Abstract
BACKGROUND
Race and geographic differences in the prevalence and predictors of hypertension in stroke survivors have been reported, but apparent treatment-resistant hypertension (aTRH) among stroke survivors by race (African ancestry vs. non-Hispanic Caucasians) and by geography (continental Africa vs. the United States) are under studied.
METHODS
This is a cross-sectional study using ethically approved stroke registries from the University of Florida and the Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. Univariate and multivariate regression was used to evaluate for differences in prevalence of aTRH and associations with clinical covariates.
RESULTS
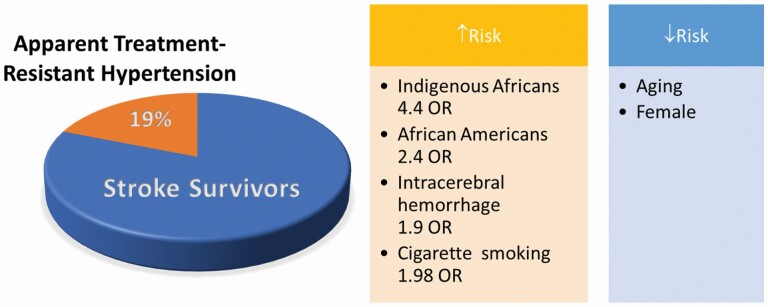
Harmonized data were available for 3,365 stroke survivors of which 943 (28.0%) were indigenous Africans, 558 (16.6%) African Americans, and 1,864 (55.4%) non-Hispanic Caucasians with median ages (interquartile range) of 59 (49–68), 61 (55–72), and 70 (62–78) years, P < 0.0001. The overall frequency of aTRH was 18.5% (95% confidence interval [CI]: 17.2%–19.8%) with 42.7% (95% CI: 39.6%–46.0%) among indigenous Africans, 16.1% (95% CI: 13.2%–19.5%) among African Americans, and 6.9% (95% CI: 5.8%–8.2%) among non-Hispanic Caucasians, P < 0.0001. Five factors associated with aTRH: age, adjusted odds ratio (95% CI) of 0.99 (0.98–0.99), female sex 0.70 (0.56–0.87), cigarette smoking 1.98 (1.36–2.90), intracerebral hemorrhage 1.98 (1.57–2.48), and Black race namely indigenous Africans 4.42 (3.41–5.73) and African Americans 2.44 (1.81–3.29).
CONCLUSIONS
Future studies are needed to investigate the contribution of socioeconomic disparities in the prevalence aTRH in those with African Ancestry to explore the long-term impact, and evaluate effective therapeutic interventions in this subpopulation.
Keywords: blood pressure, geography, hypertension, race, resistant hypertension, stroke, stroke types
Graphical Abstract
Graphical Abstract.
Hypertension is the premiere modifiable vascular risk factor for incident and recurrent strokes worldwide.1–3 In the United States, the prevalence of hypertension is higher among African Americans than other racial groups.4 The population-risk attributable to hypertension for stroke rates among Black Americans is 39% (95% confidence interval [CI]: 19%–56%)5 compared with 91% (95% CI: 88%–94%) in sub-Saharan Africa.6 Furthermore, individuals of Black race exhibit a greater proclivity for hypertension-driven stroke subtypes, including intracranial large vessel atherosclerosis, intracerebral hemorrhages, and small vessel ischemic strokes.7–9 Several emerging lines of evidence suggest that excess salt and water retention from impaired kidney function increases the propensity of Blacks to resistant hypertension.10–14
True resistant hypertension is defined as the blood pressure (BP) of a hypertensive patient that remains elevated above goal despite the concurrent use of 3 antihypertensive agents of different classes, commonly including a long-acting calcium channel blocker (CCB), a blocker of the renin–angiotensin system (angiotensin-converting enzyme inhibitor or angiotensin receptor blocker), and a diuretic.15 The term apparent treatment-resistant hypertension (aTRH) is used for population-based studies. In population-based studies, it is not feasible to remove confounders such as medication nonadherence, white-coat effect, or aberrations in BP measurement accuracy. An estimated 17.7% of adults in the United States taking antihypertensive medication had aTRH based on BP cutoff value of <140/90 mm Hg in 2008. However, using updated data collected between 2009 and 2014, the prevalence of aTRH among Americans has increased to 19.7% from the updated 2018 Scientific Statement using a BP control cutoff of <130/80 mm Hg.15,16 Resistant hypertension is an important medical condition because individuals with aTRH tend to harbor cardiovascular disease risk factors such as chronic kidney disease, diabetes mellitus (DM), and elevated 10-year predicted atherosclerotic cardiovascular disease risk, thus consequently poor cardiovascular disease outcomes.
While the treatment and control of BP remain an important goal of secondary prevention after surviving a stroke, this goal is often elusive. Resistant hypertension is probably important, albeit overlooked, a potential contributor to poor control of hypertension among stroke survivors. Although studies report differences in prevalence of hypertension in stroke survivors between those European and African ancestry,17 few studies have specifically evaluated the burden and factors contributing to presence of resistant hypertension in patients that have had a stroke, particularly within patients of African ancestry in comparison to other groups.18–20 Understanding the contribution of race and geographical location to the burden of resistant hypertension among stroke survivors may identify an important epidemiological and biological determinants for ameliorating poor outcomes for stroke survivors, so this is very important question to investigate. The main objective of this study is to evaluate the prevalence and predictors of aTRH poststroke in study subjects from the United States and Ghana, and by stroke types to determine if the previously report stroke risks reported in patients of African ancestry is associated with an increased prevalence of aTRH.
