Abstract
Background:
Systemic immunosuppression characterizing cancer patients represents a concern regarding the efficacy of anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination, and real-world evidence is needed to define the efficacy and the dynamics of humoral immune response to mRNA-based anti-SARS-CoV-2 vaccines.
Methods:
We conducted an observational study that included patients with solid tumors who were candidates for mRNA anti-SARS-CoV-2 vaccination at the Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy. The primary objective was to monitor the immunologic response to the mRNA anti-SARS-CoV-2 vaccination in terms of anti-spike antibody levels. All the patients received two doses of the mRNA-1273 vaccine or the BNT162b2 vaccine. Healthcare workers served as a control group of healthy subjects.
Results:
Among the 243 patients included in the present analysis, 208 (85.60%) and 238 (97.94%) resulted seroconverted after the first and the second dose of vaccine, respectively. Only five patients (2.06%) had a negative titer after the second dose. No significant differences in the rate of seroconversion after two vaccine doses were observed in patients as compared with the control group of healthy subjects. Age and anticancer treatment class had an independent impact on the antibody titer after the second dose of vaccination. In a subgroup of 171 patients with available data about the third timepoint, patients receiving immunotherapy with immune checkpoint inhibitors seem to have a higher peak of antibodies soon after the second dose (3 weeks after), but a more pronounced decrease at a late timepoint (3 months after).
Conclusions:
The systemic immunosuppression characterizing cancer patients did not seem to dramatically affect the humoral response to anti-SARS-CoV-2 mRNA vaccines in our population of patients with solid tumors. Further investigation is needed to dissect the interplay between immunotherapy and longitudinal dynamics of humoral response to mRNA vaccines, as well as to analyze the cellular response to mRNA vaccines in cancer patients.
Keywords: cancer patients, COVID19, mRNA vaccine, SARS-CoV-2, solid tumors, vaccination
Introduction
The emergence in December 2019 of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) resulted in devastating consequences on global health. Fortunately, the rapid and widespread adoption of anti-SARS-CoV-2 vaccination based on mRNA platforms has dramatically reduced the morbidity and mortality associated with the SARS-CoV-2-related coronavirus disease 2019 (COVID-19).1–4
Cancer patients represent a particularly vulnerable population to the adverse clinical outcomes of COVID-19, given the immunosuppressive status linked to the malignancy itself and to specific anticancer treatments (e.g. cytotoxic and myelotoxic agents). Indeed, a multicenter study performed in China at the beginning of SARS-CoV-2 spread showed that COVID-19 patients with cancer had a higher risk of severe outcomes, 5 even if another large cohort study suggested that mortality from SARS-CoV-2 infection in cancer patients appears to be mainly driven by age and comorbidities, 6 similar to the general population. Thus, international organizations, such as the European Society for Medical Oncology, have released statements and guidelines to address the issues and concerns on immunizing patients with solid and hematological malignancies, recommending that cancer patients should be vaccinated against SARS-CoV-2 regardless of any other indications (i.e. age) and positioned at high prioritization. 7 However, the systemic immunosuppression characterizing cancer patients represents a concern also regarding the efficacy of anti-SARS-CoV-2 vaccination. Recently, several reports have started to clarify the spectrum of anti-SARS-CoV-2 vaccine response among cancer patients in a real-world setting. Still, the follow-up time of most studies is limited. 8 Considering the underrepresentation of cancer patients in anti-SARS-CoV-2 vaccine trials, further evidence is needed to define the efficacy and the dynamics of the humoral immune response to mRNA-based anti-SARS-CoV-2 vaccines. Moreover, a precise dissection of the dynamics and determinants of the humoral immune response to anti-SARS-CoV-2 mRNA vaccines in cancer patients may be of particular interest since it may help to speed up the development of mRNA-based anticancer treatments, one of the most promising biotechnologies of the next-generation cancer immunotherapy. 9 Starting from these considerations, in the present study, we report on the data of a large institutional registry aimed at assessing and monitoring the immunologic response to mRNA anti-SARS-CoV-2 vaccination in patients with solid tumors.
Methods
Study design and patients’ population
This was an observational study that included patients with solid tumors who were candidates for mRNA anti-SARS-CoV-2 vaccination at the Fondazione IRCCS Istituto Nazionale dei Tumori of Milan between 1 April and 30 April 2021 according to the national guidelines and international recommendations. All the patients included in the present study had received two doses of the mRNA-1273 vaccine or the BNT162b2 vaccine at the time of the data cutoff (1 November 2021). The second dose of the mRNA-1273 and BNT162b2 vaccines was administered 24–31 days after the first dose according to the local and national guidelines. No heterologous vaccination was allowed.
As per protocol, healthcare workers at the same institution served as a control group to assess the immunogenicity after two doses of mRNA vaccine (about 35 days after the second dose) in a population of healthy subjects.
The primary objective of the study was to monitor the immunologic response to the mRNA anti-SARS-CoV-2 vaccination in terms of anti-spike antibody levels in patients with solid tumors. Secondary objectives included the following: (a) the comparison of the immunologic response to the mRNA anti-SARS-CoV-2 vaccination in terms of anti-spike antibody levels between cancer patients and a control population of healthy subjects and (b) the evaluation of the role of clinicopathological characteristics, anticancer treatment class, and different mRNA anti-SARS-CoV-2 vaccines received in the seroconversion dynamics after vaccination. The main inclusion criteria were as follows: (a) cytologically or histologically confirmed diagnosis of a solid malignancy; (b) age ⩾ 18 years; and (c) willingness to undergo mRNA anti-SARS-CoV-2 vaccination according to the national guidelines and international recommendations. The main exclusion criteria were as follows: (a) allergy to any vaccine component; (b) previous severe reactions after non-anti-SARS-CoV-2 vaccinations conditioning the exclusion from anti-SARS-CoV-2 vaccination programs; and (c) pregnancy or breast-feeding for female patients. For the present report, we excluded patients with prior known SARS-CoV-2 infection. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Fondazione IRCCS Istituto Nazionale dei Tumori of Milan (INT 119/21). All the patients and healthcare workers signed an informed consent form.
