Abstract
Introduction:
Oncogenic alterations in RET occur in 1–2% of non-small-cell lung cancers (NSCLCs). The efficacy and safety of the first-in-class, highly selective, and potent RET inhibitor selpercatinib in Chinese patients with RET fusion-positive NSCLC remains unknown.
Methods:
In this open-label, multicenter, phase II study (NCT04280081), patients with advanced RET-altered solid tumors received selpercatinib (160 mg orally twice daily) in a 28-day cycle. The primary endpoint was independent review committee (IRC)-assessed objective response rate (ORR; Response Evaluation Criteria in Solid Tumors v1.1). Secondary endpoints included duration of response, central nervous system (CNS) response, and safety. Efficacy against NSCLC was assessed in the primary analysis set (PAS; centrally confirmed RET status) and in all enrolled patients with NSCLC.
Results:
Of 77 enrolled patients, 47 had RET fusion-positive NSCLC. After 9.7 months of median follow-up, IRC-assessed ORR in the PAS (n = 26) was 69.2% [95% confidence interval (CI), 48.2–85.7] and 94.4% of responses were ongoing; the ORR was 87.5% and 61.1% in treatment-naïve and pre-treated patients, respectively. IRC-assessed ORR in all patients with NSCLC (n = 47) was 66.0% (95% CI, 50.7–79.1). Among five patients with measurable CNS metastases at baseline, four (80%) achieved an IRC-assessed intracranial response. In the safety population (n = 77), most treatment-emergent adverse events (TEAEs) were grade 1 or 2. The most common grade ⩾3 TEAE was hypertension (19.5%). Three (3.9%) patients discontinued therapy due to treatment-related AEs; no deaths occurred due to treatment-related AEs.
Conclusion:
Selpercatinib, with potent and durable antitumor activity including intracranial activity, was well tolerated in Chinese patients with RET fusion-positive NSCLC, consistent with LIBRETTO-001 (ClinicalTrials.gov: NCT04280081).
Keywords: Chinese, non-small-cell lung cancer, RET fusion, selective RET inhibitor, selpercatinib
Introduction
Lung cancer is the second most common cancer both in China and worldwide and the leading cause of cancer-related death in many countries.1–3 Non-small-cell lung cancer (NSCLC) accounts for the vast majority of lung cancer cases. Multiple oncogenic driver mutations have been identified in NSCLC. In addition to the most common oncogenic driver mutations in Chinese patients with NSCLC, including EGFR (~56%), KRAS (~12%), ALK (~3%), BRAF (~2%), HER2 (~2%), and MET (~1.3%), RET fusions have been identified in 0.6–2.0% of Chinese patients with NSCLC.4–9 This prevalence is comparable to estimates in global populations (1–3%). 10
RET encodes a transmembrane tyrosine kinase receptor, the activation of which leads to a series of signaling cascades that ultimately trigger cell growth. RET signaling is tightly controlled by various negative regulators, and activating alterations in the RET gene can result in aberrant RET signaling.11,12 Genomic alterations in RET have been implicated in the pathogenesis of several human cancers, including lung cancer. RET fusion is the most common RET alteration in patients with NSCLC; fusions of the RET kinase domain with CCDC6/PTC1, KIF5B, and NCOA4/PTC3 account for ~85% of chromosomal rearrangements in RET in NSCLC. 13 These fusions produce hybrid proteins with ligand-independent activity.13–15 In patients with lung cancer, RET fusions have been associated with a high risk of brain metastases. 16 Despite mounting evidence suggesting that RET is a promising therapeutic target in cancers harboring RET alterations, there are limited targeted therapies available for RET fusion-positive NSCLC.17–19 New targeted therapies are needed that can potently inhibit RET in tumors while sparing other kinase and non-kinase off-targets that contribute to toxicity.
Selpercatinib (formerly known as LOXO-292) is a first-in-class, highly selective, and potent small-molecule RET inhibitor with central nervous system (CNS) activity that inhibits multiple RET alterations. 20 It exhibited potent antitumor activity in vitro and in vivo in multiple, biologically relevant RET-dependent tumor models, including NSCLC harboring RET fusions.20,21 Due to its ability to penetrate the blood–brain barrier, selpercatinib has also shown antitumor activity against brain lesions in preclinical models and in the clinic.22,23 Selpercatinib has been approved in multiple countries for the treatment of metastatic RET fusion-positive NSCLC and RET-altered thyroid cancers (TCs). 23 The approval of selpercatinib was based on evidence from the global phase I/II LIBRETTO-001 trial, in which selpercatinib induced robust and durable clinical responses in patients with RET fusion-positive NSCLC who had received prior platinum chemotherapy [objective response rate (ORR): 64%; 95% confidence interval (CI), 54−73] or were treatment-naïve (ORR: 85%; 95% CI, 70−94). 24 At a median follow-up of 15.7 months and 9.8 months, 58% and 76% of responses were ongoing in pretreated and treatment-naïve patients, respectively, and the median duration of response (DOR) was 17.5 months (95% CI, 12.0–not evaluable) in pretreated patients and not reached in treatment-naïve patients.24,25 In addition, the 1-year progression-free survival (PFS) rates were 66% (95% CI, 56–74) and 68% (95% CI, 50–80) and the 2-year overall survival (OS) rates were 68% (95% CI, 55.3–77.8) and 88% (95% CI, 68.6–95.8) in pretreated and treatment-naïve patients, respectively.24,25 Among 22 patients with measurable CNS disease at baseline, the CNS ORR was 82% (95% CI, 60–95) and the median CNS DOR was not reached at a median follow-up of 9.5 months. 26 Selpercatinib was also well tolerated, and discontinuations due to treatment-related adverse events (AEs) occurred in 2% of 746 patients who received treatment in LIBRETTO-001. 25
Although the efficacy and safety of selpercatinib has been well described in the global LIBRETTO-001 trial, it has not been evaluated in Chinese patients. Herein, we present results from LIBRETTO-321 (NCT04280081), a phase II study evaluating the efficacy and safety of selpercatinib in Chinese patients with RET fusion-positive NSCLC.
