Abstract
Background
Wide-spread application of chimeric antigen receptor (CAR) T cell therapy for cancer is limited by the current use of autologous CAR T cells necessitating the manufacture of individualized therapeutic products for each patient. To address this challenge, we have generated an off-the-shelf, allogeneic CAR T cell product for the treatment of glioblastoma (GBM), and present here the feasibility, safety, and therapeutic potential of this approach.
Methods
We generated for clinical use a healthy-donor derived IL13Rα2-targeted CAR+ (IL13-zetakine+) cytolytic T-lymphocyte (CTL) product genetically engineered using zinc finger nucleases (ZFNs) to permanently disrupt the glucocorticoid receptor (GR) (GRm13Z40-2) and endow resistance to glucocorticoid treatment. In a phase I safety and feasibility trial we evaluated these allogeneic GRm13Z40-2 T cells in combination with intracranial administration of recombinant human IL-2 (rhIL-2; aldesleukin) in six patients with unresectable recurrent GBM that were maintained on systemic dexamethasone (4-12 mg/day).
Results
The GRm13Z40-2 product displayed dexamethasone-resistant effector activity without evidence for in vitro alloreactivity. Intracranial administration of GRm13Z40-2 in four doses of 108 cells over a two-week period with aldesleukin (9 infusions ranging from 2500–5000 IU) was well tolerated, with indications of transient tumor reduction and/or tumor necrosis at the site of T cell infusion in four of the six treated research subjects. Antibody reactivity against GRm13Z40-2 cells was detected in the serum of only one of the four tested subjects.
Conclusions
This first-in-human experience establishes a foundation for future adoptive therapy studies using off-the-shelf, zinc-finger modified, and/or glucocorticoid resistant CAR T cells.
Keywords: allogeneic, glioblastoma, glucocorticoid receptor, IL13Rα2-CAR T cells, off-the-shelf
Key Points.
CAR T cells were engineered for steroid-resistance via glucocorticoid receptor knockout.
Intracranially infused allogeneic CAR T cells were well tolerated in GBM patients.
Transient anti-tumor effects support future advancement of off-the-shelf strategies.
Importance of the Study.
This is a first-in-human study of locally administered, glucocorticoid resistant, allogeneic CAR T cells for the treatment of recurrent GBM as an off-the-shelf therapy. The efficiency and specificity of zinc finger nuclease-directed disruption of the glucocorticoid receptor (GR) locus enabled the engineering of dexamethasone-resistant IL13Rα2-targeted CAR T cells. These steroid-resistant CAR T cells retained effector function in the presence of dexamethasone, which was used clinically to attenuate tumor-related neuro-edema, as well as the rejection of the therapeutic allogeneic cells. The CAR T cell product displayed a defined TCR profile without evidence of graft-versus-host alloreactivity. This study demonstrates the safety and feasibility of such gene-modified cells for use as an off-the-shelf allogeneic CAR T cell product. Evidence of transient anti-tumor effects in four of the six treated GBM patients further sets the stage for future adoptive therapy studies using off-the-shelf, glucocorticoid resistant CAR T cells for the treatment of brain tumors.
Chimeric antigen receptor (CAR) T cell therapy has led to remarkable clinical responses in cancer patients,1–3 but its wide-spread application is limited by the current use of autologous CAR T cells requiring the manufacture of a new therapeutic product for each patient. While the development of allogeneic off-the-shelf CAR T cell therapy is an active area of investigation (reviewed in4,5), several challenges remain, including the avoidance of both graft-versus-host responses as well as immunologic rejection of the product (i.e., host-vs-graft responses). To address these challenges, we developed an allogeneic CAR T cell product for the treatment of glioblastoma (GBM) that was 1) oligoclonal without evidence of alloreactivity; and 2) engineered for glucocorticoid resistance to improve the function of the therapeutic cells in the presence of dexamethasone, commonly prescribed to control cerebral edema in GBM6 and used here to also reduce immune rejection of the allogeneic cells.
Prior clinical experience treating three GBM patients with an autologous first-generation “IL13-zetakine” CAR T cell therapy demonstrated safety, feasibility, and evidence for transient anti-tumor responses.7 Building on this experience, herein we report the clinical evaluation of an allogeneic CAR T cell therapy for GBM, using bi-allelic inactivation of the glucocorticoid receptor (GR) gene via zinc finger nuclease (ZFN) targeting in IL13-zetakine+ CD8+ cytolytic T-lymphocytes (CTLs). These functionally GR-negative IL13-zetakine+ CTLs maintained their cytolytic function against GBM cells and did not show allogeneic cross-reactivity against targets generated from multiple different donors. Based on these preclinical results, a first-in-human study was then conducted in which the GR-negative IL13-zetakine+ CD8+ alloclone T cell product, GRm13Z40-2, was administered intratumorally to six patients with nonresectable recurrent GBM who were receiving dexamethasone for recurrent GBM. This study is the first to demonstrate the safety and bioactivity of a ZFN-modified, allogeneic off-the-shelf CAR CTL product for the treatment of a solid tumor.
Materials and Methods
Study Design
This phase 1, open-label, uncontrolled study of patients with recurrent GBM was conducted at the City of Hope National Medical Center from March 2011 to March 2013 (NCT#01082926). The primary objective of the study was to assess the safety and tolerability of the intratumoral administration of GRm13Z40-2 cells in combination with aldesleukin. Secondary objectives included tracking the modified cells by 9-(4-fluoro-3-hydroxy-methyl-butyl) guanine (18FHBG) positron emission tomography (PET) as previously reported.8
Subjects were eligible for enrollment if they were 18 to 70 years of age with progressive/recurrent grade III or IV malignant glioma expressing IL13Rα2 and required ongoing dexamethasone therapy. IL13Rα2 expression was determined by immunohistochemistry on the most recent tumor resection tissue available prior to enrollment. The Karnofsky Performance Score (KPS) was required to be ≥50 with adequate renal (creatinine <1.6 mg/dL), liver (bilirubin <1.5 mg/dL; AST and ALT <2X upper limits of normal) and bone marrow function (WBC >2000/dL and platelets >100 000/dL). The key exclusion criteria were expected survival <4 weeks and tumors requiring decompressive craniotomy, and location of the tumor in the basal ganglia or thalamus or significant involvement of midbrain, pons, and medulla. Subjects with pulmonary (supplemental oxygen), cardiac (arrhythmia, hypotension), and neurologic disease (refractory seizure, progressive encephalopathy) were also excluded. The final protocol, amendments, and consent documents were reviewed and approved by the Institutional Review Board and Independent Ethics Committee at the City of Hope National Medical Center as well as the Recombinant DNA Advisory Committee of the National Institutes of Health. All subjects provided written informed consent.
