Abstract
Background
The NIH Revitalization Act, implemented 29 years ago, set to improve the representation of women and minorities in clinical trials. In this study, we investigate progress made in all phase therapeutic clinical trials for neuroepithelial CNS tumors stratified by demographic-specific age-adjusted disease incidence and mortality. Additionally, we identify workforce characteristics associated with clinical trials meeting established accrual benchmarks.
Methods
Registry study of published clinical trials for World Health Organization defined neuroepithelial CNS tumors between January 2000 and December 2019. Study participants were obtained from PubMed and ClinicalTrials.gov. Population-based data originated from the CBTRUS for incidence analyses. SEER-18 Incidence-Based Mortality data was used for mortality analysis. Descriptive statistics, Fisher exact, and χ 2 tests were used for data analysis.
Results
Among 662 published clinical trials representing 49 907 participants, 62.5% of participants were men and 37.5% women (P < .0001) representing a mortality specific over-accrual for men (P = .001). Whites, Asians, Blacks, and Hispanics represented 91.7%, 1.5%, 2.6%, and 1.7% of trial participants. Compared with mortality, Blacks (47% of expected mortality, P = .008), Hispanics (17% of expected mortality, P < .001) and Asians (33% of expected mortality, P < .001) were underrepresented compared with Whites (114% of expected mortality, P < .001). Clinical trials meeting accrual benchmarks for race included minority authorship.
Conclusions
Following the Revitalization Act, minorities and women remain underrepresented in therapeutic clinical trials for neuroepithelial tumors, relative to disease incidence and mortality. Study accrual has improved with time. This study provides a framework for clinical trial accrual efforts and offers guidance regarding workforce considerations associated with enrollment of underserved patients.
Keywords: clinical trials, clinical trial accrual, disparities, glioma
Key Point.
Minorities and women with brain tumor diagnosis remain significantly under-accrued for neuro-oncology clinical trials compared to Caucasians and men based on proportional disease burden and demographic-specific mortality.
Importance of the Study.
The current state of clinical trial accrual in the US for adult patients with gliomas across demographic groups has not been comprehensively studied. This study aims to quantify clinical trial accrual by age-adjusted disease incidence and mortality for gender and race during a 20-year period following the NIH Revitalization Act, and to identify workforce characteristics associated with clinical trials meeting established race and gender accrual benchmarks. Minorities and women with brain tumor diagnosis remain significantly under-accrued for neuro-oncology clinical trials compared to Caucasians and men based on proportional disease burden and demographic-specific mortality. Despite the enactment of the NIH Revitalization Act to improve the representation of women and minorities in clinical trials nearly 30 years ago, this goal remains unmet in the field of neuro-oncology. Significant work is required to continue to implement and improve interventions to increase accrual of diverse patient populations.
Patient-specific factors such as race and gender remain essential contributors to an individual’s health and wellness in the United States (US). National policies such as the 1993 National Institutes of Health (NIH) Revitalization Act established guidelines for the inclusion of women and minorities in clinical research. The statute outlined the necessary components in design, implementation, and outreach to include under-represented populations, consistent with their representation in the US population (currently 51% women, 36.3% minorities).1–3 Additional guidance offered a framework for enrollment based on disease-specific race and gender incidence.
Despite this legislation, over the last 30 years, in general, clinical trial participants remain largely young, white, and male.4–7 A review of clinical trials associated with US Food and Drug Administration (FDA) approved cancer therapies from 2008 to 2018, found that 15 years following the NIH Revitalization Act, only 63% of trials reported race, and 7.8% reports the four major racial groups in the US.8 Furthermore, within FDA approved cancer trials, Black and Hispanic patients accounted for 3.1% and 6.1% of trial participants respectively, far below cancer incidence.
Neuroepithelial brain tumors such as diffuse gliomas are the most common adult primary brain tumors in the United States, accounting for over 50% of malignant brain cancers. For these patients, many will exhaust standard of care treatment options allowing clinical trials to be widely accepted as the highest quality care, with participation being associated with improved clinical outcomes and increased survival.9 Access to clinical trials not only allows for generalizability of scientific research, but it also provides for equitable treatment of diverse patient populations. Knowledge regarding clinical trial participation among women and minorities at the population level in neuro-oncology is a critical knowledge gap.
In neuro-oncology, the importance of race and gender diversity towards ensuring equity, validity, and generalized interpretability of results is of great importance. Our goal is to provide a framework for understanding clinical trial enrollment for brain cancer patients by (1) reviewing clinical trial gender and race reporting, (2) quantifying proportion of study participants stratified by age-adjusted disease-specific incidence and mortality, (3) use population data to determine how these findings have changed over a 20-year period following institution of the NIH Revitalization Act, and (4) identify workforce characteristics associated with optimal clinical trial accrual. This study will provide a framework for investigating clinical trial participation based on disease burden through direct evaluation of incidence and mortality rates.