Materials and methods
Study design and patients
This open-label, multicenter, phase II study was conducted at 15 institutions in China (Supplemental Table S1). Eligible patients were aged ⩾18 years with a diagnosis of advanced solid tumors, including patients with RET fusion-positive NSCLC, RET-mutant medullary TC (MTC), and RET fusion-positive TC. Patients were divided into three cohorts based on tumor type and type of RET alteration. Regardless of the cohort, RET alterations in the tumor and blood were detected by polymerase chain reaction (PCR), next-generation sequencing, and/or fluorescence in situ hybridization performed in a certified local laboratory or a central laboratory. RET alterations in tumors were detected at a central laboratory using the AmoyDx® 9-in-1 PCR assay (Amoy Diagnostics Co., Ltd., Haicang District, Xiamen, Fujian, China). Cohorts 1 and 2 included patients with RET alterations in tumor rather than in blood (with the exception of patients with MTC in whom a positive germline DNA test for a RET gene mutation was accepted) and patients with measurable disease as assessed by the Investigator, respectively. Cohort 3 included patients with RET alterations in the blood, patients without measurable disease, and patients with other RET-mutant solid tumors or other RET alterations (Supplemental Figure S1). For patients with NSCLC, only those harboring RET-KIF5B, RET-CCDC6, and RET-NCOA4 fusions confirmed by a central laboratory were included in the primary efficacy analysis population/primary analysis set (PAS). Patients were also required to have an Eastern Cooperative Oncology Group performance status score of 0–2 with no sudden deterioration 2 weeks prior to the first dose of selpercatinib, a corrected QT interval of 470 msec or less, and adequate hematologic, hepatic, and renal function. Patients with tumor progression or intolerance on at least one prior line of treatment with chemotherapy, immune checkpoint inhibitors, or multitargeted kinase inhibitors (MKIs; including those with anti-RET activity) were included in this study. Patients who declined or were deemed unsuitable for standard first-line therapy in the opinion of the Investigator and those with tumors for which no standard therapy existed were also included. Patients with previously treated or untreated CNS metastases and who were either asymptomatic or had been in a neurologically stable condition for at least 2 weeks were eligible. CNS metastases at baseline were confirmed by an independent review committee (IRC) of expert radiologists.
Key exclusion criteria were as follows: no qualified RET alteration status, prior treatment with selective RET inhibitors (including investigational selective RET inhibitors), unresolved toxicities from prior therapy worse than grade 1 according to the Common Terminology Criteria for Adverse Events (CTCAE), known infection with HIV, history of active hepatitis B or C virus infection, symptomatic primary or metastatic CNS tumor, concurrent use of drugs prolonging QTc, active secondary malignancy, pregnancy, and presence of additional oncogenic drivers that could cause resistance to selpercatinib (only for cohorts 1 and 2).
The study was conducted in accordance with the principles described in the Declaration of Helsinki, Good Clinical Practice guidelines, Council for International Organizations of Medical Sciences International Ethical Guidelines, and country and local regulations. All protocols were approved by the institutional review board or independent ethics committee at each investigative site (Supplemental Table S2). Written informed consent was obtained from all patients prior to any protocol-related procedures, including screening evaluations. The study protocol was prospectively registered at ClinicalTrials.gov (NCT04280081, first registered on 21 February 2020).
Treatment and evaluations
Selpercatinib was administered orally (160 mg, twice daily) in a 28-day cycle until disease progression, death, unacceptable toxicity, or withdrawal of consent. Radiologic assessments were performed at baseline, at week 4 (±7 days, optional) and week 8 (±7 days), and then every 8 weeks (±7 days) until week 48 following Cycle 1 Day 1, and every 12 weeks (±7 days) thereafter. All responses were confirmed by a second radiologic assessment conducted at least 4 weeks after the first assessment showing a response. CNS imaging (contrast-enhanced magnetic resonance imaging or computed tomography) was performed during screening for patients with RET fusion-positive tumors or a history of CNS metastases, or if clinically indicated. Patients with CNS metastases at baseline underwent repeated CNS imaging during each response assessment. Patients lost to follow-up were censored. Patients were continuously monitored for adverse effects from the first dose of selpercatinib until 28 days (±7 days) after the last dose of selpercatinib. The severity of AEs was graded as per the CTCAE, version 5.0.
Endpoints
The primary endpoint was ORR by IRC, defined as the proportion of patients who achieved a best overall response (BOR) of complete response (CR) or partial response (PR) determined by an IRC of expert radiologists according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. 27 Secondary endpoints included the following: Investigator-assessed ORR as per RECIST v1.1; DOR by IRC and Investigator (defined as the number of months from the start date of PR or CR to the date of disease progression or death, whichever occurred earlier); clinical benefit rate (CBR) based on the proportion of patients with a BOR of CR, PR, or stable disease (SD) lasting 16 or more weeks following the initiation of selpercatinib as assessed by IRC and Investigator; time to response (TTR) defined as the number of months elapsed between the date of the first dose of selpercatinib and the first documentation of objective response (CR or PR, whichever occurred first) as per RECIST v1.1; time to best response (TTBR) defined as the number of months elapsed between the date of confirmed best response and the date of the first dose of selpercatinib; PFS by IRC and Investigator defined as the number of months elapsed between the date of the first dose and the earliest date of documented disease progression or death from any cause; OS defined as the number of months elapsed between the date of the first dose and the date of death from any cause; and safety. Intracranial ORR and DOR were assessed in patients with IRC-assessed CNS metastasis at baseline using RECIST v1.1.
Statistics
Efficacy outcomes were evaluated in the PAS, consisting of treated patients with NSCLC enrolled in cohort 1 who had RET fusion-positive status confirmed by a central laboratory (Supplemental Figure S1). To assess response in a larger population, efficacy outcomes were also evaluated in all enrolled patients with NSCLC. In addition, efficacy was also assessed for pretreated and treatment-naïve subgroups in the PAS and all patients with NSCLC. ORR was estimated based on the observed proportion of patients whose BOR was confirmed as CR or PR as determined by the IRC and the Investigator. The estimates of the ORR were accompanied by a two-sided 95% exact binomial CI calculated using the Clopper–Pearson method. The DOR, PFS, and OS were estimated using the Kaplan–Meier method. The safety population consisted of all enrolled patients who received at least one dose of selpercatinib. With an overall sample size of 77, the probability of observing one or more instances of a specific AE with a true incidence rate of 2% and 5% was approximately 80% and 98%, respectively. Treatment compliance was defined as the total dose of selpercatinib received/the total amount of selpercatinib prescribed ×100. For cohort 1, the enrollment target was at least 20 patients to provide a preliminary assessment of the antitumor activity of selpercatinib in Chinese patients with RET fusion-positive NSCLC. Based on a high observed ORR (i.e. ⩾45%) within a cohort of 20 patients, the corresponding lower limit of a two-sided exact 95% CI will exclude true response rates that are considered marginal or not clinically meaningful (<40%).
