Abstract
Introduction:
About one-third of primary biliary cholangitis (PBC) patients do not exhibit complete response to ursodeoxycholic acid (UDCA). Some of these patients were reported to benefit from the combination therapy of fibrates and UDCA, but more clinical evidence is required. In this study, we conducted a randomized, controlled trial on the safety and efficacy of fenofibrate in the treatment of patients with PBC.
Methods:
Forty-eight PBC patients with incomplete response to UDCA were enrolled and randomly assigned to two groups (24 in the experiment group and 24 in the control group). For the experimental group, the patients were administered 13–15 mg/kg/day UDCA in combination with 200 mg/day fenofibrate. For the control group, the patients continued to receive UDCA at 13–15 mg/kg/day. The patients were followed up for at least 12 months. The serum levels of alkaline phosphatase (ALP), gamma-glutamyl transferase (γ-GT), aspartate aminotransferase (AST), and other biochemical parameters were measured at 3, 6, and 12 months during the trial to assess patient conditions.
Results:
At 12 months, 20.8% of the patients in the experimental group had all three indexes of serum ALP, γ-GT, and total bilirubin normalized, while 0% of patients in the control group reached the primary outcome (difference, 20.8 percentage points; 95% CI, 4.6–37.0). 54.2% of the patients had normal ALP levels in the experimental group and 4.2% in the control group (difference, 50 percentage points; 95% CI, 28.5–71.5). The experimental group had greater improvement of ALP (p < 0.001) and IgG (p = 0.026) than the control group. The biochemical indexes of the patients in the experimental group also significantly improved during the treatment of fenofibrate.
Conclusion:
Addition of fenofibrate can improve biochemical indexes of PBC patients who had an incomplete response to UDCA. Reversible elevation of serum creatine and transaminases is observed in some patients.
The trial was registered in the Chinese Clinical Trial Registry (ChiCTR) as ChiCTR1800020160 (protocol available online: http://www.chictr.org.cn/showproj.aspx?proj=32443).
Keywords: fenofibrate, primary biliary cholangitis, ursodeoxycholic acid
Introduction
Primary biliary cholangitis (PBC), also known as primary biliary cirrhosis before 2015, 1 is a cholestatic liver disease caused by the autoimmune destruction of the intrahepatic bile ducts. The pathology features nonsuppurative inflammation, destruction, and fibrosis of the bile canaliculi. 2 Patients slowly progress to cirrhosis and even liver failure requiring liver transplantation. Ursodeoxycholic acid (UDCA) is the first-line treatment for PBC. With the standard dose of UDCA (13–15 mg/day per kilogram of body weight), some patients can achieve complete biochemical response. These patients have been reported to have better prognosis. 3 However, only one-third of the patients responded completely to UDCA. For patients who cannot respond completely to UDCA, the disease progresses faster and the survival is worse when compared with responders. The response to UDCA is usually assessed after 1-year treatment. Our previous study 4 showed that the assessment could be performed at month 6 so that the patients with incomplete response can be differentiated and receive second-line treatment earlier. However, the treatment options for these patients are limited. In 2017, the FDA approved obeticholic acid, a synthetically modified derivative of chenodeoxycholic acid and potent farnesoid X receptor (FXR) agonist, as second-line treatment for patients with incomplete response to UDCA or intolerant to UDCA, but this drug is not yet available in China and many other countries. Therefore, the development of new therapies for these patients is urgently needed.
Fibrates are a group of amphipathic carboxylic acids that have been widely used as lipid-modulating drugs for dyslipidemia. They function as agonists for peroxisome proliferator-activated receptors (PPARs) and have an overall systemic lipid-lowering effect. There are three subtypes of PPARs, named as α, β, and γ. The distribution and physiological functions of the three subtypes vary, and different fibrates target different subtypes. Bezafibrate is a pan-agonist of all three PPAR subtypes, while the activity of fenofibrate is limited to PPARα, which is the dominant subtype found in the liver. 5 Accumulating evidence indicates that bezafibrate can improve biochemical response in PBC patients with incomplete response to UDCA.6–9 In particular, Corpechot et al. reported promising findings in a randomized, placebo-controlled trial of bezafibrate. One hundred patients were enrolled and randomly assigned to either the placebo group or the bezafibrate group. After a follow-up of 2 years, 31% of the patients in the bezafibrate group had exhibited a complete response. 10 A Japanese nationwide retrospective cohort study also showed the correlation between the use of UDCA and bezafibrate and a decrease in all-cause and liver-related mortality in PBC patients. 11 Ciprofibrate, a type of fibrate acting as PPAR α agonist, was also reported to show benefit in combination with UDCA for the nonresponders. It has also been reported that fenofibrate could help to reduce the enzyme levels and improve the conditions of PBC patients,12–16 but the trials were preliminary, with short follow-up periods and small sample sizes, often with no randomization or controls. In this study, we started a randomized, controlled trial in PBC patients with incomplete responses to UDCA to assess the safety and efficacy of fenofibrate for the treatment of PBC.
