Summary
Background
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive form of brain stimulation that positively regulates the motor and non-motor symptoms of Parkinson's disease (PD). Although, most reviews and meta-analysis have shown that rTMS intervention is effective in treating motor symptoms and depression, very few have used randomised controlled trials (RCTs) to analyse the efficacy of this intervention in PD. We aimed to review RCTs of rTMS in patients with PD to assess the efficacy of rTMS on motor and non-motor function in patients with PD.
Methods
In this systematic review and meta-analysis, we searched PubMed, MEDLINE and Web of Science databases for RCTs on rTMS in PD published between January 1, 1988 to January 1, 2022. Eligible studies included sham-controlled RCTs that used rTMS stimulation for motor or non-motor symptoms in PD. RCTs not focusing on the efficacy of rTMS in PD were excluded. Summary data were extracting from those RCTs by two investigators independently. We then calculated standardised mean difference with random-effect models. The main outcome included motor and non-motor examination of scales that were used in PD motor or non-motor assessment. This study was registered with PROSPERO, CRD42022329633.
Findings
Fourteen studies with 469 patients met the criteria for our meta-analysis. Twelve eligible studies with 381 patients were pooled to analyse the efficacy of rTMS on motor function improvement. The effect size on motor scale scores was 0.51 (P < 0.0001) and were not distinctly heterogeneous (I2 = 29%). Five eligible studies with 202 patients were collected to evaluate antidepressant-like effects. The effect size on depression scale scores was 0.42 (P = 0.004), and were not distinctly heterogeneous (I2 = 25%), indicating a significant anti-depressive effect (P = 0.004). The results suggest that high-frequency of rTMS on primary motor cortex (M1) is effective in improving motor symptoms; while the dorsolateral prefrontal cortex (DLPFC) may be a potentially effective area in alleviating depressive symptom.
Interpretation
The findings suggest that rTMS could be used as a possible adjuvant therapy for PD mainly to improve motor symptoms, but could have potential efficacy on depressive symptoms of PD. However, further investigation is needed.
Funding
The National Natural Science Foundation of China (NO: 81873777, 82071414), Initiated Foundation of Zhujiang Hospital (NO: 02020318005), Scientific Research Foundation of Guangzhou (NO: 202206010005), and Science and Technology Program of Guangdong of China (NO: 2020A0505100037).
Keywords: Repetitive transcranial magnetic stimulation, Parkinson disease, Motor Function, Cognition, Depression
Research in context.
Evidence before this study
Repetitive transcranial magnetic stimulation (rTMS) is a type of non-invasive brain stimulation and has neuromodulation effects. We searched PubMed, MEDLINE, and Web of Science for systematic reviews and meta-analysis and clinical trials on rTMS intervention and its efficacy in Parkinson's disease (PD) in terms of non-pharmacological therapeutic purposes, published between January 1, 1988 to January 1, 2022. We found numerous studies, including systematic reviews and meta-analysis. Although, most systematic reviews and meta-analysis showed rTMS intervention effective in motor symptoms and depression, very few have used randomised controlled trials (RCTs) to analyse the efficacy of this intervention in PD.
Added value of this study
This is the first systematic review and meta-analysis to estimate the efficacy of rTMS intervention in motor and non-motor symptoms of PD by analyzing RCTs only. The results suggest that rTMS could be used as a possible adjuvant therapy for motor symptoms in PD and could have potential efficacy for the depressive symptoms of PD; however, further investigation is needed. Therefore, future RCTs should be carried out to investigate the efficacy of rTMS and the suitable rTMS parameters, including stimulation site and frequency, for alleviating depressive symptoms in patients with PD.
Implications of all the available evidence
rTMS could be used as a possible adjuvant therapy to treat motor symptoms and depression in patients with PD. More RCTs should be conducted to further explore evidence and suitable parameters of rTMS intervention in PD.
Alt-text: Unlabelled box
Introduction
Parkinson's disease (PD) is a neurodegenerative disease that is common in middle-aged people and results from a progressive and selective loss of dopaminergic neurons mostly in the substantia nigra pars compacta. The motor symptoms of PD include bradykinesia, tremor, hypertonia, and gait disorder; and the non-motor symptoms of PD manifest as cognitive impairment and emotional processing, including depression, apathy, visual dysfunction and even cardiovascular autonomic dysfunction.1, 2, 3, 4, 5, 6
There are many pharmacologic treatments, including levodopa therapy,1,7 to relieve PD symptoms. However, these medicines sometimes fail to provide the desired effects or sometimes even induce motor complications, such as levodopa-induced dyskinesias (LIDs), especially when used in chronic treatment.8,9 Therefore, it is necessary to explore other alternative and promising PD treatments, such as non-invasive brain stimulation (NIBS).10 Our studies have demonstrated various neuroimaging markers and their relative neuropathogenesis in PD, which appear to contribute to motor/non-motor dysfunctions and discriminative aspects.11, 12, 13 Besides, our recent studies on the genetics and anti-neuroinflammatory therapy of PD14, 15, 16, 17, 18, 19 propel me to write one meta-analysis on the non-pharmacological therapy on PD.
Repetitive transcranial magnetic stimulation (rTMS), as one of the forms of NIBS, has neuromodulation effects.10 In rTMS intervention, a wire coil is used to generate a magnetic field that can pass through the scalp and the skull to change the excitability in the cortex according to the frequency. High-frequency rTMS (≥5 Hz) induces excitability in the cortex, while low-frequency rTMS (≤1 Hz) induces an inhibitory effect. A longer duration of stimulation is likely to induce a longer duration of effect.20,21 Additionally, there are numerous choices of stimulation sites of rTMS intervention; these sites include the primary motor cortex (M1), which is used mostly for motor symptoms; the dorsolateral prefrontal cortex (DLPFC); the supplementary motor area (SMA); and, in some cases, the cerebellum.22,23
For motor symptoms of PD, rTMS is considered a possible treatment in some studies, although the results are inconsistent because of the variation in rTMS stimulation parameters.24 rTMS therapy is also considered a potential treatment for PD-related non-motor symptoms, such as depression symptoms, as this therapy has already been used in some studies for medication-resistant depression.24 However, the sample size in most clinical trials that used rTMS to improve PD symptoms was small, and the parameters of those trials were too complicated and varied. Therefore, we conducted a meta-analysis to analyse the efficacy of rTMS intervention on motor and non-motor function by evaluating multiple scales. For non-motor aspects, we tried to evaluate the efficacy of rTMS on cognitive improvement and antidepression.
Methods
Search strategy
This study was conducted in accordance with the Preferred Reporting Items of the Guidelines for Systematic Reviews and Meta-Analysis (PRISMA). In this systematic review and meta-analysis, we searched PubMed, MEDLINE, and Web of Science databases from January 1, 1988 to January 1, 2022 for randomised controlled trials (RCTs) on rTMS intervention in PD. The search keywords were “Parkinson OR Parkinsonism” AND “transcranial magnetic stimulation OR TMS OR non-invasive brain stimulation”. The articles in the reference lists for these RCTs were also searched for additional studies. Unpublished data and replies to other articles were not included. This strategy yielded 2760 studies on transcranial magnetic stimulation (TMS) treatment for PD. The initial study protocol was pre-registered at PROSPERO (CRD42022329633). This is a review and meta-analysis without original patient data; therefore, the informed consent from patients was not required.
Select criteria
We chose RCTs that evaluated the efficacy of rTMS intervention on either motor or non-motor improvement in patients with PD. The following criteria were included: (1) The manuscript was written in English. (2) The study was accepted and published. (3) Patients were more than 18 years of age. (4) Motor improvement was measured by analysing the motor section of the Unified Parkinson's Disease Rating Scale (UPDRS Part III, a.k.a. UPDRS-III) and the motor section of Movement Disorder Society-Sponsored Revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS Part III, a.k.a. MDS-UPDRS-III). (5) Non-motor aspects included cognitive improvement and antidepressant-like effect; cognitive improvement was measured by the Mini-Mental State Exam (MMSE), Montreal Cognitive Assessment (MoCA) or the Mattis Dementia Rating Scale-2 (DRS-2 or MDRS-2); and antidepressant-like effect was measured by the Beck Depression Inventory (BDI), Hamilton Depression Rating Scale (abbreviated as HDRS in this article, and sometimes abbreviated as HRSD and HAMD in other articles) or Montgomery–Asberg Depression Rating Scale (MADRS). (6) The research should include the mean and standard deviation (SD) of the scales mentioned above before and after the rTMS intervention group and the sham group.