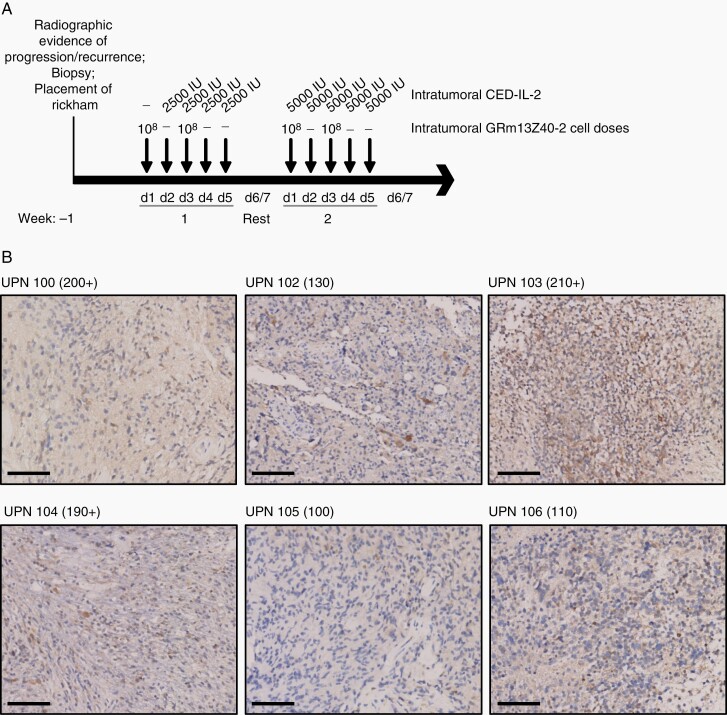
Six subjects were enrolled, assigned a unique patient number (UPN), and underwent a stereotactic biopsy to confirm GBM recurrence followed by placement of a Rickham catheter in or proximal to the MRI-defined tumor site. GRm13Z40-2 cells (108 cells per infusion) were infused locally into the tumor via the Rickham catheter on days 1, 3, 8, and 10, and aldesleukin was infused locally into the tumor via the Rickham catheter over a 3-hour period (0.5 mL/hr) on days 2, 3, 4, 5, 8, 9, 10, 11, and 12. For UPNs 100 and 102, the cells were infused over a two-hour period; however, due to cell aggregation in the tubing, the protocol was amended to manually inject the cells over a 10-min period for all subsequent participants. On the morning of each T cell infusion, the GRm13Z40-2 cells were thawed, washed, and reformulated in 0.5 mL preservative-free normal saline (PFNS)/2% human serum albumin (HSA) and delivered manually (~10 min) using a syringe to inject into the Rickham catheter. This was followed by a 1.0 mL PFNS flush by convection-enhanced delivery (CED at 0.5 mL/hr; day 1), or followed by 1.5 mL PFNS/2% HSA containing aldesleukin by CED and then the 1.0 mL PFNS flush (both at 0.5 mL/hr; days 3, 8, and 10). The intracranial dose of aldesleukin (Chiron) was 2500 IU on days 2–5 and 5000 IU on days 8–12. Subjects underwent MRI, 18FHBG PET, and 18FDG PET at baseline with additional MRIs at the end of week 1 and at weeks 4 and 8. The 18FHBG PET was repeated at week 3, and the 18FDG PET was repeated at weeks 4 and 8.
Generation of GRm13Z40-2
Peripheral blood mononuclear cells (PBMC) derived from a healthy male volunteer donor apheresis unit were subjected to electrotransfer of the IL13-zetakine CAR-containing plasmid vector followed by activation with 30 ng/mL monoclonal OKT3 (Janssen Biotech, Inc.) and selection with 0.2 mg/mL hygromycin (InvivoGen) as previously described.7,9 Briefly, a rapid expansion method (REM) was carried out by 14-day cycles of coculture with OKT3, 50 U/mL rhIL-2 (Chiron), 106/mL allogeneic irradiated (3300 rads) PBMC, and 2x105 allogeneic irradiated (5000 rads) lymphoblastic cell line (LCL10) cells. T cells were then subjected to transfection with the Ad5/F35 vector SB-313 containing the GR-specific ZFN open reading frames, followed by selection in 10-4 M Dexamethasone and further REM prior to cryopreservation of both a master cell bank and clinical batches.
Serum Antibody Response Assay
Heat inactivated serum samples were incubated at a 1:10 dilution with 7.5 x 104 GRm13Z40-2 cells in 50µL FCS buffer (PBS with 5% BSA and sodium azide) for 20 minutes at 4°C in a 96-well round-bottom plate. The cells were then washed with FCS buffer twice prior to incubation with a 1:50 dilution of FITC-conjugated goat anti-human IgG Fcγ (Jackson ImmunoResearch) for 20 minutes at 4°C in the dark. Cells were washed again and resuspended in 300 µL FCS buffer prior to data acquisition on a Guava easyCyte 5HT (Millipore Sigma) and analysis with FCS Express V3 Software.
Other Materials and Methods may be found in the Supplementary Material.
