Abstract
Study Objectives:
Poor sleep, including short sleep duration, is common among caregivers of persons with dementia. However, it is unclear whether poor sleep is consistent across both self-reported and objective measures of sleep in caregivers. This study aimed to test the role of caregiving status (caregivers vs noncaregivers) on the discrepancy between self-reported and objective sleep duration.
Methods:
This was a cross-sectional study. Study participants were community-dwelling caregivers of spouses with dementia (n = 122) and noncaregivers (n = 53). A sleep duration discrepancy index was created by subtracting objective sleep duration measured with 3 consecutive 24-hour periods of actigraphy from self-reported sleep duration measured with the Pittsburgh Sleep Quality Index. Covariates included participants’ demographic characteristics, depressive symptoms, positive and negative affects, personal mastery, and caregiving-role overload.
Results:
Caregivers showed a greater discrepancy in sleep duration than did noncaregivers (−0.46 hour vs 0.22 hour, respectively; P = .003). In a regression model, however, caregiving status was no longer associated with this sleep duration discrepancy, when covariates were accounted for. Higher positive affect was significantly associated with less sleep duration discrepancy (R2 = 11.3%, P = .014). The Sobel test of mediation showed that 26% of the effect of caregiving on this sleep discrepancy was attributable to caregivers with low positive affect.
Conclusions:
The findings suggest a potential mediating role of positive affect on the relationship between caregiving status and sleep duration discrepancy. As an aid for understanding the role of lower positive affect, use of actigraphy may help address sleep discrepancy in caregivers.
Citation:
Song Y, Moore RC, Jeste DV, et al. Discrepancy between self-reported and objective sleep duration among dementia caregivers and noncaregivers. J Clin Sleep Med. 2022;18(8):1945–1952.
Keywords: positive affect, negative affect, stress, well-being, actigraphy, insomnia, objective sleep, self-reported sleep
BRIEF SUMMARY
Current Knowledge/Study Rationale: Both objective and self-reported sleep measures show poor sleep, including shorter sleep duration, among caregivers of persons with dementia. However, the discrepancy in sleep duration as measured by actigraphy and self-report and the impact of potential mediators (positive and negative affects) on this discrepancy among caregivers are less known.
Study Impact: The combination of self-reported and objective sleep measures will help to inform the diagnosis and treatment strategies to improve sleep quality among caregivers with lack of positive affect. Concurrent assessment of positive affect during the treatment will also provide ancillary data to address potential sleep discrepancy among this vulnerable group.
INTRODUCTION
Poor sleep, whether determined by self-report or objective measures such as polysomnography (PSG) or actigraphy, is common among family caregivers of people with dementia (PwD). Between 44% and 92% of caregivers of PwD experience sleep disturbances, a range that is significantly higher than that among noncaregivers.1–5 Self-reported poor sleep has been measured with standardized questionnaires, such as Pittsburgh Sleep Questionnaire Index (PSQI),6 and/or with a sleep diary. Poor sleep reported by caregivers includes difficulty falling and staying asleep,1,5 poor sleep quality,4,7,8 frequent nighttime awakenings,9 long duration of time awake after sleep onset,10,11 and short nighttime sleep duration.4,8 Objectively measured poor sleep using PSG or actigraphy includes short sleep duration,11 low sleep efficiency (ie, percentage of time spent asleep while in bed trying to sleep at nighttime),11,12 and a long time spent awake during the nighttime.13,14
Poor sleep among caregivers of PwD is associated with higher levels of stress and fatigue, lower positive affect, and poorer perceived quality of life.1 It is also associated with elevated levels of inflammatory markers such as interleukin-6 and C-reactive protein,10 which are known risk factors for cardiovascular disease.
These significant impacts of poor sleep on caregivers’ health outcomes suggest a critical need for sleep assessment using both self-reported and objective measures to better understand the nuances of the sleep disturbances. Although these caregivers may sleep for adequate amounts of time (eg, 7–8 hours) when assessed with objective sleep measures, it is critical to address their perceived sleep (underestimated or overestimated) for several reasons. First, older adults with poor cognitive function and functional disability are more likely to present greater sleep measurement discrepancy (ie, discordance between self-reported and objective sleep measures).15 This is a more serious problem for caregivers who are older and perceive their sleep as being deficient, as this can ultimately affect not only their personal health but also the quality of care they provide to their PwD. Second, misperception of a sleep deficit may facilitate excessive concerns and distress about sleep and can contribute to greater anxiety and arousal at nighttime, thus increasing the risk of developing clinical insomnia, where both self-reported and objective sleep is deprived.16 Third, self-reported recall of sleep can be affected by feelings or mood present at the time of recall.16 Caregivers commonly experience psychological symptoms such as depression, stress, and negative affect.17 If these symptoms are present at the moment of recall, they can influence the accuracy of reported sleep.
Unfortunately, studies show only moderate association between actigraphy-measured and self-reported sleep durations.18 Potential mediating factors seem to play a role in the concordance between these 2 measures of sleep. Studies have shown that patients with insomnia tend to underestimate their perceived sleep duration in comparison with objectively measured sleep duration.19,20 Moreover, having higher depressive symptoms, poor self-reported health status, and being non-White is associated with lower correlations between actigraphy-measured and self-reported sleep duration.21,22 Both positive and negative affects may also contribute to differences in sleep measures, given the significant relationship between affect and actigraphy-measured and/or self-reported sleep characteristics in caregivers7,14,23 and other groups.24,25
Taken together, addressing the discrepancy between self-reported and objectively measured sleep is a critical part of initial sleep management, so that a health care provider can further examine if sleep is misperceived or if other factors are mediating the discrepancy. Yet, such sleep discrepancy is not fully explored in caregivers of PwD. Given the heterogeneity of caregiving for PwD and the potential impact of caregiving on caregivers’ sleep, it is important to explore sleep discrepancies and related factors. This paper focuses on discrepancy between self-reported and objectively measured sleep durations among spousal caregivers. We hypothesized that caregivers of PwD will show a greater discrepancy in sleep duration (ie, more underestimations of self-reported sleep) than noncaregivers. We also hypothesized that greater discrepancy of sleep duration among the caregivers will remain significant compared to noncaregivers after adjusting for such characteristics of participants as race/ethnicity, depression, and positive affect. As a secondary analysis, we explored potential mediating effects of the negative and positive affect covariates, given the evidence of a relationship between those affects and sleep addressed previously.7,14,23–25 Therefore, we hypothesized that both negative and positive affects mediate the relationship between caregiving status and sleep duration discrepancy.