Methods
Clinical Trial Accrual Data
Based on World Health Organization (WHO) 2016 diagnostic criteria, study enrollment included the following neuroepithelial tumor diagnosis: diffuse astrocytoma, anaplastic astrocytoma, glioblastoma, oligodendroglioma, anaplastic oligodendroglioma, ependymal tumors, glioma malignant, not otherwise specified (NOS). A systematic review of the literature was conducted through a PubMed query to identify articles published of clinical (Phase I–IV) trials of adult gliomas between January 1, 2000 and December 31, 2019. Adult (18+) participants with a primary glioma diagnosis were included for analysis. For the studies conducted in the US, “Minority” status was defined as patients belonging to any of the NIH-designated race-based underserved groups. This includes individuals with the following racial or ethnicity makeup: Asian/ Pacific Island native, African American/Black, Hispanic/Latinx, and Native American/Alaska Native. If participant demographics were not explicitly reported in the article, and the trial’s national clinical trial (NCT) number was available, a subsequent search was conducted on ClinicalTrials.gov. This study included analysis of de-identified population data and was approved by institutional review board and performed in accordance with the Declaration of Helsinki.
Incidence & Mortality Data
The following datasets were utilized and are described in detail below: CBTRUS Incidence Data: Central Brain Tumor Registry of the United States SEER*Stat Database. Centers for Disease Control (CDC) National Program of Cancer Registries (NPCR) and National Cancer Institute (NCI) Surveillance, Epidemiology and End Results (SEER) Incidence Data, 2019 submission (2000–2017).10SEER Incidence-Based Mortality Data: SEER Program (www.seer.cancer.gov) SEER*Stat Database: Incidence-Based Mortality—SEER Research Data, 18 Registries, Nov 2019 Sub (2000–2017)—Linked to County Attributes—Time-Dependent (1990–2017) Income/Rurality, 1969–2018 Counties, National Cancer Institute, DCCPS, Surveillance Research Program. Released April 2020, based on the 2019 submission.11
The Central Brain Tumor Registry of the United States (CBTRUS) database (Data provided by CDC’s NPCR and NCI’s SEER Program, 2000–2017) was used to estimate the age-adjusted incidence rates. Average annual age-adjusted incidence rates and 95% confidence intervals were estimated per 100 000 population, based on one-year age groupings and standardized to the 2000 US standard population by race and ethnicity (Supplementary Table 2).12,13 Incidence-based age-adjusted mortality rates were calculated using the data from the SEER 18 (Supplementary Table 3) by race and ethnicity.
Enrollment to Incidence Ratio (EIR) and Enrollment to Mortality Ratios (EMR) were calculated using the enrolled proportion for each demographic group as the numerator to the incident- and mortality-based disease burden, respectively as the denominator.
Diversity Data Among High Accruing Programs
Clinical trials which reported minority recruitment at or above 10% of participants (equivalent to the upper tercile of the distribution) were identified. Descriptive statistics were reported from self-reported faculty diversity in the neurology, medical oncology, and neurosurgery departments, contrasted with 2018 Association of American Medical Colleges (AAMC) National Physician Workforce data along with Census Bureau 2010–2016 City Demographic data.
Data Analysis
This study reports descriptive statistics of enrollment proportions for each demographic group for the 20-year study period, and year-over-year trends. Incidence and mortality counts, rates, and other relevant statistics were calculated using SEER*Stat 8.3.8.11 Primary enrollment disparity is reported as the difference in proportions (DF) between accrual and mortality. A secondary comparison is reported in the supplemental data examining “accrual vs incidence”, by group. Z and χ 2 tests of proportions were used to evaluate the significance of the associations in comparison groups and odds ratios are used to describe enrollment ratios. The level of significance was P < .05 for all analyses. Group level statistics were performed using STATA SE 16.
Results
Clinical Trial Reporting Accrual by Demographic Group
An initial search returned 1932 articles that met inclusion criteria. After screening, 662 full-text articles were identified that reported patient roster with demographic information of accrued participants. These 662 articles included 49 907 enrolled participants published during the 20-year period. Of these, 527 articles (including 41 933 participants) specifically reported the distribution of sex in the study. One hundred and thirty articles (including 11 943 participants) reported participants of White race, while 104 of those articles specified participants belonging to any minority racial or ethnic group (Supplementary Figure 1). Importantly, while 80% of eligible articles reported accrual by sex, only 20% of articles reported any racial breakdown, where many listed numbers of “White” participants only, and only 16% reported the number accrued of any minority race or ethnicity (Table 1).
Table 1.
