Abstract
Colorectal cancer (CRC) is associated with numerous genetic disorders and cellular abnormalities, and liver metastasis is a common health concern in patients with CRC. Exploring newer and more efficient therapies to block liver metastasis is pivotal for prolonging patient survival. Therefore, small interfering RNAs (siRNAs) are expected to be remarkable tools capable of regulating gene expression by participating in a process called RNA interference (RNAi). RNAi is a biological process among eukaryotes wherein specific messenger RNA (mRNA) molecules are destroyed and gene expression is inhibited. This technology is a promising therapeutic agent in the treatment of CRC liver metastasis (CRLM). Nevertheless, crucial problems in siRNA therapeutics, including inherent poor serum stability and nonspecific uptake into biological systems, must be recognized. For this reason, delivery systems are being developed in an attempt to solve these problems. Here, we discuss the utility of siRNA therapy for the treatment of CRCLM by targeting the major metastasis-related signaling pathways. siRNA therapy has the potential to be one of the most effective methods for CRLM treatment in the future.
Keywords: gene therapy colorectal cancer liver metastasis, RNA interference, siRNA, therapeutic targets
Introduction
Colorectal cancer (CRC) is closely associated with many genetic disorders1–3 and cellular abnormalities.4–6 Liver metastasis remains a considerable health concern of CRC throughout the world. In 2018, there were 1.8 million confirmed cases and 881 000 deaths worldwide. 7 Metastasis shortens survival time, and liver metastasis is the most common type among distant metastases. Approximately 19% of patients are initially diagnosed with concurrent liver metastases. 8 Although great efforts have been employed to detect, prevent, and treat CRC during the past decades,9–11 exploring newer and more efficient therapies to block liver metastasis is important to prolong patients’ lives.
Epidemiology, Risk Factors, and Treatments for CRC Liver Metastasis
Epidemiological studies have shown that CRC is the third most common cancer and the fourth leading cause of cancer-related deaths worldwide. 12 The incidence and mortality of CRC in men are 25% higher than those in women. CRC has higher rates in developed countries, especially Hungary and Slovakia. It is predicted that there will be 2.5 million new cases worldwide by 2035. 13 The most common metastatic site of CRC is the liver. 14 The 5-year relative survival of CRC varies according to stage, ranging from more than 90% in stage I to less than 10% in stage IV. 15
In the majority of CRC cases, westernization involving obesity, sedentarism, poor diet, alcohol consumption, and smoking can result in CRC. Individuals with germline MLH1 and APC mutations tend to develop CRC. 10 Several risk factors have also been studied. The increasing prevalence of nonalcoholic fatty liver disease (NAFLD) parallels that of obesity. Whether NAFLD is a risk factor for CRC liver metastasis (CRLM) remains unclear. 14
The currently employed treatments for CRC are surgery, radiotherapy, chemotherapy, and other treatments, but there is no valid therapy for CRLM. 16 Liver resection and chemotherapy are the gold standard treatment methods for metastatic livers. 17 However, only 25% of the patients with CRLM have opportunities for liver resection. In reality, only 50% of the patients who undergo liver resection have a 5-year survival rate. In CRC treatment, chemotherapy is the primary method for prolonging the life of patients. However, chemotherapy drugs usually lead to serious side effects and toxicity. 8 In this regard, for the development of newer and more efficient therapies, selective targeting may show potential to block liver metastasis. 18
Small Interfering RNA (siRNA) Technology in CRLM
Recently, RNA interference (RNAi) has received considerable attention for its ability to block liver metastasis. 19 RNAi is a biological process that destroys specific messenger RNA (mRNA) molecules and inhibits eukaryotic gene expression. Small interfering RNA (siRNAs) are among the most relevant therapeutic agents. 20 In cells, siRNAs are short double-stranded RNAs (dsRNAs) of approximately 25 nucleotides. 21 RNAi begins with siRNA cleavage catalyzed by the enzyme Dicer. Two single-stranded (ss) RNAs are generated from the unwinding of each siRNA. One ssRNA, the passenger strand, is degraded. Another ssRNA, called the guide strand, is incorporated into the RNA-induced silencing complex (RISC). RISC can strongly silence specific mRNAs when the guide strand sequence is complementary to an mRNA-specific sequence, thereby preventing the synthesis of undesired proteins. 20 Moreover, long short hairpin RNA (shRNAs) can be used to form siRNAs. 22 Because of the RNAi mechanism, mRNA transcript sequences extracted from human genomic data can be used to design siRNAs, which is a new way to treat cancer. Accordingly, after the discovery of RNAi and subsequent studies, numerous cancers such as CRC, 23 hepatocellular carcinoma, 24 and lung cancer 25 have been treated with siRNAs.
Therapeutic Targets for siRNA-Based Treatment of CRLM
In patients with CRC, the leading risk factor for mortality is the high incidence of liver metastasis, and although the mechanisms of CRLM are poorly understood, a variety of signaling pathways are dysregulated, which has attracted much research attention during the last few years. There are some major metastasis-related signaling pathways, including the PI3K-AKT-mTOR, RAS-ERK, NF-κB, Wnt/β-catenin, and TGFβ-SMAD pathways. From a theoretical perspective, siRNA-based treatments can be developed to target genes that are overexpressed and promote metastasis. 26 In fact, plenty of siRNAs targeting important genes involved in the CRLM have been exploited. Table 1 lists the siRNA targets of metastasis-related signaling pathways in CRLM therapy, which have been investigated in previous studies. The following sections report a number of studies designed to provide siRNA-based approaches for targeted CRLM gene therapies. These results revealed that inhibition of these genes and metastasis-related pathways has important potential to fight CRLM.
Table 1.
