Abstract
Objective
To investigate the antibody response to SARS-CoV-2 and identify associated factors in frontline and second-line healthcare workers (HCWs) at a large hospital in Mexico City during the first wave of COVID-19 pandemic.
Methods
This was a cross-sectional study of HCWs returning to work following mandatory isolation after recovering from COVID-19. Immunoglobulin (Ig) M and IgG antibodies elicited by SARS-CoV-2 were semiquantitatively measured using densitometric analysis of band intensities in lateral flow assay (LFA) devices. The mean pixel intensity (dots-per-inch [dpi]) of each band on the LFA was considered a measure of antibody titre.
Results
Of the 111 HCWs involved in the study, antibody responses were detected in 73/111 (66%) participants. Severe COVID symptoms was associated with old age. No differences in IgM intensity were observed between men and women, but IgG intensity was significantly higher in men than in women. Second-line HCWs produced a higher IgG intensity than firstline HCWs. The IgG intensity was high in severe cases.
Conclusions
For HCWs who may acquire SARS-CoV-2 infection, it is necessary to establish a routine program for detection of the virus to avoid risk of infection and spread of COVID-19.
Keywords: SARS-CoV-2, COVID-19, seroprevalence, healthcare workers
Introduction
A novel coronavirus which causes severe acute respiratory syndrome (SARS-CoV-2) emerged in Wuhan, Hubei, China, in 2019. 1 The infection it caused, Coronavirus disease 2019 (COVID-19) spread globally and in the North American continent, Mexico recorded one of the highest numbers of cases. 2 Frontline healthcare workers (HCWs) who treat patients infected with SARS-CoV-2 are at high risk of acquiring the infection. Indeed, it was reported that 97,632 Mexican HCWs were infected with SARS-CoV-2 from the beginning of the pandemic until August 23, 2020. 3
Serological tests for SARS-CoV-2 infection are an important tool for surveillance and epidemiological studies and assist in the understanding of the dynamics of virus transmission in the general population. In addition, antibody detection is an important marker for immunity in a population and indicates the level of protection and the continued endurance of protective antibodies. Antibody detection amongst HCWs is a particularly useful tool in identifying occupational risk due to high rates of subclinical infection.4,5 Moreover, evidence suggested that clinical severity of the SARS-CoV-2 infection is associated with high titres of antibodies.6,7 In a multicentre cross-sectional study involving 571 patients, peak concentrations of immunoglobulin M (IgM) were reached at day 10 and immunoglobulin G (IgG) at day 20. 7 Unlike direct viral detection methods, such as nucleic acid amplification or antigen detection tests which can detect acute infection, antibody tests can help determine if the individual being tested has previously been infected even if that person does not show any symptoms. 8
We performed a cross-sectional study among frontline and second-line HCWs at a large hospital in Mexico City during the course of the first wave of COVID-19 pandemic to investigate the antibody response to SARS-CoV-2 and identify associated factors.
Methods
Setting
This cross-sectional study was performed from June 2020 to January 2021 at the Hospital Regional “1° de Octubre” in Mexico City. During the first COVID-19 wave (i.e., March to August 2020) patients infected with SARS-CoV-2 were sent to this hospital. The reporting of this study conforms to STROBE guidelines as well as guidance established by the European Medicine Agency.9,10 All participants volunteered for the study and provided written consent. The study protocol did not require review and approval from an ethics committee because it was performed during a critical phase of the pandemic and the data were obtained from routine tests. The dataset was released by the Mexican Ministry of Health and was compiled by the General Directorate of Epidemiology (DGE) through the Epidemiological Surveillance System for Viral Respiratory Diseases.