Data analysis
The extracted variables were as follows: (1) study design; (2) demographic characteristics (including number of patients, sex, and age); (3) mean and SD of the scores of the following scales: (I) MDS-UPDRS-III or UPDRS-III, which were the motor section of the scales; (II) cognition scales, including MMSE/MoCA/DRS-2; and (III) depression scales, including BDI/HDRS/MADRS; (4) mean and SD of the scores of the scales for the follow-up evaluation, if available; and (5) rTMS parameters (frequency, intensity, sessions, and site). If the studies had multiple rTMS intervention groups with different sites or frequencies, these groups were viewed as separate studies in our analysis. Summary data were extracting from those RCTs by two investigators independently.
Heterogeneity among studies was first assessed. We evaluated the major features that contributed to the study heterogeneity; these features include study design, demographic characteristics (such as the number of patients, age, and sex), PD clinical characteristics (such as the baseline scores, duration of disease, and medication state during assessment) and treatment characteristics (rTMS parameters).
The data were evaluated by using RevMan (Review Manager software, version 5.4.1, Cochrane Collaboration, UK). For effect size, we considered the main outcome measures are continuous data, and thereby the standardised mean difference (SMD) of the change in scores of multiple scale sets was calculated for the continuous measures of scales that reflected motor and non-motor function. Considering that the effects of rTMS intervention could be varied among the recruited studies, the random effects model was applied to measure the pooled weighted effect size, which could be used to evaluate whether the mean effect size was significant (P ≤ 0.05). For studies with follow-up evaluation, one mean effect size was obtained across multiple effect sizes.
For heterogeneity analysis, Cochran's Q statistics and the I2 index were used to analyse heterogeneity in the meta-analysis. An I2 value that was greater than 50% and a probability value of P that was less than 0.05 indicated that the included studies were heterogeneous.25
For risk of bias, the Cochrane risk-of-bias tool was adopted to evaluate each included study. We used this tool to assess the inclusion of studies in terms of the following aspects: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias. Then, these studies were classified as having a low, high, or unclear risk of bias according to the assessment result. A funnel plot diagram was also adopted to evaluate publication bias when necessary.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. WJZ and QW had accessed and verified the data reported in the manuscript. The corresponding author has the final responsibility to submit for publication following approval from all co-authors.
Results
The PRISMA screening flow diagram is shown in Figure 1.26 We found 2760 publications by using the keywords mentioned above in Section 2.1, and all the manuscripts were carefully reviewed. First, we excluded 2598 records because they were (1) duplicate literature, or (2) not clinical trials, or (3) written in another language. Then, we carefully read the remaining 162 remaining references and excluded 148 references because they (1) were not RCTs, or (2) focused on other topics, or other types of non-invasive brain stimulation rather than typical rTMS stimulation, or (2) used evaluation measures other than MDS-UPDRS-III/UPDRS-III for motor sections or BDI/HDRS/MADRS/MMSE/MoCA/DRS-2 for non-motor sections, or (3) applied antidepressant medication that had depressive scale outcomes would not be included in depressive group analysis, or (4) were considered not suitable for this analysis by authors. Therefore, 14 studies27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 met the criteria for our meta-analysis. All the studies included in our analysis were randomised, and most of them were double blinded. The same article could be used for multiple assessments because one article may contain multiple scales or have multiple stimulation sites or frequencies. Twelve articles were evaluated for motor improvement, and 5 articles were used for non-motor evaluation (3 for cognition assessment, and 5 for depression assessment).
Figure 1.
Study selection.
Most adverse events in these studies were mild or moderate, although some events resulted in dropouts. However, one study reported that ischemic stroke occurred in one patient in the active group. This incident was considered as a serious adverse event, while the authors of this study determined that the incident was unrelated to their study but did not specify the reason.34
Characteristics of patients
A total of 469 patients with PD in 14 studies were included. A total of 381 of these patients were analysed for motor symptoms, and 202 of these patients were analysed for non-motor symptoms. The average age of patients in most studies was more than 60 years old. The average disease duration of patients in most studies was over 6 years. Three articles did not provide the Hoehn and Yahr Scale stage (H&Y stage) of the patients, and one article did not provide disease duration. Most patients in these studies had stable medication before and during treatment, thus leaving the medication state on. The details of the patients and the rTMS variables used in these studies can be viewed in Tables 1 and 2.
Table 1.
Characteristics of participants.
| Sample, Count | Sex (F/M) | Age Mean (SD), year |
Disease Duration Mean (SD), year |
H&Y Stage Mean (SD) | |
|---|---|---|---|---|---|
| Li et al. (2020)27 | 48 | 16/32 | 61.6 (7.6) | 6.0 (4.8) | 1.8 (0.6) |
| Chung et al. (2020)28 | 50 | 24/26 | 62.3 (6.0) | 6.5 (4.0) | 2.2 (0.3) |
| Mi et al. (2019)29 | 30 | 16/14 | 63.6 (9.9) | 8.6 (5.5) | 2.5 (0.9) |
| Khedr et al. (2019)30 | 33 | N/A | 59.5 (9.2) | 6.0 (3.8) | 3.2 (1.1) |
| Cohen et al. (2018)31 | 42 | 10/32 | 65.6 (7.5) | 5.1 (3.5) | 2.0 (0.37) |
| Buard et al. (2018)32 | 46 | 13/33 | 68.5 (7.6) | N/A | N/A |
| Yokoe et al. (2017)33 | 19 | 12/7 | 69.1 (8.4) | 9.5 (3.2) | 3.5 (0.6) |
| Brys et al. (2016)34 | 61 | 24/37 | 63.4 (10.0) | 6.9 (4.7) | 2.5 (0.6) |
| Makkos et al. (2016)35 | 44 | 20/14 | 66.5 (8.0) | 5.5 (4.8) | 2.3 (0.8) |
| Kim et al. (2015)36 | 17 | 5/12 | 64.5 (8.4) | 7.8 (4.9) | 3.0 (0.5) |
| Maruo et al. (2013)37 | 21 | 10/11 | 63.0 (11.3) | 12.0 (6.3) | 3.1 (0.5) |
| Benninger et al. (2012)38 | 26 | 6/20 | 64.1 (8.5) | 9.0 (5.5) | 2.6 (0.4) |
| Pal et al. (2010)39 | 22 | 11/11 | 68.5 (7.9) | 6.0 (4.8) / 6.5 (5)a | N/A |
| Filipović et al (2009)40 | 10 | 5/5 | 64.5 (9.6) | 15.6 (5.7) | N/A |
Active group/Sham group: Median (SD), SD converted from IQR, SD=IQR/1.35.
Abbreviations: SD, standard deviation; F/M, Female/Male, H&Y stage, Hohn and Yahr Scale stage.
Table 2.