Results
Patient characteristics
Among the 77 patients enrolled in this study between 16 March 2020 and 25 March 2021, 47 were diagnosed with RET fusion-positive NSCLC, 1 with RET fusion-positive TC, and 29 with RET-mutant medullary TC (Supplemental Figure S1). This analysis included data from patients in the PAS (n = 26) in addition to all enrolled patients with NSCLC (n = 47). The median age of patients in the PAS was 52 years (range, 26–72); 15 (57.7%) patients were women and 11 (42.3%) were men (Table 1). All patients in the PAS had RET-KIF5B, RET-CCDC6, or RET-NCOA4 fusions. The median number of lines of prior treatment was 2 (range, 0–7). Of the 26 patients in the PAS, 18 (69.2%) had received previous treatment and 8 (30.8%) were treatment-naïve. In all, 17 patients (65.4%) had been previously treated with platinum-based chemotherapy and 6 (23.1%) had received prior treatment with programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) inhibitors; none of the patients in the PAS had been previously treated with MKIs. Eight patients (30.8%) in the PAS were diagnosed with brain metastases at baseline, including five with measurable CNS lesions at enrollment.
Table 1.
Baseline patient characteristics.
| Characteristic | PAS a (n = 26) | All NSCLC b (n = 47) |
|---|---|---|
| Sex, n (%) | ||
| Female | 15 (57.7) | 26 (55.3) |
| Male | 11 (42.3) | 21 (44.7) |
| Median age, years (range) | 52 (26–72) | 54 (26–72) |
| Median weight, kg (range) | 60.6 (44.8–87.4) | 61.1 (44.8–108.0) |
| Smoking status, n (%) | ||
| Never smoked | 19 (73.1) | 33 (70.2) |
| Current smoker | 1 (3.8) | 1 (2.1) |
| Former smoker | 6 (23.1) | 13 (27.7) |
| Median prior treatment regimens, n (range) | 2 (0–7) | 2 (0–9) |
| Prior platinum-based chemotherapy, n (%) | 17 (65.4) | 34 (72.3) |
| Prior PD-1/PD-L1 inhibitor, n (%) | 6 (23.1) | 11 (23.4) |
| Prior multikinase inhibitor, n (%) | 0 | 2 (4.3) |
| Treatment naïve, n (%) c | 8 (30.8) | 11 (23.4) |
| Brain metastases, n (%) | 8 (30.8) | 17 (36.2) |
| ECOG PS, n (%) d | ||
| 0 | 2 (7.7) | 5 (10.6) |
| 1 | 23 (88.5) | 40 (85.1) |
| 2 | 1 (3.8) | 2 (4.3) |
| RET fusion gene | ||
| KIF5B/CCDC6/NCOA4 | 26 (100) | 42 (89.4) |
| Other e | 0 (0) | 5 (10.6) |
Patients with RET fusion-positive NSCLC whose RET status was confirmed by a central laboratory.
All enrolled patients with NSCLC.
Treatment-naïve patients included patients who received no prior systemic therapies or who received only adjuvant or neo-adjuvant therapies.
ECOG PS scores range from 0 to 5, with higher scores indicating greater disability.
RASGEF1A-RET; ERC1-RET; C10orf118-RET and CCDC186-RET; KIF5B-RET and PHYH-RET; and CCDC6-RET and ACBD5-RET.
ECOG PS, Eastern Cooperative Oncology Group performance status; PAS, primary analysis set; PD-1, programmed death-1; PD-L1, programmed death ligand-1.
Among all 47 enrolled patients with NSCLC, 26 (55.3%) were women and 21 (44.7%) were men (Table 1). The median age of all patients with NSCLC was 54 years (range, 26–72) and the median number of lines of prior treatment was 2 (range, 0–9). In total, 36 (76.6%) patients had been previously treated and 11 (23.4%) were treatment-naïve. In all, 34 (72.3%) patients had been previously treated with platinum-based chemotherapy, 11 (23.4%) with PD-1/PD-L1 inhibitors, and 2 (4.3%) with MKIs.
Efficacy outcomes
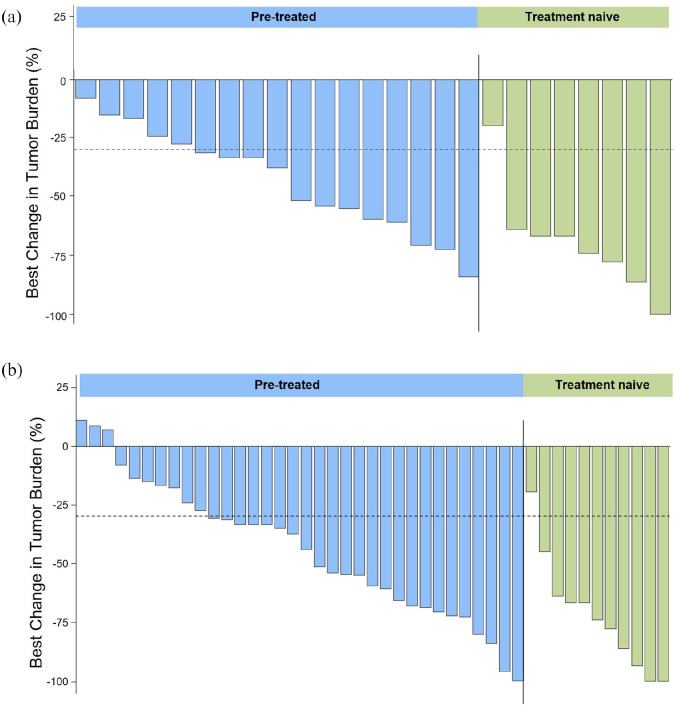
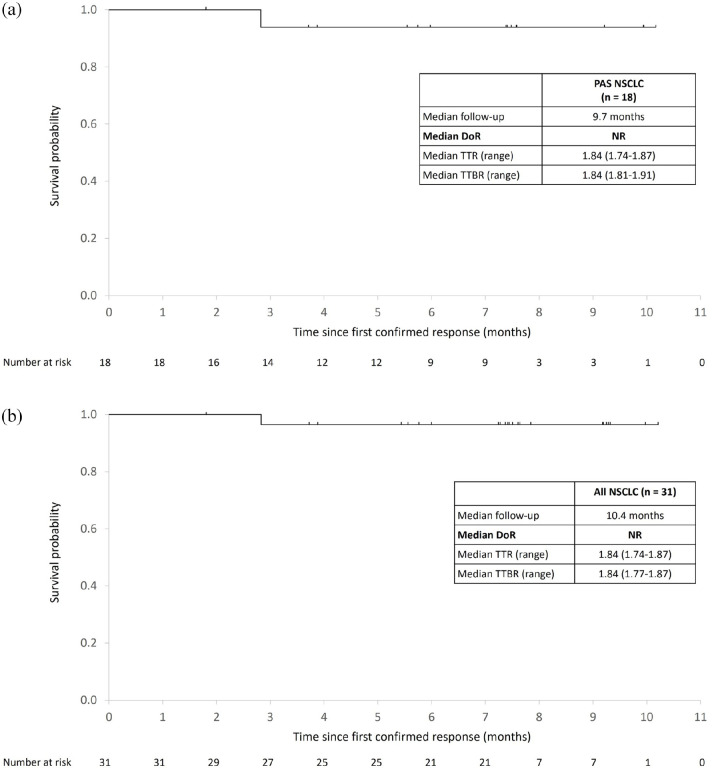
