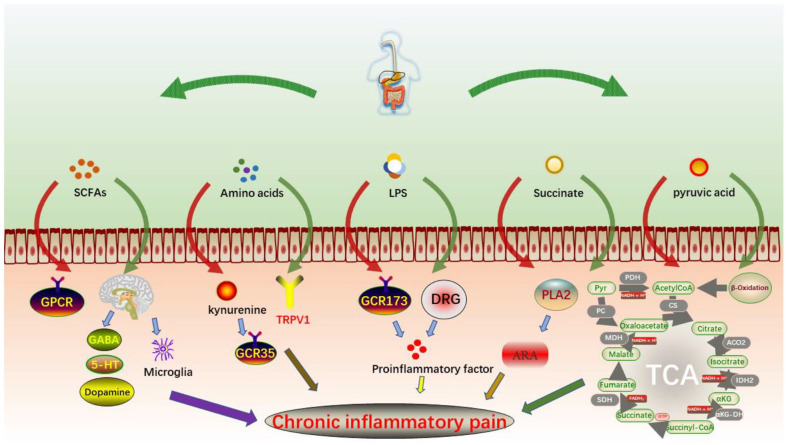
Short-chain fatty acids (SCFAs) are natural metabolites with no more than six carbon chains produced by dietary fiber in the fermentation process of anaerobic bacteria, such as Bacteroides, Bifidobacterium, Fusobacterium, and Streptococcus, which are generally distributed in mammals and mainly consist of acetic acid, propionic acid, and butyric acid. 28 SCFAs in patients with inflammatory bowel disease activate downstream signaling pathways by binding specific G-protein-coupled receptors (GPR43, GPR109A, etc.), regulating T cell activation, increasing histone acetylation, decreasing NF-κB activation, and regulating chronic inflammatory pain (Figure 2).29,30
Figure 2.
Regulation of intestinal metabolites on CP.
SCFAs can effectively alleviate osteoporosis by increasing calcium absorption and transport in mice, stimulating intestinal serotonin synthesis and directly inhibiting osteoclast differentiation and bone resorption. 31 Meanwhile, at the same time, SCFAs play a role in regulating the maturation of mouse entero-brain axis and proximal microglia and regulate the expression of nerve molecules, such as GABA, serotonin, and dopamine, to influence inflammatory pain. 32 The lack of fat in a high-fiber diet effectively alters SCFAs in the gut. High-fiber diet-induced G-protein-coupled protein activation of NLRP3 inflammasomes is directly associated with gout attacks. 33 The content of SCFAs in patients with chronic inflammatory pain may affect that not only acts on the intestinal tract to regulate the intestinal inflammatory balance but also maintains the overall balance through the blood–brain barrier, regulates the secretion of inflammatory factors, and effectively inhibits the occurrence of chronic inflammatory pain.28,30,32
Amino acid metabolites
Significant differences in amino acid metabolism from normal levels have been observed in chronic critical patients with persistent inflammation. 34 Kynurenine is a tryptophan metabolite that activates G-protein-coupled receptor 35 (GPR35) and has anti-inflammatory pain effects in acetic acid writhing experiment mice. 35 In the serum metabolism of 38 patients with angina, tryptophan degradation was found to produce serotonin, which affects the expression of nerve molecules, suggesting that tryptophan degradation inhibits pain. 36 Inflammation and pain are associated with the combined action of active metabolites p-benzoquinone and N-acetyl-p-benzoquinone imine on cysteine activation and sensitization of TRPV1 receptors. 37 The urine metabolomics of 74 patients with OA and 68 healthy subjects were compared. It was found that the expression levels of lysine metabolite aminophenediamine, serine metabolite L-methanesulfonate, and glycine metabolite 3-methylcrocodile glycine were lower than those of normal subjects (Figure 2). 38
Simultaneously, glutamate was found in the urine of 58 posttraumatic ankle arthritis patients as a major marker metabolite for posttraumatic ankle arthritis, helping to distinguish synovial fluid of patients from healthy subjects. 39 The recurrence of CP can greatly increase the risk of depression, which is often accompanied by the coexistence of two diseases. It was found that the tryptophan canine metabolic pathway was impaired in the urine metabolism of mice in both diseases, and the overall amino acid level in the high uric acid group increased, the tryptophan level decreased, and the IL-1 receptor antagonist level decreased. 40 These results indicate that tryptophan metabolites have the potential to be the effective markers of chronic inflammatory pain.
Lipopolysaccharide
Intake of high-fat and high-sugar diet is prone to obesity, diabetes, hypertension and other diseases, joint inflammation, and painful degenerative disease OA, and other complications are also constantly occurring. It can also cause postoperative pain and delay wound growth in rats. 41 Lipopolysaccharide (LPS) is the main component of the external membrane of most gram-negative bacteria, which often induces chronic inflammatory pain (Figure 2).
A model of inflammatory pain induced by injection of 0.4 ng/kg of E. coli LPS decreases pain threshold and increases serum levels of inflammatory cytokines IL-1β, TNF-α, and IL-6 in patients aged 18–45 years. It also increased expression of dorsal root ganglion (DRG) neurons, TRPA1, and TRPV1 proteins. 42 LPS-stimulated macrophage inflammatory cytokines in mice and increased exome of miRNAs mediate inflammation, thereby activating NF-κB factor. 43 The levels of pro-inflammatory cytokines IL-1β, COX-2, and PGE2 in the microglia of rats were increased. 44 LPS can reduce the activity of G-protein-coupled receptor GPR173 in human dental pulp, induce the secretion of pro-inflammatory factors, such as IL-6, activate toll-like receptor-4 (TLR-4), MyD88, NF-κB pathway, and improve inflammatory response and pain. 45
Succinic acid
Succinic acid can be used as a food material and drug synthetic material, and an intermediate product of metabolism, which has analgesic, detoxification, and antibacterial effects. 46 In the agarose egg yolk experiment, it was found that succinic acid can control the synthesis of arachidonic acid and inhibit inflammation and pain by inhibiting the activity of phospholipase A2 (PLA2). The main way is that the active site of PLA2 can interact with histidine residues. 47 Metabolism of glutamate and succinate decreased in the serum of patients with chronic urticaria, suggesting decreased metabolic efficiency of butyric acid (Figure 2). 48
Notably, it was found that succinic acid could bind to the GRP91 receptor not only intracellular but also extracellular, promoting the release of inflammatory mediators, such as IL-1β and NO, and promoting the inflammatory response by the initial neonatal rat cardiomyocytes experiment. 49 Its derivatives ammonia succinate and acetylsalicylic acid can be used in combination to regulate metabolic disorders in chronic inflammation of various joints and viscera. 50
According to human feces, Prevotellaceae and Veillonellaceae can produce succinic acid, while Odoribacteraceae and Clostridiaceae can consume succinic acid. 51 An excessive amount of succinic acid in the human body can cause some intestinal diseases, such as inflammatory bowel disease and Crohn’s disease. 52
Other metabolites
Many of the metabolites of patients with chronic inflammation are different from those of healthy people, such as elevated levels of acetone and pyruvate in patients with RA (Figure 2). In the redox environment, the transport and metabolism of iron, sulfur, zinc, and arginine are altered, 53 but the levels of taurine, urea, betaine, and hippuric acid are reduced. 54 The tricarboxylic acid cycle can regulate Candida albicans. Pyruvate participates in the tricarboxylic acid cycle and completes the transformation of three nutrients through acetyl CoA. Taurine is an essential amino acid in the body, but its synthesis in the human body is low, and it is related to nerve conduction and lipid metabolism. Probiotic therapy increased taurine in mice and significantly increased the abundance of Firmicutes, Ruminobacteriaceae, and an unidentified clostridium bacterium while decreasing the abundance of Verruciaceae and Erysipelaceae. 55 In chronic kidney disease patients with acute gout, uric acid increases, urea and creatinine decrease in the same direction. 56 At the genus level, E. coli – Shigella numbers were significantly higher in patients with high uric acid. The intestinal flora of the high uric acid group was predicted to be rich in metabolism, human disease, and LPS biosynthesis. 57 Uric acid is the end product of purine metabolites, and gout arthritis is caused by the deposition of urate in the joints. Reducing intake of high-purine food, improving their metabolic function to prevent gout arthritis has a good effect (Table 1).
