Abstract
BACKGROUND
Mean arterial pressure (MAP) drives ocular perfusion. Excessive 24-h MAP variability relates to glaucoma, however, whether this is due to dips or increases in the blood pressure (BP) is undocumented. We investigated the association of open-angle glaucoma (OAG) in relation to the 5 largest MAP dips/increases over 24-h, henceforth called dips/blips.
METHODS
In the Maracaibo Aging Study (MAS), 93 participants aged ≥40 y (women, 87.1%; mean age, 61.9 y) underwent baseline ophthalmological and 24-h ambulatory BP monitoring assessments. OAG was the presence of optic nerve damage and visual field defects. Statistical methods included logistic regression and the generalized R2 statistic. For replication, 48 OAG cases at the Leuven Glaucoma Clinic were matched with 48 controls recruited from Flemish population.
RESULTS
In the MAS, 26 participants had OAG. OAG compared to non-OAG participants experienced longer and deeper dips (116.5 vs. 102.7 minutes; to 60.3 vs. 66.6 mm Hg; −21.0 vs. −18.0 mm Hg absolute or 0.79 vs. 0.81 relative dip compared to the preceding reading). The adjusted odds ratios associated with dip measures ranged from 2.25 (95% confidence interval [CI], 1.31–4.85; P = 0.009) to 3.39 (95% CI, 1.36–8.46; P = 0.008). On top of covariables and 24-MAP level/variability, the dip measures increased the model performance (P ≤ 0.025). Blips did not associate with OAG. The case–control study replicated the MAS observations.
CONCLUSIONS
Dips rather than increases in the 24-h MAP level were associated with increased risk for OAG. An ophthalmological examination combined with 24-h BP monitoring might be precautious steps required in normotensive and hypertensive patients at risk of OAG.
Keywords: ambulatory blood pressure monitoring, blood pressure, blood pressure variability, dips, glaucomatous optic neuropathy, hypertension, open, angle glaucoma, population science
Graphical Abstract
Graphical Abstract.
Glaucoma is the leading cause of visual disability and irreversible blindness worldwide, affecting approximately 64.4 million people.1 The disease is characterized by chronic, progressive, and irremediable loss of the retinal ganglion cells, which leads to structural lesions of the optic nerve and visual field loss.2 To date, the only proven treatment for glaucoma remains lowering the intraocular pressure (IOP),2 but glaucomatous optic nerve injury can still progress or even arise when the IOP is within normal limits.2
Mean arterial pressure (MAP) drives the ocular perfusion.3 Systemic hypotension potentially aggravates the perfusion deficiency of the optic nerve head, thereby compromising oxygen supply and accelerating the loss of retinal ganglion cells.4 Moreover, excessive blood pressure variations might destabilize the ocular perfusion pressure even in patients with normal blood pressure levels.5 The 24-h ambulatory blood pressure monitoring is the state-of-the-art method to estimate BP level and variability.6,7 Although high 24-h blood pressure variability relates to open-angle glaucomatous optic damage,8,9 it is undocumented whether excessive dips or increases in the blood pressure around an averaged 24-h blood pressure level better relates to glaucomatous optic neuropathy. To test this hypothesis, we analyzed in the Maracaibo Aging Study (MAS) the association of open-angle glaucomatous optic neuropathy in relation to the 5 lowest and highest ambulatory MAP levels. We sought replication of the MAS findings in a case–control study with cases from the Leuven Eye Study10 and controls from the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO).11 Cases and controls in the replication study resided in Flanders, Belgium.
METHODS
Study participants
The MAS is a prospective population-based study of chronic age-related diseases which enrolled participants age ≥ 40 years of age from the Santa Lucia (since 1998) and the Santa Rosa de Agua (since 2010) neighborhoods of Maracaibo, Venezuela.12 The MAS focuses on ophthalmic, neurological, and cardiovascular diseases. Detailed methodology of the MAS is described elsewhere.12 The initial participation rate was 70.0%. From August 2011 until June 2016, 534 participants were additionally enrolled, using a sampling frame prioritizing the female ancestral lines with the intention to engage in genetic studies. In this study phase, we invited 130 adults, aged 40 years and older, to undergo ambulatory blood pressure monitoring and an in-depth eye examination, in which 114 (87.7%) accepted the invitation and underwent the assessments. The Institutional Review Boards of the Cardiovascular Institute at the University of Zulia, Maracaibo and Columbia University, New York, approved the MAS study, which complied with the declaration of Helsinki.13 All participants in this study phase of the MAS renewed written informed consent. We excluded 21 participants from analysis because they declined 24-h ambulatory blood pressure monitoring. Thus, the number of the MAS participants statistically analyzed totaled 93.
We sought to replicate our hypothesis using retrospective data of different cohorts of subjects. We included 48 patients with glaucoma selected from the database available at the Glaucoma Unit, University Hospitals Leuven, Belgium.10 Cases included normal- (n = 31) and high-tension primary open-angle glaucoma (n = 17) as they qualified in view of the disease continuum between the 2 entities.14,15 The only other selection criterion was the availability of a 24-h ambulatory blood pressure recording during an interval of 1.5 years between the ambulatory blood pressure assessment and glaucoma diagnosis. Exclusion criteria included patients with suspected, angle-closure, or pseudoexfoliative glaucoma, as well as ocular hypertension. These 48 glaucoma cases were matched for sex, age (±8 years), and 24-h MAP (±8 mm Hg) with healthy participants enrolled in FLEMENGHO,11 who were recruited from a defined geographical area in Northern Belgium. The Ethics Committee of the University Hospitals Leuven approved the secondary use of the data from the glaucoma patients (registration numbers, S65245 and B32220083510) as well as the secondary use of FLEMENGHO data (S58273 [ML4804] and B32220083510).
Ophthalmological examination
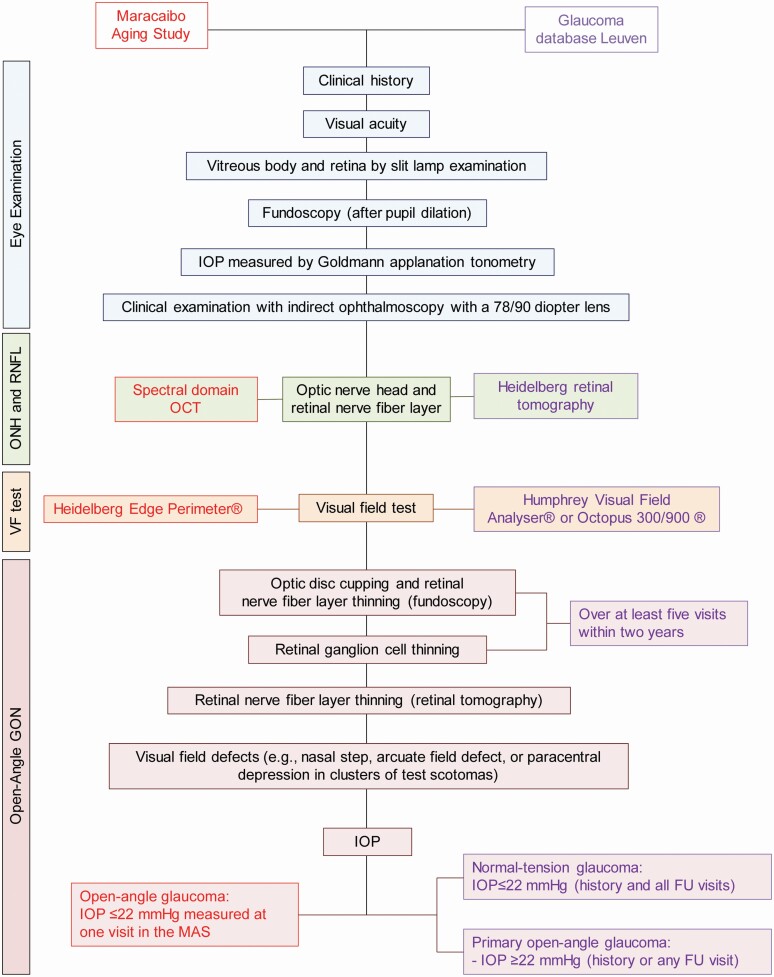
In both the MAS and Leuven Glaucoma Clinic, the eye examination was performed by an ophthalmologists specialized in glaucomatous eye disease (M.P. in the MAS). After history taking the ocular examination included measurement of visual acuity (with correction), an assessment of the lens, the vitreous body, and after pupil dilatation the visualization of the retina by slit lamp examination, fundoscopy, and indirect ophthalmoscopy with a 78/90 diopter lens (Figure 1). The optic nerve head and the retinal fiber layer was examined by spectral domain optic coherence tomography (Spectralis, Heidelberg Engineering GmbH, Heidelberg, Germany) and the Spectralis software, version 5.4.7.0 in the MAS and by Heidelberg Retinal Tomography (HRT3, Heidelberg Engineering GmbH) in Leuven (Figure 1). In the MAS, the visual field was tested using the Heidelberg Edge Perimeter and in Leuven by the Humphrey Visual Field Analyser (Carl Zeiss Meditec AG, Jena, Germany) or the Octopus 300/900 system (Haag-Streit AG, Köniz, Switzerland). In Leuven, a glaucoma expert, determined the nerve rim width or retinal ganglion cell thinning over at least 5 visits within 2 years, while in the MAS the eye examination took place at a single visit. However, all ocular tomographic images and visual field results obtained in the MAS participants were read a second time by an independent specialist (C.G.D.M.) at Columbia University, New York, resulting in κ-coefficients ≥ 0.90, indicating high inter-reader reproducibility.16
Figure 1.
