Abstract
Mucoepidermoid carcinoma (MEC) is the most common malignant tumour of the salivary gland, primarily involving the parotid gland. Here, the cases of two patients, aged 47 and 67 years, respectively, who underwent surgery for pathologically confirmed Warthin-like MEC of the parotid gland between January 2019 and December 2019 in Anyang Tumour Hospital, are described. In each case, the tumour consisted of epithelial and lymphoid cell components, covered with two or more layers of epithelium, with visible scattered mucous cells, and lymphoid stroma with a large number of lymphocytes and germinal centres formed. Most importantly, the tumours lacked the well-organized, bilayered oncocytic epithelial structure that is characteristic of Warthin’s tumour. Mastermind like transcriptional coactivator 2 (MAML2) gene rearrangements were identified in the tumour cells using break-apart fluorescence in situ hybridization (FISH) probes, confirming the diagnosis of Warthin-like MEC. Post-operatively, patients have remained disease free for 31 and 27 months, respectively. Warthin-like MEC of the parotid gland is rare and is often misdiagnosed as metaplastic Warthin’s tumour. Diagnosis depends mainly on the unique clinicopathologic features together with FISH analyses.
Keywords: Warthin’s tumour, mucoepidermoid carcinoma, parotid neoplasm, fluorescence in situ hybridization, MAML2, break-apart probes
Introduction
Mucoepidermoid carcinoma (MEC) is the most common malignant tumour of the salivary gland, most frequently involving the parotid gland. 1 Several histological variations of MEC have been reported, including Warthin-like, clear-cell, oncocytic and sclerosing variants.2–4 Warthin-like MEC was proposed by Ishibashi et al in 2015, 5 following their analyses of 15 cases diagnosed as metaplastic Warthin’s tumour, among which, five were found to possess the CREB regulated transcription coactivator 1 (CRTC1)–mastermind like transcriptional coactivator 2 (MAML2) gene fusion. Histological analyses of the fusion-positive and fusion-negative tumours revealed an almost identical morphology of the metaplastic epithelium, however, the oncocytic bilayered tumour epithelium typical of Warthin’s tumour was always found in fusion-negative tumours, but was not observed in fusion-positive tumours. 5 Consequently, the term ‘Warthin tumour-like MEC’ was proposed for tumours that are positive for this gene fusion. Because Warthin-like MEC of the parotid gland is a rare tumour that is often misdiagnosed as metaplastic Warthin’s tumour, two cases of Warthin-like MEC diagnosed by the Pathology Department of Anyang Tumour Hospital, between January 2019 and December 2019, are described. The clinicopathologic features, molecular diagnosis and differential diagnosis of Warthin-like MEC are discussed, in order to improve the understanding of this tumour type.
Case reports
Case 1
A 47-year-old Chinese male presented at Anyang Tumour Hospital, The Affiliated Anyang Tumour Hospital of Henan University of Science and Technology in June 2019, with a lump under his right ear, which he had noticed with occasional pain for more than 3 months previously. The patient was a non-smoker and had no self-reported history of radiation or any other relevant family history. Clinical examination revealed a firm lump of 20 × 20 mm in the right parotid region without tenderness. The contralateral parotid gland and surrounding lymph nodes were not enlarged.
Magnetic resonance imaging showed a 22 × 14-mm mass in the right parotid region with clear boundary and homogeneous echo inside, suggesting a benign tumour. The patient then underwent resection of the tumour, which was noted to be generally grey and solid, with a clear boundary between the tumour and the surrounding parotid gland ( Figure 1a ). The patient was initially diagnosed with metaplastic Warthin’s tumour.
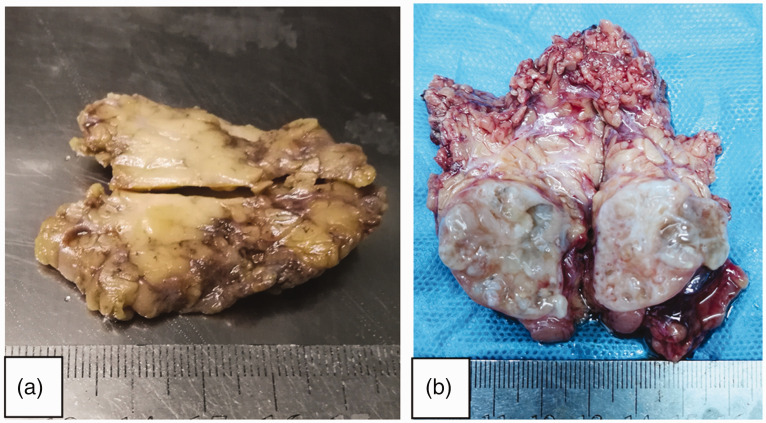
Figure 1.