After a median follow-up of 9.7 months, the IRC-assessed ORR in the PAS (pretreated and treatment-naïve patients) was 69.2% (95% CI, 48.2–85.7), with one (3.8%) confirmed CR and 17 (65.4%) confirmed PRs, and the CBR was 80.8% (95% CI, 60.6–93.4) [Table 2 and Figure 1(a)]. The IRC-assessed ORR in previously treated patients was 61.1% (95% CI, 35.7–82.7), and the CBR was 77.8% (95% CI, 52.4–93.6). Treatment-naïve patients exhibited an IRC-assessed ORR of 87.5% (95% CI, 47.3–99.7) and a CBR of 87.5% (95% CI, 47.3–99.7). Among the 18 PAS patients with RET fusion-positive NSCLC with IRC-confirmed CR or PR, the median TTR was 1.84 months [interquartile range (IQR), 1.74–1.87], the median TTBR was 1.84 months (IQR, 1.81–1.91), the median DOR was not reached, and 94.4% of responses were ongoing at a median follow-up of 9.7 months [Figure 2(a)]. Median PFS and OS were not reached for any of the patient groups.
Table 2.
Tumor responses to selpercatinib in patients with RET fusion-positive NSCLC.
| PAS a (n = 26) | All NSCLC b (n = 47) | |||||
|---|---|---|---|---|---|---|
| All (n = 26) | Pretreated (n = 18) | Treatment naïve (n = 8) | All (n = 47) | Pretreated (n = 36) | Treatment naïve (n = 11) | |
| BOR, n (%) | ||||||
| CR | 1 (3.8) | 0 (0) | 1 (12.5) | 3 (6.4) | 1 (2.8) | 2 (18.2) |
| PR | 17 (65.4) | 11 (61.1) | 6 (75.0) | 28 (59.6) | 20 (55.6) | 8 (72.7) |
| SD | 7 (26.9) | 6 (33.3) | 1 (12.5) | 14 (29.8) | 13 (36.1) | 1 (9.1) |
| SD ⩾16 weeks | 3 (11.5) | 3 (16.7) | 0 (0) | 5 (10.6) | 5 (13.9) | 0 |
| PD | 1 (3.8) | 1 (5.6) | 0 (0) | 2 (4.3) | 2 (5.6) | 0 |
| ORR, n (%) | 18 (69.2) | 11 (61.1) | 7 (87.5) | 31 (66.0) | 21 (58.3) | 10 (90.9) |
| 95% CI c | 48.2–85.7 | 35.7–82.7 | 47.3–99.7 | 50.7–79.1 | 40.8–74.5 | 58.7–99.8 |
Patients with RET fusion-positive NSCLC whose RET status was confirmed by central laboratory.
All enrolled patients with NSCLC.
Confidence intervals estimated using the Clopper–Pearson method.
BOR, best overall response; CI, confidence interval; CR, complete response; IRC, independent review committee; NSCLC, non-small-cell lung cancer; ORR, objective response rate; PAS, primary analysis set; PD, progressive disease; PR, partial response; RET, rearranged during transfection; SD, stable disease.
Figure 1.
Efficacy of selpercatinib in pretreated and treatment-naïve patients with RET fusion-positive NSCLC. Waterfall plots showing the maximum change in tumor size in all target lesions in the PAS (n = 26; a) and in all enrolled patients with NSCLC (n = 47; b) according to IRC assessment. Waterfall plots only show patients with measurable target lesions. One patient in the PAS and two patients of all enrolled patients with NSCLC had nonmeasurable disease.
IRC, independent review committee; NSCLC, non-small-cell lung cancer; PAS, primary analysis set.
Figure 2.
Duration of response. Kaplan–Meier estimates of DOR in patients with RET fusion-positive NSCLC (a) and all patients with NSCLC (b) who had a CR or PR confirmed by IRC.
CR, complete response; DOR, duration of response; IQR, interquartile range; IRC, independent review committee; NR, not reached; PR, partial response; TTBR, time to best response; TTR, time to response.
Among all enrolled patients with NSCLC (n = 47), after a median follow-up of 10.4 months, the IRC-assessed ORR was 66.0% (95% CI, 50.7–79.1); 58.3% (95% CI, 40.8–74.5) in pretreated patients and 90.9% (95% CI, 58.7–99.8) in treatment-naïve patients. The IRC-assessed CBR in all enrolled patients with NSCLC was 76.6% (95% CI, 62.0–87.7); 72.2% (95% CI, 54.8–85.8) in pretreated patients and 90.9% (95% CI, 58.7–99.8) in treatment-naïve patients [Table 2 and Figure 1(b)]. Among the patients with NSCLC who had an IRC-confirmed CR or PR (n = 31), the median TTR and TTBR were both 1.84 months and median DOR was not reached, with 96.8% of responses ongoing at a median follow-up of 10.4 months [Figure 2(b)]. The median PFS and OS were not reached.