Participant Demographics Reported in Published Glioma Clinical Trial Articles 2000–2019
| Reported demographic | No. of Articles (662) | % of Articles | No. of patients (49,907) | % of all patients | % Reported accrual |
|---|---|---|---|---|---|
| Sex | 527 | 80% | 41,933 | 84% | - |
| Male | - | - | 26,237 | 53% | 63% |
| Female | - | - | 15,696 | 31% | 37% |
| Race/Ethnicity | 130 | 20% | 11,943 | 24% | - |
| Minority (Any) | 104 | 16% | 663 | 1.3% | 5.6% |
| White | 130 | 20% | 10,806 | 22% | 90.5% |
| Black/AA | 66 | 10% | 256 | 0.5% | 2.0% |
| Asian/PI | 47 | 7% | 158 | 0.3% | 1.3% |
| Hispanic/Latino | 36 | 5% | 239 | 0.5% | 2.0% |
| American Indian/AN | 10 | 2% | 20 | 0.04% | 0.2% |
| Other | 57 | 9% | 464 | 0.9% | 4.0% |
AA: African American, PI: Pacific Islander, AN: Alaska Native
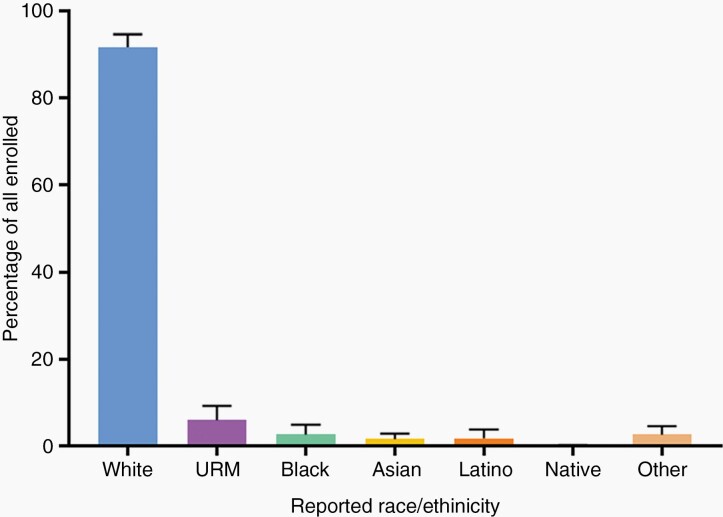
Eighty percent of studies reported sex over the 20-year period. Men accounted for an average of 63% (n = 26 237 of 41 933 participants) of accrued participants with gender reported. Regarding accrual by race and ethnicity, 11 943 of participants (24% of the total 49,907 patients) had race or ethnicity demographics reported. White participants accounted for 90.5% (n = 10 806 of 11 943 participants), Black/African Americans 2.0% (n = 256 of 11 943 participants), Asian/Pacific Islanders 1.3% (n = 158 of 11 943 participants), American Indian/Alaska Native 0.2% (n = 20 of 11 943 participants), and Hispanic/Latino participants accounted for 2.0% (n = 239 of 11 943 participants). A separate designation of race and ethnicity “other” was confirmed in 4.0% of study participants (n = 464 of 11 943 participants). Collectively, participants belonging to an NIH-designated minority group accounted for 5.6% (n = 673 of 11 943 participants) of the total participants with race and ethnicity reported (Table 1). An evaluation of the proportions (percentages) of each demographic group within each clinical trial revealed that over the 20-year period, articles reported average sample populations that were 62.3% Male (standard deviation [SD]: 1.9%), 91.7% White (SD: 2.9%) and 5.9% Minority (SD: 3.4%). The Minority group was comprised of 2.6% Black/African American (SD: 2.2%), 1.5% Asian/Pacific Islander (SD:1.3%), 1.7% Hispanic-Latino (SD: 2.1%), 0.1% American Indian/Alaska Native (SD: 0.1%). Year-by-year proportions of each sex, race, and ethnicity group can be found in Supplementary Figure 2.
Disease incidence and mortality by demographic group
CBTRUS data included patients aged ≥20 years with newly diagnosed selected primary brain and central nervous (CNS) tumors that were either microscopically or radiographically confirmed for diagnosis from 2000 to 2017. The specific WHO ICD-O-3 histology codes included under each selected histology for analysis are included in Supplementary Table 1. Incidence data included a total of 257 663 incident-cases, of these 214 057 non-Hispanic White, 15 367 non-Hispanic Black, 22 145 Hispanic (all races), 4879 Asian/Pacific Islander, and 1215 American Indian/Alaskan Native (Supplementary Table 2). Mortality data included a total of 45 765 deaths; 36 577 non-Hispanic Whites, 2537 non-Hispanic Blacks, 4468 Hispanic (all races), 2046 Asian/Pacific Islanders, and 137 American Indian/Alaskan Natives (Supplementary Table 3).