Study population
All HCWs, aged 23–63 years, with no medical restrictions associated with chronic diseases, who wanted to return to work after they had completed the mandatory 10-day COVID-19 isolation period, were included in the study. The HCWs were separated into two groups: frontline and second-line staff depending on the level of risk to which they were exposed. Frontline HCWs were defined as those who had a high risk of exposure to SARS-CoV-2 because they were directly involved in the care of patients with COVID-19 (i.e., close contact and long exposure time). 11 Second-line HCWs were defined as those at low risk because they had no direct exposure to patients or biological material infected with SARS-CoV-2.
At least 10 days after their initial diagnosis of COVID, participants underwent a real-time reverse transcriptase–polymerase chain reaction (RT-PCR) test for COVID-19 nucleic acid, a computed tomography (CT) scan, and were assessed according to clinical criteria established by WHO. 12 Only HCWs with a negative PCR test were allowed to return to work. Approximately five days after these tests, HCWs were tested for serum SARS-CoV-2 IgG and IgM using COVID-19 IgM/IgG test kits (Karmacare, KPC Biotech Inc, Corona, CA, USA). The kits which have previously been validated 13 are based on an immunochromatographic lateral flow assay (LFA) that detects antibodies against the viral nucleocapsid and spike proteins. According to the manufacturer’s instructions, for IgM, the assay has sensitivity and specificity of 90% and 98.8%, respectively; for IgG the LFA has sensitivity and specificity of 100% and 98.8%, respectively.
For the LFAs, 10 µl of blood was applied to the test strip followed immediately by two drops of test diluent. The results were read 10 minutes later. If a red band was present in zone C and a purple line in zone G, this indicated a positive result for IgG; a red line in zone C and a purple line in zone M indicated a positive result for IgM; the presence of three lines in zones C, G, and M indicated IgM/IgG positive result; the presence of a red line in zone C and the absence of lines from zones G and M indicated a negative result. Finally, the absence of a red line and the presence of bands in zones G or M indicated an invalid result.14,15
The immunochromatography paper in the LFA was used for densitometric measurements and the mean pixel intensity in dots per inch (dpi) of each band was considered a semiquantitative measure of the antibody titre. For the measurements, the chromatographic paper from each cartridge was unmounted and scanned, and the dpi intensity was measured using software gel Quant V.11.4 (Bio- Imaging Systems LTD, Jerusalem Israel). This method has been previously used in antigen rapid test development. 16
With regard to COVID-19 symptomatology, asymptomatic infection was defined as having a body temperature <37.5°C and no clinical symptoms at the time of virus detection by PCR. Mild infection was defined as having at least one sign or symptom (i.e., sputum, rhinorrhoea, cough, headache, sore throat, chest discomfort/dyspnea, myalgia, and/or febrile/chilling sensation) in association with positive PCR. Moderate infection was defined as clinical or radiographic evidence of lower respiratory tract disease with oxygen saturation (SpO2) ≥94% and a positive PCR. Severe infection was defined as SpO2 <94% in standard atmospheric conditions, respiratory rate >30 breaths/min, and/or a lung infiltrate >50%, in association with positive PCR.8,17–19
Statistical Analysis
Data were analyzed using StatView v 5.0 (SAS Institute Inc., Cary, NC, USA). A P-value <0.05 was considered to indicate statistical significance. Contingency tables were used to summarize the results and data were analyzed using repeated-measures analysis of variance (ANOVA) with post hoc analyses using the Bonferroni (Dunn) test and Scheffé’s test.
Results
In total, data were obtained from 111 HCWs (44 women, 67 men). Of these, 74/111 (67%) were frontline (high risk) staff and 37/111 (33%) were second-line (low risk) staff. Median age of the participants was 40 years (range 23–81). Overall, 32 (29%), 16 (14%), 15, (14%) and 11 (10%) HCWs, had none, asymptomatic, mild, moderate or severe symptoms, respectively. Participants with more severe disease tended to be in the older age groups. For example, mean ages (SD) for the groups were as follows: asymptomatic, 34.8 (8.6) years; mild, 41.5 (8.9) years; moderate 47.9 (11.4) years; severe, 56.4 (12.4) years (Figure 1). Indeed, analysis showed that HCWs with severe disease were statistically significantly older than HCWs with mild disease (P = 0.005) or those with no symptoms (P = 0.0005) (Figure 1). In addition, HCWs in the moderate group were statistically significantly older than those in the asymptomatic group (P = 0.05).