Work flow applied in the diagnosis of glaucomatous optic nerve neuropathy. Abbreviations: FU, follow-up; GON, glaucomatous optic neuropathy; IOP, intraocular pressure; ONH, optic nerve head; RNFL denotes retinal nerve fiber layer; VF, visual field.
All patients in the MAS and in the Leuven Glaucoma Unit database had open-angle glaucoma as determined by gonioscopy (Figure 1). Glaucomatous optic neuropathy was significant retinal nerve fiber layer thinning, measured with spectral domain optical tomography, with or without visual field defects reflecting these lesions (e.g., nasal step, arcuate field defect, or paracentral depression in clusters of test scotomas). For analysis, in the MAS, cases of glaucomatous optic neuropathy with or without visual field defects were combined.
For FLEMENGHO controls, the absence of glaucoma was ascertained via the electronic medical records at the University Hospitals Leuven and the 4 regional hospitals in the study’s catchment area, and via the medical files of the general practitioners caring for FLEMENGHO participants.
Blood pressure
Office blood pressure, measured after study participants had rested for 5 minutes or longer in the sitting position, was the average of 5 consecutive oscillometric (MAS) or auscultatory (FLEMENGHO) readings, or the average of 3 readings at the initiation of the ambulatory recordings in the patients enrolled at the Leuven Glaucoma Clinic. Office hypertension was a blood pressure of ≥ 130 mm Hg systolic or ≥ 80 mm Hg diastolic, or use of antihypertensive drugs.6 The ambulatory blood pressure was recorded with validated oscillometric recorders: 90202 or 90207 Spacelabs monitors17 in MAS and FLEMENGHO and Mobil-O-Graph devices18 at the Leuven Glaucoma Clinic. Readings were programmed at 15-minute intervals during the day and at 30-minute intervals at night in the MAS and in cases recruited at the Leuven Glaucoma Clinic, and at 20-minute intervals during daytime and 40-minute intervals during nighttime in the controls enrolled in the FLEMENGHO study (Supplementary Table S1 online). The same SAS macro processed all ambulatory recordings, which stayed largely unedited. Readings with higher diastolic than systolic blood pressure were flagged with an error code were removed. We used the oscillometric estimated MAP from the automated ambulatory blood pressure monitoring. The within-participant 24-h MAP was a time-weighted average, giving a weight to each individual reading proportional to the interval to the previous reading.
Variability independent of the mean (VIM) was computed to capture the 24-h MAP variability. VIM was calculated as the within-participant SD divided by the 24-h mean to the power × and multiplied by the population mean to the power x.19 The power × was obtained by fitting a curve through a plot of the SD against the mean, using the model: SD = a × meanx, where × was derived by non-linear regression analysis. The value of × so obtained was 0.49 in the MAS, 1.79 in controls from FLEMENGHO study, and 1.54 in glaucoma the glaucoma cases from the Leuven Glaucoma Database. In addition, blood pressure variability as captured by single readings in an ambulatory recording was expressed relative to the within-participant 24-MAP mean and relative to consecutive readings in an individual ambulatory recording. To study extreme decreases and increases in MAP, the 5 readings with the largest drop or the greatest surge compared to the previous reading in individual 24-h recordings, henceforth called dips and blips, were selected for further analysis—the time elapsed in between was used to quantify the duration of dips/blips.
Statistical analysis
For database management and statistical analysis, we used SAS software, version 9.4, maintenance Level 5. We compared means by t-tests or Wilcoxon–Mann–Whitney, and proportions by Fisher’s exact test. Pearson correlations were computed between MAP level and variability and IOP. We identified potential covariables based on their biological relevance to glaucoma or their possible role as confounders.2,20 The association of glaucomatous optic neuropathy with MAP level and variability and the MAP dips/blips were estimated by logistic regression analysis. We constructed heat maps to visualize the contribution of 24-h MAP level and VIM to the probability of having glaucomatous optic neuropathy. Improvement in the fit of nested logistic models was assessed by the log likelihood-ratio and the generalized R2 statistic. The normal approximation was applied for between-group comparisons in the odds ratios. In sensitivity analyses, we constructed models additionally adjusted for the night-to-day MAP ratio or the nighttime MAP level. Significance was a 2-tailed α-level of ≤ 0.05.
RESULTS
Characteristics of MAS participants
Among 93 participants (Table 1), 26 (27.9%) had open-angle glaucomatous optic neuropathy in one (n = 3) or both (n = 23) eyes. Of participants with one eye affected, one had visual field defects; among participants with both eyes affected, visual field defects were present in at least one eye in 56.5%. The study population had a mean age of 62 years (range, 5th–95th percentile interval, 43–82 years) and included 81 women (87.1%). All participants had an office IOP within the normal range, and only one participant with glaucomatous optic neuropathy was under treatment with an IOP lowering collyrium (dorzolamide 2% plus Timolol 0.5%). Compared to participants with normal eyes (Table 1), glaucoma participants were older (73 vs. 58. years), less educated (6 vs. 9 years), tended to be leaner (26.6 vs. 28.8 kg/m2), had a higher high-density lipoprotein (HDL) serum cholesterol (47 vs. 43 mg/dl) and a higher prevalence of refractive error (57.7 vs. 34.3%). In the whole study population, 48.4% of participants were taking antihypertensive drugs as monotherapy (42.2%) or in combination (17.2%). The antihypertensive agents used (Supplementary Table S2 online) were diuretics in four (4.3%) participants, β-blockers in 9 (9.7%), calcium-channel blockers in 11 (11.8%), and angiotensin-conversion enzyme inhibitors in 31 (33.3%). Antihypertensive treatment did not differ between MAS participants with and without glaucoma (Supplementary Table S2 online). The 24-h heart rate levels were similar between participants with and without glaucoma (P = 0.258).
Table 1.