Results
Generation of allogeneic IL13-zetakine+ CTL product with ZFN-targeted disruption of the GR locus
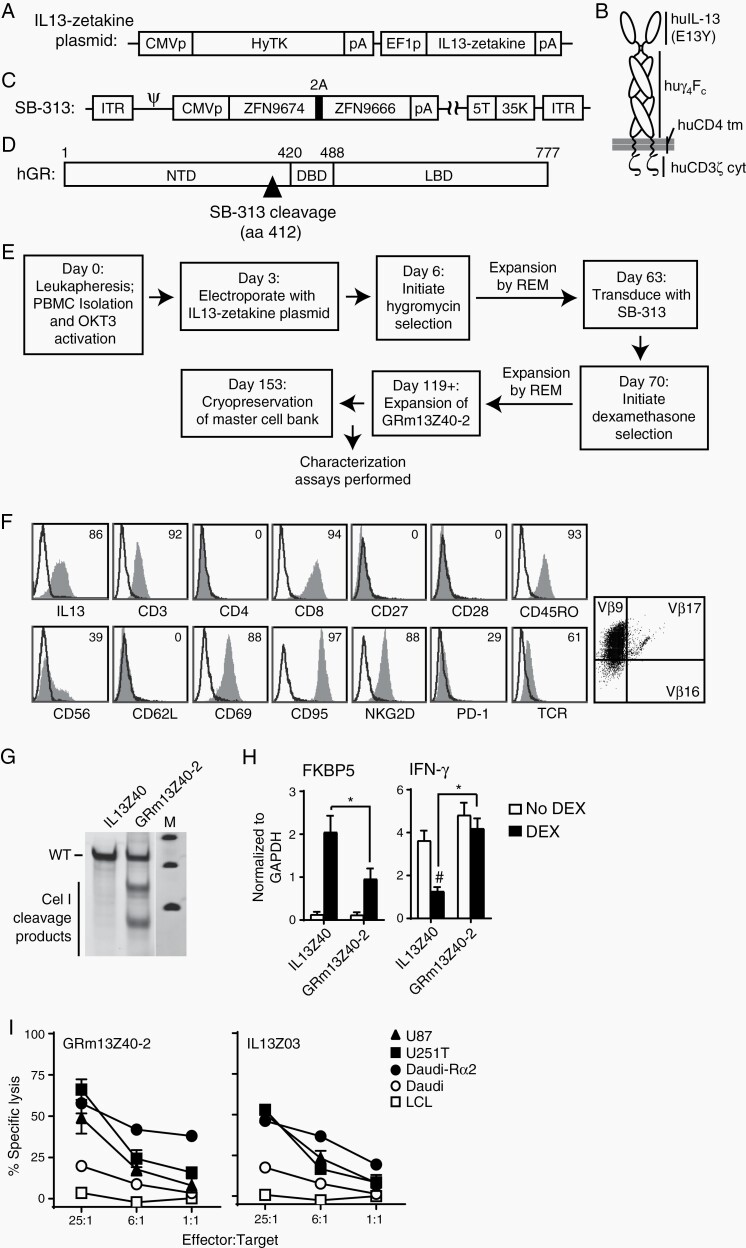
From a healthy male donor, leukapheresis was performed to isolate peripheral blood mononuclear cells and, following OKT3 T cell activation, the cells were electroporated with plasmid (Figure 1A) that would confer expression of the first-generation IL13-zetakine CAR, consisting of an IL13(E12Y) mutein for IL13Rα2-targeting (Figure 1B) as previously described.7 Subsequently, cells were transduced with the Ad5/F35 vector SB-313 containing the two GR-specific ZFN open reading frames (ZFN9674 and ZFN9666) (Figure 1C) that knock out GR by cleaving the locus at a site within the N-terminal domain (Figure 1D). Cells were then selected for glucocorticoid resistance by the addition of 10–4 M dexamethasone. This cell population (clinical product GRm13Z40-2) was then further expanded by rapid expansion method (REM), prior to cryopreservation (Figure 1E). Flow cytometric analysis of the T cell product revealed that it was IL13-zetakine+, CD8+, and oligoclonal, with TCR Vβ analysis revealing that the cells predominantly expressed Vβ9 (Figure 1F).
Fig. 1.
Characterization of GRm13Z40-2 cell product. (A) Schematic of the IL13-zetakine plasmid containing the hygromycin phosphotransferase-HSV thymidine kinase selection/PET reporter fusion gene (HyTK) with poly-adenosine tail (pA) under the control of the cytomegalovirus immediate-early promoter (CMVp), and the IL13-zetakine receptor gene under the control of the human elongation factor 1α promoter (EF1p). (B) Illustration of the IL13-zetakine receptor, where the IL13Rα2-specific ligand IL13(E13Y), IgG4Fc linker (huγ 4Fc), CD4 transmembrane domain (huCD4 tm), and CD3ζ cytoplasmic signaling domain (huCD3ζ cyt) are depicted. (C) Schematic of the GR-ZFN-containing Ad5/35 vector SB-313, where the inverted terminal repeats (ITR), the psi packaging sequence (ψ), the zinc finger nuclease (ZFN) expression cassette containing the CMV promoter (CMVp), the two GR-locus targeting ZFN open reading frames (ZFN9674, ZFN9666) separated by the 2A ribosomal skip sequence, and the chimeric fiber protein sequence containing the shaft and knob domain from adenovirus serotype 35 (35K) in place of that for adenovirus serotype 5 are indicated. (D) Schematic of the human glucocorticoid receptor (hGR) protein sequence, where the N-terminal domain (NTD), DNA binding domain (DBD), ligand binding domain (LBD), and SB-313 target site at amino acid 412 are indicated. (E) Diagram of the manufacturing process, with day of each step indicated. PBMC, peripheral blood mononuclear cells; OKT3, a CD3 agonistic antibody used to activate T cells; REM, rapid expansion method involving 14-day cycles of stimulation with OKT3, rhIL-2 and irradiated feeders as previously described.9 (F) GRm13Z40-2 cells were stained with fluorochrome-conjugated reagents specific for the indicated markers (shaded histograms), with IL13-specific staining used to detect the CAR, and the IOTest® Beta Mark TCR V-beta Repertoire Kit used to determine TCR Vβ9 predominance. Percentages of positive cells above isotype control staining (open histograms) are indicated. Isotype control staining was used to set the quadrants in right-most histogram. (G) Sequence diversity of the GR locus as determined with PCR products incubated with the Cel-1 mismatch specific nuclease, with band analysis showing a 36% frequency of GR locus modification. Depicted are parental IL13Z40 cells (nontransduced with SB-313), and GRm13Z40-2 clinical product cells. M, marker lane. (H) Same cells as in (G) were either treated with 10-6 M dexamethasone (DEX) or left untreated (no DEX) for 20 hours and message RNA levels of glucocortoid induced gene FKBP5 (left) and glucocorticoid-repressed gene IFN-γ (right) were analyzed by RT-PCR. Mean ± S.D. of 4 RT-PCR runs are depicted. *, P ≤ .0089 using a paired Student’s t-test; #, P = .0067 when compared to untreated cells using a paired Student’s t-test. (I) GRm13Z40-2 cells or control IL13Z03 (nontransduced with SB-313) cells were used as effectors in a 4-hour 51Cr release assay using the indicated E:T ratios. The IL13Rα2-positive tumor targets were U87 and U251T cells, and Daudi cells engineered to express IL13Rα2 (Daudi-Rα2); the negative tumor target controls were the LCL and Daudi parental cells. Mean ± S.E.M. of triplicate wells are depicted.
The success of the SB-313 mediated GR-modification was confirmed by incubation of GR locus PCR products from GRm13Z40-2 cells with the Cel-1 mismatch specific nuclease (Figure 1G). In a similar assay, the specificity of the SB-313 ZFNs for the GR locus was supported by the lack of cleavage in the PCR amplified sequences of 15 predicted off-target sites (Supplementary Figure S1). Furthermore, after stimulation with dexamethasone for 20 hours, both induction of the glucocorticoid-induced gene FKBP5 and inhibition of the glucocorticoid-repressed gene IFN-γ were observed to be decreased and/or absent in the SB-313 transduced cells (i.e., GRm13Z40-2 cells) compared to parental controls (Figure 1H). To ensure that GR-modification of the IL13-zetakine+ CTLs did not affect their ability to target and kill IL13Rα2-expressing tumor targets, chromium release assays were performed and showed that GRm13Z40-2 cells exhibited IL13Rα2-targeted cytolytic activity against a panel of tumor cell lines (Figure 1I) that was equivalent to that seen with non-GR-modified IL13-zetakine+ CTL controls. The GRm13Z40-2 master cell bank was determined to be stable with regards to viability, expression of TCRαβ and CD8/CD3, as well as IL13Rα2-targeted cytolytic activity for up to 3.5 years (Supplementary Table S1).