Characteristics of rTMS variables.
| Site | Frequency | Intensity | Session | Treatment Duration | Post-rTMS Evaluation | Medication state | |
|---|---|---|---|---|---|---|---|
| Li et al. (2020)27 | M1 | 20 Hz | 80% RMT | 5 | 5 d | immediately, 2wk, 4wk | On |
| Chung et al. (2020)28 | M1 | 1 / 25 Hz | 80% RMT | 12 | 3 wk | 1 d, 1 mon, 3 mon | On |
| Mi et al. (2019)29 | SMA | 10 Hz | 90% RMT | 10 | 2 wk | 5th, 10th, 2wk, 4wk | On & Off |
| Khedr et al. (2019)30 | M1 | 20 Hz | 90% RMT | 10 | 10 d | immediately, 1 mon, 2 mon, 3 mon | On |
| Cohen et al. (2018)31 | M1+PFC | 1 Hz+10 Hz | 110% +100% MT | 24 | 3 mon | immediately | On |
| Buard et al. (2018)32 | DLPFC | 20 Hz | 90% RMT | 10 | 2 wk | Unclear | On |
| Yokoe et al. (2017)33 | M1 /DLPFC /SMA | 10 Hz | 100% RMT | 4 | 3 d | 1 h | On |
| Brys et al. (2016)34 | M1 /DLPFC | 10 Hz | 120 V | 2 | 10 d | 1 mon | On & Off |
| Makkos et al. (2016)35 | M1 | 5 Hz | 90% RMT | 10 | 10 d | 1 d, 30d | On |
| Kim et al. (2015)36 | M1 | 10 Hz | 90% RMT | 5 | 1 wk | 5 d, 12 d | On |
| Maruo et al. (2013)37 | M1 | 10 Hz | 100% MT | 3 | 3 d | 1 h | Off |
| Benninger et al. (2012)38 | M1 | 50 Hz | 80% RMT | 8 | 2 wk | 1 d, 1 mon | On & Off |
| Pal et al. (2010)39 | DLPFC | 5 Hz | 90% RMT | 10 | 10 d | 1 d | On & Off |
| Filipović et al (2009)40 | M1 | 1 Hz | 90% RMT | 4 | 4 d | 1 d | On |
Abbreviations: DLPFC, dorsolateral prefrontal cortex; M1, primary motor cortex; MT, motor threshold; PFC, prefrontal cortex; RMT, resting motor threshold; rTMS, repetitive transcranial magnetic stimulation; SMA, supplementary motor area.
Quality evaluation
Most articles documented completed patient information, including age and duration of illness. All the studies applied randomised allocation. Thirteen studies were double-blinded, and one study was single-blinded. Twelve studies reported the exact number of dropouts, and some studies specified that dropouts of these studies had completed the primary evaluation; the remaining two studies did not state whether dropouts occurred. Twelve studies reported whether patients in these studies had encountered adverse events, but three of these studies did not specify the number.
The risk of bias assessment results for all studies are shown in eFigure 1 in the supplement. Four of 14 studies had a low risk of bias across all seven aspects. According to the quality evaluation results, all 14 studies were RCTs, and all the studies had a low risk of bias.
Assessment of motor function improvement
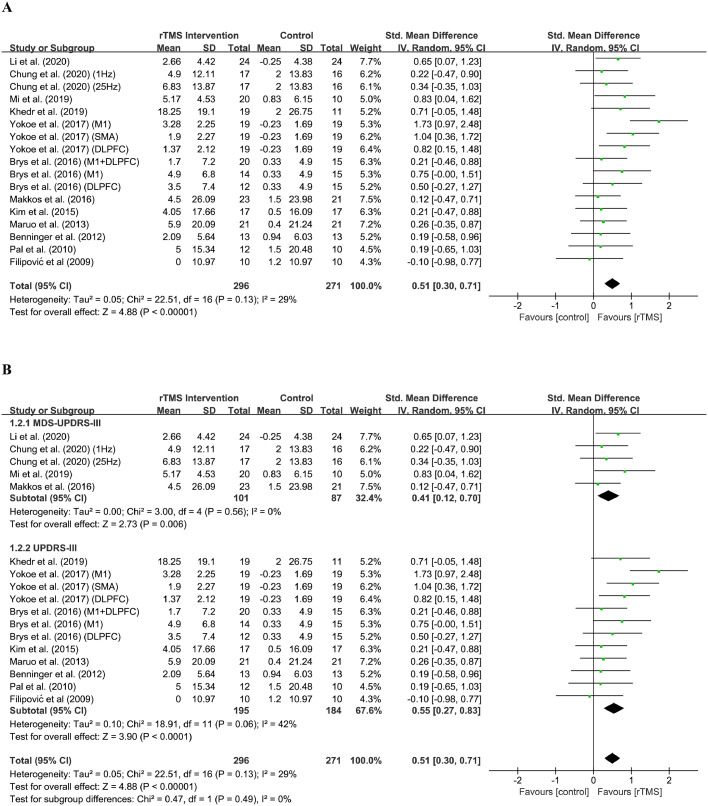
The 12 eligible studies27, 28, 29, 30,33, 34, 35, 36, 37, 38, 39, 40 with 381 patients assessed the efficacy of rTMS intervention on motor improvement. The total effect size of rTMS on motor scale (MDS-UPDRS-III27, 28, 29,35 and UPDRS-III30,33,34,36, 37, 38, 39, 40) scores was 0.51 (95% CI, 0.30 to 0.71), which indicated that the effect size favored the active rTMS group over the sham group (Z = 4.88; P < 0.0001). The mean change in the motor scale score for the active rTMS group was 4.61 (13.3), which indicated a moderately clinically important difference.41 The distribution of overall and effect sizes can be seen in Figure 2A.
Figure 2.
Forest plot showing the standardised mean difference (SMD) and 95% CI of differences in motor scale scores between the repetitive transcranial magnetic stimulation (rTMS) group and control group.
(A) Overall analysis.
(B) subgroup analysis.
For heterogeneity analysis, the results indicated that these studies were not distinctly different (Chi2 = 22.51; P = 0.13; I2 = 29%). Additionally, Figure 2B shows that these two scales were not statistically heterogeneous (Chi2 = 0.47; P = 0.49; I2 = 0%), thus implying that these two chosen scales did not cause a certain deviation. In addition, the results of the funnel plot of the motor scale evaluation (eFigure 2 in the supplement) illustrated that both the left and right sides were roughly symmetrical, thus indicating the insignificance of publication bias.
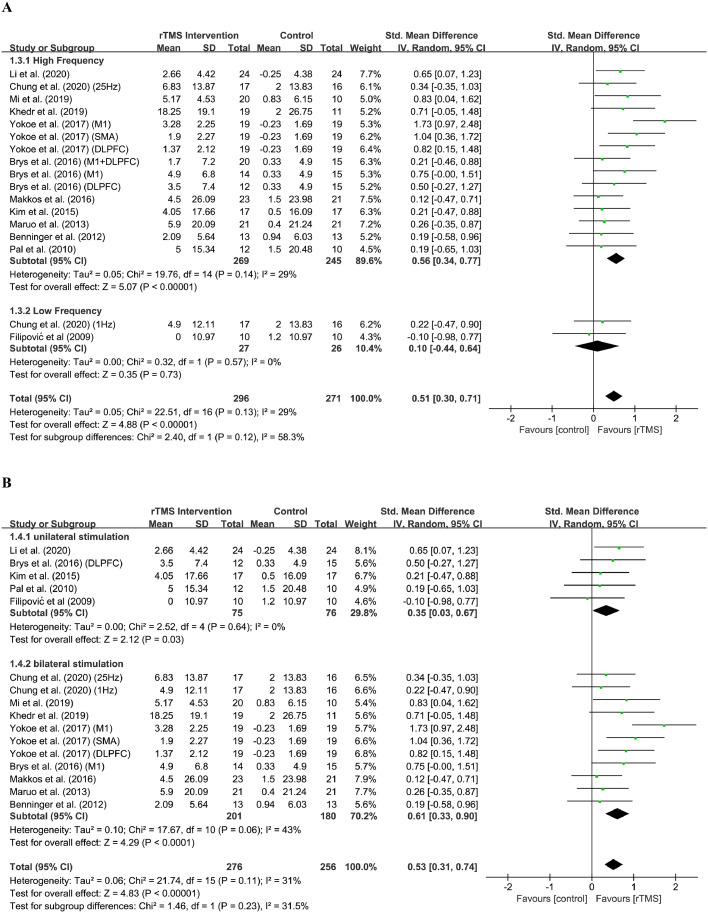