MATERIALS AND METHODS
Study design
This is a cross-sectional study using the review board and ethically approved stroke registry data from the University of Florida institutional AHA Get With The Guidelines (GWTG) Database in the United States and the Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. Data from the University of Florida were collected between January 2014 and November 2019, while data from Ghana were collected between January 2018 and March 2020. This work represents the authors’ independent analysis of local or multicenter data gathered using the AHA GWTG Patient Management Tool but is not an analysis of the national GWTG dataset and does not represent findings from the AHA GWTG National Program.
Stroke data collection and vascular risk factor harmonization for the US University of Florida GWTGS stroke database and Ghanaian cohort
Data included in the GWTG stroke registry was obtained from clinical record of patients admitted at the University of Florida based using the nationally accepted GWTG stroke metrics. For the Ghanaian cohort, stroke type was identified using head CT scans acquired up to 10-day poststroke, divided into the following subtypes including subarachnoid hemorrhage, intracerebral hemorrhage, ischemic stroke, or undefined for those lacking neuroimaging data in Ghana. Subjects without neuroimaging were diagnosed with stroke based on the World Health Organization definition,21 using the 8-item questionnaire for verifying stroke-free status (QVSFS) as previously described.22
We also collected demographic data such as age, sex, location of residence (urban or rural), and lifestyle factors such as alcohol use and cigarette smoking. As previously describe, presence of DM was based on clinical history, medication use for glucose management, fasting glucose, or hemoglobin A1c values.20,23 Similarly, patients were determined to have hypercholesterolemia based on National Cholesterol Education Program (NCEP) guidelines low-density lipoprotein (LDL)-C levels,24 clinical history, or prestroke statin use. As previously described, the diagnosis of hypertension was given to patients that had a BP ≥140/90 mm Hg or were taking on antihypertensives prestroke.20 As previously note, a BP reading ≥140/90 mm Hg while taking 3 or more antihypertensives or being prescribed 4 or more antihypertensive medications regardless of BP was criteria for aTRH.15
Statistical analysis
Significant differences in patient demographics between study participates by race or aTRH diagnosis for nonnormally distributed variables were compared using nonparametric t-tests (Kruskal–Wallis test for >2 groups or the Mann–Whitney’s U-test), and differences in proportions assessed using the chi-squared tests or Fisher’s exact test when appropriate. Logistic regression was used to identify variables significantly associated with aTRH, including variables in the model obtained selected from empirical data after bivariate analyses and prespecified known risk factors for aTRH. Variables included in our unadjusted regression models included: age, gender, stroke type, DM, high LDL, location of residence (urban vs. rural), alcohol use, and smoking. Factors attaining a P value <0.20 in bivariate analyses were included in the adjusted multivariate logistic regression models. We have also conducted a sensitivity analysis with a BP cutoff of 130/80 mm Hg to account for temporal differences that be present by the nature of data collection between sites occurred in the context of clinical guideline updates. Two-tailed P values <0.05 were considered significant. Statistical analysis was performed using SAS software version 15.1.
RESULTS
Comparison of demographic and clinical features by race and geographical location of stroke survivors
Harmonized data were available for 3,365 stroke survivors, of which 943 (28.0%) were indigenous Africans from Ghana, 558 (16.6%) African Americans, and 1,864 (55.4%) non-Hispanic Caucasians. Table 1 shows a comparison of demographic and clinical characteristics by race and geographic location of stroke survivors. Briefly, the median age (interquartile range) of Ghanaian stroke survivors was 59 (49–68) years, compared with 61 (55–72) years for African Americans and 70 (62–78) years for non-Hispanic Caucasians, P < 0.0001. African American stroke survivors (64%) were more likely to reside in urban residence followed by non-Hispanic Caucasians (57%) and then indigenous Africans (56%). The proportion of ischemic strokes were 75% among indigenous Africans, 79% among African Americans, and 80% among non-Hispanic Caucasians. African Americans most frequently reported cigarette smoking, most likely to have DM and elevated LDL-cholesterol, while indigenous Africans were most likely to have hypertension and report alcohol use (Table 1). These findings were also significant when comparing Indigenous Africans are also compared with African Americans in Table 1.
Table 1.