GRm13Z40-2 Lack Alloreactivity
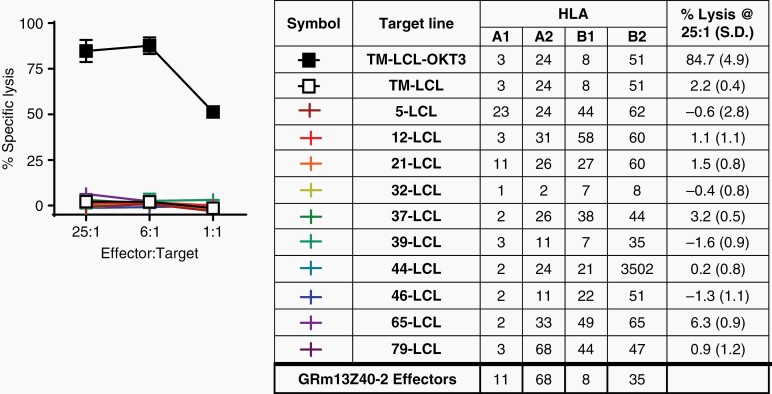
A primary challenge of an allogeneic CAR T cell platform is the risk of graft-versus-host disease (GVHD) due to mismatched human leukocyte antigen (HLA). To evaluate the potential alloreactivity of the oligoclonal GRm13Z40-2 cell product, a panel of EBV-transformed lymphoblastic cell lines (LCL) generated from healthy donors of different HLA subtypes were used as targets in a chromium release assay. Regardless of the HLA A1, A2, B1, or B2 subtype expressed by the target cells, the GRm13Z40-2 effectors lacked reactivity except against positive control targets expressing the CD3-agonist OKT3 (Figure 2). The lack of alloreactivity in this preclinical assay supported the potential safety of this GR-modified, IL13-zetakine+ CTL line.
Fig. 2.
GRm13Z40-2 cells lack alloreactivity. Alloreactivity was tested using a 4-hour 51Cr-release assay with GRm13Z40-2 effectors against EBV-transformed lymphoblastic cell lines (LCL) generated from donors of different allogeneic HLA types (indicated in Table at right). TM-LCL-OKT3 targets expressed the CD3 agonist OKT3 and acted as a positive control.
Patient Characteristics and Treatment Overview
Allogeneic GRm13Z40-2 cells were administered to 6 subjects with unresectable recurrent GBM, as an off-the-shelf therapy, in a single-center, open-label, uncontrolled study (Table 1). All of the subjects were Caucasian and had a mean (+SD) age of 56.2 + 10.7 years (range: 36–66 years). The mean (+SD) duration since histological diagnosis of GBM to enrollment on this trial was 24 + 27.3 months (range: 5.8–79 months). Four of the six subjects had undergone at least one surgical resection, with two of them having had 3 resections. All of the subjects had received prior temozolomide and radiotherapy. The Karnofsky Performance Score of the subjects at enrollment ranged from 60 to 80 (Table 1).
Table 1.
Patient and Outcome Summary
| UPN | Gender | Age at Enrollment (yrs) | Time from Diagnosis to Enrollment (months) |
Prior Therapies | KPS at Enrollment | Dex Dose Per Day* | DLTs** | Serum Ab Response^ |
Evidence for Radiographic Response | KPS at Last Contact | Survival Post Treatment (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | Female | 62 | 5.8 | 1surgery-b#, RT/TMZ |
60 | 12 mg | None | NE | Local necrosis | 30 | 2.9 |
| 102 | Male | 36 | 79 | 3 surgeries-r/r/r, RT/TMZ, 4 other¥, BVZ |
70 | 12 mg | None | NE | None | 70 | 2.9 |
| 103 | Female | 52 | 15.9 | 3 surgeries-r/b/b, RT/TMZ, 1 other, BVZ |
80 | 4 mg | None | + | None | 70 | 6.9 |
| 104 | Male | 61 | 17.9 | 3surgeries-r/r/r, RT/TMZ |
80 | 4–6 mg§ | None | - | Local necrosis | 70 | 2.3 |
| 105 | Male | 66 | 14.4 | 1surgery-r, RT/TMZ, 2 other |
70 | 6 mg | None | - | None | 60 | 11.3 |
| 106 | Male | 60 | 11 | 1 surgery-b, RT/TMZ |
70 | 12 mg | None | - | None | 40 | 2.5 |
BVZ, bevacizumab; Dex, dexamethasone; DLTs, dose limiting toxicities; RT, radiation therapy; TMZ, temozolomide.
#Surgeries were performed for either biopsy (b) or resection (r) as indicated.
*Dexamethasone dose during the two-week treatment period is provided.
**Toxicities described further in Supplementary Table S2.
^Serum Ab response described further in Supplementary Table S3.
¥Other therapies may include clinical trials, chemotherapies and/or laser ablation.
§Patient received 4 mg dexamethasone for two days, then 6 mg for 11 days before reducing back to 4 mg again.
After placement of a Rickham catheter in or proximal to the MRI-defined tumor site, GRm13Z40-2 cells (4 infusions of 108 cells) and IL-2 (aldesleukin; 9 infusions ranging from 2500–5000 IU) were locally infused via the catheter over a two-week period (Figure 3A). All patients were on a daily dexamethasone dose of ≥ 4 mg. (Table 1). While prior documented IL13Rα2 expression on archival tissue was an enrollment criterion, intermediate IL13Rα2 expression was also detected in the recurrent GBM of all patients (Figure 3B; i.e., biopsies from each patient exhibited an H score of 100 or greater using a formula modified from11).
Fig. 3.
Treatment schema and target antigen expression of excised tumors. (A) Intratumoral treatment overview for the 4 cycles of 108 GRm13Z40-2 cells administered twice a week, with IL-2 administered by convection-enhanced delivery (CED) on days 2-5 of week one, and days 1-5 of week 2. (B) Recurrent tumors of each UPN resected at time of Rickham placement underwent immunohistochemical (IHC) staining using IL13Rα2-specific DAB with hematoxylin counterstain. Scale bars indicate 100 µm. H scores are indicated in parenthases, and were obtained by the formula: (3 x percentage of strongly staining cells) + (2 x percentage of moderately staining cells) + percentage of weakly staining cells, giving a range of 0 to 300 (Modified from11); plus signs indicate that membranous staining was observed.
