Abstract
Wound represents a significant socioeconomic burden for both affected individuals and as a whole healthcare system. Accordingly, stem cells have garnered attention due to their differentiation capacity and ability to aid tissue regeneration by releasing biologically active molecules, found in the cells’ cultivated medium which known as conditioned medium (CM) or secretomes. This acellular approach provides a huge advantage over conventional treatment options, which are mainly used cellular treatment at wound closure. Interestingly, the secretomes contained the cell-secreted proteins such as growth factors, cytokines, chemokines, extracellular matrix (ECM), and small molecules including metabolites, microvesicles, and exosomes. This review aims to provide a general view on secretomes and how it is proven to have great potential in accelerating wound healing. Utilizing the use of secretomes with its secreted proteins and suitable biomaterials for fabrications of acellular skin substitutes can be promising in treating skin loss and accelerate the healing process.
Keywords: Secretomes, conditioned medium, secreted protein, wound healing, tissue regeneration
Introduction
Skin is important as a protective barrier against the aggression of external microorganisms and dehydration. It also has the role of covering and protecting the underlying organs from ultraviolet radiation, mechanical and chemical injury, microorganisms’ infection, and water loss. 1 Skin injury is widespread and is closely related to a high mortality and morbidity rate due to breaks off the skin’s barrier function and changes of the sensitivity of the pain, temperature, and sense of touch. Because of this crucial role, it is necessity for the wounds to be successfully healed within a swift time frame. Skin damage can occur because of burns, cuts, abrasions, and an ulcer depends on degrees of severity. Without proper treatments, any holes or breakage of the skin may disturb its barrier function and expose the body’s tissues to infectious or damages. 2
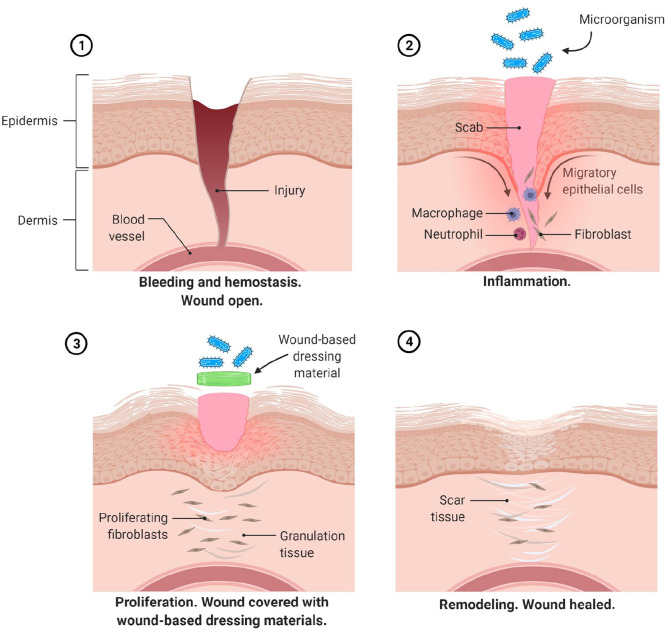
Wound healing is a process that usually requires a well-orchestrated integration of composite cellular and molecular events that occur after the onset of a tissue lesion in order to restore the damaged tissue. It is a normal biological and spontaneous self-repairing process in human body that achieved through four sequential stages including hemostasis, inflammation, proliferation (migration or tissue formation), and remodeling. 3 Once an injury occurs, immediately the first phase of hemostasis begins at the onset of injury and its main objective is to stop the bleeding with vascular disruption and fibrin clot formation. The thrombin, an enzyme will initiates the formation of fibrin mesh and the platelets aggregate and become lodged in the matrix to form clot-like structure. When the bleeding progress is controlled within the first 24 h post injury, chemotaxis event occurs where the inflammatory cells are migrated toward the wound and encourage the inflammatory phase. As the inflammatory phase relieves accompanied by apoptosis of immune cells, the proliferation phase begins. This phase is the stage when the wound actually closed and mainly described by epithelial proliferation and migration (re-epithelialization), formation of blood vessels (angiogenesis) and reestablishment of the extracellular matrix (ECM). The underlying mechanism that responsible for accelerating wound healing is normally due to the rapid re-epithelialization which may occurred because of the regulation of collagen expression. 4 After an extensive amount of collagen has deposited into the wound, the fibroblast will apoptose and remodeling phase can begin. This stage is maturation phase where the new tissue slowly gains strength and flexibility. The ECM and collagen fibers prepared in the proliferation phase are remodeled and realigned.
The common problems with wound healing in human demonstrated by the delayed wound healing such as in diabetes or radiation exposure or excessive healing, that may cause hypertrophic scar or keloid scars or deprivation of skin appendages. Earlier on, the fasters of wound closure were the primary goal of wound treatment. However, the focus in the field of wound repair nowadays is to achieve optimum or perfect healing with a higher quality of the regenerated skin, that can restore the anatomical and functionality of the skin. Therefore, there is a need to establish and determine the best practice to enhancing wound healing. 5
The healing of cutaneous wounds requires interactions between cell and tissue components of the surrounding damaged skin which includes the dermal and epidermal cells (fibroblast and keratinocytes), ECM, and the nervous and vascular components.6,7 But sadly, the healing of skin wounds, particularly in the older generation or in diabetic patients, is often slowed and weakened, causing increased morbidity. The fetuses and adults skin tissues have different responses toward injury and mechanisms for wound healing process. Usually, there is absence of contraction and scarring for fetal skin during healing process as compared to adult skin that involves intense inflammation and scarring. Dermal fibroblasts are the primary cells involved in cutaneous regeneration, which are different between fetal and adults’ skin in many aspects including cells proliferation and migration rates, ability to form myofibroblasts, synthesis of ECM and responses to inflammatory cues. 8
Regenerative medicine, which uses tissue engineering methods, is a promising new multidisciplinary field of medicine aiming at regenerating and guiding the restoration and augmentation of organ and tissue functioning, ultimately enhancing the overall quality of life. 9 Tissue engineering that employs stem cell-based therapies is currently explored in numerous research fields and shows a great deal of potential in regenerative medicine.10 –13 Stem cells have garnered a lot of attention in regenerative medicine in recent years because of their differentiation capacity and ability to aid tissue regeneration by releasing biologically active molecules known as secretomes. The conditioned medium (CM) is also known as secretomes which can be found in the spent medium used for culturing the stem cells. A secretome is normally has cell-secreted proteins such as growth factors, cytokines, chemokines, ECM, and small molecules, including metabolites, ions, peptides, microvesicles, and exosomes. 14 Several studies on secreted proteins of stem cells have shown that the used of these secreted factors alone, without the cell, can trigger healing process in injured tissues/organs. 15 Mocchi et al. have also shown in their review that most recent studies focused on using cell-secreted proteins for therapeutic purposes. With this in mind, the aim of this review is to provide a broad view on how secretomes is proven to have great potential in accelerating wound healing, alternative uses for cell secretomes, the methods for collecting secretomes, as well as safety and ethical issues concerning the use of secretomes. 16
Standard treatment for tissue regeneration
Autografts remain the standard of care in the treatment of skin defects which are caused by burns injuries, traumatic akin loss, disease such as diabetic foot ulcers and tumor excision.17 –21 The commonly used treatment for this skin loss is autogenous and allogeneic skin grafting. 22 Split-thickness skin grafting (SSG) is the standard treatment where the harvest of SSG consisting of epidermal and dermal tissue creates a partial-thickness wound that involves pain management, wound care and healing time.23,24 However, SSG have several disadvantages, including the potential creating of secondary skin injury from the donor site, high risk of infections, post-implantation failure, increase scarring formation, and difficulty in treating patients with severe skin loss due to the limited amount of skin to be harvested.25,26 Besides that, constituting a further challenge to the use of autologous grafts is the limitation of donor-site availability in patients who at the extremes of age such as very young and very old patients.27 –29
Hence, skin substitutes may offer another option for wound management and they have also been considered as one of the treatment options for significant skin loss. Skin substitutes consist of variety group of biologics, synthetic, or biosynthetic materials that can provide temporary or permanent coverage of open skin wounds. In the treatment of burns, skin substitutes are primarily used to treat full-thickness skin loss and can also be used to cover skin defects that may result following release of post-burn contractures. 30 Previously study by Rheinwald and Green 31 who are first expanded human epidermal keratinocytes into cultured epithelial autografts for transplantation onto burn injuries. Up to date, commercially available skin substitutes may be categorized as acellular and cellular, or by source including human (allograft), animal (xenograft), or synthetic origin. 32 Acellular substitutes serve as skin scaffolding for granulation tissue formation and revascularization. Meanwhile, cellular substitutes consist of cells that secrete the ECM proteins and growth factors.33,34
Therapeutic approaches using tissue engineered products
Wound dressing-based therapy
Wound management has become a crucial problem in the healthcare industry as it is a constantly progressing process involving a complete range of medicinal methods, interventions, and holistic clinical measures, in the care of patients with wounds. Various methods of wound dressing-based therapy have been introduced to help mitigate this issue. These are include old traditional methods (plasters, gauze, and bandages) that require frequent changing, unique skin substitutes (allograft, autografts, and tissue engineered derivatives) that accelerate the wound closure and replaces the role the skin has to promote wound healing, interactive materials (gels, foams, and hydrofibres) that promote wound healing and mitigate or prevent bacterial infection and finally bioactive dressings (collagen, growth factors, and elastin) that were prepared from biomaterials to enhance the wound healing. Moreover, these wound-based dressing biomaterials would have eco-friendly nature and cost-effective for the preparation of wound care materials in order to resist microbial infection and promote healing as well (Figure 1). These wound dressings are categorized in two types, primary dressings which are directly placed on the wound and secondary dressings that are covered on the primary and act as a supplement. Additionally, dressings can also be categorized as either bioactive/interactive dressings or inert/passive dressings depending on the type of interaction with the biological tissue. Bioactive/interactive dressings are readily available for application in acute and subacute wounds however; inert/passive dressings are a bit unconventional and include antimicrobial dressing’s steps.
Figure 1.
Schematic image of the human skin barrier function and protection with wound-based dressing material against wound infection bacteria. An ideal dressing should be biocompatible and biodegradable, maintain the moisture, be permeable to oxygen, enable the exudate removal, prevent the wound from pathogens and mechanical irritation, improve cell behaviors, and promote wound healing. Image created with Biorender.com.
Recent developments in wound dressing-based therapy have introduced and incorporated the use of nanoparticles and nanomaterials in biomedical applications to help create natural polymeric-based dressings. 35 This is due to their amazing properties such as non-allergenic nature, biocompatibility, biodegradability, and low toxicity. Examples are electrospun nanofibers which have been useful because of their nanoscale diameter as well as their ability to form a nonwoven structure or membrane-based materials with suitable porosity. 36 Structure-wise, they are similar to that of collagen fibers in tissues, becoming a good choice for skin substitutes. 37 These nanomaterials are used found in silk fibroin which has been carefully studied on their outstanding traits for wound dressing such as their robust mechanical properties, high water and oxygen uptake, low immunogenicity as well as biocompatibility and biodegradability. 38
Various hurdles regarding wound healing arise; causes apart from infections are still not completely explained. These include the size and location of the wound, perceived levels of pain felt by patients as well as the limitations of certain models of wound based dressing therapy. Ideally the development of new wound-based dressings should also explore the patient-related consequences including but not limited to; improved ease of a dressing application or removal, quality of live improvements as well as perceptions of the painful in wound care management and how to reduce it. An ideal wound-based therapy should address the flaws of currently available wound dressings, also feedback from physicians and healthcare workers as well as patients who have been administered to these treatments. 39 Current advancements of this field have suggested new-material based dressings on hydrogel or bacterial nanocellulose which comes close fixing the problems of current treatments and is step toward the ideal wound-based therapy.40,41 However numerous complications still arise and recent studies revealed a relationship between healing progressions with certain biomarkers such as pH, temperature inflammatory mediators toward the formation of biofilm. 42 Henceforward, other methods of wound healing that provide an alternative to wound dressing able to offer their own unique benefits.