Evaluation of anti-SARS-CoV-2-spike antibody serum levels
Anti-SARS-CoV-2-spike antibody serum levels were evaluated at the following timepoints: (1) after the administration of the first dose and prior to the administration of the second dose, T1; (2) from 2 to 6 weeks after the administration of the second vaccine dose, T2; and (3) about 3 months after the administration of the second vaccine dose, T3. A T0 timepoint, prior to the administration of the first dose, was analyzed to exclude the suspected previous infections of SARS-CoV-2. The Roche Elecsys® Anti-SARS-CoV-2 S (Roche S tAb, Roche Diagnostics International Ltd, Rotkreuz, Switzerland) was used to quantitatively measure the level of antibodies to the receptor-binding domain of the spike (S) protein of the SARS-CoV-2 according to the manufacturer’s instructions. The anti-SARS-CoV-2 S antibodies concentration was expressed in units per milliliter (U/mL). A concentration <0.80 U/mL was interpreted as negative for the presence of anti-SARS-CoV-2 S antibodies, whereas a concentration ⩾0.80 U/mL was interpreted as positive. 10
Statistical analyses
Continuous variables were reported as median and range or interquartile range (IQR) and categorical variables as proportions. Associations between categorical variables were tested by the Chi-square statistic or Fisher’s exact test whenever appropriate. For each patient, the percentage change in the anti-SARS-CoV-2 antibody titer with respect to T(i − 1) (with i = 2, 3; i.e. 100*[T2 or T3 value] − [T1 or T2]/[T1 or T2]) was computed and a graphical representation of the median values according to the variables of interest was performed. Due to the highly positive skewed distributions of the data, the subsequent analysis was conducted on the log-transformation IgG data.
To investigate the effects of all the available clinicopathological characteristics, anticancer treatment class and different mRNA anti-SARS-CoV-2 vaccines received on the anti-spike antibody levels at T2, a one-way analysis of variance (ANOVA) was carried out. Anticancer treatments were classified as follows: biological therapy (including monoclonal antibodies other than immune checkpoint inhibitors, antibody–drug conjugates, tyrosine kinase inhibitors and other small molecules, hormone therapy, mTOR inhibitors), chemotherapy, immunotherapy, chemotherapy + immunotherapy, biological therapy + immunotherapy, radiotherapy/chemotherapy + radiotherapy. A multivariate initial model including all of the variables that were statistically significant at univariate analysis was implemented. A more parsimonious final model was then obtained using a backward selection procedure that retained only those variables reaching the conventional level of significance of 5%. The same approach was applied using the difference between the anti-spike antibody levels at T2 and T3 on their logarithmic scale (ΔT3–T2).
The time trends profiles of the anti-spike antibody levels were assessed by resorting to mixed models and by considering antibody levels (on a logarithmic scale) as a function of time (fixed factor, T1, T2, and T3) and subjects (random factor). In addition, the time trends were studied even by considering the following covariates: sex, age (dichotomized at 50 years), and class of anticancer treatment and their possible interactions with the time factor. The most appropriate matrix of variance–covariance for each model was selected according to the Akaike Information Criterion.
All statistical analyses were performed with SAS software (Version 9.4.; SAS Institute, Inc., Cary, NC, USA), adopting a nominal significance level of α = 0.05.
Results
Patients’ characteristics
Among the 325 patients with solid tumors included in the registry, 243 matched the following criteria: (i) having received the two doses of mRNA anti-SARS-CoV-2 vaccine, (ii) no previous SARS-CoV-2 infection, and (iii) having a non-missing value for the anti-spike antibody level at both T1 and T2 (Supplemental Figure 1). The subjects’ clinical and pathological characteristics, anticancer treatment class, and different mRNA anti-SARS-CoV-2 vaccines received are shown in Table 1. Details about specific anticancer treatments are reported in Supplemental Table 1. In particular, patients receiving immunotherapy or chemo-immunotherapy were all treated with anti-programmed cell 1/programmed cell death ligand 1 antibodies as the immunotherapeutic agents (Supplemental Table 1). The percentages of patients receiving the mRNA-1273 vaccine and the BNT162b2 vaccine were similar. The median age of patients was 62 years (range: 24–84), almost 60% of the patients were women and more than 70% had a metastatic tumor. The most frequent tumor types were breast and lung cancers followed by melanoma (20.99%, 16.87%, and 16.46%, respectively). The majority of patients were receiving a biological anticancer therapy at the time of the first dose of vaccine, and about 6% of patients were not under active anticancer treatment.
Table 1.
Patients’ characteristics.
| Variable | Patients in the analysis (n = 243) | |
|---|---|---|
| N | % | |
| Age | ||
| Median (range) | 62 (24–84) | |
| Sex | ||
| Male | 100 | 41.15 |
| Female | 143 | 58.85 |
| Stage of tumor | ||
| Metastatic | 173 | 71.19 |
| Localized | 70 | 28.81 |
| Type of tumor | ||
| Lung | 41 | 16.87 |
| Breast | 51 | 20.99 |
| Gynecologic | 20 | 8.23 |
| Melanoma | 40 | 16.46 |
| NET | 9 | 3.7 |
| Sarcoma | 18 | 7.41 |
| Gastrointestinal | 17 | 7 |
| Head and neck | 11 | 4.53 |
| Genitourinary | 24 | 9.88 |
| Other | 12 | 4.94 |
| Treatment | ||
| Biological therapy | 106 | 43.62 |
| Chemotherapy | 62 | 25.51 |
| Chemotherapy + immunotherapy | 9 | 3.7 |
| RT/chemo + RT | 2 | 0.82 |
| Immunotherapy | 34 | 13.99 |
| Biological therapy + immunotherapy | 5 | 2.06 |
| None | 15 | 6.17 |
| – | 10 | 4.12 |
| Type of vaccine | ||
| mRNA-1273 | 126 | 51.85 |
| BNT162b2 | 117 | 48.15 |
NET, neuroendocrine tumor; RT, radiotherapy.