Characteristics of participants by glaucomatous neuropathy status
| Characteristic | All | GON | Normal eyes | P value |
|---|---|---|---|---|
| Number in group | 93 | 26 | 67 | |
| Characteristics of participants | ||||
| Women, n (%) | 81 (87.1) | 23 (88.5) | 58 (86.6) | 0.807 |
| Current smoking, n (%) | 6 (6.5) | 1 (3.9) | 5 (7.6) | 0.514 |
| Drinking alcohol, n (%) | 9 (9.7) | 3 (11.5) | 6 (9.0) | 0.705 |
| Refractive error, n (%) | 38 (40.9) | 15 (57.7) | 23 (34.3) | 0.040 |
| Office hypertension, n (%) | 78 (83.9) | 23 (88.5) | 55 (82.1) | 0.453 |
| Treated hypertension, n (%) | 44 (73.3) | 14 (70.0) | 30 (75.0) | 0.432 |
| Diabetes mellitus, n (%) | 13 (14.0) | 5 (19.2) | 8 (11.9) | 0.363 |
| Previous CV disease | 1 (1.08) | 0 (0.0) | 1 (1.50) | 0.531 |
| Mean/median of characteristics | ||||
| Age, y | 61.9 ± 13.3 | 70.9 ± 12.1 | 58.4 ± 12.1 | <0.001 |
| Years of education, y | 6 (4, 11) | 6 (3, 8) | 9 (4, 11) | 0.012 |
| Body mass index, kg/m2 | 28.2 ± 5.2 | 26.6 ± 5.9 | 28.8 ± 4.7 | 0.007 |
| Fasting serum glucose, mg/dl | 106.3 ± 27.5 | 105.8 ± 22.9 | 106.4 ± 29.3 | 0.871 |
| HbA1c, % | 5.92 ± 0.75 | 6.10 ± 1.01 | 5.86 ± 0.62 | 0.231 |
| Total serum cholesterol, mg/dl | 200.7 ± 47.7 | 198.8 ± 50.4 | 201.4 ± 46.9 | 0.813 |
| HDL serum cholesterol, mg/dl | 44.6 ± 11.2 | 47.2 ± 9.3 | 43.6 ± 11.8 | 0.042 |
| Serum creatinine, mg/dl | 0.92 ± 0.27 | 1.01 ± 0.31 | 0.88 ± 0.24 | 0.168 |
| Office systolic BP pressure, mm Hg | 141.0 ± 22.8 | 148.7 ± 26.1 | 138.0 ± 20.38 | 0.056 |
| Office diastolic BP pressure, mm Hg | 76.0 ± 8.3 | 74.4 ± 50.4 | 76.6 ± 7.5 | 0.250 |
| 24-h systolic BP, mm Hg | 122.2 ± 15.6 | 122.3 ± 14.8 | 122.1 ± 17.8 | 0.955 |
| 24-h diastolic BP, mm Hg | 70.6 ± 8.7 | 67.4 ± 8.8 | 71.9 ± 8.4 | 0.024 |
| 24-h heart rate, beats per minute | 71.9 ± 9.4 | 73.7 ± 10.5 | 71.2 ± 8.9 | 0.258 |
| 24-h MAP level, mm Hg | 89.0 ± 10.4 | 87.5 ± 12.1 | 89.5 ± 9.6 | 0.413 |
| 24-h MAP VIM, mm Hg | 10.3 ± 2.0 | 11.4 ± 2.1 | 9.9 ± 1.8 | <0.001 |
| Office IOP, mm Hg | 12.6 ± 2.8 | 13.1 ± 3.2 | 12.4 ± 2.6 | 0.385 |
Smoking and drinking habits and years of education were assessed by questionnaire. The biochemical measurements were performed by a single certified laboratory. Office hypertension was a blood pressure of ≥ 130 mm Hg systolic or ≥ 80 mm Hg diastolic, or use of antihypertensive drugs. Diabetes mellitus was a fasting glucose of 126 mg/dl or use of antidiabetic drugs. The central tendency and the spread of continuously distributed variables are represented by the arithmetic mean (±SD) or the median (interquartile range) and compared between GON patients and participants with normal eyes using a t-test or the Wilcoxon–Mann–Whitney test, as appropriate. To convert total and HDL serum cholesterol to mmol/l, multiply by 0.0259; to convert glucose to mmol/l, multiply by 0.056. Abbreviations: BP, blood pressure; CV, cardiovascular; GON, glaucomatous optic neuropathy; HDL, high-density lipoprotein; IOP, intraocular pressure; MAP, mean arterial pressure; VIM, variability independent of the mean.
24-H MAP level and variability in MAS
The median number of readings in the 24-h recordings was 70 (5th–95th percentile interval, 56–81; Supplementary Table S1 online). The correlation coefficients of IOP with 24-h MAP and VIMMAP were 0.042 (P = 0.690) and 0.061 (P = 0.561), respectively (Supplementary Table S3 online). The 24-h MAP level was similar in participants with and without glaucoma (87.5 vs. 89.5 mm Hg; Table 1), whereas VIMSBP (13.4 vs. 11.4 mm Hg; P = 0.002), VIMDBP (10.6 vs. 9.5 mm Hg; P = 0.012), and VIMMAP was higher in glaucoma patients (11.4 vs. 9.9 mm Hg; P < 0.001).
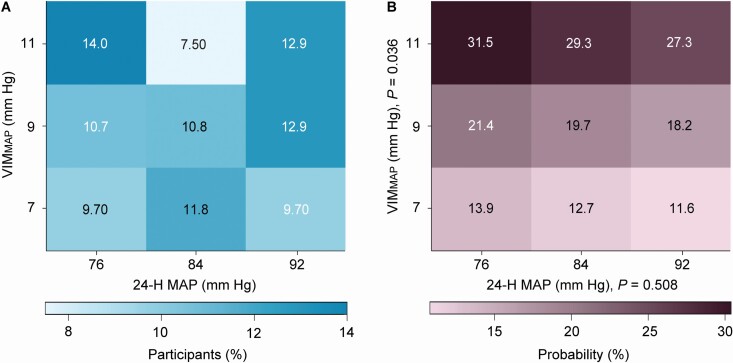
Without any adjustment, the risk of glaucomatous optic neuropathy was not associated with 24-h MAP, but increased with VIMMAP; the odds ratios, expressed per 1-SD increment in the explanatory variable and given with 95% confidence interval were 0.91 (0.72–1.14; P = 0.410) for 24-h MAP and 2.16 (1.31–3.55; P = 0.002) for VIMMAP. With adjustments applied for sex, age, body mass index, HDL serum cholesterol, years of education, office IOP refractive error, and use of antihypertensive drugs, these odds ratios were 0.92 (0.70–1.21; P = 0.528) and 1.84 (1.04–3.28; P = 0.037), respectively. In fully adjusted models, these risk estimates were 0.91 (0.69–1.20; P = 0.508) for 24-h MAP additionally adjusted for VIMMAP, and 1.85 (1.04–3.31; P = 0.036) for VIMMAP additionally adjusted for 24-h MAP. A heatmap (Figure 2) combining the MAP level and VIMMAP showed that along the vertical axis, the probability of glaucomatous optic neuropathy increased with higher VIMMAP (P = 0.036), whereas along the horizontal axis the probability was not associated with higher 24-h MAP (P = 0.508). In a sensitivity analysis, the participants were stratified according to age (<60 vs. ≥60 years). The fully-adjusted odds ratios were similar in the younger and older participants: 0.67 vs. 0.89 (P = 0.688) and 1.80 vs. 1.77 (P = 0.991) for 24-h MAP and VIMMAP, respectively.
Figure 2.
Heatmap depicting the probability of glaucomatous optic neuropathy in MAS participants in relation to 24-h mean arterial pressure level (MAP) and variability (VIMMAP). (A) The percentage of participants contributing to each cell of the cross-classification between MAP and VIMMAP. The heat map (B) was derived by multivariable logistic regression. Panel B shows that along the vertical axis, the probability of glaucomatous optic neuropathy increased with higher VIMMAP (P = 0.034), whereas along the horizontal axis the probability was not significantly associated with higher 24-h MAP (P = 0.321). The probability was standardized to the average of the distributions in the whole study population of sex, age, body mass index, high-density lipoprotein serum cholesterol, years of education, office intraocular pressure, refractive error, and use of antihypertensive drugs.