Representative images showing gross morphology of Warthin-like mucoepidermoid carcinoma tumours of the parotid gland: (a) the tumour in Case 1 was grey and solid with a clear boundary between the tumour and the surrounding parotid gland; and (b) the tumour in Case 2 was a 30-mm large, tan-white, cystic solid mass located in the left parotid gland.
The resected tumour tissue was further analysed by the pathology department of Anyang Tumour Hospital, The Affiliated Anyang Tumour Hospital of Henan University of Science and Technology. The specimen was fixed in 10% buffered formalin, and processed and embedded in paraffin following standard protocols. Tissue sections of 4-µm thickness were cut and stained with haematoxylin and eosin (H&E). Immunohistochemistry was performed using automated equipment and the following prediluted primary antibodies (all Roche Diagnostics, Rotkreuz, Switzerland) according to the manufacturer’s instructions: mouse anti-human p63, mouse anti-human cytokeratin (CK)5/6, mouse anti-human CK7, mouse anti-human carcinoembryonic antigen (CEA), and mouse anti-human E3 ubiquitin-protein ligase MIB1 (MIB1)/proliferation marker protein Ki-67 (Ki67). Negative and positive controls were included. Positive expression was detected by adding substrate (Roche Diagnostics) to induce a chromogenic reaction, shown by brown and yellow staining of appropriate cell components: CK5/6, CK7 and CEA were stained in the cytoplasm, while p40 (p63 subtype), p63 and Ki67 were stained in the nucleus. To further classify the tumour type, tissue sections were analysed for MAML2(11q21) rearrangement by fluorescence in situ hybridization (FISH) using a commercially available MAML2(11q21) dual-colour break-apart probe (Wuhan Kanglu Biotechnology Co., Ltd, Wuhan, China), according to the manufacturer’s instructions.
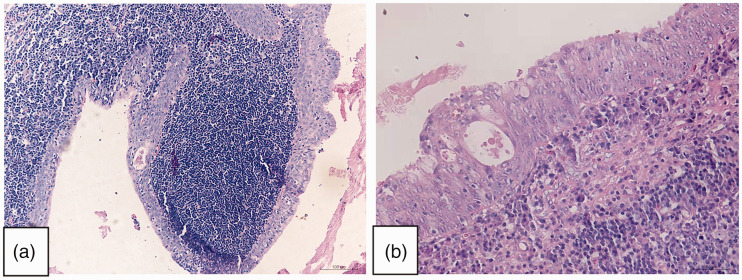
Histological analysis revealed that the tumour had a clear boundary, part of which was cystic in structure and consisted of epithelial and lymphoid cell components. The tumour was covered with two or more layers of epithelium, with visible scattered mucous cells, and lymphoid stroma with a large number of lymphocytes and germinal centres. Fibrosis could be observed in the background of the focal area, and the cystic structure of stratified epithelium showed an infiltrative pattern ( Figure 2a–d ). The tumour lacked the well-organized, bilayered oncocytic epithelial organization that is characteristic of Warthin’s tumour.
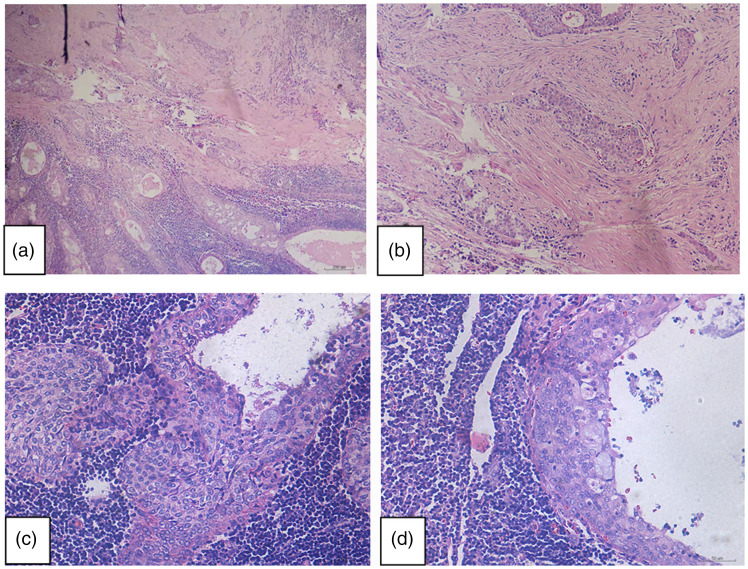
Figure 2.
