Abstract
Background
This multinational study was conducted to report clinical presentations and treatment strategies in patients with intracranial germinomas across selected Asian centers, including failure patterns, risk factors, and outcomes.
Methods
A retrospective data collection and analysis of these patients, treated between 1995 and 2015 from eight healthcare institutions across four countries was undertaken.
Results
From the results, 418 patients were analyzed, with a median follow-up of 8.9 years; 79.9% of the patients were M0, and 87.6% had β-human chorionic gonadotropin values <50 mIU/mL. The 5/10-year overall survival (OS) and recurrence-free survival (RFS) rates were 97.2%/96.2% and 89.9%/86.9%, respectively. RFS was predicted by the radiotherapy (RT) field, with focal RT having the worst outcome, whereas chemotherapy usage had no impact on survival. Among patients who received chemotherapy, response to chemotherapy did not predict survival outcomes. In M0 patients, primary basal ganglia tumors predicted a worse RFS. In patients with bifocal tumors, an extended field RT was associated with better outcomes. In multivariable analysis, only RT fields were associated with RFS. In relapsed patients, salvage rates were high at 85.7%. Additionally, patients who received salvage RT had a better outcome (91.6% vs. 66.7%).
Conclusions
Survival outcomes of patients with germinoma were excellent. Thus, the focus of treatment for intracranial germinoma should be on survivorship. Further studies are warranted to find the optimal intensity and volume of radiation, including the role of chemotherapy in the survival of patients with intracranial germinomas, considering age, primary tumor location, and extent of disease.
Keywords: chemotherapy, intracranial germinoma, radiotherapy, outcomes, treatment
Key Points.
While treatment strategies varied across Asian countries, survival outcomes were similarly excellent.
Tumor location and radiation field predicted recurrence.
Treatment for intracranial germinoma should be focused on survivorship.
Importance of the Study.
Although intracranial germinomas are chemo- and radio-sensitive and have good outcomes, it remains unclear whether the use of chemotherapy to reduce radiation doses improve disease control and quality of life. In addition, treatment strategies for basal ganglia or bifocal disease have not yet been established. As intracranial germinoma has an Asian prevalence, we conducted an Asian multicenter retrospective study and reported the data of 418 patients from eight institutions in four Asian countries. We confirmed that the overall survival of germinomas was excellent with a 5-year overall survival of 97.2%, although a spectrum of practice among the different institutions varied. Tumor location and radiation field predicted recurrence with focal radiotherapy giving the worst outcome, whereas chemotherapy use had no impact on survival. Primary basal ganglia tumors predicted a worse outcome. In patients with bifocal tumors, extended field radiotherapy was associated with better outcomes. Considering excellent tumor control, we recommend that future trials need to focus on survivorship through optimization of treatment intensity without overtreatment.
Intracranial germ cell tumors (ICGCTs) present with a varied epidemiological distribution. In Western countries, ICGCT represents 0.5–3% of pediatric CNS tumors but represents 10% in Asia.1 It typically occurs as midline lesions around the third ventricle, most commonly involving the pineal gland and the suprasellar region of the brain. Less commonly, tumors occur in the basal ganglia or thalamic nuclei. There are reports of ethnic differences in the location of primary tumors, prognosis, and epidemiology.2
ICGCT is divided into germinomas and nongerminomatous germ cell tumors. Germinomas are more prevalent with a much better prognosis. Additionally, the treatment paradigm has less emphasis on chemotherapy, the usage of which is institution-dependent.3 As germinomas are chemo- and radio-sensitive and have good treatment outcomes, the focus is more on reducing long-term side effects. A series of chronologically spaced publications from one large Korean institute showed that the best treatment results were obtained through craniospinal irradiation (CSI).4,5 It is also well recognized that CSI dose can be safely reduced to 19.5–24 Gy in 1.5–1.8 Gy per fraction 4,6–9. However, subsequent studies have been continuously undertaken as well to reduce radiotherapy fields, with results showing that replacing CSI with the whole brain (WB) or whole ventricular irradiation (WVI) in patients with localized germinomas resulted in a spinal failure rate of less than 10%.10–14
International Society of Pediatric Oncology (SIOP) CNS GCT 96 compared chemotherapy followed by 40 Gy focal radiotherapy (RT) vs 24 Gy CSI with an additional boost of 16 Gy alone in 190 patients with localized germinoma. The 5-year event-free survival for patients receiving chemotherapy and focal RT was less than for those receiving RT to a larger field without chemotherapy (88% vs. 94%). Additionally, the pattern of relapse suggests that the ventricles should be included in the radiation field. Furthermore, for patients with metastatic germinoma, CSI alone was enough to cure the disease and did not demonstrate additional benefit of chemotherapy. Given the aforementioned studies, the latest international consensus publication agreed that at least WVI was necessary to control localized germinoma.14,15 Recently, early results from the SIOP CNS GCT II trial also suggested that 24 Gy WVI without boost was sufficient in localized germinomas after a complete radiological response from chemotherapy.16
Areas of controversies and literature gaps still exist. It remains unproven with empirical data if using chemotherapy to reduce radiation doses leads to better disease control and quality of life. Some studies have highlighted possible worse outcomes with basal ganglia primaries.17 The staging of bifocal disease and the cut-off thresholds for human chorionic gonadotropin (HCG) are still being debated as well. Data has also suggested that larger tumors were risk factors.18,19 Therefore, as ICGCT has an Asian prevalence, this study was undertaken to report treatment policies and outcomes in Asia.