Dynamics of seroconversion in patients with solid tumors
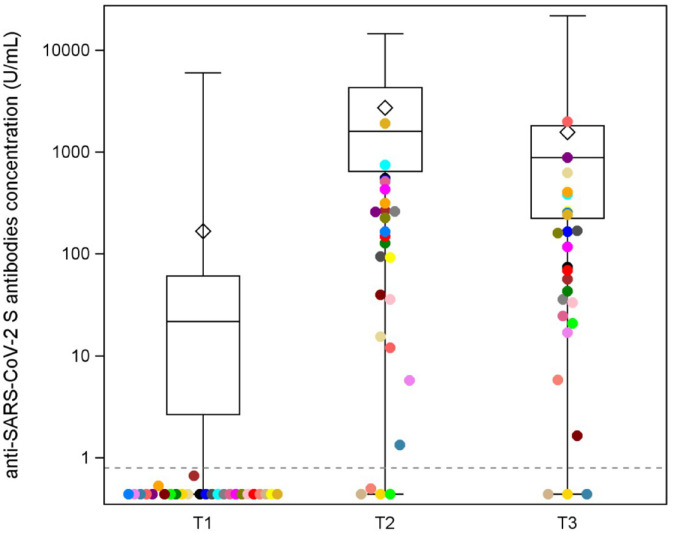
Among the 243 patients included in the present analysis, 208 (85.60%) and 238 (97.94%) resulted seroconverted at T1 and T2, respectively; only five patients (2.06%) had a negative titer after the second dose of vaccine. The median value of the anti-spike antibody titer was equal to 15.4 U/mL (IQR: 2.45–52.6) at T1 and 1422.0 U/mL (IQR: 555.0–4066.0) at T2.
To compare the immunological response to the mRNA anti-SARS-CoV-2 vaccination in terms of anti-spike antibody levels at T2 between cancer patients and the control population of healthy subjects, we performed a sex- and age-matched analysis, comparing the response rate and the antibody titer in 164 patients and in 164 healthy workers (96 females, median age 56.5 years, range: 47–62 in both case and control). No significant differences were observed in the rate of seroconversion after the second dose of vaccination in our population of patients with solid tumors compared with the control group of healthy workers. In the control group, the seroconversion rate after the second dose of vaccine was 99.4% (95% confidence interval [CI]: 96.7–100%) compared with a 98.8% rate (95% CI: 95.7–99.9%) in the patients’ group (p = 0.5619). The median level of anti-spike antibody at T2 was equal to 1812.5 U/mL (IQR: 721.0–4456.5) in the patients’ group compared with 1129.5 U/mL (IQR: 667.5–1840.5) in the control group (p = 0.6594 adjusted for the dose administration time).
In a subgroup of 171 patients, we had available information about the anti-spike antibody level at T3 (Supplemental Figure 1). The distributions of the antibody titer at each timepoint (T1, T2, and T3) are shown in Figure 1. Of these 171 patients, 168 patients (98.25%) were seroconverted at T3. Among the three patients with a negative titer at T3, only one patient had a positive titer at T2 and became seronegative at T3. The seroconversion dynamics after each dose and the corresponding clinicopathological characteristics, anticancer treatment class, and different mRNA anti-SARS-CoV-2 vaccines received are reported in Supplemental Table 2.
Figure 1.
Distribution of the anti-spike antibody levels at T1, T2, and T3. Distributions of the antibody titer of the 171 patients at different timepoints (T1, T2, and T3). Each box indicates the 25th and 75th percentiles. The horizontal line and the diamond inside the box indicate the median and the mean, respectively. Whiskers indicate the extreme measured values. Patients with a negative titer after the first dose (T1) are represented with different colors to identify them and evaluate their dynamics of seroconversion in T2 and T3.
Determinants of humoral immune response after mRNA anti-SARS-CoV-2 vaccination in patients with solid tumors
The ANOVA results on the roles of the variables of interest on the titer after the second dose of vaccine are reported in Table 2. Age, tumor type, and anticancer treatment class had a significant effect on the anti-spike antibody levels at T2. Younger patients had higher levels of antibodies (Supplemental Figure 2(a)) as compared with older patients, whereas patients with melanoma had the highest median levels, followed by patients with gastrointestinal tumors and neuroendocrine tumors (Supplemental Figure 2(b)). Moreover, patients receiving chemotherapy (alone or in combination with immunotherapy or radiotherapy) showed a lower level of antibodies as compared with those undergoing immunotherapy alone (contrast p = 0.0006), biological therapy (alone or in combination with immunotherapy) (contrast p = <0.0001), or those who were not receiving any anticancer treatment (contrast p = 0.0093) (Supplemental Figure 2(c)). Of note, the type of vaccine had no impact on the anti-spike antibody levels at T2 (Table 2). Following a backward procedure, the final multivariate model for the titer after the second dose of vaccine included only age and anticancer treatment class (p = 0.0230 and p < 0.0001, respectively) (Table 2). By pursuing the analysis according to the type of chemotherapy (platinum based versus non-platinum based), we did not find any difference in terms of antibody levels (p = 0.0833) as well as in terms of seroconversion rate (p = 0.2575) (Supplemental Figure 3(a) and (b)). Similarly, no statistically significant differences were observed between chemo-immunotherapy and chemotherapy alone subgroups, both in terms of antibody levels (p = 0.9069) and seroconversion rate (p = 0.4259) (Supplemental Figure 3(c) and (d)). In addition, by focusing on the specific classes of biological therapies (Supplemental Figure 3(e)), we found that patients treated with poly (ADP-ribose) polymerase (PARP) inhibitors (N = 6) showed the lowest median value of antibody levels, and a statistically significant difference was observed when compared with tyrosine kinase inhibitors (TKIs) (contrast p = 0.0042), monoclonal antibodies/antibody–drug conjugates (contrast p = 0.0061), or somatostatin analogs (contrast p = 0.0031).
Table 2.