METHODS
Study design and participants
This study used a cross-sectional design and baseline data from the University of California San Diego (UCSD) Alzheimer’s Caregiver Study, which aimed to investigate mental and physical health among spousal Alzheimer’s caregivers in comparison to noncaregivers in a longitudinal study design.26 Participants were eligible for the study if they were at least 55 years old and married to and resided with their spouse in community settings in the San Diego, CA area. Caregivers were required to be providing primary care for their spouse with a physician-based chart diagnosis of dementia (eg, Alzheimer disease). Potential participants (both caregivers and noncaregivers) were excluded from the study if they had cognitive impairment (ie, Mini-Mental State Examination score < 27),27 or other medical factors that might, independently of caregiving status, affect outcomes of interest (eg, biomarkers of inflammation or cardiovascular risk) in the parent study. Examples of exclusions included current cancer treatment, severe hypertension (ie, blood pressure > 200/120 mm Hg), and treatment with steroids, nonselective beta blockers, or oral anticoagulants. If noncaregivers provided care for a spouse with any type of chronic disease, they were excluded from the study. Noncaregivers were recruited from senior centers, senior health fairs, and referrals from the participants already enrolled in our study. Caregivers were recruited from local caregiver support groups, community health fairs, and referrals from local caregiver agencies and other participants. All study processes and protocols were approved by the UCSD institutional review board. All study participants provided informed written consent. Our final analytic sample size was 175 participants (n = 122 caregivers and n = 53 noncaregivers).
Measures
Sociodemographic data collected from participants included age, sex, caregiver status, race/ethnicity, years of education, years married, and employment status. Self-reported comorbidities of participants included history of hypertension and diabetes. Perceived health status was rated from poor to excellent. Body mass index, history of taking antidepressant medications over the past 30 days (yes/no), and duration of daily moderate exercise were also asked.
The 10-item Center for Epidemiologic Studies–Depression (CESD) scale was used to measure depressive symptoms.28 It asks about the frequency of depressive symptoms that occurred during the previous week, with a higher score indicating worse depressive symptoms. The Epworth Sleepiness Scale was used to assess daytime sleepiness by asking about the chance of dozing off or falling asleep in 8 different situations.29 A high score indicates a greater degree of daytime sleepiness. Sense of mastery was measured with the Personal Mastery Scale,30 which asks participants about the level of agreement or disagreement with 7 statements (eg, “There is really no way I can solve some of the problems I have.”). Response choices range from 0 (strongly disagree) to 3 (strongly agree). A higher score indicates better perceived control. Role-related stress was measured with the Pearlin Role Overload scale.31 It asks participants to rate their energy level in 4 situations (eg, “You are exhausted when you go to bed at night.”), using a 4-point Likert scale ranging from 0 (not at all) to 3 (completely).
Positive and negative affects were measured with the Positive and Negative Affect Schedule (PANAS).32 It consists of 20 mood adjectives (10 items of positive affect and 10 items of negative affect) from the original PANAS. Participants rated each item on a 5-point scale ranging from 1 (very slightly or not at all) to 5 (extremely). Total scores for each component of affect were calculated by summing each item. The present study used the total scores of positive and negative affects.
Sleep
The PSQI was used to assess self-reported sleep quality during the past week.6 It consists of 19 items, which measure 7 components: sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. A global score > 5 yields high sensitivity (89.6%) and specificity (86.5%) in distinguishing good and poor sleepers (kappa = 0.75).6 We used 1 item in the PSQI that asks about sleep duration (“During the past week, how many hours of actual sleep did you get at night?”).
Actigraphy was used to measure objective sleep. Participants wore the SleepWatch-O actigraph (Ambulatory Monitoring, Inc., Ardsley, NY) on the nondominant wrist for 3 consecutive days and nights, which is consistent with current clinical and research guidelines of a minimum of 72 hours of actigraphy.33 The actigraph is a small, unobtrusive, watch-sized device useful in longitudinal and naturalistic assessment of sleep-wake patterns. Criterion validity is well-established, with correlations between actigraphy and PSG reported for total sleep time (r = .81 to .91) and for percent sleep (r = .61 to .78) in older adults.34 A sleep duration measured with an actigraph was defined as the total time of scored sleep between sleep onset time and sleep end time.
We defined discrepancy of sleep duration by subtracting total sleep time measured with actigraphy (objective sleep duration) from total sleep time reported on the PSQI (self-reported sleep duration). Therefore, a negative value of this discrepancy variable would suggest underestimated self-reported sleep, whereas a positive value would indicate overestimated self-reported sleep. Our approach to calculating the sleep duration discrepancy index has been used in prior studies.15,20
Data analysis
Descriptive statistics were calculated for all variables. Pearson correlations were calculated to test the association between discrepancy of sleep duration and other variables (age; the CESD, excluding the sleep item; personal mastery; positive affect; negative affect). A Student’s t test was calculated to test the association between sleep discrepancy and caregiving status (yes/no). Results of this bivariate testing that yielded P-values at ≤ .20 were used to select potential covariates for inclusion in a multiple regression model. Then we ran a multiple regression model with sleep duration discrepancy as a dependent variable, caregiving status (yes/no) as an independent variable, and other selected covariates. We also tested the mediating effect of a covariate (specifically, negative and positive affect) on sleep duration discrepancy and caregiving status using a Sobel test.35 For these statistical tests, P < .05 was considered statistically significant. Analyses were conducted using Stata version 15 (Stata Corp., College Station, TX).