Methods
Participants
Patients aged 18–65 years diagnosed with PBC and exhibiting incomplete response to UDCA were recruited at the Peking Union Medical College Hospital (PUMCH, Beijing, China). The diagnosis of PBC was made according to the 2009 American Association for the Study of Liver Diseases practice guidelines for PBC and confirmed by two specialists. 17 The complete response to UDCA was defined as the normalization of alkaline phosphatase (ALP), gamma-glutamyl transferase (γ-GT), and total bilirubin (TBil) after the standard treatment of UDCA (13–15 mg/kg/day) for at least 6 months. Patients with at least one of the three biochemical indexes above the upper limit of normal range (ULN) (i.e. ALP > ULN, or γ-GT > ULN, or TBil > ULN) were considered nonresponders and recruited for this study. All the patients who participated in the study had dyslipidemia and met the indication for the use of fenofibrate. All the patients enrolled were stage 2 or 3 (for patients without a liver biopsy, they should have biochemical abnormalities or symptoms, with no evidence of cirrhosis), while patients with cirrhosis were excluded from the study. Patients with the following conditions were also excluded: (1) patients with comorbidity of other hepatic diseases (viral hepatitis, autoimmune hepatitis, etc.); (2) patients with other systematic autoimmune diseases like rheumatoid arthritis, angiitis, etc.; (3) patients with severe organ dysfunction; (4) patients with severe infections; (5) women during pregnancy or breastfeeding; (6) patients with history of severe allergy; (7) patients with Mayo risk score greater than 7.8; (8) patients participating or having been on other clinical trials within 3 months prior to recruitment. Written informed consent was obtained from all the patients enrolled in the study.
Study design
In this randomized controlled open-label study, the patients were randomly divided into two groups and assigned to either the experimental group or the control group at a 1:1 ratio using a computer program. For the experimental group, patients received fenofibrate 200 mg/day in combination with UDCA 13–15 mg/kg/day, while the control group continued their original UDCA treatment. By the end of the first month, all patients had their hepatic biochemicals tested for the early detection of possible liver impairment by fenofibrate. If there is an abnormal read, they would report it to the investigators, who would decide whether the patients discontinue or reduce the dose of fenofibrate according to the degree of liver damage. They were followed up until the end of the trial. The follow-up period was 12 months. The serum levels of ALP, γ-GT, alanine aminotransferase (ALT), aspartate aminotransferase (AST), TBil, cholesterol, triglycerides, high-density lipoprotein cholesterol (HDLC), low-density lipoprotein cholesterol (LDLC), as well as immunological indexes such as immunoglobulin A (IgA), IgM, and IgG of the patients, were measured at the beginning and at months 3, 6, and 12 of the trial for assessment of biochemical response and adverse effects. Symptoms, signs, and abnormalities of lab tests of the patients were examined and recorded during the hospital visit every 3 months. Patients were encouraged to report any discomfort with emails and WeChat messages at any time. Pruritis was assessed using the Visual Analogue Scale. Each patient was given a Medication Administration Record chart to keep track of their adherence to medication. The medications were given to them at every inspection and the bottles were retrieved and the remaining pills were counted during every hospital inspection. Treatment was discontinued if the patient experienced serious adverse events. The trial was registered in the Chinese Clinical Trial Registry (ChiCTR) as ChiCTR1800020160 (protocol available at www.chictr.org.cn).
Outcomes
The primary outcome was the percentage of patients with a complete biochemical response (i.e. normalization of ALP, γ-GT, and TBil levels) at 12 months. Secondary outcomes included the percentage of patients with a complete response at any time point; the percentage of patients with normalized ALP levels at any time point, and changes in the levels of ALP, γ-GT, ALT, AST, TBil, and other biochemical as well as immunological indexes.
Safety reports
The investigators evaluated the patients’ tolerance to fenofibrate based on their reports of abnormal biochemical measures during the first month. After that, the patients visited the hospital every 3 months for blood tests and other evaluations. Liver function, blood lipid levels, and serum creatine levels were measured at each visit. Pruritus, fatigue, nausea, and other side effects reported by patients were also recorded.
Sample size
To calculate the sample size, based on our previous research and published studies, 9 we expected that 33% of the patients in the experimental group would have a complete biochemical response to the combination therapy of fenofibrate and UDCA, while in the control group, the response rate would be 1%. Assuming a 20% loss to follow-up and withdrawal of consent, we calculated that a total of 48 patients would be enrolled for the study to have 90% power at a two-sided significance level of 5%.
Statistical analysis
The data were analyzed at the end of the trial in the intention-to-treat population. All analyses were performed without imputation of the missing data. The Chi-square test or Fisher’s exact test was used to compare the response rates between the two groups when appropriate [with 95% confidence intervals (CIs)]. Quantitative data are expressed as means with standard deviations or medians and interquartile ranges when appropriate (with 95% CIs). For the differences in the changes in biochemical and immunological indexes between the two groups at different time points, two-way repeated measures ANOVA was used when appropriate. Analyses were performed using SPSS Statistics version 23 (IBM). The graphs were generated using GraphPad Prism 8 (California Corp.).
Results
A total of 48 patients (24 in each group) were enrolled from 20 December 2018 to 31 December 2020. The baseline characteristics of the participants are shown in Table 1.
Table 1.
Baseline characters of patients.