Table 1.
Effects of intestinal microorganisms and metabolites on various indexes in vivo.
| Metabolites | Targets | Biological effects (References) |
|---|---|---|
| SCFAs | Human | Bind-specific G protein-coupled receptor29,30
Activate T cells29,30 Reduce NF-κB protein expression29,30 |
| Mice | Increase calcium absorption
31
Stimulate intestinal serotonin synthesis31,32 Inhibition of osteoclast differentiation and bone resorption 31 |
|
| Amino acid metabolites | Human | Serotonin production
36
Activate the TRPV1 receptor 37 |
| Mice | Activate G protein-coupled receptor 3535
Impaired tryptophan canine metabolic pathway35,40 |
|
| LPS | Human | Reduce pain threshold
42
Increase inflammatory factor IL-1β, TNF-α, and IL-6 levels 42 Increase the expression of DRG neurons, TRPA1, and TRPV1 proteins 42 |
| Rat | Increase postoperative pain and delay wound growth
41
Increase inflammatory factor IL-1β, COX-2, and PGE2 levels 44 |
|
| Succinic acid | Yolk | Inhibit the activity of PLA247 |
| Rat | Bind to GRP91 receptor and promote IL-1β and NO release 49 |
DRG, dorsal root ganglion; LPS, Lipopolysaccharide; NF-κB, nuclear factor kappa-B; SCFA, short-chain fatty acid
Intestinal microorganisms and their metabolites associated with chronic inflammatory pain include SCFAs, amino acids, LPSs, succinic acid, and pyruvate. SCFAs bind to some G-protein-coupled receptors to reduce NF-κB activation. It can also regulate nerve molecules, such as GABA, 5-hydroxytryptamine, and dopamine to relieve pain. Kynurenine activates GPR35 to exert an analgesic effect. LPS can reduce the pain threshold, which is mainly used to induce the secretion of inflammatory factors, reduce the activity of G-protein-coupled receptor GPR173, and increase pain sensation. Succinic acid mainly reduces pain by inhibiting PLA2 activity. Pyruvate is involved in the tricarboxylic acid cycle mainly to enhance nerve conduction and lipid metabolism to regulate pain.
Mechanism of drugs intervention on chronic inflammatory pain and its effect on intestinal microflora
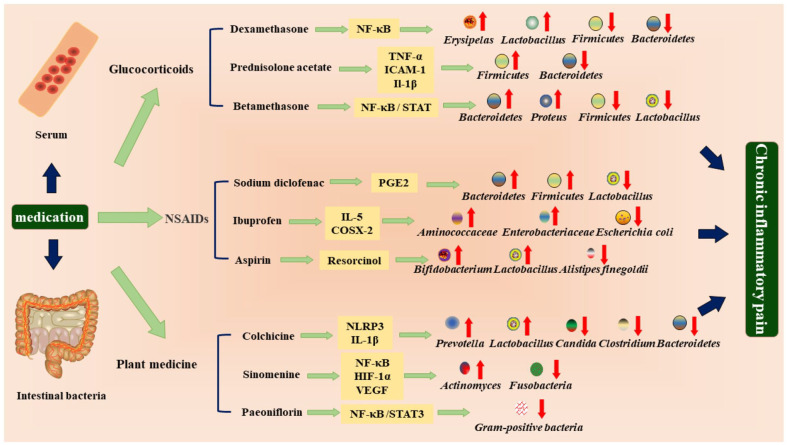
Patients with chronic inflammatory pain have a variety of options for treatment drugs, with the current use of non-steroidal anti-inflammatory drugs, glucocorticoids, traditional Chinese herbal plants, and other biological agents.58,59 The above drugs affect intestinal flora through different channels in vivo and are closely related to glucose and lipid metabolism, protein metabolism, inflammatory response, and intercellular signal molecule transmission. 60 It is difficult to cure chronic inflammation and pain, while intestinal microorganism is a relatively stable environment. To clarify how drugs change intestinal microorganisms, find the correlation between the two, change intestinal microecology, to regulate the occurrence of an inflammatory response, reduce pain, and reduce recurrence. It is helpful for the radical treatment of chronic inflammatory pain (Figure 3).
Figure 3.
Improvement mechanisms of anti-inflammatory analgesic drugs based on the regulation of intestinal bacteria and inflammatory factors.