Safety of GRm13Z40-2 Administration
All research subjects received the entire 2-week treatment cycle without any dose limiting toxicities (Table 1). All adverse events (AEs) considered at least possibly related to GRm13Z40-2 cells and/or IL-2 treatment were ≤ grade 3, with no AEs considered probably related to the treatment, and only one grade 1 injection site reaction (UPN 100) and one grade 1 fever (UPN 104) considered definitely related to the treatment (Supplementary Table S2). The most common AEs that were possibly related to the treatment included fatigue (UPNs 100, 103, 104, 106), confusion (UPNs 100, 104, 105), sinus tachycardia (UPNs 100, 105, 106), and headache (UPNs 100, 102, 103, 105, 106). One patient, UPN 100, experienced the most AEs that were possibly related to the treatment, including a stroke distant from the T cell injection site nearly two weeks after the final infusion; however, the stroke was considered probably related to dehydration. Overall, an acceptable safety profile was observed in all 6 patients and all treatment-related AEs were transient and/or manageable.
Evidence of Transient Antitumor Bioactivity Following GRm13Z40-2 Administration
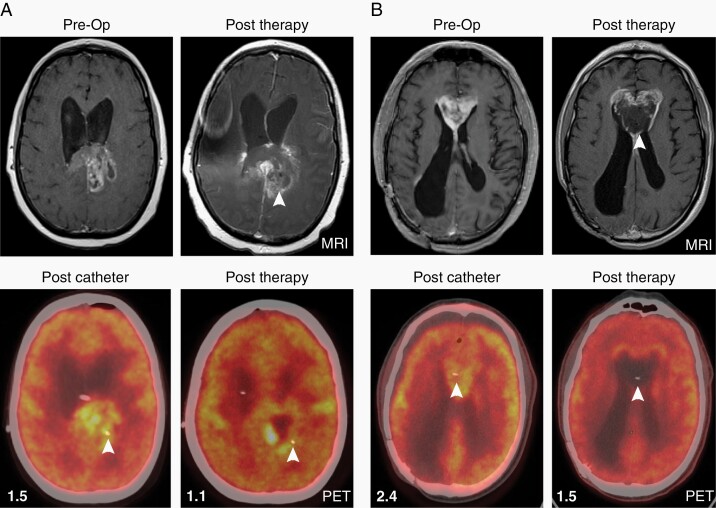
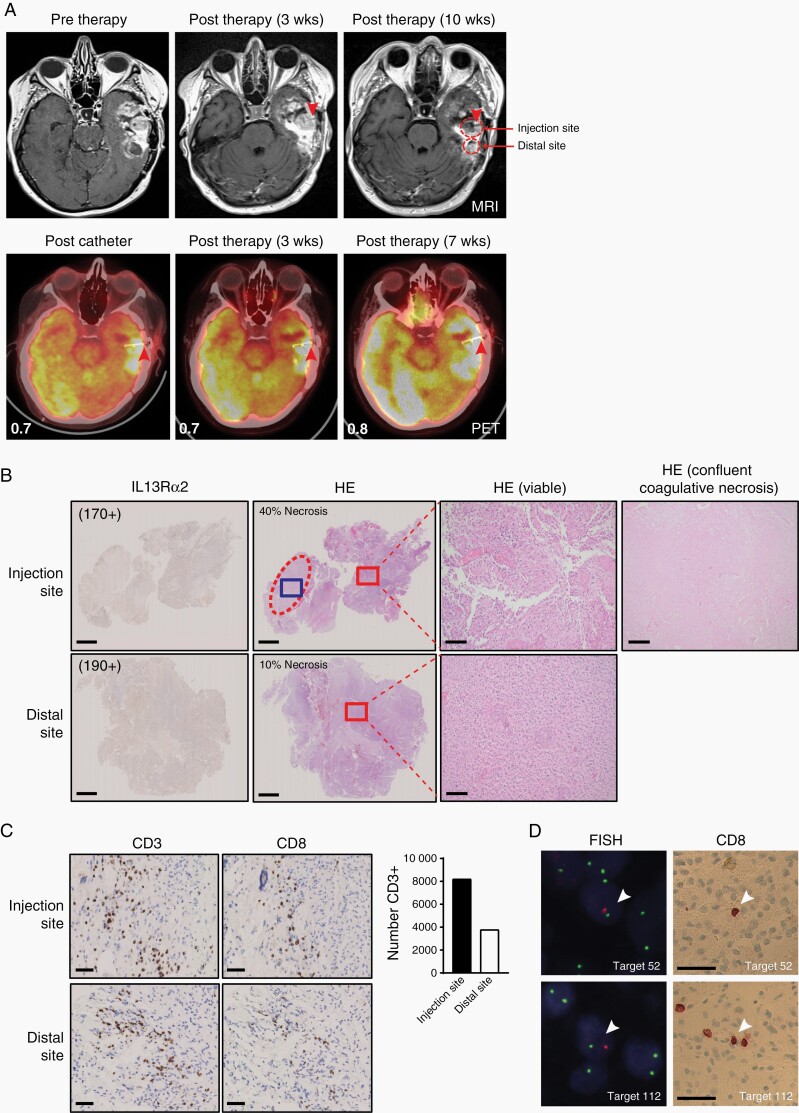
Serial radiographic imaging with magnetic resonance imaging (MRI) and positron emission tomography (PET) was conducted to evaluate for tumor regression (Figures 4, 5A, Supplementary Figures S2A and S3). Two research subjects (UPNs 100 and 104) had radiographic evidence of enhanced tumor necrosis at 4 weeks at the site of GRm13Z40-2 cell administration as detected by MRI and 18F-fluorodeoxyglucose (FDG) PET imaging (Table 1 and Figure 4). Furthermore, two patients (UPNs 103 and 106) who underwent a subsequent craniotomy for symptomatic tumor progression and lacked evidence of anti-tumor responses on post-therapy images (Figure 5A and Supplementary Figure S2A), had histological features suggestive of local treatment responses near the site of catheter placement as indicated by large areas of confluent coagulative necrosis observed exclusively in proximity to the T cell infusion site (Figure 5B and Supplementary Figure S2D). Further immunohistochemical analysis of the pathology specimens from these two patients (UPNs 103 and 106) revealed maintenance of IL13Rα2 expression (Figure 5B and Supplementary Figure S2B), as well as the presence of CD8+ T cells in the tumor microenvironment (Figure 5C and Supplementary Figure S2C), which appeared higher at the injection site vs. a more distal tumor biopsy site that was collected from UPN 103 (Figure 5C). However, using fluorescence in situ hybridization (FISH) with an XY probe to determine the genotype of the CD8+ cells in female patient UPN 103 (Figure 5D), only two CD8+ male cells were identified in the injection site sample (117 fields evaluated), and one CD8+ male cell was identified in the distal site sample (124 fields evaluated), suggesting that only a few injected GRm13Z40-2 cells had persisted 10 weeks after the final T cell infusion.
Fig. 4.