| Demographic and clinical characteristics at baseline | Experimental group (UDCA + fenofibrate) |
Control group (UDCA alone) |
p value |
|---|---|---|---|
| Age (year) | 51.5 ± 11.7 | 50.4 ± 11.2 | 0.79 |
| Female no. (%) | 19 (79.2) | 24 (100) | 0.0496 |
| Median ALP (IQR)-U/l | 174 (137–253.5) | 221 (165.75–267.75) | 0.117 |
| Median γ-GT (IQR)-U/l | 218 (123.25–413.25) | 169 (110.5–295.25) | 0.398 |
| Median ALT (IQR)-U/l | 39.5 (26.75–99.25) | 47 (29.5–97.25) | 0.529 |
| Median AST (IQR)-U/l | 45 (33–66.75) | 54.5 (42–106.25) | 0.085 |
| Median TBil (IQR)-umol/L | 15.45 (10.75–23.525) | 14.05 (10.125–21.925) | 0.592 |
| AMA positive no. (%) | 22 (91.7) | 23 (95.8) | 1.000 |
| AMA-M2 positive no. (%) | 21 (87.5) | 23 (95.8) | 0.6085 |
| ACA positive no. (%) | 0 (0) | 2 (8.3) | 0.4894 |
| Anti-gp210 Ab positive no. (%) | 3 (12.5) | 5 (20.8) | 0.7008 |
| Anti-sp100 Ab positive no. (%) | 5 (20.8) | 4 (16.7) | 1.000 |
| ANA positive no. (%) | 24 (100) | 24 (100) | 1.000 |
| Median cholesterol (IQR) (mmol/l) | 5.98 (5.63–7.29) | 5.49 (4.91–6.53) | 0.02 |
| Median triglycerides (IQR) (mmol/l) | 1.76 (1.27–2.35) | 1.26 (1.12–2.34) | 0.327 |
| Median HDLC (IQR) (mmol/l) | 1.63 (1.30–2.04) | 1.62 (1.28–1.89) | 0.781 |
| Median LDLC (IQR) (mmol/l) | 3.64 (3.05–4.31) | 3.15 (2.42–3.86) | 0.071 |
| Median Alb (IQR) (g/l) | 43 (41–46) | 42 (38–43) | 0.015 |
Ab, antibody; ACA, anti-centromere antibody; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMA, anti-mitochondrial antibody; ANA, antinuclear antibody; AST, aspartate aminotransferase; HDLC, high-density lipoprotein cholesterol; IQR, interquartile range; LDLC, low-density lipoprotein cholesterol; TBil, total bilirubin; UDCA, ursodeoxycholic acid; γ-GT, gamma-glutamyl transferase.
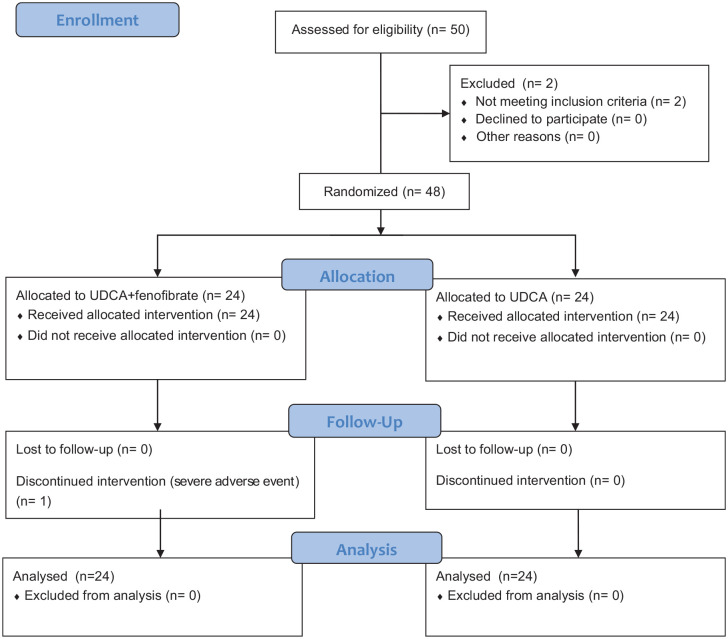
None of the patients showed signs of significant liver damage during the first month. One patient in the experimental group had ALT levels over 3 times the upper limit of the normal range in the tests at month 3 and had half the dose of fenofibrate. One patient had half the dose of fenofibrate due to elevated creatine at month 3. The tests improved and they both continued the half dose until the end of the 12th month. Another patient in the experimental group stopped fenofibrate at month 3 with ALT elevated more than 10 times the upper limit of the normal range. The patient received glucuronolactone 100 mg 3 times a day and polyene phosphatidylcholine 456 mg 3 times a day. The patient’s ALT level was still over 3 times the upper limit of the normal range at month 12, and the fenofibrate was not added back till the end of the study. None of the patients in the control group experienced severe adverse events. Figure 1 shows the entire trial procedure.
Figure 1.
Flowchart of the trial.
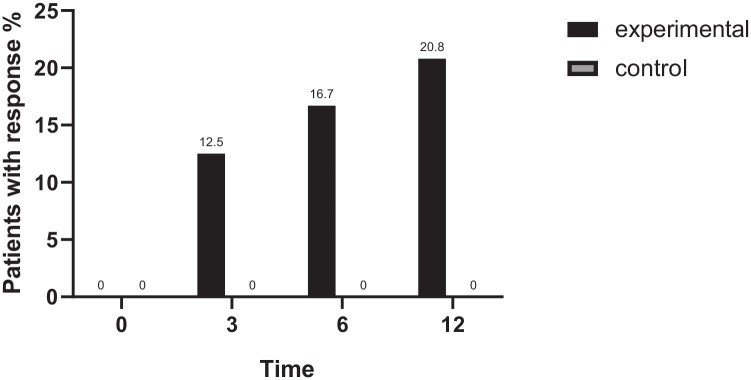
At 12 months, the percentage of patients with complete biochemical response in the experimental group was 20.8%, while the percentage of patients reaching the primary outcome in the control group was 0% (difference, 20.8%; 95% CI, 4.6–37.0; p = 0.025). No statistically significant difference in the response rate was observed between the two groups at either 3 or 6 months (p > 0.05). The response rates of the two groups at different time points are shown in Figure 2. We also evaluated the effect of fenofibrate using several published criteria, such as Rochester,18,19 Toronto, 20 Barcelona, 3 Paris 1, 21 and Paris 2. 22 Except for Paris 1 criteria (ALP < 3×ULN, AST < 2×ULN, and TBil < 17.1μmol/l), by the definition of which over half of the patients enrolled in the study already had a complete response, the patients in the experimental group showed a significantly higher rate of response than the patients in the control group by rest of the criteria (shown in Supplementary Figure 1).
Figure 2.
Response rates at different time-points (n = 24 for each group). The percentage of patients reaching the primary outcome was statistically significant between the experimental group and the control group (p < 0.05), while no significant difference was observed in the response rates at 3 and 6 months.
