Abstract
The introduction of extended factor IX (FIX) products has significantly facilitated the treatment of hemophilia B patients. However, optimal perioperative management remains a topic of hot debate, particularly in surgeries with high bleeding risk. For the first time, we report here a patient with mild hemophilia B and degenerative aneurysms of aortic root and ascending aorta undergoing elective Bentall’s operation with full cardiopulmonary bypass, who was successfully managed with eftrenonacog alfa (Alprolix®), a recombinant FIX Fc fusion protein (rFIXFc). rFIXFc could safely be monitored using the Pathromtin SL aPTT-reagent. No significant bleeding was noted intraoperatively despite systemic heparinization as well as postoperatively. Higher doses of rFIXFc were inevitable to reach target FIX levels intraoperatively, whereas in the post-surgery setting stable FIX concentrations were maintained with only few rFIXFc injections facilitating fast wound healing and remobilization of the patient.
Keywords: Bentall’s operation, case report, eftrenonacog alfa
Introduction
Hemophilia B is a chromosome X linked diathesis as a result of coagulation factor IX (FIX) deficiency characterized by excess bleeding and increased morbidity and mortality. 1 Standard treatment with plasma-derived or recombinant FIX concentrates effectively reduces the risk of bleeding and has contributed to a greatly enhanced life expectancy in affected patients. 2 Consequently, age-related degenerative cardiac health conditions will likely accumulate in daily clinical practice.
However, data on how to optimally approach hemophilia B patients undergoing cardiac surgery are lacking, and perioperative management remains a topic of ongoing debate. 3
For the first time, we report here the case of a patient with mild hemophilia B and degenerative aneurysms of aortic root and ascending aorta undergoing elective Bentall’s operation with full cardiopulmonary bypass and systemic heparinization for 172 min, who was successfully managed with eftrenonacog alfa (Alprolix), a recombinant FIX Fc fusion protein (rFIXFc) with extended terminal half-life. 4
Preoperatively, the 60-year-old Caucasian patient (100 kg body weight) was referred to our comprehensive care hemophilia center to plan target FIX activity level, dosage, and duration of FIX substitution. Baseline FIX activity level was 11%, and only traumatic bleeding complications (i.e. knife cutting) were reported in the past. Hence, the patient has received FIX replacement only on demand. FIX inhibitors were not detected, and there was no evidence for chronic liver disease. Two years ago, the patient was perioperatively treated with standard recombinant FIX for elective extraction of three teeth without any bleeding complications, and FIX recovery was determined 1% per IU FIX and kg body weight by plasma-derived FIX (Nonacog alfa) at this time.
Due to extended risk of bleeding and thrombosis associated with perioperative full cardiopulmonary bypass and systemic heparinization during Bentall’s procedure, we opted for substitution with rFIXFc with extended terminal half-life to help maintain more stable FIX concentrations with less frequent perioperative intravenous infusion demand. The treatment plan was discussed with our colleagues from the departments of anesthesiology and cardiac surgery as well as with the patient.
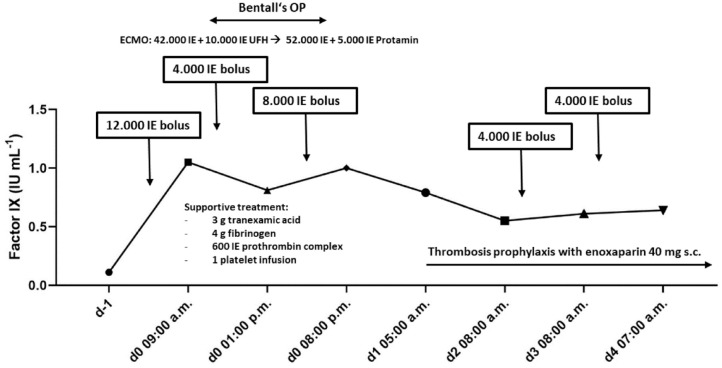
Just 30 min before surgery (09:00 a.m., hemoglobin level 14.1 g/dl), a 12.000 IU rFIXFc loading dose (120 IU/kg body weight) was administered resulting in a FIX level activity of 100%. With an initial bedside activated clotting time of 113 s, intraoperative systemic heparinization and full cardiopulmonary bypass priming were accomplished with 42.000 IU and 10.000 IU of unfractionated heparin intravenously, respectively.
Four hours after start of surgery (1:00 p.m.), another 4.000 IU of rFIXFc were given after the decline of the FIX level to 81% and additional 8.000 IU of rFIXFc due to prolonged bleeding at the end of surgery (4:00 p.m., hemoglobin level 11.3 g/dl). Concomitantly, the patient received 3 g tranexamic acid, 4 g fibrinogen, 600 IU prothrombin complex concentrates and one platelet infusion. CellSaver was used to avoid intraoperative red cell transfusion. These supportive treatments were indicated and administered under control of the surgeon and anesthesiologist as usual in our center. Systemic heparinization was neutralized with 52.000 IU of protamine, and another 5.000 IU were given adapted to prolonged activated clotting time (138 s). In summary, the bleeding was comparable to non-hemophilic patients undergoing this procedure. Besides hemophila-specific FIX administration, the patient received coagulation factor replacement according to the local treatment standards during these procedures.