Imaging demonstrates increased necrosis near site of CAR T cell injection. Axial T1 with contrast MRI images show preoperative recurrent tumors extending into left splenium of UPN 100 (A) and into the corpus callosum of UPN 104 (B), with PET imaging obtained after catheter placement demonstrating hypermatabolic tumors at each site. One month after treatment initiation (“Post Therapy” panels at right), local necrosis is noted in areas of previous MRI enhancement (white arrow heads); and PET imaging shows hypometabolic tumors, consistent with necrosis, surrounding each catheter site (white arrow heads). Mean standardized uptake values (SUVs) of tumor site normalized to background SUVs are indicated in each PET panel.
Fig. 5.
Comparison of injection and distal tumor sites post-therapy. (A) Axial T1 with contrast MRI images show recurrent tumor of UPN 103, with no significant radiological changes noted adjacent to catheter (red arrow head) at 3 weeks after initiation of therapy, but more heterogenous enhancement of the tumor bulk surrounding the catheter at 10 weeks after initiation of therapy (top panels). PET imaging obtained after catheter insertion (red arrow head) and at 3 and 7 weeks after initiation of therapy shows hypermetabolic tumor (bottom panels). Biopsy sites collected at 10 weeks are indicated with red outlines. Mean SUVs of tumor site normalized to background SUVs are indicated in each PET panel. (B) IL13Rα2 IHC and hematoxylin and eosin (HE) staining of biopsies collected at 10 weeks after initiation of therapy. H scores for IL13Rα2 staining are indicated in parentheses, and were obtained as described in Figure 3 legend. Injection site shows 40% necrosis, which is chiefly represented by large areas of confluent coagulative necrosis (dashed oval, blue box magnified in far-right panel). Distal site shows predominantly viable tissue with foci of pseudo-palisading necrosis representing 10% of the resected tissue. Red boxes outline areas of viable tumor as magnified in the subsequent panels. Scale bars indicate 2.5 mm (images at left) and 100 µm (magnified images at right). (C) Tumor biopsies of injection and distal sites collected at 10 weeks after initiation of therapy were examined for the presence of CD3+ and CD8+ T cells by IHC. Scale bars indicate 50 µm. Enumeration of CD3+ cells in the 2.5 x 108 μm2 area of each biopsy IHC section is depicted at the right. (D) Detection of male CAR T cells in biopsies collected at 10 weeks after initiation of therapy. Upon alignment of FISH and CD8 IHC brightfield images, two CD8+ male cells (Target 52 depicted, white arrowhead; Target 110 not shown) were identified in the injection site biopsy section, and one CD8+ male cell (Target 112 depicted, white arrowhead) was identified in the distal site biopsy section. Scale bars indicate 50 µm.
The median survival of the research participants treated on this study was 2.9 months after T cell therapy with the longest survival being 11.3 months in a patient (UPN 105) who did not have radiographic evidence of response (Table 1). Overall mean survival from initial GBM diagnosis was 29 months, with a range of 8.6 to 82 months, for this patient cohort. In summary, while evidence of antitumor bioactivity following CAR T cell and adeleuskin therapy was observed, no objective clinical responses or significant survival benefit could be documented in this patient population with advanced GBM.
Assessment of Anti-GRm13Z40-2 Humoral Responses
To assess the induction of host immune responses against the allogeneic cells, antibody response assays were carried out with the serum collected from four patients (Table 1 and Supplementary Table S3). Antibody reactivity against the surface-expressed CAR transgene using a flow cytometry-based assay was observed in only one of the four tested participants. This response detected in UPN 103 started at week 3 (i.e., the week after completion of all four GRm13Z40-2 administration cycles), and while it did not coincide with any adverse events of grade 3 or higher, it does suggest that in some cases an immune rejection response may be mounted by patients. However, this patient also received the lowest dose of dexamethasone (i.e., 4 mg/day), so it may be possible that a higher dose of dexamethasone would have prevented this immune rejection.
Discussion
GBM and other brain tumors are an attractive clinical setting for the application of CAR T cell therapy.12,13 For many patients with malignant brain tumors, clinical urgency limits patients from waiting for a new therapy such as the time needed to engineer an autologous cell-based therapy. Moreover, the immune-privileged nature of the central nervous system (CNS) may provide a particularly attractive clinical setting in which to test allogeneic CAR T cell products. Foundational studies reveal that immune rejection of allografts occurs more slowly in the CNS versus periphery,14 a feature that may provide a window of opportunity for off-the-shelf therapies for malignant brain tumors. To further protect against immune rejection of the allogeneic CAR T cell product, we took advantage of the immunosuppressive effects of the glucocorticoid dexamethasone, which is a standard adjuvant treatment used to mitigate cerebral edema in brain tumor patients and documented to suppress immune responses in brain tumors.15,16 Although attractive as an approach, clinical validation for safety and feasibility of an allogeneic CAR T cell off-the-shelf approach has not previously been reported in the setting of malignant brain tumors. Here we present the first-in-human evaluation of a locally administered, glucocorticoid resistant, allogeneic CAR T cell product for the treatment of recurrent GBM.
To bypass glucocorticoid signaling in our engineered CTLs and bestow steroid-resistant effector function, we used ZFNs to target a site in exon 3 of the GR locus to permanently disrupt expression of the full-length receptor (see Figure 1D). ZFN-induced DNA cleavage followed by non-homologous end joining leads to the small insertion or deletion of nucleotides at the cleavage site, which will in most cases alter the reading frame to include a premature stop codon, thus generating a truncated protein variant and reduced protein levels (reviewed in17). The truncated GR protein generated by the GR ZFNs would be expected to lack the DNA binding domain, ligand binding domain, and nuclear localization signal, and therefore not possess any gene regulatory activity. Indeed, our findings support that GR-disrupted CAR T cell products do retain effector function in the presence of dexamethasone.
The use of ZFN-targeted genetic modification has been investigated by others in the manufacture of T cells for adoptive transfer. The general efficiency of ZFN-mediated gene editing of CD4+ and CD8+ T cells by homology-directed repair using Adeno-associated virus vector to deliver the homologous donor template has been described by Wang et al.18 We considered two features of such ZFN-targeted technology to be especially useful when designing our CAR T cell product for this clinical trial. First, the endogenous GR locus sequence can be modified at a high frequency on both alleles, so that there is no residual functional GR expression and gene modification is maintained and transmitted stably. Second, ZFN expression is required only transiently, which allows expression of the ZFN from a nonintegrating delivery vector (i.e., Ad5/F35) and avoids the hazards associated with vector and transgene insertion and long-term instability of transgene expression. However, other gene editing techniques that have been successfully used in the development of allogeneic CAR T cell products include transcription activator-like effector nucleases (TALENs) and the clustered regularly interspaced short palindromic repeats (CRISPR) system (reviewed in 4,5). Independent of the gene-editing approach employed, our study highlights the potential of GR-locus knockout for off-the-shelf CAR T cell development, and provides the first clinical proof-of-concept for its application.