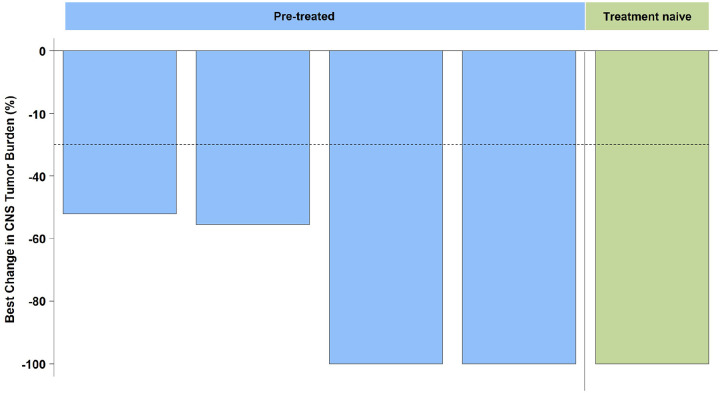
Selpercatinib also exhibited antitumor activity against intracranial lesions. In the CNS population (n = 8), the ORR was 62.5% (95% CI, 24.5–91.5; 5/8). Among five patients with measurable CNS metastasis at enrollment, the IRC-assessed intracranial ORR was 80%, including one patient (20%) with an intracranial CR, three (60%) with an intracranial PR, and one patient (20%) with intracranial SD (Figure 3). The median DOR was not reached, and 100% of responses were ongoing at a median follow-up of 9.3 months. Taken together, these data show the marked and sustained responses associated with selpercatinib in Chinese patients with NSCLC, consistent with findings in the global population and East Asians 28 included in LIBRETTO-001. 24
Figure 3.
Antitumor activity of selpercatinib against metastatic brain lesions in patients with RET fusion-positive NSCLC. Waterfall plot showing the percent change in brain target lesion size in the CNS population according to IRC evaluation. Waterfall plots only show patients with measurable target lesions. Among the eight patients with brain metastases at baseline, three had nonmeasurable disease.
CNS, central nervous system; IRC, independent review committee; NSCLC, non-small-cell lung cancer.
Safety
The safety population consisted of 77 enrolled patients with RET fusion-positive NSCLC and RET-altered TCs, all of whom received selpercatinib. The median treatment compliance was 106.84% (Q1–Q3, 102.73–110.24), and the median number of cycles received was 10 (range, 1–13). After a median follow-up of 9.7 months (95% CI, 9.0–10.2), 84.4% of patients remained on treatment (Supplemental Table S3). The median duration of therapy was 40.29 weeks (range, 2.29–51.29 weeks), and the median relative dose intensity was 100.0% (Q1–Q3, 85.27–106.84). Of the 77 patients, 75 (97.4%) experienced at least one treatment-emergent AE (TEAE) of which the majority were manageable or reversible. The most common TEAEs of any grade were as follows: increased levels of alanine aminotransferase (ALT) (64.9%), aspartate aminotransferase (AST) (61.0%), and blood bilirubin (39.0%); thrombocytopenia (39.0%); hypertension (36.4%); and hypoalbuminemia (33.8%) (Table 3). In total, 46 (59.7%) patients experienced at least one grade ⩾3 TEAE. The most common grade ⩾3 TEAEs were hypertension (19.5%); increased levels of ALT (15.6%) and AST (15.6%); thrombocytopenia (10.4%); and electrocardiogram QT prolonged (7.8%). Grade ⩾3 treatment-related AEs were highly consistent with TEAEs (Table 3).
Table 3.
Summary of TEAEs in the safety population.
| Adverse event | TEAEs | TEAEs related to study drug a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Any Grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Any grade | |
| Number of patients (%) | ||||||||||
| Alanine aminotransferase increased b | 30 (39.0) | 8 (10.4) | 11 (14.3) | 1 (1.3) | 50 (64.9) | 28 (36.4) | 8 (10.4) | 11 (14.3) | 1 (1.3) | 48 (62.3) |
| Aspartate aminotransferase increased b | 31 (40.3) | 4 (5.2) | 12 (15.6) | 0 (0) | 47 (61.0) | 31 (40.3) | 4 (5.2) | 12 (15.6) | 0 | 47 (61.0) |
| Blood bilirubin increased | 21 (27.3) | 9 (11.7) | 0 (0) | 0 (0) | 30 (39.0) | 21 (27.3) | 9 (11.7) | 0 | 0 | 30 (39.0) |
| Thrombocytopenia b | 18 (23.4) | 4 (5.2) | 6 (7.8) | 2 (2.6) | 30 (39.0) | 17 (22.1) | 4 (5.2) | 6 (7.8) | 2 (2.6) | 29 (37.7) |
| Hypertension b | 2 (2.6) | 11 (14.3) | 15 (19.5) | 0 (0) | 28 (36.4) | 2 (2.6) | 12 (15.6) | 12 (15.6) | 0 | 26 (33.8) |
| Hypoalbuminemia | 18 (23.4) | 6 (7.8) | 2 (2.6) | 0 (0) | 26 (33.8) | 14 (18.2) | 5 (6.5) | 1 (1.3) | 0 | 20 (26.0) |
| Diarrhea b | 21 (27.3) | 3 (3.9) | 1 (1.3) | 0 (0) | 25 (32.5) | 18 (23.4) | 3 (3.9) | 1 (1.3) | 0 | 22 (28.6) |
| White blood cell count decreased | 11 (14.3) | 11 (14.3) | 3 (3.9) | 0 (0) | 25 (32.5) | 10 (13.0) | 11 (14.3) | 3 (3.9) | 0 | 24 (31.2) |
| Dry mouth b | 22 (28.6) | 0 (0) | 0 (0) | 0 (0) | 22 (28.6) | 21 (27.3) | 0 | 0 | 0 | 21 (27.3) |
| Blood alkaline phosphatase increased | 14 (18.2) | 6 (7.8) | 1 (1.3) | 0 (0) | 21 (27.3) | 14 (18.2) | 4 (5.2) | 1 (1.3) | 0 | 19 (24.7) |
| Bilirubin conjugated increased | 13 (16.9) | 5 (6.5) | 2 (2.6) | 0 (0) | 20 (26.0) | 13 (16.9) | 5 (6.5) | 2 (2.6) | 0 | 20 (26.0) |
| Neutrophil count decreased | 7 (9.1) | 10 (13.0) | 3 (3.9) | 0 (0) | 20 (26.0) | 7 (9.1) | 9 (11.7) | 3 (3.9) | 0 | 19 (24.7) |
| Electrocardiogram QT prolonged b | 12 (15.6) | 1 (1.3) | 6 (7.8) | 0 (0) | 19 (24.7) | 9 (11.7) | 1 (1.3) | 5 (5.6) | 0 | 15 (19.5) |
| Hyperuricemia | 19 (24.7) | 0 (0) | 0 (0) | 0 (0) | 19 (24.7) | 16 (20.8) | 0 | 0 | 0 | 16 (20.8) |
| Blood creatinine increased b | 11 (14.3) | 7 (9.1) | 0 (0) | 0 (0) | 18 (23.4) | 11 (14.3) | 7 (9.1) | 0 | 0 | 18 (23.4) |
| Blood lactate dehydrogenase increased | 16 (20.8) | 2 (2.6) | 0 (0) | 0 (0) | 18 (23.4) | 14 (18.2) | 2 (2.6) | 0 | 0 | 16 (20.8) |
| Weight increased | 7 (9.1) | 11 (14.3) | 0 (0) | 0 (0) | 18 (23.4) | 3 (3.9) | 6 (7.8) | 0 | 0 | 9 (11.7) |
| Gamma-glutamyltransferase increased | 10 (13.0) | 5 (6.5) | 2 (2.6) | 0 (0) | 17 (22.1) | 10 (13.0) | 4 (5.2) | 2 (2.6) | 0 | 16 (20.8) |
| Oedema b | 13 (16.9) | 4 (5.2) | 0 (0) | 0 (0) | 17 (22.1) | 10 (13.0) | 4 (5.2) | 0 | 0 | 14 (18.2) |
| Pyrexia b | 15 (19.5) | 2 (2.6) | 0 (0) | 0 (0) | 17 (22.1) | 10 (13.0) | 2 (2.6) | 0 | 0 | 12 (15.6) |
Only TEAEs occurring in 20% of study subjects are shown. One patient had grade 5 acute pancreatitis deemed by the Investigator to be unrelated to selpercatinib.