Growth factor therapy
In wound healing process, the secretion mediators or growth factors are crucial for the biological process by activating or inhibiting the respective signaling. Therefore, these secretion mediators have the potential to be used as supplementary therapies in wound treatment. The main growth factors that play role in re-epithelialization during healing are transforming growth factor (TGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and granulocyte-macrophage colony-stimulating factor (GM-CSF).43,44 Besides that, becaplermin (Regranex gel, Johnson & Johnson Wound Management, Somerville, NJ, USA), which contains human PDGF, an important angiogenesis and granulation growth factor, 45 was approved by the US FDA for treating diabetic neuropathic ulcers because it enhances the rate of wound healing. 46 Meanwhile, epidermal growth factor (EGF) and keratinocyte growth factor-2 are the recommended treatment for venous ulcer. 47 Dermatopontin (DPT), a non-collagenous matrix protein that mainly expressed in the dermis, is vital in wound healing, especially during re-epithelialization, promoting keratinocyte migration in vitro via paracrine action, while IGF-1 and EGF influence the keratinocyte components that regulate wound epithelialization speed.48,49 The major growth factors and cytokines involved in wound healing are listed in Table 1. At present, researchers are focusing on the proteins secreted by cells in culture medium and their effects on wound healing in vitro and in vivo. However, the use of growth factors or conditioned medium (CM) in promoting wound healing remains unclear. Its success depends on the interaction between the growth factors, cells and ECM, and a suitable delivery system and dosage to ensure a satisfactory wound healing rate.
Table 1.
List of major growth factors and cytokines involve in wound healing.
| Growth factor | Cells | Functions | References |
|---|---|---|---|
| EGF | Platelets, Macrophages, Fibroblasts | Mitogenic for keratinocytes and fibroblasts, re-epithelialization | Haase et al., 49 Bhora et al. 50 |
| FGF-2 | Keratinocytes, Mast Cells, Fibroblasts, Endothelial cells, Smooth muscle cells, Chondrocytes | Granulation tissue formation, re-epithelialization, matrix formation, remodeling, angiogenesis, wound contraction, and matrix deposition | Shipley et al., 51 Sogabe et al. 52 |
| TGF-β (including isoform β1, β2, and β3) | Platelets, Keratinocytes, Macrophages, Lymphocytes, Fibroblasts, endothelial cells | Inflammation, granulation tissue formation, re-epithelialization, matrix formation, remodeling, stimulates tissue inhibitor metalloproteinases (TIMP) synthesis, keratinocytes migration, angiogenesis and fibroplasia, inhibit production of metalloproteinases (MMPs) and keratinocytes proliferation | Penn et al., 53 Pakyari et al. 54 |
| PDGF | Platelets, Keratinocytes, Macrophages, Endothelial cells, Fibroblasts | Inflammation, granulation tissue formation, re-epithelialization, matrix formation and remodeling, stimulate angiogenesis and wound contraction | Barrientos et al., 43 Pierce et al. 55 |
| VEGF | Platelets, Neutrophils, Macrophages, Endothelial cells, Smooth muscle cells, Fibroblasts | Granulation tissue formation, mitogenic for endothelial cells | Barrientos et al., 43 Bao et al., 56 Johnson and Wilgus 57 |
| IL-1 | Neutrophils, Monocytes, Macrophages Keratinocytes | Inflammation, re-epithelialization | Sauder et al., 58 Ishida et al. 59 |
| IL-6 | Neutrophils, Macrophages | Inflammation, re-epithelialization | Lin et al., 60 McFarland-Mancini et al. 61 |
| TNF-a | Neutrophils, Macrophages | Inflammation, re-epithelialization | Barrientos et al., 43 Ashcroft et al. 62 |
Tissue engineered skin substitutes
The culturing of human skin cells and development of tissue-engineered skin substitutes have been started since 1975 and skin substitutes have been used in most hospital for treating major skin loss, especially due to chronic wounds. 46 Currently, many skin substitutes that are commercially available were classified according to the presence (cellular) or absence (acellular) of viable cells, the use of cells or tissue that is autologous (from the same patient), allogeneic (from different human donor), or xenogeneic (from animal), use of biomaterials that are either biological or synthetic, and involve physical stimulation to create a living substitute. 63 Skin substitutes are suitable for alleviating non-healing chronic wounds, as such wounds are deeper and involve a more wide total surface area, or for non-healing smaller wounds, due to other factors. 64 Re-epithelialization is only activated when fibroblasts begin to produce collagen and matrix and recreate the dermis.
Biological skin substitutes are used as permanent replacements or temporary dressing before normal skin regeneration or healing occurs. 65 The transplanted skin substitute acts as a normal autologous skin graft by supporting the wounded area and providing the normal function of the skin in term of physiological and mechanical strength. The best skin substitute should have no immune or toxicity effect; have biocompatibility, and low risk of disease transmission. Besides, the other advantages of skin substitutes which includes: (a) protection against microbial infection and slow down the loss of vapor, water, proteins and electrolytes; (b) procrastination: covering the wound after wound debridement until the wound is ready and closed by the skin grafts; (c) promotion: by providing the dermal matrix, and releasing its components such as cytokines, chemokines and growth factors, can promotes and enhances the healing process; and (d) provision: of new anatomical skin structures and re-establishment the skin function by using different biomaterials or ECM such as dermal collagen or cultured cells that are incorporated into the wound during healing process.66,67
Skin substitutes are used in treating acute wounds, although no substitute can fully replace the damaged skin.68,69 Each skin substitute product has its advantages and disadvantages, relying on the patient’s clinical condition. The tissue-engineered skin substitutes are divided into four groups based on the layer: (a) epidermal, (b) dermal, (c) bilayer, and (d) amniotic membrane. The epidermal can be classified to the first degree injuries, superficial, or deep dermal referred to the second degree injuries, bilayer or full thickness denoted to the third degree injuries meanwhile the amniotic is for acute wounds. 70 Shortly after the findings, it is clear that the skin substitute or skin replacements provide speedy wound protection solutions that may require a less vascular layer of the wound bed, increase in the dermal component of healed wound, reduce or eliminate factors that impede wound healing, reduce the inflammatory response and subsequent scarring.65,71 The list also shows the combination of acellular, cellular or biomaterials, which are derived from human only, human with animal-derived products or human-/animal-derived products with synthetic products. The high demand for skin substitutes has inspired most of the scientists and specialist in this field to collaborate in developing the suitable skin substitutes that can promote healing, as an alternative to SSG.
Cellular approaches
The use of cellular therapy in the treatment of skin wounds is currently an active area of investigation. The cell-based treatment is simple and reduces the surgical burden for the patients in the acute wounds repair. Therapeutically delivered cells may participate directly in regenerative capacity and become attractive choice due to cells have a large proliferative potential, the ability to differentiate into different cell types and produce a variety cytokines and growth factors which are important to wound healing. 72 However, each method has advantages and some limitations since that in this case, most cell-based skin substitutes require long production time and thus, patients endure long waiting times. Table 2 showed the list of the commercial tissue-engineered skin substitutes with living cells and their applications with approval by US Food and Drug Administration (FDA).
Table 2.
Tissue-engineered skin substitutes with living cells.
| Product | Description | Indication | Nature of FDA Approval(s) and year(s) |
|---|---|---|---|
| Cellular autologous skin substitute | |||
| Epicel™ | • Autologous keratinocytes from skin on petrolatum gauze
backing. • Permanent use |
• For treatment of partial and full thickness burns, chronic ulcers and congenital nevi | Unregulated device (1988) Humanitarian use device Exemption (HDE) (2007) |
| Epidex™ | • Autologous keratinocytes from outer root sheath of hair follicles on a silicone membrane. | • As epidermal substitute for treatment of partial and full-thickness burns and chronic ulcers | (2003) No FDA Designation |
| Laserskin™ or Vivoderm™ | • Autologous keratinocytes seeded directly on esterified
laser-perforated hyaluronic acid
matrix. • Permanent use |
• For treatment of partial and full thickness burns, chronic venous and pressure ulcers, vitiligo | (2002)–510(k) |
| BioSeed-S™ | • Autologous oral mucosal cells on a fibrin matrix | • For treatment of partial-thickness burns and chronic ulcers | (2002) - No FDA approval |
| Myskin™ | • Autologous keratinocytes seeded on coated silicone sheet | • For treatment of partial-thickness burns and chronic ulcers | (2004) - No FDA approval |
| CellSpray™or ReCell® | • Cultured autologous keratinocytes suspension delivered via spray | • For treatment of partial-thickness burns and chronic ulcers | Premarket Approval (2018) |
| PermaDerm® | • Autologous keratinocytes and fibroblast seeded onto dermal substitute of absorbable bovine collagen matrix | • For treatment of full-thickness burns | Orphan Approval by FDA (2012) |
| LOEX skin | • Autologous keratinocytes seeded on top of dermal substitute of collagen gel containing autologous fibroblasts | • For treatment of full- or partial-thickness burns and chronic ulcers | No FDA approval |
| Hyalograft 3D™ | • Autologous fibroblasts seeded on hyaluronic acid matrix | • For treatment of full- and partial-thickness wounds, foot ulcer | 510(k)-(2003) |
| MyDerm™ | • Autologous keratinocytes and fibroblasts in autologous fibrin matrix | • For treatment of full- and partial-thickness wounds | No FDA approval |
| Cellular allogenenic skin substitute | |||
| Dermagraft™ | • Allogeneic dermal substitute using cryopreserved human
fibroblasts from neonatal foreskin cultured on
bioabsorbable polyglactin mesh matrix • Dermal replacement or temporary skin substitute • Biosynthetic |
• For treatment of full-thickness chronic wound and diabetic ulcer | PMA-(2001) |
| TransCyte™ | • Silicone membrane as temporary epidermal
barrier • Biosynthetic • Human fibroblasts from neonatal foreskin on silicone with nylon mesh and combined with synthetic epidermal layer. |
• Temporary wound coverage • For treatment of partial- and full-thickness burns |
PMA-(1998) |
| ICX-SKN™ | • Allogeneic fibroblasts seeded on a freeze-dried natural human collagen matrix | • For treatment of full-thickness burns | No FDA Approval |
| PriMatrix™ (TEI Biosciences) | • Processed from fetal bovine dermal matrix (Xenogenic) | • For treatment of partial and full-thickness of chronic and diabetic wounds; | 510(k)-(2006) |
| SurgiMend® PRS (TEI Biosciences) | • Processed from fetal bovine dermis (Xenogenic) | • Trial for breast reconstruction. | 510(k)-(2017) |
| OrCel™ | • Human keratinocytes and fibroblasts from neonatal
foreskin seeded on bovine collagen sponge
matrix • Duration of contact- permanent |
• For treatment of skin graft donor sites and partial thickness wound. | PMA-(1998) |
| Apligraf™ | • Similar to Orcel™ • Bilayer skin substitute • Human keratinocytes upper layer whilst bottom layer fibroblast consist of fibroblast with bovine collagen- to produce matrix • Duration of contact permanent |
• For treatment of venous and diabetic foot ulcers. | PMA-(1998) |
| TheraSkin™ | • Cryopreserved human native skin allograft consists of both epidermal and dermal layers. | • For treatment of chronic wounds | HCT/Ps - (2011) Compliance with the AATB and FDA guidelines |
| EpiFix® | • Dehydrated human amniotic membrane | • For acute and chronic wound care | HCT/Ps - (2013) |
| AmnioFix | • Dehydrated human amniotic membrane | • For acute and chronic wound care • Reduce scar tissue formation, enhance healing and acts as a barrier. |
HCT/Ps - (2018) |
| Grafix®Core | • Cryopreserved placental membrane comprised of an extra-cellular matrix and cells native to the tissue. | • For the treatment of acute and chronic wounds | - |
Acellular approaches
Basically, wound healing process involved dynamic interaction between cells, ECM and growth factors. The non-cellular component which is ECM become a major constituent of the skin layer and it is composed of structural proteins such as fibronectin, collagen, laminin, glycosaminoglycans, proteoglycans, and other non-structural matricellular proteins. In wounds, proteolytic dysregulation has leads to degradation of ECM components, receptors and growth factors in which are essential for healing and regeneration. Thus, it can provides a structural support for cells and act as a reservoir for growth factors that are rapidly to mobilized to stimulated cell proliferation and migration upon injury. 76
In addition to developing tissue engineered skin substitutes, there have been numerous efforts as to mimic structural and functional aspects of the native ECM. Once placed it in the wound, the tissue engineered skin substitute would be able to withstand the harsh proteolytic wound micro environment and act as a temporary matrix that allows cell infiltration so that normal cellular activity can occur to promote matrix deposition, angiogenesis, and ultimately wound healing. 77 Table 3 showed the list of the commercial tissue-engineered skin substitutes without living cells and their applications with approval by US Food and Drug Administration (FDA).