Glucocorticoids
Dexamethasone
Dexamethasone is a synthetic substitute for cortisol, commonly used as an anti-inflammatory and also used to simulate stress state. 61 Dexamethasone can alleviate joint swelling and pain by improving the expression of inflammatory cytokines and transcription factors, such as IL-1β, IL-17, TNF-α, and MMP-3 in primary synovial tissue cells of CIA rats. Studies have shown that dexamethasone can downregulate the expression of the NF-κB signaling pathway in the serum of CIA rats, effectively treat knee OA, stabilize oxidative stress, and relieve pain.62,63 Dexamethasone protects glucocorticoid receptor knockout mice from TNF injection by promoting dimerization of glucocorticoid receptors. 64
After intraperitoneal injection of dexamethasone in 40 albino rats, it was found that a high concentration of 10 mg/kg dexamethasone regulated the intestinal flora, with total aerobic bacteria and lactic acid bacteria as the dominant flora, while a low concentration of 0.5 mg/kg dexamethasone regulated the number of anaerobic bacteria. 65 Dexamethasone also increased the abundance of Erysipelas and Lactobacillus in the feces of asthmatic rats, 66 while the relative abundance of Firmicutes, Bacteroidetes, α-Proteobacteria, γ-Proteobacteria, and Actinobacteria decreased in the feces of circadian rats. 67
Prednisolone acetate
Prednisolone acetate is mainly used in the clinical treatment of allergic and autoimmune inflammatory diseases, such as rheumatism and RA, to inhibit cellular immune response. However, it cannot repair articular cartilage tissue and even has the side effect of osteoblastic apoptosis. As a long-term but short-acting drug, long-term use can lead to osteoporosis and bone loss, and adverse reactions increase after drug withdrawal.68,69 The levels of TNF-α, IL-1β, and ICAM-1 in the serum of rats with RA were significantly decreased by intragastric administration of prednisolone. 70 Prednisolone destroyed the microbial community structure of immunosuppressed C57BL/6J mouse feces, decreased the Bacteroidetes, and increased the Firmicutes in feces. It also increased glutathione content and decreased catalase activity in the colon of Colitis rats.71,72
Betamethasone
Betamethasone is widely used clinically as an anti-inflammatory and immunosuppressive agent. At present, the combination of betamethasone with some non-steroidal anti-inflammatory drugs and injectable Gellan Gel (GG) hydrogels has been widely used in clinical studies at home and abroad in the treatment of arthritis, and compared with the efficacy of single-use, it can effectively treat knee OA and gout arthritis.73–75 Betamethasone can downregulate signal transduction and transcriptional activators (STAT) 3 and nuclear factor κB (nuclear factor-κB) in CHON-002 human chondrocytes, inactivate the NF-κB /STAT3 signaling pathway, and significantly downregulate inflammatory cytokines IL-6 and IL-8. 76 One point of concern is that the CTX-II and COMP of the degradation products of betamethasone cartilage increased as the effect decreased in CIA rat models, suggesting that betamethasone can eliminate local inflammation but alter the cellular metabolic structure of its articular cartilage. 77
Looking at the gut microbes, betamethasone increased the fecal abundance of Bacteroides and Proteus but decreased the fecal abundance of Lactobacillus and Firmicutes in Balb/ C mice in an experiment to evaluate gut sensitivity to the colon. 78
Non-steroidal anti-inflammatory drugs
Sodium diclofenac
Diclofenac sodium has been found to inhibit prostaglandin synthesis in the plasma of 18- to 65-year-olds with RA, resulting in analgesic and anti-inflammatory effects and effective relief of joint pain and swelling. 79 It can inhibit glycolysis of fibroblast synoviocytes in human RA, and decrease the serum levels of rheumatoid factor (RF), high-sensitivity C-reactive protein (hs-CRP), erythrocyte sepulchration rate (ESR), disease activity score-28 (DAS-28), and joint symptom and sign the score. 80
Mice treated with diclofenac sodium increased β-glucuronidase (GUS) activity in fecal samples, while GUS was 60% from Firmicutes and 21% from Bacteroidetes. 81 Sodium diclofenac may increase the intestinal flora of Firmicutes and Bacteroidetes. In rats, sodium diclofenac is mainly excreted into bile in the form of glucuronic acid conjugates to participate in the hepatointestinal circulation, which may affect the metabolic level of glutamate.82,83 Diclofenac sodium increased the abundance of Proteobacteria and Bacteroidetes in the fecal stool of enteric rats and eventually consumed Lactobacillus. 84
Ibuprofen
Ibuprofen has a strong anti-inflammatory and analgesic effect in clinical practice, which can mediate its therapeutic effect by inhibiting cyclooxygenase and subsequently by inhibiting the production of prostacyclin. 85 Ibuprofen improved joint inflammation induced by type II collagen in DBA/1 mice and increased the secretion of the typical Th2 cytokine IL-5 in their serum. 86 Ibuprofen can reduce the mRNA expressions of MMP-3, MMP-13, COX-2, ADAMTS-5, IL-6, and TNF-α in synovial cells of OA rats stimulated by sodium iodoacetate through porous microspheres. 87
In an experiment on the effects of drugs on the composition of intestinal bacteria in 155 adults, 62 chose NSAIDs, in which ibuprofen increased the abundance of bacteria in the families of Aminococcaceae, Enterobacteriaceae, Propioniaceae, Pseudomonas, Pomegranates, and Salicaceae. 88 At the same time, ibuprofen and its degradation products were found to be toxic to Lactobacillus acidophilus, E. coli, and Clostridium fischeri by in vitro bacterial culture experiments, and inhibited their growth. 89
Aspirin
Aspirin has a good anti-inflammatory effect, but its specificity is not strong. It is not only used in the treatment of arthritis but also against inflammatory diseases, such as endometrium and digestive tract, and a cancer treatment. Aspirin irreversibly acetylates COX-1 and prevents arachidonic acid from entering the active site, thereby reducing the production of thromboxane A2 and resulting in thrombocytopenia. 90 Aspirin promotes the production of resorcinol, an anti-inflammatory and pro-decomposition lipid medium. Aspirin also acetylated the COX-2-activated pathway for enzymatic production and combined with choline had good effects on foot swelling induced by carrageenan in mice and inflammatory pain induced by LPS in mice.91,92 The expression of TNF-α, IL-6, IL-1β, IL-10, and other inflammatory factors in synovial fluid of AIA rats was decreased by aspirin Elisa kit. Western blot results showed that it also contributed to the decrease in NF-κB/p38MAPK signaling pathway-related proteins and phosphorylated proteins. 93
By using antibiotics to reduce some intestinal bacteria in mice, it was found that normal mice taking aspirin had fewer colon tumors than sterile mice taking aspirin. The results of the 16S rDNA technique showed that the feces samples of mice given aspirin were rich in Bifidobacterium and Lactobacillus, while Alistipes finegoldii and Bacteroides fragile were decreased.94,95
Plant medicine
Colchicine
Colchicine is one of the earliest drugs used to treat gouty arthritis. In vitro cell experiments, the micromolar dose of colchicine can effectively relieve acute gout pain by inhibiting the crystal activation of NLRP3 induced by monosodium urate (MSU) and blocking the release of IL-1β.96,97
Colchicine has certain toxicity to the gastrointestinal tract, so it can regulate the composition of intestinal flora. It was found that colchicine could significantly increase the relative abundance of Firmicutes and reduce the relative abundance of Bacteroidetes at the concentration of 2.5 mg/kg. In general, with colchicine intervention, the abundance of Bacteroidetes, Candida, Rickneria, and Clostridium decreased significantly. However, the abundance of Lactobacillus and Prevotella increased significantly. 98
Sinomenine
Sinomenine is an alkaloid isolated from the root of Sabia japonica, which is a traditional Chinese medicine for the treatment of RA. 99 In Elisa’s test of serum of adjuvant arthritis rats, 10 and 20 mg/kg oral dose of sinomenine can decrease TNF-α, IL-6, and IL-1β in a dose-dependent manner. In terms of protein expression, the expression of p-NF-κBp65, P-IκBα, and RIP140 was significantly downregulated by the above two doses. 100 In the same Elisa experiment, at the dose of 30, 100, and 300 mg/kg, the levels of HIF-1α, VEGF, and ANG-1 in synovial membrane and peripheral blood of CIA mice were reduced in a dose-dependent manner, providing important theoretical support for the treatment of RA. 101 Zhengqing Fengtongning is a proprietary Chinese medicine made from a sinomenine monomer, which reduces PGE2 synthesis by inhibiting mPGES-1 to treat RA. 102 The use of leflunomide and methotrexate in combination with proprietary Chinese medicine effectively reduced side effects. 103
Sinomenine plays a broad role in the intestinal flora of zebrafish. After injection of 80 mg/kg sinomenine, the abundance of Fusobacteria in intestinal contents of zebrafish was decreased and the abundance of Actinomyces was increased. Meanwhile, the increase in Bacteroidetes/Firmicutes ratio was inhibited. 104
Paeoniflorin
It was found that paeoniflorin at a dose of 50 μM could treat and prevent OA of the knee by inhibiting the activation of the NF-κB pathway, chondrocyte inflammation and degeneration, and osteoclast differentiation in osteoclast cells of CIA mice. 105 The dose of 0.388 g/kg/d for 21 days can downregulate the inflammatory factors IL-21, IL-6, and TNF-α in the serum of CIA mice. In terms of protein, it mainly inhibits the differentiation of SPLEEN TFH cells and the formation of germinal center response (GC) through the STAT3 signaling pathway. 106
At 25, 50, and 100 mg/kg/d for 5 days, paeoniflorin decreased the abundance of gram-positive bacteria in feces and infiltration of gram-positive bacteria in intestinal tissue of C57/BL/6 colitis mice, inhibited the MDP-NOD2 pathway dependent on gram-positive bacteria, and alleviated inflammation (Table 2). 107
Table 2.