Summary of Some siRNA Targets in CRLM Treatment
| Target | Notes | References |
|---|---|---|
| PI3K-AKT-mTOR pathway | ||
| AKT | siRNA targeting AKT gene induces apoptosis | 27 |
| RAS-ERK pathway | ||
| RAGE | siRNA targeting against RAGE inhibits cancer cell stemness and development | 28 |
| Wnt/β-catenin pathway | ||
| CTNNB1 | siRNA against CTNNB1 significantly inhibits tumor growth | 29 |
| Bag-1 | Inhibition of overexpressed Bag-1 in colon cancer leads to the increase of apoptosis | 30 |
| NF-κB pathway | ||
| MUC13 | MUC13-specific siRNA reduces the cancer stem cells and lowers the resistance to therapy | 31 |
| IL-8 | Silencing of IL-8 expression through modulation of MDR1 leads to sensitizing of CRC cells to doxorubicin | 32 |
| Rac1 | Rac1 knockdown by siRNA causes suppression of migration and sensitization of colon cancer cells to dihydroartemisinin-induced cell cycle arrest | 33 |
| TGFβ/SMAD pathway | ||
| TGF-β1 | Downregulation of TGF-β1 decreases the volume and number of CRC metastatic foci and suppresses CRLM in vivo by optimization of the immune microenvironment | 22 |
| PAR2 | Application of siRNA against PAR2 inhibits the migration and EMT | 34 |
| MEGF6 | siRNA against MEGF6 suppresses the EMT | 35 |
| Combined targeting | ||
| PIK3CA and KRAS | Targeted delivery of siRNA against PIK3CA and KRAS inhibits the growth of human CRC xenograft tumor | 36 |
| Arl4c | Arl4c silencing by using specific siRNAs reduces the tumor growth in vivo | 37 |
Targeting the PI3K-AKT-mTOR Pathway
Among the various dysregulated signaling pathways, one attractive pathway is the PI3K-AKT-mTOR pathway, which belongs to the epidermal growth factor receptor (EGFR) signaling cascade. EGFR is a tyrosine kinase receptor (RTK) that is causally linked to the progression and metastasis of CRC. Activation of RTK stimulates specific pathways that directly affect the proliferation, migration, and survival of tumor cells. Abnormal regulation of the EGFR pathway is common in advanced CRC and liver metastasis. 38 Currently, cetuximab, an EGFR receptor blocker, is used to treat advanced CRC with an activated EGFR mutant. The crucial mechanism of resistance to anti-EGFR monoclonal antibodies is activation of oncogenic pathways downstream of EGFR. Therefore, the PI3K-AKT-mTOR pathway has attracted attention as a candidate molecular target. 39 Protein kinase B (AKT) is one of the most crucial proto-oncogenes. AKT is abnormally expressed in many cancers, including CRC. 40 Kang et al. demonstrated that siRNA targeting AKT in animal models of CRLM reduces AKT2 production and initiates apoptosis in cancer cells. 27 The PI3K/AKT pathway is significantly downregulated by targeting AKT. In addition, cleaved caspase 9 and Bax are upregulated, which marks the beginning of apoptosis. 27 These reports indicate that siRNA-based treatments may become a reality for CRLM.
Targeting the RAS-ERK Pathway
Abnormal activation of the RAS-extracellular signal regulated kinase (ERK) pathway plays an important role in CRC. 41 Variations in the RAS-ERK pathway are related to the shortest overall survival after patients are diagnosed with CRC metastasis. 42 KRAS mutations, which are involved in the RAS-ERK pathway, have been shown to play important roles in colorectal tumorigenesis. These mutations can significantly increase the number and growth rate of tumors and even induce cancer stem cell markers, leading to liver metastasis in a mouse xenograft model. 43 In one study, a direct association between receptor for advanced glycation end products (RAGE) and KRAS is found after HMGB1 exposure in CRC cells. RAGE promotes RAS-dependent Yap1 to drive CRC stemness and development. siRNA-mediated silencing is used to inhibit the harmful effects of this gene, and positive results show a bright future of the underlying target. 28
Targeting the Wnt/β-Catenin Pathway
The Wnt/β-catenin pathway is an important regulatory pathway in CRC. 44 It regulates epithelial-to-mesenchymal transition (EMT) and intestinal stem cell (ISCs) homeostasis. In CRC, EMT affects a variety of malignant phenotypes related to metastasis. In adult intestine, the Wnt/β-catenin pathway maintains the crypt stem cells homeostasis. Dysregulation of pathway activity can induce tumor stem cell formation. The plasticity and differentiation ability of tumor stem cells enable them to better adapt to the microenvironment through metastasis. 45 β-catenin is a member of the Wnt signaling pathway that promotes tumor metastasis. β-catenin protein levels are raised in 70% to 80% of colon cancer cells. 46 CTNNB1 encodes β-catenin. Ganesh et al. 29 evaluated the therapeutic effects of siRNAs against CTNNB1 in vitro and in vivo. Their results suggest that silencing CTNNB1 significantly inhibits tumor growth. They concluded that the siRNA silencing of CTNNB1 might be an attractive gene therapy. Moreover, in another experiment, Rudzinski et al. focused on β-catenin, which contributes to tumor progression. 46 In this study, the entry of anti-β-catenin siRNA, confirmed by confocal microscopy and western blot analysis in cultured colon cancer cells, is negatively correlated with β-catenin protein levels. 46 Although this study lacks biological functional tests on cells, it may prove that humans may benefit from targeting β-catenin for colon cancer therapy. In addition, Bcl-2 plays a critical role in anti-apoptosis, 47 and Bcl-2 associated athanogene (Bag-1) is a positive regulator of apoptosis. 48 Overexpression of this regulatory gene has been found in many tumor tissues, especially in colon cancer. 49 Huang et al. evaluated the therapeutic effects of siRNA targeting Bag-1 in vitro. Their results suggest that silencing of Bag-1 significantly increases the apoptosis rate. Further studies showed that β-catenin, the major molecule in the Wnt/β-catenin pathway, is reduced after Bag-1 was silenced. 30 The authors concluded that siRNA silencing of Bag-1 might be an attractive gene therapy.
Targeting the NF-κB Pathway
NF-κB is a characteristic heterodimer transcription factor composed of p50 and p65 that plays a central role in the metastasis of CRC. Inactivated NF-κB/p65 is present in the cytosol and binds to the inhibitory protein IκBα. Through a variety of extracellular signals, IκB kinase (IKK) is stimulated and phosphorylates IκBα. The phosphorylation of IκBα leads to ubiquitination, dissociation of the complex, and degradation of IκBα. Activated NF-κB/p65 is subsequently translocated into the nucleus where it activates target gene transcription. Constitutively activated NF-κB is involved in the invasive behavior of CRC. 50 MUC13 is a transmembrane mucin glycoprotein overexpressed in many cancers. MUC13 is responsible for activation of the NF-κB pathway via 2 distinct pathways, resulting in the upregulation of BCL-xL (BCL-extra-large). One pathway promotes tumor necrosis factor (TNF)-induced NF-κB activation, and the other promotes genotoxin-induced NF-κB activation. Moreover, elevated cytoplasmic MUC13 and NF-κB levels facilitate CRC metastasis. Silencing of MUC13 lowers the resistance of CRC cells to cytotoxic drugs and inflammatory signals, reduces chemotherapy-induced cancer stem cells, impends xenograft growth in vivo by a specific siRNA, and induces tumor regression combined with 5-fluoruouracil. Hence, CRLM treatment can be accelerated by a combination therapy with chemotherapy and MUC13 antagonism. 31 Du et al. showed that inhibition of IL-8 by siRNA modulates multidrug resistance 1 (MDR1) via IKK-β/p65 signaling and sensitizes tumor cells to doxorubicin in CRC cells. 32 Rac1 is overexpressed in colon carcinoma. It is closely associated with the progression and metastasis of colon cancer. Rac1 also plays an important role in activating NF-κB-mediated transcription. Han et al. reported that Rac1 siRNA suppresses the NF-κB pathway. It was observed that colon cancer cells are sensitized to dihydroartemisinin-induced cell cycle arrest and cell migration is inhibited. 33