Figure 1.
Severity of COVID infection versus patient age.
Participants with more severe disease tended to be in the older age groups. The mean (standard deviation [SD]) age of the severe group (56.4 [12.4] years) was statistically significantly higher than that for the mild group (41.5 [8.9] years; **P = 0.005) and the asymptomatic group (34.8 [8.6] years; ***P = 0.0005). In addition, the mean age of the moderate group (47.9 [11.4] years) was statistically significantly higher than that for the asymptomatic group (*P = 0.05).
Overall, 48 (43%), 37 (33%), 15, (14%) and 11 (10%) health care workers (HCWs), had none, mild, moderate or severe symptoms, respectively.
A positive antibody response on LFA was detected in 73/111 (66%) participants (Table 1). Approximately, half of the participants (48/111; 43%) were positive for SARS- CoV-2 (i.e., confirmed by PCR, CT scan and symptoms) and also had positive LTAs. However, 34 (31%) participants were free of the infection (i.e., negative for the three diagnostic methods and LFAs). Interestingly, 8 (7%) participants had a positive PCR, developed antibodies and were asymptomatic, and, while 2 (2%) were positive for COVID-19 in all three diagnostic tests but did not develop antibodies. One participant had a positive PCR test but was asymptomatic and did not develop antibodies, suggesting this was a false positive result. In total, there were 59 positive PCR tests. By contrast, 13 (12%) participants had a negative PCR test but had symptoms and developed antibodies. A further 4 (4%) participants had negative PCR, were asymptomatic, but had antibodies, which suggests false negative results since the sensitivity and specificity of the antibody test is >96%. In total, 17 participants had negative PCR test results but had positive antibody responses. Lastly, one participant had a negative PCR, did not develop antibodies but had symptoms suggesting that the individual had some other respiratory pathology.
Table 1.
Diagnostic test results for COVID-19 in healthcare workers (n = 111).
| PCR | CT | Clinical presentation | Antibody detection by LFA | Number (%) |
|---|---|---|---|---|
| + | + | + | + | 48 (43%) |
| + | – | – | + | 8 (7%) |
| + | + | + | – | 2 (2%) |
| + | – | – | – | 1 (1%) |
| – | – | – | – | 34 (31%) |
| – | + | + | + | 13 (12%) |
| – | – | – | + | 4 (4%) |
| – | + | + | – | 1 (1%) |
Abbreviations: PCR, polymerase chain reaction; CT, computed tomography, LFA, lateral flow assay.
RT-PCR on nasopharyngeal samples is considered the ‘gold standard’ for COVID-19 testing. We evaluated the diagnostic accuracy of the different diagnostic tests used in this study by comparing their sensitivities, specificities, positive and negative predictive values, positive and negative likelihood ratios (LR) and Youden’s Index (Table 2). By comparison with PCR, detection of antibodies with LFA had a sensitivity of 95% and specificity of 67%. By comparison, positive clinical data vs. PCR had a lower sensitivity at 85% but a higher specificity at 73%. Compared with positive antibodies as a confirmatory diagnosis, PCR and clinical data had the highest specificities at 92%. With regard to the positive predictive value (PPV), compared with presence of antibodies, PCR and clinical symptoms obtained the highest values (95%). Regarding the negative predictive value (NPV), the presence of antibodies obtained the highest values with respect to PCR and clinical data (92% in both cases). For positive LR (LR+), compared to the detection of antibodies, clinical data and PCR had the highest scores (10.6 and 9.7, respectively). Interestingly, the lowest value for LR+ (2.9) was obtained by the detection of antibodies compared to the ‘gold-standard’ method, PCR. The highest negative LR (LR−) value (0.27) was obtained when PCR data was compared against clinical data; the lowest values were obtained when presence of antibodies were compared to PCR (0.08) or clinical data (0.06).