Effects of different drugs on intestinal microflora of patients with chronic inflammatory pain and its therapeutic mechanism.
| Drug type | Experimental model | Dosage of administration | Treatment
mechanism (references) |
Intestinal microbiota
regulation (references) |
|---|---|---|---|---|
| Dexamethasone | Synovial cells of CIA rat fibroblasts | Administer Dex Intraperitoneally (1 mg/kg) three times a week for more than 2 weeks |
Reduce IL-1β, IL-17, TNF-α, MMP-3 inflammatory factors 62 | Increase the abundance of Erysipelas
and Lactobacillus
reduce the abundance of Firmicutes, Bacteroidetes, α-proteobacteria, γ-Proteobacteria, and Actinobacteria66,67 |
| Synovial tissue of CIA rat | The knee joint was injected 1 mg/kg twice a week for 3 weeks |
Decrease NF-κB pathway expression 63 | ||
| GRmt/mt, GRdim/dim mice | Intraperitoneal injection of 10 mg/kg | Promote glucocorticoid receptor synthesis 64 | ||
| Prednisolone acetate | CIA rat serum | Intragastric administration of 0.1 mL/10 g lasted for 10 days | Decrease the levels of TNF-α, IL-1β, and ICAM-1 inflammatory factors 70 | Decrease the abundance of Bacteroidetes
increased the abundance of Firmicutes71,72 |
| Betamethasone | CHON-002 human chondrocyte cell | Treatment with 10 μM for 48 h | Inhibit the expression of NF-κB/STAT3 signaling pathway
76
Downregulate inflammatory cytokines IL-6 and IL-876 |
Increase the abundance of Bacteroides
and Proteus
Decrease the abundance of Lactobacillus and Firmicutes 78 |
| Sodium diclofenac | 45 patients (38 female, 7 male; mean age 47.88 years) with RA |
Orally 50 mg tablets twice daily for 8 weeks | Inhibit prostaglandin synthesis 79 | Increase the abundance of Firmicutes,
Bacteroidetes, and
Proteobacteria
Reduce the abundance of Lactobacillus82,83 |
| Ibuprofen | Male DBA/1 mice | Ibuprofen was taken orally twice daily for 2 weeks | Promote IL-5 secretion 86 | Increase the abundance of
Aminococcaceae,
Enterobacteriaceae,
Propioniaceae,
Pseudomonas,
Pomegranates, and
Salicaceae
consume Lactobacillus Acidophilus, Escherichia coli, and Clostridium Fischeri88,89 |
| Rat synovial cells | Polylactic acid–glycolic acid copolymer was loaded with 300 μg/mL, 900 μg/mL, 1500 μg/mL mixed medium for 72 h | Decrease mRNA expression of MMP-3, MMP-13, COX2, ADAMTS-5, and other factors 87 | ||
| Aspirin | Synovial fluid of AIA rats | 0.1 mmol/kg was administered orally once a day for 2 weeks | Decrease the expression of TNF-α, IL-6, IL-1β, and
IL-10 Reduce the protein expression of NF-κB/p38MAPK signaling pathway 93 |
Increase the abundance of
Bifidobacterium and
Lactobacillus
Decrease the abundance of Alistipes finegoldii and Bacteroides fragile94,95 |
| Colchicine | J774 cells | 5–15 µM | Inhibit the activation of NLRP3 crystals Block IL-1 β release96,97 |
Increase the abundance of Firmicutes,
Lactobacillus, and
Prevotella
Decrease the abundance of Bacteroidetes, Candida, Rickneria, and Clostridium 98 |
| Sinomenine | Arthritis induced by Freund’s adjuvant in rats | Gavage of 10 and 20 mg/kg lasted for 1 week | Downregulate the protein expression of p-NF-κBp65, p-IκBα, and RIP140100 | Increase the abundance of Actinomyces
Decrease the abundance of Fusobacteria 104 |
| Osteoporotic vertebral fractures in rats | Zhengqing Fengtongning injection was injected at L3 Jiaji point in doses of 0.5 and 2.0 mg | Inhibition of mPGES-1 reduces PGE2 synthesis 102 | ||
| Paeoniflorin | DBA/1 male mice and CIA rats | Daily gavage of 0.388 g/kg lasted for 3 weeks | Downregulate inflammatory cytokines IL-21, IL-6, and
TNF-α Inhibit the expression of STAT3 signaling pathway 106 |
Decrease the richness of gram-negative bacteria 107 |
NF-κB, nuclear factor kappa-B; STAT-3, Signal Transducer and Activator of Transcription.
Drug intervention changed the composition of intestinal bacteria. Most of the Bacteroides were decreased in CP, while most of the Firmicutes and Lactobacillus were increased. Drug therapy reduced the secretion of inflammatory factors, such as TNF-α, IL-1β, and IL-6 in serum and reduced the inflammatory response through the corresponding pathways, so that the pain signal received by the brain was weakened.