Comparison of trial accrual and disease burden by demographic group
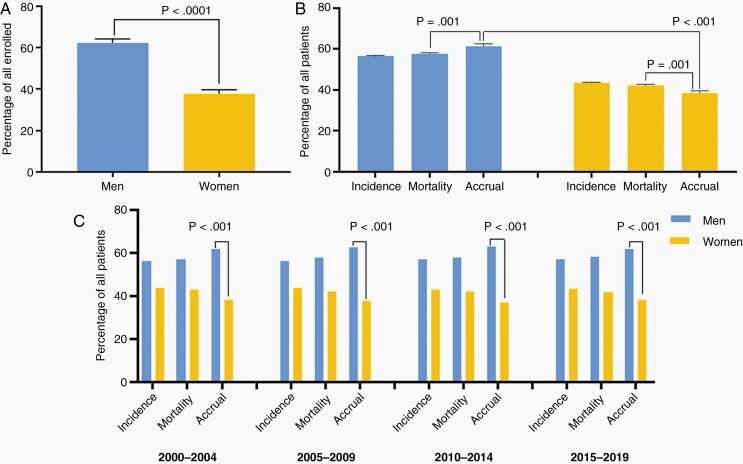
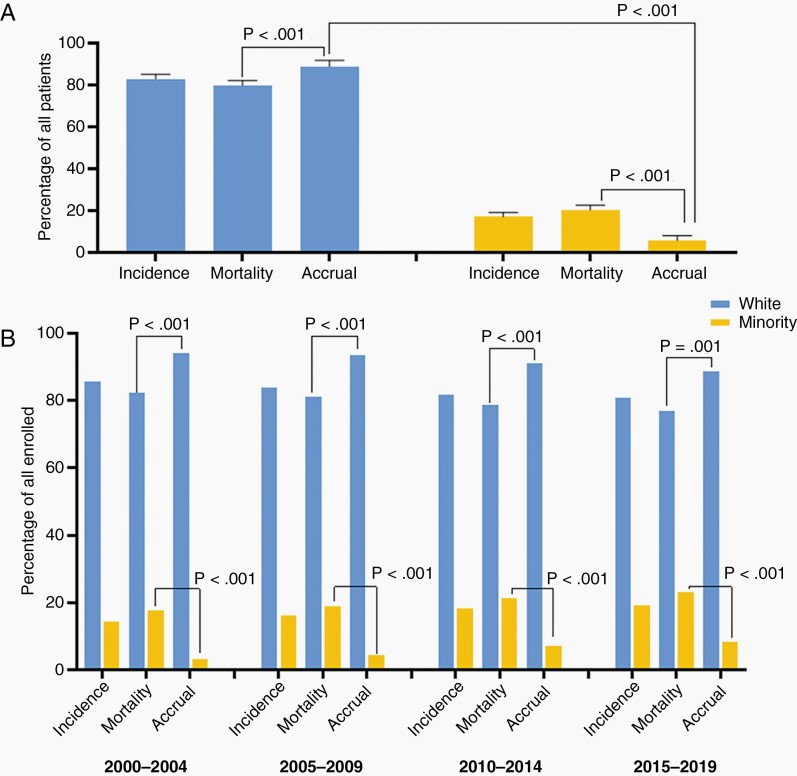
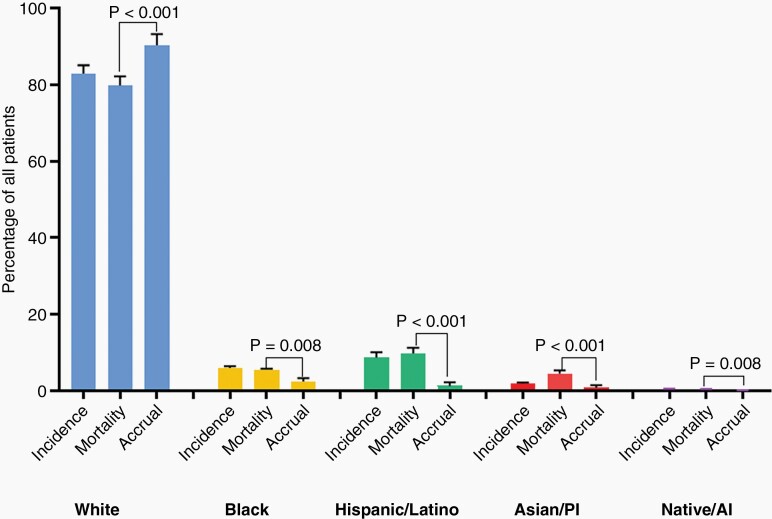
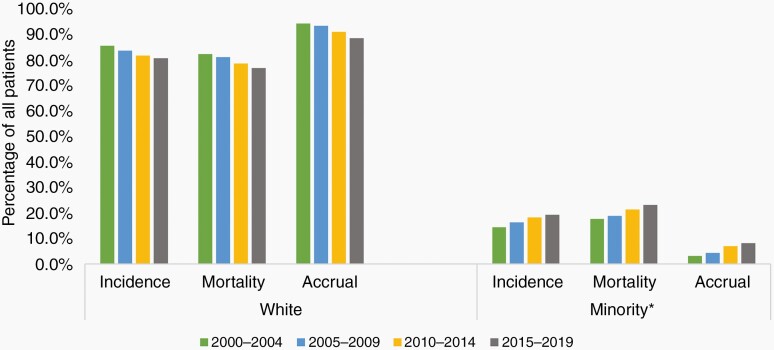
Over the 20-year period, men on average accounted for 62.3% of trial accrual, 55.9% of incident cases, and 55.8% of disease mortality, while women accounted for 37.7% of trial accrual, 44.1% of incident cases, and 44.2% of disease mortality (Figure 1.A). There was a statistically significant difference between accrual of men and women (DF: 23%, CI: 19–26%, P < .001) and between accrual and mortality for men (DF: 3.78 CI: 1.7–5.8%, P = .001, positive direction) and women (DF: –3.78 CI: –5.8 to 11.7%, P = .001, negative direction) (Figure 1.B), which remained significant across 5-year trends (Figure 1.C). White participants accounted for 91.7% of trial accrual, 83.1% of incident cases, and 79.9% of deaths. The minority group at large, accounted for 5.9% of accrual, 16.9% of incident cases, and 20.1% of deaths. There was a statistically significant difference in accrual of Whites and Minorities (DF: 84% (CI: 77%–92%, P =< .001)(Figure 2.A.). There was a statistically significant difference between accrual and mortality for White (DF: 9% CI: 7%–11%, P < .001, positive direction), the Minority group (DF:16% CI: 14%–18%, P < .001) which were consistent across 5-year trends (Figure 2.B). Breakdown by race and ethnicity showed Black/African American accounted for 3.3% of trial accrual, 6.0% of incident cases, and 5.5% of mortalities; Hispanic/Latino: 1.8% of trial accrual, 8.6% of incidence, 9.8% of deaths, Asian/Pacific-Islander: 1.3% of trial accrual, 1.9% of incident cases, and 4.5% of deaths, American Indian/Alaska-Native:.06% of trial accrual,.5% of incident cases and.3% of deaths (accrual proportions in Figure 3). All minority racial/ethnic backgrounds showed significant under-accrual compared to their mortality burden; Black (DF: 3.1%, CI: 1.5–4.6%, P = .008), Hispanic/Latino (DF: 8.5%, CI: 7.6–9.5%, P < .001), Asian (DF: 3.7%, CI: 3.1–4.2%, P < .001), and Native (DF:0.3%, CI: 0.2–0.5%, P = .008) (Figure 4). There has been modest upward improvement in accrual over the last 20 years. This includes, minority accrual just below 3% between 2000–2004 and 8% between 2015–2019 (Figure 5).