RESULTS
Characteristics of study participants
Mean age of the study participants was 74.63 years (standard deviation 7.48). Approximately 70% were women. Participant characteristics by caregiving status are shown in Table 1. Caregivers were more likely to be White and perceive their health to be worse than noncaregivers. Caregivers spent less time on moderate exercise, reported more depressive symptoms, higher levels of role-related stress and negative affect, less sense of mastery, and lower levels of positive affect than did noncaregivers. Caregivers had a significantly higher PSQI total score than did noncaregivers (6.62 ± 3.59 vs 4.47 ± 2.62, respectively, P = .001), whereas actigraphy-assessed sleep efficiency was similar between the 2 groups (87.2% vs 87.9%, respectively). Seventy-nine percent of participants who underestimated sleep duration (negative value of sleep discrepancy) had a PSQI total score > 5. No differences were found in either self-reported or objective sleep duration between caregivers and noncaregivers.
Table 1.
Study participant characteristics (n = 175).
| Characteristics | Mean (SD) or n (%) | t or χ2 | df | P | |
|---|---|---|---|---|---|
| Caregivers (n = 122) | Noncaregivers (n = 53) | ||||
| Age, mean (SD) | 74.31 (8.01) | 75.37 (6.07) | 0.86 | 173 | .387 |
| Female, n (%) | 86 (70.49%) | 35 (66.04%) | 0.34 | 1 | .558 |
| Race/ethnicity | |||||
| Hispanic, n (%) | 10 (8.20%) | 7 (13.20%) | 1.06 | 1 | .304 |
| White, n (%) | 116 (95.08%) | 45 (84.90%) | 5.20 | 1 | .023 |
| Years of education | 15.12 (3.04) | 15.55 (3.07) | 0.85 | 173 | .398 |
| Years married | 42.88 (16.64) | 43.15 (16.42) | 0.10 | 173 | .923 |
| Employed, n (%) | 20 (16.39%) | 6 (11.32%) | 0.75 | 1 | .386 |
| Body mass index (kg/m2) | 26.55 (4.74) | 26.31 (6.21) | −0.28 | 173 | .783 |
| Comorbidities | |||||
| History of hypertension | 69 (56.56%) | 23 (43.30%) | 2.57 | 1 | .109 |
| History of diabetes† | 15 (12.30%) | 2 (3.77%) | 3.06 | 1 | .064 |
| Taking antidepressant | 32 (26.23%) | 12 (22.64%) | 0.25 | 1 | .615 |
| Self-reported health status† | 24.51 | 4 | < .001 | ||
| Poor | 4 (3.28%) | 1 (1.89%) | |||
| Fair | 13 (10.66%) | 1 (1.89%) | |||
| Good | 48 (39.34%) | 8 (15%) | |||
| Very good | 42 (34.43%) | 22 (41.51%) | |||
| Excellent | 15 (12.30%) | 21 (39.62%) | |||
| ≥ 30 minutes of moderate exercise/d | 66 (54.10%) | 40 (75.47%) | 7.07 | 1 | .008 |
| CESD, excluding the sleep item | 8.99 (3.88) | 7.04 (2.24) | −3.42 | 173 | < .001 |
| ESS total score | 5.58 (3.96) | 5.21 (4.90) | −0.53 | 173 | .594 |
| Personal Mastery Scale score | 11.49 (3.32) | 15.15 (3.82) | 6.39 | 173 | < .001 |
| Pearlin Role Overload score | 5.14 (3.19) | 1.43 (1.99) | −7.82 | 173 | < .001 |
| PANAS, Negative affect | 17.89 (6.10) | 13.57 (5.34) | −4.47 | 173 | < .001 |
| PANAS, Positive affect | 31.81 (7.50) | 36.72 (6.87) | 4.08 | 173 | < .001 |
| PSQI total score | 6.62 (3.59) | 4.47 (2.62) | −3.93 | 173 | < .001 |
| PSQI total sleep time, hour | 6.84 (1.37) | 7.15 (1.05) | 1.48 | 173 | .140 |
| Actigraphy total sleep time, hour | 7.27 (1.13) | 6.97 (0.91) | −1.71 | 164 | .089 |
†Fisher’s exact test. CESD = Center for Epidemiological Studies–Depression scale, ESS = Epworth Sleepiness Scale, PANAS = Positive and Negative Affect Schedule, PSQI = Pittsburgh Sleep Quality Index, SD = standard deviation.
Association between caregiving status and discrepancy of sleep duration
As shown in Table 2, sleep duration discrepancy was significantly different between caregivers and noncaregivers. Based on actigraphy scores, caregivers significantly underestimated their sleep duration, whereas noncaregivers overestimated their sleep duration (–0.46 hour vs 0.22 hour, respectively; P = .003). In a multiple regression model (Table 3), however, caregiving status was no longer significantly associated with discrepancy of sleep duration when adjusting for covariates (ie, White; the CESD, excluding the sleep item; mastery; positive affect; and negative affect). Positive affect was the only significant factor to predict sleep duration discrepancy in the model (P = .037).
Table 2.