Conclusion
Due to the unknown onset time and mechanism of chronic inflammatory pain and the rejuvenation of the disease, the research on chronic inflammatory pain has been carried out and deepened. The results show that the occurrence and regression of chronic inflammation cannot be separated from the balance of intestinal microecology, and the key factors to ensure the balance are the intake of diet and drugs, the good foundation of genetic metabolism, and a healthy lifestyle to restore the microecology balance. From the gut–brain axis, it is found that inflammatory pain is related to the brain nerve and the intestinal nerve, and the differences between the intestinal flora and metabolites in patients with chronic inflammatory pain and normal people, and the effects of metabolites on patients with CP are analyzed. The relationship between CP and intestinal flora and metabolites is proposed. From the aspect of CP treatment drugs, the commonly used drugs not only change the characteristics of chronic inflammatory pain but also change the intestinal microorganisms in the body.
It is still not realistic to fundamentally solve chronic inflammatory pain from the intestinal microbiological level. What we need is a large number of experimental studies and more precise instruments. This article aims to explore the relationship between chronic inflammatory pain and intestinal microorganisms, find out the potential targets of intestinal microorganisms and their metabolites and provide a theoretical basis for the development of more symptomatic and safer therapeutic drugs.
Footnotes
ORCID iD: Shu-Lan Su  https://orcid.org/0000-0002-2008-5887
https://orcid.org/0000-0002-2008-5887
Contributor Information
Jia-Shang Li, Jiangsu Collaborative Innovation Center of Chinese Medicinal Resources Industrialization, National and Local Collaborative Engineering Center of Chinese Medicinal Resources Industrialization and Formulae Innovative Medicine, and Jiangsu Key Laboratory for High Technology Research of TCM Formulae, Nanjing University of Chinese Medicine, Nanjing, P.R. China.
Shu-Lan Su, Jiangsu Collaborative Innovation Center of Chinese Medicinal Resources Industrialization, National and Local Collaborative Engineering Center of Chinese Medicinal Resources Industrialization and Formulae Innovative Medicine, and Jiangsu Key Laboratory for High Technology Research of TCM Formulae, Nanjing University of Chinese Medicine, 138 Xianlin Road, Nanjing 210023, P.R. China.
Zhuo Xu, Jiangsu Collaborative Innovation Center of Chinese Medicinal Resources Industrialization, National and Local Collaborative Engineering Center of Chinese Medicinal Resources Industrialization and Formulae Innovative Medicine, and Jiangsu Key Laboratory for High Technology Research of TCM Formulae, Nanjing University of Chinese Medicine, Nanjing, P.R. China.
Li-Hui Zhao, Jiangsu Collaborative Innovation Center of Chinese Medicinal Resources Industrialization, National and Local Collaborative Engineering Center of Chinese Medicinal Resources Industrialization and Formulae Innovative Medicine, and Jiangsu Key Laboratory for High Technology Research of TCM Formulae, Nanjing University of Chinese Medicine, Nanjing, P.R. China.
Ruo-Ying Fan, Jiangsu Collaborative Innovation Center of Chinese Medicinal Resources Industrialization, National and Local Collaborative Engineering Center of Chinese Medicinal Resources Industrialization and Formulae Innovative Medicine, and Jiangsu Key Laboratory for High Technology Research of TCM Formulae, Nanjing University of Chinese Medicine, Nanjing, P.R. China.
Jian-Ming Guo, Jiangsu Collaborative Innovation Center of Chinese Medicinal Resources Industrialization, National and Local Collaborative Engineering Center of Chinese Medicinal Resources Industrialization and Formulae Innovative Medicine, and Jiangsu Key Laboratory for High Technology Research of TCM Formulae, Nanjing University of Chinese Medicine, Nanjing, P.R. China.
Da-Wei Qian, Jiangsu Collaborative Innovation Center of Chinese Medicinal Resources Industrialization, National and Local Collaborative Engineering Center of Chinese Medicinal Resources Industrialization and Formulae Innovative Medicine, and Jiangsu Key Laboratory for High Technology Research of TCM Formulae, Nanjing University of Chinese Medicine, Nanjing, P.R. China.
Jin-Ao Duan, Jiangsu Collaborative Innovation Center of Chinese Medicinal Resources Industrialization, National and Local Collaborative Engineering Center of Chinese Medicinal Resources Industrialization and Formulae Innovative Medicine, and Jiangsu Key Laboratory for High Technology Research of TCM Formulae, Nanjing University of Chinese Medicine, 138 Xianlin Road, Nanjing 210023, P.R. China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Jia-Shang Li: Conceptualization; Investigation; Visualization; Writing – original draft; Writing – review & editing.
Shu-Lan Su: Funding acquisition; Project administration; Resources; Supervision; Writing – review & editing.
Zhuo Xu: Formal analysis; Validation.
Li-Hui Zhao: Investigation; Methodology.
Ruo-Ying Fan: Writing – review & editing.
Jian-Ming Guo: Resources; Supervision.
Da-Wei Qian: Resources; Supervision.
Jin-Ao Duan: Funding acquisition; Project administration; Resources; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Natural Science Foundation of China, No. 30973885; Excellent Talents Program of The Ministry of Education, No.NCET-13-0873; Open Project of Jiangsu Provincial Key Laboratory of High Technology Research on Prescriptions, No.FJGJS-2020-19.