Fig. 1.
Proportions of men and women enrolled in a clinical trial 2000–2019, compared to incidence and mortality burden. A. Clinical trial accrual proportions in men and women over the 20-year period, 2000–2019. Men represented 62.3% of accrued participants, women 37.7% (P < .0001) B. Proportions of accrued participants as compared to disease incidence and mortality. Men were disproportionately accrued compared to their disease burden (P = .001), and women were under-accrued compared to their disease burden (P = .001). C. Five-year trends from 2000 to 2019 show consistently significant results across the time period. *Data Source: Incidence—CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2000–2017, Mortality—Incidence-Based Mortality SEER Research Data, (2000–2017), Accrual—Systematic review of the literature published of clinical (Phase I–IV) trials of adult gliomas (2000–2019).
Fig. 2.
Patient proportions for incidence, mortality, and accrual according to minority* identity, 20-year average and five-year trends 2000–2019. A. Patient proportions for incidence, mortality, and accrual according to Minority* identity, 2000–2019. White participants represented 91.7% of accrued participants, Minority 5.9% (P < .001). White patients were disproportionately accrued compared to their disease burden (P < .001), and Minority patients were under-accrued compared to their disease burden (P < .001). B. Five-Year Trends from 2000 to 2019 show consistently significant results across the time period. *Data Source: Incidence—CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2000–2017, Mortality—Incidence-Based Mortality SEER Research Data, (2000–2017), Accrual—Systematic review of the literature published of clinical (Phase I–IV) trials of adult gliomas (2000–2019). **Minority status determined by identification with an NIH-defined group.
Fig. 3.
Clinical trial enrollment by race and ethnicity in articles published from 2000 to 2019. White participants, on average, accounted for 91.7% of enrolled participants over the 20-year period. Under-represented minorities as a group, accounted for 5.9% of enrolled participants (Black/African American: 2.6%, Asian/Pacific Islander: 1.5%, Hispanic/Latino: 1.7%, Native American/Alaska Native: 0.1%). URM: Under-represented minority.
Fig. 4.
Patient proportions for incidence, mortality, and accrual according to race and ethnicity, 2000–2019. Over the 20-year period, White patients were disproportionately-accrued compared to their mortality burden 114% of expected (P < .001), their minority counterparts were significantly under-accrued; Black/African-American 47% of expected (P = .008), Hispanic/Latino 17% of expected (P < .001), Asian/Pacific Islander 33% of expected (P < .001) and Native American/Alaska Native 20% of expected (P = .008). PI: Pacific Islander, AI: Alaska Native. *Data Source: Incidence—CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2000–2017, Mortality—Incidence-Based Mortality SEER Research Data, (2000–2017), Accrual—Systematic review of the literature published of clinical (Phase I–IV) trials of adult gliomas (2000–2019).
Fig. 5.
Five-year grouped trend data for incidence, mortality and accrual by minority* status 2000–2019. Five-Year Trends from 2000 to 2019 shows increased accrual for minority patients from 3.2% (2000–2004) to 8.2% (2015–2019) over the 20 year period. *Data Source: Incidence—CBTRUS: Data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2000–2017, Mortality—Incidence-Based Mortality SEER Research Data, (2000–2017), Accrual—Systematic review of the literature published of clinical (Phase I–IV) trials of adult gliomas (2000–2019).
We computed a measure of enrollment by demographic group using Enrollment Incidence Ratio (EIR) and Enrollment Mortality Ratio (EMR). The overall EIR was 1.10 in men, 0.89 in women, 1.1 in White patients, and 0.35 in Minority patients (0.44 in Black, 0.211 in Hispanic/Latino, 0.78 in Asian/PI, 0.13 in Native/AI). By EIR, men had 1.25 greater odds of enrollment compared to women, while White patients had 3.95 greater odds of enrollment compared to their Minority counterparts. The overall EMR was 1.08 in men, 0.89 in women, 1.15 in White patients, and 0.29 in Minority patients (0.47 in Black, 0.17 in Hispanic/Latino, 0.33 in Asian/PI, 0.20 in Native/AI). By EMR, men had 1.20 greater odds of enrollment compared to women, while White patients had 3.76 greater odds of enrollment compared to their Minority counterparts.