Comparison of demographic and clinical characteristics of stroke survivors by race and geographical location
| Characteristic | Indigenous Africans (IA) N = 943 |
African Americans (AA) N = 558 |
Non-Hispanic Caucasians N = 1,864 |
P value (comparison of IA vs. AA) | P value (comparison of 3 groups) |
|---|---|---|---|---|---|
| Age in years, median (IQR) | 59 (49–68) | 61 (55–72) | 70 (62–78) | <0.0001 | <0.0001 |
| Male, n (%) | 475 (50.4) | 292 (52.3) | 1,019 (54.8) | 0.4400 | 0.0821 |
| Urban location, n (%) | 514 (55.8) | 352 (63.9) | 1,041 (56.6) | 0.0019 | 0.0043 |
| Stroke types, n (%) | 0.0626 | 0.0220 | |||
| Ischemic stroke | 710 (75.3) | 443 (79.4) | 1,486 (79.7) | ||
| Intracerebral hemorrhage | 233 (24.7) | 115 (20.6) | 378 (20.3) | ||
| Smoking, n (%) | 47 (5.3) | 123 (22.0) | 353 (18.9) | <0.0001 | <0.0001 |
| Alcohol use, n (%) | 218 (24.4) | 75 (13.4) | 153 (8.2) | <0.0001 | <0.0001 |
| High blood pressure at discharge, n (%) | 610 (64.7) | 222 (39.8) | 698 (37.5) | <0.0001 | <0.0001 |
| Diabetes mellitus, n (%) | 253 (26.8) | 246 (44.1) | 589 (31.6) | <0.0001 | <0.0001 |
| High LDL-cholesterol, n (%) | 342 (36.3) | 237 (42.5) | 658 (35.3) | 0.0201 | 0.0081 |
Abbreviation: IQR, interquartile range.
Prevalence and characteristics of aTRH
The overall frequency of aTRH is 18.5% (95% CI: 17.2%–19.8%) being 42.7% (95% CI: 39.6%–46.0%) among indigenous Africans, 16.1% (95% CI: 13.2%–19.5%) among African Americans, and 6.9% (95% CI: 5.8%–8.2%) among non-Hispanic Caucasians, P < 0.0001. Stroke survivors with aTRH were significantly younger with a median (interquartile range) age of 58 (49–70) years compared with 67 (57–76) years those without aTRH, P < 0.0001; were significantly more likely to report alcohol use (20.5% vs. 11.9%), but less likely to report smoking (7.7% vs. 17.6%) than those without aTRH (Table 2).
Table 2.
Comparison of patient demographic and clinical characteristics of stroke survivors according to apparent treatment-resistant hypertension status by race and geographical location
| Characteristic | Apparent treatment-resistant hypertension N = 622 |
No apparent treatment-resistant hypertension N = 2,743 |
P value |
|---|---|---|---|
| Age in years, median (IQR) | 58 (49–70) | 67 (57–76) | <0.0001 |
| Male, n (%) | 352 (56.6) | 1,434 (52.3) | 0.05 |
| Cohort, n (%) | <0.0001 | ||
| Ghana (indigenous Africans) | 403 (64.8) | 540 (19.7) | |
| United States (African Americans) | 90 (14.5) | 468 (17.1) | |
| United States (non-Hispanic Caucasians) | 129 (20.7) | 1,735 (63.3) | |
| Urban location of residence, n (%) | 326 (53.7) | 1,581 (58.5) | 0.03 |
| Smoker, n (%) | 46 (7.7) | 477 (17.6) | <0.0001 |
| Alcohol use, n (%) | 123 (20.5) | 323 (11.9) | <0.0001 |
| Stroke types | <0.0001 | ||
| Ischemic stroke | 418 (67.2) | 2,221 (81.0) | |
| Intracerebral hemorrhage | 204 (32.8) | 522 (19.0) | |
| Diabetes mellitus comorbidity, n (%) | 203 (32.6) | 885 (32.3) | 0.86 |
| High LDL, n (%) | 234 (37.6) | 1,003 (36.6) | 0.62 |
| Classes of antihypertensive | |||
| ACE-I | 269 (43.3) | 1,248 (45.5) | 0.31 |
| ARB | 323 (51.9) | 523 (19.1) | <0.0001 |
| Beta blockers | 372 (59.8) | 1,239 (45.2) | <0.0001 |
| Calcium channel blockers | 600 (96.5) | 1,217 (44.4) | <0.0001 |
| Other hypertensive agents | 501 (80.6) | 348 (12.7) | <0.0001 |
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; IQR, interquartile range.
Risk factors for aTRH poststroke and stroke types
In a multivariable logistic regression analysis, we identified 5 independent factors associated with aTRH. The factors adversely associated with aTRH include being an indigenous African or African American, intracerebral hemorrhage compared with ischemic stroke, and cigarette smoking while being female, and increasing age protective against aTRH (Table 3). Among those with ischemic strokes, 4 factors, namely age, Black race, DM, and smoking, were independently associated with aTRH (Table 4). Among stroke survivors with intracerebral hemorrhage, 3 factors: female sex, Black race, and cigarette smoking, were associated with aTRH (Table 5).
Table 3.
Factors associated with apparent treatment-resistant hypertension among stroke survivors in the United States and Ghana
| Factor | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
|---|---|---|---|---|
| Age, each year rise | 0.97 (0.96–0.97) | <0.0001 | 0.99 (0.98–0.99) | 0.0015 |
| Female sex | 0.86 (0.72–1.03) | 0.11 | 0.70 (0.56–0.87) | 0.0013 |
| Rural residence | 1.19 (1.00–1.43) | 0.05 | 1.16 (0.94–1.43) | 0.1709 |
| Racial (vs. non-Hispanic Caucasian) | ||||
| Indigenous African | 9.87 (7.89–12.35) | <0.0001 | 4.42 (3.41–5.73) | <0.0001 |
| African American | 2.54 (1.90–3.39) | <0.0001 | 2.44 (1.81–3.29) | <0.0001 |
| Intracerebral hemorrhage | 2.07 (1.70–2.52) | <0.0001 | 1.98 (1.57–2.48) | <0.0001 |
| Diabetes mellitus | 1.01 (0.83–1.22) | 0.94 | ||
| High LDL | 0.94 (0.78–1.12) | 0.47 | ||
| Alcohol use | 0.52 (0.42–0.66) | <0.0001 | 0.91 (0.68–1.23) | 0.5374 |
| Smoking | 2.66 (1.92–3.67) | <0.0001 | 1.98 (1.36–2.90) | 0.0004 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Table 4.