Table 2.
Diagnostic accuracy of the different diagnostic methods of COVID-19 in healthcare workers (n = 111).
| Antibodies vs. PCR(95% CI) | Antibodies vs. Clinical data(95% CI) | Clinical data vs PCR(95% CI) | Clinical data vs. Antibodies(95% CI) | PCR vs. Antibodies(95% CI) | PCR vs. Clinical data (95% CI) | |
|---|---|---|---|---|---|---|
| Sensitivity, % | 95 (86, 99) | 95 (87, 99) | 85 (73, 93) | 84 (73, 91) | 77 (65, 86) | 78 (66, 87) |
| Specificity, % | 67 (53, 80) | 74 (60, 86) | 73 (59, 84) | 92 (79, 98)) | 92 (79, 98) | 81 (67, 91) |
| Positive Predictive Value, % | 77 (65, 86) | 84 (73, 91) | 78 (66, 87) | 95 (87, 99) | 95 (86, 99) | 85 (73, 93) |
| Negative Predictive Value, % | 92 (79, 98) | 92 (79, 98) | 81 (67, 91) | 74 (60, 86) | 67 (53, 80) | 73 (59, 84) |
| Positive Likelihood Ratio | 2.9 (2, 4.3) | 3.7 (2.3, 6.1) | 3.2 (2, 5) | 10.6 (3.6, 32) | 9.7 (3.3, 29) | 4.08 (2.2, 7.5) |
| Negative Likelihood Ratio | 0.08 (0.02, 0.23) | 0.06 (0.02, 0.19) | 0.21 (0.11, 0.39) | 0.18 (0.11, 0.30)) | 0.25 (0.17, 0.39) | 0.27 (0.17, 0.44) |
| Accuracy/ Effectiveness, % | 82 (74, 89) | 86 (79, 92) | 79 (71, 86) | 86 (79, 92)) | 82 (74, 89) | 79 (71, 86) |
| Youden’s Index, % | 62 (39, 79) | 70 (47, 85) | 57 (32, 77) | 76 (52, 90) | 69 (44, 84) | 59 (33, 78) |
Abbreviations: CI, confidence intervals; PCR, polymerase chain reaction.
The highest values for accuracy/effectiveness corresponded to antibody/clinical comparisons (86%), followed by the antibody/PCR comparison (82%) and by clinical/PCR comparison (79%). For Youden’s index, which reflects the performance of the diagnostic tests, the highest value was achieved for the clinical/antibody comparison (76%); for PCR/antibody comparison the value was 62% and for PCR/clinical the value was 57%.
Using densitometric analysis for the quantification of band intensity on the chromatography paper of the LFAs, there was no difference between men and women in mean pixel intensity (dpi) of the IgM bands (Figure 2). Mean (SD) pixel intensity of IgM bands for men was 24.7 (29.3) and for women was 20.0 (22.9). However, for IgG, there was a statistically significant difference between men and women (P = 0.0005). Mean (SD) pixel intensity of IgG bands for men was 88.1 (26.2) and for women was 57.6 (41.39).
Figure 2.
Densitometric measurements of IgM and IgG.
Densitometric analysis of the immunochromatography paper for the quantification of band intensity in dots per inch (dpi) of IgM showed no difference between male (n = 67) and female (n = 44) health care workers (HCWs). Mean (SD) pixel intensity of IgM bands for men was 24.7 (29.3) and for women was 20.0 (22.9). However, for IgG, there was a statistically significant difference between men and women. Mean (SD) pixel intensity of IgG bands for men was 88.1 (26.2) and for women was 57.6 (41.39) (***P = 0.0005).