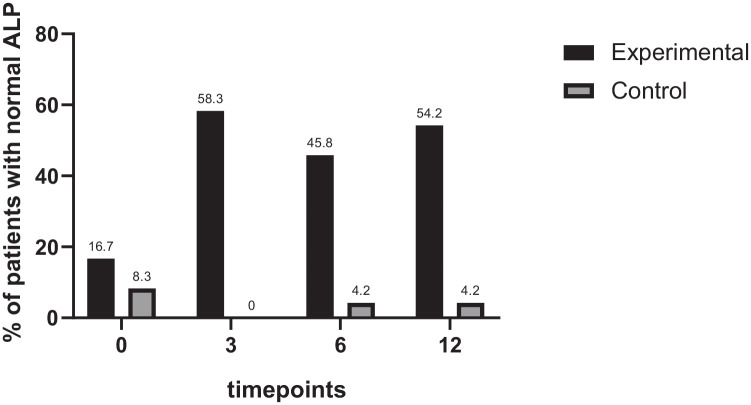
The percentage of patients with ALP levels returning to the normal range in both groups was also calculated. By the end of the third month, more patients in the experimental group had ALP levels within the normal range compared with the control group (58.3% of the experimental group and 0% of the control group), and the difference was statistically significant (p < 0.001). The pattern was sustained at months 6 and 12 (both with p < 0.001), as shown in Figure 3.
Figure 3.
Percentage of patients with their ALP returning normal range (n = 24 for each group). The percentage of patients reaching the secondary outcome for the two groups was comparable at baseline but showed a significant difference between the groups after 3 months (p < 0.05).
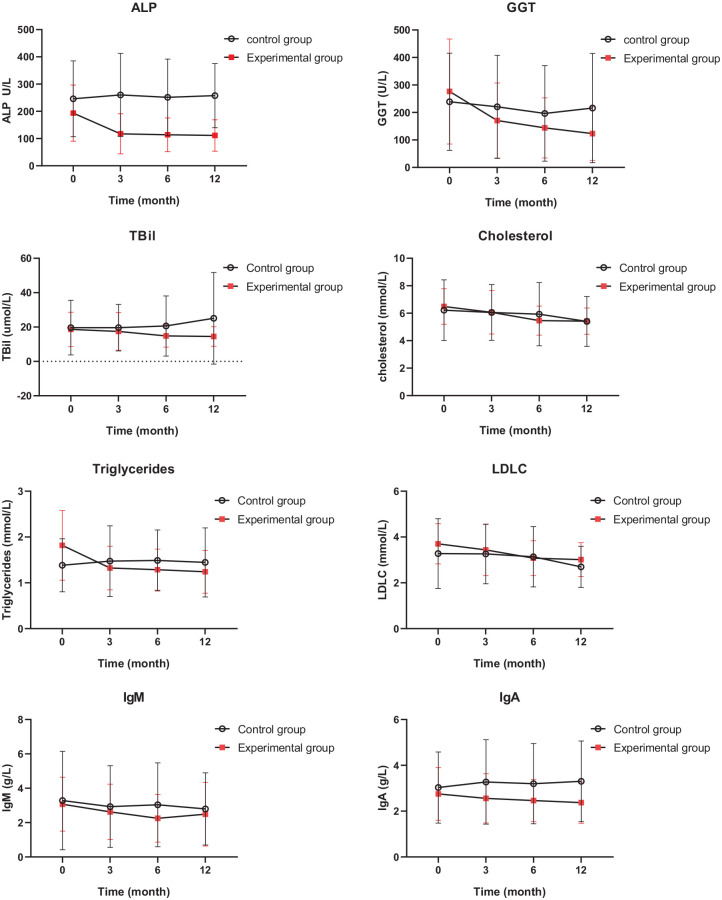
The biochemical indexes of the patients during the trial are shown in Figure 4. The change in ALP levels at different time points between the experimental group and the control group was significantly different (p < 0.001). There was also a statistically significant difference in the change in IgG levels at different time points between the two groups (p = 0.026). The patients in the experimental group also showed a trend of decreasing TBil levels when compared with patients in the control group (p = 0.036). For the other biochemical indexes such as ALT, AST, cholesterol, triglycerides, high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C), as well as immunological indexes such as IgM, IgA, and IgG; however, no significant difference was observed between the two groups. When investigating the experimental group separately, we also found significant differences in the ALP, γ-GT, and TBil levels at different time points (p < 0 .001 for ALP and γ-GT, p = 0.006 for TBil). Cholesterol, triglycerides, LDL-C, IgM, and IgA levels also showed significant changes for the experimental group (p < 0.001 for cholesterol, p < 0.001 for triglycerides, p < 0.001 for LDL-C, p = 0.001 for IgM and p < 0.001 for IgA). On the other hand, the control group did not show a significant difference in the indexes at different time points.
Figure 4.
Biochemical and immunological indexes of patients in the two groups at different time-points.
Discussion
About 1/3 of the patients fail to respond completely to UDCA, which is still the first-line treatment for PBC. For these patients, the options for second-line therapies are limited. Recently, the USFDA approved obeticholic acid in combination with UDCA for patients with inadequate response to UDCA monotherapy, but it remains unavailable in China and many other countries. Adverse events, such as pruritus, have also been reported. Several old and new medications had previously been administered to these patients, including fibrates, glucocorticoids, immunosuppressants, mesenchymal stem cells, biological agents (anti-interleukin-12, cytotoxic T-lymphocyte antigen 4 immunoglobulin, anti-CD20), and antifibrotic drugs. 23 Most of these therapies were only tested in small populations and rarely had randomized, controlled trials to validate their safety and efficacy. Obeticholic acid has been approved by USFDA as second-line treatment of PBC for patients who are intolerant to UDCA or fail to have complete response to UDCA. The real-world data also showed the efficacy of this medication, but pruritus was a major complaint. 24 There are also observational studies and pilot-controlled studies on other FXR agonists, but the population was relatively small, and the follow-up period was not long enough. 25 Anti-fibrotic therapies targeting fibroblast growth factor-19 (FGF-19) are also considered promising. An engineered FGF19 analog has shown promising results in a 28-day, double-blind, placebo-controlled phase 2 trial. 26 Fibrates or PPAR agonists, among other options, had more evidence accumulated. High-quality RCTs of bezafibrate for PBC have been published. 10 In addition, other new PPAR agonists such as pemafibrate, 27 elafibranor, 28 and other newly developed drugs 29 have also been tested in PBC patients, and promising results have been reported, however, more careful evaluation is needed. Fenofibrate is a relatively old drug, but its performance in patients with PBC is yet to be further assessed. Thus, our group conducted this study to assess the safety and efficacy of fenofibrate.