Drug relationship was assessed by the Investigator.
Consolidated adverse event term.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; TEAEs, treatment-emergent adverse events.
Only 4 of 77 (5.2%) patients discontinued selpercatinib due to TEAEs, of which 3 (3.9%) were considered to be related to selpercatinib by Investigator assessment: hypersensitivity, platelet count decreased, and abnormal liver function (in one patient each). TEAEs led to dose reductions in 32.5% (n = 25) of patients. The most common TEAEs leading to dose reductions were hypersensitivity (9.1%; n = 7); increased levels of AST (7.8%; n = 6) and ALT (6.5%; n = 5); and decreased platelet count (5.2%; n = 4). By the data cutoff date on 25 March 2021, there was one (1.3%) grade 5 TEAE of acute pancreatitis considered unrelated to selpercatinib, which occurred in a patient with RET-mutant MTC.
Safety was also evaluated in all enrolled patients with NSCLC (n = 47). Overall, the safety profile of selpercatinib was similar in the two patient populations. In all, 46 (97.9%) patients experienced at least one TEAE, and most TEAEs were grade 1 or 2. In total, 29 (61.7%) patients experienced at least one grade ⩾3 TEAE. The most common grade ⩾3 TEAEs were increased level of AST (21.3%; n = 10), hypertension (19.1%; n = 9), increased level of ALT (17.0%; n = 8), and thrombocytopenia (17.0%; n = 8). TEAEs led to discontinuation of selpercatinib in three (6.4%) patients, two (4.3%) of which were considered to be related to selpercatinib: decreased platelet count and abnormal liver function (in one patient each). TEAEs resulted in dose reductions in 18 (38.3%) patients. The most common TEAEs leading to dose reductions were increased level of AST (12.8%; n = 6), hypersensitivity (12.8%; n = 6), decreased platelet count (8.5%; n = 4), and increased level of ALT (6.4%; n = 3). There were no deaths due to TEAEs in this population. Patients with NSCLC who had received prior immunotherapy (n = 11) or were immunotherapy naïve (n = 36) had a comparable incidence of TEAEs of any grade (100% and 97.2%) and grade ⩾3 (63.6% and 61.1%) and the most common TEAEs of any grade in both subgroups were increased levels of ALT and AST and decreased platelet count (Supplemental Table S4). However, these findings should be interpreted cautiously due to the relatively small sample size. Overall, these safety data suggest that selpercatinib was well tolerated and the safety profile of selpercatinib in Chinese patients with RET-altered tumors is consistent with the findings in the global population and East Asians 28 included in LIBRETTO-001. 24
Discussion
In this phase II trial, we investigated the efficacy and safety of the selective RET inhibitor selpercatinib in Chinese patients with advanced NSCLC harboring RET fusions. Selpercatinib demonstrated robust and durable antitumor activity in RET fusion-positive patients with NSCLC, providing an IRC-assessed ORR of 69.2% for patients in the PAS (87.5% for treatment-naïve patients and 61.1% for previously treated patients) and 94.4% of responses were ongoing at a median follow-up of 9.7 months. Notably, selpercatinib provided a clinical benefit in this cohort regardless of the number of lines of prior treatment.
MKIs with some degree of anti-RET activity, in addition to targeting other kinases (e.g. cabozantinib and vandetanib), have received regulatory approval for the treatment of advanced MTC (irrespective of the presence of a RET mutation). 20 Preliminary data suggest moderate antitumor activity for MKIs with anti-RET activity in RET fusion-positive lung cancer, with response rates of 16–53% (depending on the specific MKI and patient population), and a median PFS of only 3.6–7.3 months.29–32 The limited efficacy of these MKIs in tumors harboring RET alterations might be due to incomplete inhibition of RET, poor pharmacokinetics, and significant toxicity from stronger inhibition of other targets (e.g. KDR/VEGFR2, EGFR, MET) requiring dose interruptions, reductions, or treatment cessation. However, head-to-head comparisons of outcomes in cohorts with different baseline characteristics and prior treatments are challenging and should be made with caution.