Risk factors of aTRH among stroke survivors by ischemic stroke
| Factor | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
|---|---|---|---|---|
| Age, each year rise | 0.97 (0.97–0.98) | <0.0001 | 0.98 (0.97–0.99) | 0.0007 |
| Female sex | 1.05 (0.85–1.30) | 0.67 | ||
| Rural residence | 1.05 (0.85–1.31) | 0.64 | ||
| Racial (vs. non-Hispanic Caucasian) | ||||
| Indigenous African | 10.88 (8.27–14.33) | <0.0001 | 4.81 (3.54–6.55) | <0.0001 |
| African American | 2.83 (2.00–4.02) | <0.0001 | 2.54 (1.78–3.61) | <0.0001 |
| Diabetes mellitus | 0.79 (0.63–0.98) | 0.04 | 0.62 (0.48–0.79) | 0.0002 |
| High LDL | 1.05 (0.84–1.31) | 0.65 | ||
| Alcohol use | 0.66 (0.49–0.89) | 0.01 | 0.88 (0.61–1.26) | 0.4826 |
| Smoking | 2.63 (1.80–3.83) | <0.0001 | 1.56 (1.02–2.39) | 0.0406 |
Abbreviations: aTRH, apparent treatment-resistant hypertension; CI, confidence interval; OR, odds ratio.
Table 5.
Risk factors of aTRH among stroke survivors by intracerebral hemorrhage
| Factor | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
|---|---|---|---|---|
| Age, each year rise | 0.96 (0.95–0.97) | <0.0001 | 0.99 (0.98–1.01) | 0.42 |
| Female sex | 0.54 (0.39–0.76) | 0.0005 | 0.60 (0.40–0.89) | 0.01 |
| Rural residence | 1.47 (1.05–2.04) | 0.02 | 1.39 (0.96–2.01) | 0.09 |
| Racial (vs. non-Hispanic Caucasian) | ||||
| Indigenous African | 7.91 (5.30–11.82) | <0.0001 | 5.97 (3.68–9.70) | <0.0001 |
| African American | 2.08 (1.22–3.54) | 0.007 | 1.92 (1.09–3.37) | 0.02 |
| Diabetes mellitus | 1.52 (0.98–2.37) | 0.06 | 1.14 (0.69–1.87) | 0.61 |
| High LDL | 0.49 (0.35–0.71) | 0.0001 | 0.72 (0.48–1.08) | 0.11 |
| Alcohol use | 0.43 (0.29–0.63) | <0.0001 | 0.78 (0.47–1.32) | 0.36 |
| Smoking | 2.23 (1.18–4.23) | 0.01 | 2.27 (1.09–4.72) | 0.03 |
Abbreviations: aTRH, apparent treatment-resistant hypertension; CI, confidence interval; OR, odds ratio.
Prevalence and factors associated with aTRH according to new AHA/ACC BP cutoffs
Using the new ACC/AHA guideline BP control cutoff of <130/80 mm Hg, the prevalence of aTRH was 22.1%, being 48% among indigenous Africans, 20.8% among African Americans, and 9.3% among non-Hispanic Caucasians, P < 0.0001. Using the new guideline, we identified 4 independent factors: increasing age, non-Hispanic Caucasians, DM, and cigarette smoking (Table 6).
Table 6.
Risk factors of aTRH among stroke survivors by new HTN guidelines
| Factor | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
|---|---|---|---|---|
| Age, each year rise | 0.97 (0.96–0.97) | <0.0001 | 0.99 (0.98–0.99) | 0.0002 |
| Female sex | 0.92 (0.78–1.09) | 0.33 | ||
| Rural residence | 1.16 (0.98–1.37) | 0.09 | 1.11 (0.94–1.39) | 0.1709 |
| Racial (vs. non-Hispanic Caucasian) | ||||
| Indigenous African | 8.93 (7.27–10.97) | <0.0001 | 3.69 (2.91–4.67) | <0.0001 |
| African American | 2.52 (1.95–3.27) | <0.0001 | 2.40 (1.84–3.14) | <0.0001 |
| Intracerebral hemorrhage | 2.42 (2.02–2.92) | <0.0001 | 2.30 (1.87–2.84) | <0.0001 |
| Diabetes mellitus | 1.00 (0.84–1.19) | 0.99 | ||
| High LDL | 1.01 (0.85–1.20) | 0.93 | ||
| Alcohol use | 0.54 (0.43–0.67) | <0.0001 | 0.84 (0.64–1.11) | 0.2130 |
| Smoking | 2.34 (1.77–3.11) | <0.0001 | 1.79 (1.28–2.50) | 0.0007 |
Abbreviations: aTRH, apparent treatment-resistant hypertension; CI, confidence interval; OR, odds ratio.