Bivariate Pearson Correlations between participant characteristics and sleep duration discrepancy (n = 166).
| Discrepancy, Mean (SD) | P | |
|---|---|---|
| Sex | .215 | |
| Female (n = 113) | −0.34 (1.39) | |
| Male (n = 53) | −0.06 (1.21) | |
| Caregiving status | .003 | |
| Caregiver (n = 115) | −0.46 (1.36) | |
| Noncaregiver (n = 51) | 0.22 (1.18) | |
| Ethnicity | .384 | |
| Hispanic (n = 16) | −0.53 (1.90) | |
| Non-Hispanic (n = 150) | −0.22 (1.27) | |
| Race | .163 | |
| White (n = 153) | −0.29 (1.34) | |
| Others (n = 13) | 0.25 (1.23) | |
| Employment status | .470 | |
| Employed (n = 26) | −0.07 (1.38) | |
| Unemployed/retired (n = 140) | −0.28 (1.33) | |
| History of hypertension | .798 | |
| Yes (n = 88) | −0.22 (1.34) | |
| No (n = 78) | −0.28 (1.34) | |
| History of diabetes | .550 | |
| Yes (n = 17) | −0.43 (1.28) | |
| No (n = 149) | −0.23 (1.35) | |
| Taking antidepressant | .340 | |
| Yes (n = 43) | −0.40 (1.32) | |
| No (n = 123) | −0.20 (1.34) | |
| Moderate exercise/d | .784 | |
| < 30 minutes/d (n = 66) | −0.28 (1.32) | |
| ≥ 30 minutes/d (n = 100) | −0.23 (1.35) | |
| Pearson Correlation Coefficient | ||
| Age | −0.013 | .865 |
| Years of education | 0.083 | .287 |
| Years married | 0.042 | .593 |
| Body mass index | 0.002 | .976 |
| Self-reported health status | 0.091 | .245 |
| CESD, excluding the sleep item | −0.130 | .095 |
| ESS total score | 0.062 | .429 |
| Personal Mastery Scale score | 0.194 | .012 |
| Pearlin Role Overload score | −0.198 | .011 |
| PANAS, Negative affect | −0.123 | .115 |
| PANAS, Positive affect | 0.254 | .001 |
CESD = Center for Epidemiological Studies–Depression scale, ESS = Epworth Sleepiness Scale, PANAS = Positive and Negative Affect Schedule, SD = standard deviation.
Table 3.
A multiple regression model predicting sleep duration discrepancy (n = 166).
| Independent Variables | Discrepancy, Beta (95% CI) | P |
|---|---|---|
| Being a caregiver | −0.35 (−0.88, 0.18) | .198 |
| White vs others | −0.40 (−1.17, 0.36) | .299 |
| Personal Mastery Scale score | 0.014 (−0.06, 0.08) | .690 |
| Pearlin Role Overload score | −0.03 (−0.11, 0.05) | .499 |
| CESD score† | −0.02 (−0.09, 0.06) | .655 |
| PANAS, Negative affect | 0.01 (−0.03, 0.06) | .588 |
| PANAS, Positive affect | 0.03 (0.00, 0.06) | .037 |
F (7, 158) = 2.58; P = .015, R2 = 10.3%; Adjusted R2 = 6.3%. †Excluding the sleep item. CESD = Center for Epidemiological Studies–Depression scale, CI = confidence interval, PANAS = Positive and Negative Affect Schedule.
A mediating effect of positive affect on sleep duration discrepancy and caregiving status
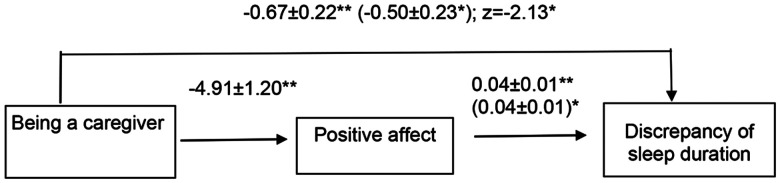
We ran a Sobel test to determine whether there was an indirect effect of caregiving status on the sleep duration discrepancy by positive affect. The result showed a significant difference from zero (z = −2.13, P = .033), which confirmed that positive affect partially mediated the relationship between caregiving status and discrepancy of sleep duration. Using the formula provided by MacKinnon and Dwyer,36 we determined that approximately 26% of the effect of caregiving on underestimated sleep is attributable to caregivers with low positive affect. The mediational model is shown in Figure 1.
Figure 1. Mediational model for association between caregiver status and discrepancy of sleep duration.
*P < .05; **P < .01. Values are presented as standardized coefficient ± SE. Values outside parentheses are zero-order betas. Values inside parentheses are partial regression coefficients when both caregiving status and positive affect are included in the model. z = Sobel test.
DISCUSSION
The current study evaluated the cross-sectional relationship between caregiving status and discrepancy between self-reported and objective sleep durations at night. We also tested the mediating role of positive affect and negative affect in the relationship between caregiving status and discrepancy of sleep duration. Our hypotheses were partially supported, such that being a caregiver was significantly associated with more underestimated self-reported sleep duration. The group difference in discrepancy of sleep duration may be the result of poorly perceived sleep quality among the caregivers (PSQI total score > 5, on average). It also aligns with existing knowledge of the higher degree of sleep discrepancy among patients with insomnia.19,37 In a large, population-based study, sleep misperception was prevalent in insomnia patients with objectively measured normal sleep duration but not in those with short sleep duration.37 Their sleep misperception was significantly associated with depressive, anxious-ruminative personality traits, and poor coping resources.
In our study, the relationship between sleep duration discrepancy and caregiving status was no longer significant when accounting for the following covariates: participants’ race/ethnicity, mastery, role overload, depressive symptoms, and positive and negative affects. Additionally, we found that low positive affect, in part, mediated the relationship between caregiving status and discrepancy of sleep duration.