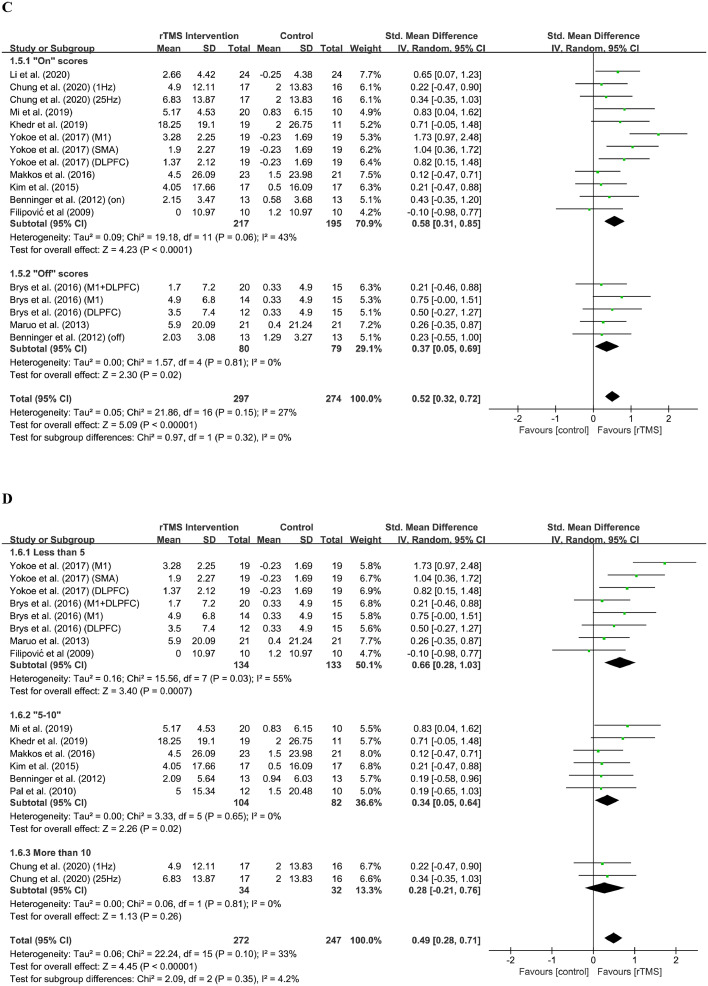
For parameters analysis, the frequency, stimulation site, medication state and number of rTMS session were evaluated. For frequency analysis (Figure 3A), the effect size of high frequency group was 0.56 (95% CI, 0.34 to 0.77) and the result was significant (Z = 5.07; P < 0.0001), while the effect size of low frequency group was 0.10 (95% CI, -0.44 to 0.64) and the result was insignificant (Z = 0.35; P = 0.73). The test of subgroup differences indicated that there were subgroup differences but insignificant (Chi2 = 2.40; I2 = 58.3%, P = 0.12). For stimulation site analysis (Figure 3B), the effect size of unilateral group was 0.35 (95% CI, 0.03 to 0.67) and the result was significant (Z = 2.12; P = 0.03), while the effect size of bilateral group was 0.61 (95% CI, 0.33 to 0.90) and the result was also significant (Z = 4.29; P < 0.0001). The test of subgroup differences indicated subgroup differences were insignificant (Chi2 = 1.46; I2 = 31.5%, P = 0.23). For medication state analysis (Figure 3C), the effect size of “on” group was 0.58 (95% CI, 0.31 to 0.85) and the result was significant (Z = 4.23; P < 0.0001), while the effect size of “off” group was 0.37 (95% CI, 0.05 to 0.69) and the result was also significant (Z = 2.30; P = 0.02). The test of subgroup differences indicated subgroup differences were not significant (Chi2 = 0.97; I2 = 0%, P = 0.32). For the number of rTMS sessions analysis (Figure 3D), the effect size of “Less than 5” group is 0.66 (95% CI, 0.28 to 1.03) and the result was significant (Z = 3.40; P = 0.0007); the effect size of “5 to 10” group is 0.34 (95%CI, 0.05 to 0.64) and the result was significant (Z = 2.26; P = 0.02). The effect size of “More than 10” group is 0.28 (95%CI, -0.21 to 0.76) and the result was not significant (Z = 1.13; P = 0.26). The test of subgroup differences indicated subgroup differences were not significant (Chi2 = 2.09; I2 = 4.2%, P = 0.35).
Figure 3.
Forest plot showing the standardised mean difference (SMD) and 95% CI of differences in motor scale scores between the repetitive transcranial magnetic stimulation (rTMS) group with different parameters and control group.
(A) Frequencies.
(B) Stimulation sites.
(C) Medication states.
Assessment of non-motor symptoms
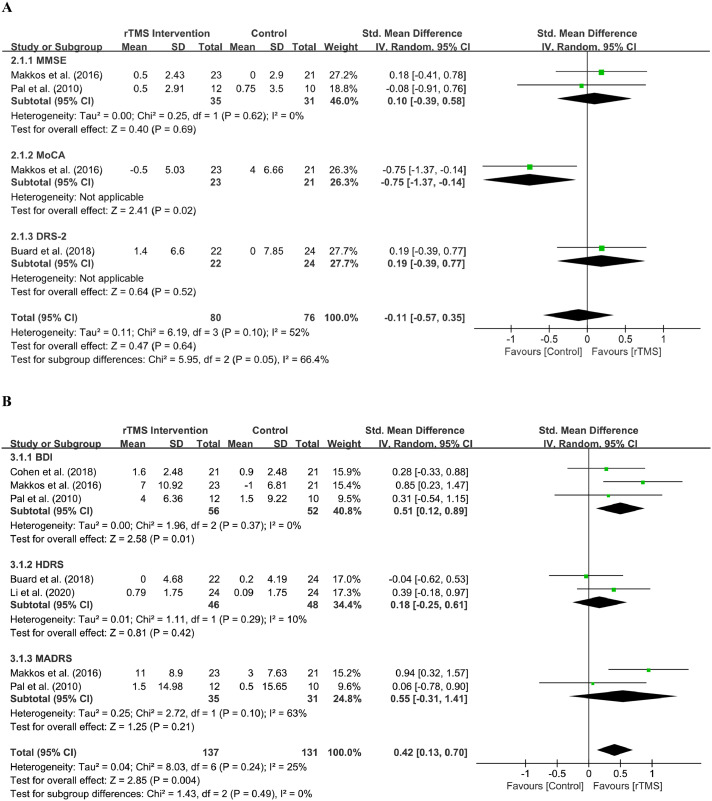
Five eligible27,31,32,35,39 studies with 202 patients assessed the efficacy of rTMS intervention on non-motor improvement, including cognitive improvement and antidepressant-like effects.
Cognition
Three studies32,35,39 included 112 patients in this evaluation section. The cognition scale subgroup (MMSE, MoCA, DRS-2) analysis used a random effect model. The effect size of rTMS on cognition scale scores was -0.11 (95% CI, -0.57 to 0.35), indicating that there was not enough evidence to determine the efficacy of rTMS intervention on cognitive function (Z = 0.47; P = 0.64). The results of the heterogeneity analysis showed that the heterogeneity among these studies was not significant (Chi2 = 6.19; P = 0.10; I2 = 52%).
The results of the heterogeneity analysis also showed that the effect size of the MMSE group score was 0.10 (95% CI, -0.39 to 0.58, P = 0.69), indicating an insignificant improvement (Z = 0.40; P = 0.69) without heterogeneity (Chi2 = 0.25; P = 0.62; I2 = 0%). The results of the other two cognition scales, namely, MoCA and DRS-2, could not determine heterogeneity because of insufficiency of the included studies. In addition, the test of subgroup differences may indicate that subgroup differences were significant (Chi2 = 5.95; I2 = 66.4%, P = 0.05), and the heterogeneity of subgroups may cause a certain deviation. The distribution of overall and subgroup effect sizes can be seen in Figure 4A.
Figure 4.
Forest plot showing the standardised mean difference (SMD) and 95% CI of differences in non-motor scale scores between the repetitive transcranial magnetic stimulation (rTMS) and control group, and subgroup analysis.
(A) Cognitive scales.
(B) Depressive scales.