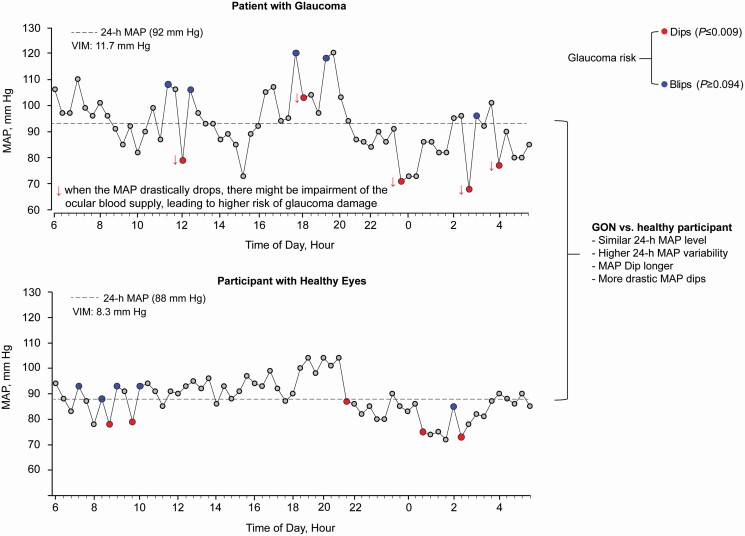
Dips and blips in MAS
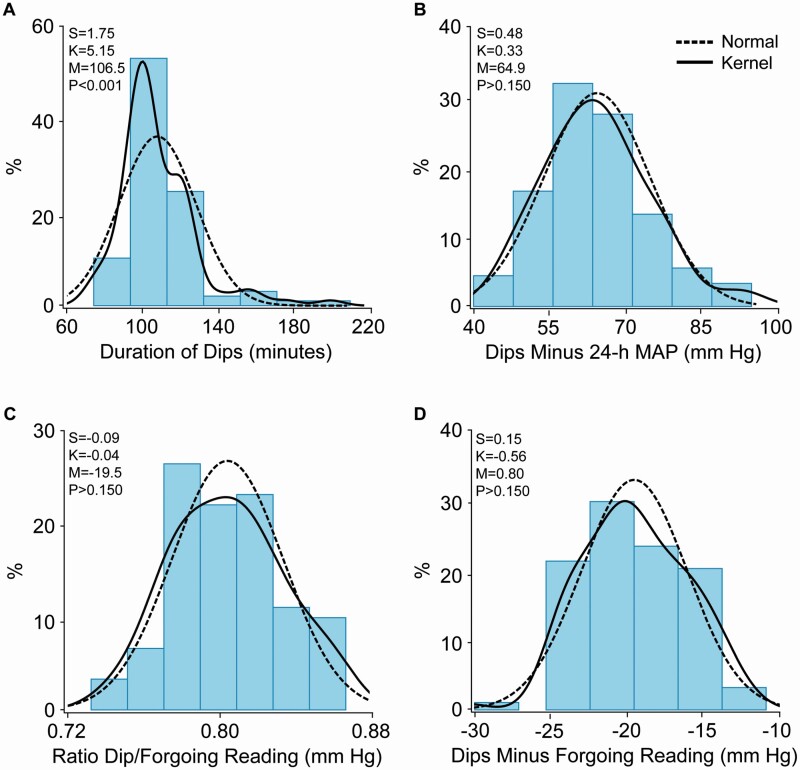
Of the total number of dips, 20% occurred during nighttime (11 pm to 6 am). The dip measures were normally distributed (Figure 3). Patients with glaucoma, compared with participants with normal eyes (Table 2), dipped for a longer period (116.5 vs. 102.7 minutes) and more profoundly relative to the 24-h MAP level (60.3 vs. 66.6 mm Hg) and the forgoing reading (difference, −21.0 vs. −18.0 mm Hg; ratio, 0.79 vs. 0.81). In multivariable-adjusted logistic regression (Table 3), in models including covariables and the 24-h MAP level, the risk of glaucomatous optic neuropathy increased with the periods covered by the dips and the magnitude of the dips relative to the 24-h MAP level and the forgoing reading. The odds ratios, expressed per 1-SD increment in the explanatory variable ranged from 2.25 (P = 0.009) for the period covered by the dips to 3.39 for the dips minus 24-MAP level (P = 0.008).
Figure 3.
Distributions of the duration of dips (A) and their intensity expressed relative to the 24 h mean arterial pressure (B), or the foregoing reading expressed as ratio (C) or differences (D). The red and blue dotted lines represent the normal and kernel density distributions. The P values are for departure of the actually observed distribution from normality according to the Shapiro–Wilk statistic. Skewness and kurtosis were computed as the third and fourth moments about the mean divided by the cube of the standard deviation. Abbreviations: M, mean; S, skewness; K, kurtosis.
Table 2.
Dips and blips in mean arterial pressure in MAS participants
| Variable | All | GON | Healthy eyes | P value |
|---|---|---|---|---|
| Number in group | 93 | 26 | 67 | |
| Dips | ||||
| Nighttime dips, % of total | 20 (0–20) | 20 (20–40) | 20 (0–20) | 0.227 |
| Duration of dips, minutes | 106.6 ± 20.9 | 116.5 ± 31.4 | 102.7 ± 13.5 | 0.003 |
| 24-MAP minus dips, mm Hg | 64.8 ± 10.7 | 60.3 ± 10.6 | 66.6 ± 10.3 | 0.011 |
| Dips minus forgoing reading | −19.6 ± 3.7 | −21.0 ± 3.9 | −18.0 ± 3.4 | 0.031 |
| Ratio dip/forgoing reading | 0.80 ± 0.03 | 0.79 ± 0.03 | 0.81 ± 0.03 | 0.004 |
| Blips | ||||
| Daytime blips, % of total | 80 (80–100) | 80 (80–100) | 80 (80–100) | 0.636 |
| Duration of blips, minutes | 115.5 ± 33.9 | 121.4 ± 47.9 | 113.2 ± 26.7 | 0.303 |
| Blips minus 24-MAP, mm Hg | 64.8 ± 10.9 | 61.0 ± 12.8 | 66.3 ± 9.7 | 0.035 |
| Blips minus forgoing reading | 19.8 ± 4.7 | 20.9 ± 4.4 | 19.4 ± 4.7 | 0.171 |
| Ratio blip/forgoing reading | 1.25 ± 0.06 | 1.27 ± 0.06 | 1.24 ± 0.06 | 0.039 |
Dips and blips refer to the 5 readings with the largest drop or the greatest surge compared to the previous reading within-in individual 24-h MAP recordings. Daytime and nighttime refer to the intervals from 6 am to 11 pm and from 11 pm to 6 am, respectively. Values are arithmetic mean ± SD or median (interquartile range). P values denote the significance of the difference between GON patients and participants with normal eyes. Abbreviations: GON, participants with glaucomatous optic neuropathy; MAP, mean arterial pressure.
Table 3.
Glaucomatous optic neuropathy in MAS in relation to dips and blips in mean arterial pressure
| Variable | Univariable | Multivariable-adjusted | Fully-adjusted | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Dips | ||||||
| Duration of dips, +20 minutes | 1.94 (1.17–3.21) | 0.010 | 1.91 (1.15–3.17) | 0.013 | 2.25 (1.23–4.14) | 0.009 |
| 24-MAP minus dips, +2 mm Hg | 1.88 (1.14–3.13) | 0.014 | 2.97 (1.40–6.34) | 0.004 | 3.39 (1.36–8.46) | 0.008 |
| Dips minus forgoing reading, +3.6 mm Hg | 1.69 (1.03–2.77) | 0.036 | 2.28 (1.25–4.15) | 0.007 | 2.54 (1.26–5.13) | 0.009 |
| Ratio dip/forgoing reading, 0.03 mm Hg | 2.10 (1.23–3.58) | 0.006 | 2.07 (1.21–3.53) | 0.008 | 2.52 (1.31–4.85) | 0.006 |
| Blips | ||||||
| Duration of blips, +33 minutes | 1.25 (0.81–1.93) | 0.315 | 1.29 (0.82–2.01) | 0.272 | 1.08 (0.65–1.81) | 0.762 |
| Blips minus 24-MAP, +2 mm Hg | 0.91 (0.82–0.99) | 0.039 | 0.86 (0.75–0.98) | 0.026 | 0.87 (0.75–1.02) | 0.094 |
| Blips minus forgoing reading, +4.7 mm Hg | 1.37 (0.87–2.16) | 0.173 | 1.65 (0.98–2.77) | 0.061 | 1.45 (0.79–2.66) | 0.227 |
| Ratio blip/forgoing reading, +0.06 mm Hg | 1.60 (1.01–2.54) | 0.045 | 1.58 (0.99–2.52) | 0.053 | 1.63 (0.93–2.89) | 0.091 |
MAP indicates mean arterial pressure. Dips and blips refer to the 5 readings with the largest drop or the greatest surge compared to the previous reading within-in individual 24-h MAP recordings. Values are unadjusted and multivariable-adjusted odds ratios (OR), given with 95% confidence interval, and express the risk per 1-SD increment in the independent variable. Multivariable-adjusted models accounted for sex, age, body mass index, high-density lipoprotein serum cholesterol, years of education, office intraocular pressure, refractive error, and use of antihypertensive drugs. Fully adjusted models additionally included the 24-h MAP level.