Although IgM band intensities (i.e., titres) tended to be higher in the severe COVID symptom group compared with all other groups, only the comparison with the mild symptom group was statistically significant (P = 0.005) (Figure 3a). Mean (SD) band intensities for IgM were, 18.3 (20.0), 16.6 (25.9), 23.7 (23.8) and 41.8 (26.2) for HCWs with none, mild, moderate and severe symptoms, respectively.
Figure 3.
Densitometric measurements of IgM and IgG band intensities according to severity of COVID symptoms. (a) Although pixel intensity (dots per inch [dpi]) of IgM bands (i.e., titres) tended to be higher in the severe COVID symptom group compared with all other groups, only the comparison with the mild symptom group was statistically significant (P = 0.005). Mean (SD) band intensities for IgM were, 18.3 (20.0), 16.6 (25.9), 23.7 (23.8) and 41.8 (26.2) for health care workers (HCWs) with none, mild, moderate and severe symptoms, respectively and (b) By comparison, IgG band intensities (i.e., titres) in severe cases were statistically significantly higher than that for asymptomatic (P = 0.0005) and mild (P = 0.05) cases but did not differ from moderate cases. In addition, IgG band intensities for moderate cases were statistically significantly higher than that for asymptomatic cases (P = 0.05). IgG band intensities for mild cases were also statistically significantly higher than asymptomatic cases (P = 0.05). Mean (SD) band intensities for IgG were, 37.1 (26.6), 68.6 (35.0), 79.9 (45.0) and 104.9 (20.0) for HCWs with none, mild, moderate and severe symptoms, respectively.
By comparison, IgG band intensities (i.e., titres) in severe cases were statistically significantly higher than that for asymptomatic (P = 0.0005) and mild (P = 0.05) cases but did not differ from moderate cases (Figure 3b). In addition, IgG band intensities for moderate cases were statistically significantly higher than that for asymptomatic cases (P = 0.05). IgG band intensities for mild cases were also statistically significantly higher than asymptomatic cases (P = 0.05). Mean (SD) band intensities for IgG were, 37.1 (26.6), 68.6 (35.0), 79.9 (45.0) and 104.9 (20.0) for HCWs with none, mild, moderate and severe symptoms, respectively.
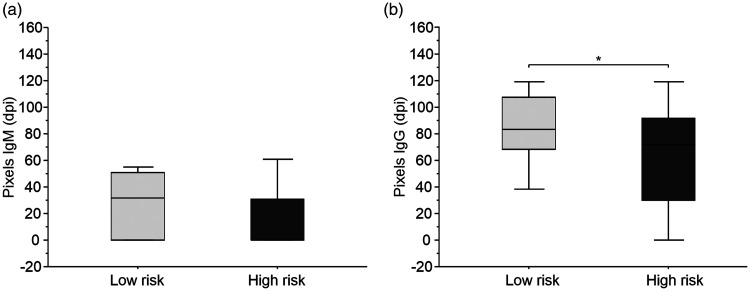
Densitometric analysis of band intensities showed that IgM production was similar in high risk (frontline) and low risk (second-line) HCW groups (Figure 4a). However, band intensities for IgG were statistically significantly higher (P = 0.05) in the low risk group compared with the high risk group (Figure 4b).
Figure 4.
Densitometric measurements of IgM and IgG band intensities according to risk of exposure. (a) Densitometric analysis of band intensities showed that IgM production was similar in high risk (frontline, n = 74) and low risk (second-line, n = 37) healthcare workers (HCWs) and (b) However, band intensities for IgG were statistically significantly higher (P = 0.05) in the low risk group compared with the high risk group.