Depression
Five studies27,31,32,35,39 included 202 patients in this evaluation section. The depression scale subgroup (BDI, HDRS, and MADRS) analysis used a random effect model. The effect size of rTMS intervention on depression scale scores was 0.42 (95% CI, 0.13 to 0.70), which indicated a potential minor effect size favoring the active group, and the effect was significant (Z = 2.85; P = 0.004). The subgroup analysis showed that the effect size of the BDI group score was 0.51 (95% CI, 0.12 to 0.89), which indicated a minor but significant improvement (Z = 2.58; P = 0.01). The effect size of the HDRS group score was 0.18 (95% CI, -0.25 to 0.61) and the improvement is insignificant (Z = 0.81; P = 0.42). The effect size of the MADRS group score was 0.55 (95% CI, -0.31 to 1.41) and the improvement is also insignificant (Z = 1.25; P = 0.21).
The heterogeneity analysis of these studies these studies were not distinctly different (Chi2 = 8.03; P = 0.24; I2 = 25%). The BDI group showed no heterogeneity (Chi2 = 1.96; P = 0.37; I2 = 0%), and HDRS also showed no heterogeneity (Chi2 = 1.11; P = 0.29; I2 = 10%). The heterogeneity analysis of studies that use the MADRS also showed insignificant heterogeneity (Chi2 = 2.72; P = 0.10; I2 = 63%). Additionally, the test of subgroup differences may indicate that there were no subgroup differences (Chi2 = 1.43; P = 0.49; I2 = 0%). The distribution of overall and subgroup effect sizes can be seen in Figure 4B.
Discussion
Numerous relevant general conclusions could be drawn based on the results of this meta-analysis. Overall, the results of this analysis indicated a positive effect of rTMS intervention on motor improvement, but the efficacy on cognitive improvement and antidepression remained unclear.
For the motor aspect, the random effects across 12 studies showed a significant effect size (SMD = 0.51) in favor of the active rTMS group, thus indicating the improvement of motor function and a reduction of the relevant symptoms of PD; these results had been demonstrated in numerous reviews and meta-analyses.42, 43, 44 Interestingly, according to Table 2, 10 of those studies chose high-frequency stimulation only. Our subgroup analysis for frequency showed that the effect size of high frequency group (SMD = 0.56) was larger than low frequency group (SMD = 0.10), and the effect size of low frequency group was insignificant. We assumed that high-frequency stimulation could serve as an effective stimulation parameter. For stimulation site, previous studies have already pointed out that rTMS targeting M1 was effective in reducing the motor symptoms of PD compared with other stimulation sites.44 Meanwhile, further investigation showed that bilateral group (SMD = 0.61) was larger than unilateral group (SMD = 0.35), but the subgroup differences were not significant, and therefore future study may investigate whether bilateral stimulation is beneficial. However, according to Lefaucheur et al. (2020), high-frequency rTMS (HF-rTMS) of the M1 in patients with PD has been recommended for the treatment of motor symptoms of PD.45 According to Table 2, nine of 12 studies were included in our motor group containing HF-rTMS of the M1 simulation mode, which explained the good results on motor symptoms. But we also notice that the good results of UPDRS-III scores sometimes cannot translate to the improvement in quality of life as measured by the Parkinson's Disease Questionnaire (PDQ-39) like some studies mentioned, thus probably resulting the conflicting outcomes.
For medication state, our subgroup analysis showed that the effect size of “on” group (SMD = 0.58) was larger than “off” group (SMD = 0.37), but the subgroup differences were not significant. Interestingly, several studies indicated that rTMS-induced cortical inhibition could be affected by anti-PD pharmacotherapy including dopamine agonist, which may imply that dopamine treatment could modify the effect of rTMS intervention.46 However, there were four studies where dopamine agonist was used in some or all of the participants during the study,30,35,39,40 while the rest of studies provided levodopa equivalent daily dose and oral medication. Meanwhile, most studies did not mention the exact duration of pharmacotherapy, but describe like “during the experiment”, or “for at least a time before starting the study”. Therefore, the insufficient detail of oral medication hindered us to further evaluate the potential role of duration and type of anti-PD treatment in rTMS intervention. Therefore, it would be better for future study to examine the duration and type of pharmacotherapy treatment, rather than simply describe as ON/OFF state, and to further investigate the role of dopamine treatment in the efficacy of rTMS intervention.
For the number of rTMS sessions analysis, the subgroup differences were not significant, which indicated that the number of rTMS session may not be the significant indicator of the efficacy of rTMS. This result was consistent with Chou et al. (2015),44 which indicated that the number of pulses per or across sessions, rather than the number of sessions, could improve the efficacy. However, Lefaucheur et al. (2020) indicated that increased number of sessions may optimize the efficacy. This inconsistency requires further investigation.45
In addition, heterogeneity analysis showed that the scores of the UPDRS-III and MDS-UPDRS-III were not statistically heterogeneous. However, for limitation of MDS-UPDRS-III, some studies indicated that, in motor symptoms of early PD, psychometric limitations of MDS-UPDRS-III may result in limitation of precision and insensitivity, compared with traditional UPDRS-III.47,48 This limitation may prevent MDS-UPDRS from wider use of PD assessment, and more investigation is needed to explore the possible direction of improvement.
Besides, there are four manuscripts in our meta-analysis that used PDQ-39 but the results were not consistent.27,33, 34, 35 Li et al. (2020) and Makkos et al. (2016) indicated that PDQ-39 was improved in the active group, while Brys et al. (2016) and Yokoe et al. (2017) indicated no correlation of improvement in UPDRS-III translated into improvement in quality of life as measured by PDQ-39. Interestingly, we found a conference abstract by Lokhandwala et al. (2019) who showed that MDS-UPDRS-III may correlated with PDQ-39.49 Although the rest of manuscripts in our meta-analysis did not include PDQ-39 and hindered us from further analyzing, we think that further clinical studies and meta-analysis should evaluate if the improvement in UPDRS-III can translate into improvement in quality of life.
Moreover, freezing of gait (FOG) is one of the common symptoms of PD. Three studies included in our meta-analysis investigated whether rTMS intervention could improve FOG. Mi et al. (2019) indicated that HF-rTMS of SMA could improves FOG in PD.29 Kim et al. (2015) pointed out that HF-rTMS of the lower leg primary motor cortex of the dominant hemisphere (M1-LL) could also improve FOG.36 However, in Benninger's study (2012) the scores of Freezing of Gait Questionnaire (FOGQ) did not have significant improvement in HF-rTMS of M1 group.38 Given that the insufficient number of studies for further investigation, we searched for other reviews for reference. Gao et al. (2020)50 pointed out that rTMS intervention is beneficial for the improvement of FOG in PD and SMA may be a potential stimulation site. Chen et al. (2019)24 also drew a similar conclusion. However, a conference abstract by Gao et al. (2019) pointed out that HF-rTMS of SMA cannot alleviate the sequence effect (SE) in patients with PD with FOG.51 Therefore, future study to investigate whether FOG can be improved by HF-rTMS of SMA is needed.
In addition, levodopa-induced dyskinesias (LIDs) is a side effect of levodopa therapy in patients with PD. Although this meta-analysis mainly focused on the efficacy of rTMS in the symptoms resulted from PD and most of the included studies did not mention this complication, we do notice that some studies indicated that simulation of SMA52 and cerebellar53 could help reduced LIDs. Therefore, we hope that future studies could evaluate the efficacy of rTMS in LIDs.
For non-motor aspects, the effect size of rTMS intervention on cognition scale scores was -0.11 (P = 0.64), and the effect size of rTMS on depression scale scores was 0.42 (P = 0.004).
For cognition, although there were multiple studies32,35,39,54, 55, 56, 57, 58, 59 assessing the efficacy of rTMS intervention on cognitive function in patients with PD, most studies were not high-quality RCTs, and there were mixed designs, cognition scales and results among those studies. In this study, we tried to pool RCTs to analyse the efficacy of rTMS intervention on cognitive function, but the number of RCTs with suitable cognition scales before and after rTMS intervention was insufficient. Some reviews and meta-analyses considered that rTMS intervention may have limited but positive effects on executive function60 or working memory.61 However, Lawrence et al. (2017) considered the results were insufficient to determine whether rTMS intervention was effective, but the authors could not exclude the ceiling effect of the potential therapeutic effect of the intervention because the number of included studies with rTMS intervention was insufficient.62 Therefore, more high-quality RCTs with suitable cognition scales are needed to further assess the potential efficacy of rTMS intervention on cognitive function.