Of the total number of blips, 80% occurred during daytime (6 am to 11 pm). Blips in patients with glaucoma compared to their counterparts with normal eyes (Table 2) were smaller relative to the 24-h MAP level (61.0 vs. 66.3 mm Hg), but expressed as a ratio relative to the forgoing reading were steeper (1.27 vs. 1.24). However, in multivariable-adjusted logistic regression (Table 3), the risk of glaucomatous optic neuropathy was not associated with any of the measures capturing the blips (P ≥ 0.091).
The odds ratios associated with dips and blips were similar (P ≥ 0.234) in patients with and without visual field defects (Supplementary Table S4 online), except for the depth of the dips relative to the 24-h MAP level (P = 0.001). These MAS observations were also consistent if in all participants the odds ratios were additionally adjusted for the night-to-day MAP ratio, combined or not with the 24-h MAP level (Supplementary Table S5 online).
Performance of variability indexes in MAS
Compared with a basic model (R2 = 22.2%; Supplementary Table S6 online), that included sex, age, body mass index, high-density lipoprotein serum cholesterol, office IOP, refractive error, and antihypertensive treatment, 24-h MAP level did not improve the explained variance (R2 = +0.43%; P = 0.524), whereas VIMMAP did (R2 = +4.82%; P = 0.032). On top of 24-h MAP level and VIMMAP, the dip measures increased the model performance with R2 estimates ranging from 4.46% to 7.48 (P ≤ 0.039).
Replication study
Of the 48 glaucoma cases in the replication study, 31 (64.6%) were normal-tension glaucoma and 17 (35.4%) were primary open-angle. A total of 33 glaucoma patients were on topical (68.7%) treatment to lower IOP (no systemic IOP lowering treatment was registered). Office IOP averaged 14.3 ± 3.2 mm Hg (range 8.0–21 mm Hg). The replication study included 56 women (58.3%). Age and 24-h MAP averaged 63.2 years and 91.3 mm Hg, respectively, and were similar in cases and controls (P ≥ 0.630), whereas VIMMAP was 1.6 mm Hg (95% confidence interval, 0.51–2.53 mm Hg; P = 0.004) higher in cases than controls (Supplementary Table S7 online).
In models adjusted for sex, age, body mass index, current smoking and alcohol intake, diabetes mellitus, previous cardiovascular disease, and use of antihypertensive drugs, the risk of glaucomatous optic neuropathy was not associated with 24-h MAP, but increased with VIMMAP; the odds ratios, expressed per 1-SD increment in the explanatory variable were 0.99 (0.62–1.61; P = 0.991) for 24-h MAP and 2.04 (1.17–3.57; P = 0.012) for VIMMAP. In fully-adjusted models, these risk estimates were 0.74 (0.43–1.27; P = 0.274) for 24-h MAP additionally adjusted for VIMMAP, and 2.32 (1.25–4.32: P = 0.007) for VIMMAP additionally adjusted for 24-h MAP. A heatmap (Supplementary Figure S1 online) combining the MAP level and VIMMAP showed that along the vertical axis, the probability of glaucomatous optic neuropathy increased with higher VIMMAP (P = 0.009), whereas along the horizontal axis the probability was not associated with higher 24-h MAP (P = 0.254).
Cases compared with controls (Supplementary Table S8 online) dipped deeper relative to the forgoing reading (difference, −17.3 vs. −15.6 mm Hg; ratio, 0.81 vs. 0.84; P ≤ 0.006). In multivariable-adjusted logistic regression (Supplementary Table S9 online), the risk of glaucomatous optic neuropathy, increased with the magnitude of the dips relative to the 24-h MAP level and the forgoing reading (P ≤ 0.025). Blips in cases compared to controls (Supplementary Table S8 online) covered a shorter period (108.1 vs. 120.6 minutes; P = 0.019) were smaller relative to the 24-h MAP level (61.0 vs. 66.3 mm Hg), but expressed as a ratio relative to the forgoing reading were steeper (1.22 vs. 1.21; P = 0.021). In multivariable-adjusted logistic regression (Supplementary Table S9 online), the risk of glaucoma was inversely associated with the period covered by blips (odds ratio, 0.40; P = 0.003).
DISCUSSION
Our study showed that 24-h excessive MAP dips, but not the 24-h MAP level or MAP blips, were associated with an increased risk of open-angle glaucomatous optic neuropathy. Our study moves the field forward in several ways. First, MAP was directly recorded over 24-h, not computed from diastolic and systolic blood pressure. The software embedded in oscillometric devices draws an envelope around the pressure oscillations in the brachial cuff and estimates MAP as the cuff pressure at the point of maximal oscillations.21 MAP captures the risk associated with both systolic and diastolic blood pressure, which respectively represent the pulsatile and steady components of blood flow.22 MAP is the driving force of organ perfusion and is similar across the circulation from the central arteries up to the small arterioles,23 a property which is used to compute ocular perfusion pressure from MAP and IOP.3,24,25 As index of blood pressure variability, VIMMAP was applied. In contrast to other indexes of blood pressure variability, such as the SD, the coefficient of variation, average real variability, and the minimum–maximum blood pressure difference, VIMMAP is not correlated with the blood pressure level (Supplementary Table S3 online), and thereby avoids issues of collinearity in regression analyses.19 In addition to VIMMAP as index of blood pressure variability, the current study also included a reading-to-reading analysis of the 24-h MAP recordings, which was implemented by the same macro for all ambulatory recordings. This reading-to-reading analysis excluded blood pressure increases (blips), as being associated with glaucomatous optic nerve lesions. Finally, extreme dips in MAP were additive to VIMMAP in their association with glaucomatous optic neuropathy, an observation hitherto not reported before. Finally, the MAS observations were replicated in a case–control study conducted in Flanders, Belgium, which included well-documented normal-tension (n = 31, 64.6%) and primary open-angle (n = 17, 35.4%) glaucoma cases from the Leuven Glaucoma Clinic10 and controls enrolled and followed-up in FLEMENGHO.11
There is an abundant literature26–30 associating glaucomatous optic neuropathy with the systemic blood pressure level and variability. Most studies focused on the nocturnal blood pressure decline. A systematic review of the literature published in 2015,27 identified 5 studies31–35 published from 199631,32 to 2001,35 which reported on the ambulatory blood pressure with separate data for daytime and nighttime blood pressure, the nocturnal blood pressure fall, and an assessment of visual fields over a period of at least 2 years. There was no difference in mean systolic or diastolic diurnal or nocturnal blood pressure between patients with or without progressive visual field loss. The odds ratios for deteriorating visual fields over 2 years with nocturnal dips of 10% or greater in systolic or diastolic blood pressure were 3.32 (CI, 1.84–6.00) and 2.09 (CI, 1.20–3.64), respectively.27 However, the pooled estimates derived by fixed-effect meta-analysis were not adjusted for any confounder. An additional issue of particular concern was the application of blood pressure variability indexes, which are closely correlated with the blood pressure level, such as the SD28,30 or coefficient of variation28 across visit-to-visit28 or ambulatory28,30 blood pressure readings.
Several studies addressed the variability in IOP or ocular perfusion pressure (OPP), as determined from MAP and IOP.26,29,36–38 The number of patients (eyes) ranged from 22 (22)38 to 301 (301).37 In 2 prospective studies, the median follow-up was 7.237 and 8.726 years. In keeping with the current findings, most studies suggested that greater diurnal variation in the IOP36,37 or ocular perfusion pressure26 was a factor in the prevalence or progression of open-angle glaucoma. However, 2 studies failed to show association of the severity or progression of glaucomatous lesions with the diurnal amplitude of IOP38 or the habitual IOP or 24-h OPP.29 The methodological challenges in the aforementioned studies principally rely on the unavailability of continuous IOP measurements. Although implantable IOP monitors combined with continuous blood pressure monitoring using finger cuff devices39 or cuffless blood pressure monitors40 might be the innovative approach that might revolutionize glaucoma research, wearable IOP sensors provide intermittent IOP readings and cause corneal deformation and some degree of discomfort.41 Moreover, implantable IOP monitors are still being developed42 and cuffless blood pressure devices are not supported by hypertension specialists (https://medaval.ie/services-for-manufacturers).