Targeting the TGFβ/SMAD Pathway
Transforming growth factor β1 (TGF-β1) is a tumor-related growth factor that inhibits systemic immune function and host immunosurveillance. 51 SMADs are trimeric proteins that form the main intracellular substrates of activated TGF-β receptors. An increasing number of studies in metastatic models of CRC suggest that targeting the TGF-β pathway reduces metastasis. The process is possibly mediated by direct action on tumor cells and by interference with the pernicious intercellular communication occurring in the tumor microenvironment. It is well known that levels of TGF-β are elevated in patients with advanced CRC. In addition, high levels of circulating TGF-β are precursors of liver metastasis after CRC resection. 52 It was shown that siRNA against TGF-β1 leads to reduction of volume and number of CRC metastatic foci and inhibition of CRLM in vivo by downregulation of TGF-β1 expression, inhibiting the formation of tumor-associated macrophages and improving the immune microenvironment. 22 TGF-β also mediates EMT in tumor cells. 34 TGF-β-mediated EMT is found to be related to protease-activated receptor 2 (PAR2) after 5-FU treatment. siRNA-mediated knockdown of PAR2 suppresses cell migration. 34 Multiple epidermal growth factor-like domain protein 6 (MEGF6) promotes CRC metastasis by inducing EMT through the TGFβ/SMAD signaling pathway. 35 The use of siRNA directed against MEGF6 in human colorectal cell lines results in the downregulation of the target, inhibition of cell proliferation, and promotion of apoptosis. 35
Combined Targeting
A comprehensive multidisciplinary approach is now the backbone of successful outcomes in CRLM treatment. 17 Given that a variety of signaling pathways are involved in CRLM, targeting 2 or more signaling pathways may be more effective. KRAS mutations are found in approximately 40% of cases resistant to anti-EGFR antibodies. 39 In one study, the downregulation of PIK3CA and KRAS via cetuximab-complexed and endoribonuclease-prepared specific siRNA pools interfere with the growth of human CRC xenograft tumors. 36 In addition, known downstream factors, such as p-ERK, p-AKT, and c-MYC, are decreased. Accordingly, antibody-endoribonuclease-prepared-siRNA complexes may be used as a new treatment option for mutations in EGFR signaling. In addition, the expression of ADP-ribosylation factor (ARF)-like 4c (Arl4c) is increased in tissue specimens from patients with CRC compared to that in nontumor regions. 37 In HCT116 CRC cells, Arl4c mRNA levels increase upon activation of Wnt/β-catenin or epidermal growth factor/Ras signaling. Arl4c-depleted cancer cells inhibit Rac activity and prevent its nuclear localization. Cells with Arl4c knockdown show decreased migration, invasion, and proliferation capabilities both in vitro and in vivo. 37 To further confirm the function of this gene in CRC, Fujii et al. used siRNA targeting Arl4c genes in HCT116 cell-derived tumors in immunodeficient mice. 37 These injections suppress tumor growth in vivo. These data suggest that Arl4c is a novel therapeutic target.
siRNA in Clinical Settings for CRLM Treatment
To date, the number of clinical trials of RNAi-based therapies has grown rapidly. Most DNA sequences in the genome are transcribed into noncoding transcripts. Hence, RNA therapies can expand the range of drug targets. 20 In addition to cancer, it has been applied to treat multiple diseases, such as hypercholesterolemia, cardiovascular diseases, inflammatory bowel disease, and familial amyloid polyneuropathy.53–56 Table 2 lists clinical trials of siRNA therapeutics in CRLM, and these data from the clinical trials enrich the knowledge and experience and pave the way for a successful application of siRNA in CRLM therapy.
Table 2.
Summary of siRNA Therapies in CRLM Clinical Trials.
| Intervention | Target | Administration route | Status | Phase | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| Drug: ALN-VSP02 | VEGF and KSP | IV | Completed (2011) Completed (2012) | Phase 1 | NCT00882180 NCT01158079 |
| Drug: Atu027 | PKN3 | IV | Completed (2012) | Phase 1 | NCT00938574 |
| Drug: TKM 080301 | PLK1 | Catheter | Completed (2012) | Phase 1 | NCT01437007 |
| Biological: APN401 | Cblb | IV | Completed (2020) | Phase 1 | NCT03087591 |
| Drug: NBF-006 | GSTP1 | IV | Recruiting | Phase 1 | NCT03819387 |
| Drug: siRNA-EphA2-DOPC | EphA2 | IV | Active, not recruiting | Phase 1 | NCT01591356 |
| Drug: CALAA-01 | RRM2 | IV | Terminated (2012) | Phase 1 | NCT00689065 |
| Drug: DCR-MYC | MYC | IV | Terminated (2016) | Phase 1 | NCT02110563 |
Targeting VEGF and KSP
For CRLM, a 2 siRNA molecules formulation, ALN-VSP02, targeting VEGF and kinesin spindle protein (KSP), was used in the first human clinical trial. The results showed that the drug is detected in tumor biopsies, mRNA cleavage in the liver is mediated by siRNA, and pharmacodynamic target downregulation and antitumor activity are present. In the trial, ALN-VSP02 was administered through the IV route biweekly and was safe and well tolerated (clinicaltrials.gov identifier number: NCT00882180, NCT01158079). 57 These data can be recognized as a milestone in siRNA treatment in humans.
Targeting PKN3
Atu027 contains siRNAs that target protein kinase N3 (PKN3). PKN3 is expressed in the vascular endothelium, and silencing PKN3 has been shown to inhibit local tumor invasion and metastasis to lymph nodes and lungs in mouse cancer models. A phase 1 clinical trial of the drug was conducted and showed a favorable safety profile in patients with advanced solid cancer (clinicaltrials.gov identifier number: NCT00938574). 58
Targeting PLK1
In a phase 1 clinical trial, another new drug, TKM 080301 (clinicaltrials.gov identifier number: NCT01437007), which is a product of lipid nanoparticle-formulated (LNP) siRNA against the polo-like kinase-1 (PLK1) gene, was investigated in patients with CRLM. PLK1 plays a crucial role in the progression of various cancers.59–61 In metastatic hepatocellular carcinoma, the PLK1-mediated pathway is aberrant and affects the fate of metastatic cells. 62 Downregulation of PLK1 in proliferating cancer cells leads to mitotic arrest and apoptosis. Unfortunately, the trial enrolled only one participant, and no results were posted.
Targeting Cblb
Peripheral blood mononuclear cells (APN401) transfected with siRNA were used in a phase 1 trial in patients with recurrent or metastatic solid tumors that could not be treated by surgery, including CRC (clinicaltrials.gov identifier number: NCT03087591). APN401 is an autologous cell therapy that uses RNAi technology to inhibit the immune checkpoint Cblb. Although this trial has been completed, the results have not been reported.
Targeting GSTP1
NBF-006 was designed for patients with CRC. It consists of siRNA that inhibits glutathione S-transferase P1(GSTP1). Abnormal GSTP1 expression is associated with multiple tumor types. In CRC, it promotes the proliferation, invasion, and metastasis of cells. 63 Phase 1 clinical trial is conducted by intravenous infusion of NBF-006 into patients (clinicaltrials.gov identifier number: NCT03819387). This trial is currently ongoing.