DISCUSSION
aTRH was prevalent among 18.5% (95% CI: 17.2%–19.8%) of stroke survivors from the United States and Ghana. This overall prevalence is partitioned into 43% among indigenous Africans, 16% among African Americans, and 7% among non-Hispanic Caucasians. Using the updated guideline definition with a BP cutoff of 130/80 mm Hg from the AHA/ACC, we obtained a prevalence of 22% along the same gradient of 48% among indigenous Africans, 21% among African Americans, and 9% among Caucasians. Our data are largely in consonance with the 2018 Scientific Statement from the AHA on the prevalence of aTRH based on the National Health and Nutrition Examination Survey, which found a prevalence of 17.7% using a BP cutoff of >140/90 mm Hg in 2008, increasing to 19.7% using >130/80 mm Hg in 2018.16
Resistant hypertension is characterized by the clustering of unique demographics, comorbidities, physiological perturbations, and metabolic abnormalities. In a large ethnically diverse hypertension population in the United States, demographic covariates linked to resistant hypertension included Black race, male sex, and older age.19 Regarding the racial contribution of resistant hypertension among the population of interest in the present study (i.e., stroke survivors), we found that indigenous Africans and African Americans had adjusted odds ratios of 4.4 and 2.4, respectively, relative to Caucasians. The associations between Black race and risk of aTRH persisted in sensitivity analyses by stroke types. While, on the one hand, racial/genetic underpinnings may explain the higher burden of aTRH among Blacks than Caucasians, on the other hand, ecological and social determinants may account for the substantially greater burden of aTRH among indigenous Africans relative to African Americans. Hence, the inordinately higher burden of resistant hypertension among indigenous Africans compared with African Americans could be due to contextual differences in healthcare access for treatment of hypertension, physician practices, adherence to medications, and sociocultural practices, potential confounders which were not measured in our study. Indeed the contribution of psychosocial determinants to resistant hypertension has been previously documented in the Jackson Heart Study.25 In addition, over the past 3 decades, there has been a steady improvement in population-level control of BP in the United States which may contribute to a lower prevalence of uncontrolled BP in the US cohort unlike the situation in sub-Saharan Africa.26 In sub-Saharan Africa, the awareness, detection and control of hypertension are abysmal and is a strong contributor to the significant surge in stroke burden on the continent.27
Intracerebral hemorrhage is generally more closely associated with hypertension than ischemic stroke. Hence, the observed association between resistant hypertension and intracerebral hemorrhage (ICH) relative to ischemic is intriguing and may provide some insights into why some individuals with hypertension are more prone to the more devastating ICH stroke type rather than ischemic stroke. Although other mechanisms such as structural malformations, medication use, and amyloid angiopathy may all contribute to the occurrence of intracerebral hemorrhage, hypertension is by far the most potent risk factor for this stroke type. It is tempting to speculate that resistant forms of hypertension may potentially accentuate a predisposition towards intracerebral hemorrhage relative to ischemic strokes. Furthermore, cigarette smoking was associated with 85% higher odds of aTRH among stroke survivors overall, with odds being 63% higher among ischemic stroke survivors and 127% higher among intracerebral hemorrhage survivors. Cigarette smoking promotes both atherosclerosis and arteriolosclerosis, which are implicated in the pathophysiology of the 2 primary stroke types. We rather found an inverse association between age and risk of resistant hypertension among stroke survivors, probably due to the higher representation of indigenous Africans, who experience stroke 15 years earlier than those in the United States.6,17,28,29 Probably, for similar demographic reasons, DM was however found to be protective against resistant hypertension in the present study, given the lower prevalence of DM among indigenous Africans with stroke relative to the US cohort.
Implications
Several studies have now explicitly demonstrated adverse associations between aTRH and risk of cardiovascular mortality and indeed all-cause mortality.30–35 In fact, it has been worryingly shown in a recent study that the risk of cardiovascular disease deaths remains significantly higher even among individuals with controlled BP on 3 classes of antihypertensive medications as well as those with controlled resistant hypertension on 4 or more antihypertensive medications.36 If the outcomes among individuals with resistant hypertension without cardiovascular disease events are worse as these previous studies suggest, then one could speculate that outcomes among stroke survivors with resistant hypertension may be even dire. Unfortunately, the literature is deficient in the short- and long-term cardiovascular, neurocognitive, quality of life, and mortality outcomes of resistant hypertension, specifically among stroke survivors. Although the technicalities of defining true resistant hypertension instead of apparent resistant hypertension (RH) require that adherence, medication doses, and white-coat hypertension be ruled out, apparent RH nonetheless is lethal as borne out by previous studies. Hence, clinicians managing stroke survivors should adopt an aggressive approach to achieving control of BP among those with aTRH while more studies are encouraged to understand the biology, treatment, and outcomes of resistant hypertension. Given that using a cutoff BP of 140/90 mm Hg for defining resistant hypertension is associated with poorer outcomes, it would be an interesting proposition to consider the longer-term outcomes of resistant hypertension using the lower BP cutoff of 130/80 mm Hg as recommended by the AHA/ACC.