Graft-versus-host (GVH) disease is a potential severe adverse complication of allogenic CAR T cell products. In our study, we avoided GVH activity by selecting for an oligoclonal T cell population that was evaluated prior to clinical administration for its alloreactivity. GRm13Z40-2 cells did not exhibit GVH reactivity when tested against a panel of donor-derived cell lines with different HLA subtypes, and while this panel was not fully comprehensive, our clinical data further supports the safety of repetitive delivery of an allogeneic CAR T cell product that was derived from healthy donor PBMC with defined TCR specificity. This safety profile was observed despite the fact that patient HLA screening (i.e., to match that of the subtypes in the test panel) was not a part of enrollment. Other cell sources actively being investigated for their utility as an off-the-shelf product with reduced risk of GVH include use of virus-specific memory T cells with restricted TCR repertoire, or non-TCRαβ cells such as NK cells, NKT cells, or TCRγδ T cells (reviewed in 4). Alternatively, gene knockout of the T cell receptor alpha locus (TRAC) using CRISPR and other gene-editing technologies4,19 is also a strategy to overcome the risk of GVH. Indeed, Torikai et al were the first to report the ZFN-mediated targeting of the TRAC locus as an approach to avoid GVH responses.20 Use of induced pluripotent stem cells (iPSCs) is another attractive source of T cells that our group is actively investigating, as they would provide unlimited self-renewal and can be banked indefinitely.21 A bank of iPSCs with common HLA haplotypes might be generated to minimize the risk of allo-rejection, and gene editing might be used to eliminate TCR to avoid GVH reactivity.
We had conducted an earlier Phase I trial evaluating autologous cloned CD8+ CTLs, also genetically modified to express the first-generation IL13-zetakine, but with intact GR (NCT00730613).7 Each of the patients treated on that trial experienced brain inflammation at the site of T cell infusion, which is in contrast to the trial reported here, where we attribute the lack of neuroinflammation to the regular administration of dexamethasone. Regardless, for both clinical protocols, CAR T cells were delivered intracranially through an implanted reservoir/catheter system that allowed repetitive dosing of the cells directly into the resected tumor cavity or the site of the tumor. No immediate or acute device-related adverse events (e.g., occlusion, malfunction, infection) were detected. Together, the safety profiles, as well as evidence of local tumor necrosis, support the strategy of targeting IL13Rα2 in the context of CAR T cell immunotherapy.
Despite the local transient activity, tumors eventually recurred in all patients, and the T cells did not persist in the tumor microenvironment. We cannot rule out allo-rejection, which might benefit from stricter patient HLA screening/selection. Further, the impact of dexamethasone on the endogenous immune system may also have played a role in limiting the therapeutic effectiveness of this strategy. We previously reported a case in which IL13Rα2-CAR T cells mediated a complete response in a patient with actively progressing recurrent multifocal GBM, and have shown that this response was associated with activation of host immunity.3,22,23 Other successful adoptive T cell therapies are increasingly understood to be impacted by engagement of endogenous immune responses.24–26 Indeed, in a report by Keskin et al., vaccine-driven T cell responses were observed only in patients who did not receive dexamethasone during neoantigen priming.16 The immunosuppressive effects of the GBM microenvironment27 might also have limited the effectiveness of the therapy and combination approaches targeting the tumor microenvironment, such as combining CAR T cell therapy with checkpoint inhibition, may improve patient outcomes.28
While the trial design might be improved by addressing issues of patient selection, engagement of endogenous immunity, and/or the suppressive tumor microenvironment, it must also be acknowledged that the GRm13Z40-2 cells represent first-generation CAR T cells, in which persistence has been noted to be low and has since been improved by including costimulatory domains.29 Further, it has been shown that the IgG4-Fc linker within this first-generation CAR can lead to off-target interactions with Fc gamma receptors which reduce CAR T cell persistence, and can be overcome by mutating that portion of the CAR design.30 Finally, these GRm13Z40-2 cells had also undergone extensive in vitro culture and expansion, which is known to be detrimental to CAR T cell fitness.31 Thus, the lack of CAR T persistence in this trial is likely at least partly due to specific features of the CAR T cell product.
More recently, to improve IL13Rα2-targeted CAR T cell persistence and potency, we have both optimized the CAR to contain a 4-1BB costimulatory domain, and modified our manufacturing platform by engineering CD62L+ naïve and memory cells, and reducing the ex vivo expansion time to < 14 days (29 and unpublished data). These IL13BBζ-CAR T cells exhibit significantly improved antitumor activity and persistence compared to first-generation IL13ζ-CAR CTL. Based on these preclinical results and the clinical safety reported for first-generation IL13Rα2-targeted CAR CTL in this study and previously,7 we have initiated a first-in-human clinical trial [NCT02208362] evaluating the safety, feasibility, and bioactivity of weekly intracranial infusions of autologous second-generation IL13BBζ-CAR T cells without GR disruption in patients with recurrent IL13Rα2+ high-grade glioma. While this study is ongoing, there is evidence of clinical activity in a subset of patients, including one patient that achieved a complete response.3 Together, these studies support the future development of allogeneic second-generation IL13Rα2-targeted CAR T cell products for the treatment of GBM.
Overall, we demonstrate safety and feasibility of GR-modified cells for use as an off-the-shelf allogeneic CAR T cell product. This first-in-human experience thus establishes a foundation for future adoptive therapy studies using off-the-shelf, glucocorticoid resistant CAR T cells for the treatment of brain tumors.
Supplementary Material
Acknowledgments
We thank Anita Kurien for regulatory support and David DiGiusto for insightful discussion in the design of this trial. In the memory of Xin (Cindy) Yang and Dale Ando, we would like to acknowledge their contributions to this research.
Contributor Information
Christine E Brown, Department of Hematology & Hematopoietic Cell Transplantation (T Cell Therapeutics Research Laboratories), City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Analiz Rodriguez, Department of Neurosurgery, City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Joycelynne Palmer, Department of Computational and Quantitative Medicine, City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Julie R Ostberg, Department of Hematology & Hematopoietic Cell Transplantation (T Cell Therapeutics Research Laboratories), City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Araceli Naranjo, Department of Hematology & Hematopoietic Cell Transplantation (T Cell Therapeutics Research Laboratories), City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Jamie R Wagner, Department of Hematology & Hematopoietic Cell Transplantation (T Cell Therapeutics Research Laboratories), City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Brenda Aguilar, Department of Hematology & Hematopoietic Cell Transplantation (T Cell Therapeutics Research Laboratories), City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Renate Starr, Department of Hematology & Hematopoietic Cell Transplantation (T Cell Therapeutics Research Laboratories), City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Lihong Weng, Department of Hematology & Hematopoietic Cell Transplantation (T Cell Therapeutics Research Laboratories), City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Timothy W Synold, Department of Cancer Biology, City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Vivi Tran, Department of Cancer Biology, City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Shelley Wang, Sangamo Therapeutics, Inc., Richmond, California, USA.