Competing interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
References
- 1. Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom of a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019; 160: 19–27. [DOI] [PubMed] [Google Scholar]
- 2. Magistro G, Wagenlehner FM, Grabe M, et al. Contemporary management of chronic prostatitis/chronic pelvic pain syndrome. Eur Urol 2016; 69: 286–297. [DOI] [PubMed] [Google Scholar]
- 3. Appiya Santharam M, Khan FU, Naveed M, et al. Interventions to chronic prostatitis/Chronic pelvic pain syndrome treatment. Where are we standing and what’s next? Eur J Pharmacol 2019; 857: 172429. [DOI] [PubMed] [Google Scholar]
- 4. van der Woude D, van der Helm-van Mil AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol 2018; 32: 174–187. [DOI] [PubMed] [Google Scholar]
- 5. Vitkov L, Hannig M, Minnich B, et al. Periodontal sources of citrullinated antigens and TLR agonists related to RA. Autoimmunity 2018; 51: 304–309. [DOI] [PubMed] [Google Scholar]
- 6. Harth M, Nielson WR. Pain and affective distress in arthritis: relationship to immunity and inflammation. Expert Rev Clin Immunol 2019; 15: 541–552. [DOI] [PubMed] [Google Scholar]
- 7. Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol 2020; 16: 380–390. [DOI] [PubMed] [Google Scholar]
- 8. Mahon OR, Dunne A. Disease-associated particulates and joint inflammation; mechanistic insights and potential therapeutic targets. Front Immunol 2018; 9: 1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther 2017; 19: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo R, Chen LH, Xing C, et al. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br J Anaesth 2019; 123: 637–654. [DOI] [PubMed] [Google Scholar]
- 11. Russo R, Cristiano C, Avagliano C, et al. Gut-brain axis: role of lipids in the regulation of inflammation, pain and CNS diseases. Curr Med Chem 2018; 25: 3930–3952. [DOI] [PubMed] [Google Scholar]
- 12. Hills RD, Jr, Pontefract BA, Mishcon HR, et al. Gut microbiome: profound implications for diet and disease. Nutrients 2019; 11: 1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma Q, Li Y, Li P, et al. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed Pharmacother 2019; 117: 109138. [DOI] [PubMed] [Google Scholar]
- 14. Wang HX, Wang YP. Gut microbiota-brain axis. Chin Med J (Engl) 2016; 129: 2373–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patterson E, Ryan PM, Cryan JF, et al. Gut microbiota, obesity and diabetes. Postgrad Med J 2016; 92: 286–300. [DOI] [PubMed] [Google Scholar]
- 16. Wang PX, Deng XR, Zhang CH, et al. Gut microbiota and metabolic syndrome. Chin Med J (Engl) 2020; 133: 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nijs J, Tumkaya Yilmaz S, Elma Ö, et al. Nutritional intervention in chronic pain: an innovative way of targeting central nervous system sensitization. Expert Opin Ther Targets 2020; 24: 793–803. [DOI] [PubMed] [Google Scholar]
- 18. Maldonado Galdeano C, Cazorla SI, Lemme Dumit JM, et al. Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab 2019; 74: 115–124. [DOI] [PubMed] [Google Scholar]
- 19. Traina G. Mast cells in gut and brain and their potential role as an emerging therapeutic target for neural diseases. Front Cell Neurosci 2019; 13: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang QB, Tang ML, Li W, et al. Correlation between intestinal microbiome imbalance and inflammatory response in rheumatoid arthritis. Chin J Gerontol 2021; 41: 812–815. [Google Scholar]
- 21. Shoskes DA, Wang H, Polackwich AS, et al. Analysis of gut microbiome reveals significant differences between men with chronic prostatitis/chronic pelvic pain syndrome and controls. J Urol 2016; 196: 435–441. [DOI] [PubMed] [Google Scholar]
- 22. Alipour B, Homayouni-Rad A, Vaghef-Mehrabany E, et al. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: a randomized double-blind clinical trial. Int J Rheum Dis 2014; 17: 519–527. [DOI] [PubMed] [Google Scholar]
- 23. Guo Z, Zhang J, Wang Z, et al. Intestinal microbiota distinguish gout patients from healthy humans. Sci Rep 2016; 6: 20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sánchez Romero EA, Meléndez Oliva E, Alonso Pérez JL, et al. Relationship between the gut microbiome and osteoarthritis pain: review of the literature. Nutrients 2021; 13: 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Szychlinska MA, Di Rosa M, Castorina A, et al. A correlation between intestinal microbiota dysbiosis and osteoarthritis. Heliyon 2019; 5: e01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guida F, Boccella S, Belardo C, et al. Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain Behav Immun 2020; 85: 128–141. [DOI] [PubMed] [Google Scholar]
- 27. Ticinesi A, Tana C, Nouvenne A, et al. Gut microbiota, cognitive frailty and dementia in older individuals: a systematic review. Clin Interv Aging 2018; 13: 1497–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blaak EE, Canfora EE, Theis S, et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes 2020; 11: 411–455. [DOI] [PubMed] [Google Scholar]
- 29. Lührs H, Gerke T, Müller JG, et al. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol 200; 37: 458–466. [DOI] [PubMed] [Google Scholar]
- 30. Shao BZ, Xu ZQ, Han BZ, et al. NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol 2015; 6: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li J, Yang M, Lu C, et al. Tuna bone powder alleviates glucocorticoid-induced osteoporosis via coregulation of the NF-κB and Wnt/β-Catenin signaling pathways and modulation of gut microbiota composition and metabolism. Mol Nutr Food Res 2020; 64: e1900861. [DOI] [PubMed] [Google Scholar]
- 32. Patterson E, Ryan PM, Wiley N, et al. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci Rep 2019; 9: 16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vieira AT, Macia L, Galvão I, et al. A role for gut microbiota and the metabolite-sensing receptor GPR43 in a murine model of gout. Arthritis Rheumatol 2015; 67: 1646–1656. [DOI] [PubMed] [Google Scholar]
- 34. Horn DL, Bettcher LF, Navarro SL, et al. Persistent metabolomic alterations characterize chronic critical illness after severe trauma. J Trauma Acute Care Surg 2021; 90: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cosi C, Mannaioni G, Cozzi A, et al. G-protein coupled receptor 35 (GPR35) activation and inflammatory pain: studies on the antinociceptive effects of kynurenic acid and zaprinast. Neuropharmacology 2011; 60: 1227–1231. [DOI] [PubMed] [Google Scholar]
- 36. Cai X, Du J, Li L, et al. Clinical metabolomics analysis of therapeutic mechanism of Tongmai Yangxin Pill on stable angina. J Chromatogr B Analyt Technol Biomed Life Sci 2018; 1100–1101: 106–112. [DOI] [PubMed] [Google Scholar]