Our finding on the contribution of positive affect to sleep discrepancy among caregivers is in concordance with prior studies of sleep and positive affect. In a cross-sectional study, caregivers with high levels of positive affect reported fewer sleep problems than those with low levels of positive affect; however, this association was not statistically significant among noncaregivers.7 In our previous work, increased levels of positive affect were longitudinally associated with better self-reported sleep quality (measured with PSQI) among spousal caregivers of PwD. This relationship remained significant when controlling for negative affect, female sex, caregiver health conditions, caregiving-related burden, and dementia-related behaviors. However, there was no significant relationship between actigraphy-measured sleep parameters (sleep duration, sleep efficiency, and wake after sleep onset) and positive affect.14 In another study of caregivers of PwD, self-reported and objective total wake time and perceived sleep quality were not significantly associated with positive affect at both within- (day-to-day) and between-person levels. Instead, lower self-reported total wake time and higher perceived sleep quality significantly predicted lower negative affect (accounting for 43% of day-to-day variance and 21% of average variance in negative affect) among this group.23 Nevertheless, none of the studies investigated discrepancy between self-reported and objective sleep duration among caregivers of PwD. Similar results were found in prior studies of other groups (eg, noncaregivers). For example, among older adults, using 14 days of data assessment, positive affect was associated with self-reported sleep quality but not with objective sleep quality.24 Findings on the relationship between positive affect and sleep duration were inconsistent across studies of other groups.38,39
Our study did not explicitly test how low levels of positive affect are different from negative affect. Some studies addressed whether positive affect and negative affect may not reflect opposite aspects of emotion. Instead, they may play independent or different roles given a particular situation or moment.40 Future studies are needed to investigate the role of negative affect or the mutual effect of positive and negative affects in this population, for example, by measuring both types of affect multiple times during the day using ecological momentary assessment.
There are a few possible underlying mechanisms for the findings. First, stressful caregiving situations may lower positive affect, which may bias retrieval of memory for sleep duration.16 Second, memory of sleep may be biased based on emotional feelings present at the time of recall.16 Third, cognitive-emotional hyperarousal at bedtime, combined with rumination during presleep and coping strategies focusing on less positive affect,41 may have contributed to misperception of sleep by creating a tendency toward interpreting sleep as wakefulness. Last, it is possible that objective sleep duration measured with actigraphy may have been simply overestimated. Studies have shown that actigraphy overestimates sleep duration15,42; however, it provides relatively unobtrusive sleep monitoring over time and is largely acceptable to patients with sleep disorders.43
Large cohort studies that have compared sleep duration have used different instruments for measurement. For example, in a study of middle-to-old adults, self-reported sleep duration (measured with the PSQI) was only modestly associated with the sleep duration estimated by in-home PSG, whereas the correlations of PSG with actigraphy-assessed sleep duration was stronger.21 In that study, PSG-measured sleep duration was shorter than self-reported sleep duration estimates but longer compared with actigraphy-measured sleep duration. PSQI-assessed sleep duration was almost an hour longer than actigraphy-measured sleep duration,21 which was similar in another study.22 Further studies are needed to compare sleep duration across multiple measures, including PSG, actigraphy, PSQI, and sleep diaries in caregivers of PwD.
In our study, daytime sleepiness measured with the Epworth Sleepiness Scale was not different between caregivers and noncaregivers. There were no significant differences between daytime sleepiness and actigraphy-measured daytime sleep durations, which suggest a possible discrepancy between perceived daytime sleepiness and objectively measured daytime duration. Additionally, less daytime sleepiness was significantly associated with both increased PSQI- and actigraphy-measured sleep durations but not with nighttime sleep duration discrepancy. Although increased sleep duration at night can reduce the self-reported feeling of sleepiness during the daytime, future studies exploring the relationship between self-reported-objective sleep discrepancy and daytime sleepiness or other symptoms of daytime impairment (eg, fatigue) among caregivers are needed.
We did not find a notable role of negative affect on sleep duration discrepancy. This may be explained by low levels of negative affect among both spousal caregivers and noncaregivers, and the relationship between negative affect and positive affect was only modestly significant in our study. Another possible reason may be in part that caregiving appraisal among spousal caregivers is different from that of adult-child caregivers. Spousal caregivers may accept their commitment to caregiving more readily than adult-child caregivers.44 In 1 study, caregiving satisfaction was a significant factor in positive affect among spousal caregivers but not among adult-child caregivers.44 This suggests a potential role of positive affect on spousal caregiving appraisal among spousal caregivers. Some studies reported that higher levels of burden were significantly associated with worse depressive symptoms among adult-child caregivers than among spousal caregivers of PwD.45 Another study also showed that adult-child caregivers reported more negative perceptions of the quality of life of PwD than did spousal caregivers.46 Such negative caregiving experience and perceptions regarding PwD may increase levels of negative affect among adult-child caregivers. Future studies are warranted to investigate sleep discrepancy in relationship to affect among different types of caregivers (eg, spousal caregivers, adult-child caregivers).
Findings of this study have clinical implications. Caregivers with higher levels of positive affect more accurately reported their sleep compared to objectively measured sleep. In other words, caregivers with lower levels of positive affect may benefit from objective sleep assessments due to their potential tendency to inaccurately report their sleep. The accuracy of estimations of self-reported sleep duration increased in accordance with objective sleep durations when participants experienced higher levels of positive affect. This suggests that positive affect may play a role as a protective factor on misperception of sleep, particularly among spousal caregivers. While use of objective sleep measures is not required for routine insomnia diagnosis, using both self-reported and objective sleep tools may help assess and monitor treatment response when caregivers complain of poor sleep. Studies have shown significant improvement in sleep discrepancy among insomnia patients after they received cognitive behavioral therapy for insomnia (CBTI), suggesting that underestimated self-reported sleep can be modified through CBTI and may be related to improvement in poor sleep.19 Longitudinal sleep monitoring along with concurrent assessment of positive and negative affects can also guide optimal treatment strategies, for example, through the addition of approaches to enhance positive affect to cognitive behavioral therapy–based recommendations.