High-Accruing Studies
Seventeen studies were identified as upper tercile, in which minority recruitment met or exceeded 10% of enrollment. Within these studies, 55% of papers included a primary and/or senior author who self-identified as representing an NIH-defined minority group. These programs were in geographic areas with 60.5% minority population on average (2010-2016 Census Data). Their respective departments of Neurology and Neurosurgery were diverse, on average consisting of 43% minorities, exceeding the national average for US medical school faculty diversity (28.5% minorities) and physician workforce diversity in neurology and neurosurgery combined (22.7% minorities; 24% in neurology, and 22% in neurosurgery).
Discussion
Within the US significant differences in health outcomes exist between specific patient demographics. While health disparities are often interpreted as differences in outcomes between racial or ethnicity groups, disparities can exist across many dimensions, including gender, age, sexual orientation, socioeconomics, and disability status. The goal of this study was to analyze whether clinical trial accrual within neuro-oncology differentiates from patterns of disease-specific incidence and mortality during the 20-year period following the NIH Revitalization Act. The present study demonstrates that White males remain disproportionately represented in clinical trials for adult neuroepithelial CNS tumors compared to women. Minority patients are diagnosed with neuroepithelial tumors at a lower rate compared with White patients; however, they suffer from a striking underrepresentation in trial accrual based on incidence and mortality.
Clinical trial accrual for women and minorities remains below established benchmarks 27 years since the implementation of the NIH Revitalization Act. This study demonstrates that the disparity in clinical trial participation for CNS neuroepithelial tumors remain significant for all minority groups, however the trajectory of accrual over time for underrepresented populations is slowly improving. Between 2000–2019 clinical trial race and gender reporting was poor and when reported, accrual did not meet the burden of mortality or incidence for Black/African-Americans, Hispanic/Latinos, Asian/Pacific-Islanders, or American-Indian/Alaska Natives. There has been modest upward improvement in accrual over the last 20 years. Specifically, we found minority accrual just below 3% between 2000–2004 and 8% between 2015–2019. While a notable improvement, 8% accrual remains below disease specific mortality accrual benchmarks which would establish a 17–23% accrual target (Figure 5). With respect to gender, the inclusion of women in North American clinical trials has seen steady gains over the last two decades.4 Perhaps the best example is Murthy et al in which patients with breast, colorectal, lung, and prostate cancer enrolled in non-surgical therapeutic clinical trials were studied between 1996–2002.4 These results demonstrated that men and women 30 to 64 years of age with colorectal or lung cancers were equally likely to participate in trials. With advancing age, however, older men enrolled at a higher rate. Meinert et al. systematically reviewed clinical trials published in Annals of Internal Medicine, British Medical Journal, Journal of the American Medical Association, and New England Journal of Medicine between 1985–1995 noting that for cancer clinical trials, female-only trials (20.8%) outnumbered male-only trials (8.7%) by a substantial margin.14 By the early 2000’s, policies appeared to be making an impact towards increasing female participation in clinical trials. In 2001, the Government Accountability Office (GAO) reported that for investigational drugs, women represented 52% of study participants for all new drug applications between 1998 and 2000 (results not limited to cancer indications).15 Within neuro-oncology however gender enrollment disparities have remained sluggish when compared with men.3 Furthermore, only a fraction of clinical trials reported race and ethnicity data, and among those that did report, there were inconsistencies in the manner in which demographic information was presented.
Disparities persist in delivery of standard of care and experimental cancer-directed therapies in neuro-oncology. For example, non-Hispanic Blacks and Hispanics remain less likely to receive chemoradiation when compared with non-Hispanic Whites.16 A recent review of NCI-sponsored clinical trials found persistent and significant under-reporting and under-enrollment of minorities in cancer studies. The authors found that less than 2% of clinical trials had a primary purpose of investigating cancer in minority populations.3 There are a number of obstacles to be confronted in efforts to improve accrual of minorities in clinical trials. The conceptual framework first conceived by Ford, et. al and later adapted by Napoles et. Al., provides an example of the complexity in understanding patient-level decision making in considering participation in a clinical trial.5 In fact, published results in cancer clinical trials have identified studies that were able to engage minority participants. Clinical trial accrual for diverse population is a consequence of limited access in general. There remain numerous barriers to minority enrollment including those of awareness, knowledge, and opportunity, all of which remain formidable challenges in clinical trial design and implementation. There are however known multi-dimensional strategies focused on recruitment of underserved patient populations. These efforts include ensuring the availability of interpreter services, sharing published results with local community members, culturally appropriate outreach and education, and allocation of resources for infrastructure supporting stakeholders serving underrepresented communities.5
Transparency in reporting results of clinical trials is needed in order to accomplish meaningful change in accrual in neuro-oncology, particularly for subgroup analyses by race and ethnicity. Collective, purposeful efforts are needed to standardize the manner in which clinical research data is collected and reported in published studies. Achieving race, ethnic, and gender equity in scientific research is not just a moral cause. Diversity in clinical trials, and clinical research at large, is paramount to strengthening our ability to affirm validity of findings that advance both mechanistic understanding of disease and medical interventions. Appropriate inclusion of all affected demographic groups, at all levels of investigation, is central to the path of both healthcare equity and precision medicine.