Representative photomicrographs from histological examination of haematoxylin and eosin-stained Warthin-like mucoepidermoid carcinoma tumour tissue in Case 1: (a and b) the tumour displayed a cystic structure comprising epithelial and lymphoid cell components. Fibrosis background can be seen in the focal area, in which the cystic structure of stratified epithelium showed infiltrative growth (original magnification, × 100); (c and d) the tumour was covered with two or more layers of epithelium, with visible scattered mucous cells and lymphoid stroma with a large number of lymphocytes and germinal centres (original magnification, × 200).
Immunohistochemical analysis showed that the tumour cells were positive for p40, p63, CK5/6 and CK7, and scattered mucous cells showed CEA-positive staining ( Figure 3 ). Ki67 expression was present in <2% of the tumour cells. MAML2 gene rearrangement was identified in the tumour cells by the FISH break-apart probes ( Figure 4 ). The patient was subsequently diagnosed with Warthin-like MEC, confirmed by at least two senior pathologists (RL and HY).
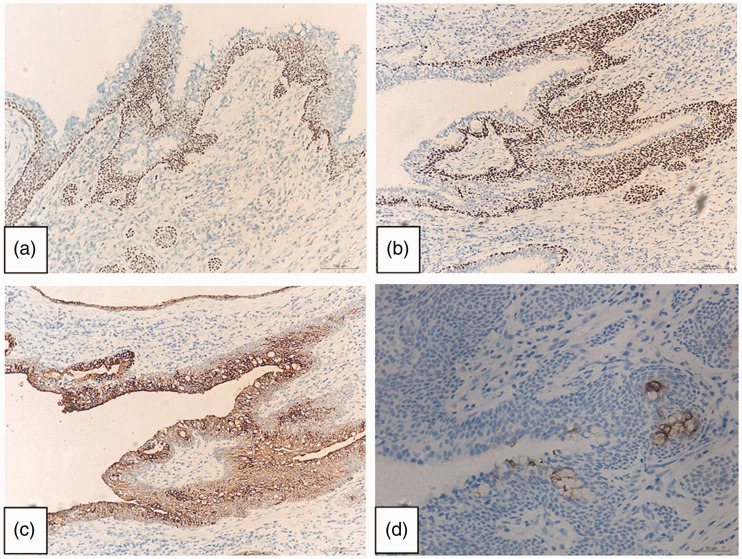
Figure 3.
Representative photomicrographs from immunohistochemistry of the Warthin-like mucoepidermoid carcinoma tumour tissue: (a) positive p40 staining; (b) positive p63 staining; (c) positive cytokeratin 7 staining (original magnification, × 100); and (d) positive carcinoembryonic antigen staining showing scattered mucus cells (original magnification, × 200).
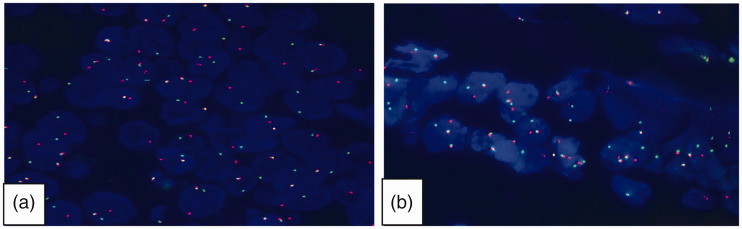
Figure 4.
Mastermind like transcriptional coactivator 2 (MAML2) gene rearrangements were identified in the tumour cells of (a) case 1 and (b) case 2 using fluorescence in situ hybridization break-apart probes. Most tumour cells showed a yellow, red and green signal, indicating rearrangements of the MAML2 gene (original magnification, × 1000 by oil immersion lens).
At the time of writing, the patient has remained disease free during 31 months of post-operative follow-up.