Targeting EphA2
The Ephrin type-A receptor 2 (EphA2) gene is upregulated in many cancers. Although its main functions are not fully understood, tumor-based models suggest that it plays an important role in proliferation, survival, migration, invasion, and angiogenesis. 64 siRNA-EphA2-DOPC is currently in a phase 1 clinical trial, where it is administered intravenously to patients with advanced or recurrent solid tumors, including CRLM (clinicaltrials.gov identifier number: NCT01591356). 65
Targeting RRM2
CALAA-01 is a siRNA drug used to treat solid tumors. It contains siRNA that inhibits the expression of the M2 subunit of ribonucleotide reductase (RRM2). RRM2 is crucial for DNA replication and cell division and is overexpressed in multiple tumor types. 66 CALAA-01 was administered intravenously. Nevertheless, the phase 1 clinical trial was terminated because of dose-limiting toxicity and other side effects (clinicaltrials.gov identifier number: NCT00689065). 67
Targeting MYC
DCR-MYC inhibits the oncogene MYC and is designed for a variety of cancers such as solid tumors, multiple myeloma, and lymphoma. MYC is considered an important oncogenic target owing to its function in angiogenesis and metastasis. 68 Data from a phase 1 clinical trial showed that DCR-MYC has satisfactory clinical and metabolic responses (clinicaltrials.gov identifier number: NCT02110563). 69 However, the clinical trial was terminated based on the sponsor's decision.
Challenges of siRNA Technology
For the use of siRNA in treatment, the challenges include the intrinsic lack of serum stability, nonspecific uptake, difficulty in reaching the required siRNA levels, and activation of the immune response.27,70 These challenges necessitate the development of more efficient delivery systems. Different delivery materials have been developed and are mainly divided into 2 categories: viral and nonviral vectors. Viral vectors have high target specificity and transfection efficiency but may cause serious potential unknown effects when repeatedly applied to the body. 71 Among nonviral vectors, polymers and lipids are widely studied because they are biocompatible and able to carry or deliver siRNAs.72–76 Exosomes are cell-derived vesicles that are regarded as cell-created drug delivery systems that participate in siRNA delivery.77,78 At the same time, routes of administration are considered a solution to the issues brought about by siRNA. Local delivery, such as the intravitreal or intranasal routes, is the current administration of most siRNAs in clinical trials, and systemic delivery is also under consideration. 79 Some researchers have introduced the oral (PO) route because of its convenience and ease of multiple dosing.27,80,81 Another point of note is the toxicity of the drug. The 4 major sources of toxicity include immunogenic reactions to siRNA, toxicity of excipients, unintended siRNA activity, and on-target siRNA activity in nontarget tissues. Modifications of delivery systems may solve the problem of immunogenic reactions. 82 In comparison, excipient chemicals also have toxic effects, which have troubled nanoparticle drug formulation and are related to dose-limiting toxicities in many studies. 83 Restricting the excipients to a smaller number may be an important method. Off-target siRNA silencing can be eliminated by massive testing to ameliorate overlaps between the gene of concern and the target sites. To avoid siRNA activity in nontarget tissues, highly disease-selective genes are carefully identified, and delivery routes are modified. 82 For clinical applications, efficient and safe delivery systems and convenient routes of administration have important effects on the use of siRNA.
Discussion
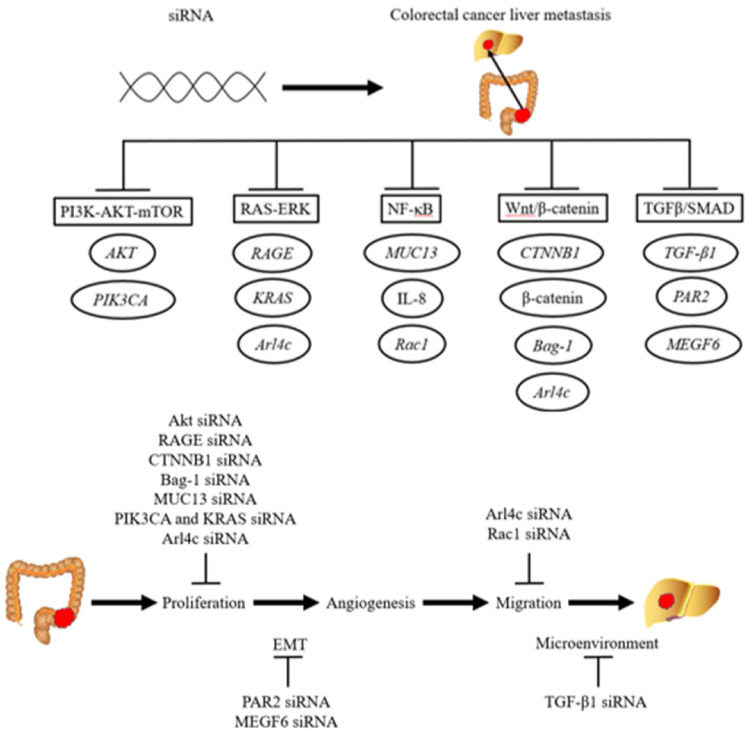
CRC is one of the most widespread cancers, with high mortality rates worldwide. During the course of CRC, metastases occur in more than half of patients. Approximately one-fifth of patients with CRC are diagnosed with synchronous liver metastases, and in the coming 2 to 3 years, other 25% to 30% of patients with CRC will develop liver metastases. 84 The treatments available for patients with liver metastases are less effective than those for patients without metastases. Newer and less invasive therapies are necessary based on the current treatments, including surgery, radiotherapy, and chemotherapy. Among these new treatments, siRNA therapy has attracted considerable attention. siRNAs have been studied for the treatment of CRLM by targeting the CRLM-promoting genes in many studies. Five classical pathways, the PI3K-AKT-mTOR, RAS-ERK, NF-κB, Wnt/β-catenin, and TGFβ-SMAD pathways, have been found to be involved in CRLM (Figure 1). Therefore, silencing the genes involved in metastasis-related pathways, which are important and connected to clinical settings, has contributed to precision treatment of CRLM and may reduce patient mortality.
Figure 1.
Schematic diagram of the siRNA-mediated downregulation of genes related to colorectal cancer liver metastasis signaling pathways.
A multi-step tumorigenesis model is the basis of the traditional paradigm of CRLM. 85 The common steps involved are proliferation, epithelial-mesenchymal transition, angiogenesis, and cell migration.86–88 Proliferation is the basis of tumor metastasis. Therefore, the majority of siRNA therapies for CRLM affect the proliferation of tumor cells by regulating the cell cycle, promoting apoptosis, and inhibiting tumor stem cells. Other studies have attempted to influence tumor metastasis by altering EMT, tumor cell migration, or the tumor microenvironment. Contemporarily, treatment is usually ineffective when a single drug is used. Therefore, effective treatments require blocking 2 or more steps of metastasis. The goal can be realized by combining 2 or more targets or therapeutic methods that incorporate a variety of mechanisms. In this case, combining siRNA with an antibody or chemotherapeutic drug in the same delivery system may produce a better therapeutic effect. It is also important to identify additional metastasis-related molecules.