Nevertheless, this study has several limitations. Most of our study participants were White and their education level was relatively high; therefore, both perceived sleep and levels of affect may be different in other racial/ethnic groups or among those with a lower level of education. Given the eligibility criteria of our study, the study participants were relatively healthy. Thus, caregivers and noncaregivers with more severe medical conditions may represent their sleep or affect differently. We cannot avoid the possibility that noncaregivers may have provided their spouses or other family members with care as well, for old age or other reasons. Although both the sleep questionnaire (PSQI) and actigraphy were collected over the same 3-day period, the time points reflecting sleep were different between the 2 instruments—ie, our study participants were asked about self-reported sleep (measured with the PSQI) retrospectively, based on the past week, whereas objective sleep was measured prospectively for 3 nights. Although caregiver participants showed poor global sleep quality (mean PSQI total score > 5), we did not assess insomnia disorder that was clinically diagnosed based on the Diagnostic and Statistical Manual of Mental Disorders, fifth edition,47 and/or International Classification of Sleep Disorders, third edition.48 Other sleep disorders, such as sleep apnea, were not assessed in the present study. Given the evidence of greater sleep discrepancy among patients with sleep disorders,19,37 further assessment of participants’ clinical criteria for sleep disorder may help to identify clinically relevant at-risk groups, particularly among caregivers with underestimated sleep duration. Moreover, it is possible that caregivers diagnosed with concurrent insomnia and sleep apnea may underestimate their sleep duration at a greater level49 and therefore experience higher levels of negative affect. Our study did not measure night-to-night variability in sleep discrepancy, which has shown a significant relationship with insomnia symptoms in older adults in prior studies.50 Particularly, sleep monitoring containing both days of full-time and part-time caregiving (eg, days when care recipients were in an adult day care program) would have elucidated potential variability in sleep discrepancy within and between the groups (ie, caregivers vs noncaregivers). Night-to-night sleep measures, along with simultaneous assessment of positive and negative affects, may provide better information about sleep discrepancy linked to fluctuation of caregiver affect. Sample sizes of the caregiver and noncaregiver groups are considerably different in our study. Thus, the control group might not be as representative of the general population as the caregiver group is. We cannot avoid type 1 error on testing factors associated with sleep duration discrepancy. Additionally, we did not measure other covariates that may play a critical role in sleep discrepancy. For example, in a study of patients with major depression, underestimated self-reported sleep duration was significantly associated with a higher percentage of N1 stage of sleep, more time awake after sleep onset, and lower percentage of slow-wave sleep.51 This may suggest that sleep discrepancy may be associated with certain medical or psychiatric conditions and that assessing sleep duration using only 1 measure may bias true association.
In summary, caregivers of PwD perceived their total sleep duration as being less than when it was measured objectively, compared to noncaregivers. Measuring both self-reported and objective sleep duration and addressing its discrepancy are clinically important for caregivers with poor sleep, particularly when they have low levels of positive affect. Future studies are needed to understand the potential mechanisms underlying sleep discrepancy among caregivers. These may provide us with practical guidance on optimal measures of sleep in this vulnerable group. Further studies are warranted to investigate sleep discrepancy in relationship to both caregiving experiences (eg, nighttime caregiving responsibilities awakening caregivers, positive and negative caregiving aspects) and factors related to care recipients, such as dementia-related problematic behaviors.
DISCLOSURE STATEMENT
All authors read and approved the final manuscript and contributed to critical revision of the manuscript for important intellectual content. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs, National Institutes of Health, or the US Government. Work for this study was supported by the National Institute on Aging (R01AG015301, R01AG031090, I. Grant; K23AG055668, Y. Song). R.C. Moore’s effort was supported by the National Institute on Aging (R01AG062387; R01AG061066) and the Shiley-Marcos Alzheimer’s Disease Research Center (ADRC). B.T. Mausbach’s effort was supported by the National Institute on Aging (R01AG061941, RF1AG015301). Additional support was provided by the VA Greater Los Angeles Healthcare System, Geriatric Research, Education and Clinical Center (Y. Song) and Sam and Rose Stein Institute for Research on Aging (D. Jeste). Dr. Moore is a cofounder of KeyWise AI, Inc., and a consultant for NeuroUX. The terms of these arrangements have been reviewed and approved by UC San Diego in accordance with its conflict-of-interest policies. Dr. Ancoli-Israel is a consultant for Eisai Co., Ltd., Idorsia, and Merck. The other authors report no conflicts of interest.