Our current study adds to the literature by clarifying that MAP dips—not blips—are the blood pressure variability component associated with the risk of glaucomatous optic neuropathy. From a clinical viewpoint, 2 groups of patients should be considered to be at risk of glaucomatous optic neuropathy. First, normotensive patients with high blood pressure variability—reflecting impaired baroreflexes,43 as they are more likely to experience excessive dips compromising the ocular perfusion pressure. Second, as several studies have demonstrated that high blood pressure positively associates with glaucoma,44 hypertensive patients might be at risk as a consequence of an impaired autoregulation of the ocular microcirculation to maintain the perfusion pressure45 when the blood pressure excessively drops, as for instance induced by medication. This issue is critical, because (i) approximately 50% of glaucoma patients suffer from hypertension,46 and (ii) because current hypertensive guidelines do not propose how to guide antihypertensive therapy in glaucoma patients.6,7 The ACC/AHA 2017 guideline recommended an office systolic/diastolic blood pressure of less than 130/80 mm Hg, based on the findings from the SPRINT study, which did not include glaucomatous optic damage as an adverse outcome47,48 and in which patients in the intensive-treatment group experienced more serious hypotension and syncope events.49 The Thessaloniki Eye Study reported that the association between antihypertensive medication and optic nerve structures was driven by controlling diastolic blood pressure to less than 90 mm Hg.50 As a measure of precaution, patients in whom intensive blood pressure lowering treatment is indicated, such as those with diabetes or a history of cardiovascular or renal disease, should be referred for an ophthalmological examination prior to intensification of antihypertensive treatment. Likewise, both normotensive and hypertensive glaucoma patients experiencing progressive glaucomatous damage despite controlled or low intraocular pressures, should be followed with standardized protocols regarding blood pressure measurements and antihypertensive therapy by hypertensive specialists.47 Consensus guidelines to balance the ocular and cardiovascular risk in patients with OAG are still an unmet clinical need.47,48
Strengths and limitations
In the MAS study, 2 experienced ophthalmologists graded glaucomatous optic neuropathy independent from one another and achieved an intergrader κ-statistics of 0.90. The number of readings in ambulatory recordings met the guideline-endorsed number of at least 48 measurements over 24-h (Supplementary Table S1 online). The MAS observation were independently replicated using normal-tension and primary open-angle glaucoma cases examined at the Leuven Eye Clinic10 and matched controls in the FLEMENGHO study.11 However, the current study must also be interpreted within the context of its limitations. First, as per design, women were overrepresented in the MAS study sample. This was also the case in the replication case–control study. However, the prevalence of glaucomatous optic neuropathy was similar in Maracaibo and in a sex-balanced sample of Hispanics of Caribbean origin residing in New York.51 Second, cross-sectional association studies, such as the current study, do not allow drawing causal inferences. However, application of the Bradford–Hill criteria52 suggest that hypoperfusion might be a causal factor in primary-open angle glaucoma, given the plausibility of the hypothesis,4 consistency of the findings,26,29,36–38 and hypotension preceding the disease.27 Finally, a single office intraocular pressure measurement might limit the generalizability of the MAS findings. However, the case–control study replicated the MAS observations along the continuum from normal-tension to primary open-angle glaucoma.14,15
We found that the association between 24-h MAP variability and open-angle glaucomatous optic neuropathy was driven by dips rather than increases in the MAP. Autoregulation of blood flow in the retina and optic nerve head occurs over a large range of intraocular pressures and blood pressures. Antihypertensive drugs not only interfere with the systemic blood pressure, but potentially also with the autoregulation of the retinal blood flow. To our knowledge, none of the trials of intensive vs. usual blood pressure lowering49,53,54 reported on the incidence of glaucomatous optic damage in the intervention vs. the control group. Glaucoma being the first cause of irreversible blindness worldwide55 and given the ever decreasing blood pressure targets in primary49 and secondary56 cardiovascular prevention, an ophthalmological examination combined with 24-h ambulatory blood pressure monitoring are precautious steps advisable in patients at risk of glaucoma. However, to date, no specific recommendation can be proposed to limit dips, which might occur instantly and unexpectedly during daytime as well as during nighttime (Supplementary Figure S2 online). In the absence of any solid evidence and consolidated guidelines, a tailored management of each individual patient is warranted, always balancing the risks of cardiovascular and ocular complications.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the participants and assessment team of the MAS in both Santa Lucía and Santa Rosa. We also acknowledge the fellows Drs. Federico Flores, Rubén Torre Alba, Roxana Chacón, and David Santana, who collected the ophthalmological information, and the support from Dr Doris Molina. We gratefully acknowledge Rosa Pirela (South Texas Diabetes and Obesity Institute, University of Texas Rio Grande Valley, Brownsville, TX) for supporting the preparation of this report and Vera De Leebeeck and Renilde Wolfs (Research Unit Hypertension and Cardiovascular Epidemiology, Department of Cardiovascular Sciences, University of Leuven) for expert clerical assistance.
Contributor Information
Jesus D Melgarejo, Studies Coordinating Centre, Research Unit Hypertension and Cardiovascular Epidemiology, KU Leuven Department of Cardiovascular Sciences, University of Leuven, Leuven, Belgium; Laboratory of Neurosciences, Faculty of Medicine, University of Zulia, Maracaibo, Zulia, Venezuela.
Jan V Eijgen, Department of Ophthalmology, University Hospitals UZ Leuven, Leuven, Belgium; Department of Neurosciences, Research Group Ophthalmology, University of Leuven, Leuven, Belgium.
Gladys E Maestre, Laboratory of Neurosciences, Faculty of Medicine, University of Zulia, Maracaibo, Zulia, Venezuela; Rio Grande Valley Alzheimer’s Disease Resource Center for Minority Aging Research (RGV AD-RCMAR), University of Texas Rio Grande Valley, Brownsville, Texas, USA; Institute for Neuroscience, School of Medicine, University of Texas Rio Grande Valley, Harlingen, Texas, USA; Department of Human Genetics, School of Medicine, University of Texas Rio Grande Valley, Brownsville, Texas, USA.
Lama A Al-Aswad, Department of Ophthalmology, New York University (NYU) Grossman School of Medicine, NYU Langone Health, New York, New York, USA.
Lutgarde Thijs, Studies Coordinating Centre, Research Unit Hypertension and Cardiovascular Epidemiology, KU Leuven Department of Cardiovascular Sciences, University of Leuven, Leuven, Belgium.
Luis J Mena, Department of Informatics, Universidad Politécnica de Sinaloa, Mazatlán, Mexico.
Joseph H Lee, Taub Institute for Research in Alzheimer’s Disease and the Aging Brain, G.H. Sergievsky Center, Columbia University Medical Center, New York, New York, USA; Departments of Epidemiology and Neurology, Columbia University Medical Center, New York, New York, USA.
Joseph D Terwilliger, Department of Genetics and Development, Columbia University, New York, New York, USA; Department of Psychiatry, G.H. Sergievsky Center, Columbia University, New York, New York, USA; Division of Medical Genetics, New York State Psychiatric Institute, New York, New York, USA; Division of Public Health Solutions, National Institute for Health and Welfare, Helsinki, Finland.
Michele Petitto, Glaucoma and Retina Units, Eye Clinic of Maracaibo, Maracaibo, Zulia, Venezuela.
Carlos A Chávez, Laboratory of Neurosciences, Faculty of Medicine, University of Zulia, Maracaibo, Zulia, Venezuela.
Miguel Brito, Instituto Docente de Especialidades Oftalmológicas (IDEO), Maracaibo, Zulia, Venezuela.
Gustavo Calmon, Laboratory of Ambulatory Recordings, Cardiovascular Institute (IECLUZ), University of Zulia, Maracaibo, Zulia, Venezuela.
Egle Silva, Laboratory of Ambulatory Recordings, Cardiovascular Institute (IECLUZ), University of Zulia, Maracaibo, Zulia, Venezuela.