Table 3.
Tissue-engineered skin substitutes without living cells.
| Product | Description | Indication | Nature of FDA Approval(s) and year(s) |
|---|---|---|---|
| Acellular skin substitute | |||
| Integra™ | • Acellular • Bilayer substitutes that contains epidermal substitute of silicone polymer polysiloxane on top of dermal matrix made of bovine collagen and chondroitin-6 sulfate or glycosaminoglycan (biosynthetic) • Temporary |
• For treatment of partial- or full-thickness burns | PMA-(1996) 510(k)-(2002) |
| Biobrane™ | • Ultrathin silicone as epidermal and nylon membrane as
dermal chemically bound to porcine collagen
(biosynthetic) • Temporary |
• For treatment of partial-thickness burns and wounds | 510(k)-(2008) |
| Alloderm™ | • Skin tissue from cadavers • Acellular (freeze-dried) allogeneic dermis • Duration of contact- permanent |
• For treatment of full- and partial-thickness wounds, Burns | HCT/Ps-(2011) |
| Oasis™ | • Acellular porcine small intestine
submucosa • Matrix composed of acellular collagen acts as a wound covering and dermal scaffold for tissue growth |
• For treatment of partial- and full-thickness pressure, venous and diabetic wounds, burns | 510(k)-(2006) |
| EZ-Derm™ | • Aldehyde-crosslinked porcine dermal collagen to provide strength and durability | • For treatment of partial- and full- thickness pressure, venous and diabetic wounds, burns | 510(k)-(1994) |
| Permacol™ (Covidien) | • Composed of cross-linked porcine dermal
collagen. • Crosslinking improves the tensile strength and long-term durability, but decreases pliability (Xenogenic) |
• Soft tissue repair | 510(k)-(2006) |
| FortaFlex™ | • Acellular porcine small intestine submucosa | • For treatment of full- and partial-thickness burns, venous and diabetic ulcers | 510(k)-(2002) |
| Repliform™ | • Acellular human dermal allograft | • Urological plastic surgery applications | Discontinued |
| Cymetra™ | • Micronized particulate acellular cadaveric dermal matrix | • Wound filler in plastic surgery | HCT/Ps-(2009) |
| Matriderm™ | • Acellular scaffold made with bovine collagen types I, III, V and elastin | • For treatment of partial- or full-thickness burns | 510(k) -(2000) |
| DermACELL™ | • Decellularized regenerative human tissue matrix allograft | • Chronic non-healing wound | HCT/Ps - (2011) |
| DermaMatrix™ | • Freeze-dried acellular dermis (allograft) derived from donated human skin tissue. | • Replacement, repair or reinforcement of soft tissues for grafting procedure | No FDA approval |
| DermaPure™ | • A single layer decellularized human dermal allograft | • For the treatment of acute and chronic wounds | 510(k) - (2016) |
| Graftjacket® | • Acellular regenerative tissue matrix that has been processed from human skin | • For treatment of full- and partial-thickness chronic ulcers | HCT/Ps - (2006) |
| Strattice™ | • Non-cross-linked porcine-derived acellular dermal matrix (xenogenic) | • Support tissue regeneration | 510(k) - (2007) |
| XenoMem™ | • Decellularized porcine peritoneal membrane | • For treatment of partial and full- thickness wounds; venous ulcers, diabetic ulcers, chronic vascular ulcers. | 510(k) - (2015) |
| Allopatch® | • Aseptically processed human reticular dermal tissue for use as a chronic or acute wound covering. | • For the treatment of acute wounds, chronic wounds, diabetic ulcers, pressure ulcers. | - |
Secretomes
Secretomes refers to the used/waste/spent medium of cultured cells after specific incubation time that contains proteins, includes growth factors, cytokines, chemokines, and ECM secreted by the cells. Besides that, small molecules including metabolites, ions, peptides, microvesicles, exosomes, etc. are also released by the cells. Proteins secretion is one of the most fundamental biological processes as they are involved in the regulation of numerous cellular functions. 14 The use of secretomes is attractive because it can be used allogenically and contains proteins that enhance cell proliferation, differentiation, migration and tissue repair. Several studies have revealed that the secretory proteins in secretomes are involved in intercellular connection and have substantial in regulating biological effects. 78 This is due to the properties of living cells being unable to interact directly with foreign material but require attachment to soluble matrix proteins to establish cellular interaction. 79 Secretomes analysis also identifies the proteins in CM, which can be used as biomarker identification for specific diseases and in drug identification and development. 80 Therefore, studies on secretomes provide insight into tissue engineering and regenerative medicine.
Secretomes is collected from various cell types after exposure to serum-free medium or hypoxic conditions. More than 10 sources of mesenchymal stem cells (MSC), were used to treat skin conditions including isolated from bone marrow (BM), umbilical cord or Wharton’s jelly, are the most common cells used in the study of secretomes. The umbilical cord or Wharton jelly mesenchymal stem cells (MSCs) is the easiest obtainable cell source which has known with low immunogenicity thus give advantages to wound healing process for allogenic application. 81
The secretomes of cells may also vary depending on the age of the cells. 82 Transplanted MSC secrete growth factors and cytokines thus create a better microenvironment for tissue regeneration.1,83,84 CM from MSC (MSC-CM) contains various ECM components (e.g. collagen, fibronectin, lumican, periostin), growth factors (e.g. bFGF, TGF-β1, pigment epithelium-derived factor [PEDF]), cytokines and chemokines.1,85,86 The secretory proteins enhance cellular migration, proliferation, and angiogenesis, and accelerate wound healing in vitro and in vivo.87 –89 Interestingly, the factors also have anti-inflammatory, anti-fibrotic and immunomodulatory actions. In a diabetes-like microenvironment, MSC-CM can reduce glucose levels, activate extracellular signaling, and promote keratinocyte proliferation and migration. 8
Another alternative to get the secretomes is by using the human adipose-derived stem cells (ADSC) due to the ease of access in human tissue and the great potential of secretory proteins in stem cell therapy.90 –92 ADSC-CM is good for wound healing therapy because it contains growth factors and cytokines vital to wound healing by promoting granulation and vascularization and enhancing macrophage recruitment. 93 The growth factors include FGF, TGF-β1, and PDGF, which promote ECM synthesis, deposition, and organization of collagen and fibronectin besides contributing to cell signaling and tissue remodeling. 94 The possible signaling pathways stimulated by ADSC-CM on skin cells are involved in cell motility, migration, differentiation, response to wound injury, and angiogenesis. 85 Moreover, the secretory factors in ADSC-CM such as VEGF, HGF and IGF have therapeutic effects on hair regeneration, promoting hair growth in a clinical study. 2
Although in vitro and animal models have their place, by far the better models are based on humans that provide physiologically accurate mechanisms on which new treatments. 95 Most recently, secretomes have been used in pre-clinical studies as a substitute for numerous cellular based therapies including wound healing. It is accepted that the use of human models offers the best opportunity to understand the factors that influence secretomes in wound healing as well as to evaluate efficacy of treatments applied to wounds. Recent clinical study has shown the wound healing process of a 17-year-old man treated with Wharton’s Jelly-derived MSCs-CM topical gel improved significantly, the lesion has reduced in size and turned into a closed wound. 96
Embryonic stem cells (ESC) also have the potential to secrete growth factors in secretomes, which is essential in angiogenesis and wound healing. Topical application and subcutaneous injection of ESC-CM into wounds accelerate wound healing via granulation tissue formation and re-epithelialization and increase the regenerated skin tensile strength. Furthermore, ESC-CM enhances keratinocyte and fibroblast proliferation and migration in vitro. 2 Moreover, dental pulp stem cells (DPSC), which is an available stem cell source, secrete mediators that enhance angiogenesis, ECM synthesis and deposition and activate fibroblast proliferation and migration.97 –99
Keratinocyte CM (KCM) has favorable effects in regulating ECM degradation and remodeling. The mediators expressed by keratinocytes include IL-1, IL-6, IL-7, IL-8, IL-10, IL-12, IL-15, IL-18 IL-20; TNF-α, interferon (IFN), TGF-β1, and PDGF. Keratinocytes indirectly stimulate the expression of autocrine anti-fibrogenic factor to control deposition of ECM which may cause scarring formation during healing.100,101 The scar formation or contracture during healing should be controlled because it affects the patient’s quality of life.
Interestingly, secretomes is rich in various growth factors, cytokines and tissue regenerative agents secreted by the stem cells that will satisfy regulatory requirements. This is because the cell’s secreted products are likely to have less risk or safety issues than stem cell therapy products. The most common secreted factors found in secretomes collected from stem cells are VEGF, PDGF, Hepatocyte growth factor (HGF), bFGF, Macrophage stimulating protein (MSP), Keratinocyte growth factor (KGF), Insulin-like growth factor 1 (IGF-1), and many more. 102 These secreted factors play essential role in regeneration and angiogenesis during healing process.
The different microenvironments for preconditioning different cell types yield different secretory profile outcomes. Secretomes from different cell sources was studied extensively to evaluate the potential of secretomes on skin regeneration and wound healing. In order to establish a suitable method for collecting the enriched secreted proteins from cells, optimization of the pre-conditioned environment is important including the culture medium, cell confluence and incubation time. Therefore, medium that was commonly selected were due to their role on the cell growth and their potential to enhance the secretion of essential proteins into culture medium. 103 Table 4 shows the secretomes derived from difference sources and the relevant effects involved in wound healing. Though, the preparation, composition and concentration of the secreted proteins as well differ with culture conditions and environmental factors. One of the extracellular membrane vesicles is exosome which secreted from various cells and contain proteins, lipids and nucleic acids to coordinate intercellular communication.
Table 4.