Four hours after the end of surgery (19:57 p.m.), FIX level activity was 100% and 79% in the morning hours of postoperative Day 1 (05:00 a.m.), respectively. On Day 2, the patient received 4.000 IU of rFIXFc to keep FIX level above 60% according to World Federation of Hemophilia (WFH) guidelines (FIX activity: 55% at 08:00 a.m.). On Day 3, one last infusion of 4.000 IU rFIXFc was administered for cardiac surgery drain removal (FIX level activity: 72%).
Periprocedural FIX level activity with regard to rFIXFc administrations is illustrated in Figure 1.
Figure 1.
Bolus dosing of eftrenonacog alfa versus FIX levels (IU mL–1) in a patient (100 kg body weight) with hemophilia B undergoing cardiac surgery.
In the post-surgery period, the patient experienced neither thrombotic nor bleeding complications, which was evaluated by physical examination and sonography. Thrombosis prophylaxis with enoxaparin 40 mg subcutaneously was given starting on postoperative Day 1. Post-surgery thrombosis prophylaxis was given as long as the patient was on intensified FIX product prophylaxis, which is standard protocol at our center. Of note, laboratory analysis did not detect FIX inhibitor formation 6 weeks after surgery.
Perioperative substitution with FIX concentrates has decreased surgery-associated mortality to approximately 1% in hemophilia B patients. 5 However, lacking evidence-based recommendations, optimal perioperative treatment remains under hot debate. Management can be complicated by excess bleeding, thrombosis formation, and inhibitor development. 3 rFIXFc demonstrated a prolonged half-life and lower consumption compared with conventional recombinant FIX, maintaining appropriate FIX levels with reduced infusion frequency. 4 In particular, subgroup analysis of the pivotal phase III study confirmed safety and efficacy with prolonged dosing intervals for perioperative hemostasis in patients undergoing major surgery, mostly orthopedic procedures. 6 For major cardiac surgery, however, an issue likely becoming more prevalent in an aging hemophilia population, data are scarce and clinical decision-making is based on few case reports and personal experience.
Previous case reports included off-pump coronary artery bypass grafting (CABG) with rFIX bolus substitution hypothesizing that off-pump surgery would interfere less with hemostasis than traditional CABG 7 and a biological aortic valve replacement for aortic stenosis with continuous recombinant FIX infusion in order to avoid broad fluctuations in factor levels. 8 Another patient undergoing CABG with recombinant FIX bolus substitution experienced aortic dissection on Day 2 post-surgery with a quite low FIX level of 37%. 9 For the first time, we report the successful management of a patient who received rFIXFc for extensive cardiac surgery requiring full cardiopulmonary bypass and systemic heparinization.
The WFH recommends a target FIX level of 40–60% for the first 3 days following major surgery, then 30–50% on Days 4–6, and 20–40% on Days 7–14. These threshold values, however, are mainly derived from data of orthopedic interventions, and specific recommendations for cardiac surgeries are missing. 10
Given the extended bleeding risk associated with Bentall’s operation, we selected 100% as target FIX level after loading dose, although supranormal FIX levels may increase the thromboembolic risk. 11
In accordance with WFH guidelines, factor dosing thereafter did not rely on specific target-level activity thresholds, but it was rather determined by clinical need during and after surgery ahead of laboratory results. rFIXFc with prolonged half-life and longer dosing intervals was preferred to rFIX in order to avoid wide fluctuations in factor-level activity perioperatively.
Adequate FIX activity monitoring is critical during high-risk surgeries. The FIX activity in our patient was monitored using the Pathromtin SL aPTT-reagent (Siemens, Marburg, Germany). Acceptable recovery values for rFIXFc have been previously shown, with a trend to underestimate values by approximately 20–25% in target dilutions of 20–150%. 12 Due to this encouraging reliability and availability of the test, rFIXFc was preferred to other FIX products with prolonged half-life.
Relatively higher rFIXFc doses than expected were necessary to reach WFH-recommended target FIX levels in our patient, at least in part related to the large volume of distribution of the rFIXFc and possibly a result of inevitably surgery-associated bleeding episodes and dilution effects from crystalloid fluids. Hence, no additional benefit may be derived from rFIXFc when used in surgeries with inherently higher bleeding. On the contrary, only one dose application required for drain removal, wound healing, and mobilization in our patient may illustrate a beneficial role in the post-surgery period when stable FIX concentrations seem favorable to allow adequate tissue repair.
In summary, our case report suggests feasibility and safety of perioperative FIX substitution with rFIXFc in hemophilia B patients undergoing cardiac surgery. Considering the higher cost of the product, however, higher drug doses necessary for intraoperative coagulation compensation than expected with prolonged half-life may point toward a beneficial role rather in the post-surgery setting than within surgeries when associated with a generally higher risk of bleeding.