We conducted an Asian multi-institutional retrospective study, comparing the patterns of care and reporting clinical outcomes from the various treatment strategies. We also analyzed failure patterns and risk factors in intracranial germinoma cases. This study is the first report of ICGCT from different countries and institutions in Asia–Korea, Taiwan, Singapore, and Japan.
Materials and Methods
Methods
A retrospective analysis of patients’ medical records from eight institutions in Korea, Taiwan, Singapore, and Japan was performed. The institutions were Asan Medical Center, CHA Bundang Medical Center, Yonsei University Severance Hospital, National Cancer Center Singapore/KK Women’s and Children’s Hospital, National Center for Child Health and Development Japan, Seoul National University Hospital, Taipei Veterans General Hospital, and National Cancer Center Korea. Patients were treated from January 1, 1995 to December 31, 2015. Data was collected retrospectively by each study member from the existing registries of participating hospitals, using a standardized data collection form. The anonymized dataset was sent to National Cancer Center Korea for analysis. The study was approved by the Institutional Review Board from each institution.
The inclusion criteria were as follows: (1) clinically or pathologically diagnosed with pure germinoma with or without teratoma; (2) aged 0–40 years; (3) treated with curative intent; and (4) available for the analysis of radiotherapy and chemotherapy. Patients with missing key information such as tumor location, data on surgery, chemotherapy, and radiotherapy, and post-treatment evaluation were excluded. If histology was available and showed elements other than germinoma or teratoma, patients were ineligible. Furthermore, patients with elevated βHCG levels were allowed only if the biopsy showed pure germinoma. Patients with a clinical diagnosis based on neuroimaging characteristics and response to RT were allowed to be included.
The data collected included demographic and clinical characteristics (pathology, serum and CSF tumor markers, CSF cyctology, brain and spine MRI), treatment (chemotherapy, RT, surgery, response to chemotherapy), and outcome (recurrence, survival, and secondary malignancy). M stage was determined by abnormal CSF cytology or imaging evidence of tumor in the brain or spine. Response to chemotherapy was retrospectively graded uniformly according to the standard criteria as completer response (disappearance of tumor on MRI), very good partial response (suprasellar tumor decreased to <4 mm and pineal tumor decreased to <10 mm), partial response (>50% reduction in tumor size, as measured by the sum of the products of the maximum diameters), stable disease (<50% reduction in tumor size), and progressive disease (>25% increase in the tumor size or appearance of a new lesion). The recurrence of tumor was determined by MRI findings.
Statistical Analysis
Overall survival (OS) was calculated from the treatment start date (either chemotherapy or radiotherapy) to death date or censored at the last follow-up. Recurrence-free survival (RFS) was also calculated from the treatment start to recurrence date and censored at the last follow-up. Kaplan–Meier’s method was used to estimate survival probabilities, while, univariate and multivariate analyses were performed using Cox proportional hazard models. Mann–Whitney’s U test was conducted to compare dose parameters. Pearson’s Chi-square or Fisher`s exact tests were used to compare categorical characteristics. SAS v9.4 (SAS Institute Inc., Cary, NC, USA) and R v3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) was used as well for statistical analysis.
Results
Baseline Demographics and Disease Characteristics
Four hundred and eighteen patients were eligible and analyzed, with a median age of 14.4 years (range 3.8–39.1), of which 320 were males (76.6%). The most common primary location of tumors was suprasellar (34.0%), followed by the pineal (31.1%), bifocal (16.5%), basal ganglia (12.7%), and others (5.7%). Most tumors were localized (M0) at presentation (79.9%). Furthermore, serum βHCG levels were cut off at two levels; 10 mIU/mL and 50 mIU/mL for analysis. Most patients had serum βHCG values of <10 mIU/mL (77.3%) and <50 mIU/mL (87.6%) (range 0.0–712; interquartile range, 0.8–3.0) (Table 1).