- 37. Eberhardt MJ, Schillers F, Eberhardt EM, et al. Reactive metabolites of acetaminophen activate and sensitize the capsaicin receptor TRPV1. Sci Rep 2017; 7: 12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abdelrazig S, Ortori CA, Doherty M, et al. Metabolic signatures of osteoarthritis in urine using liquid chromatography-high resolution tandem mass spectrometry. Metabolomics 2021; 17: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adams SB, Jr, Nettles DL, Jones LC, et al. Inflammatory cytokines and cellular metabolites as synovial fluid biomarkers of posttraumatic ankle arthritis. Foot Ankle Int 2014; 35: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 40. Sakurai M, Yamamoto Y, Kanayama N, et al. Serum Metabolic Profiles of the Tryptophan-Kynurenine Pathway in the high risk subjects of major depressive disorder. Sci Rep 2020; 10: 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Song Z, Xie W, Strong JA, et al. High-fat diet exacerbates postoperative pain and inflammation in a sex-dependent manner. Pain 2018; 159: 1731–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wegner A, Elsenbruch S, Rebernik L, et al. Inflammation-induced pain sensitization in men and women: does sex matter in experimental endotoxemia. Pain 2015; 156: 1954–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McDonald MK, Tian Y, Qureshi RA, et al. Functional significance of macrophage-derived exosomes in inflammation and pain. Pain 2014; 155: 1527–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hsieh CT, Lee YJ, Dai X, et al. Systemic lipopolysaccharide-induced pain sensitivity and spinal inflammation were reduced by minocycline in neonatal rats. Int J Mol Sci 2018; 19: 2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun G, Ren Q, Bai L, et al. Phoenixin-20 suppresses lipopolysaccharide-induced inflammation in dental pulp cells. Chem Biol Interact 2020; 318: 108971. [DOI] [PubMed] [Google Scholar]
- 46. Nissen MD, Lau ETL, Cabot PJ, et al. Baltic amber teething necklaces: could succinic acid leaching from beads provide anti-inflammatory effects? BMC Complement Altern Med 2019; 19: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nidamarthi HVK, Choudhury M, Velmurugan D. Understanding the binding mechanism of succinic acid against phospholipase A2 from bee venom. J Biochem Mol Toxicol 2021: e22715. [DOI] [PubMed] [Google Scholar]
- 48. Wang D, Guo S, He H, et al. Gut microbiome and serum metabolome analyses identify unsaturated fatty acids and butanoate metabolism induced by gut microbiota in patients with chronic spontaneous urticaria. Front Cell Infect Microbiol 2020; 10: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li J, Yang YL, Li LZ, et al. Succinate accumulation impairs cardiac pyruvate dehydrogenase activity through GRP91-dependent and independent signaling pathways: therapeutic effects of ginsenoside Rb1. Biochim Biophys Acta Mol Basis Dis 2017; 1863: 2835–2847. [DOI] [PubMed] [Google Scholar]
- 50. Saratikov AS, Bulatnikov AP, Vengerovskiĭ AI, et al. Vliianie ammoniia suktsinata na farmacologicheskie éffekty kisloty atsetilsalitsilovoĭ [Effect of ammonium succinate on pharmacological effects of acetylsalicylic acid]. Eksp Klin Farmakol 2000; 63: 56–58. [PubMed] [Google Scholar]
- 51. Fernández-Veledo S, Vendrell J. Gut microbiota-derived succinate: friend or foe in human metabolic diseases. Rev Endocr Metab Disord 2019; 20: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Connors J, Dawe N, Van Limbergen J. The role of succinate in the regulation of intestinal inflammation. Nutrients 2018; 11: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang X, Zhang D, Jia H, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med 2015; 21: 895–905. [DOI] [PubMed] [Google Scholar]
- 54. Cheng B. Study on the mechanism of Danggui Sini Decoction on rheumatoid arthritis based on metabonomics and network pharmacology. Nanning, China: Guangxi Medical University, 2018. [Google Scholar]
- 55. Ahmadi S, Wang S, Nagpal R, et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 2020; 5: e132055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Johnson RJ, Nakagawa T, Jalal D, et al. Uric acid and chronic kidney disease: which is chasing which. Nephrol Dial Transplant 2013; 28: 2221–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang W, Wang T, Guo R, et al. Variation of serum uric acid is associated with gut microbiota in patients with diabetes mellitus. Front Cell Infect Microbiol 2022; 11: 761757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim GW, Lee NR, Pi RH, et al. IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch Pharm Res 2015; 38: 575–584. [DOI] [PubMed] [Google Scholar]
- 59. Zhang Q, Liu J, Zhang M, et al. Apoptosis induction of fibroblast-like synoviocytes is an important molecular-mechanism for herbal medicine along with its active components in treating rheumatoid arthritis. Biomolecules 2019; 9: 795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee JY, Mannaa M, Kim Y, et al. Comparative analysis of fecal microbiota composition between rheumatoid arthritis and osteoarthritis patients. Genes (Basel) 2019; 10: 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hua C, Geng Y, Chen Q, et al. Chronic dexamethasone exposure retards growth without altering the digestive tract microbiota composition in goats. BMC Microbiol 2018; 18: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang Q, Xu B, Fan K, et al. Inflammation suppression by dexamethasone via inhibition of CD147-mediated NF-κB pathway in collagen-induced arthritis rats. Mol Cell Biochem 2020; 473: 63–76. [DOI] [PubMed] [Google Scholar]
- 63. Wang QS, Xu BX, Fan KJ, et al. Dexamethasone-loaded thermosensitive hydrogel suppresses inflammation and pain in collagen-induced arthritis rats. Drug Des Devel Ther 2020; 14: 4101–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ballegeer M, Van Looveren K, Timmermans S, et al. Glucocorticoid receptor dimers control intestinal STAT1 and TNF-induced inflammation in mice. J Clin Invest 2018; 128: 3265–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Unsal H, Balkaya M, Unsal C, et al. The short-term effects of different doses of dexamethasone on the numbers of some bacteria in the ileum. Dig Dis Sci 2008; 53: 1842–1845. [DOI] [PubMed] [Google Scholar]
- 66. Kong YH, Shi Q, Han N, et al. Structural modulation of gut microbiota in rats with allergic bronchial asthma treated with recuperating lung decoction. Biomed Environ Sci 2016; 29: 574–583. [DOI] [PubMed] [Google Scholar]
- 67. Wu T, Yang L, Jiang J, et al. Chronic glucocorticoid treatment induced circadian clock disorder leads to lipid metabolism and gut microbiota alterations in rats. Life Sci 2018; 192: 173–182. [DOI] [PubMed] [Google Scholar]
- 68. Zhang M, Hu X. Mechanism of chlorogenic acid treatment on femoral head necrosis and its protection of osteoblasts. Biomed Rep 2016; 5: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Qiao J, Liu A, Liu J, et al. Salvianolic acid B (Sal B) alleviates the decreased activity induced by prednisolone acetate on osteoblasts by up-regulation of bone formation and differentiation genes. Food Funct 2019; 10: 6184–6192. [DOI] [PubMed] [Google Scholar]
- 70. Zhang R, Guo LH, Yin Y, et al. Effect of electroacupuncture on serum TNF-α, IL-1β and intercellular adhesion molecule 1 levels in rheumatoid arthritis rats. Zhen Ci Yan Jiu 2016; 41: 51–54 (in Chinese). [PubMed] [Google Scholar]
- 71. Tourret J, Willing BP, Dion S, et al. Immunosuppressive treatment alters secretion of ileal antimicrobial peptides and gut microbiota, and favors subsequent colonization by uropathogenic escherichia coli. Transplantation 2017; 101: 74–82. [DOI] [PubMed] [Google Scholar]