Discussion
Using LFAs, we analyzed antibody responses to SARS-CoV-2 in 111 frontline and second-line HCWs (i.e., doctors, nurses, psychologists, orderlies, technicians, and secretaries) returning to work following mandatory isolation after recovering from COVID-19 during the first wave of the pandemic. We decided to use LFAs because of the feasibility of being able to perform the assay easily, the small amount of sample required (10 µl), and the immediacy of the results. Moreover, lateral flow test chromatography has been evaluated versus enzyme-linked immunosorbent assay (ELISA) and chemiluminescent immunoassay (CLIA) and has demonstrated an accurate equivalent performance. 20
We used a semiquantitative method for evaluating antibody responses. We conducted densitometric measurements of the band intensities on the immunochromatography paper in the LFAs and considered the mean pixel intensity of each band as a measure of antibody titre. This method has been used elsewhere. 16 Using this method, we found a seroprevalence of 66% (73/111) in the HCWs. However, this value was well above seroprevalence levels previously reported. For example, a study from Japan reported only 0.74% seroprevalence, followed by Germany with 0.83%, Italy 7.4% and China 17%. 21 In a study from Iran, seroprevalence of 6% for IgG was reported in HCWs from two hospitals and out of the 42 positive PCR tests, only 29 (69%) were associated with IgG production. By contrast, in our study, of the 59 positive PCR tests, 56 (95%), were positive for antibodies. Differences in the results from seroprevalence studies may reflect differences in the various methodologies used.
Consistent with previous findings, the most intense antibody response was observed in HCWs with severe COVID symptoms. 22 We believe that this result is possibly related to triggering of the immune response and exacerbation of the inflammatory response. The immune pathogenesis of SARS-CoV-2 has been extensively studied and it is known that when infection occurs there is a prompt innate and adaptive immune response which results in viral clearance and recovery. However, when viral clearance is not effective, the overexpression of proinflammatory cytokines is triggered and the humoral response is impaired, which can lead to severe complications.23–26
We observed that 17 HCWs who were infected but were PCR-negative exhibited an antibody response. Therefore, while it is important to evaluate PCR results and clinical data, the detection of antibodies may be useful in improving the diagnosis, prognosis, and follow-up treatment for COVID. Our HCW population was separated into low and high risk groups based on level of exposure to the virus. Interestingly, there were no differences in IgM titres between the two groups suggesting that there was no difference in the level of initial antibody production. However, the IgG titres were higher than the IgM titres suggesting that this group of HCWs may have had a prolonged exposure to SARS-CoV-2. In addition, there was a significant difference in IgG titres between groups with the low risk group producing a more intense response than the high risk group. This result may have been related to measures taken to protect high risk front-line HCWs (i.e., the use of protective equipment, prior education on the management of infected patients, etc.). 17 Our findings suggest that there is a need for similar measures to be extended to second-line HCWs.
Additionally, we analyzed the clinical severity of infection by patient age, and found an association between older age groups and increased severity of the illness. Our results are consistent with previous observations. 27 When we analyzed the antibody titre (i.e., band intensities) with respect to sex, we found no difference between male and female HCWs in IgM response, suggesting a similar initial immune response. However, there was a significant difference between men and women in IgG titres (i.e., band intensities) with men having a greater response. This finding is consistent with previous reports that have observed more severe disease in men compared with women. 22
Antibody responses indicate the level of immunological protection against viral infection. For example, the production of specific anti-SARS-CoV-2 antibodies leads to titres that persist for several weeks and slowly decline over time. 28 In our study, some participants that produced both IgG and IgM antibodies showed a slow decline in the levels of both antibodies (data not shown). Although this slow decline was observed in only a small number of cases, it may suggest that, in such cases, it is possible to observe a longer antibody response than has previously been reported. 29 We hypothesized that this prolonged response was due to the continuous exposure of HCWs to SARS-CoV-2 after they had developed an initial antibody response, or possibly to reinfection that had gone unnoticed. 30 However, we could not continue monitoring our participants long-term because their vaccination program had begun.
Our study had several limitations including a small sample size, cross sectional design, and no control group. Also, more studies are required to confirm the validity of using densitometric measurements of band intensities on immunochromatography paper from the LFAs as a measure of antibody titres.