Both bezafibrate and fenofibrate are members of the fibric acid family. Fibrates can act as agonists for PPARs and regulate lipid levels in the blood. How they improve cholestasis in PBC patients, however, has not been fully elucidated. Some studies have suggested a possible role of activated PPARs and associated pathways. After the activation of PPAR, the cholesterol 7-alpha-hydroxylase (also known as cytochrome P450 7A1, CYP7A1) was suppressed, leading to decreased synthesis of bile acids. In addition, the translation of efflux transporters such as MDR3 (multiple drug resistance 3, encoded by ABCB4, that is, ATP binding cassette subfamily B member 4) was also up-regulated, which increased the excretion of bile by the hepatocytes. Some studies also reported downregulation of NF-κB downstream of PPAR activation, which reduced the inflammation of the bile canaliculi. 30 Bezafibrate, a pan-agonist of all three PPAR subtypes, has been shown to improve the response of PBC patients to UDCA. However, fenofibrate mainly activates PPARα, the dominant subtype found in the liver. To our knowledge, our study is the first randomized, controlled trial for fenofibrate in PBC patients with incomplete response to our knowledge, and we provide data for the Chinese population for the first time.
PBC is a slowly progressing disease, and the progression to cirrhosis often takes tens of years, which requires long follow-up periods to observe the effects of a treatment histologically. However, it has been revealed that the improvements in the biochemical indexes are positively correlated to the survival of the patients. Therefore, the response to treatment is often measured biochemically as a substituent of the ‘hard endpoint’ of survival or survival without liver transplantion, although the definition for ‘complete response’ varies.3,21,22 A previous study on bezafibrate used the Paris 2 criteria for incomplete response (i.e. a serum level of ALP or AST > 1.5 times the upper limit of the normal range or an abnormal total bilirubin level after 6 months or more of treatment). 10 We also used the complete biochemical response as the primary outcome, but our criteria for complete response were stricter, with serum levels of ALP, γ-GT, and TBil all returned within the normal range. Thus, more patients who failed to respond adequately to UDCA were eligible for our trial. Moreover, normalization of ALP levels as well as TBil levels of no more than 0.6×ULN (the upper limit of the normal range) were found to be associated with a low rate of liver transplantation or death. 31 Patients who reached the primary outcome in our study were more likely to have a better prognosis. For those who did not reach the primary outcome, biochemical improvement at different time points of the study was measured as the secondary outcome.
Our study shows that patients in the experimental group on the combination therapy UDCA and fenofibrate had a higher response rate than those on the UDCA single therapy at the end of the 12-month follow-up period, and the patients in the experimental group also showed a trend toward better biochemical improvement compared with the control group. Our findings are consistent with those of other studies in which bezafibrate9,32,33 and fenofibrate14,15 were used. We reported a response rate of 20.8% in the experimental group, while the response rate for bezafibrate reported in BEZURSO (bezafibrate in combination with ursodeoxycholic acid in primary biliary cholangitis, NCT01654731) was 31% (95% CI 10–50%). 10 Other studies also reported improvements in biochemical indexes with the addition of fenofibrate or bezafibrate.
Concerning the safety of fibrates, the most frequently reported severe adverse events include creatine elevation, ALT elevation, gastrointestinal tract irritation, creatine kinase elevation, and rhabdomyolysis. The BEZURSO trial reported that 20% of the patients experienced myalgia, and that 3 out of 50 patients discontinued the medicine due to excessive (over 5 times the upper limit of the normal range) elevation of ALT. In our study 48 patients were enrolled: among the 24 patients in the experimental group, one patient had to cease the trial due to severe liver damage, while two others had abnormal metabolic panels but were able to continue the trial with half dose of fenofibrate. There were reports about long latency fenofibrate induced liver injury, 34 mostly within 18–56 weeks after the beginning of medication. In our study, 2 patients had liver injury at month 3, but no more cases were found during the 12-month follow-up period. Previous studies showed up to 17% of fenofibrate intolerance, in our study, the number was 12.5%. The patients included in our study were at earlier stage of the disease, which could partly explain the relatively lower rate of adverse effects. The sample size of our study was also relatively small. In general, the occurrence of adverse events in this study was comparable with published studies, revealing fenofibrate as a relatively safe treatment; however, liver damage should be considered.
We also investigated other measures, such as immunoglobulins and blood lipids. There was a decreasing trend in the IgM and IgA levels in the experimental group. Some studies have revealed that the elevation of serum IgM is associated with the destruction of the bile canaliculi, lymphocytic piecemeal necrosis of the hepatocytes, and inflammatory response of the lobule. Other studies also reported a decrease in IgM in patients on fenofibrate, 12 indicating a possible mechanism by which fenofibrate contributes to the improvement of liver inflammation. In addition, most PBC patients also have hyperlipidemia, and the elevation of triglycerides and cholesterol was found to be related to disease progression. 35 The lowering of cholesterol, which is the substrate for the synthesis of bile acids, can help to reduce cholestasis. Statins were also found to improve the ALP, γ-GT, and IgM levels in some patients.36–38 However, statins can also cause acute cholestatic hepatitis and are thus not suitable for patients with PBC. Fenofibrate, on the other hand, was observed to lower the triglycerides and cholesterol levels in PBC patients, which consolidated the use of fenofibrate for lipid regulation in PBC patients.