The total random effect of depressive scale scores across five studies showed a significant effect size (SMD = 0.42), and the random effects of BDI scale scores across three studies showed a significant effect size (SMD = 0.51) in favor of active rTMS over sham rTMS. The result indicated a potential antidepression-like effect for patients with PD. However, we noticed that the effect of total depressive scale scores and BDI score were significant, but HDRS and MADRS had insignificant improvement. Therefore, we propose that the model we used in our analysis may cause this inconsistency. We chose random-effect model based on the heterogeneity analysis in the original version of meta-analysis. However, Borenstein et al. (2010) pointed out that such a strategy should be strongly discouraged.63 Although random-effect model is generally a more plausible match, we tried the fixed-effect model because the insufficient number of studies included in those groups may cause deviation in random-effect model. We speculated that the standard error of the summary effect and the confidence intervals were narrowed when the analysis moved to fix-effect model. Interestingly, the fix-effect model (eFigure 3) provided slightly different result. The effect size of MADRS scale scores was 0.63 (95% CI, 0.13 to 1.13), and the result became significant (Z = 2.45; P = 0.01). This result indicated that the model we chose may cause deviation because of the insufficient numbers of studies in those groups. However, we also found that most studies seem to prefer random-effect model because they consider that the treatment effects of rTMS intervention may be varied among the included studies.
Multiple reviews and meta-analyses have demonstrated that rTMS intervention could reduce depression scale scores, and thus indicating that this intervention may have potential antidepression-like effects.45,64, 65, 66 Meanwhile, some studies showed that rTMS intervention had antidepressant-like effects similar to that of oral medications such as selective serotonin reuptake inhibitors (SSRIs) that were clinically used for antidepression therapy.59,64 To our knowledge, rTMS intervention could be applied to not only the treatment of PD-related depression, but also other depressive disorders. McClintock et al. (2018) illustrated the antidepressant effect of rTMS therapy in patient with major depressive disorder, and high frequency over left DLPFC and low frequency rTMS over right DLPFC had antidepressant effects.67 Interestingly, Lefaucheur et al. (2020) pointed out that HF-rTMS of the left DLPFC in patients with PD could provide probable antidepressant efficacy, which is similar to our findings.
Therefore, we considered rTMS intervention to be effective for antidepression, and DLPFC could be a potential target site, while the suitable stimulation site (left or right DLPFC, unilateral or bilateral) and frequency are needed to be further investigated.
This study had several limitations. First, this meta-analysis did not further analyse the specific stimulation site of rTMS intervention for motor section. Meanwhile, we acknowledge that the absence of this separate evaluation is one of the limitations of our study, and future clinical study and meta-analysis should take it into consideration.
Second, the insufficiency of high-quality RCTs on non-motor aspects hindered us from further assessing the efficacy of rTMS on cognitive and depressive aspects and resulted in the deviation of our choice of these scales. Therefore, we did not further analyse the change in various cognitive aspects as other reviews and meta-analyses.60,61 Meanwhile, we acknowledge that the insufficient numbers of studies included in depressive groups may cause inconsistency of results among subgroups. Besides, we agreed that analysis separating the patients with PD as young vs early stage is beneficial.68 However, unfortunately, three studies in our analysis seem to have insufficient information for such an analysis. Pal et al. (2010)39 does not provide H&Y Stage, and Buard et al. (2018)32 does not provide H&Y Stage and Disease Duration. Such an additional analysis seems to be difficult based on the current studies in our meta-analysis. We acknowledge that lacking such an analysis is one of the limitations of our study, and future study is needed to evaluate the role of this factor in cognition change and the efficacy of rTMS treatment.
Moreover, the sex difference was not considered a comparable factor in our meta-analysis, while Oltra et al. (2022) reported that the sex effect may significantly affect cognitive impairment in patients with PD.69 Sex differences may require attention in future studies of cognitive assessment in patients with PD. Meanwhile, some studies pointed out that other factors like diabetes mellitus could affect the progression of PD and its motor and non-motor symptoms, especially the cognition symptoms,46,70, 71, 72 which may affect the efficacy of rTMS intervention and the result of this meta-analysis. In addition, we did not include studies that used antidepressant medications, such as SSRIs, as a control group.
Third, we did not evaluate other non-motor symptoms, such as sleep disorders, that may serve as an early PD symptom,73 while Babiloni et al. (2021) demonstrated a potential positive effect of rTMS intervention on sleep disturbances.74
Forth, although we focused mainly on rTMS intervention in this study, several types of new TMS protocols, such as theta burst stimulation (TBS); paired associative stimulation (PAS); and other types of NIBS, including transcranial direct current stimulation (tDCS), started developing rapidly and are worth attention; these new protocols introduced innovative approaches to modulate cortical excitability.75, 76, 77, 78
Lastly, due to the limitation of the software we used, not all p values could provide two significant figures.
This study showed that rTMS intervention with proper parameters could positively affect the improvement of motor symptoms in patients with PD and may have potential antidepression-like effects, but we cannot determine the efficacy on cognitive improvement. Therefore, rTMS treatment can be used as an adjuvant therapy for PD with motor symptoms, and more studies of parameters are needed to further improve the efficacy. Meanwhile, some studies had pointed out that rTMS intervention was effective and DLPFC could be a possible target for stimulation in patients with PD who have depression. Thus, more RCTs should be carried out to further investigate its efficacy and suitable parameter including stimulation site and frequency on depressive aspects in patients with PD.
Contributors
Conceived and designed the study: WJZ, HZ, BD and QW. Performed the study: WJZ, HZ, BD and QW. Revised the paper for intellectual content: FX, AS, JFG, HJ and BST. Data statistics and analysis: WJZ, FX and QW. Wrote the paper: WJZ and QW. WJZ and QW had accessed and verified the data reported in the manuscript. All authors read and approved the final manuscript.
Data sharing statement
Extracted data are from published studies and are available in the paper, and the dataset are not subject to embargo or restrictions. The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Declaration of interests
All authors declare no competing interests.
Acknowledgments
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (NO: 81873777, 82071414), Initiated Foundation of Zhujiang Hospital (NO: 02020318005), Scientific Research Foundation of Guangzhou (NO: 202206010005) and Science and Technology Program of Guangdong of China (NO: 2020A0505100037) to QW.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101589.