The SARS-CoV-2 pandemic has raised several questions about how to better define its diagnosis and prognosis and improve treatment of post-COVID-19 sequelae.31,32 HCWs are a group of individuals who are at continuous risk of contact with infectious diseases and so adherence to prevention and control measures is vitally important. 33 Therefore, it is essential for hospital staff to follow guidelines established for personal protection and help reduce the spread within the hospital or from the hospital to the community. 34 However, despite measures taken to control SARS-CoV-2, the number of HCWs infected around the world has been high. 35 The dynamics of SARS-CoV-2 infection make it difficult to detect the initial day of infection. Therefore, in the case of HCWs who may acquire SARS-CoV-2 infection in hospital, it is necessary to establish a routine program for detection of the virus in order to avoid risk of infection and spread of COVID-19 to close contacts.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605221099458 for Features of antibody responses after SARS-COV-2 infection in healthcare workers in the first wave of COVID-19 pandemic in Mexico City by Maria del Rocío Thompson-Bonilla, Jorge A León, Martha Beatriz Cárdenas-Turrent, Alba Peña-Thompson, Diana Hanessian-De la Garza, Sergio Zavala-Vega, Juan Xicohtencatl-Cortes, Sara A Ochoa, Ariadnna Cruz-Córdova and José Arellano-Galindo in Journal of International Medical Research
Acknowledgments
The authors are grateful to KPC Biotech-USA for the support received in terms of providing the consumables and Direction of Research at the Hospital Infantil de México Federico Gómez HIM/2021/089.
Footnotes
ORCID iD: José Arellano-Galindo https://orcid.org/0000-0001-7289-0821
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding Sources
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a donation from KPC Biotech USA, No Mex/100 and Direction of Research at the Hospital Infantil de México Federico Gómez HIM/2021/089, and finally we appreciate the trust of all the H. R. “1° de Octubre” HCWs, who decided to participate.
References
- 1.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579: 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control. [Internet]. 2019. [cited 2021 Apr 25]. Available from: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases
- 3.Agren D. Understanding Mexican health worker COVID-19 deaths. Lancet 2020; 396: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varona JF, Madurga R, Peñalver F, et al. Seroprevalence of SARS-CoV-2 antibodies in over 6000 healthcare workers in Spain. Int J Epidemiol 2021; 50: 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tadj A, Lahbib SSM. Our Overall Current Knowledge of Covid 19: An Overview. Microbes, Infection and Chemotherapy 2021; 1: e1262. [Google Scholar]
- 6.Chvatal-Medina M, Mendez-Cortina Y, Patiño PJ, et al. Antibody Responses in COVID-19: A Review. Front Immunol 2021; 12: 633184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin X, Shen J, Dai E, et al. The seroprevalence and kinetics of IgM and IgG in the progression of COVID-19. BMC Immunol 2021; 22: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choe PG, Kim K, Kang CK, et al. Antibody Responses 8 Months after Asymptomatic or Mild SARS-CoV-2 Infection. Emerg Infect Dis 2021; 27: 928–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Medicines Agency. (2020). Guidance on the management of clinical trials during the COVID-19 (Coronavirus) pandemic. [Internet]. 2020 [cited 2022 Apr 28]. Available from (https://ec.europa.eu/health/system/files/2022-02/guidanceclinicaltrials_covid19_en_1.pdf)
- 10.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 11. Characteristics of Health Care Personnel with COVID-19 — United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Living guidance for clinical management of COVID-19. 2021. [Internet publication].
- 13.Montesinos I, Gruson D, Kabamba B, et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J.Clin.Virol 2020; 128: 104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serology Test Evaluation Report for “Karmacare COVID-19 IgM/IgG Rapid Diagnostic Test for the Detection of SARS-CoV-2 IgM/IgG Ab” from Access Bio Inc [Internet]. 2020. [cited 2020 Jun 23]. Available from: https://karmacare.biotech.com
- 15.The Karmacare™ COVID-19 IgM/IgG. Package Insert: [Internet]. 2021 [cited 2021 Jun 15]. Available from: https://karmacare.biotech.com Karmacare COVID-19 IgM/IgG–Instructions for Use (fda.gov).