Andreas Reik, Sangamo Therapeutics, Inc., Richmond, California, USA.
Massimo D’Apuzzo, Department of Pathology, City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Julie A Ressler, Department of Diagnostic Radiology, City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Yuanyue Zhou, Sangamo Therapeutics, Inc., Richmond, California, USA.
Matthew Mendel, Sangamo Therapeutics, Inc., Richmond, California, USA.
Philip D Gregory, Sangamo Therapeutics, Inc., Richmond, California, USA.
Michael C Holmes, Sangamo Therapeutics, Inc., Richmond, California, USA.
Winson W Tang, Sangamo Therapeutics, Inc., Richmond, California, USA.
Stephen J Forman, Department of Hematology & Hematopoietic Cell Transplantation (T Cell Therapeutics Research Laboratories), City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Michael C Jensen, Ben Town Center for Childhood Cancer, Seattle Children’s Research Institute, Seattle, Washington, USA.
Behnam Badie, Department of Neurosurgery, City of Hope Beckman Research Institute and Medical Center; Duarte, California, USA.
Funding
This work was supported by the National Institutes of Health’s National Cancer Institute grants R01CA155769 (BB), R01CA236500 (CEB), and R01CA254271 (BB, CEB); the Liam McGee Brain Tumor Fund (BB); and the California Institute of Regenerative Medicine grant CLIN2-10248 (CEB). Research reported in this article included work in the GMP Manufacturing, Pathology Research Services and Analytical Pharmacology Cores, which were supported by National Institutes of Health’s National Cancer Institute grant P30CA033572.
Conflict of interest
AR, YZ and MM are current employees of Sangamo Therapeutics, Inc. SW, PDG, MCH and WWT were employed by Sangamo Therapeutics, Inc. when this work was conducted. SJF and CEB received royalty payments from Mustang Bio. CEB received royalty payments and research support from Chimeric Therapeutics. No other disclosures were reported.
Author contributions
Conceptualization: PDG, MCH, MCJ, BB. Methodology: CEB, JP, TS, ARe, MCJ, BB. Investigation: CEB, ARo, JP, AN, JW, BA, RS, LW, VT, SW, MDA, JAR, YZ, MM, WWT. Visualization: ARo, JRO,. Funding acquisition: CEB, SJF, MCJ, BB. Supervision: CEB, ARe, PDG, MCH, SJF, MCJ, BB. Writing—original draft: ARo, JP, JRO. Writing—review & editing: CEB, JP, JRO, MDA, BB
References
- 1. Maude SL, Laetsch TW, Buechner J, et al. . Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neelapu SS, Locke FL, Bartlett NL, et al. . Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown CE, Alizadeh D, Starr R, et al. . Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26):2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19(3):185–199. [DOI] [PubMed] [Google Scholar]
- 5. Morgan MA, Büning H, Sauer M, Schambach A. Use of cell and genome modification technologies to generate improved “off-the-shelf” CAR T and CAR NK cells. Front Immunol. 2020;11:1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith LK, Cidlowski JA. Glucocorticoid-induced apoptosis of healthy and malignant lymphocytes. Prog Brain Res. 2010;182:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown CE, Badie B, Barish ME, et al. . Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res. 2015;21(18):4062–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keu KV, Witney TH, Yaghoubi S, et al. . Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci Transl Med. 2017;9(373): eaag2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jensen MC, Clarke P, Tan G, et al. . Human T lymphocyte genetic modification with naked DNA. Mol Ther. 2000;1(1):49–55. [DOI] [PubMed] [Google Scholar]
- 10. Bird AG, McLachlan SM, Britton S. Cyclosporin A promotes spontaneous outgrowth in vitro of Epstein-Barr virus-induced B-cell lines. Nature. 1981;289(5795):300–301. [DOI] [PubMed] [Google Scholar]
- 11. Ishibashi H, Suzuki T, Suzuki S, et al. . Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab. 2003;88(5):2309–2317. [DOI] [PubMed] [Google Scholar]
- 12. Akhavan D, Alizadeh D, Wang D, et al. . CAR T cells for brain tumors: lessons learned and road ahead. Immunol Rev. 2019;290(1):60–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chuntova P, Downey KM, Hegde B, Almeida ND, Okada H. Genetically engineered T-cells for malignant glioma: overcoming the barriers to effective immunotherapy. Front Immunol. 2018;9:3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Medawar PB. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to anterior chamber of the eye. Br J Exp Pathol 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 15. Iorgulescu JB, Gokhale PC, Speranza MC, et al. . Concurrent dexamethasone limits the clinical benefit of immune checkpoint blockade in glioblastoma. Clin Cancer Res. 2021;27(1):276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keskin DB, Anandappa AJ, Sun J, et al. . Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang YF, Imam JS, Wilkinson MF. The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem. 2007;76:51–74. [DOI] [PubMed] [Google Scholar]
- 18. Wang J, DeClercq JJ, Hayward SB, et al. . Highly efficient homology-driven genome editing in human T cells by combining zinc-finger nuclease mRNA and AAV6 donor delivery. Nucleic Acids Res. 2016;44(3):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stadtmauer EA, Fraietta JA, Davis MM, et al. . CRISPR-engineered T cells in patients with refractory cancer. Science 2020;367(6481): eaba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Torikai H, Reik A, Liu PQ, et al. . A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012;119(24):5697–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Themeli M, Kloss CC, Ciriello G, et al. . Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31(10):928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alizadeh D, Wong RA, Gholamin S, et al. . IFNγ is critical for CAR T cell-mediated myeloid activation and induction of endogenous immunity. Cancer Discov. 2021;11(9):2248–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jonsson VD, Ng RH, Dullerud N, et al. . CAR T cell therapy drives endogenous locoregional T cell dynamics in a responding patient with glioblastoma. bioRxiv 2021; doi: 10.1101/2021.09.22.460392. [DOI] [Google Scholar]
- 24. Bachireddy P, Azizi E, Burdziak C, et al. . Mapping the evolution of T cell states during response and resistance to adoptive cellular therapy. Cell Rep. 2021;37(6):109992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hegde M, Joseph SK, Pashankar F, et al. . Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma. Nat Commun. 2020;11(1):3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hunder NN, Wallen H, Cao J, et al. . Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358(25):2698–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol. 2011;2011:732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamath SD, Kumthekar PU. Immune checkpoint inhibitors for the treatment of Central Nervous System (CNS) metastatic disease. Front Oncol. 2018;8:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown CE, Aguilar B, Starr R, et al. . Optimization of IL13Rα2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol Ther. 2018;26(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jonnalagadda M, Mardiros A, Urak R, et al. . Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Mol Ther. 2015;23(4):757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Itzhaki O, Hovav E, Ziporen Y, et al. . Establishment and large-scale expansion of minimally cultured “young” tumor infiltrating lymphocytes for adoptive transfer therapy. J Immunother. 2011;34(2):212–220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.