Our study had some limitations. First, the follow-up period was only 12 months, which was sufficient for the observation of biochemical response, as we found in our previous studies, 4 but its adequacy for the determination of the correlation between the biochemical improvement after adding fibrates and the actual prognosis of the patients remains controversial.39–42 We will follow up with these patients in the future for long-term effects. Second, the patients enrolled in our study were all early stage: they had not developed cirrhosis, and their serum albumin level and prothrombin time were still within the normal range at the end of the observation. Therefore, the UK-PBC and other scoring tools were not suitable for the comparison of the prognosis between the two groups of patients. This was partly due to the slow progression of the disease, but also because bezafibrate is forbidden for use in patients with cirrhosis, and the safety of fenofibrate in these patients is not yet clear. We are planning a larger real-world study to further assess the use of fenofibrate in PBC patients and compare it with bezafibrate as well as obeticholic acid and other new regimens. Another limitation of this study is that the criteria for complete response in our study were different from other published studies. Patients with γ-GT increase only were also included as non-responders. We included γ-GT in the criteria because it is related to the injury of bile canaliculi. There are concerns that γ-GT is not a good marker of disease activity as alkaline phosphatase and bilirubin and this might be a confounding factor. There were three patients in the experimental group with increased GGT only and two patients in the control group. All three patients with γ-GT increased only in the experimental group had decreased γ-GT by the end of month 12, while the γ-GT of the two patients in the control group showed no improvement. There were also clinical observations that some AMA positive patients with normal ALP levels but elevated γ-GT also had PBC. 43 A correlation between γ-GT level and the risk of liver transplantation or death in PBC patients was also reported. 44 More research is needed to explore the γ-GT level and the prognosis of PBC. Third, the efficacy of fenofibrate would also be better confirmed if we had obtained liver biopsy samples of the patients showing histological improvement. However, since liver biopsy is an invasive procedure, we chose not to perform it on patients for safety concerns. Finally, we are aware of the importance of patient-reported outcomes such as pruritus and other discomfort caused both by the disease and the treatment. However, pruritus is difficult to quantify and assess at every visit. Therefore, the improvement of pruritus was not recorded in our study, although no patient reported worsening of pruritus.
Conclusion
This randomized, controlled open-label study shows that fenofibrate can be used for PBC patients with incomplete response to UDCA, both effectively and safely, although the influence on the histological features and long-term prognosis still needs further investigation. With induction of new therapies, there will be more hope for PBC patients with incomplete responses.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221114198 for A randomized, controlled trial on fenofibrate in primary biliary cholangitis patients with incomplete response to ursodeoxycholic acid by Chunlei Li, Kunyu Zheng, Yiran Chen, Chengmei He, Suying Liu, Yunjiao Yang, Mengtao Li, Xiaofeng Zeng, Li Wang and Fengchun Zhang in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors thank Editage (www.editage.cn) for English language editing.
Footnotes
ORCID iDs: Chunlei Li  https://orcid.org/0000-0002-3840-4751
https://orcid.org/0000-0002-3840-4751
Chengmei He  https://orcid.org/0000-0003-4711-8068
https://orcid.org/0000-0003-4711-8068
Suying Liu  https://orcid.org/0000-0002-4386-9895
https://orcid.org/0000-0002-4386-9895
Mengtao Li  https://orcid.org/0000-0003-4252-2889
https://orcid.org/0000-0003-4252-2889
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Chunlei Li, Department of Internal Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China.
Kunyu Zheng, Department of Internal Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China; Department of Rheumatology and Clinical Immunology, Chinese Academy of Medical Sciences & Peking Union Medical College, National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID), Ministry of Science & Technology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital (PUMCH), Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Yiran Chen, Department of Internal Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China; Department of Rheumatology and Clinical Immunology, Chinese Academy of Medical Sciences & Peking Union Medical College, National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID), Ministry of Science & Technology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital (PUMCH), Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Chengmei He, Department of Internal Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China; Department of Rheumatology and Clinical Immunology, Chinese Academy of Medical Sciences & Peking Union Medical College, National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID), Ministry of Science & Technology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital (PUMCH), Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Suying Liu, Department of Internal Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China; Department of Rheumatology and Clinical Immunology, Chinese Academy of Medical Sciences & Peking Union Medical College, National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID), Ministry of Science & Technology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital (PUMCH), Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Yunjiao Yang, Department of Internal Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China; Department of Rheumatology and Clinical Immunology, Chinese Academy of Medical Sciences & Peking Union Medical College, National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID), Ministry of Science & Technology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital (PUMCH), Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Mengtao Li, Department of Internal Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China; Department of Rheumatology and Clinical Immunology, Chinese Academy of Medical Sciences & Peking Union Medical College, National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID), Ministry of Science & Technology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital (PUMCH), Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Xiaofeng Zeng, Department of Internal Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China; Department of Rheumatology and Clinical Immunology, Chinese Academy of Medical Sciences & Peking Union Medical College, National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID), Ministry of Science & Technology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital (PUMCH), Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Li Wang, Department of Internal Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China; Department of Rheumatology and Clinical Immunology, Chinese Academy of Medical Sciences & Peking Union Medical College, National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID), Ministry of Science & Technology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital (PUMCH), Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, No.1 Shuaifuyuan, Dongcheng District, Beijing 100730, China.
Fengchun Zhang, Department of Internal Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China; Department of Rheumatology and Clinical Immunology, Chinese Academy of Medical Sciences & Peking Union Medical College, National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID), Ministry of Science & Technology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital (PUMCH), Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Beijing, China.