Limitations
This study has limitations worth noting. First, we cannot draw causal associations between factors identified for resistant hypertension among stroke survivors due to the cross-sectional design of the study. Second, the doses of antihypertensive medications and adherence to therapy were not available for analysis; hence, we have assessed aTRH, terminology used in epidemiological studies when such data are unavailable. Thirdly, data on kidney function such as estimated glomerular filtration rate were not available for us to include in our models. It is possible, given the racial differences in predisposition to renal dysfunction, that the differences in the prevalence of resistant hypertension by race observed in our study may be differential exposure to kidney disease. There may be other unmeasured confounders such as differences in care provider practices in the 2 countries, availability of and access to antihypertensive medications may differ as well as assessments of behavioral patterns such as alcohol use, dietary practices, and so forth. These unmeasured factors may potentially have biased our study findings and should be borne in mind in the interpretation of our findings. For example, the data from the Ghanaian cohort emanated from a neurology clinic where follow-up care for BP management is under the auspices of neurology trainee residents and 1 neurologist. In other centers in Ghana, stroke survivors are cared for by general practitioners. Thus said, clinicians in the practices in Ghana and even the United States may be vary in the rigor of their investigative approach for diagnosis of potential causes of resistant hypertension. In spite of these limitations, a major strength of our study is its transcontinental design, the inclusion of both ischemic and hemorrhagic stroke types, and large sample size. Also, since Florida is known to have a significant prevalence of hypertension with a prevalence of 37.9% in non-Hispanic Caucasians and 35.9% in African Americans,37 and highest in the North Florida region which our institution primarily serves,38 we are able to evaluate for differences within our databases. We highlight that resistant hypertension is highly prevalent among indigenous Africans and could only worsen the already poor outcomes of stroke in Africa. It is worth also mentioning that the prevalence of resistant hypertension may also vary in prevalence within different regions within African and has changed overtime.37,39 Among Ghanaian stroke survivors, resistant hypertension has been recently demonstrated to be independently associated with long-term survival.40 Further studies are needed to characterize the genetic and epigenetic ancestry41,42 and to tailor treatment of resistant hypertension using renin–aldosterone profile in those with perturbations on this axis. Also, more studies are needed to investigate how prenatal care and maternal fetal health may play a role in the development of resistant hypertension in those of African ancestry.43
There are significant racial and geographic disparities in the burden of resistant hypertension among stroke survivors which may in part explain the poorer cardiovascular and neurocognitive outcomes of individuals of the Black race. For stroke survivors with aTRH, opportunities exist for pharmacological tailoring of antihypertensive therapy and lifestyle interventions to help bring BP under control. Further studies on the patient-level, practice gaps, and policy-level determinants of resistant forms of hypertension are needed to address this severe phenotype of hypertension across the globe.
Contributor Information
Fred Stephen Sarfo, Department of Medicine, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Esther Olasoji, Department of Neurology, University of Florida, Gainesville, Florida, USA.
Grant P Banfill, Department of Neurology, University of Florida, Gainesville, Florida, USA.
Bruce Ovbiagele, Department of Neurology, University of California, San Francisco, San Francisco, California, USA.
Alexis N Simpkins, Department of Neurology, University of Florida, Gainesville, Florida, USA.
FUNDING
F.S.S. and B.O. are support by a grant from the National Heart, Lung, and Blood Institute (R01HL152188).
DISCLOSURES and CONFLICTS of INTEREST
The authors have nothing to disclose and no conflicts of interest to report.
REFERENCES
- 1. Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, Mensah GA, Norrving B, Shiue I, Ng M, Estep K, Cercy K, Murray CJL, Forouzanfar MH. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol 2016; 15:913–924. [DOI] [PubMed] [Google Scholar]
- 2. O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, Lopez-Jaramillo P, Damasceno A, Langhorne P, McQueen MJ, Rosengren A, Dehghan M, Hankey GJ, Dans AL, Elsayed A, Avezum A, Mondo C, Diener HC, Ryglewicz D, Czlonkowska A, Pogosova N, Weimar C, Iqbal R, Diaz R, Yusoff K, Yusufali A, Oguz A, Wang X, Penaherrera E, Lanas F, Ogah OS, Ogunniyi A, Iversen HK, Malaga G, Rumboldt Z, Oveisgharan S, Al Hussain F, Magazi D, Nilanont Y, Ferguson J, Pare G, Yusuf S. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 2016; 388:761–775. [DOI] [PubMed] [Google Scholar]