Case 2
A 67-year-old Chinese female presented at Anyang Tumour Hospital, The Affiliated Anyang Tumour Hospital of Henan University of Science and Technology in October 2019, with a lump in the left neck, which she had noticed inadvertently for the previous 3 months. The patient was a non-smoker with no self-reported history of radiation or any other relevant family history. Clinical examination revealed a solid cystic lump of 30 × 20 mm in the left parotid gland. Upon examination, the right parotid gland was found to be normal and peripheral lymph nodes were not enlarged.
Computed tomography of the left parotid region showed a lesion with relatively clear boundary, measuring 2.5 × 2 cm, with an uneven internal echo, suggesting a benign tumour ( Figure 1b ). Thus, the lesion was considered to be a benign tumour of the left parotid gland and the patient underwent surgical resection of the tumour. Gross examination of the surgical specimen showed a 30 mm, tan-white, solid cystic mass located in the left parotid gland ( Figure 1b ), and the patient was initially diagnosed with metaplastic Warthin’s tumour.
The resected tumour tissue was further analysed by the pathology department of Anyang Tumour Hospital as described for Case 1. Similar to Warthin’s tumour, the tumour in this case comprised multilocular cystic epithelium surrounded by prominent lymphoid stroma and germinal centres. However, at high magnification, some areas of the lining epithelial cells showed features of stratified squamous cells and mucocytes with mild cytological atypia and focal complex architecture ( Figure 5a and b ). No lymphovascular invasion, perineural invasion, or lymph node metastasis was identified. The tissue lacked the bilayered oncocytic epithelial organization that is characteristic of Warthin’s tumour.
Figure 5.
Representative photomicrographs from histological examination of haematoxylin and eosin-stained Warthin-like mucoepidermoid carcinoma tumour tissue in Case 2: (a and b) the tumour was composed of multilocular cystic epithelial hyperplasia, surrounded by prominent lymphoid matrix, germinal centre, stratified squamous cells and scattered mucous cells (A [original magnification, × 100] and B [original magnification, × 200]).
Immunohistochemical analyses, performed as described for Case 1, showed that the tumour cells were positive for p40, p63, CK5/6 and CK7, with scattered mucous cells that were positive for CEA ( Figure 3 ). As found in Case 1, <2% of the tumour cells were positive for Ki67, and MAML2 rearrangements were revealed by FISH ( Figure 4 ). A diagnosis of Warthin-like MEC was confirmed by at least two senior pathologists (RL and HY).
At the time of writing, the patient has remained disease free during 27 months of post-operative follow-up.
The study was approved by the Institutional Research Ethics Committee of Anyang Tumour Hospital (approval No. 2022WZ06K01). Written informed consent was obtained from both patients for publication of the case reports and any accompanying images. The reporting of this study conforms to CARE guidelines. 6
Discussion
Warthin-like MEC is an uncommon entity first described by Ishibashi et al. 5 in 2015, following their analyses of 15 tumours initially diagnosed as metaplastic Warthin’s tumours. Among the 15 cases, five were found to be positive for CRTC1–MAML2 gene fusions, which they proposed to be termed ‘Warthin tumour-like MEC’.
Clinical features
Warthin-like MEC appears to be most common in women aged 17–60 years. 7 The patients described in the present study, one male and one female, were at the older end of the spectrum, inadvertently finding the tumour in the parotid gland area, and the cut surface of each tumour was tan or grey-white.
Diagnosis
In the present cases, each tumour had a clear boundary and was characterised by multiloculated cystic epithelial proliferation surrounded by prominent lymphoid stroma with germinal centres. Stratified squamous cells and scattered mucous cells were observed in the epithelial components, and fibrosis was noted in the background of the focal area with infiltrative growth of stratified epithelium. Perineural invasion and necrosis have been observed in some cases, but these features are rare. 8 Most importantly, Warthin-like MEC lacks the well-organized, bilayered oncocytic epithelial organization that is characteristic of Warthin’s tumor. 9
Immunohistochemically, the tumour cells in the present cases were positive for p40, p63, CK5/6 and CK7. Scattered mucous cells were shown to express CEA, and Ki67 highlighted <2% of the tumour cells. Both cases were initially misdiagnosed as metaplastic Warthin’s tumour, and were later proved to be Warthin-like MEC by FISH detection of MAML2 gene rearrangements. When squamous cells and mucocytes are present in a Warthin-like tumour with atypical oncocytic bilayered epithelium, a diagnosis of Warthin-like MEC should be considered. The diagnosis of MEC should be confirmed using FISH to detect the presence of MAML2 rearrangements.