ABBREVIATIONS
- CESD
Center for Epidemiologic Studies–Depression
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- PwD
people/person with dementia
REFERENCES
- 1. Cupidi C, Realmuto S, Lo Coco G, et al . Sleep quality in caregivers of patients with Alzheimer’s disease and Parkinson’s disease and its relationship to quality of life . Int Psychogeriatr. 2012. ; 24 ( 11 ): 1827 – 1835 . [DOI] [PubMed] [Google Scholar]
- 2. Fonareva I, Amen AM, Zajdel DP, Ellingson RM, Oken BS . Assessing sleep architecture in dementia caregivers at home using an ambulatory polysomnographic system . J Geriatr Psychiatry Neurol. 2011. ; 24 ( 1 ): 50 – 59 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McKibbin CL, Ancoli-Israel S, Dimsdale J, et al . Sleep in spousal caregivers of people with Alzheimer’s disease . Sleep. 2005. ; 28 ( 10 ): 1245 – 1250 . [DOI] [PubMed] [Google Scholar]
- 4. Peng HL, Lorenz RA, Chang YP . Factors associated with sleep in family caregivers of individuals with dementia . Perspect Psychiatr Care. 2019. ; 55 ( 1 ): 95 – 102 . [DOI] [PubMed] [Google Scholar]
- 5. Flaskerud JH, Carter PA, Lee P . Distressing emotions in female caregivers of people with AIDS, age-related dementias, and advanced-stage cancers . Perspect Psychiatr Care. 2000. ; 36 ( 4 ): 121 – 130 . [DOI] [PubMed] [Google Scholar]
- 6. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research . Psychiatry Res. 1989. ; 28 ( 2 ): 193 – 213 . [DOI] [PubMed] [Google Scholar]
- 7. Fredman L, Gordon SA, Heeren T, Stuver SO . Positive affect is associated with fewer sleep problems in older caregivers but not noncaregivers . Gerontologist. 2014. ; 54 ( 4 ): 559 – 569 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simpson C, Carter P . Dementia behavioural and psychiatric symptoms: effect on caregiver’s sleep . J Clin Nurs. 2013. ; 22 ( 21–22 ): 3042 – 3052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Willette-Murphy K, Todero C, Yeaworth R . Mental health and sleep of older wife caregivers for spouses with Alzheimer’s disease and related disorders . Issues Ment Health Nurs. 2006. ; 27 ( 8 ): 837 – 852 . [DOI] [PubMed] [Google Scholar]
- 10. von Känel R, Ancoli-Israel S, Dimsdale JE, et al . Sleep and biomarkers of atherosclerosis in elderly Alzheimer caregivers and controls . Gerontology. 2010. ; 56 ( 1 ): 41 – 50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mausbach BT, Ancoli-Israel S, von Känel R, et al . Sleep disturbance, norepinephrine, and D-dimer are all related in elderly caregivers of people with Alzheimer disease . Sleep. 2006. ; 29 ( 10 ): 1347 – 1352 . [DOI] [PubMed] [Google Scholar]
- 12. Figueiro MG, Hunter CM, Higgins P, et al . Tailored lighting intervention for persons with dementia and caregivers living at home . Sleep Health. 2015. ; 1 ( 4 ): 322 – 330 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spira AP, Friedman L, Beaudreau SA, et al . Sleep and physical functioning in family caregivers of older adults with memory impairment . Int Psychogeriatr. 2010. ; 22 ( 2 ): 306 – 311 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. von Känel R, Mausbach BT, Ancoli-Israel S, et al . Positive affect and sleep in spousal Alzheimer caregivers: a longitudinal study . Behav Sleep Med. 2014. ; 12 ( 5 ): 358 – 372 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Den Berg JF, Van Rooij FJ, Vos H, et al . Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons . J Sleep Res. 2008. ; 17 ( 3 ): 295 – 302 . [DOI] [PubMed] [Google Scholar]
- 16. Harvey AG, Tang NK . (Mis)perception of sleep in insomnia: a puzzle and a resolution . Psychol Bull. 2012. ; 138 ( 1 ): 77 – 101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sheehan OC, Haley WE, Howard VJ, Huang J, Rhodes JD, Roth DL . Stress, burden, and well-being in dementia and nondementia caregivers: insights from the Caregiving Transitions Study . Gerontologist. 2021. ; 61 ( 5 ): 670 – 679 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cespedes EM, Hu FB, Redline S, et al . Comparison of self-reported sleep duration with actigraphy: results from the Hispanic Community Health Study/Study of Latinos Sueño Ancillary Study . Am J Epidemiol. 2016. ; 183 ( 6 ): 561 – 573 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kay DB, Buysse DJ, Germain A, Hall M, Monk TH . Subjective-objective sleep discrepancy among older adults: associations with insomnia diagnosis and insomnia treatment . J Sleep Res. 2015. ; 24 ( 1 ): 32 – 39 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scarlett S, Nolan HN, Kenny RA, O’Connell MDL . Discrepancies in self-reported and actigraphy-based sleep duration are associated with self-reported insomnia symptoms in community-dwelling older adults . Sleep Health. 2021. ; 7 ( 1 ): 83 – 92 . [DOI] [PubMed] [Google Scholar]
- 21. Matthews KA, Patel SR, Pantesco EJ, et al . Similarities and differences in estimates of sleep duration by polysomnography, actigraphy, diary, and self-reported habitual sleep in a community sample . Sleep Health. 2018. ; 4 ( 1 ): 96 – 103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ . Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008. ; 19 ( 6 ): 838 – 845 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCrae CS, Dzierzewski JM, McNamara JP, Vatthauer KE, Roth AJ, Rowe MA . Changes in sleep predict changes in affect in older caregivers of individuals with Alzheimer’s dementia: a multilevel model approach . J Gerontol B Psychol Sci Soc Sci. 2016. ; 71 ( 3 ): 458 – 462 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCrae CS, McNamara JP, Rowe MA, et al . Sleep and affect in older adults: using multilevel modeling to examine daily associations . J Sleep Res. 2008. ; 17 ( 1 ): 42 – 53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu J, Zhu L, Liu C . Sleep quality and self-control: the mediating roles of positive and negative affects . Front Psychol. 2020. ; 11 : 607548 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. von Känel R, Mills PJ, Mausbach BT, et al . Effect of Alzheimer caregiving on circulating levels of C-reactive protein and other biomarkers relevant to cardiovascular disease risk: a longitudinal study . Gerontology. 2012. ; 58 ( 4 ): 354 – 365 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Folstein MF, Folstein SE, McHugh PR . “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician . J Psychiatr Res. 1975. ; 12 ( 3 ): 189 – 198 . [DOI] [PubMed] [Google Scholar]