In line with the findings of the present analysis, previous early-stage clinical investigations showed that selpercatinib demonstrated robust and durable antitumor and CNS activities in patients with cancers harboring RET alterations. Based on the early results from LIBRETTO-001, selpercatinib received Food and Drug Administration (FDA) Breakthrough Therapy Designation for the treatment of RET fusion-positive NSCLC in 2018. On 8 May 2020, the FDA granted accelerated approval to selpercatinib for the treatment of adult patients with metastatic RET fusion-positive NSCLC. In Europe, selpercatinib was granted conditional marketing authorization in 2020 for the treatment of adults with advanced RET fusion-positive NSCLC who require systemic therapy following prior treatment with immunotherapy and/or platinum-based chemotherapy. 33 LIBRETTO-001 was a global phase I/II study evaluating the efficacy of selpercatinib in patients with RET-altered solid tumors: RET fusion-positive NSCLC, RET-mutant MTC, and RET fusion-positive TC. 24 In 105 patients with RET fusion-positive NSCLC previously treated with platinum chemotherapy, the IRC-assessed ORR was 64% (95% CI, 53.9–73.0) and the median DOR by IRC was 17.5 months (95% CI, 12.1–not reached). In 48 patients with treatment-naïve RET fusion-positive NSCLC, the IRC-assessed ORR was 85% (95% CI, 72.2–93.9), and the median DOR by IRC was not reached (95% CI, 12.0–not reached). 25 In pretreated patients, the 1-year PFS rate was 66% (95% CI, 56–74) and the 2-year OS rate was 68% (95% CI, 55.3–77.8); among treatment-naïve patients, the rates were 68% (95% CI, 50–80) and 88% (95% CI, 68.6–95.8), respectively. 25
The lifetime prevalence of CNS metastases in patients with RET fusion-positive NSCLC is approximately 50% and is a significant cause of morbidity and mortality in this patient population. 16 In this study, 4/5 patients with measurable CNS metastasis at baseline achieved an intracranial CR or PR, highlighting the intracranial activity of selpercatinib. This finding is supported by previous results showing significant and rapid CNS penetration for selpercatinib (intracranial ORR of 82%; 95% CI, 60–95) in 22 patients with NSCLC and measurable intracranial disease at baseline. 26 Consistent with the safety profile observed in the present study, selpercatinib previously exhibited acceptable tolerability in the global population and East Asians 28 included in LIBRETTO-001. 24 In LIBRETTO-001 and the present study, the most common AEs were increased blood ALT/AST and bilirubin levels, thrombocytopenia, and hypertension. These AEs were manageable and reversible with dose interruptions and reductions or with the addition of concomitant medications. In the present study, only 3.9% of patients discontinued therapy due to treatment-related AEs, and there were no deaths due to treatment-related AEs. In contrast to MKIs, selpercatinib selectively binds to RET’s adenosine triphosphate binding site at nanomolar potency and has a limited binding affinity to other kinase and non-kinase targets at similar concentrations. This high selectivity of selpercatinib may explain its relatively low toxicity. 20
This study has some important limitations. First, this was a single-arm study with an open-label design, which may have resulted in possible bias in the results. Furthermore, at the time of analysis, many patients remained progression free, and responses were ongoing. Therefore, survival data were not mature, and median PFS and OS could not be estimated. Finally, the study included a relatively small number of patients, especially for the CNS population, and interpretation of the results should be made with caution.
In conclusion, in the phase II LIBRETTO-321 study, selpercatinib exhibited robust and durable antitumor activity, including CNS activity, in Chinese patients with RET fusion-positive NSCLC, providing high ORRs of clinically relevant duration regardless of prior treatment line. Furthermore, selpercatinib was well tolerated and associated with mild and manageable adverse effects. The findings of this study suggest that selpercatinib is a promising treatment option for Chinese patients with locally advanced or metastatic RET fusion-positive NSCLC, who currently have limited treatment options. Selpercatinib is currently being evaluated in patients with advanced or metastatic RET fusion-positive NSCLC in the phase III trial LIBRETTO-431 (NCT04194944).
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221105020 for Efficacy and safety of selpercatinib in Chinese patients with advanced RET fusion-positive non-small-cell lung cancer: a phase II clinical trial (LIBRETTO-321) by Shun Lu, Ying Cheng, Dingzhi Huang, Yuping Sun, Lin Wu, Chengzhi Zhou, Ye Guo, Jingxin Shao, Wanli Zhang and Jianying Zhou in Therapeutic Advances in Medical Oncology
Supplemental material, sj-jpg-2-tam-10.1177_17588359221105020 for Efficacy and safety of selpercatinib in Chinese patients with advanced RET fusion-positive non-small-cell lung cancer: a phase II clinical trial (LIBRETTO-321) by Shun Lu, Ying Cheng, Dingzhi Huang, Yuping Sun, Lin Wu, Chengzhi Zhou, Ye Guo, Jingxin Shao, Wanli Zhang and Jianying Zhou in Therapeutic Advances in Medical Oncology
Acknowledgments
Editorial support for this article was provided by Christos Evangelou, MSD, PhD and Jake Burrell, PhD (Rude Health Consulting) and paid for by Eli Lilly and Company.
Footnotes
ClinicalTrials.gov Identifier: NCT04280081 (first registered February 21, 2020)
ORCID iDs: Shun Lu  https://orcid.org/0000-0001-8833-7262
https://orcid.org/0000-0001-8833-7262
Dingzhi Huang  https://orcid.org/0000-0002-2798-9459
https://orcid.org/0000-0002-2798-9459
Chengzhi Zhou  https://orcid.org/0000-0002-1758-0035
https://orcid.org/0000-0002-1758-0035
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Shun Lu, Department of Oncology, Shanghai Lung Cancer Center, Shanghai Chest Hospital, Shanghai JiaoTong University, Shanghai, China.
Ying Cheng, Department of Thoracic Oncology, Jilin Cancer Hospital, Changchun, Jilin, 130012, China.
Dingzhi Huang, Department of Thoracic Oncology, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China.
Yuping Sun, Department of Oncology, Jinan Central Hospital Affiliated to Shandong University, Jinan, China.
Lin Wu, Department II of Thoracic Medicine, Hunan Cancer Hospital, Changsha, China.
Chengzhi Zhou, Respiratory Medicine Department, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Ye Guo, Department of Medical Oncology, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, China.
Jingxin Shao, Oncology, Eli Lilly and Company, Shanghai, China.
Wanli Zhang, Oncology, Eli Lilly and Company, Shanghai, China.
Jianying Zhou, Department of Respiratory Diseases, First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Shun Lu: Conceptualization; Data curation; Investigation; Writing – original draft; Writing – review & editing.
Ying Cheng: Data curation; Investigation; Writing – review & editing.
Dingzhi Huang: Data curation; Investigation; Writing – review & editing.
Yuping Sun: Data curation; Investigation; Writing – review & editing.
Lin Wu: Data curation; Investigation; Writing – review & editing.
Chengzhi Zhou: Data curation; Investigation; Writing – review & editing.
Ye Guo: Data curation; Investigation; Writing – review & editing.
Jingxin Shao: Conceptualization; Formal analysis; Writing – original draft; Writing – review & editing.