Results of univariate and multivariate analyses.
| T2 analysis (n = 243) | ∆T3-T2 analysis (n = 171) | |||
|---|---|---|---|---|
| Univariate analysis | Final multivariate model | Univariate analysis | Final multivariate model | |
| p Value | p Value | p Value | p Value | |
| Sex | 0.1665 | – | 0.0042 | 0.0017 |
| Type of vaccine | 0.0832 | – | 0.0381 | 0.0068 |
| Stage of tumor | 0.6744 | – | 0.4440 | – |
| Type of tumor | 0.0072 | – | 0.0102 | – |
| Treatment | 0.0001 | <0.0001 | 0.0114 | 0.0118 |
| Age | 0.0174 | 0.0230 | 0.7397 | – |
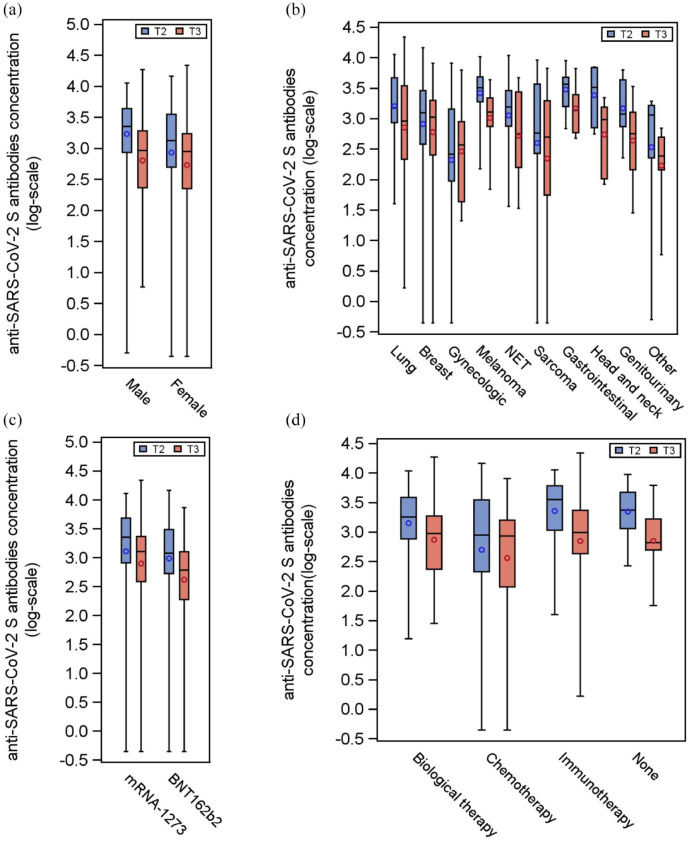
By analyzing the difference between antibody levels at T3 and T2, we found that sex, type of vaccine, type of tumor, and class of antitumor treatment significantly contributed to the modulation of the antibody levels at T3 (Table 2 and Figure 2). We observed a global decrease in the titer of antibodies between T2 and T3. The decrease was slighter in women, in patients receiving the mRNA-1273 vaccine and in patients with types of tumors that predominantly affect women (Supplemental Figure 4(a)–4(c)). Interestingly, patients receiving immunotherapy or not receiving any anticancer treatment had a more pronounced decrease in antibody levels compared with patients undergoing chemotherapy (contrast p = 0.0026; contrast p = 0.0348, respectively) (Supplemental Figure 4(d)).
Figure 2.
Distributions of the anti-spike antibody levels at T2 and T3 according to selected variables. Distributions of the antibody titer of the 171 patients at T2 (blue box) and T3 (red box), according to sex (panel a), type of tumor (panel b), type of vaccine (panel c), and anticancer treatment class (panel d). Each box indicates the 25th and 75th percentiles. The horizontal line and the circle inside the box indicate the median and the mean, respectively. Whiskers indicate the extreme measured values.
The final multivariate model for the difference between antibody levels between T3 and T2, following a backward procedure, included the following variables: sex, type of vaccine, and anticancer treatment class (p = 0.0017, p = 0.0068, and p = 0.0118, respectively) (Table 2).
We then resorted to a mixed model and we found that the time factor (as well as each time contrast) resulted in a statistically significant (p < 0.0001) longitudinal effect (as depicted in Supplemental Figure 5(a)), with a significant difference in the trends between younger and older patients (p = 0.0082, see Supplemental Figure 5(b)). Interestingly, we found significant interactions between time and sex (p = 0.0120), as well as between time and different anticancer treatment class (p = 0.0484) as represented in Supplemental Figure 5(c) and 5(d).
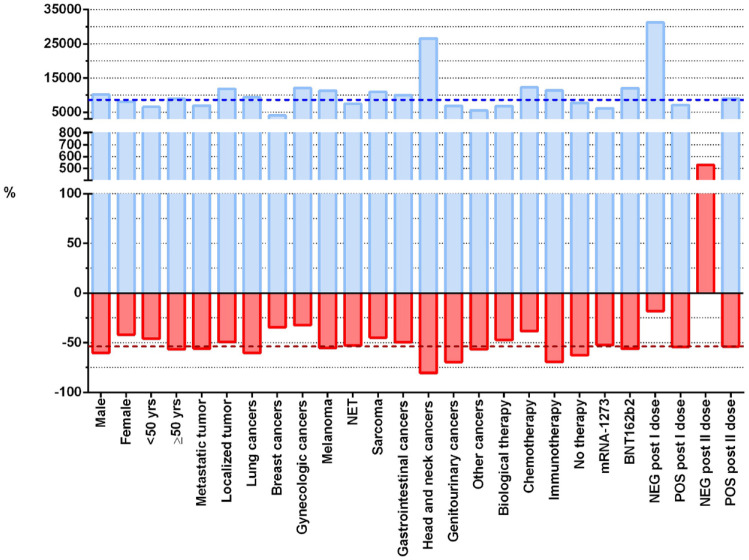
Figure 3 shows the median percentage changes in the antibody levels between T1 and T2 and between T2 and T3 for each category of the variables of interest. Considering as reference the overall median percentage changes, the highest increment between T1 and T2 was observed for patients with a negative titer after the first dose, followed by patients with head and neck cancer and patients undergoing chemotherapy. Interestingly, head and neck cancer patients were also those with the highest decrement of titer between T2 and T3, followed by patients with genitourinary tumors and patients receiving immunotherapy.
Figure 3.
Percentage changes in the antibody levels between T1, T2 and T2, T3. Each blue and red bar represents the median percentage change between T2 and T1 or between T3 and T2, respectively, in each category of the variables of interest. The blue and red dotted reference lines indicate the overall median percentage change for the T2 − T1 and T3 − T2 differences, respectively.