Secretomes derived from different cell sources that are used in wound healing.
| Cell sources of secretomes | Culture medium | Preparation of secretomes | Secretome contents | Application/outcome | References |
|---|---|---|---|---|---|
| • Mesenchymal Stem Cells-CM • Bone marrow-CM • Umbilical cord -CM or Wharton’s jelly-CM |
• Serum free DMEM/F12 medium supplemented with insulin
transferring selenium (ITS-X, Invitrogen) and
antibiotics • Serum-free DMEM |
• Incubated for 24, 48, and 72 h, cellular debris was
centrifuged and filtrated. • Then, it was frozen and stored at −80°C until use |
• α-SMA • Collagen type-I |
• In-vitro skin wound healing model. • Promote wound repair • Induce hair follicle regeneration. • Faster wound closure. • Less granulation tissue area. • More neovascularization • Increased proliferation and migration of dermal fibroblasts. |
Dong et al., 1 Shrestha et al., 6 Walter et al., 87 Mishra et al., 104 Kim et al., 105 Li et al. 106 |
| • Adipose Derived Stem Cells-CM | • Serum-free DMEM • Serum free DMEM/F12 medium |
• Incubated for 24 h. • Hypoxic conditions with 2% oxygen for 2 week. • Micro- filtered and freeze-dried |
• Collagen type-I • Ell3 |
• In vitro skin wound healing model. • Promote hair growth in vitro |
Shin et al., 2 Zhou et al., 107 Lee et al., 108 Kim et al. 109 |
| • Keratinocyte-conditioned medium (KCM) | • Keratinocytes serum free medium supplemented with bovine
pituitary extract and epidermal growth
factor. • Keratinocytes medium (CnT basal medium1 with supplements A, B, C; CELLnTEC) |
• Incubated for 24 h and 48 h, filtered and stored at −20°C until use | • Collagen type-I • β-actin • Pro-α1(I) |
• Regulate ECM collagen type I. • Promote viability of dermal fibroblast. • However, no effect on the fibroblast expansion |
Ghaffari et al., 101 Bukowska et al. 110 |
| • Fibroblast-conditioned medium (F-CM) | • Serum-free high-glucose DMEM supplemented with 1% P/S F-CM | • Incubated for 48 h • Centrifuged at 300 × g, 5 min • Filtration using 0.2 μm syringe filter |
• Pro-collagen type I | • Accelerate wound contraction and re-epithelialization | Hur et al. 94 |
| • Dermal Fibroblast Conditioned medium (DFCM) | • Serum-free keratinocyte-specific media (KM1 or KM2) and
serum-free fibroblast-specific medium (FM). • KM1 (Gibco, EpiLife™ Medium), KM2 (Gibco, Defined Keratinocyte Serum-free Medium), FM (Sigma, F12: DMEM without serum) |
• Incubated for 72 h, centrifuged to remove the cell debris, concentrated by ultrafiltration using centrifugal filter units with cut-off 1 kDa (Milipore, MA), filtration using 0.2 μm syringe filter, frozen and stored at −80°C until use. | • Fibronectin • Serotransferrin • Serpine • Collagen type-I |
• Enhances cells attachment, proliferation and migration | Maarof et al., 111 Maarof et al., 112 Maarof et al. 113 |
| • Multipotent adult progenitor cells-conditioned medium (MAPC-CM) | • MAPC culture media, low-glucose DMEM (Thermo Fisher Scientific, VIC, Australia) | • Incubated for 24 h under hypoxic
conditions. • Centrifuged at 350 × g for 10 min and sterilized using 0.22 μm filters. |
• Interleukins (IL): IL-1β, IL-2, IL-6,
IL-8 • MCP-1 • FGF-2 • VEGF • HGF • MMP-1 |
• Enhances collagens I and III deposition of
fibroblasts. • Increase the proliferation and migration of fibroblasts, keratinocytes and endothelial cells. |
Ahangar et al. 114 |
| • Antler stem cell-conditioned medium (ASC-CM) | • Cultured in DMEM + 10% FBS (Gibco, Australia), 500 U/mL penicillin, and 500 μg/mL streptomycin | • Incubated for 48 h, centrifuged at 1000 × g, and filtered using 0.22-μm filters. | • Col3A1/Col1A2 • TGF-β3/TGF-β1 |
• Accelerate wound closure rate and enhanced quality of wound healing. | Rong et al., 115 Seo et al. 116 |
Extracellular vesicles (EVs) and exosomes
Tissue engineering and regenerative medicine are strongly associated with the use of controlled cells for wound healing therapy. An important recent development in the understanding of intercellular communication is the appreciation of the role of extracellular vesicles (EVs). The EVs consist of variety of subtypes, including of exosomes, microvesicles (MVs), ectosomes, oncosomes and apoptotic bodies where they are offered unique potential to be used as a delivery vehicle in personalized medicine. 117 Its role to be an important cell-based component therapy and become a primary design consideration in tissue engineering specifically as the potential impact of culture conditions and cell-biomaterials.118,119 Interestingly in the field of EVs research, the starting material for EVs isolation is cell culture CM. Furthermore, EVs isolation from cell culture and EVs production as well are constantly involve the use of CM which contains EVs released from cultured cells along with other proteins and metabolites. 120 Thus, most researchers added fetal bovine serum (FBS) as a supplement in the culture media as to keep the culture cells in a healthy condition so that they can continue to release EVs. Regarding the methods used for the isolation and purification of EVs, the most widely used approach is ultracentrifugation, complemented by other techniques such as precipitation, size exclusion chromatography, affinity isolation and density gradient centrifugation. 121
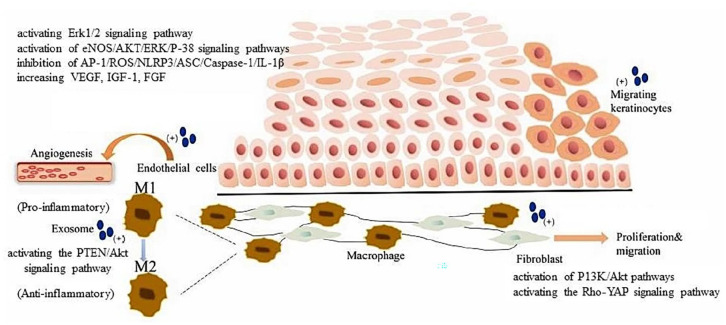
As mentioned above, exosomes are one of the several subtypes from various of membrane vesicles and have unique characteristics including specialized components and biogenesis. 122 Basically, they are called EVs and their diameter ranges from 30 to 150 nm. Previous studies proved that exosomes are participants in intercellular communication and also indicated that exosomes can diagnose diseases and predict prognosis as biomarkers.123,124 Furthermore, exosomes also able to modulate the function of cells which involved in wound healing in particularly promote neovascular growth,125,126 stimulate collagen deposition 127 and inhibit inflammation, 128 thus accelerating wound healing. Recently, Li and Wu 124 reported on the mechanism of exosomes in the cells involved in diabetic wound healing including endothelial cells, fibroblasts, macrophages and keratinocytes. Figure 2 represented the mechanism by which exosomes regulate the processes in order to enhance and promote diabetic wound healing.
Figure 2.
Exosomes and important cells involved in diabetic wound healing. Exosomes can promote endothelial cell function recovery and angiogenesis through activating the Erk1/2signaling pathway or activation of eNOS/AKT/ERK/P-38 signaling pathways, inhibition of AP-1/ROS/NLRP3/ASC/Caspase-1/IL-1β, and increasing the secretion of VEGF, IGF-1, and FGF. Exosomes inhibit inflammation by promoting phenotypic changes of macrophages by activating the PTEN/Akt signaling pathway. Exosomes promote proliferation and migration of fibroblasts by activation of PI3K/Akt pathways or activating the Rho-YAP signaling pathway exosomes promote the proliferation and migration of keratinocytes. The figure is reprinted (adapted) with permission from reference 124 under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Application of secretomes
The secretomes have a promising prospect for tissue engineering and regenerative medicine due to the abundance of factors that can enhance tissues and organs’ regeneration. Several studies on stem cell-derived secretory factors showed that secreted factors alone, without stem cells can promote tissue repair when applied in different conditions. 14 The secretomes contain cell-secreted products but without cellular components demonstrated not only for therapeutic benefits for regenerative treatment application meanwhile, the cell free therapy is relatively safe to human body.
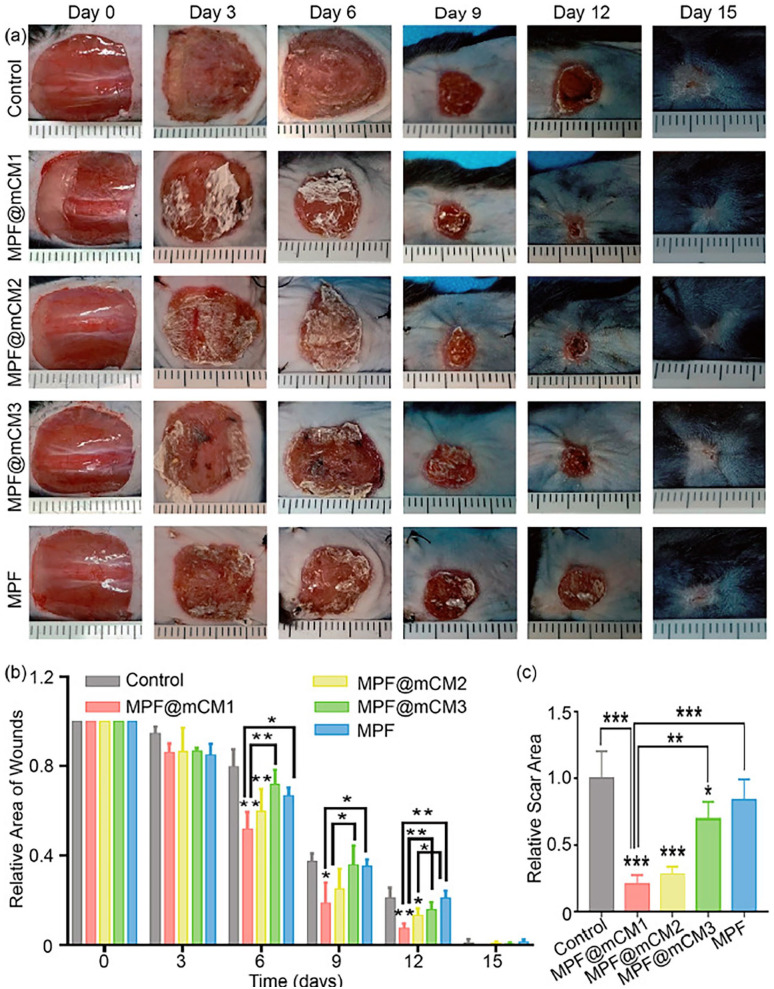
Recently, a study that pioneered in secretomes with biomaterials showed significant acceleration in wound closure and improved regeneration outcome. Based on the above-mentioned promoting regeneration effect, a study proposed by Chen et al. demonstrated that the use of secretomes to treat wounds during in vivo assays significantly accelerated wound closure and improved regeneration outcome as shown in Figure 3. Wounds of all groups were almost closed after 15 days. They were indicated that secretomes had significant effect on promoting normal skin regeneration, inhibiting fibrosis, and quickly restoring the appearance and function of damaged skin in vivo. 129 Gomes et al. demonstrate secretome derived from adipose tissue has shown to have regenerative properties by inducing neurite outgrowth. 130 In some research, it shows that MSC-CM can intensify migration of skin cells in in vitro scratch assays. 94 Besides that, the PEDF in MSC-CM play roles in enhancing migration of fibroblasts to the injured tissues. MSC-CM also have a significant effects on the keratinocytes migration and proliferation by activating the extracellular signal regulated kinase (ERK) signaling pathway in diabetes-like microenvironment. 14 Therefore, preconditioning of MSCs or any related cells in the suitable microenvironment are important to exploit the therapeutic potential of the secretomes in wound healing.
Figure 3.
Secretomes promoted wound healing and inhibits scar formation in vivo. (a) Compared with the control and MPF, MPF@CM promotes skin regeneration in vivo. Statistical analysis of the healing of (b) skin defect and (c) scar area (Average ± SD, n = 3 for each group, *denotes p < 0.05, **denotes p < 0.01, and ***denotes p < 0.0001 by One-way ANOVA followed by Tukey’s multiple comparison test). Figure (a–c) reprinted (adapted) with permission from reference 129 under the terms of the CC-BY-NC-ND license (http>//creativecommons.org/license/BY-NC-ND/4.0/).