In clinical settings, siRNA-based therapies for CRLM are in progress. A series of targets have been explored, and clinical trials have been conducted to determine their efficacy and safety, to make the clinical application of siRNA as soon as possible. However, it is worth noting that the targets involved in these clinical trials are focused on applications in solid tumors and not exclusively in CRLM. At the same time, all clinical trials were phase 1 trials, in which relatively few participants were enrolled. These 2 factors may interfere with the results of siRNA therapies for CRLM. Therefore, developing specific targets for CRLM and conducting corresponding clinical trials could produce more accurate results regarding siRNA therapies. To identify specific targets, we need to combine the findings of basic research on metastasis-related signal pathways and CRLM pathogenesis.
siRNA therapy is expected to expand the range of drug targets, because most DNA sequences in the human genome are transcribed into nonprotein-coding transcripts. 89 Simultaneously, with progress in the molecular biological basis of malignant tumor metastasis, it is possible to design individualized treatment plans using siRNA therapy, which will make the treatment more targetable and benefit more patients with cancer.
Nevertheless, some limitations restrict the therapeutic application of siRNAs. To solve their intrinsically poor serum stability, efficient delivery systems and appropriate routes of administration have been investigated to protect siRNA. To ensure safety, many strategies, including identifying key genes and restricting excipients, have promoted the development of siRNA in CRLM therapy. At the same time, in vivo experiments, clinical trials, and corresponding evaluation criteria regarding the efficacy and safety of siRNAs are urgently needed.
Limitations
Several therapeutic targets for the siRNA-based treatment of CRLM were not discussed in this review. Only therapeutic targets linked with corresponding signaling pathways in CRLM therapy were discussed in the hope of providing inspiration to explore more powerful targets from the perspective of metastasis-related signaling pathways.
Conclusion
We have identified therapeutic targets associated with metastasis-related signaling pathways for siRNA-mediated treatment of CRLM. This technology has shown therapeutic effects not only in vivo but also in clinical trials. We expect to translate this promising technology into clinical treatment by overcoming the current challenges.
Acknowledgments
The authors gratefully acknowledge Prof. Yu Zhu and Prof. Yuenan Wang for editing the manuscript. Meanwhile, we would like to thank Editage (www.editage.com) for English language editing.
Abbreviations
- AKT
protein kinase B
- ARF
ADP-ribosylation factor
- Arl4c
ADP-ribosylation factor-like 4c
- Bag-1
Bcl-2-associated athanogene 1
- BCL-xL
BCL-extra-large
- CRC
colorectal cancer
- CRLM
colorectal cancer liver metastasis
- dsRNA
double-stranded RNA
- EGFR
epidermal growth factor receptor
- EMT
epithelial-to-mesenchymal transition
- EphA2
ephrin type-A receptor 2
- ERK
extracellular signal regulated kinase
- GSTP1
glutathione S-transferase P1
- IKK
IκB kinase
- ISCs
intestinal stem cells
- KSP
kinesin spindle protein
- LNP
lipid nanoparticle-formulated
- MDR
multidrug resistance
- MEGF6
multiple epidermal growth factor-like domains protein 6
- mRNA
messenger RNA
- NAFLD
nonalcoholic fatty liver disease
- PAR2
protease-activated receptor 2
- PLK1
polo-like kinase-1
- PKN3
protein kinase N3
- PO
oral
- RAGE
receptor for advanced glycation end products
- RNAi
RNA interference
- RRM2
M2 subunit of ribonucleotide reductase
- RTK
tyrosine kinase receptor
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- ss
single-stranded
- TGF-β1
transforming growth factor β1;
- TNF
tumor necrosis factor
Footnotes
Ethical Approval: The manuscript is a review and not directly related to the animal and human studies, ethical statement is not required in the submission.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Shenzhen Science and Technology Innovation Commission Project (grant numbers GJHZ20200731095207023, KCXFZ202002011010508, ZDSYS20190902092855097).
ORCID iD: Junlin Xie https://orcid.org/0000-0001-8812-6967
References
- 1.Valle L, de Voer RM, Goldberg Y, et al. Update on genetic predisposition to colorectal cancer and polyposis. Mol Aspects Med. 2019;69:10-26. doi: 10.1016/j.mam.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 2.Ahluwalia P, Mondal AK, Bloomer C, et al. Identification and clinical validation of a novel 4 gene-signature with prognostic utility in colorectal cancer. Int J Mol Sci. 2019;20(15):3818. doi: 10.3390/ijms20153818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tu X, Hong D, Jiang Y, et al. FH535 Inhibits proliferation and migration of colorectal cancer cells by regulating CyclinA2 and Claudin1 gene expression. Gene. 2019;690:48-56. doi: 10.1016/j.gene.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 4.Yang ZH, Dang YQ, Ji G. Role of epigenetics in transformation of inflammation into colorectal cancer. World J Gastroenterol. 2019;25(23):2863-2877. doi: 10.3748/wjg.v25.i23.2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin J, Yu X, Xie L, et al. eIF6 promotes colorectal cancer proliferation and invasion by regulating AKT-related signaling pathways. J Biomed Nanotechnol. 2019;15(7):1556-1567. doi: 10.1166/jbn.2019.2792 [DOI] [PubMed] [Google Scholar]
- 6.Liang Z, Li X, Liu S, Li C, Wang X, Xing J. MiR-141-3p inhibits cell proliferation, migration and invasion by targeting TRAF5 in colorectal cancer. Biochem Biophys Res Commun. 2019;514(3):699-705. doi: 10.1016/j.bbrc.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 7.Arnold M, Abnet CC, Neale RE, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159(1):335-349. doi: 10.1053/j.gastro.2020.02.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong Y, Rao Y. Current status of nanoscale drug delivery systems for colorectal cancer liver metastasis. Biomed Pharmacother. 2019;114:108764. doi: 10.1016/j.biopha.2019.108764 [DOI] [PubMed] [Google Scholar]