Discussion
Understanding the dynamics of the humoral immune response in cancer patients after mRNA anti-SARS-CoV-2 vaccination is crucial to optimally plan the next steps of an effective SARS-CoV-2 mitigation/control strategy in such population of fragile patients. 11 In the present study, we observed an optimal response after two doses of mRNA vaccine in patients with solid tumors, with a rate of seroconversion that was comparable to that of a matched control population of healthy subjects, in line with the recently reported data of a prospective, multicenter, non-inferiority trial. 12 Of note, the 97.94% rate of seroconversion after the second dose of vaccination observed in our study population was higher than the rates reported in similar populations.13–15 The efficacy of anti-SARS-CoV-2 vaccination in patients with solid tumors (in terms of seroconversion and humoral response) seems better than in patients with hematological malignancies, as an indirect comparison with our previously reported data suggests (seroconversion rate in patients with hematological malignancies: 64.6% after two doses of mRNA vaccine), 16 and in line with the literature data. 17 These differences may be explained by the peculiar suppression of the B-cell immune response that characterizes hematological malignancies (as compared with solid tumors) and that is driven by intrinsic biological features and specific anticancer treatments (i.e. anti-CD20 monoclonal antibodies used in lymphoid malignancies). 16 Regarding the impact of concomitant anticancer treatments on the efficacy of mRNA vaccination in our population of patients with solid tumors, we did not observe a difference in the rate of seroconversion according to the type of concomitant treatment, even if the titer of antibodies after the second dose of vaccine was lower for patients receiving cytotoxic chemotherapy, consistently with the recent data on the negative impact of multiple-agent cytotoxic chemotherapy on post-vaccination anti-SARS-CoV-2 IgG titer in cancer patients.18–22 In the subset of patients treated with biological therapies, patients treated with PARP inhibitors showed the lowest median value of anti-SARS-CoV-2 IgG levels, in line with the evidence that PARP deficiency may impair peripheral B-cell homeostasis and humoral response. 23 Interestingly, even if immunotherapy does not seem to affect the rate of seroconversion in cancer patients as previously reported,24,25 the more pronounced decrease in antibody levels in the late timepoint that we observed in patients receiving immune checkpoint inhibitors was consistent with two other reports about sustained antibody levels in cancer patients receiving immunotherapy-based treatments at the time of vaccination.26,27 A possible explanation is that immune checkpoint inhibitors may have a positive acute effect, favoring a higher peak of antibody levels soon after the second dose, thus exaggerating the decrease in antibodies at late timepoints. However, there is a need to fully understand the interplay between immune checkpoint inhibitors and the humoral immune response to mRNA vaccination in cancer patients. Finally, regarding the impact of the type of tumor, the observed highest median levels of anti-spike antibody levels at T2 in patients with melanoma may be explained, at least in part, by the fact that none of these patients received chemotherapy (associated with a low titer of antibodies at T2), whereas about 40% of the patients received immune checkpoint inhibitors (associated with a high titer of antibody at T2). We acknowledge that (i) the lack of data on cellular anti-SARS-CoV-2 immunity upon vaccination and (ii) the lack of data on the dynamics of antibodies titer modulation after a third booster dose of vaccine are the major limitations of the present study, but the availability of data regarding a late timepoint (three months) after the second dose of vaccination adds new valuable insights on the longitudinal dynamics of humoral response to mRNA vaccines in patients with solid tumors.
In conclusion, the systemic immunosuppression characterizing cancer patients did not seem to dramatically affect the humoral response to anti-SARS-CoV-2 mRNA vaccines in our population of patients with solid tumors. The seroconversion rate of cancer patients was very high and comparable to that of healthy subjects. Our data confirm that the class of concomitant anticancer treatment may modulate the degree of the humoral response (in terms of antibody titer) but does not affect the seroconversion rate. Further investigations are needed to dissect the interplay between current immunotherapy (i.e. immune checkpoint inhibitors) and the longitudinal dynamics of humoral response to mRNA vaccines, as well as to analyze the cellular response to mRNA vaccines in cancer patients.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221108687 for Efficacy of mRNA anti-SARS-CoV-2 vaccination and dynamics of humoral immune response in patients with solid tumors: results from the institutional registry of an Italian tertiary cancer center by Giovanni Fucà, Mara Lecchi, Chiara Maura Ciniselli, Arianna Ottini, Andrea Spagnoletti, Laura Mazzeo, Daniele Morelli, Paola Frati, Martina Stroscia, Elisabella Ebrahem, Elisa Sottotetti, Giulia Galli, Maria Grazia D’Elia, Riccardo Lobefaro, Monika Ducceschi, Lorenza Di Guardo, Sherrie Bhoori, Salvatore Provenzano, Marco Platania, Monica Niger, Elena Colombo, Federico Nichetti, Matteo Duca, Licia Rivoltini, Roberta Mortarini, Paolo Baili, Giovanni Apolone, Filippo de Braud, Paolo Verderio and Silvia Damian in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359221108687 for Efficacy of mRNA anti-SARS-CoV-2 vaccination and dynamics of humoral immune response in patients with solid tumors: results from the institutional registry of an Italian tertiary cancer center by Giovanni Fuc’, Mara Lecchi, Chiara Maura Ciniselli, Arianna Ottini, Andrea Spagnoletti, Laura Mazzeo, Daniele Morelli, Paola Frati, Martina Stroscia, Elisabella Ebrahem, Elisa Sottotetti, Giulia Galli, Maria Grazia D’Elia, Riccardo Lobefaro, Monika Ducceschi, Lorenza Di Guardo, Sherrie Bhoori, Salvatore Provenzano, Marco Platania, Monica Niger, Elena Colombo, Federico Nichetti, Matteo Duca, Licia Rivoltini, Roberta Mortarini, Paolo Baili, Giovanni Apolone, Filippo de Braud, Paolo Verderio and Silvia Damian