- 16.Salcedo N, Reddy A, Gomez AR, Bosch I and Herrera BB. Monoclonal antibody pairs against SARS-CoV-2 for rapid antigen test development. PLoS Neglect Trop D, 2022; 16(3): e0010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.COVID-19 Treatment Guidelines. Clinical Spectrum of SARS-CoV-2 Infection. [Internet]. 2020 [cited 2021 Oct 19]. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- 18.Gandhi RT, Lynch JB and Del Rio C. Mild or moderate Covid-19. New Eng J Med. 2020; 383(18): 1757–1766. [DOI] [PubMed] [Google Scholar]
- 19.Choe PG, Kang EK, Lee SY, et al. Selecting coronavirus disease 2019 patients with negligible risk of progression: early experience from non-hospital isolation facility in Korea. Korean J Intern Med 2020; 35: 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montesinos I, Gruson D, Kabamba B, et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J.Clin.Virol 2020; 128: 104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagheri K, Behnam H, Navid O, et al. Prevalence of Anti-SARS-CoV-2 Antibody in Hospital Staff in Double-Center Setting: A preliminary Report of a Cohort Study From Iran. Shiraz E- Med J 2021; 22: e112681. Available from: https://brieflands.com/articles/semj-112681.html. [Google Scholar]
- 22.Kowitdamrong E, Puthanakit T, Jantarabenjakul W, et al. Antibody responses to SARS-CoV-2 in patients with differing severities of coronavirus disease 2019. PloS one 2020; 15: e0240502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020; 20: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu C, Chen X, Cai Y, et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 2020; 180: 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA J Am Med Assoc 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilk AJ, Rustagi A, Zhao NQ, et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med 2020; 26: 1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020; 183: 996–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lumley SF, Wei J, O'Donnell D, et al. The duration, dynamics and determinants of SARS-CoV-2 antibody responses in individual healthcare workers. Clin Infect Dis 2021; 73: e699–e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Post N, Eddy D, Huntley C, et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS One 2020; 15: e0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta V, Bhoyar RC, Jain A, et al. Asymptomatic Reinfection in 2 Healthcare Workers From India With Genetically Distinct Severe Acute Respiratory Syndrome Coronavirus 2. Clin Infect Dis 2021; 73: e2823–e2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babiker A, Marvil CE, Waggoner JJ, et al. The importance and challenges of identifying SARS-CoV-2 reinfections. J Clin Microbiol 2021; 59: e02769–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang FC, Benson CA, Del Rio C, et al. Covid-19—lessons learned and questions remaining. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 2020; 72; 2225–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houghton C, Meskell P, Delaney H, et al. Barriers and facilitators to healthcare workers’ adherence with infection prevention and control (IPC) guidelines for respiratory infectious diseases: a rapid qualitative evidence synthesis. Cochrane Database Syst Rev 2020; 4: CD013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chughtai AA, Seale H, Islam MS, et al. Policies on the use of respiratory protection for hospital health workers to protect from coronavirus disease (COVID-19). Int J Nurs 2020; 105: 103567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erdem H, Lucey DR. Healthcare worker infections and deaths due to COVID-19: A survey from 37 nations and a call for WHO to post national data on their website. Int J Infect Dis 2021; 102: 239–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605221099458 for Features of antibody responses after SARS-COV-2 infection in healthcare workers in the first wave of COVID-19 pandemic in Mexico City by Maria del Rocío Thompson-Bonilla, Jorge A León, Martha Beatriz Cárdenas-Turrent, Alba Peña-Thompson, Diana Hanessian-De la Garza, Sergio Zavala-Vega, Juan Xicohtencatl-Cortes, Sara A Ochoa, Ariadnna Cruz-Córdova and José Arellano-Galindo in Journal of International Medical Research