Declarations
Ethics approval and consent to participate: Ethics Committee approval was obtained from the Institutional Ethics Committee of Chinese Academy of Medical Sciences, Peking Union Medical College Hospital to the commencement of the study (approval number JS-1739). The study was conducted under the supervision of the same committee. Written informed consent for participation was obtained from all the patients enrolled in the study.
Consent for publication: Not applicable.
Author contributions: Chunlei Li: Data curation; Formal analysis; Investigation; Visualization; Writing – original draft.
Kunyu Zheng: Data curation; Investigation; Visualization; Writing – review & editing.
Yiran Chen: Investigation; Writing – review & editing.
Chengmei He: Investigation; Writing – review & editing.
Suying Liu: Investigation; Writing – review & editing.
Yunjiao Yang: Investigation; Writing – review & editing.
Mengtao Li: Investigation; Supervision; Writing – review & editing.
Xiaofeng Zeng: Investigation; Supervision; Writing – review & editing.
Li Wang: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Fengchun Zhang: Conceptualization; Funding acquisition; Resources; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Science (Grant Number 2019XK320022), CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant Number 2021-I2M-1-005), National Natural Science Fund (Grant Numbers 81771764, 81501414), and Capital Health development Research Special Fund (Grant Number 2016-4-14011).
Competing interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Beuers U, Gershwin ME, Gish RG, et al. Changing nomenclature for PBC: from ‘cirrhosis’ to ‘cholangitis’. J Hepatol 2015; 63: 1285–1287. [DOI] [PubMed] [Google Scholar]
- 2. Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet 2015; 386: 1565–1575. [DOI] [PubMed] [Google Scholar]
- 3. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology 2006; 130: 715–720. [DOI] [PubMed] [Google Scholar]
- 4. Zhang LN, Shi TY, Shi XH, et al. Early biochemical response to ursodeoxycholic acid and long-term prognosis of primary biliary cirrhosis: results of a 14-year cohort study. Hepatology 2013; 58: 264–272. [DOI] [PubMed] [Google Scholar]
- 5. Willson TM, Brown PJ, Sternbach DD, et al. The PPARs: from orphan receptors to drug discovery. J Med Chem 2000; 43: 527–550. [DOI] [PubMed] [Google Scholar]
- 6. Zhang Y, Chen K, Dai W, et al. Combination therapy of bezafibrate and ursodeoxycholic acid for primary biliary cirrhosis: a meta-analysis. Hepatol Res 2015; 45: 48–58. [DOI] [PubMed] [Google Scholar]
- 7. Weber-Freissmuth C, Kozbial K, Staettermayer AF, et al. Effect of fibrate-add-on treatment in primary biliary cholangitis patients with an insufficient response to ursodeoxycholic acid (UDCA). Unit Euro Gastroenterol J 2016; 4: A547. [Google Scholar]
- 8. Honda A, Tanaka A, Komori A, et al. Bezafibrate improves GLOBE and UK-PBC scores and long-term outcomes in patients with primary biliary cholangitis. Hepatology 2017; 66: 42A–43A. [DOI] [PubMed] [Google Scholar]
- 9. Corpechot C, Chazouillères O, Rousseau A, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Eng J Med 2018; 378: 2171–2181. [DOI] [PubMed] [Google Scholar]
- 10. Corpechot C, Chazouilleres O, Rousseau A, et al. A 2-year multicenter, double-blind, randomized, placebocontrolled study of bezafibrate for the treatment of primary biliary cholangitis in patients with inadequate biochemical response to ursodeoxycholic acid therapy (Bezurso). J Hepatol 2017; 66: S89. [Google Scholar]
- 11. Tanaka A, Hirohara J, Nakano T, et al. Association of bezafibrate with transplant-free survival in patients with primary biliary cholangitis. J Hepatol 2021; 75: 565–571. [DOI] [PubMed] [Google Scholar]
- 12. Dohmen K, Mizuta T, Nakamuta M, et al. Fenofibrate for patients with asymptomatic primary biliary cirrhosis. W J Gastroenterol 2004; 10: 894–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levy C, Peter JA, Keach JC, et al. Fenofibrate improves liver biochemistries in primary biliary cirrhosis. Hepatology 2009; 50: 995A–996A. [Google Scholar]
- 14. Liberopoulos EN, Florentin M, Elisaf MS, et al. Fenofibrate in primary biliary cirrhosis: a pilot study. Open Cardiovasc Med J 2010; 4: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Han XF, Wang QX, Liu Y, et al. Efficacy of fenofibrate in Chinese patients with primary biliary cirrhosis partially responding to ursodeoxycholic acid therapy. J Dig Dis 2012; 13: 219–224. [DOI] [PubMed] [Google Scholar]
- 16. Grigorian AY, Mardini HE, Corpechot C, et al. Fenofibrate is effective adjunctive therapy in the treatment of primary biliary cirrhosis: a meta-analysis. Clin Res Hepatol Gastroenterol 2015; 39: 296–306. [DOI] [PubMed] [Google Scholar]
- 17. Lindor KD, Gershwin ME, Poupon R, et al. Primary biliary cirrhosis. Hepatology 2009; 50: 291–308. [DOI] [PubMed] [Google Scholar]
- 18. Angulo P, Lindor KD, Therneau TM, et al. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ursodeoxycholic acid. Liver 1999; 19: 115–121. [DOI] [PubMed] [Google Scholar]
- 19. Momah N, Silveira MG, Jorgensen R, et al. Optimizing biochemical markers as endpoints for clinical trials in primary biliary cirrhosis. Liver Int 2012; 32: 790–795. [DOI] [PubMed] [Google Scholar]
- 20. Kumagi T, Guindi M, Fischer SE, et al. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol 2010; 105: 2186–2194. [DOI] [PubMed] [Google Scholar]