Differences in the quality of health care that are not due to clinical need, access-related factors, or patient preferences are considered health care disparities. The underlying causes of gender, race, and ethnicity disparities for cancer patients are numerous. Factors such as systemic racism within the healthcare system, physician bias, patient mistrust, lack of a diverse provider workforce, and communication shortcomings between patient and provider are a few of the most commonly cited examples.17,18 We acknowledge that the impact of race/ethnicity as a social determinant of health does not exist in a vacuum and is profoundly impacted by and alongside various socioeconomic, environmental, and structural factors. With limited access to healthcare for certain patient groups, disparities in translational research participation will likely remain. These facts are particularly true in cancer research and treatment. For example, given that only 20% of published articles in neuro-oncology included detailed breakdowns of race and/or ethnicity of participants, when considering the accrual of Hispanic/Latino participants prior to the introduction of formal accounting for ethnicity, we cannot determine with certainty, the articles that did not report specific minority groups, truly did not enroll a considerable number of Hispanic/Latino participants. However, it would be expected that race and ethnicity would be similarly represented across reported studies. This idea is supported by the relatively narrow variability in the incidence of minority enrollment across studies that report race demographics. Nonetheless, specific clinical trial reporting standards should be observed.
The finding that within neuro-oncology, diverse investigator teams accrue greater numbers of diverse patients in therapeutic brain cancer clinical trials supports efforts towards diversifying the healthcare workforce. In this study, we discovered that neuro-oncology studies which were able to recruit more than 10% minorities, appeared to have diverse department faculty and are located in geographic areas with higher proportions of minorities patients. Additional systems and provider interventions thought to advance recruitment of minority patients in cancer research include (1) targeted community outreach and marketing, (2) routine educational efforts for both patients and providers about the importance of cancer clinical trials, (3) patient-facing programs to increase access to surveillance imaging and cancer screening, (4) resources to mitigate transportation barriers, (5) education program to raise awareness about cancer treatment inequities, and (6) funding agency requirements for study protocols to have enrollment plans for women and minority accrual.5,6 In order to address the central issues of healthcare disparities in cancer research, we must begin with both accurate and precise data collection along with continued emphasis on recruiting and retaining diverse populations that meet the needs of demographic-specific disease burden.
Conclusion
Despite the NIH mandate, introduced almost three decades ago, a clear disparity remains between the accrual of minorities and women in clinical trials for adult neuroepithelial tumors as compared to their respective disease burden and representation in the US population. The gap in enrollment for women has improved substantially in the general cancer population and gender-based enrollment differences persist in neuro-oncology trials. Representation of minorities in clinical trials also remains significantly below disease burden. The slight upward trends in accrual over time for both women and minority groups, are a positive sign that efforts towards improving participation and standardizing reporting may be moving the needle toward equitable participation. The quality of scientific research and the knowledge base in neuro-oncology can only be strengthened by increased diversity. With improved representation comes immense potential to improve the clinical outcomes for groups that often bear disproportionate burden.
Supplementary Material
Acknowledgments
The CBTRUS data were provided through an agreement with the Centers for Disease Control’s National Program of Cancer Registries. In addition, CBTRUS used data from the research data files of the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results Program, and the National Center for Health Statistics National Vital Statistics System. CBTRUS acknowledges and appreciates these contributions to this report and to cancer surveillance in general. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the NCI.
Contributor Information
Sheantel J Reihl, University of California, San Francisco, Department of Neurosurgery, San Francisco, California, USA.
Nirav Patil, Central Brain Tumor Registry of the United States (CBTRUS), Hinsdale, Illinois, USA; University Health System, Research and Education Institute, Cleveland, Ohio, USA; University Hospitals Health System, Research Health Analytics and Informatics, Cleveland, Ohio, USA.
Ramin A Morshed, University of California, San Francisco, Department of Neurosurgery, San Francisco, California, USA.
Mulki Mehari, University of California, San Francisco, Department of Neurosurgery, San Francisco, California, USA.
Alexander Aabedi, University of California, San Francisco, Department of Neurosurgery, San Francisco, California, USA.
Ugonma N Chukwueke, Center for Neuro-Oncology, Dana-Farber Cancer Institute, Department of Neurology, Harvard Medical School, Boston, Massachusetts, USA.
Alyx B Porter, Mayo Clinic, Division of Neuro-Oncology, Department of Neurology, Phoenix, Arizona, USA.
Valy Fontil, University of California San Francisco, Division of General Internal Medicine, San Francisco, California, USA; University of California San Francisco, Center for Vulnerable Populations, Zuckerberg San Francisco General Hospital, San Francisco, California, USA.
Gino Cioffi, Division of Cancer Epidemiology and Genetics, Trans-Divisional Research Program, National Cancer Institute, Bethesda, Maryland, USA; Central Brain Tumor Registry of the United States (CBTRUS), Hinsdale, Illinois, USA.
Kristin Waite, Division of Cancer Epidemiology and Genetics, Trans-Divisional Research Program, National Cancer Institute, Bethesda, Maryland, USA; Central Brain Tumor Registry of the United States (CBTRUS), Hinsdale, Illinois, USA.