Appendix. Supplementary materials
References
- 1.Nemade D, Subramanian T, Shivkumar V. An update on medical and surgical treatments of Parkinson's disease. Aging Dis. 2021;12(4):1021. doi: 10.14336/AD.2020.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirmani BF, Shapiro LA, Shetty AK. Neurological and neurodegenerative disorders: novel concepts and treatment. Aging Dis. 2021;12(4):950. doi: 10.14336/AD.2021.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendricks RM, Khasawneh MT. A systematic review of Parkinson's disease cluster analysis research. Aging Dis. 2021;12(7):1567. doi: 10.14336/AD.2021.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu S, Li H, Xu X, et al. The pathogenesis and treatment of cardiovascular autonomic dysfunction in Parkinson's disease: what we know and where to go. Aging Dis. 2021;12(7):1675. doi: 10.14336/AD.2021.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilat M, Ginis P, Zoetewei D, et al. A systematic review on exercise and training-based interventions for freezing of gait in Parkinson’s disease. npj Parkinson’s Disease. 2021;7(1):81. doi: 10.1038/s41531-021-00224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Z, Hu W, Liao H, et al. Association of visual impairment with risk for future Parkinson's disease. eClin Med. 2021;42 doi: 10.1016/j.eclinm.2021.101189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease. JAMA. 2014;311(16):1670. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 8.Fahn S. The history of dopamine and levodopa in the treatment of Parkinson's disease. Movement Disord. 2008;23(S3):S497–S508. doi: 10.1002/mds.22028. [DOI] [PubMed] [Google Scholar]
- 9.Turcano P, Mielke MM, Bower JH, et al. Levodopa-induced dyskinesia in Parkinson disease. Neurology. 2018;91(24):e2238–e2243. doi: 10.1212/WNL.0000000000006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanches C, Stengel C, Godard J, et al. Past, present, and future of non-invasive brain stimulation approaches to treat cognitive impairment in neurodegenerative diseases: time for a comprehensive critical review. Front Aging Neurosci. 2021;12:578339. doi: 10.3389/fnagi.2020.578339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu S, Deng B, Huang Z, et al. “Hot cross bun” is a potential imaging marker for the severity of cerebellar ataxia in MSA-C. npj Parkinson’s Disease. 2021;7(1):15. doi: 10.1038/s41531-021-00159-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu S, Li H, Deng B, et al. Various diseases and clinical heterogeneity are associated with “hot cross bun”. Front Aging Neurosci. 2020;12:592212. doi: 10.3389/fnagi.2020.592212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin A, Zheng W, He Y, et al. Gut microbiota in patients with Parkinson's disease in southern China. Parkinsonism Relat D. 2018;53:82–88. doi: 10.1016/j.parkreldis.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Yang W, Chang Z, Que R, et al. Contra-directional expression of plasma superoxide dismutase with lipoprotein cholesterol and high-sensitivity C-reactive protein as important markers of parkinson’s disease severity. Front Aging Neurosci. 2020;12:53. doi: 10.3389/fnagi.2020.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Luo Y, Ray CK, Reynolds R, Tan EK, Pettersson S. The role of gut dysbiosis in Parkinson's disease: mechanistic insights and therapeutic options. Brain. 2021;144(9):2571–2593. doi: 10.1093/brain/awab156. [DOI] [PubMed] [Google Scholar]
- 16.Yang W, Deng B, Huang Y, et al. Two heterozygous progranulin mutations in progressive supranuclear palsy. Brain. 2021;144(3):e27. doi: 10.1093/brain/awaa428. [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Huang Z, Feng C, et al. Multimodal analysis of gene expression from postmortem brains and blood identifies synaptic vesicle trafficking genes to be associated with Parkinson’s disease. Brief Bioinform. 2021;22(4):1–15. doi: 10.1093/bib/bbaa244. [DOI] [PubMed] [Google Scholar]
- 18.Que R, Zheng J, Chang Z, et al. Dl-3-n-butylphthalide rescues dopaminergic neurons in Parkinson’s disease models by inhibiting the NLRP3 inflammasome and ameliorating mitochondrial impairment. Front Immunol. 2021;12:794770. doi: 10.3389/fimmu.2021.794770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou J, Chen Z, Wei X, et al. Cystatin C as a potential therapeutic mediator against Parkinson's disease via VEGF-induced angiogenesis and enhanced neuronal autophagy in neurovascular units. Cell Death Dis. 2017;8(6):e2854. doi: 10.1038/cddis.2017.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunoni AR, Chaimani A, Moffa AH, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes. Jama Psychiat. 2017;74(2):143. doi: 10.1001/jamapsychiatry.2016.3644. [DOI] [PubMed] [Google Scholar]
- 21.Milev RV, Giacobbe P, Kennedy SH, et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder. Can J Psychiatry. 2016;61(9):561–575. doi: 10.1177/0706743716660033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol, Neurosurg Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 23.Shin H, Hallett M, Sohn YH. Cerebellar repetitive transcranial magnetic stimulation for patients with essential tremor. Parkinsonism Relat D. 2019;64:304–307. doi: 10.1016/j.parkreldis.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Chen KHS, Chen R. Invasive and noninvasive brain stimulation in parkinson's disease: clinical effects and future perspectives. Clin Pharmacol Ther. 2019;106(4):763–775. doi: 10.1002/cpt.1542. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Mi T, Zhu B, et al. High-frequency repetitive transcranial magnetic stimulation over the primary motor cortex relieves musculoskeletal pain in patients with Parkinson's disease: a randomized controlled trial. Parkinsonism Relat D. 2020;80:113–119. doi: 10.1016/j.parkreldis.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Chung CLH, Mak MKY, Hallett M. Transcranial magnetic stimulation promotes gait training in parkinson disease. Ann Neurol. 2020;88(5):933–945. doi: 10.1002/ana.25881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mi T, Garg S, Ba F, et al. High-frequency rTMS over the supplementary motor area improves freezing of gait in Parkinson's disease: a randomized controlled trial. Parkinsonism Relat D. 2019;68:85–90. doi: 10.1016/j.parkreldis.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Khedr EM, Mohamed KO, Soliman RK, Hassan AMM, Rothwell JC. The effect of high-frequency repetitive transcranial magnetic stimulation on advancing parkinson's disease with dysphagia: double blind randomized clinical trial. Neurorehab Neural Re. 2019;33(6):442–452. doi: 10.1177/1545968319847968. [DOI] [PubMed] [Google Scholar]
- 31.Cohen OS, Rigbi A, Yahalom G, et al. Repetitive deep TMS for Parkinson disease. J Clin Neurophysiol. 2018;35(2):159–165. doi: 10.1097/WNP.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 32.Buard I, Sciacca DM, Martin CS, et al. Transcranial magnetic stimulation does not improve mild cognitive impairment in Parkinson's disease. Movement Disord. 2018;33(3):489–491. doi: 10.1002/mds.27246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokoe M, Mano T, Maruo T, et al. The optimal stimulation site for high-frequency repetitive transcranial magnetic stimulation in Parkinson's disease: a double-blind crossover pilot study. J Clin Neurosci. 2018;47:72–78. doi: 10.1016/j.jocn.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Brys M, Fox MD, Agarwal S, et al. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease. Neurology. 2016;87(18):1907–1915. doi: 10.1212/WNL.0000000000003279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makkos A, Pál E, Aschermann Z, et al. High-frequency repetitive transcranial magnetic stimulation can improve depression in Parkinson's disease: a randomized, double-blind, placebo-controlled study. Neuropsychobiology. 2016;73(3):169–177. doi: 10.1159/000445296. [DOI] [PubMed] [Google Scholar]
- 36.Kim MS, Chang WH, Cho JW, et al. Efficacy of cumulative high-frequency rTMS on freezing of gait in Parkinson's disease. Restor Neurol Neuros. 2015;33(4):521–530. doi: 10.3233/RNN-140489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maruo T, Hosomi K, Shimokawa T, et al. High-frequency repetitive transcranial magnetic stimulation over the primary foot motor area in parkinson's disease. Brain Stimul. 2013;6(6):884–891. doi: 10.1016/j.brs.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Benninger DH, Iseki K, Kranick S, Luckenbaugh DA, Houdayer E, Hallett M. Controlled study of 50-Hz repetitive transcranial magnetic stimulation for the treatment of parkinson disease. Neurorehab Neural Re. 2012;26(9):1096–1105. doi: 10.1177/1545968312445636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pal E, Nagy F, Aschermann Z, Balazs E, Kovacs N. The impact of left prefrontal repetitive transcranial magnetic stimulation on depression in Parkinson's disease: a randomized, double-blind, placebo-controlled study. Movement Disord. 2010;25(14):2311–2317. doi: 10.1002/mds.23270. [DOI] [PubMed] [Google Scholar]