- 9.Marcuello M, Vymetalkova V, Neves RPL, et al. Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol Aspects Med. 2019;69:107-122. doi: 10.1016/j.mam.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 10.Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713-732. doi: 10.1038/s41575-019-0189-8 [DOI] [PubMed] [Google Scholar]
- 11.Buccafusca G, Proserpio I, Tralongo AC, Rametta Giuliano S, Tralongo P. Early colorectal cancer: diagnosis, treatment and survivorship care. Crit Rev Oncol Hematol. 2019;136:20-30. doi: 10.1016/j.critrevonc.2019.01.023 [DOI] [PubMed] [Google Scholar]
- 12.Araghi M, Soerjomataram I, Jenkins M, et al. Global trends in colorectal cancer mortality: projections to the year 2035. Int J Cancer. 2019;144(12):2992-3000. doi: 10.1002/ijc.32055 [DOI] [PubMed] [Google Scholar]
- 13.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394(10207):1467-1480. doi: 10.1016/S0140-6736(19)32319-0 [DOI] [PubMed] [Google Scholar]
- 14.Lv Y, Patel N, Zhang HJ. The progress of non-alcoholic fatty liver disease as the risk of liver metastasis in colorectal cancer. Expert Rev Gastroenterol Hepatol. 2019;13(12):1169-1180. doi: 10.1080/17474124.2019.1697231 [DOI] [PubMed] [Google Scholar]
- 15.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490-1502. doi: 10.1016/S0140-6736(13)61649-9 [DOI] [PubMed] [Google Scholar]
- 16.Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kow AWC. Hepatic metastasis from colorectal cancer. J Gastrointest Oncol. 2019;10(6):1274-1298. doi: 10.21037/jgo.2019.08.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchiò S, Arap W, Pasqualini R. Targeting the extracellular signature of metastatic colorectal cancers. Expert Opin Ther Targets. 2009;13(3):363-379. doi: 10.1517/14728220902762910 [DOI] [PubMed] [Google Scholar]
- 19.Abedini F, Ismail M, Hosseinkhani H, et al. Effects of CXCR4 siRNA/dextran-spermine nanoparticles on CXCR4 expression and serum LDH levels in a mouse model of colorectal cancer metastasis to the liver. Cancer Manag Res. 2011;3:301-309. doi: 10.2147/CMR.S11678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu AM, Jian C, Yu AH, Tu MJ. RNA Therapy: are we using the right molecules? Pharmacol Ther. 2019;196:91-104. doi: 10.1016/j.pharmthera.2018.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaradoddi JS, Kontro MH, Ganachari SVet al. et al. RNA Nanotechnology. In: Martínez L, Kharissova O, Kharisov B, eds. Handbook of Ecomaterials. Springer; 2018:1-14. doi: 10.1007/978-3-319-48281-1_193-1 [DOI] [Google Scholar]
- 22.Fang JK, Chen L, Lu XG, et al. Optimization of transforming growth factor-β1 siRNA loaded chitosan-tripolyphosphate nanoparticles for the treatment of colorectal cancer hepatic metastasis in a mouse model. J Biomed Nanotechnol. 2016;12(7):1489-1500. doi: 10.1166/jbn.2016.2265 [DOI] [PubMed] [Google Scholar]
- 23.Salguero-Aranda C, Sancho-Mensat D, Canals-Lorente B, et al. STAT6 Knockdown using multiple siRNA sequences inhibits proliferation and induces apoptosis of human colorectal and breast cancer cell lines. PLoS One. 2019;14(5):e0207558. doi: 10.1371/journal.pone.0207558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao Y, Wang T, Liu Y, Zhang N. Co-delivery of sorafenib and VEGF-siRNA via pH-sensitive liposomes for the synergistic treatment of hepatocellular carcinoma. Artif Cells Nanomed Biotechnol. 2019;47(1):1374-1383. doi: 10.1080/21691401.2019.1596943 [DOI] [PubMed] [Google Scholar]
- 25.Liu B, Cao W, Qiao G, et al. Effects of gold nanoprism-assisted human PD-L1 siRNA on both gene down-regulation and photothermal therapy on lung cancer. Acta Biomater. 2019;99:307-319. doi: 10.1016/j.actbio.2019.08.046 [DOI] [PubMed] [Google Scholar]
- 26.Sousa AR, Oliveira AV, Oliveira MJ, Sarmento B. Nanotechnology-based siRNA delivery strategies for metastatic colorectal cancer therapy. Int J Pharm. 2019;568:118530. doi: 10.1016/j.ijpharm.2019.118530 [DOI] [PubMed] [Google Scholar]
- 27.Kang SH, Revuri V, Lee SJ, et al. Oral siRNA delivery to treat colorectal liver metastases. ACS Nano. 2017;11(10):10417-10429. doi: 10.1021/acsnano.7b05547 [DOI] [PubMed] [Google Scholar]
- 28.Qian F, Xiao J, Gai L, Zhu J. HMGB1-RAGE Signaling facilitates Ras-dependent Yap1 expression to drive colorectal cancer stemness and development. Mol Carcinog. 2019;58(4):500-510. doi: 10.1002/mc.22944 [DOI] [PubMed] [Google Scholar]
- 29.Ganesh S, Koser ML, Cyr WA, et al. Direct pharmacological inhibition of β-catenin by RNA interference in tumors of diverse origin. Mol Cancer Ther. 2016;15(9):2143-2154. doi: 10.1158/1535-7163.MCT-16-0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang W, Liu Z, Zhou G, Tian A, Sun N. Magnetic gold nanoparticle-mediated small interference RNA silencing Bag-1 gene for colon cancer therapy. Oncol Rep. 2016;35(2):978-984. doi: 10.3892/or.2015.4453 [DOI] [PubMed] [Google Scholar]
- 31.Sheng YH, He Y, Hasnain SZ, et al. MUC13 Protects colorectal cancer cells from death by activating the NF-κB pathway and is a potential therapeutic target. Oncogene. 2017;36(5):700-713. doi: 10.1038/onc.2016.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du J, He Y, Li P, Wu W, Chen Y, Ruan H. IL-8 regulates the doxorubicin resistance of colorectal cancer cells via modulation of multidrug resistance 1 (MDR1). Cancer Chemother Pharmacol. 2018;81(6):1111-1119. doi: 10.1007/s00280-018-3584-x [DOI] [PubMed] [Google Scholar]
- 33.Han P, Luan Y, Liu Y, et al. Small interfering RNA targeting Rac1 sensitizes colon cancer to dihydroartemisinin-induced cell cycle arrest and inhibited cell migration by suppressing NFκB activity. Mol Cell Biochem. 2013;379(1–2):171-180. doi: 10.1007/s11010-013-1639-1 [DOI] [PubMed] [Google Scholar]
- 34.Quan Q, Zhong F, Wang X, Chen K, Guo L. PAR2 Inhibition enhanced the sensitivity of colorectal cancer cells to 5-FU and reduced EMT signaling. Oncol Res. 2019;27(7):779-788. doi: 10.3727/096504018X15442985680348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu H, Wang M, Wang H, et al. MEGF6 Promotes the epithelial-to-mesenchymal transition via the TGFβ/SMAD signaling pathway in colorectal cancer metastasis. Cell Physiol Biochem. 2018;46(5):1895-1906. doi: 10.1159/000489374 [DOI] [PubMed] [Google Scholar]