Wanli Zhang: Conceptualization; Formal analysis; Writing – original draft; Writing – review & editing.
Jianying Zhou: Data curation; Investigation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Eli Lilly and Company.
Competing Interests: Jingxin Shao and Wanli Zhang are employees of Eli Lilly and Company. The other authors have no conflicts of interest to declare.
Availability of data and material: Not applicable.
References
- 1. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health 2019; 85: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer 2019; 10: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao S, Li N, Wang S, et al. Lung cancer in People’s Republic of China. J Thorac Oncol 2020; 15: 1567–1576. [DOI] [PubMed] [Google Scholar]
- 4. Meng H, Guo X, Sun D, et al. Genomic profiling of driver gene mutations in Chinese patients with non-small cell lung cancer. Front Genet 2019; 10: 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang K, Chen H, Wang Y, et al. Clinical characteristics and molecular patterns of RET-rearranged lung cancer in Chinese patients. Oncol Res 2019; 27: 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol 2012; 30: 4352–4359. [DOI] [PubMed] [Google Scholar]
- 7. Tsuta K, Kohno T, Yoshida A, et al. RET-rearranged non-small-cell lung carcinoma: a clinicopathological and molecular analysis. Br J Cancer 2014; 110: 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai W, Su C, Li X, et al. KIF5B-RET fusions in Chinese patients with non-small cell lung cancer. Cancer 2013; 119: 1486–1494. [DOI] [PubMed] [Google Scholar]
- 9. Xu C, Wang WX, Wang D, et al. 415P Real-world fusion landscape of RET gene fusions and its response to cabozantinib in Chinese non-small cell lung cancer (NSCLC) using next generation sequencing. Ann Oncol 2020; 31: S1404. [Google Scholar]
- 10. Li AY, McCusker MG, Russo A, et al. RET fusions in solid tumors. Cancer Treat Rev 2019; 81: 101911. [DOI] [PubMed] [Google Scholar]
- 11. Fielder GC, Yang TW, Razdan M, et al. The GDNF family: a role in cancer? Neoplasia 2018; 20: 99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mulligan LM. GDNF and the RET receptor in cancer: new insights and therapeutic potential. Front Physiol 2019; 9: 1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santoro M, Moccia M, Federico G, et al. RET gene fusions in malignancies of the thyroid and other tissues. Genes (Basel) 2020; 11: 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Drilon A, Hu ZI, Lai GGY, et al. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 2018; 15: 151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gautschi O, Milia J, Filleron T, et al. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol 2017; 35: 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drilon A, Lin JJ, Filleron T, et al. Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J Thorac Oncol 2018; 13: 1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stinchcombe TE. Current management of RET rearranged non-small cell lung cancer. Ther Adv Med Oncol 2020; 12: 1758835920928634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alexander M, Kim SY, Cheng H. Update 2020: management of non-small cell lung cancer. Lung 2020; 198: 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drusbosky LM, Rodriguez E, Dawar R, et al. Therapeutic strategies in RET gene rearranged non-small cell lung cancer. J Hematol Oncol 2021; 14: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol 2018; 29: 1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Subbiah V, Konda B, Bauer T, et al. Efficacy and safety of selpercatinib in RET fusion–positive cancers other than lung or thyroid cancers. In: Proceedings of the 112th annual meeting of the American association for cancer research, 10–15 April 2021. Philadelphia, PA: AACR. Abstract nr CT011. [Google Scholar]
- 22. Subbiah V, Gainor JF, Oxnard GR, et al. Intracranial activity of selpercatinib (LOXO-292) in RET fusion-positive non-small cell lung cancer (NSCLC) patients on the LIBRETTO-001 trial. J Clin Oncol 2020; 38: 9516. [Google Scholar]
- 23. Bradford D, Larkins E, Mushti SL, et al. FDA approval summary: selpercatinib for the treatment of lung and thyroid cancers with RET gene mutations or fusions. Clin Cancer Res 2021; 27: 2130–2135. [DOI] [PubMed] [Google Scholar]
- 24. Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. N Engl J Med 2020; 383: 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Besse B, Drilon A, Solomon BJ, et al. Updated overall efficacy and safety of selpercatinib in patients (pts) with RET fusion+ non-small cell lung cancer (NSCLC): poster 9065. J Clin Oncol 2021; 39: 9065. [Google Scholar]
- 26. Subbiah V, Gainor JF, Oxnard GR, et al. Intracranial efficacy of selpercatinib in fusion-positive non–small cell lung cancers on the LIBRETTO-001 trial. Clin Cancer Res 2021; 15: 4160–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 28. Loong H, Goto K, Park K, et al. FP14.10 efficacy and safety of selpercatinib (LOXO-292) in East Asian patients with RET fusion-positive NSCLC. J Thorac Oncol 2021; 16: S231–S232. [Google Scholar]
- 29. Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol 2016; 17: 1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hida T, Velcheti V, Reckamp KL, et al. A phase 2 study of lenvatinib in patients with RET fusion-positive lung adenocarcinoma. Lung Cancer 2019; 138: 124–130. [DOI] [PubMed] [Google Scholar]
- 31. Lee S-H, Lee J-K, Ahn M-J, et al. A phase II study of vandetanib in patients with non-small cell lung cancer harboring RET rearrangement. J Clin Oncol 2016; 34: 9013. [Google Scholar]
- 32. Yoh K, Seto T, Satouchi M, et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med 2017; 5: 42–50. [DOI] [PubMed] [Google Scholar]
- 33. Retsevmo. Summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/retsevmo-epar-product-information_en.pdf (accessed December 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221105020 for Efficacy and safety of selpercatinib in Chinese patients with advanced RET fusion-positive non-small-cell lung cancer: a phase II clinical trial (LIBRETTO-321) by Shun Lu, Ying Cheng, Dingzhi Huang, Yuping Sun, Lin Wu, Chengzhi Zhou, Ye Guo, Jingxin Shao, Wanli Zhang and Jianying Zhou in Therapeutic Advances in Medical Oncology
Supplemental material, sj-jpg-2-tam-10.1177_17588359221105020 for Efficacy and safety of selpercatinib in Chinese patients with advanced RET fusion-positive non-small-cell lung cancer: a phase II clinical trial (LIBRETTO-321) by Shun Lu, Ying Cheng, Dingzhi Huang, Yuping Sun, Lin Wu, Chengzhi Zhou, Ye Guo, Jingxin Shao, Wanli Zhang and Jianying Zhou in Therapeutic Advances in Medical Oncology