- 28. Andresen EM, Malmgren JA, Carter WB, Patrick DL . Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) . Am J Prev Med. 1994. ; 10 ( 2 ): 77 – 84 . [PubMed] [Google Scholar]
- 29. Johns MW . A new method for measuring daytime sleepiness: the Epworth sleepiness scale . Sleep. 1991. ; 14 ( 6 ): 540 – 545 . [DOI] [PubMed] [Google Scholar]
- 30. Pearlin LI, Schooler C . The structure of coping . J Health Soc Behav. 1978. ; 19 ( 1 ): 2 – 21 . [PubMed] [Google Scholar]
- 31. Pearlin LI, Mullan JT, Semple SJ, Skaff MM . Caregiving and the stress process: an overview of concepts and their measures . Gerontologist. 1990. ; 30 ( 5 ): 583 – 594 . [DOI] [PubMed] [Google Scholar]
- 32. Watson D, Clark LA, Tellegen A . Development and validation of brief measures of positive and negative affect: the PANAS scales . J Pers Soc Psychol. 1988. ; 54 ( 6 ): 1063 – 1070 . [DOI] [PubMed] [Google Scholar]
- 33. Smith MT, McCrae CS, Cheung J, et al . Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine Clinical Practice Guideline . J Clin Sleep Med. 2018. ; 14 ( 7 ): 1231 – 1237 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ancoli-Israel S, Clopton P, Klauber MR, Fell R, Mason W . Use of wrist activity for monitoring sleep/wake in demented nursing-home patients . Sleep. 1997. ; 20 ( 1 ): 24 – 27 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sobel ME . Asymptotic Confidence Intervals for Indirect Effects in Structural Equation Models . In: Leinhardt S , ed. Sociological Methodology. Washington, DC: : American Sociological Association; ; 1982. : 290 – 312 . [Google Scholar]
- 36. Mackinnon DP, Dwyer JH . Estimating mediated effects in prevention studies . Eval Rev. 1993. ; 17 ( 2 ): 144 – 158 . [Google Scholar]
- 37. Fernandez-Mendoza J, Calhoun SL, Bixler EO, et al . Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles . Psychosom Med. 2011. ; 73 ( 1 ): 88 – 97 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gray EK, Watson D . General and specific traits of personality and their relation to sleep and academic performance . J Pers. 2002. ; 70 ( 2 ): 177 – 206 . [DOI] [PubMed] [Google Scholar]
- 39. Sin NL, Wen JH, Klaiber P, Buxton OM, Almeida DM . Sleep duration and affective reactivity to stressors and positive events in daily life . Health Psychol. 2020. ; 39 ( 12 ): 1078 – 1088 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Larsen JT, Hershfield HE, Stastny BJ, Hester N . On the relationship between positive and negative affect: Their correlation and their co-occurrence . Emotion. 2017. ; 17 ( 2 ): 323 – 336 . [DOI] [PubMed] [Google Scholar]
- 41. Hantsoo L, Khou CS, White CN, Ong JC . Gender and cognitive-emotional factors as predictors of pre-sleep arousal and trait hyperarousal in insomnia . J Psychosom Res. 2013. ; 74 ( 4 ): 283 – 289 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martin JL, Hakim AD . Wrist actigraphy . Chest. 2011. ; 139 ( 6 ): 1514 – 1527 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith MT, McCrae CS, Cheung J, et al . Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-Analysis, and GRADE assessment . J Clin Sleep Med. 2018. ; 14 ( 7 ): 1209 – 1230 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lawton MP, Moss M, Kleban MH, Glicksman A, Rovine M . A two-factor model of caregiving appraisal and psychological well-being . J Gerontol. 1991. ; 46 ( 4 ): 181 – 189 . [DOI] [PubMed] [Google Scholar]
- 45. Reed C, Belger M, Dell’agnello G, et al . Caregiver burden in Alzheimer’s disease: differential associations in adult-child and spousal caregivers in the GERAS observational study . Dement Geriatr Cogn Disord Extra. 2014. ; 4 ( 1 ): 51 – 64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Conde-Sala JL, Garre-Olmo J, Turró-Garriga O, Vilalta-Franch J, López-Pousa S . Quality of life of patients with Alzheimer’s disease: differential perceptions between spouse and adult child caregivers . Dement Geriatr Cogn Disord. 2010. ; 29 ( 2 ): 97 – 108 . [DOI] [PubMed] [Google Scholar]
- 47. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: : American Psychiatric Association; ; 2013. . [Google Scholar]
- 48. American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed . Darien, IL: : American Academy of Sleep Medicine; ; 2014. . [Google Scholar]
- 49. Ma Y, Goldstein MR, Davis RB, Yeh GY . Profile of subjective-objective sleep discrepancy in patients with insomnia and sleep apnea . J Clin Sleep Med. 2021. ; 17 ( 11 ): 2155 – 2163 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kay DB, Dzierzewski JM, Rowe M, McCrae CS . Greater night-to-night variability in sleep discrepancy among older adults with a sleep complaint compared to noncomplaining older adults . Behav Sleep Med. 2013. ; 11 ( 2 ): 76 – 90 . [DOI] [PubMed] [Google Scholar]
- 51. Tsuchiyama K, Nagayama H, Kudo K, Kojima K, Yamada K . Discrepancy between subjective and objective sleep in patients with depression . Psychiatry Clin Neurosci. 2003. ; 57 ( 3 ): 259 – 264 . [DOI] [PubMed] [Google Scholar]