Table 1.
Baseline Characteristics and Outcomes of Germinoma Patients (n = 418)
| Variables | Number | % | |
|---|---|---|---|
| Sex | Male | 320 | 76.6 |
| Female | 98 | 23.4 | |
| Age at diagnosis (years) | Median (min–max) | 14.4 (3.8–39.1) | |
| Location | Suprasellar | 142 | 34 |
| Pineal | 130 | 31.1 | |
| Bifocal | 69 | 16.5 | |
| Basal ganglia | 53 | 12.7 | |
| Other (thalamus, etc.) | 24 | 5.7 | |
| M stage | M0 | 334 | 79.9 |
| M+ | 84 | 20.1 | |
| Operation type | No surgery | 100 | 23.9 |
| Biopsy | 249 | 59.6 | |
| Tumor removal (+biopsy) | 69 | 16.5 | |
| Chemotherapy | Yes | 260 | 62.2 |
| No | 158 | 37.8 | |
| Second malignancy | No | 414 | 99 |
| Yes | 4 | 1 | |
| Serum βHCG (mIU/mL) | Median (min–max) | 1.2 (0.0–712.0) | |
| <10 | 336 | 87.6 | |
| ≥10 | 50 | 12.0 | |
| <50 | 366 | 87.6 | |
| ≥50 | 20 | 4.8 | |
| Missing | 32 | 7.7 | |
| Serum AFP (ng/mL) | Median (min–max) | 1.5 (0.0–1090.0) | |
| Missing | 28 | 6.7 | |
| F/U time (years) | Median (min–max) | 8.9 (0.5–25.1) | |
| Recurrence | No | 368 | 88 |
| Yes | 50 | 12 | |
| Survival | Alive | 400 | 95.7 |
| Death | 18 | 4.3 |
Abbreviations: M, metastasis; HCG, human chorionic gonadotropin; AFP, alpha-fetoprotein; F/u, follow-up.
Grouped according to age at diagnosis, male predominance was not observed under 10 years of age (male: female = 44: 34), whereas significant male predominance was observed above 10 years of age (male: female = 276: 64) (P < .001). Additionally, while suprasellar tumor was more common in patients younger than 10 years (36/78, 46.2% vs. 106/340, 31.2%), pineal and bifocal tumors were more common in patients older than 10 years old (18/78, 23.1% vs. 112/340, 32.9% for the pineal tumor, and 6/78, 7.7% vs. 63/340, 18.5% for bifocal tumor) (P = .014). Results also showed that more patients over 10 years had M+ disease, compared with patients under 10 years old (7.7% in patients under 10 years old vs. 22.9% in patients over 10 years old, P = .002). However, more patients had teratoma components in the younger patient group compared with the older patient group (4/74, 5.1% vs. 2/338, 0.6%, P = .013) (Supplementary Table S1).
Treatment Characteristics
Most patients had a biopsy (59.6%), 16.5% had a resection, while 23.9% had neither. Most patients had chemotherapy (56.9% pre-RT, 5.3% post-RT, 37.8% no chemotherapy). Various chemotherapy regimens were used, which differed by the institution. The most common chemotherapy regimen was platinum-based and a few received additional bleomycin. Specifically, 145 patients received an alternating regimen of carboplatin/etoposide and cyclophosphamide/etoposide, 21 patients received an alternating regimen of cisplatin/etoposide and cyclophosphamide/vincristine, 20 patients received an alternating regimen of carboplatin/etoposide and ifosfamide/etoposide, 20 other patients received a combination of carboplatin/etoposide, 20 patients received a combination of cisplatin/etoposide, 13 patients received a combination of cisplatin/etoposide/bleomycin, and 16 patients received other regimens. The detailed regimen was unknown in four patients.
In pre-RT chemotherapy patients, radiological treatment response to chemotherapy was documented in 238 patients, with 100 complete responses (42.0%) being reported. Furthermore, 11.3% had very good partial responses, 31.9% partial responses, 7.6% stable disease, whereas, 2.1% experienced progression.
Among the different countries, varied approaches were used (Supplementary Table S2). The usage of chemotherapy was most prevalent in Japan (100%), followed by Singapore (85.7% of M+ and 76.7% of M0), Korea (78.9% of M+, 61.7% of M0), and Taiwan (50% of M+, 10.8% of M0). Furthermore, in M+ patients, CSI was most prevalent in Korea (82.5%), followed by Singapore (71.4%), and Taiwan (40.0%). In M0 patients, WVI was most prevalent in Taiwan (83.8%), then Japan (63.6%), Singapore (53.3%), and Korea (35.2%).