- 40.Filipović SR, Rothwell JC, van de Warrenburg BP, Bhatia K. Repetitive transcranial magnetic stimulation for levodopa-induced dyskinesias in Parkinson's disease. Movement Disord. 2009;24(2):246–253. doi: 10.1002/mds.22348. [DOI] [PubMed] [Google Scholar]
- 41.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson's disease rating scale. Archives Neurol. 2010;67(1):64. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 42.Fregni F. Non-invasive brain stimulation for Parkinson's disease: a systematic review and meta-analysis of the literature. J Neurol, Neurosurg Psychiatry. 2005;76(12):1614–1623. doi: 10.1136/jnnp.2005.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elahi B, Elahi B, Chen R. Effect of transcranial magnetic stimulation on Parkinson motor function-Systematic review of controlled clinical trials. Movement Disord. 2009;24(3):357–363. doi: 10.1002/mds.22364. [DOI] [PubMed] [Google Scholar]
- 44.Chou Y, Hickey PT, Sundman M, Song AW, Chen N. Effects of repetitive transcranial magnetic stimulation on motor symptoms in parkinson disease. Jama Neurol. 2015;72(4):432. doi: 10.1001/jamaneurol.2014.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lefaucheur J, Aleman A, Baeken C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018) Clin Neurophysiol. 2020;131(2):474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Lang N, Speck S, Harms J, Rothkegel H, Paulus W, Sommer M. Dopaminergic potentiation of rTMS-induced motor cortex inhibition. Biol Psychiat. 2008;63(2):231–233. doi: 10.1016/j.biopsych.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 47.Regnault A, Boroojerdi B, Meunier J, Bani M, Morel T, Cano S. Does the MDS-UPDRS provide the precision to assess progression in early Parkinson's disease? Learnings from the Parkinson's progression marker initiative cohort. J Neurol. 2019;266(8):1927–1936. doi: 10.1007/s00415-019-09348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tosin MHS, Stebbins GT, Comella C, et al. Does MDS-UPDRS provide greater sensitivity to mild disease than UPDRS in De Novo Parkinson's disease? Movement Disord Clin Practice. 2021;8(7):1092–1099. doi: 10.1002/mdc3.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lokhandwala T., Lee A., Yue B., Thach A., Navia B. Correlation between PDQ-39 and MDS-UPDRS scores in a phase 3 randomized controlled trial of patients with Parkinson’s disease [abstract] Mov Disord. 2019;34(suppl 2):S389–S390. [Google Scholar]
- 50.Gao C, Liu J, Tan Y, Chen S. Freezing of gait in Parkinson’s disease: pathophysiology, risk factors and treatments. Transl Neurodegener. 2020;9(1):12. doi: 10.1186/s40035-020-00191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao L., Mi T., Wu T., Chan P. Repetitive transcranial magnetic stimulation does not improve the sequence effect in freezing of gait [abstract] Mov Disord. 2019;34(suppl 2):S263–S264. doi: 10.1155/2019/2196195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brusa L, Versace V, Koch G, et al. Low frequency rTMS of the SMA transiently ameliorates peak-dose LID in Parkinson's disease. Clin Neurophysiol. 2006;117(9):1917–1921. doi: 10.1016/j.clinph.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 53.Koch G, Brusa L, Carrillo F, et al. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology. 2009;73(2):113–119. doi: 10.1212/WNL.0b013e3181ad5387. [DOI] [PubMed] [Google Scholar]
- 54.Dagan M, Herman T, Mirelman A, Giladi N, Hausdorff JM. The role of the prefrontal cortex in freezing of gait in Parkinson's disease: insights from a deep repetitive transcranial magnetic stimulation exploratory study. Exp Brain Res. 2017;235(8):2463–2472. doi: 10.1007/s00221-017-4981-9. [DOI] [PubMed] [Google Scholar]
- 55.Srovnalova H, Marecek R, Kubikova R, Rektorova I. The role of the right dorsolateral prefrontal cortex in the Tower of London task performance: repetitive transcranial magnetic stimulation study in patients with Parkinson's disease. Exp Brain Res. 2012;223(2):251–257. doi: 10.1007/s00221-012-3255-9. [DOI] [PubMed] [Google Scholar]
- 56.Srovnalova H, Marecek R, Rektorova I. The role of the inferior frontal gyri in cognitive processing of patients with Parkinson's disease: a pilot rTMS study. Movement Disord. 2011;26(8):1545–1548. doi: 10.1002/mds.23663. [DOI] [PubMed] [Google Scholar]
- 57.Benninger DH, Lomarev M, Wassermann EM, et al. Safety study of 50Hz repetitive transcranial magnetic stimulation in patients with Parkinson's disease. Clin Neurophysiol. 2009;120(4):809–815. doi: 10.1016/j.clinph.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Epstein CM, Evatt ML, Funk A, et al. An open study of repetitive transcranial magnetic stimulation in treatment-resistant depression with Parkinson's disease. Clin Neurophysiol. 2007;118(10):2189–2194. doi: 10.1016/j.clinph.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boggio PS, Fregni F, Bermpohl F, et al. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson's disease and concurrent depression. Movement Disord. 2005;20(9):1178–1184. doi: 10.1002/mds.20508. [DOI] [PubMed] [Google Scholar]
- 60.Jiang Y, Guo Z, McClure MA, He L, Mu Q. Effect of rTMS on Parkinson’s cognitive function: a systematic review and meta-analysis. Bmc Neurol. 2020;20(1):377. doi: 10.1186/s12883-020-01953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Begemann MJ, Brand BA, Ćurčić-Blake B, Aleman A, Sommer IE. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis. Psychol Med. 2020;50(15):2465–2486. doi: 10.1017/S0033291720003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lawrence BJ, Gasson N, Bucks RS, Troeung L, Loftus AM. Cognitive training and noninvasive brain stimulation for cognition in Parkinson's disease: a meta-analysis. Neurorehab Neural Re. 2017;31(7):597–608. doi: 10.1177/1545968317712468. [DOI] [PubMed] [Google Scholar]
- 63.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 64.Xie C, Chen J, Wang X, et al. Repetitive transcranial magnetic stimulation (rTMS) for the treatment of depression in Parkinson disease: a meta-analysis of randomized controlled clinical trials. Neurol Sci. 2015;36(10):1751–1761. doi: 10.1007/s10072-015-2345-4. [DOI] [PubMed] [Google Scholar]
- 65.Lesenskyj AM, Samples MP, Farmer JM, Maxwell CR. Treating refractory depression in Parkinson’s disease: a meta-analysis of transcranial magnetic stimulation. Transl Neurodegener. 2018;7(1):8. doi: 10.1186/s40035-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Randver R. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex to alleviate depression and cognitive impairment associated with Parkinson's disease: a review and clinical implications. J Neurol Sci. 2018;393:88–99. doi: 10.1016/j.jns.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 67.McClintock SM, Reti IM, Carpenter LL, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1):35–48. doi: 10.4088/JCP.16cs10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8(5):464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 69.Oltra J, Uribe C, Campabadal A, et al. Sex differences in brain and cognition in de novo Parkinson’s Disease. Front Aging Neurosci. 2022;13:791532. doi: 10.3389/fnagi.2021.791532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bohnen NI, Kotagal V, Müller MLTM, et al. Diabetes mellitus is independently associated with more severe cognitive impairment in Parkinson disease. Parkinsonism Relat D. 2014;20(12):1394–1398. doi: 10.1016/j.parkreldis.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun Y, Chang Y, Chen H, Su Y, Su H, Li C. Risk of Parkinson disease onset in patients with diabetes. Diabetes Care. 2012;35(5):1047–1049. doi: 10.2337/dc11-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ong M, Foo H, Chander RJ, et al. Influence of diabetes mellitus on longitudinal atrophy and cognition in Parkinson's disease. J Neurol Sci. 2017;377:122–126. doi: 10.1016/j.jns.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 73.Horsager J, Andersen KB, Knudsen K, et al. Brain-first versus body-first Parkinson's disease: a multimodal imaging case-control study. Brain. 2020;143(10):3077–3088. doi: 10.1093/brain/awaa238. [DOI] [PubMed] [Google Scholar]
- 74.Herrero Babiloni A, Bellemare A, Beetz G, et al. The effects of non-invasive brain stimulation on sleep disturbances among different neurological and neuropsychiatric conditions: a systematic review. Sleep Med Rev. 2021;55 doi: 10.1016/j.smrv.2020.101381. [DOI] [PubMed] [Google Scholar]
- 75.Huang Y, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 76.Di Lazzaro V, Profice P, Pilato F, Dileone M, Oliviero A, Ziemann U. The effects of motor cortex rTMS on corticospinal descending activity. Clin Neurophysiol. 2010;121(4):464–473. doi: 10.1016/j.clinph.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 77.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 78.Liu X, Liu H, Liu Z, et al. Transcranial direct current stimulation for parkinson’s disease: a systematic review and meta-analysis. Front Aging Neurosci. 2021;13:746797. doi: 10.3389/fnagi.2021.746797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.