Carol Kruchko, Central Brain Tumor Registry of the United States (CBTRUS), Hinsdale, Illinois, USA.
Quinn Ostrom, Central Brain Tumor Registry of the United States (CBTRUS), Hinsdale, Illinois, USA; Department of Neurosurgery, Duke University School of Medicine, Durham, North Carolina, USA; The Preston Robert Tisch Brain Tumor Center, Duke University School of Medicine, Durham, North Carolina, USA; Duke Cancer Institute, Duke University Medical Center, Durham, North Carolina, USA.
Jill Barnholtz-Sloan, Central Brain Tumor Registry of the United States (CBTRUS), Hinsdale, Illinois, USA; Center for Biomedical Informatics and Information Technology, National Cancer Institute, Bethesda, Maryland, USA.
Shawn L Hervey-Jumper, University of California, San Francisco, Department of Neurosurgery, San Francisco, California, USA.
Funding
National Institute of Health, Award Number: TL1TR001871-05 (SR). National Center for Advancing Translational Sciences of the NIH (RAM), Neurosurgery Research and Education Foundation (RAM), Robert Wood Johnson Foundation 74259 (SHJ), NINDS K08 110919-01 (SHJ), Loglio Collective (SHJ). Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under Contract No. 75D30119C06056, the American Brain Tumor Association, The Sontag Foundation, Novocure, the Musella Foundation, National Brain Tumor Society, the Pediatric Brain Tumor Foundation, the Uncle Kory Foundation, the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations.
Conflicts of interest.
The authors report no conflict of interest.
Authorship statement.
Research Concept and Plan: SJR, UNC, ABP, VF, SLHJ. Data collection & analysis: SJR, NP, RAM, MM, GC, KM, CK, QO, JBS, SLHJ. Manuscript preparation: all authors.
References
- 1. Freedman LS, Simon R, Foulkes MA, et al. Inclusion of women and minorities in clinical trials and the NIH Revitalization Act of 1993–the perspective of NIH clinical trialists. Control Clin Trials. 1995;16(277–279):293–309. [DOI] [PubMed] [Google Scholar]
- 2. NIH. Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. Bethesda: National Institute of Health; 2001. [Google Scholar]
- 3. Chen MS Jr, Lara PN, Dang JH, Paterniti DA, Kelly K. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer. 2014;120Suppl 7(07):1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. [DOI] [PubMed] [Google Scholar]
- 5. Napoles A, Cook E, Ginossar T, Knight KD, Ford ME. Applying a conceptual framework to maximize the participation of diverse populations in cancer clinical trials. Adv Cancer Res. 2017;133:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228–242. [DOI] [PubMed] [Google Scholar]
- 7. Hussain-Gambles M, Atkin K, Leese B. Why ethnic minority groups are under-represented in clinical trials: a review of the literature. Health Soc Care Community. 2004;12(5):382–388. [DOI] [PubMed] [Google Scholar]
- 8. Loree JM, Anand S, Dasari A, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019;5(10):e191870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Field KM, Drummond KJ, Yilmaz M, et al. Clinical trial participation and outcome for patients with glioblastoma: multivariate analysis from a comprehensive dataset. J Clin Neurosci. 2013;20(6):783–789. [DOI] [PubMed] [Google Scholar]
- 10. Central Brain Tumor Registry of the United States SEER*Stat Database. CDC National Program of Cancer Registries and NCI Surveillance, Epidemiology and End Results Incidence Data. 2019. submission (2000–2017). [Google Scholar]
- 11. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat software version 8.3.6. 2020. www.seer.cancer.gov/seerstat. Accessed February 2020.
- 12. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence-Based Mortality - SEER Research Data, 18 Registries, Nov 2019 Sub (2000-2017) - Linked To County Attributes - Time Dependent (1990-2017) Income/Rurality, 1969-2018 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2020, based on the November 2019 submission.www.seer.cancer.gov. Accessed April 2, 2021.
- 13. Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547–569. [DOI] [PubMed] [Google Scholar]
- 14. Meinert CL, Gilpin AK, Unalp A, Dawson C. Gender representation in trials. Control Clin Trials. 2000;21:462–475. [DOI] [PubMed] [Google Scholar]
- 15. United States General Accounting Office. Women sufficiently represented in new drug testing, but FDA oversight needs improvement, 2001. https://www.gao.gov/new.items/d01754.pdf. Accessed May, 30 2020.
- 16. Ostrom QT, Krebs HL, Patil N, Cioffi G, Barnholtz-Sloan JS. Racial/ethnic disparities in treatment pattern and time to treatment for adults with glioblastoma in the US. J Neurooncol. 2021;152:603–615. doi: 10.1007/s11060-021-03736-4. [DOI] [PubMed] [Google Scholar]
- 17. Morshed RA, Reihl SJ, Molinaro AM, et al. The influence of race and socioeconomic status on therapeutic clinical trial screening and enrollment. J Neurooncol. 2020;148(1):131–139. [DOI] [PubMed] [Google Scholar]
- 18. Lee EQ, Chukwueke UN, Hervey-Jumper SL, et al. Barriers to accrual and enrollment in brain tumor trials. Neuro Oncol. 2019;21(9):1100–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.