in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-3-tam-10.1177_17588359221108687 for Efficacy of mRNA anti-SARS-CoV-2 vaccination and dynamics of humoral immune response in patients with solid tumors: results from the institutional registry of an Italian tertiary cancer center by Giovanni Fuc’, Mara Lecchi, Chiara Maura Ciniselli, Arianna Ottini, Andrea Spagnoletti, Laura Mazzeo, Daniele Morelli, Paola Frati, Martina Stroscia, Elisabella Ebrahem, Elisa Sottotetti, Giulia Galli, Maria Grazia D’Elia, Riccardo Lobefaro, Monika Ducceschi, Lorenza Di Guardo, Sherrie Bhoori, Salvatore Provenzano, Marco Platania, Monica Niger, Elena Colombo, Federico Nichetti, Matteo Duca, Licia Rivoltini, Roberta Mortarini, Paolo Baili, Giovanni Apolone, Filippo de Braud, Paolo Verderio and Silvia Damian in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-4-tam-10.1177_17588359221108687 for Efficacy of mRNA anti-SARS-CoV-2 vaccination and dynamics of humoral immune response in patients with solid tumors: results from the institutional registry of an Italian tertiary cancer center by Giovanni Fuc’, Mara Lecchi, Chiara Maura Ciniselli, Arianna Ottini, Andrea Spagnoletti, Laura Mazzeo, Daniele Morelli, Paola Frati, Martina Stroscia, Elisabella Ebrahem, Elisa Sottotetti, Giulia Galli, Maria Grazia D’Elia, Riccardo Lobefaro, Monika Ducceschi, Lorenza Di Guardo, Sherrie Bhoori, Salvatore Provenzano, Marco Platania, Monica Niger, Elena Colombo, Federico Nichetti, Matteo Duca, Licia Rivoltini, Roberta Mortarini, Paolo Baili, Giovanni Apolone, Filippo de Braud, Paolo Verderio and Silvia Damian in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-5-tam-10.1177_17588359221108687 for Efficacy of mRNA anti-SARS-CoV-2 vaccination and dynamics of humoral immune response in patients with solid tumors: results from the institutional registry of an Italian tertiary cancer center by Giovanni Fuc’, Mara Lecchi, Chiara Maura Ciniselli, Arianna Ottini, Andrea Spagnoletti, Laura Mazzeo, Daniele Morelli, Paola Frati, Martina Stroscia, Elisabella Ebrahem, Elisa Sottotetti, Giulia Galli, Maria Grazia D’Elia, Riccardo Lobefaro, Monika Ducceschi, Lorenza Di Guardo, Sherrie Bhoori, Salvatore Provenzano, Marco Platania, Monica Niger, Elena Colombo, Federico Nichetti, Matteo Duca, Licia Rivoltini, Roberta Mortarini, Paolo Baili, Giovanni Apolone, Filippo de Braud, Paolo Verderio and Silvia Damian in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-6-tam-10.1177_17588359221108687 for Efficacy of mRNA anti-SARS-CoV-2 vaccination and dynamics of humoral immune response in patients with solid tumors: results from the institutional registry of an Italian tertiary cancer center by Giovanni Fuc’, Mara Lecchi, Chiara Maura Ciniselli, Arianna Ottini, Andrea Spagnoletti, Laura Mazzeo, Daniele Morelli, Paola Frati, Martina Stroscia, Elisabella Ebrahem, Elisa Sottotetti, Giulia Galli, Maria Grazia D’Elia, Riccardo Lobefaro, Monika Ducceschi, Lorenza Di Guardo, Sherrie Bhoori, Salvatore Provenzano, Marco Platania, Monica Niger, Elena Colombo, Federico Nichetti, Matteo Duca, Licia Rivoltini, Roberta Mortarini, Paolo Baili, Giovanni Apolone, Filippo de Braud, Paolo Verderio and Silvia Damian in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-7-tam-10.1177_17588359221108687 for Efficacy of mRNA anti-SARS-CoV-2 vaccination and dynamics of humoral immune response in patients with solid tumors: results from the institutional registry of an Italian tertiary cancer center by Giovanni Fuc’, Mara Lecchi, Chiara Maura Ciniselli, Arianna Ottini, Andrea Spagnoletti, Laura Mazzeo, Daniele Morelli, Paola Frati, Martina Stroscia, Elisabella Ebrahem, Elisa Sottotetti, Giulia Galli, Maria Grazia D’Elia, Riccardo Lobefaro, Monika Ducceschi, Lorenza Di Guardo, Sherrie Bhoori, Salvatore Provenzano, Marco Platania, Monica Niger, Elena Colombo, Federico Nichetti, Matteo Duca, Licia Rivoltini, Roberta Mortarini, Paolo Baili, Giovanni Apolone, Filippo de Braud, Paolo Verderio and Silvia Damian in Therapeutic Advances in Medical Oncology
Footnotes
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Giovanni Fucà: Data curation; Investigation; Methodology; Visualization; Writing – original draft.
Mara Lecchi: Formal analysis; Methodology; Visualization; Writing – original draft.
Chiara Maura Ciniselli: Formal analysis; Methodology; Visualization; Writing – original draft.
Arianna Ottini: Data curation; Investigation; Writing – review & editing.
Andrea Spagnoletti: Data curation; Investigation; Writing – review & editing.
Laura Mazzeo: Data curation; Investigation; Writing – review & editing.
Daniele Morelli: Data curation; Investigation; Writing – review & editing.
Paola Frati: Data curation; Investigation; Writing – review & editing.
Martina Stroscia: Data curation; Investigation; Writing – review & editing.
Elisabella Ebrahem: Data curation; Investigation; Writing – review & editing.
Elisa Sottotetti: Data curation; Investigation; Writing – review & editing.
Giulia Galli: Data curation; Investigation; Writing – review & editing.
Maria Grazia D’Elia: Data curation; Investigation; Writing – review & editing.
Riccardo Lobefaro: Data curation; Investigation; Writing – review & editing.
Monika Ducceschi: Data curation; Investigation; Writing – review & editing.
Lorenza Di Guardo: Data curation; Investigation; Writing – review & editing.
Sherrie Bhoori: Data curation; Investigation; Writing – review & editing.
Salvatore Provenzano: Data curation; Investigation; Writing – review & editing.
Marco Platania: Data curation; Investigation; Writing – review & editing.
Monica Niger: Data curation; Investigation; Writing – review & editing.
Elena Colombo: Data curation; Investigation; Writing – review & editing.
Federico Nichetti: Data curation; Investigation; Writing – review & editing.
Matteo Duca: Data curation; Investigation; Writing – review & editing.
Licia Rivoltini: Data curation; Investigation; Writing – review & editing.
Roberta Mortarini: Data curation; Investigation; Writing – review & editing.
Paolo Baili: Data curation; Investigation; Writing – review & editing.
Giovanni Apolone: Conceptualization; Supervision; Writing – review & editing.
Filippo de Braud: Conceptualization; Supervision; Writing – review & editing.
Paolo Verderio: Conceptualization; Formal analysis; Methodology; Supervision; Writing – original draft.
Silvia Damian: Conceptualization; Data curation; Investigation; Methodology; Supervision; Writing – original draft.