Dong-Mei Wei, Studies Coordinating Centre, Research Unit Hypertension and Cardiovascular Epidemiology, KU Leuven Department of Cardiovascular Sciences, University of Leuven, Leuven, Belgium.
Ella Cutsforth, Biomedical Science Group, Faculty of Medicine, University of Leuven, Leuven, Belgium.
Karel V Keer, Department of Ophthalmology, University Hospitals UZ Leuven, Leuven, Belgium; Department of Neurosciences, Research Group Ophthalmology, University of Leuven, Leuven, Belgium.
C Gustavo De Moraes, Department of Ophthalmology, Columbia University, New York, New York, USA.
Thomas Vanassche, Centre for Molecular and Vascular Biology, KU Leuven Department of Cardiovascular Sciences, University of Leuven, Leuven, Belgium.
Stefan Janssens, Division of Cardiology, Department of Internal Medicine, University Hospitals UZ Leuven, Leuven, Belgium.
Ingeborg Stalmans, Department of Ophthalmology, University Hospitals UZ Leuven, Leuven, Belgium; Department of Neurosciences, Research Group Ophthalmology, University of Leuven, Leuven, Belgium.
Peter Verhamme, Centre for Molecular and Vascular Biology, KU Leuven Department of Cardiovascular Sciences, University of Leuven, Leuven, Belgium.
Jan A Staessen, Biomedical Science Group, Faculty of Medicine, University of Leuven, Leuven, Belgium; Research Institute Alliance for the Promotion of Preventive Medicine, Mechelen, Belgium.
Zhen-Yu Zhang, Studies Coordinating Centre, Research Unit Hypertension and Cardiovascular Epidemiology, KU Leuven Department of Cardiovascular Sciences, University of Leuven, Leuven, Belgium.
FUNDING
This report is supported by the Gene-Environment Interaction in Cognition in Venezuela Families project founded by the National Institute on Aging-National Institutes of Health under Award Number R01AG036469 and 1 R03AG054186-01 (G.E.M., J.D.T., W.L.). Internal Funds KU Leuven (STG-18-00379) supported the Research Unit Hypertension and Cardiovascular Epidemiology, Department of Cardiovascular Sciences, Leuven. The Non-Profit Research Institute Alliance for the Promotion of Preventive Medicine (URL: http://www.appremed.org), Mechelen, Belgium, received a non-binding grant from OMRON Healthcare, Kyoto, Japan.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014; 121:2081–2090. [DOI] [PubMed] [Google Scholar]
- 2. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma. A review. J Am Med Assoc 2014; 311:1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Keer K, Breda JB, Pinto LA, Stalmans I, Vandewalle E. Estimating mean ocular perfusion pressure using mean arterial pressure and intraocular pressure. Invest Ophthalmol Vis Sci 2016; 57:2260. [DOI] [PubMed] [Google Scholar]
- 4. Leeman M, Kestelyn P. Glaucoma and blood pressure. Hypertension 2019; 73:944–950. [DOI] [PubMed] [Google Scholar]
- 5. Choi J, Kim KH, Jeong J, Cho H, Lee CH, Kook MS. Circadian fluctuation of mean ocular perfusion pressure is a consistent risk factor for normal-tension glaucoma. Invest Ophthalmol Vis Sci 2007; 48:104–111. [DOI] [PubMed] [Google Scholar]
- 6. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr.. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 7. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018; 39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 8. Sung KR, Lee S, Park SB, Choi J, Kim ST, Yun SC, Kang SY, Cho JW, Kook MS. Twenty-four hour ocular perfusion pressure fluctuation and risk of normal-tension glaucoma progression. Invest Ophthalmol Vis Sci 2009; 50:5266. [DOI] [PubMed] [Google Scholar]
- 9. Melgarejo JD, Maestre GE, Mena LJ, Lee J, Petitto M, Chavez CA, Calmon G, Silvia E, Thijs L, Al-Aswad LA, Terwilliger JD, De Moraes CG, Wei FF, Vanassche T, Verhamme P, Staessen JA, Zhang ZY. Normal-tension glaucomatous optic neuropathy is related to blood pressure variability in the Maracaibo Aging Study. Hypertens Res 2021; 44:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pinto LA, Willekens K, Van Keer K, Shibesh A, Molenberghs G, Vandewalle E, Stalmans I. Ocular blood flow in glaucoma—the Leuven Eye Study. Acta Ophthalmol 2016; 94:592–598. [DOI] [PubMed] [Google Scholar]
- 11. Wei FF, Zhang ZY, Thijs L, Yang WY, Jacobs J, Cauwenberghs N, Gu YM, Kuznetsova T, Allegaert K, Verhamme P, Li Y, Struijker-Boudier HA, Staessen JA. Conventional and ambulatory blood pressure as predictors of retinal arteriolar narrowing. Hypertension 2016; 68:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maestre GE, Pino-Ramirez G, Molero AE, Silva ER, Zambrano R, Falque L, Gamero MP, Sulbarán TA. The Maracaibo Aging Study: population and methodological issues. Neuroepidemiology 2002; 21:194–201. [DOI] [PubMed] [Google Scholar]
- 13. World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Am Med Assoc 2013; 310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 14. Shields MB. Normal-tension glaucoma: is it different from primary open-angle glaucoma. Curr Opin Ophthalmol 2008; 19:85–88. [DOI] [PubMed] [Google Scholar]
- 15. Mroczkowska S, Benavente-Perez A, Negi A, Sung V, Patel SR, Gherghel D. Primary open-angle glaucoma vs normal-tension glaucoma. JAMA Ophthalmol 2013; 131:36–43. [DOI] [PubMed] [Google Scholar]
- 16. Siegel S, Castellan NJJ. Nominally scaled data and the kappa statistic κ. In Nonparametric Statistics for the Behavioral Sciences. 2nd edn. McGraw-Hill: New York, NY, 1988, pp. 284–291. [Google Scholar]
- 17. Groppelli A, Omboni S, Parati G, Mancia G. Evaluation of noninvasive blood pressure monitoring devices Spacelabs 90202 and 90207 versus resting and ambulatory 24-hour intra-arterial blood pressure. Hypertension 1992; 20:227–232. [DOI] [PubMed] [Google Scholar]
- 18. Weiß W, Tölle M, Zidek W, van der Giet M. Validation of the Mobil-O-Graph: 24 h-blood pressure measurement device. Blood Press Monit 2010; 15:225–228. [DOI] [PubMed] [Google Scholar]
- 19. Rothwell PM, Howard SC, Dolan E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010; 375:895–905. [DOI] [PubMed] [Google Scholar]
- 20. Melgarejo JD, Lee JH, Petitto M, Yépez JB, Murati FA, Jin Z, Chávez CA, Pirela RV, Calmón GE, Lee W, Johnson MP, Mena LJ, Al-Aswad LA, Terwilliger JD, Allikmets R, Maestre GE, De Moraes CG. Glaucomatous optic neuropathy associated with nocturnal dip in blood pressure: findings from the Maracaibo Aging Study. Ophthalmology 2018; 125:807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Babbs CF. Oscillometric measurement of systolic and diastolic blood pressures validated in a physiologic mathematical model. Biomed Eng Online 2012; 11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Melgarejo JD, Yang WY, Thijs L, Yan L, Asayama K, Hansen TW, Wei FF, Kikuya M, Ohkubo T, Dolan E, Stolarz-Skrzypek K, Huang QF, Tikhonoff V, Malyutina S, Casiglia E, Lind L, Sandoya E, Filipovský J, Gilis-Malinowska N, Narkiewicz K, Kawecka-Jaszcz K, Boggia J, Wang JG, Imai Y, Vanassche T, Verhamme P, Janssens S, O’Brien E, Maestre GE, Staessen JA, Zhang ZY. Association of fatal and nonfatal cardiovascular outcomes with 24-hour mean arterial pressure. Hypertension 2021; 77:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boggia J, Luzardo L, Lujambio I, Sottolano M, Robaina S, Thijs L, Olascoaga A, Noboa O, Struijker-Boudier HA, Safar ME, Staessen JA. The diurnal profile of central hemodynamics in a general Uruguayan population. Am J Hypertens 2016; 29:737–746. [DOI] [PubMed] [Google Scholar]