There are several advantages of secretomes compared to the use of cells where it can be manufactured and formulated in various form depending on the suitability, application, and can be packed or transported more easily. Since the collected secretomes are in a liquid form, a scaffold is required as a carrier for the secretomes to be used for skin treatment. The scaffold is one of the main components of a delivery system for sustained release of supplementary proteins. The scaffold also can provide a native microenvironment like the ECM to support tissue regeneration.131,132 Accordingly, a scaffold fortified with supplementary proteins could be suitable for fabricating acellular tissue substitutes, for example an acellular skin patch. A scaffold is fabricated using biological or synthetic biomaterials to regenerate different tissues and organs. 133 Biomaterials interact with biological systems to repair, maintain or replace any tissue or organ to restore the body function. 134 The selection of the fabrication method and biomaterial is crucial for producing a scaffold that mimics the desired native tissue.
To the best of our knowledge, secretomes from MSC involves collagen synthesis which suggests that MSC-CM may also become a potential candidate for preventing UV-induced skin damage. 129 Collagen is a dominant protein found in the human body, including in the skin, tendons, and ligaments.135 –137 The ECM components are essential in providing the mechanical strength and extracellular architecture that support cellular growth, migration and differentiation for tissue regeneration and healing. 138 There are 29 types of collagens; type I collagen is the main collagen found in the body. Type I collagen is found in the skin, tendon, and bone, meanwhile type III collagen is found alongside it. Type II collagen is the main component of cartilage tissue, type IV collagen forms the basal lamina and type V collagen is present in cell surfaces such as hair and placenta. 139 Due to its properties such as biocompatibility, biodegradability and low immunogenicity, collagen is widely used in tissue engineering.140,141 Many collagen scaffolds are available for in vivo use, such as skin replacement, tissue fillers and artificial vascular structures.
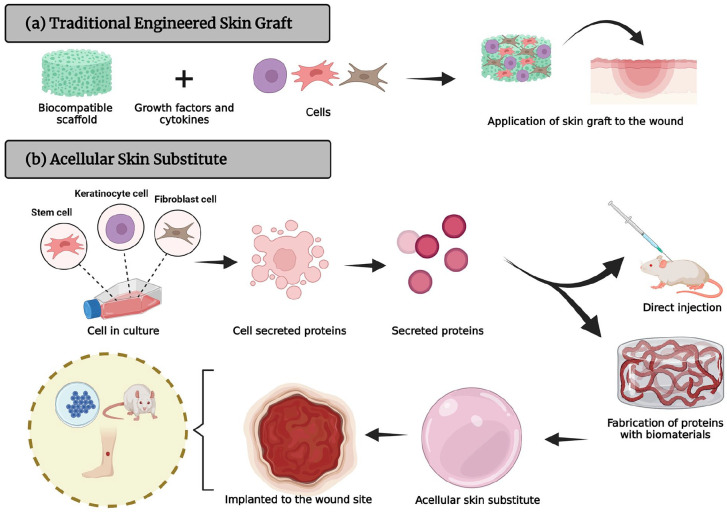
Collagen can be extracted from human cadavers or mammalian sources (such as bovine or porcine skin, tendon, or bone), fish and marine species, amphibians, plants or recombinant sources 138 and is widely used in biomedical and clinical applications. However, it stimulates the immune response in patients. 142 In addition, there is the risk of prion transmission such as that responsible for bovine spongiform encephalopathy (BSE), foot and mouth disease (FMD), and transmissible spongiform encephalopathy (TSE). 143 For that reason, alternative animals such as goat or sheep, which are less susceptible to infectious diseases and acceptable to all communities, are suitable for collagen extraction. 144 Type I collagen is the major collagen extracted from sheep tendon. 145 Marine collagen could also be a potential source of type I collagen for clinical use, nevertheless extensive studies need to be performed to ensure its biocompatibility. A three-dimensional (3D) tissue model is useful to simulate the physiology of the cells in in vivo environment to develop interaction between cell, protein, and materials. Collagen are the principle structural component of ECM cross-linked networks and they can be designed into many forms (e.g. sheets, tubes, sponges, powders, fleeces, injectable solutions, hydrogel, dispersions) for different purposes.146,147 The secretomes can be incorporated with biomaterials to fabricate a 3D acellular skin patch as an alternative approach for immediate treatment of skin loss, as illustrated in Figure 4. The role of collagen in wound healing is to attract fibroblasts and promote the deposition of new collagen into the wound site. In the aspect of skin substitute toward wound healing and tissue engineering, collagen-based dressing helps stimulate new tissue growth, while promoting autolytic debridement, promote cell proliferation, angiogenesis, re-epithelialization, provide a non-immunogenic, resorbable scaffold for cellular migration and matrix deposition, guide organization of ECM deposition.148,149 There is no doubt that collagen compounds meet many of these criteria and have several distinct advantages because they are biocompatible and non-toxic to various types of tissues.
Figure 4.
A staged schematic of (a) a traditional engineered skin graft implanted onto the injured skin formation and (b) fabrication of secretomes with biomaterials for of 3D acellular skin substitute.
Wnt-CM that is derived from umbilical cord-MSC (UC-MSC) was capable of enhancing the healing of full-thickness skin wounds in mice. 1 Previously, study using dermal fibroblasts conditioned medium (DFCM) that was produced by culturing human dermal fibroblasts (HDF) showed the presence of multiple proteins that enhance the expansion of keratinocytes and accelerate wound healing in vitro and in vivo .111 –113 Study by Shrestha et al. 6 also showed that the transplantation of UC-MSC and their secretomes to accelerate wound closure in diabetic mice which provided a detail account on the common use of secretomes in wound healing. The researchers had injected US-MSC and its’ secretomes subcutaneously around full-thickness dermal wound that were created on the diabetic mice. The wound closure was significantly expedited as compared to control group and the healing process was consistent until 14 days. 1
Adult stem cells were the main cell source used in studies of tissue regeneration. These cells are mostly originated from hematopoietic, mesenchymal, epithelial, and neural cell lineages. MSC are the most generally used source in cell therapy due to its high proliferation ability, paracrine effect, and multipotent differentiation potential immunomodulatory capacity.150,151 The use of stem cells in treating tissue regeneration have yield high efficacy results due to stem cells’ ability to facilitate the healing of skin wounds by enhancing neovascularization and promoting microvascular remodeling.152,153 Study by Hashemi et al. 154 showed the route of administration of MSC and secretomes in diabetic mice was done by injecting intraperitoneally or intravenously into mice. The outcome for both administrations showed the decrease in blood glucose, recover pancreatic islets, and increase the levels of insulin-producing cells. Previous study on DFCM fortified with collagen hydrogel as an acellular 3D skin patch demonstrated the possibility of using allogenic fibroblast secretory factors for the immediate treatment of full-thickness skin loss X91. Another study by Zhao et al. 155 showed that the administration of MSC-CM via intranasal has therapeutic effect in stroke rat’s model. The MSC-CM was administrated 24 h after middle cerebral artery occlusion in rats and the results showed significantly enhances blood brain barrier functional integrity and promotes functional outcome.
Since the secretomes only consists of cell secreted proteins, the used of secretomes for treatment have low or no risk of Graft Versus Host Disease (GvHD) and side effects, provided that the source tissue donor was properly screened, tested and are free from infectious diseases. 129 However, other issues still linger regarding secretomes on its uses, disadvantages and known issues regarding it.
Underlying mechanism of using secretomes in wound healing
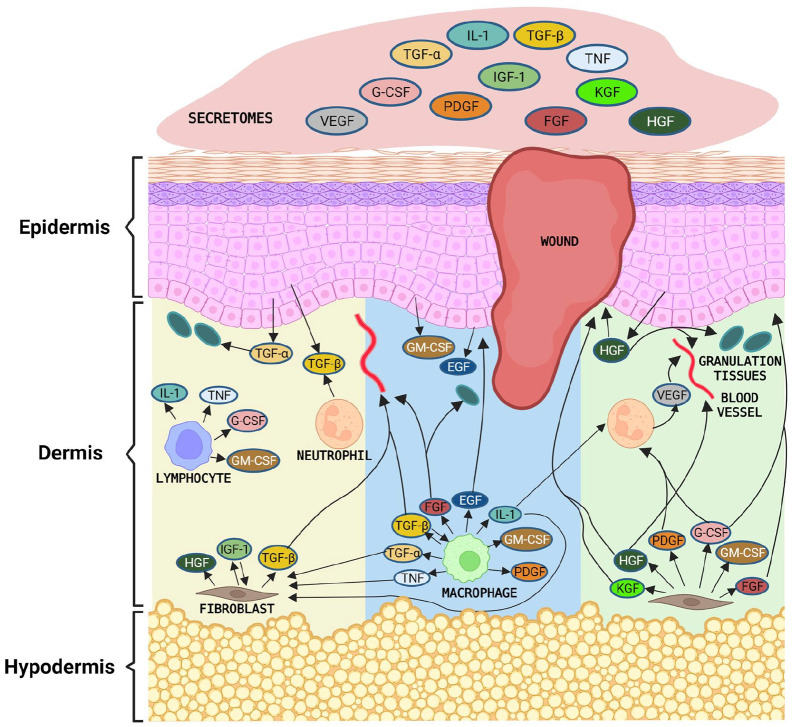
In general, skin wound healing process will takes around 2 weeks depending on the wound severity either acute or chronic wounds.156,157 Once injury occurs, the blood vessel damage and become leakage of blood constituents into the wound site. Hemostasis begins immediately after wounding. The migration of inflammatory cells into the wound by chemotaxis starts with the infiltration of neutrophils, macrophages and lymphocytes. 158 These cells are a major source of growth factors through phosphoinositide 3-kinase (PI3K) PI3K/Akt, Janus kinase, Signal Transducer and Activator of Transcription (Jak-STAT) pathways. For instance, neutrophils initiate VEGF and TGF-β, meanwhile lymphocytes initiate tumor necrosis factor (TNF), granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF), whereas IL-1 and macrophage secretes bFGF, EGF, PDGF, GM-CSF, TGF-α, TGF-β, IL-1, and TNF. However, the duration of wound healing process depends on the individuals as the expression of these groups of cytokines and growth factors.
The introduction of secretomes to the site of injury will accelerate the recovery process. Accordingly apart from the host tissue, secretomes have a wide range of cytokines and growth factor which are directly related to the wound healing development. Following that, angiogenesis is the next phase of recovery, whereby the molecules such as VEGF, bFGF, EGF, and TGF-β. They are promote new blood vessel, sustain the newly formed granulation tissue and help in the survival of endogenous keratinocytes. 98 Meanwhile for the final phase of wound healing process, growth factors including EGF, GM-CSF, and hepatocyte growth factor (HGF) will prompt keratinocytes to migrate from the basal population around the wound edge to over lesion and differentiate into squamous keratinizing epidermal cells 69 (Figure 5).
Figure 5.
Mechanism of wound healing using secretomes. The stem cell secretomes may promote wound healing by stimulating the proliferative and migratory abilities of skin cells.
Ethics, drawbacks, and issues of secretomes
The focus of using stem cells is not only for alternative treatments of wound healing and cures but also for other diseases. However, this process is interrupted by important ethical debates. Side effects and potential risks do come with the uses of stem cells, and many of these risks cannot be fully solved with the use of secretomes. There are a variety of other ethics and regulatory issues associated with the stem cell research and treatment. These ethical matters will affect the donors, scientific requirement and qualification for clinical testing, translation of stem cell-based therapies from research to clinical use, and appropriate and equitable access to emerging therapies. Besides the risks of stem cells, possible drawbacks can come from the secreted proteins on the conditioned medium.