ORCID iDs: Laura Mazzeo  https://orcid.org/0000-0001-9226-8861
https://orcid.org/0000-0001-9226-8861
Maria Grazia D’Elia  https://orcid.org/0000-0003-1467-0147
https://orcid.org/0000-0003-1467-0147
Elena Colombo  https://orcid.org/0000-0003-3132-844X
https://orcid.org/0000-0003-3132-844X
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Our research is supported by the Scientific Directorate of Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Data availability: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Giovanni Fucà, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Mara Lecchi, Unit of Bioinformatics and Biostatistics, Department of Applied Research and Technological Development, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Chiara Maura Ciniselli, Unit of Bioinformatics and Biostatistics, Department of Applied Research and Technological Development, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Arianna Ottini, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Andrea Spagnoletti, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Laura Mazzeo, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Daniele Morelli, Unit of Bioinformatics and Biostatistics, Department of Applied Research and Technological Development, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; Department of Pathology and Laboratory Medicine, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Paola Frati, Unit of Immunotherapy of Human Tumors, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Martina Stroscia, Unit of Immunotherapy of Human Tumors, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Elisabella Ebrahem, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Elisa Sottotetti, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Giulia Galli, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Maria Grazia D’Elia, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Riccardo Lobefaro, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Monika Ducceschi, Department of Gynecologic Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Lorenza Di Guardo, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Sherrie Bhoori, Hepato-Pancreatic-Biliary Surgery and Liver Transplantation, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Salvatore Provenzano, Medical Oncology Unit 2, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Marco Platania, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Monica Niger, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Elena Colombo, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Federico Nichetti, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; Computational Oncology, Molecular Diagnostics Program, National Center for Tumor Diseases and German Cancer Research Center, Heidelberg, Germany.
Matteo Duca, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Licia Rivoltini, Unit of Immunotherapy of Human Tumors, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Roberta Mortarini, Human Tumors Immunobiology Unit, Department of Research, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Paolo Baili, Unit of Bioinformatics and Biostatistics, Department of Applied Research and Technological Development, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; Scientific Directorate, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Giovanni Apolone, Scientific Directorate, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Filippo de Braud, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy Oncology and Hemato-Oncology Department, University of Milan, Milan, Italy.
Silvia Damian, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, Via Giacomo Venezian, 1, Milan 20133, Italy.
References
- 1. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thompson MG, Burgess JL, Naleway AL, et al. Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med 2021; 385: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021; 385: 1774–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov 2020; 10: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 2020; 395: 1919–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curigliano G, Banerjee S, Cervantes A, et al. Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol 2020; 31: 1320–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corti C, Antonarelli G, Scotté F, et al. Seroconversion rate after vaccination against COVID-19 in patients with cancer: a systematic review. Ann Oncol 2022; 33: 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer 2021; 20: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riester E, Findeisen P, Hegel JK, et al. Performance evaluation of the Roche Elecsys Anti-SARS-CoV-2 S immunoassay. J Virol Methods 2021; 297: 114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun L, Warner JL, Parikh RB. Immune responses to SARS-CoV-2 among patients with cancer: what can seropositivity tell us? JAMA Oncol 2021; 7: 1123–1125. [DOI] [PubMed] [Google Scholar]
- 12. Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol 2021; 22: 1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cavanna L, Citterio C, Biasini C, et al. COVID-19 vaccines in adult cancer patients with solid tumours undergoing active treatment: seropositivity and safety. A prospective observational study in Italy. Eur J Cancer 2021; 157: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shmueli ES, Itay A, Margalit O, et al. Efficacy and safety of BNT162b2 vaccination in patients with solid cancer receiving anticancer therapy: a single centre prospective study. Eur J Cancer 2021; 157: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Massarweh A, Eliakim-Raz N, Stemmer A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol 2021; 7: 1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marasco V, Carniti C, Guidetti A, et al. T-cell immune response after mRNA SARS-CoV-2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br J Haematol 2022; 196: 548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Addeo A, Shah PK, Bordry N, et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell 2021; 39: 1091–1098.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruggeri EM, Nelli F, Fabbri A, et al. Antineoplastic treatment class modulates COVID-19 mRNA-BNT162b2 vaccine immunogenicity in cancer patients: a secondary analysis of the prospective Vax-On study. ESMO Open 2021; 7: 100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naranbhai V, Pernat CA, Gavralidis A, et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in patients with cancer: the CANVAX cohort study. J Clin Oncol 2022; 40: 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peeters M, Verbruggen L, Teuwen L, et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open 2021; 6: 100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grinshpun A, Rottenberg Y, Ben-Dov IZ, et al. Serologic response to COVID-19 infection and/or vaccine in cancer patients on active treatment. ESMO Open 2021; 6: 100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goshen-Lago T, Waldhorn I, Holland R, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol 2021; 7: 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galindo-Campos MA, Bedora-Faure M, Farres J, et al. Coordinated signals from the DNA repair enzymes PARP-1 and PARP-2 promotes B-cell development and function. Cell Death Differ 2019; 26: 2667–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma Y, Liu N, Wang Y, et al. Immune checkpoint blocking impact and nomogram prediction of COVID-19 inactivated vaccine seroconversion in patients with cancer: a propensity-score matched analysis. J Immunother Cancer. 