- 36.Bäumer N, Rehkämper J, Appel N, et al. Downregulation of PIK3CA via antibody-esiRNA-complexes suppresses human xenograft tumor growth. PLoS One. 2018;13(7):e0200163. doi: 10.1371/journal.pone.0200163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujii S, Matsumoto S, Nojima S, Morii E, Kikuchi A. Arl4c expression in colorectal and lung cancers promotes tumorigenesis and may represent a novel therapeutic target. Oncogene. 2015;34(37):4834-4844. doi: 10.1038/onc.2014.402 [DOI] [PubMed] [Google Scholar]
- 38.Herynk MH, Radinsky R. The coordinated functional expression of epidermal growth factor receptor and c-Met in colorectal carcinoma metastasis. In Vivo. 2000;14(5):587-596. [PubMed] [Google Scholar]
- 39.Tsujii M. Search for novel target molecules for the effective treatment or prevention of colorectal cancer. Digestion. 2012;85(2):99-102. doi: 10.1159/000334678 [DOI] [PubMed] [Google Scholar]
- 40.Weisner J, Landel I, Reintjes C, et al. Preclinical efficacy of covalent-allosteric AKT inhibitor borussertib in combination with trametinib in KRAS-mutant pancreatic and colorectal cancer. Cancer Res. 2019;79(9):2367-2378. doi: 10.1158/0008-5472.CAN-18-2861 [DOI] [PubMed] [Google Scholar]
- 41.Jeong WJ, Ro EJ, Choi KY. Interaction between Wnt/β-catenin and RAS-ERK pathways and an anti-cancer strategy via degradations of β-catenin and RAS by targeting the wnt/β-catenin pathway. NPJ Precis Oncol. 2018;2(1):5. doi: 10.1038/s41698-018-0049-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urosevic J, Blasco MT, Llorente A, et al. ERK1/2 Signaling induces upregulation of ANGPT2 and CXCR4 to mediate liver metastasis in colon cancer. Cancer Res. 2020;80(21):4668-4680. doi: 10.1158/0008-5472.CAN-19-4028 [DOI] [PubMed] [Google Scholar]
- 43.Lee SK, Hwang JH, Choi KY. Interaction of the Wnt/β-catenin and RAS-ERK pathways involving co-stabilization of both β-catenin and RAS plays important roles in the colorectal tumorigenesis. Adv Biol Regul. 2018;68:46-54. doi: 10.1016/j.jbior.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 44.Xu C, Tian G, Jiang C, et al. NPTX2 Promotes colorectal cancer growth and liver metastasis by the activation of the canonical Wnt/β-catenin pathway via FZD6. Cell Death Dis. 2019;10(3):217. doi: 10.1038/s41419-019-1467-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang M, Miao F, Huang R, et al. RHBDD1 Promotes colorectal cancer metastasis through the Wnt signaling pathway and its downstream target ZEB1. J Exp Clin Cancer Res. 2018;37(1):60. doi: 10.1186/s13046-018-0687-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudzinski WE, Palacios A, Ahmed A, Lane MA, Aminabhavi TM. Targeted delivery of small interfering RNA to colon cancer cells using chitosan and PEGylated chitosan nanoparticles. Carbohydr Polym. 2016;147:323-332. doi: 10.1016/j.carbpol.2016.04.041 [DOI] [PubMed] [Google Scholar]
- 47.Dawe GB, Yu H, Gu S, et al. Α7 nicotinic acetylcholine receptor upregulation by anti-apoptotic Bcl-2 proteins. Nat Commun. 2019;10(1):2746. doi: 10.1038/s41467-019-10723-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kizilboga T, Baskale EA, Yildiz J, et al. Bag-1 stimulates bad phosphorylation through activation of Akt and Raf kinases to mediate cell survival in breast cancer. BMC Cancer. 2019;19(1):1254. doi: 10.1186/s12885-019-6477-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takayama S, Krajewski S, Krajewska M, et al. Expression and location of Hsp70/Hsc-binding anti-apoptotic protein BAG-1 and its variants in normal tissues and tumor cell lines. Cancer Res. 1998;58(14):3116-3131. [PubMed] [Google Scholar]
- 50.Lu YX, Ju HQ, Wang F, et al. Inhibition of the NF-κB pathway by nafamostat mesilate suppresses colorectal cancer growth and metastasis. Cancer Lett. 2016;380(1):87-97. doi: 10.1016/j.canlet.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 51.Lodyga M, Hinz B. TGF-β1 - A truly transforming growth factor in fibrosis and immunity. Semin Cell Dev Biol. 2020;101:123-139. doi: 10.1016/j.semcdb.2019.12.010 [DOI] [PubMed] [Google Scholar]
- 52.Villalba M, Evans SR, Vidal-Vanaclocha F, Calvo A. Role of TGF-β in metastatic colon cancer: it is finally time for targeted therapy. Cell Tissue Res. 2017;370(1):29-39. doi: 10.1007/s00441-017-2633-9 [DOI] [PubMed] [Google Scholar]
- 53.Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376(15):1430-1440. doi: 10.1056/NEJMoa1615758 [DOI] [PubMed] [Google Scholar]
- 54.Zhang T, Liang X, Shi L, et al. Estrogen receptor and PI3K/Akt signaling pathway involvement in S-(-)equol-induced activation of Nrf2/ARE in endothelial cells. PLoS One. 2013;8(11):e79075. doi: 10.1371/journal.pone.0079075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki K, Yokoyama J, Kawauchi Y, et al. Phase 1 clinical study of siRNA targeting carbohydrate sulphotransferase 15 in Crohn's disease patients with active mucosal lesions. J Crohns Colitis. 2017;11(2):221-228. doi: 10.1093/ecco-jcc/jjw143 [DOI] [PubMed] [Google Scholar]
- 56.Adams D, Gonzalez-Duarte A, O'Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11-21. doi: 10.1056/NEJMoa1716153 [DOI] [PubMed] [Google Scholar]
- 57.Tabernero J, Shapiro GI, LoRusso PM, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3(4):406-417. doi: 10.1158/2159-8290.CD-12-0429 [DOI] [PubMed] [Google Scholar]
- 58.Schultheis B, Strumberg D, Santel A, et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J Clin Oncol. 2014;32(36):4141-4148. doi: 10.1200/JCO.2013.55.0376 [DOI] [PubMed] [Google Scholar]
- 59.Shakil S, Baig MH, Tabrez S, et al. Molecular and enzoinformatics perspectives of targeting Polo-like kinase 1 in cancer therapy. Semin Cancer Biol. 2019;56:47-55. doi: 10.1016/j.semcancer.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 60.Hu M, Zhang Q, Tian XH, Wang JL, Niu YX, Li G. lncRNA CCAT1 is a biomarker for the proliferation and drug resistance of esophageal cancer via the miR-143/PLK1/BUBR1 axis. Mol Carcinog. 2019;58(12):2207-2217. doi: 10.1002/mc.23109 [DOI] [PubMed] [Google Scholar]
- 61.De Blasio C, Zonfrilli A, Franchitto M, et al. PLK1 Targets NOTCH1 during DNA damage and mitotic progression. J Biol Chem. 2019;294(47):17941-17950. doi: 10.1074/jbc.RA119.009881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang XQ, Zhu YQ, Lui KS, et al. Aberrant Polo-like kinase 1-Cdc25A pathway in metastatic hepatocellular carcinoma. Clin Cancer Res. 2008;14(21):6813-6820. doi: 10.1158/1078-0432.CCR-08-0626 [DOI] [PubMed] [Google Scholar]