Acknowledgments
The authors thank the patient who kindly approved the publication of this case report.
Footnotes
ORCID iDs: Jan-Paul Bohn  https://orcid.org/0000-0002-2066-7377
https://orcid.org/0000-0002-2066-7377
Clemens Feistritzer  https://orcid.org/0000-0001-5182-7217
https://orcid.org/0000-0001-5182-7217
Contributor Information
Jan-Paul Bohn, Department of Internal Medicine V, Hematology and Oncology, Medical University of Innsbruck, Anichstrasse 35, A-6020 Innsbruck, Austria.
Anna Fiala, Department of Anesthesiology and Critical Care Medicine, Medical University of Innsbruck, Innsbruck, Austria.
Sebastian Bachmann, Central Institute of Clinical and Chemical Laboratory Diagnostics, University Hospital of Innsbruck, Innsbruck, Austria.
Christian Irsara, Central Institute of Clinical and Chemical Laboratory Diagnostics, University Hospital of Innsbruck, Innsbruck, Austria.
Dominik Wolf, Department of Internal Medicine V, Hematology and Oncology, Medical University of Innsbruck, Innsbruck, Austria.
Clemens Feistritzer, Department of Internal Medicine V, Hematology and Oncology, Medical University of Innsbruck, Innsbruck, Austria.
Declarations
Ethics approval and consent to participate: The presented work complies with the guidelines for human studies, and the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The patient provided written informed consent for publication of the case presentation. According to the local regulations, approval by the local ethical committee is not required for publication of case reports.
Consent for publication: The patient provided written informed consent for publication of the case presentation.
Author contribution(s): Jan-Paul Bohn: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Validation; Visualization; Writing – original draft; Writing – review & editing.
Anna Fiala: Data curation; Formal analysis; Investigation; Resources; Supervision; Validation; Writing – original draft; Writing – review & editing.
Sebastian Bachmann: Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Christian Irsara: Data curation; Investigation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Dominik Wolf: Supervision; Validation; Writing – review & editing.
Clemens Feistritzer: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: CF reports research grant, reimbursement for attending symposium, and consulting fees from Sobi. The remaining authors declare that they have no conflict of interest.
Availability of data and materials: The data are not publicly available due to privacy data protection regulations.
References
- 1. Giangrande P. Haemophilia B: Christmas disease. Exp Opin Pharmacother 2005; 6: 1517–1524. [DOI] [PubMed] [Google Scholar]
- 2. Panicker J, Warrier I, Thomas R, et al. The overall effectiveness of prophylaxis in severe haemophilia. Haemophilia 2003; 9: 272–278. [DOI] [PubMed] [Google Scholar]
- 3. Neufeld EJ, Solimeno L, Quon D, et al. Perioperative management of haemophilia B: a critical appraisal of the evidence and current practices. Haemophilia 2017; 23: 821–831. [DOI] [PubMed] [Google Scholar]
- 4. Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med 2013; 369: 2313–2323. [DOI] [PubMed] [Google Scholar]
- 5. Bastounis E, Pikoulis E, Leppäniemi A, et al. General surgery in haemophiliac patients. Postgrad Med J 2000; 76: 494–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Powell JS, Apte S, Chambost H, et al. Long-acting recombinant factor IX Fc fusion protein (rFIXFc) for perioperative management of subjects with haemophilia B in the phase 3 B-LONG study. Br J Haematol 2015; 168: 124–134. [DOI] [PubMed] [Google Scholar]
- 7. Fernández-Caballero M, Martinez MF, Oristrell G, et al. Off-pump technique and replacement therapy for coronary artery bypass surgery in a patient with hemophilia B. J Thromb Thrombolysis 2019; 48: 299–302. [DOI] [PubMed] [Google Scholar]
- 8. Krakow EF, Walker I, Lamy A, et al. Cardiac surgery in patients with haemophilia B: a case report and review of the literature. Haemophilia 2009; 15: 108–113. [DOI] [PubMed] [Google Scholar]
- 9. Donahue BS, Emerson CW, Slaughter TF. Elective and emergency cardiac surgery on a patient with hemophilia B. J Cardiothorac Vasc Anesth 1999; 13: 92–97. [DOI] [PubMed] [Google Scholar]
- 10. Srivastava A, Santagostino E, Dougall A, et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020; 26: 1–158. [DOI] [PubMed] [Google Scholar]
- 11. Girolami A, Ferrari S, Sambado L, et al. Myocardial infarctions and other acute coronary syndromes in rare congenital bleeding disorders: a critical analysis of all reported cases. Clin Appl Thromb/Hemos 2014; 21: 359–364. [DOI] [PubMed] [Google Scholar]
- 12. Sinegre T, Trayaud A, Tardieu M, et al. Measurements of eftrenonacog alfa by 19 different combinations reagents/instrument: a single-centre study. Haemophilia 2020; 26: 543–552. [DOI] [PubMed] [Google Scholar]