2021; 9: e003712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thakkar A, Pradhan K, Jindal S, et al. Patterns of seroconversion for SARS-CoV2-IgG in patients with malignant disease and association with anticancer therapy. Nat Cancer 2021; 2: 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eliakim-Raz N, Massarweh A, Stemmer A, et al. Durability of response to SARS-CoV-2 BNT162b2 vaccination in patients on active anticancer treatment. JAMA Oncol 2021; 7: 1716–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Figueiredo JC, Merin NM, Hamid O, et al. Longitudinal SARS-CoV-2 mRNA vaccine-induced humoral immune responses in patients with cancer. Cancer Res 2021; 81: 6273–6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221108687 for Efficacy of mRNA anti-SARS-CoV-2 vaccination and dynamics of humoral immune response in patients with solid tumors: results from the institutional registry of an Italian tertiary cancer center by Giovanni Fucà, Mara Lecchi, Chiara Maura Ciniselli, Arianna Ottini, Andrea Spagnoletti, Laura Mazzeo, Daniele Morelli, Paola Frati, Martina Stroscia, Elisabella Ebrahem, Elisa Sottotetti, Giulia Galli, Maria Grazia D’Elia, Riccardo Lobefaro, Monika Ducceschi, Lorenza Di Guardo, Sherrie Bhoori, Salvatore Provenzano, Marco Platania, Monica Niger, Elena Colombo, Federico Nichetti, Matteo Duca, Licia Rivoltini, Roberta Mortarini, Paolo Baili, Giovanni Apolone, Filippo de Braud, Paolo Verderio and Silvia Damian in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359221108687 for Efficacy of mRNA anti-SARS-CoV-2 vaccination and dynamics of humoral immune response in patients with solid tumors: results from the institutional registry of an Italian tertiary cancer center by Giovanni Fuc’, Mara Lecchi, Chiara Maura Ciniselli, Arianna Ottini, Andrea Spagnoletti, Laura Mazzeo, Daniele Morelli, Paola Frati, Martina Stroscia, Elisabella Ebrahem, Elisa Sottotetti, Giulia Galli, Maria Grazia D’Elia, Riccardo Lobefaro, Monika Ducceschi, Lorenza Di Guardo, Sherrie Bhoori, Salvatore Provenzano, Marco Platania, Monica Niger, Elena Colombo, Federico Nichetti, Matteo Duca, Licia Rivoltini, Roberta Mortarini, Paolo Baili, Giovanni Apolone, Filippo de Braud, Paolo Verderio and Silvia Damian in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-3-tam-10.1177_17588359221108687 for Efficacy of mRNA anti-SARS-CoV-2 vaccination and dynamics of humoral immune response in patients with solid tumors: results from the institutional registry of an Italian tertiary cancer center by Giovanni Fuc’, Mara Lecchi, Chiara Maura Ciniselli, Arianna Ottini, Andrea Spagnoletti, Laura Mazzeo, Daniele Morelli, Paola Frati, Martina Stroscia, Elisabella Ebrahem, Elisa Sottotetti, Giulia Galli, Maria Grazia D’Elia, Riccardo Lobefaro, Monika Ducceschi, Lorenza Di Guardo, Sherrie Bhoori, Salvatore Provenzano, Marco Platania, Monica Niger, Elena Colombo, Federico Nichetti, Matteo Duca, Licia Rivoltini, Roberta Mortarini, Paolo Baili, Giovanni Apolone, Filippo de Braud, Paolo Verderio and Silvia Damian in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-4-tam-10.1177_17588359221108687 for Efficacy of mRNA anti-SARS-CoV-2 vaccination and dynamics of humoral immune response in patients with solid tumors: results from the institutional registry of an Italian tertiary cancer center by Giovanni Fuc’, Mara Lecchi, Chiara Maura Ciniselli, Arianna Ottini, Andrea Spagnoletti, Laura Mazzeo, Daniele Morelli, Paola Frati, Martina Stroscia, Elisabella Ebrahem, Elisa Sottotetti, Giulia Galli, Maria Grazia D’Elia, Riccardo Lobefaro, Monika Ducceschi, Lorenza Di Guardo, Sherrie Bhoori, Salvatore Provenzano, Marco Platania, Monica Niger, Elena Colombo, Federico Nichetti, Matteo Duca, Licia Rivoltini, Roberta Mortarini, Paolo Baili, Giovanni Apolone, Filippo de Braud, Paolo Verderio and Silvia Damian in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-5-tam-10.1177_17588359221108687 for Efficacy of mRNA anti-SARS-CoV-2 vaccination and dynamics of humoral immune response in patients with solid tumors: results from the institutional registry of an Italian tertiary cancer center by Giovanni Fuc’, Mara Lecchi, Chiara Maura Ciniselli, Arianna Ottini, Andrea Spagnoletti, Laura Mazzeo, Daniele Morelli, Paola Frati, Martina Stroscia, Elisabella Ebrahem, Elisa Sottotetti, Giulia Galli, Maria Grazia D’Elia, Riccardo Lobefaro, Monika Ducceschi, Lorenza Di Guardo, Sherrie Bhoori, Salvatore Provenzano, Marco Platania, Monica Niger, Elena Colombo, Federico Nichetti, Matteo Duca, Licia Rivoltini, Roberta Mortarini, Paolo Baili, Giovanni Apolone, Filippo de Braud, Paolo Verderio and Silvia Damian in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-6-tam-10.1177_17588359221108687 for Efficacy of mRNA anti-SARS-CoV-2 vaccination and dynamics of humoral immune response in patients with solid tumors: results from the institutional registry of an Italian tertiary cancer center by Giovanni Fuc’, Mara Lecchi, Chiara Maura Ciniselli, Arianna Ottini, Andrea Spagnoletti, Laura Mazzeo, Daniele Morelli, Paola Frati, Martina Stroscia, Elisabella Ebrahem, Elisa Sottotetti, Giulia Galli, Maria Grazia D’Elia, Riccardo Lobefaro, Monika Ducceschi, Lorenza Di Guardo, Sherrie Bhoori, Salvatore Provenzano, Marco Platania, Monica Niger, Elena Colombo, Federico Nichetti, Matteo Duca, Licia Rivoltini, Roberta Mortarini, Paolo Baili, Giovanni Apolone, Filippo de Braud, Paolo Verderio and Silvia Damian in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-7-tam-10.1177_17588359221108687 for Efficacy of mRNA anti-SARS-CoV-2 vaccination and dynamics of humoral immune response in patients with solid tumors: results from the institutional registry of an Italian tertiary cancer center by Giovanni Fuc’, Mara Lecchi, Chiara Maura Ciniselli, Arianna Ottini, Andrea Spagnoletti, Laura Mazzeo, Daniele Morelli, Paola Frati, Martina Stroscia, Elisabella Ebrahem, Elisa Sottotetti, Giulia Galli, Maria Grazia D’Elia, Riccardo Lobefaro, Monika Ducceschi, Lorenza Di Guardo, Sherrie Bhoori, Salvatore Provenzano, Marco Platania, Monica Niger, Elena Colombo, Federico Nichetti, Matteo Duca, Licia Rivoltini, Roberta Mortarini, Paolo Baili, Giovanni Apolone, Filippo de Braud, Paolo Verderio and Silvia Damian in Therapeutic Advances in Medical Oncology