- 72. Scarminio V, Fruet AC, Witaicenis A, et al. Dietary intervention with green dwarf banana flour (Musa sp AAA) prevents intestinal inflammation in a trinitrobenzenesulfonic acid model of rat colitis. Nutr Res 2012; 32: 202–209. [DOI] [PubMed] [Google Scholar]
- 73. Liu Y, Wu JS, Tang YL, et al. Multiple treatment meta-analysis of intra-articular injection for temporomandibular osteoarthritis. J Oral Maxillofac Surg 2020; 78: 373.e1–373.e18. [DOI] [PubMed] [Google Scholar]
- 74. Iannitti T, McDermott MF, Laurino C, et al. Corticosteroid transdermal delivery significantly improves arthritis pain and functional disability. Drug Deliv Transl Res 2017; 7: 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oliveira IM, Gonçalves C, Shin ME, et al. Enzymatically crosslinked tyramine-gellan gum hydrogels as drug delivery system for rheumatoid arthritis treatment. Drug Deliv Transl Res 2021; 11: 1288–1300. [DOI] [PubMed] [Google Scholar]
- 76. Sun F, Zhang Y, Li Q. Therapeutic mechanisms of ibuprofen, prednisone and betamethasone in osteoarthritis. Mol Med Rep 2017; 15: 981–987. [DOI] [PubMed] [Google Scholar]
- 77. Huang ZX, Wang M, Deng WM, et al. Effects of betamethasone injection on bone and cartilage metabolism in collagen-induced arthritis rats. J New Med 2019; 50: 105–109. [Google Scholar]
- 78. Verdú EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 2006; 55: 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res 2012; 26: 1719–1725. [DOI] [PubMed] [Google Scholar]
- 80. Hongwu Y, Yan Z, Yuzhen P, et al. Clinical efficacy of moxibustion as supplement on rheumatoid arthritis and the exploration on its mechanism. Zhongguo Zhen Jiu 2016; 36: 17–20 (in Chinese). [PubMed] [Google Scholar]
- 81. Biernat KA, Pellock SJ, Bhatt AP, et al. Structure, function, and inhibition of drug reactivating human gut microbial β-glucuronidases. Sci Rep 2019; 9: 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kojima S, Nadai M, Kitaichi K, et al. Possible mechanism by which the carbapenem antibiotic panipenem decreases the concentration of valproic acid in plasma in rats. Antimicrob Agents Chemother 1998; 42: 3136–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Feng L, Yang Y, Huo X, et al. Highly selective NIR probe for intestinal β-glucuronidase and high-throughput screening inhibitors to therapy intestinal damage. ACS Sens 2018; 3: 1727–1734. [DOI] [PubMed] [Google Scholar]
- 84. Colucci R, Pellegrini C, Fornai M, et al. Pathophysiology of NSAID-associated intestinal lesions in the rat: luminal bacteria and mucosal inflammation as targets for prevention. Front Pharmacol 2018; 9: 1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Huber G, Garg U. Quantitation of ibuprofen in blood using gas chromatography-mass spectrometry (GC-MS). Methods Mol Biol 2010; 603: 289–296. [DOI] [PubMed] [Google Scholar]
- 86. Chintalacharuvu SR, Urankar-Nagy N, Petersilge CA, et al. Treatment of collagen induced arthritis by proteolytic enzymes: immunomodulatory and disease modifying effects. J Rheumatol 2001; 28: 2049–2059. [PubMed] [Google Scholar]
- 87. Park JW, Yun YP, Park K, et al. Ibuprofen-loaded porous microspheres suppressed the progression of monosodium iodoacetate-induced osteoarthritis in a rat model. Colloids Surf B Biointerfaces 2016; 147: 265–273. [DOI] [PubMed] [Google Scholar]
- 88. Rogers MAM, Aronoff DM. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect 2016; 22: 178.e1–178.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ellepola N, Ogas T, Turner DN, et al. A toxicological study on photo-degradation products of environmental ibuprofen: ecological and human health implications. Ecotoxicol Environ Saf 2020; 188: 109892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Driver B, Marks DC, van der Wal DE. Not all (N)SAID and done: effects of nonsteroidal anti-inflammatory drugs and paracetamol intake on platelets. Res Pract Thromb Haemost 2020; 4: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Norling LV, Perretti M. The role of omega-3 derived resolvins in arthritis. Curr Opin Pharmacol 2013; 13: 476–481. [DOI] [PubMed] [Google Scholar]
- 92. Pan ZY, Wang H. Synergistic interaction between choline and aspirin against acute inflammation induced by carrageenan and lipopolysaccharide. Int Immunopharmacol 2014; 20: 229–237. [DOI] [PubMed] [Google Scholar]
- 93. Zhang N, Liu Z, Luo H, et al. FM0807 decelerates experimental arthritis progression by inhibiting inflammatory responses and joint destruction via modulating NF-κB and MAPK pathways. Biosci Rep 2019; 39: BSR20182263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhao R, Coker OO, Wu J, et al. Aspirin reduces colorectal tumor development in mice and gut microbes reduce its bioavailability and chemopreventive effects. Gastroenterology 2020; 159: 969–983. [DOI] [PubMed] [Google Scholar]
- 95. Zhang J, Sun Y, Wang R, et al. Gut microbiota-mediated drug-drug interaction between amoxicillin and aspirin. Sci Rep 2019; 9: 16194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pascart T, Richette P. Colchicine in gout: an update. Curr Pharm Des 2018; 24: 684–689. [DOI] [PubMed] [Google Scholar]
- 97. Misawa T, Takahama M, Kozaki T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol 2013; 14: 454–460. [DOI] [PubMed] [Google Scholar]
- 98. Shi Y, Li J, Yang P, et al. Colchicine increases intestinal permeability, suppresses inflammatory responses, and alters gut microbiota in mice. Toxicol Lett 2020; 334: 66–77. [DOI] [PubMed] [Google Scholar]
- 99. Li S, Han J, Wang DS, et al. Sinomenine attenuates chronic inflammatory pain in mice. Metab Brain Dis 2017; 32: 211–219. [DOI] [PubMed] [Google Scholar]
- 100. Lan Z, Wei M, Chen L, et al. Role of sinomenine on complete Freund’s adjuvant-induced arthritis in rats. IUBMB Life 2016; 68: 429–435. [DOI] [PubMed] [Google Scholar]
- 101. Feng ZT, Yang T, Hou XQ, et al. Sinomenine mitigates collagen-induced arthritis mice by inhibiting angiogenesis. Biomed Pharmacother 2019; 113: 108759. [DOI] [PubMed] [Google Scholar]
- 102. Luo X, Chen DS, Li L, et al. Effect of Zhengqing Fengtongning on osteoporotic vertebral fracture in rats. World Sci Tech – Modern Trad Chinese Med 2018; 20: 224–228. [Google Scholar]
- 103. Wang W, Zhou H, Liu L. The role of Chinese herbal medicine in the management of adverse drug reactions of leflunomide in treating rheumatoid arthritis. Phytomedicine 2020; 68: 153136. [DOI] [PubMed] [Google Scholar]
- 104. Chen Z, Zhijie C, Yuting Z, et al. Antibiotic-driven gut microbiome disorder alters the effects of sinomenine on morphine-dependent zebrafish. Front Microbiol 2020; 11: 946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Xu H, Cai L, Zhang L, et al. Paeoniflorin ameliorates collagen-induced arthritis via suppressing nuclear factor-κB signalling pathway in osteoclast differentiation. Immunology 2018; 154: 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Li H, Cao XY, Dang WZ, et al. Total Glucosides of Paeony protects against collagen-induced mouse arthritis via inhibiting follicular helper T cell differentiation. Phytomedicine 2019; 65: 153091. [DOI] [PubMed] [Google Scholar]
- 107. Luo X, Wang X, Huang S, et al. Paeoniflorin ameliorates experimental colitis by inhibiting gram-positive bacteria-dependent MDP-NOD2 pathway. Int Immunopharmacol 2021; 90: 107224. [DOI] [PubMed] [Google Scholar]