- 63.Wang F, Zhang C, Zhu X, et al. Overexpression of GSTP1 promotes colorectal cancer cell proliferation, invasion and metastasis by upregulating STAT3. Adv Clin Exp Med. 2022;31(2):139-149. doi: 10.17219/acem/142461 [DOI] [PubMed] [Google Scholar]
- 64.Wagner MJ, Mitra R, McArthur MJ, et al. Preclinical mammalian safety studies of EPHARNA (DOPC nanoliposomal EphA2-targeted siRNA). Mol Cancer Ther. 2017;16(6):1114-1123. doi: 10.1158/1535-7163.MCT-16-0541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strumberg D, Schultheis B, Traugott U, et al. Phase I clinical development of Atu027, a siRNA formulation targeting PKN3 in patients with advanced solid tumors. Int J Clin Pharmacol Ther. 2012;50(1):76-78. doi: 10.5414/cpp50076 [DOI] [PubMed] [Google Scholar]
- 66.Davis ME, Zuckerman JE, Choi CH, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067-1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuckerman JE, Gritli I, Tolcher A, et al. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc Natl Acad Sci U S A. 2014;111(31):11449-11454. doi: 10.1073/pnas.1411393111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma L, Young J, Prabhala H, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12(3):247-256. doi: 10.1038/ncb2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tolcher AW, Papadopoulos KP, Patnaik A, et al. Safety and activity of DCR-MYC, a first-in-class dicer-substrate small interfering RNA (DsiRNA) targeting MYC, in a phase I study in patients with advanced solid tumors. J Clin Oncol. 2015;33(15_suppl):11006-11006. doi: 10.1200/jco.2015.33.15_suppl.11006. [DOI] [Google Scholar]
- 70.Lee SY, Yang CY, Peng CL, et al. A theranostic micelleplex co-delivering SN-38 and VEGF siRNA for colorectal cancer therapy. Biomaterials. 2016;86:92-105. doi: 10.1016/j.biomaterials.2016.01.068 [DOI] [PubMed] [Google Scholar]
- 71.Pinto C, Silva G, Ribeiro AS, et al. Evaluation of AAV-mediated delivery of shRNA to target basal-like breast cancer genetic vulnerabilities. J Biotechnol. 2019;300:70-77. doi: 10.1016/j.jbiotec.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 72.Farra R, Maruna M, Perrone F, et al. Strategies for delivery of siRNAs to ovarian cancer cells. Pharmaceutics. 2019;11(10):547. doi: 10.3390/pharmaceutics11100547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saw PE, Yao H, Lin C, Tao W, Farokhzad OC, Xu X. Stimuli-responsive polymer-prodrug hybrid nanoplatform for multistage siRNA delivery and combination cancer therapy. Nano Lett. 2019;19(9):5967-5974. doi: 10.1021/acs.nanolett.9b01660 [DOI] [PubMed] [Google Scholar]
- 74.Jyotsana N, Sharma A, Chaturvedi A, et al. Lipid nanoparticle-mediated siRNA delivery for safe targeting of human CML in vivo. Ann Hematol. 2019;98(8):1905-1918. doi: 10.1007/s00277-019-03713-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu S, Morris VB, Labhasetwar V. Effectiveness of small interfering RNA delivery via arginine-rich polyethylenimine-based polyplex in metastatic and doxorubicin-resistant breast cancer cells. J Pharmacol Exp Ther. 2019;370(3):902-910. doi: 10.1124/jpet.119.256909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmed R, Amreddy N, Babu A, Munshi A, Ramesh R. Combinatorial nanoparticle delivery of siRNA and antineoplastics for lung cancer treatment. Methods Mol Biol. 2019;1974:265-290. doi: 10.1007/978-1-4939-9220-1_20 [DOI] [PubMed] [Google Scholar]
- 77.Familtseva A, Jeremic N, Tyagi SC. Exosomes: cell-created drug delivery systems. Mol Cell Biochem. 2019;459(1–2):1-6. doi: 10.1007/s11010-019-03545-4 [DOI] [PubMed] [Google Scholar]
- 78.Faruqu FN, Xu L, Al-Jamal KT. Preparation of exosomes for siRNA delivery to cancer cells. J Vis Exp. 2018(142):e58814. doi: 10.3791/58814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bao Y, Guo H, Lu Y, et al. Blocking hepatic metastases of colon cancer cells using an shRNA against Rac1 delivered by activatable cell-penetrating peptide. Oncotarget. 2016;7(47):77183-77195. doi: 10.18632/oncotarget.12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng H, Tang C, Yin C. Oral delivery of shRNA based on amino acid modified chitosan for improved antitumor efficacy. Biomaterials. 2015;70:126-137. doi: 10.1016/j.biomaterials.2015.08.024 [DOI] [PubMed] [Google Scholar]
- 81.He C, Yin L, Song Y, Tang C, Yin C. Optimization of multifunctional chitosan-siRNA nanoparticles for oral delivery applications, targeting TNF-α silencing in rats. Acta Biomater. 2015;17:98-106. doi: 10.1016/j.actbio.2015.01.041 [DOI] [PubMed] [Google Scholar]
- 82.Setten RL, Rossi JJ, Han SP. The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov. 2019;18(6):421-446. doi: 10.1038/s41573-019-0017-4 [DOI] [PubMed] [Google Scholar]
- 83.Zuckerman JE, Davis ME. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat Rev Drug Discov. 2015;14(12):843-856. doi: 10.1038/nrd4685 [DOI] [PubMed] [Google Scholar]
- 84.Chen C, Yao X, Xu Y, et al. Dahuang Zhechong Pill suppresses colorectal cancer liver metastasis via ameliorating exosomal CCL2 primed pre-metastatic niche. J Ethnopharmacol. 2019;238:111878. doi: 10.1016/j.jep.2019.111878 [DOI] [PubMed] [Google Scholar]
- 85.Rudmik LR, Magliocco AM. Molecular mechanisms of hepatic metastasis in colorectal cancer. J Surg Oncol. 2005;92(4):347-359. doi: 10.1002/jso.20393 [DOI] [PubMed] [Google Scholar]
- 86.Oh BY, Hong HK, Lee WY, Cho YB. Animal models of colorectal cancer with liver metastasis. Cancer Lett. 2017;387:114-120. doi: 10.1016/j.canlet.2016.01.048 [DOI] [PubMed] [Google Scholar]
- 87.LeGolvan MP, Resnick M. Pathobiology of colorectal cancer hepatic metastases with an emphasis on prognostic factors. J Surg Oncol. 2010;102(8):898-908. doi: 10.1002/jso.21817 [DOI] [PubMed] [Google Scholar]
- 88.Radinsky R, Ellis LM. Molecular determinants in the biology of liver metastasis. Surg Oncol Clin N Am. 1996;5(2):215-229. [PubMed] [Google Scholar]
- 89.Habban Akhter M, Sateesh Madhav N, Ahmad J. Epidermal growth factor receptor based active targeting: a paradigm shift towards advance tumor therapy. Artif Cells Nanomed Biotechnol. 2018;46(sup2):1188-1198. doi: 10.1080/21691401.2018.1481863 [DOI] [PubMed] [Google Scholar]