- 21. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology 2008; 48: 871–877. [DOI] [PubMed] [Google Scholar]
- 22. Corpechot C, Chazouilleres O, Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J Hepatol 2011; 55: 1361–1367. [DOI] [PubMed] [Google Scholar]
- 23. Li YM, Wang QX, Ma X. Advances in the treatment of primary biliary cholangitis [Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi]. Chin J Hepatol 2017; 25: 805–809. [DOI] [PubMed] [Google Scholar]
- 24. Roberts SB, Ismail M, Kanagalingam G, et al. Real-world effectiveness of obeticholic acid in patients with primary biliary cholangitis. Hepatol Commun 2020; 4: 1332–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trauner M, Gulamhusein A, Hameed B, et al. The nonsteroidal farnesoid X receptor agonist cilofexor (GS-9674) improves markers of cholestasis and liver injury in patients with primary sclerosing cholangitis. Hepatology 2019; 70: 788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mayo MJ, Wigg AJ, Leggett BA, et al. NGM282 for treatment of patients with primary biliary cholangitis: a multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Commun 2018; 2: 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joshita S, Umemura T, Yamashita Y, et al. Biochemical and plasma lipid responses to pemafibrate in patients with primary biliary cholangitis. Hepatol Res 2019; 49: 1236–1243. [DOI] [PubMed] [Google Scholar]
- 28. Schattenberg JM, Pares A, Kowdley KV, et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J Hepatol 2021; 74: 1344–1354. [DOI] [PubMed] [Google Scholar]
- 29. Jones D, Boudes PF, Swain MG, et al. Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol 2017; 2: 716–726. [DOI] [PubMed] [Google Scholar]
- 30. Ghonem NS, Assis DN, Boyer JL. Fibrates and cholestasis. Hepatology 2015; 62: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murillo Perez CF, Harms MH, Lindor KD, et al. Goals of treatment for improved survival in primary biliary cholangitis: treatment target should be bilirubin within the normal range and normalization of alkaline phosphatase. Am J Gastroenterol 2020; 115: 1066–1074. [DOI] [PubMed] [Google Scholar]
- 32. Itakura J, Izumi N, Nishimura Y, et al. Prospective randomized crossover trial of combination therapy with bezafibrate and UDCA for primary biliary cirrhosis. Hepatol Res 2004; 29: 216–222. [DOI] [PubMed] [Google Scholar]
- 33. Iwasaki S, Ohira H, Nishiguchi S, et al. The efficacy of ursodeoxycholic acid and bezafibrate combination therapy for primary biliary cirrhosis: a prospective, multicenter study. Hepatol Res 2008; 38: 557–564. [DOI] [PubMed] [Google Scholar]
- 34. Ahmad J, Odin JA, Hayashi PH, et al. Identification and characterization of fenofibrate-induced liver injury. Dig Dis Sci 2017; 62: 3596–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pares A, Reig A, Perez-Cormenzana M, et al. Serum metabolomic profile of patients with primary biliary cirrhosis treated with bezafibrate and changes following itching relief. Hepatology 2015; 62: 517A. [Google Scholar]
- 36. Cash WJ, O’Neill S, O’Donnell ME, et al. Randomized controlled trial assessing the effect of simvastatin in primary biliary cirrhosis. Liver Int 2013; 33: 1166–1174. [DOI] [PubMed] [Google Scholar]
- 37. Trauner M, Boyer JL. Cholestatic syndromes. Curr Opin Gastroenterol 2003; 19: 216–231. [DOI] [PubMed] [Google Scholar]
- 38. Ritzel U, Leonhardt U, Näther M, et al. Simvastatin in primary biliary cirrhosis: effects on serum lipids and distinct disease markers. J Hepatol 2002; 36: 454–458. [DOI] [PubMed] [Google Scholar]
- 39. Cheung AC, Meza-Cardona JM, Kowgier M, et al. Fenofibrates do not improve transplant-free survival despite biochemical response in patients with primary biliary cirrhosis. Hepatology 2014; 60: 345A–346A. [Google Scholar]
- 40. Reig A, Llovet LP, Sesé P, et al. Long-term bezafibrate therapy does not prevent the progression of primary biliary cholangitis in patients with advanced disease. J Hepatol 2019; 70: 407. [Google Scholar]
- 41. Hegade VS, Khanna A, Walker LJ, et al. Long-term fenofibrate treatment in primary biliary cholangitis improves biochemistry but not the UK-PBC risk score. Digest Dis Sci 2016; 61: 3037–3044. [DOI] [PubMed] [Google Scholar]
- 42. Reig A, Sesé P, Pares A. Long-term therapy with bezafibrate and ursodeoxycholic acid is insufficient for preventing disease progression in patients with advanced primary biliary cirrhosis. J Hepatol 2015; 62: S806. [Google Scholar]
- 43. Terziroli Beretta-Piccoli B, Stirnimann G, Mertens J, et al. Primary biliary cholangitis with normal alkaline phosphatase: a neglected clinical entity challenging current guidelines. J Autoimmun 2021; 116: 102578. [DOI] [PubMed] [Google Scholar]
- 44. Gerussi A, Bernasconi DP, O’Donnell SE, et al. Measurement of gamma glutamyl transferase to determine risk of liver transplantation or death in patients with primary biliary cholangitis. Clin Gastroenterol Hepatol 2021; 19: 1688–1697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221114198 for A randomized, controlled trial on fenofibrate in primary biliary cholangitis patients with incomplete response to ursodeoxycholic acid by Chunlei Li, Kunyu Zheng, Yiran Chen, Chengmei He, Suying Liu, Yunjiao Yang, Mengtao Li, Xiaofeng Zeng, Li Wang and Fengchun Zhang in Therapeutic Advances in Chronic Disease