Differential diagnosis
Warthin’s tumour with mucinous and squamous metaplasia (metaplastic Warthin’s tumour)
Warthin’s tumour is the second most common benign neoplasm of the salivary glands, composed of ducts lined with oncocytic epithelial cells, and papillary and cystic structures in a lymphoid stroma. It usually arises as a slow-growing, painless mass in the parotid area of older male smokers. The epithelial component is formed of inner columnar and outer cuboidal cells, and limited foci of squamous, mucous, ciliated and sebaceous cells can be present. 10 However, the presence of both squamous and mucinous metaplasia in Warthin’s tumour is extremely rare, at an incidence of 0.2%. 11 A Warthin’s tumour that shows large areas of squamous metaplasia is called metaplastic or infarct Warthin’s tumour, and the presence of typical bilayered epithelium is considered to be the most useful distinguishing morphological feature in such cases.5,7 Most importantly, all metaplastic Warthin’s tumours are negative for MAML2 gene fusions, and MAML2 gene rearrangement is a useful diagnostic marker for distinguishing between Warthin’s tumour and Warthin-like MEC. 12
Branchial cleft cyst
Branchial cleft cyst is a rare disease of the head and neck, and although present at birth, many cases do not become apparent until later in childhood or adolescence. 13 The term ‘branchial cleft cyst’ refers to lesions that can be considered synonymous with the cervical lymphoepithelial cyst. 14 Considering the embryogenic origin, the location of the first branchial cleft cyst will be in the vicinity of the external auditory canal, parotid gland or the angle of the mandible. 15 Macroscopically, the lesion is usually a small round nodule with complete capsule, with either a single, or multiple, capsule cavities. Microscopically, branchial cleft cyst is not related to the parotid gland, and is mainly composed of cystic and lymphatic stroma, with few epithelial components. The epithelium lining the cyst is composed of flat or thin squamous epithelial cells or columnar epithelium, and the fibrous capsule wall comprises a large amount of lymphatic stroma that may be accompanied by the formation of lymphatic follicles and germinal centres.
Lymphoepithelial carcinoma
Lymphoepithelial carcinoma is characterized by a syncytial growth pattern and dense, non-neoplastic lymphoid infiltration, and its occurrence may sometimes be related to Epstein-Barr virus infection. Microscopically, tumours usually have no capsule and invade the surrounding normal tissues. Tumour epithelial cells are arranged in infiltrative sheet, nest and island structures, with characteristic dense lymphoid stroma, often accompanied by reactive lymphoid follicular formation. Individual cells or islands/sheets of cells display a classic and characteristic pattern, arranged as a syncytium of crowded, large, undifferentiated cells with open nuclear chromatin, adjacent to, or blended with, a lymphoplasmacytic stroma. 16 In most cases, tumour cells are atypical, and prone to mitosis and necrosis, accompanied by a high Ki67-positive index. 16
Sebaceous lymphadenoma
Sebaceous lymphadenoma tumours are comprised of lymphoid tissue with epithelial islands of varying size and shape, some of which form small cysts. Within rare epithelial islands, groups of sebaceous cells may be observed. The background lymphoid portion of the lesion is mainly composed of small lymphocytes with occasional germinal centre formation. 17
Non-sebaceous lymphadenoma
In cases of non-sebaceous lymphadenoma, there is a clear boundary between the tumour and the salivary gland. Tubuloglandular structures and many lymphocytes are observed in the tumour, some of which form reactive lymph follicles with germinal centres. Small to medium-sized cysts and intraepithelial lymphocytes are common, and may be accompanied by eosinophilic, hyaline, and basement membrane-like material. 18
Treatment and prognosis
In conclusion, the present report describes two cases of Warthin-like variants of MEC, both of which were initially misdiagnosed as metaplastic Warthin’s tumour, and were later proved to be Warthin-like MEC by FISH detection of MAML2 gene rearrangement using break-apart probes. When squamous cells and mucocytes are present in a Warthin-like tumour with atypical oncocytic multilayered epithelium, a diagnosis of Warthin-like MEC should be considered. The presence of MAML2 rearrangement should be detected by FISH to confirm the MEC diagnosis. Of note, MAML2 gene fusion has been shown to occur mostly in cases of low-level MEC. 19 To date, only one case of recurrence has been reported in the published literature, therefore, there is no need for aggressive treatment after the tumour is completely removed.