Various studies used different cell types and doses of secretomes. This is in turn leads to a lack of standardization to produce secretomes, cultured medium and secretomes’s processing. The mode of delivery as well as volume of secretomes also plays a crucial role. This is vital to use secretomes for research or treatment on various human diseases. This can begin by focusing on a standard for production method such as the type of cell and number required to produce the secretomes. Other data that is important to factor in is the diseases that were treated or studied on, details on the patient (age, gender, weight,) or experiment model (species, age, gender, body weight) and what type of secretomes, volume of secretomes used and mode of application in their treatment. The standardization of secretomes can lead to mass production by pharmaceuticals companies for use in treatments. It is also crucial to note and analyze possible cytokine contents from certain secretomes. The cytokine content must be validated and have a reason for its use before application is done.
Scaling up production of secretomes—manufacturing challenges
Secretomes and its cell-secreted proteins have been shown by recent studies for its therapeutic purposes. Due to their distinctive characteristics and potential therapeutic use, there will be increasing in future demand. However, to produce a large scale of concentrated secretomes requires lots of cell expansion. This will increase production, workload and labor costs, as well as spaces for culturing processes. Furthermore, there are several scaling up processes use in manufacturing and each has its own challenges. For instance, in scaling up the production MSC, microcarriers in the stirred-tank bioreactors have been widely utilized instead of conventional planar tissue culture. Though, the used of microcarrier-expanded MSC were differ from standard culture vessel (dish- or flask) for expansion of cells in term of size, morphology, proliferation, viability, surface markers, gene expression, differentiation potential, and secretome, which will lead to alteration in immunosuppressive properties and clinical effectiveness of MSC.159,160 On the other hand, the scientists just have to depend on the donor samples in which they are coming with varies batch-to-batch samples. To reduce the variation, we have to pool all the batches. The major challenge in scaling up the cell cultures and cell-based therapy is to make sure their therapeutic potency is well-maintained. At the same time, it is to standardize bioprocessing parameters on the differentiation, migratory ability, and secretory function of the manufactured cell-based therapy. The others point is the mixture itself, in which including of proteins, metabolites and wastes secreted by cells. If the metabolites are increased, then it will be toxic to cells. Therefore, we are suggested to lyophilized secretomes to remove metabolites and small molecules.
Conclusions and future perspective
The utilization of skin substitutes and the use of secretomes with its secreted proteins can be promising in treating skin loss and aid in the healing process. Due to the limitation of treatment like SSG in treating significant skin loss, skin substitutes now can be considered as a treatment option. Besides, currently there is ongoing research on skin substitutes in treating skin loss that shows promising results. However, each skin substitute product has its advantages and disadvantages, depending on the patient’s clinical condition, hence more studies need to be done to analyze its therapeutic efficacy and safety. Secretomes and its secreted factor have lots of potential uses in tissue engineering and regenerative medicine. The released factor alone, without the stem cell, can trigger tissue repair in conditions involving skin defect. The use of stem cell secretomes in wound healing has potential as an alternative approach to overcoming the limitations of cell-based therapy. Nevertheless, there is still lack of standardization in the production of secretomes, studies on mode of delivery of secretomes and its secreted factor.
On the other hand, the future for secretomes is bright with its potential to be utilized properly in tissue regenerative medicine, wound closure, tissue engineered products and treatment of burn victims. The uses of secretomes outside tissue healing have also been carefully studied and making significant progress as well. This gives a good picture for secretomes to be used more broadly and is expected to become a more frequent sight in clinical treatments. If given a good appeal to pharmaceutical companies, we can see more commonly uses of secretomes in hospitals and private clinics.
Acknowledgments
All the authors would like to express our gratitude to the Faculty of Medicine, UKM for the guidance and resources to complete this review.
Footnotes
Author contributions: Conceptualization, M.M. and N.I.M.F.; methodology, N.I.M.F.; validation, M.M. and N.I.M.F.; formal analysis, N.I.M.F.; investigation, N.I.M.F.; data curation, N.I.M.F.; writing—original draft preparation, N.I.M.F., M.S.M.A.K.J, M.A.I.B.H., N.S.R. and S.A.S.; writing—review and editing M.M and M.B.F.; visualization, N.I.M.F.; supervision, M.M.; project administration, M.M.; funding acquisition, M.M.; BioRenders software, M.H.N. All authors have read and agreed to the published version of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded through the fundamental grant provided by Faculty of Medicine UKM, FF-2021-106.
Institutional review board statement: This study was approved by the National University of Malaysia (UKM) Research ethics committee with the approval code of UKM PPI/111/8/JEP-2021-205.
ORCID iD: Manira Maarof  https://orcid.org/0000-0002-2154-7852
https://orcid.org/0000-0002-2154-7852
References
- 1. Dong L, Hao H, Liu J, et al. A conditioned medium of umbilical cord mesenchymal stem cells overexpressing Wnt7a promotes wound repair and regeneration of hair follicles in mice. Stem Cells Int 2017; 2017: 3738071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shin H, Ryu HH, Kwon O, et al. Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: a retrospective case series study. Int J Dermatol 2015; 54: 730–735. [DOI] [PubMed] [Google Scholar]
- 3. Zhao Y, Zhang Z, Pan Z, et al. Advanced bioactive nanomaterials for biomedical applications. Exploration 2021; 1: 20210089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fadilah NIM, Rahman MBA, Yusof LM, et al. The therapeutic effect and in vivo assessment of palmitoyl-GDPH on the wound healing process. Pharmaceutics 2021; 13: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noverina R, Widowati W, Ayuningtyas W, et al. Growth factors profile in conditioned medium human adipose tissue-derived mesenchymal stem cells (CM-hATMSCs). Clin Nutr Exp 2019; 24: 34–44. [Google Scholar]
- 6. Shrestha C, Zhao L, Chen K, et al. Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. Int J Endocrinol 2013; 2013: 592454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fadilah NIM, Ahmad H, Abdul Rahman MB, et al. Synthesis and in vitro biological evaluations of novel tetrapeptide as therapeutic agent for wound treatment. J Saudi Chem Soc 2020; 24: 606–619. [Google Scholar]
- 8. Li M, Zhao Y, Hao H, et al. Mesenchymal stem cell–conditioned medium improves the proliferation and migration of keratinocytes in a diabetes-like microenvironment. Int J Low Extrem Wounds 2015; 14: 73–86. [DOI] [PubMed] [Google Scholar]
- 9. Atala A. Advances in tissue and organ replacement. Curr Stem Cell Res Ther 2008; 3: 21–31. [DOI] [PubMed] [Google Scholar]
- 10. Ahangar P, Mills SJ, Cowin AJ. Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. Int J Mol Sci 2020; 21: 7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benavides-Castellanos MP, Garzón-Orjuela N, Linero I. Effectiveness of mesenchymal stem cell-conditioned medium in bone regeneration in animal and human models: a systematic review and meta-analysis. Cell Regen 2020; 9(1): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bermudez MA, Sendon-Lago J, Seoane S, et al. Anti-inflammatory effect of conditioned medium from human uterine cervical stem cells in uveitis. Exp Eye Res 2016; 149: 84–92. [DOI] [PubMed] [Google Scholar]
- 13. Caneparo C, Baratange C, Chabaud S, et al. Conditioned medium produced by fibroblasts cultured in low oxygen pressure allows the formation of highly structured capillary-like networks in fibrin gels. Sci Rep 2020; 10(1): 9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int 2014; 2014: 965849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sole A, Spriet M, Galuppo LD, et al. Scintigraphic evaluation of intra-arterial and intravenous regional limb perfusion of allogeneic bone marrow-derived mesenchymal stem cells in the normal equine distal limb using (99m) Tc-HMPAO. Equine Vet J 2012; 44: 594–599. [DOI] [PubMed] [Google Scholar]
- 16. Mocchi M, Dotti S, Bue MD, et al. Veterinary regenerative medicine for musculoskeletal disorders: can mesenchymal stem/stromal cells and their secretome be the new frontier? Cells 2020; 9: 1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brusselaers N, Pirayesh A, Hoeksema H, et al. Skin replacement in burn wounds. J Trauma 2010; 68: 490–501. [DOI] [PubMed] [Google Scholar]
- 18. Paggiaro AO, Bastianelli R, Carvalho VF, et al. Is allograft skin, the gold-standard for burn skin substitute? A systematic literature review and meta-analysis. J Plast Reconstr Aesthet Surg 2019; 72: 1245–1253. [DOI] [PubMed] [Google Scholar]
- 19. Rezaei E, Beiraghi-Toosi A, Ahmadabadi A, et al. Can skin allograft occasionally act as a permanent coverage in deep burns? A pilot study. World journal of plastic surgery 2017; 6: 94. [PMC free article] [PubMed] [Google Scholar]
- 20. Purdue GF, Arnoldo BD, Hunt JL. Acute assessment and management of burn injuries. Phys Med Rehabil Clin N Am 2011; 22: 201–212. [DOI] [PubMed] [Google Scholar]
- 21. Liu T, Qiu C, Ben C, et al. One-step approach for full-thickness skin defect reconstruction in rats using minced split-thickness skin grafts with Pelnac overlay. Burns Trauma 2019; 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Donegan RJ, Schmidt BM, Blume PA. An overview of factors maximizing successful split-thickness skin grafting in diabetic wounds. Diabetic Foot Ankle 2014; 5: 24769. [Google Scholar]
- 23. Serebrakian AT, Pickrell BB, Varon DE, et al. Meta-analysis and systematic review of skin graft donor-site dressings with future guidelines. Plast Reconstr Surg Glob Open 2018; 6: e1928–20180924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Utami Nike D, Md Fadilah NI, Sallehuddin N, et al. Genipin-crosslinking effects on biomatrix development for Cutaneous wound healing: a concise review. Front Bioeng Biotechnol 2022; 10: 726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reddy S, El-Haddawi F, Fancourt M, et al. The incidence and risk factors for lower limb skin graft failure. Dermatol Res Pract 2014; 2014: 582080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Asuku M, Yu TC, Yan Q, et al. Split-thickness skin graft donor-site morbidity: a systematic literature review. Burns 2021; 47: 1525–1546. [DOI] [PubMed] [Google Scholar]
- 27. King A, Balaji S, Keswani SG. Biology and function of fetal and pediatric skin. Facial Plast Surg Clin North Am 2013; 21(1): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Farage MA, Miller KW, Elsner P, et al. Characteristics of the aging skin. Adv Wound Care 2013; 2: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goverman J, Kraft CT, Fagan S, et al. Back grafting the split-thickness skin graft donor site. J Burn Care Res 2017; 38: e443–e449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Figus A, Leon-Villapalos J, Philp B, et al. Severe multiple extensive postburn contractures: a simultaneous approach with total scar tissue excision and resurfacing with dermal regeneration template. J Burn Care Res 2007; 28: 913–917. [DOI] [PubMed] [Google Scholar]
- 31. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 1975; 6: 331–343. [DOI] [PubMed] [Google Scholar]