- 24. Barbosa-Breda J, Abegao-Pinto L, Van Keer K, Jesus DA, Lemmens S, Vandewalle E, Rocha-Sousa A, Stalmans I. Heterogeneity in arterial hypertension and ocular perfusion pressure definitions: towards a consensus on blood pressure-related parameters for glaucoma studies. Acta Ophthalmol 2019; 97:e487–e492. [DOI] [PubMed] [Google Scholar]
- 25. Costa VP, Harris A, Anderson D, Stodtmeister R, Cremasco F, Kergoat H, Lovasik J, Stalmans I, Zeitz O, Gugleta K, Schmetterer L. Ocular perfusion pressure in glaucoma. Acta Ophthalmol 2014; 92:e252–e266. [DOI] [PubMed] [Google Scholar]
- 26. Choi J, Lee JR, Lee Y, Lee KS, Na JH, Han S, Kook MS. Relationship between 24-hour mean ocular perfusion pressure fluctuations and rate of paracentral visual field progression in normal-tension glaucoma. Invest Ophthalmol Vis Sci 2013; 54:6150–6157. [DOI] [PubMed] [Google Scholar]
- 27. Bowe A, Grunig M, Schubert J, Demir M, Hoffmann V, Kutting F, Pelc A, Steffen HM. Circadian variation in arterial blood pressure and glaucomatous optic neuropathy - systematic review and meta-analysis. Am J Hypertens 2015; 28:1077–1082. [DOI] [PubMed] [Google Scholar]
- 28. Lee NY, Junf Y, Han K, Park CK. Fluctuations in systolic blood pressure is a major systemic risk factor factor for development of primary open-angle glaucoma. Sci Rep 2017; 7:43734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quaranta L, Katsanos A, Riva I, Dastiridou A, Oddone F, Roberti G, Konstas AGP. Twenty-four-hour intraocular pressure and ocular perfusion pressure characteristics in newly diagnosed patients with normal tension glaucoma. Eye 2016; 30:1491–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jin SW, Seo HR, Rho SS, Rho SH. The effects of nocturnal dip and blood pressure variability on paracentral scotoma in early open-angle glaucoma. Sem Ophthalmol 2017; 32:504–510. [DOI] [PubMed] [Google Scholar]
- 31. Bresson-Dumont H, Bechetoille A. Role of arterial blood pressure in the development of glaucomateus lesions. J Fr Ophthalmol 1996; 19:435–442. [PubMed] [Google Scholar]
- 32. Detry M, Boschi A, Ellinghaus G, De Plaen JF. Simultaneous 24-hour monitoring of intraocular pressure and arterial blood pressure in patients with progressive and non-progressive open-angle glaucoma. Eur J Ophthalmol 1996; 6:273–278. [DOI] [PubMed] [Google Scholar]
- 33. Collignon N, Dewe W, Guillaume S, Collignon-Brach J. Ambulatory blood pressure monitoring in glaucoma patients. The nocturnal systolic dip and its relationship with disease progression. Int Ophthalmol 1998; 22:19–25. [DOI] [PubMed] [Google Scholar]
- 34. Graham SL, Drance SM. Nocturnal hypotension: role in glaucoma progression. Surv Ophthalmol 1999; 43:S10–S16. [DOI] [PubMed] [Google Scholar]
- 35. Kashiwagi K, Hosaka O, Kashiwagi F, Taguchi K, Mochizuki J, Ishii H, Ijiri H, Tamura K, Tsukahara S. Systemic circulatory parameters: comparison between patients with normal tension glaucoma and normal subjects using ambulatory monitoring. Jpn J Ophthalmol 2001; 45:388–396. [DOI] [PubMed] [Google Scholar]
- 36. Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma 2000; 9:134–142. [DOI] [PubMed] [Google Scholar]
- 37. Caprioli J, Coleman AL. Intraocular pressure fluctuation: a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology 2008; 115:1123–1129. [DOI] [PubMed] [Google Scholar]
- 38. Renard E, Palombi K, Gronfier C, Pepin JL, Noel C, Chiquet C, Romanet JP. Twenty-four hour (nyctohemeral) rhythm of intraocular pressure and ocular perfusion pressure in normal-tension glaucoma. Invest Ophthalmol Vis Sci 2010; 51:882–889. [DOI] [PubMed] [Google Scholar]
- 39. Imholz BPM, Langewouters GJ, van Montfrans GA, Parati G, van Goudoever J, Wesseling KH, Wieling W, Mancia G. Feasibility of ambulatory, continuous 24-hour finger arterial pressure recording. Hypertension 1993; 21:65–73. [DOI] [PubMed] [Google Scholar]
- 40. Schoot TS, Weenk M, van de Belt TH, Engelen LJ, van Goor H, Bredie SJ. A new cuffless device for measuring blood pressure: a real-life validation study. J Med Internet Res 2016; 18:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sanchez I, Martin R. Advances in diagnostic applications for monitoring intraocular pressure in glaucoma: a review. J Optometry 2019; 12:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Molaei A, Karamzadez V, Safi S, Esfandiari H, Dargahi J, Khosravi MA. Upcoming methods and specifications of continuous intraocular pressure monitoring systems for glaucoma. J Ophthalmic Vis Res 2018; 13:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Visontai Z, Mersich B, Holló G. Carotid artery elasticity and baroreflex sensitivity in patients with glaucoma. J Glaucoma 2005; 14:30–35. [DOI] [PubMed] [Google Scholar]
- 44. Asefa NG, Neustaeter A, Jansonius NM, Snieder H. Autonomic dysfunction and blood pressure in glaucoma patients: the Lifelines Cohort Study. Invest Ophthalmol Vis Sci 2020; 61:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Metzner Serra L, Renard JP, Stefánsson E. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 2002; 21:359–393. [DOI] [PubMed] [Google Scholar]
- 46. Kuang TM, Xirasagar S, Kao YW, Shia BC, Lin HC. Association of systemic hypertension with primary open-angle glaucoma: a population-based case-control study. Am J Ophthalmol 2020; 218:99–104. [DOI] [PubMed] [Google Scholar]
- 47. Levine RM, Yang A, Brahma V, Martone JF. Management of blood pressure in patients with glaucoma. Curr Cardiol Rep 2017; 19:1–8. [DOI] [PubMed] [Google Scholar]
- 48. Skrzypecki J, Ufnal M, Szaflik JP, Filipiak KJ. Blood pressure and glaucoma: at the crossroads between cardiology and ophthalmology. Cardiol J 2019; 26:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. The SPRINT Research Group. Final report of a trial of intensive versus standard blood-pressure control. N Engl J Med 2021; 384:1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harris A, Topouzis F, Wilson MR, Founti P, Kheradiya NS, Anastasopoulos E, Gong G, Yu F, Jonescu-Cuypers CP, Pappas T. Association of the optic disc structure with the use of antihypertensive medications: the Thessaloniki eye study. J Glaucoma 2013; 22:526–531. [DOI] [PubMed] [Google Scholar]
- 51. Al-Aswad LA, Joiner DB, Wang X, De Moraes CG, Popplewell D, Amaro-Quireza ML, Shabsigh M, Taher N. Screening for glaucoma in populations at high risk: the Eye Screening New York Project. Cogent Med 2017; 4:1367059. [Google Scholar]
- 52. Fedak KM, Bernal A, Capashaw ZA, Gross S. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg Themes Epidemiol 2015; 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Verdecchia P, Staessen JA, Angeli F, de Simone G, Achilli A, Ganau A, Mureddu G, Pede S, Maggioni AP, Lucci D, Reboldi G; on behalf of the Cardio-Sis Investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet 2009; 374:525–533. [DOI] [PubMed] [Google Scholar]
- 54. The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang W, He M, Li Z, Huang W. Epidemiological variations and trends in health burden of glaucoma worldwide. Acta Ophthalmol 2019; 97:e349–e355. [DOI] [PubMed] [Google Scholar]
- 56. Yan C, Thijs L, Cao Y, Trenson S, Zhang ZY, Janssens S, Staessen JA, Feng YM. Opportunities of antidiabetic drug in cardiovascular medicine: a meta-analysis and perspectives for trial design. Hypertension 2020; 76:420–431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.