Footnotes
Declaration of conflicting interest: The author declares that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Ruixue Lei https://orcid.org/0000-0002-1802-6589
Data accessibility
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
References
- 1.Brandwein-Gensler M, Bell D, Inagaki H, et al. Mucoepidermoid carcinoma. In: El-Naggar AK, Chan JK, Grandis JR, Takata T, Slootweg PJ (eds). WHO Classification of Head and Neck Tumours: WHO Classification of Tumours. 4th ed, vol 9. Lyon: IARC, 2017, pp.163–164.
- 2.Liao X, Haghighi P, Coffey CS, et al. Rare case of exclusively oncocytic mucoepidermoid carcinoma with MAML2 translocation. Rare Tumors 2016; 8: 6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ide F, Mishima K, Saito I. Mucoepidermoid carcinoma with spindle cell change: a low-grade lesion potentially mistaken for sarcomatoid dedifferentiation. Head Neck Pathol 2008; 2: 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian W, Yakirevich E, Matoso A, et al. IgG4+ plasma cells in sclerosing variant of mucoepidermoid carcinoma. Am J Surg Pathol 2012; 36: 973–979. [DOI] [PubMed] [Google Scholar]
- 5.Ishibashi K, Ito Y, Masaki A, et al. Warthin-like mucoepidermoid carcinoma: a combined study of fluorescence in situ hybridization and whole-slide imaging. Am J Surg Pathol 2015; 39: 1479–1487. [DOI] [PubMed] [Google Scholar]
- 6.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 7.Zhang D, Liao X, Tang Y, et al. Warthin-like mucoepidermoid carcinoma of the parotid gland: unusual morphology and diagnostic pitfalls. Anticancer Res 2019; 39: 3213–3217. [DOI] [PubMed] [Google Scholar]
- 8.Heatley N, Harrington KJ, Thway K. Warthin tumor-like mucoepidermoid carcinoma. Int J Surg Pathol 2018; 26: 31–33. [DOI] [PubMed] [Google Scholar]
- 9.Bishop JA, Cowan ML, Shum CH, et al. MAML2 rearrangements in variant forms of mucoepidermoid carcinoma: ancillary diagnostic testing for the ciliated and Warthin-like variants. Am J Surg Pathol 2018; 42: 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Naggar A, Chan J, Grandis J, et al (eds). WHO classification of head and neck tumours: WHO classification of tumours. 4th ed, vol 9. Lyon: IARC, 2017, p.189. [Google Scholar]
- 11.Rotellini M, Paglierani M, Pepi M, et al. MAML2 rearrangement in Warthin’s tumor: a fluorescent in situ hybridisation study of metaplastic variants. J Oral Pathol Med 2012; 41: 615–620. [DOI] [PubMed] [Google Scholar]
- 12.Bieńkowski M, Kunc M, Iliszko M, et al. MAML2 rearrangement as a useful diagnostic marker discriminating between Warthin tumour and Warthin-like mucoepidermoid carcinoma. Virchows Arch 2020; 477: 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Perez LM, Prats-Golczer VE, Montes Carmona JF, et al. Bilateral first branchial cleft anomaly with evidence of a genetic aetiology. Int J Oral Maxillofac Surg 2014; 43: 296–300. [DOI] [PubMed] [Google Scholar]
- 14.Thomaidis V, Seretis K, Tamiolakis D, et al. Branchial cysts. A report of 4 cases. Acta Dermatovenerol Alp Pannonica Adriat 2006; 15: 85–89. [PubMed] [Google Scholar]
- 15.Panchbhai AS, Choudhary MS. Branchial cleft cyst at an unusual location: a rare case with a brief review. Dentomaxillofac Radiol 2012; 41: 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whaley RD, Carlos R, Bishop JA, et al. Lymphoepithelial carcinoma of salivary gland EBV-association in endemic versus non-endemic patients: a report of 16 cases. Head Neck Pathol 2020; 14: 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maruyama S, Cheng J, Inoue T, et al. Sebaceous lymphadenoma of the lip: report of a case of minor salivary gland origin. J Oral Pathol Med 2002; 31: 242–243. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, He J, Zhang C, et al. Lymphadenoma of the salivary gland: report of 10 cases. Oncol Lett 2014; 7: 1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérez‐de-Oliveira ME, Wagner VP, Araújo ALD, et al. Prognostic value of CRTC1‐MAML2 translocation in salivary mucoepidermoid carcinoma: systematic review and meta-analysis. J Oral Pathol Med 2020; 49: 386–394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.