- 32. Lu KW, Khachemoune A. Skin substitutes for the management of mohs micrographic surgery wounds: a systematic review. Arch Dermatol Res. Epub ahead of print 15 February 2022. DOI: 10.1007/s00403-022-02327-1 [DOI] [PubMed] [Google Scholar]
- 33. Boyce ST, Lalley AL. Tissue engineering of skin and regenerative medicine for wound care. Burns Trauma 2018; 6: 4–20180124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Veen VC, van der Wal MB, van Leeuwen MC, et al. Biological background of dermal substitutes. Burns 2010; 36: 305–321. [DOI] [PubMed] [Google Scholar]
- 35. Fadilah NIM, Isa ILM, Zaman WSWK, et al. The effect of nanoparticle-incorporated natural-based biomaterials towards cells on activated pathways: A Systematic Review. Polymers 2022; 14: 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fadilah NIM, Ahmad H, Abd Rahman MF, et al. Electrospun poly (vinyl alcohol) nanofibers doped with mesoporous silica nanoparticles for controlled release of hydrophilic model drug. Malaysian J Anal Sci 2019; 23: 7. [Google Scholar]
- 37. Repanas A, Andriopoulou S, Glasmacher B. The significance of electrospinning as a method to create fibrous scaffolds for biomedical engineering and drug delivery applications. J Drug Deliv Sci Tech 2016; 31: 137–146. [Google Scholar]
- 38. Farokhi M, Mottaghitalab F, Fatahi Y, et al. Overview of silk fibroin use in wound dressings. Trends Biotechnol 2018; 36: 907–922. [DOI] [PubMed] [Google Scholar]
- 39. Carta T, Gawaziuk JP, Diaz-Abele J, et al. Properties of an ideal burn dressing: a survey of burn survivors and front-line burn healthcare providers. Burns 2019; 45: 364–368. [DOI] [PubMed] [Google Scholar]
- 40. Poonguzhali R, Khaleel Basha S, Sugantha Kumari V. Novel asymmetric chitosan/PVP/nanocellulose wound dressing: in vitro and in vivo evaluation. Int J Biol Macromol 2018; 112: 1300–1309. [DOI] [PubMed] [Google Scholar]
- 41. Chen Z, Lv Z, Zhang Z, et al. Advanced microfluidic devices for fabricating multi-structural hydrogel microsphere. Exploration 2021; 1: 20210036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nischwitz SP, Hofmann E, Kamolz LP. The ideal wound dressing - Beyond the ideal: a short comment on ‘Properties of an ideal burn dressing: a survey of burn survivors and front-line burn healthcare providers’ by Carta T., Gawaziuk J.P., et al. Burns 2019; 45: 1485–1486. [DOI] [PubMed] [Google Scholar]
- 43. Barrientos S, Stojadinovic O, Golinko MS, et al. Perspective Article: Growth factors and cytokines in wound healing. Wound Repair Regen 2008; 16: 585–601. [DOI] [PubMed] [Google Scholar]
- 44. Li Y, Fan J, Chen M, et al. Transforming Growth Factor-alpha: a major human serum factor that promotes human keratinocyte migration. J Investig Dermatol 2006; 126: 2096–2105. [DOI] [PubMed] [Google Scholar]
- 45. Raines EW, Dower SK, Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science 1989; 243: 393–396. [DOI] [PubMed] [Google Scholar]
- 46. Snyder D, Sullivan N, Margolis D, et al. AHRQ technology assessments. Skin substitutes for treating chronic wounds. Rockville, MD: Agency for Healthcare Research and Quality, 2020. [PubMed] [Google Scholar]
- 47. Falanga V. The chronic wound: impaired healing and solutions in the context of wound bed preparation. Blood Cells Mol Dis 2004; 32: 88–94. [DOI] [PubMed] [Google Scholar]
- 48. Krishnaswamy VR, Korrapati PS. Role of dermatopontin in re-epithelialization: implications on keratinocyte migration and proliferation. Sci Rep 2014; 4: 7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haase I, Evans R, Pofahl R, et al. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J Cell Sci 2003; 116: 3227–3238. [DOI] [PubMed] [Google Scholar]
- 50. Bhora FY, Dunkin BJ, Batzri S, et al. Effect of growth factors on cell proliferation and epithelialization in human skin. J Surg Res 1995; 59: 236–244. [DOI] [PubMed] [Google Scholar]
- 51. Shipley GD, Keeble WW, Hendrickson JE, et al. Growth of normal human keratinocytes and fibroblasts in serum-free medium is stimulated by acidic and basic fibroblast growth factor. J Cell Physiol 1989; 138: 511–518. [DOI] [PubMed] [Google Scholar]
- 52. Sogabe Y, Abe M, Yokoyama Y, et al. Basic fibroblast growth factor stimulates human keratinocyte motility by Rac activation. Wound Repair Regen 2006; 14: 457–462. [DOI] [PubMed] [Google Scholar]
- 53. Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma 2012; 2: 18–28. [PMC free article] [PubMed] [Google Scholar]
- 54. Pakyari M, Farrokhi A, Maharlooei MK, et al. Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care 2013; 2: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pierce GF, Mustoe TA, Altrock BW, et al. Role of platelet-derived growth factor in wound healing. J Cell Biochem 1991; 45: 319–326. [DOI] [PubMed] [Google Scholar]
- 56. Bao P, Kodra A, Tomic-Canic M, et al. The role of vascular endothelial growth factor in wound healing. J Surg Res 2009; 153: 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care 2014; 3: 647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sauder DN, Kilian PL, McLane JA, et al. Interleukin-1 enhances epidermal wound healing. Lymphokine Res 1990; 9: 465–473. [PubMed] [Google Scholar]
- 59. Ishida Y, Mukaida N, Kondo T. The roles of IL-1 receptor antagonist in skin wound healing. Inflamm Regen 2008; 28: 31–35. [Google Scholar]
- 60. Lin Z-Q, Kondo T, Ishida Y, et al. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol 2003; 73: 713–721. [DOI] [PubMed] [Google Scholar]
- 61. McFarland-Mancini MM, Funk HM, Paluch AM, et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol 2010; 184: 7219–7228. [DOI] [PubMed] [Google Scholar]
- 62. Ashcroft GS, Jeong M-J, Ashworth JJ, et al. Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen 2012; 20: 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng 2011; 2: 403–430. [DOI] [PubMed] [Google Scholar]
- 64. Auger FA, Lacroix D, Germain L. Skin substitutes and wound healing. Skin Pharmacol Physiol 2009; 22: 94–102. [DOI] [PubMed] [Google Scholar]
- 65. Halim AS, Khoo TL, Mohd Yussof SJ. Biologic and synthetic skin substitutes: an overview. Indian J Plast Surg 2010; 43: S23–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shakespeare PG. The role of skin substitutes in the treatment of burn injuries. Clin Dermatol 2005; 23: 413–418. [DOI] [PubMed] [Google Scholar]
- 67. Manira M, Khairul Anuar K, Seet WT, et al. Comparison of the effects between animal-derived trypsin and recombinant trypsin on human skin cells proliferation, gene and protein expression. Cell Tissue Bank 2014; 15: 41–49. [DOI] [PubMed] [Google Scholar]
- 68. MacNeil S. Progress and opportunities for tissue-engineered skin. Nature 2007; 445: 874–880. [DOI] [PubMed] [Google Scholar]
- 69. Metcalfe AD, Ferguson MW. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 2007; 4: 413–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Goodarzi P, Falahzadeh K, Nematizadeh M, et al. Tissue engineered skin substitutes. Cell Biol Transl Med 2018; 3: 143–188. [DOI] [PubMed] [Google Scholar]
- 71. Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care 2007; 20: 493–508. [DOI] [PubMed] [Google Scholar]
- 72. Kim JY, Suh W. Stem cell therapy for dermal wound healing. Int J Stem Cells 2010; 3: 29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Debels H, Hamdi M, Abberton K, et al. Dermal matrices and bioengineered skin substitutes: a critical review of current options. Plast Reconstr Surg Glob Open 2015; 3: e284–e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Alrubaiy L, Al-Rubaiy KK. Skin substitutes: a brief review of types and clinical applications. Oman Med J 2009; 24: 4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mohamed Haflah NH, Ng MH, Mohd Yunus MH, et al. The use of engineered bilayered skin (MyDerm–) in the management of massive skin defect in Grade III Gustilo-Anderson Open Fracture. Int J Low Extrem Wounds 2017; 16: 212–216. [DOI] [PubMed] [Google Scholar]
- 76. Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009; 17: 153–162. [DOI] [PubMed] [Google Scholar]
- 77. Cho H, Blatchley MR, Duh EJ, et al. Acellular and cellular approaches to improve diabetic wound healing. Adv Drug Deliv Rev 2019; 146: 267–288. [DOI] [PubMed] [Google Scholar]
- 78. Thorsell A, Faijerson J, Blomstrand F, et al. Proteome analysis of serum-containing conditioned medium from primary astrocyte cultures. J Proteomics Bioinform 2008; 1: 128–142. [Google Scholar]
- 79. Salmerón-Sánchez M, Altankov G. Cell-protein-material interaction in tissue engineering. Tissue engineering 2010; 1: 77–103. [Google Scholar]
- 80. Thio CL, Yusof R, Ashrafzadeh A, et al. Differential analysis of the secretome of WRL68 cells infected with the Chikungunya virus. PLoS One 2015; 10: e0129033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li C, Zhao H, Cheng L, et al. Allogeneic vs. Autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci 2021; 11: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Montero-Vilchez T, Sierra-Sánchez Sanchez-Diaz M, et al. Mesenchymal stromal cell-conditioned medium for skin diseases: a systematic review. Front Cell Dev Biol 2021; 9: 654210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wong VW, Levi B, Rajadas J, et al. Stem cell niches for skin regeneration. Int J Biomater 2012; 2012: 926059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis 2014; 10: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Collawn SS, Mobley JA, Banerjee NS, et al. Conditioned Media from adipose-derived stromal cells accelerates healing in 3-Dimensional skin cultures. Ann Plast Surg 2016; 76: 446–452. [DOI] [PubMed] [Google Scholar]
- 86. Sagaradze G, Grigorieva O, Nimiritsky P, et al. Conditioned medium from human mesenchymal stromal cells: towards the clinical translation. Int J Mol Sci 2019; 20: 1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Walter MN, Wright KT, Fuller HR, et al. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res 2010; 316: 1271–1281. [DOI] [PubMed] [Google Scholar]
- 88. Akram KM, Samad S, Spiteri MA, et al. Mesenchymal stem cells promote alveolar epithelial cell wound repair in vitro through distinct migratory and paracrine mechanisms. Respir Res 2013; 14: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tamari M, Nishino Y, Yamamoto N, et al. Acceleration of wound healing with stem cell-derived growth factors. Oral Craniofacial Tissue Eng 2011; 1: 181–187. [DOI] [PubMed] [Google Scholar]
- 90. Cheng K-H, Kuo T-L, Kuo K-K, et al. Human adipose-derived stem cells: isolation, characterization and current application in regeneration medicine. Genom Med Biomark Health Sci 2011; 3: 53–62. [Google Scholar]
- 91. Dai R, Wang Z, Samanipour R, et al. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int 2016; 2016: 6737345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Guo X, Schaudinn C, Blume-Peytavi U, et al. Effects of adipose-derived stem cells and their conditioned medium in a human ex vivo wound model. Cells 2022; 11: 1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Banyard DA, Salibian AA, Widgerow AD, et al. Implications for human adipose-derived stem cells in plastic surgery. J Cell Mol Med 2015; 19: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hur W, Lee HY, Min HS, et al. Regeneration of full-thickness skin defects by differentiated adipose-derived stem cells into fibroblast-like cells by fibroblast-conditioned medium. Stem Cell Res Ther 2017; 8: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wilhelm KP, Wilhelm D, Bielfeldt S. Models of wound healing: an emphasis on clinical studies. Skin Res Technol 2017; 23: 3–12. [DOI] [PubMed] [Google Scholar]
- 96. Chandra CC, Pratiwi YI, Tan ST. Potential application of Wharton’s jelly-derived mesenchymal stem cells conditioned medium (WJMSCs-CM) on delayed wound healing: a case report. J Pharm Res Int 2022; 34: 1–6. [Google Scholar]
- 97. Ueda M, Nishino Y. Cell-based cytokine therapy for Skin Rejuvenation. J Craniofac Surg 2010; 21: 1861–1866. [DOI] [PubMed] [Google Scholar]
- 98. Jayaraman P, Nathan P, Vasanthan P, et al. Stem cells conditioned medium: a new approach to skin wound healing management. Cell Biol Int 2013; 37: 1122–1128. [DOI] [PubMed] [Google Scholar]