- 3. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014; 129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clark D III, Colantonio LD, Min YI, Hall ME, Zhao H, Mentz RJ, Shimbo D, Ogedegbe G, Howard G, Levitan EB, Jones DW, Correa A, Muntner P. Population-attributable risk for cardiovascular disease associated with hypertension in black adults. JAMA Cardiol 2019; 4:1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Owolabi MO, Sarfo F, Akinyemi R, Gebregziabher M, Akpa O, Akpalu A, Wahab K, Obiako R, Owolabi L, Ovbiagele B. Dominant modifiable risk factors for stroke in Ghana and Nigeria (SIREN): a case-control study. Lancet Glob Health 2018; 6:e436–e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2005; 111:1327–1331. [DOI] [PubMed] [Google Scholar]
- 8. Markus HS, Khan U, Birns J, Evans A, Kalra L, Rudd AG, Wolfe CD, Jerrard-Dunne P. Differences in stroke subtypes between black and white patients with stroke: the South London Ethnicity and Stroke Study. Circulation 2007; 116:2157–2164. [DOI] [PubMed] [Google Scholar]
- 9. Flaherty ML, Woo D, Haverbusch M, Sekar P, Khoury J, Sauerbeck L, Moomaw CJ, Schneider A, Kissela B, Kleindorfer D, Broderick JP. Racial variations in location and risk of intracerebral hemorrhage. Stroke 2005; 36:934–937. [DOI] [PubMed] [Google Scholar]
- 10. Batuman V. Salt and hypertension: an evolutionary perspective. J Hypertens Open Access 2012; 1:1–3. doi: 10.4172/2167-1095.1000e106 [DOI] [Google Scholar]
- 11. Grim CE, Robinson M. Salt, slavery and survival—hypertension in the African diaspora. Epidemiology 2003; 14:120–122; discussion 124–126. [DOI] [PubMed] [Google Scholar]
- 12. Jones ES, Spence JD, McIntyre AD, Nondi J, Gogo K, Akintunde A, Hackam DG, Rayner BL. High frequency of variants of candidate genes in Black Africans with low renin-resistant hypertension. Am J Hypertens 2017; 30:478–483. [DOI] [PubMed] [Google Scholar]
- 13. Bochud M, Staessen JA, Maillard M, Mazeko MJ, Kuznetsova T, Woodiwiss A, Richart T, Norton G, Thijs L, Elston R, Burnier M. Ethnic differences in proximal and distal tubular sodium reabsorption are heritable in black and white populations. J Hypertens 2009; 27:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tu W, Eckert GJ, Hannon TS, Liu H, Pratt LM, Wagner MA, Dimeglio LA, Jung J, Pratt JH. Racial differences in sensitivity of blood pressure to aldosterone. Hypertension 2014; 63:1212–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526. [DOI] [PubMed] [Google Scholar]
- 16. Carey RM, Sakhuja S, Calhoun DA, Whelton PK, Muntner P. Prevalence of apparent treatment-resistant hypertension in the United States. Hypertension 2019; 73:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Owolabi M, Sarfo F, Howard VJ, Irvin MR, Gebregziabher M, Akinyemi R, Bennett A, Armstrong K, Tiwari HK, Akpalu A, Wahab KW, Owolabi L, Fawale B, Komolafe M, Obiako R, Adebayo P, Manly JM, Ogbole G, Melikam E, Laryea R, Saulson R, Jenkins C, Arnett DK, Lackland DT, Ovbiagele B, Howard G. Stroke in indigenous Africans, African Americans, and European Americans: interplay of racial and geographic factors. Stroke 2017; 48:1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Howard VJ, Tanner RM, Anderson A, Irvin MR, Calhoun DA, Lackland DT, Oparil S, Muntner P. Apparent treatment-resistant hypertension among individuals with history of stroke or transient ischemic attack. Am J Med 2015; 128:707–714.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sim JJ, Bhandari SK, Shi J, Liu IL, Calhoun DA, McGlynn EA, Kalantar-Zadeh K, Jacobsen SJ. Characteristics of resistant hypertension in a large, ethnically diverse hypertension population of an integrated health system. Mayo Clin Proc 2013; 88:1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sarfo FS, Ovbiagele B. Apparent treatment resistant hypertension among stroke survivors in Ghana. J Stroke Cerebrovasc Dis 2020; 29:105401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health O. Cerebrovascular Disorders: A Clinical and Research Classification. Geneva: World Health Organization, 1978. https://apps.who.int/iris/handle/10665/37194 [Google Scholar]
- 22. Sarfo F, Gebregziabher M, Ovbiagele B, Akinyemi R, Owolabi L, Obiako R, Akpa O, Armstrong K, Akpalu A, Adamu S, Obese V, Boa-Antwi N, Appiah L, Arulogun O, Mensah Y, Adeoye A, Tosin A, Adeleye O, Tabi-Ajayi E, Phillip I, Sani A, Isah S, Tabari N, Mande A, Agunloye A, Ogbole G, Akinyemi J, Laryea R, Melikam S, Uvere E, Adekunle G, Kehinde S, Azuh P, Dambatta A, Ishaq N, Saulson R, Arnett D, Tiwari H, Jenkins C, Lackland D, Owolabi M. Multilingual validation of the questionnaire for verifying stroke-free status in West Africa. Stroke 2016; 47:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15:539–553. [DOI] [PubMed] [Google Scholar]
- 24. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421. [PubMed] [Google Scholar]
- 25. Shallcross AJ, Butler M, Tanner RM, Bress AP, Muntner P, Shimbo D, Ogedegbe G, Sims M, Spruill TM. Psychosocial correlates of apparent treatment-resistant hypertension in the Jackson Heart Study. J Hum Hypertens 2017; 31:474–478. [DOI] [PubMed] [Google Scholar]