- 99. Martínez-Sarrà E, Montori S, Gil-Recio C, et al. Human dental pulp pluripotent-like stem cells promote wound healing and muscle regeneration. Stem Cell Res Ther 2017; 8: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Martin P. Wound healing – aiming for perfect skin regeneration. Science 1997; 276: 75–81. [DOI] [PubMed] [Google Scholar]
- 101. Ghaffari A, Kilani RT, Ghahary A. Keratinocyte-conditioned media regulate collagen expression in dermal fibroblasts. J Investig Dermatol 2009; 129: 340–347. [DOI] [PubMed] [Google Scholar]
- 102. Mintz PJ, Huang K-W, Reebye V, et al. Exploiting human CD34+ stem cell-conditioned medium for tissue repair. Mol Ther 2014; 22: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Genier FS, Bizanek M, Webster TJ, et al. Increased viability of fibroblasts when pretreated with ceria nanoparticles during serum deprivation. Int J Nanomedicine 2018; 13: 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mishra PJ, Mishra PJ, Banerjee D. Cell-free derivatives from mesenchymal stem cells are effective in wound therapy. World J Stem Cells 2012; 4: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kim JY, Song S-H, Kim KL, et al. Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplant 2010; 19: 1635–1644. [DOI] [PubMed] [Google Scholar]
- 106. Li M, Luan F, Zhao Y, et al. Mesenchymal stem cell-conditioned medium accelerates wound healing with fewer scars. Int Wound J 2017; 14: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhou B-R, Xu Y, Guo S-L, et al. The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. Biomed Res Int 2013; 2013: 519126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lee J-Y, Oh N, Park K-S. Ell3 modulates the wound healing activity of conditioned medium of adipose-derived stem cells. Dev Reprod 2017; 21: 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kim MH, Wu WH, Choi JH, et al. Galectin-1 from conditioned medium of three-dimensional culture of adipose-derived stem cells accelerates migration and proliferation of human keratinocytes and fibroblasts. Wound Repair Regen 2018; 26: S9–S18. [DOI] [PubMed] [Google Scholar]
- 110. Bukowska J, Kopcewicz M, Kur-Piotrowska A, et al. Effect of TGFβ1, TGFβ3 and keratinocyte conditioned media on functional characteristics of dermal fibroblasts derived from reparative (Balb/c) and regenerative (Foxn1 deficient; nude) mouse models. Cell Tissue Res 2018; 374: 149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Maarof M, Lokanathan Y, Ruszymah HI, et al. Proteomic analysis of human dermal fibroblast conditioned medium (DFCM). Protein J 2018; 37: 589–607. [DOI] [PubMed] [Google Scholar]
- 112. Maarof M, Mh Busra MF, Lokanathan Y, et al. Safety and efficacy of dermal fibroblast conditioned medium (DFCM) fortified collagen hydrogel as acellular 3D skin patch. Drug Deliv Transl Res 2019; 9: 144–161. [DOI] [PubMed] [Google Scholar]
- 113. Maarof M, Chowdhury SR, Saim A, et al. Concentration dependent effect of human dermal fibroblast conditioned medium (DFCM) from three various origins on keratinocytes wound healing. Int J Mol Sci 2020; 21: 2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ahangar P, Mills SJ, Smith LE, et al. Human multipotent adult progenitor cell-conditioned medium improves wound healing through modulating inflammation and angiogenesis in mice. Stem Cell Res Ther 2020; 11: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rong X, Chu W, Zhang H, et al. Antler stem cell-conditioned medium stimulates regenerative wound healing in rats. Stem Cell Res Ther 2019; 10: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Seo M, Kim J-C, Kim H-K, et al. A novel secretory vesicle from deer antlerogenic mesenchymal stem cell-conditioned media (DaMSC-CM) promotes tissue regeneration. Stem Cells Int 2018; 2018: 3891404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lamichhane TN, Sokic S, Schardt JS, et al. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B Rev 2015; 21: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sahoo S, Klychko E, Thorne T, et al. Exosomes from human CD34+ stem cells mediate their proangiogenic paracrine activity. Circ Res 2011; 109: 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010; 4: 214–222. [DOI] [PubMed] [Google Scholar]
- 120. Pham CV, Midge S, Barua H, et al. Bovine extracellular vesicles contaminate human extracellular vesicles produced in cell culture conditioned medium when ‘exosome-depleted serum’ is utilised. Arch Biochem Biophys 2021; 708: 108963. [DOI] [PubMed] [Google Scholar]
- 121. Furi I, Momen-Heravi F, Szabo G. Extracellular vesicle isolation: present and future. Ann Transl Med 2017; 5: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yokoi A, Ochiya T. Exosomes and extracellular vesicles: rethinking the essential values in cancer biology. Semin Cancer Biol 2021; 74: 79–91. [DOI] [PubMed] [Google Scholar]
- 123. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 2020; 367: eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Li D, Wu N. Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabetes Res Clin Pract 2022; 187: 109882. [DOI] [PubMed] [Google Scholar]
- 125. Xu J, Bai S, Cao Y, et al. miRNA-221-3p in endothelial progenitor cell-derived exosomes accelerates skin wound healing in diabetic mice. Diabetes Metab Syndr Obes 2020; 13: 1259–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kim H, Wang SY, Kwak G, et al. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv Sci 2019; 6: 1900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wang J, Wu H, Peng Y, et al. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnol 2021; 19: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ti D, Hao H, Tong C, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med 2015; 13: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chen L, Cheng L, Wang Z, et al. Conditioned medium-electrospun fiber biomaterials for skin regeneration. Bioact Mater 2021; 6: 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Gomes ED, Mendes SS, Assunção-Silva RC, et al. Co-transplantation of adipose tissue-derived stromal cells and olfactory ensheathing cells for spinal cord injury repair. Stem Cells 2018; 36: 696–708. [DOI] [PubMed] [Google Scholar]
- 131. Zhu M, Li W, Dong X, et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat Commun 2019; 10: 4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Leng L, Ma J, Sun X, et al. Comprehensive proteomic atlas of skin biomatrix scaffolds reveals a supportive microenvironment for epidermal development. J Tissue Eng 2020; 11: 2041731420972310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zhou J, Zhang Z, Joseph J, et al. Biomaterials and nanomedicine for bone regeneration: Progress and future prospects. Exploration 2021; 1: 20210011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today 2011; 14: 88–95. [Google Scholar]
- 135. Cheema U, Ananta M, Mudera V. Collagen: applications of a natural polymer in regenerative medicine. In: Eberli D. (ed.) Regenerative medicine and tissue engineering-cells and biomaterials. London: IntechOpen, 2011, pp.287–300. [Google Scholar]
- 136. Busra FM, Chowdhury SR, Saim AB, et al. Genotoxicity and cytotoxicity of ovine collagen on human dermal fibroblasts. Saudi Med J 2011; 32: 1311–1312. [PubMed] [Google Scholar]
- 137. Awang MA, Firdaus MA, Busra MB, et al. Cytotoxic evaluation of biomechanically improved crosslinked ovine collagen on human dermal fibroblasts. Biomed Mater Eng 2014; 24: 1715–1724. [DOI] [PubMed] [Google Scholar]
- 138. Shoseyov O, Posen Y, Grynspan F. Human collagen produced in plants: more than just another molecule. Bioengineered 2014; 5: 49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials 2010; 3: 1863–1887. [Google Scholar]
- 140. Nair LS, Laurencin CT. Polymers as biomaterials for tissue engineering and controlled drug delivery. In: Lee K, Kaplan D. (eds) Tissue Engineering I. Berlin, Heidelberg: Springer, 2006, pp.47–90. [DOI] [PubMed] [Google Scholar]
- 141. Liu H, Wang C, Li C, et al. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv 2018; 8: 7533–7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Pollack SV. Silicone, fibre, and collagen implantation for facial lines and wrinkles. J Dermatol Surg Oncol 1990; 16: 957–961. [DOI] [PubMed] [Google Scholar]
- 143. Pammer J, Weninger W, Tschachler E. Human keratinocytes express cellular prion-related protein in vitro and during inflammatory skin diseases. Am J Pathol 1998; 153: 1353–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Banerjee I, Mishra D, Das T, et al. Caprine (goat) collagen: a potential biomaterial for skin tissue engineering. J Biomater Sci Polym Ed 2012; 23: 355–373. [DOI] [PubMed] [Google Scholar]
- 145. Fauzi MB, Lokanathan Y, Aminuddin BS, et al. Ovine tendon collagen: extraction, characterisation and fabrication of thin films for tissue engineering applications. Mater Sci Eng C Mater Biol Appl 2016; 68: 163–171. [DOI] [PubMed] [Google Scholar]
- 146. Masri S, Zawani M, Zulkiflee I, et al. Cellular interaction of human skin cells towards natural bioink via 3D-bioprinting technologies for chronic wound: a comprehensive review. Int J Mol Sci 2022; 23: 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bai H, Kyu-Cheol N, Wang Z, et al. Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers. J Tissue Eng 2020; 11: 2041731420947242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gould LJ. Topical collagen-based biomaterials for chronic wounds: rationale and clinical application. Adv Wound Care 2016; 5: 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sallehuddin N, Md Fadilah NI, Hwei NM, et al. Characterization and cytocompatibility of collagen-gelatin-elastin (CollaGee) acellular skin substitute towards human dermal fibroblasts: in vitro assessment. Biomedicines 2022; 10: 1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Trounson A, Thakar RG, Lomax G, et al. Clinical trials for stem cell therapies. BMC Med 2011; 9: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Liu S, Zhou J, Zhang X, et al. Strategies to optimize adult stem cell therapy for tissue regeneration. Int J Mol Sci 2016; 17: 982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Choi YS, Zhang Y, Xu M, et al. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell 2013; 13: 720–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Dulmovits BM, Herman IM. Microvascular remodeling and wound healing: a role for pericytes. Int J Biochem Cell Biol 2012; 44: 1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hashemi SM, Hassan ZM, Hossein-Khannazer N, et al. Investigating the route of administration and efficacy of adipose tissue-derived mesenchymal stem cells and conditioned medium in type 1 diabetic mice. Inflammopharmacology 2020; 28: 585–601. [DOI] [PubMed] [Google Scholar]
- 155. Zhao Q, Hu J, Xiang J, et al. Intranasal administration of human umbilical cord mesenchymal stem cells-conditioned medium enhances vascular remodeling after stroke. Brain Res 2015; 1624: 489–496. [DOI] [PubMed] [Google Scholar]
- 156. Szpaderska AM, Egozi EI, Gamelli RL, et al. The effect of thrombocytopenia on dermal wound healing. J Investig Dermatol 2003; 120: 1130–1137. [DOI] [PubMed] [Google Scholar]
- 157. Kim HS, Chen J, Wu LP, et al. Prevention of excessive scar formation using nanofibrous meshes made of biodegradable elastomer poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J Tissue Eng 2020; 11: 2041731420949332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Gosain A, DiPietro LA. Aging and wound healing. World J Surg 2004; 28: 321–326. [DOI] [PubMed] [Google Scholar]
- 159. Tsai A-C, Jeske R, Chen X, et al. Influence of microenvironment on mesenchymal stem cell therapeutic potency: from planar culture to microcarriers. Front Bioeng Biotechnol 2020; 8: 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Cherian DS, Bhuvan T, Meagher L, et al. Biological considerations in scaling up therapeutic cell manufacturing. Front Pharmacol 2020; 11: 654. [DOI] [PMC free article] [PubMed] [Google Scholar]