- 26. Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation 2011; 124:1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sarfo FS. Associations between hypertension knowledge, awareness, and treatment and stroke occurrence across the globe: time to act on what we know. Heart 2020; 107:264–5. doi: 10.1136/heartjnl-2020-318019 [DOI] [PubMed] [Google Scholar]
- 28. Sarfo FS, Ovbiagele B, Gebregziabher M, Wahab K, Akinyemi R, Akpalu A, Akpa O, Obiako R, Owolabi L, Jenkins C, Owolabi M. Stroke among young West Africans: evidence from the SIREN (Stroke Investigative Research and Educational Network) large multisite case-control study. Stroke 2018; 49:1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sarfo FS, Ovbiagele B, Gebregziabher M, Akpa O, Akpalu A, Wahab K, Ogbole G, Akinyemi R, Obiako R, Komolafe M, Owolabi L, Lackland D, Arnett D, Tiwari H, Markus HS, Akinyemi J, Oguntade A, Fawale B, Adeoye A, Olugbo O, Ogunjimi L, Osaigbovo G, Jenkins C, Chukwuonye I, Ajose O, Oyinloye L, Mutiso F, Laryea R, Calys-Tagoe B, Salaam A, Amusa G, Olowookere S, Imoh C, Mande A, Arulogun O, Adekunle F, Appiah L, Balogun O, Singh A, Adeleye O, Ogah O, Makanjuola A, Owusu D, Kolo P, Adebayo O, Agunloye A, Shidali V, Faniyan M, Lakoh S, Diala S, Iheonye H, Efidi C, Sanya E, Sunmonu T, Akintunde A, Owolabi M. Unraveling the risk factors for spontaneous intracerebral hemorrhage among West Africans. Neurology 2020; 94:e998–e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muntner P, Davis BR, Cushman WC, Bangalore S, Calhoun DA, Pressel SL, Black HR, Kostis JB, Probstfield JL, Whelton PK, Rahman M. Treatment-resistant hypertension and the incidence of cardiovascular disease and end-stage renal disease: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension 2014; 64:1012–1021. [DOI] [PubMed] [Google Scholar]
- 31. Smith SM, Huo T, Gong Y, Handberg E, Gulati M, Merz CN, Pepine CJ, Cooper-DeHoff RM. Mortality risk associated with resistant hypertension among women: analysis from three prospective cohorts encompassing the spectrum of women’s heart disease. J Womens Health (Larchmt) 2016; 25:996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith SM, Huo T, Delia Johnson B, Bittner V, Kelsey SF, Vido Thompson D, Noel Bairey Merz C, Pepine CJ, Cooper-Dehoff RM. Cardiovascular and mortality risk of apparent resistant hypertension in women with suspected myocardial ischemia: a report from the NHLBI-sponsored WISE Study. J Am Heart Assoc 2014; 3:e000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bangalore S, Fayyad R, Laskey R, Demicco DA, Deedwania P, Kostis JB, Messerli FH. Prevalence, predictors, and outcomes in treatment-resistant hypertension in patients with coronary disease. Am J Med 2014; 127:71–81.e1. [DOI] [PubMed] [Google Scholar]
- 34. Sim JJ, Bhandari SK, Shi J, Reynolds K, Calhoun DA, Kalantar-Zadeh K, Jacobsen SJ. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int 2015; 88:622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Irvin MR, Booth JN III, Shimbo D, Lackland DT, Oparil S, Howard G, Safford MM, Muntner P, Calhoun DA. Apparent treatment-resistant hypertension and risk for stroke, coronary heart disease, and all-cause mortality. J Am Soc Hypertens 2014; 8:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaczmarski KR, Sozio SM, Chen J, Sang Y, Shafi T. Resistant hypertension and cardiovascular disease mortality in the US: results from the National Health and Nutrition Examination Survey (NHANES). BMC Nephrol 2019; 20:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gunderson J MD. 2019 Florida Behavioral Risk Factor Surveillance System (BRFSS) Data Book. Florida Department of Health Division of Community Health Promotion Public Health Research Section, 2019. https://www.floridahealth.gov/statistics-and-data/survey-data/florida-youth-survey/florida-youth-tobacco-survey/2019BRFSSDataBookFinal.pdf [Google Scholar]
- 38. Smith SM, McAuliffe K, Hall JM, McDonough CW, Gurka MJ, Robinson TO, Sacco RL, Pepine C, Shenkman E, Cooper-DeHoff RM. Hypertension in Florida: data from the OneFlorida clinical data research network. Prev Chronic Dis 2018; 15:E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Breet Y, Lackland DT, Ovbiagele B, Owolabi MO, Ogedegbe G, Kruger IM, Schutte AE. Is the cardiovascular health of South Africans today comparable with African Americans 45 years ago? J Hypertens 2019; 37:1606–1614. [DOI] [PubMed] [Google Scholar]
- 40. Sarfo FS, Ovbiagele B. Key determinants of long-term post-stroke mortality in Ghana. J Neurol Sci 2021; 434:120123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simpkins AN, Neeland IJ, Lavie CJ. Tipping the scales for older adults: time to consider body fat assessment and management for optimal atherosclerotic cardiovascular disease and stroke prevention? J Am Heart Assoc 2021; 10:e021307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Simpkins AN, Janowski M, Oz HS, Roberts J, Bix G, Doré S, Stowe AM. Biomarker application for precision medicine in stroke. Transl Stroke Res 2020; 11:615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Udenze IC. Association of pre-eclampsia with metabolic syndrome and increased risk of cardiovascular disease in women: a systemic review. Niger J Clin Pract 2016; 19:431–435. [DOI] [PubMed] [Google Scholar]