In M0 patients, the median dose of radiation was 21.0 Gy (CSI) and 23.4 Gy (WVI), whereas, in M + patients, the median dose was 23.4 Gy (CSI) and 24.0 Gy (WVI), respectively. The median total tumor dose was 36.0 Gy.
In the combined chemotherapy and radiotherapy group (n = 260), 43 patients (16.5%) received focal RT, 99 patients (38.1%) received WVI, 114 patients (43.8%) received CSI, and 4 patients (1.5%) received WB radiotherapy. However, in the RT-only group (n = 158), the distribution of the radiation field was significantly different (P = .036), with 15 patients (9.5%) receiving focal RT, 81 (51.3%) patients receiving WVI, 59 patients (37.3%) receiving CSI, and 3 patients (1.9%) receiving WB RT.
Furthermore, among patients who received WVI, the median dose of radiation for patients who received combined chemotherapy and radiotherapy was 19.8 Gy. This value was significantly lower than that (24 Gy) of patients receiving radiotherapy only (P < .001). Also, among patients who received CSI, the median dose of radiation for the combined chemotherapy and radiotherapy group was 19.8 Gy, which was not significantly different from that (21 Gy) of the RT-only group. (p = .129). Additionally, tumor dose in the combined chemotherapy and radiotherapy group was higher than that in the RT-only group. (39.3 Gy vs. 35.5. Gy, P < .001). This phenomenon was due to patients with less than complete response post-chemotherapy receiving a higher dose of radiotherapy boost (Table 2).
Table 2.
Radiation Doses and Volumes According to Chemotherapy Responses in Patients who Received Chemotherapy
| Response | |||||||
|---|---|---|---|---|---|---|---|
| CR | VGPR | PR | SD | PD | P value | ||
| RT volume | Focal | 16(16.0) | 7(25.9) | 9(11.8) | 6(33.3) | 1(20) | 0.700 |
| Whole ventricle | 44(44.0) | 10(37.0) | 29(38.2) | 4(22.2) | 2(40) | ||
| Craniospinal | 39(39.0) | 10(37.0) | 37(48.7) | 8(44.4) | 2(40) | ||
| Whole brain | 1(1.0) | 0(0) | 1(1.3) | 0(0) | 0(0) | ||
| Tumor dose | Median (min–max) | 30.6 (19.8–54.0) | 39.6 (25.15–54.0) | 39.6 (3.2–59.15) | 39.6 (23.4–55.8) | 39.6 (90.6–45.0) | 0.002 |
| CSI dose | Median (min–max) | 23.4 (19.8–39.0) | 23.4 (19.8–30.0) | 20.25 (16.2–36.0) | 21.9 (19.5–36.0) | 19.65 (19.5–19.8) | 0.614 |
| WVI dose | Median (min–max) | 19.8 (16.2–39.3) | 24.0 (19.8–30.0) | 19.8 (19.8–45.0) | 21.9(19.8–24.0) | 19.8 (18.0–24.9) | 0.038 |
Abbreviations: RT, radiotherapy; CR, complete response; VGPR, very good partial response; PR, partial response; SD, stable disease; PD, progressive disease; CSI, craniospinal irradiation; WVI, whole ventriclar irradiation.
Outcomes
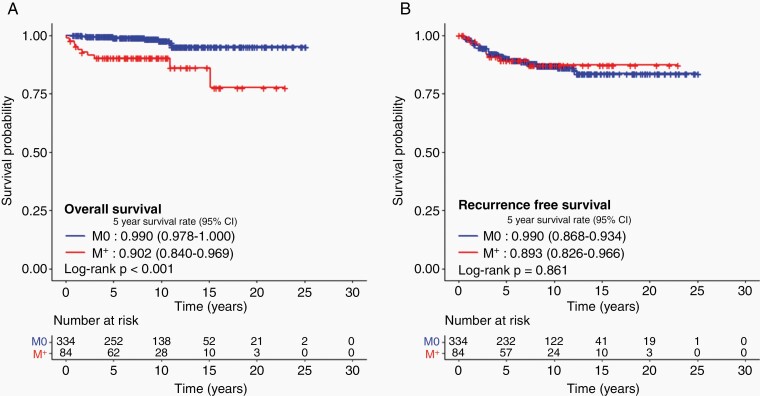
After a median follow-up of 8.9 years, 368 patients did not experience any recurrence, and 399 patients were alive. Five and 10-year OS were 97.2% (95% confidence interval [CI], 95.6%–98.9%) and 96.2% (95% CI, 94.2%–98.4%), with a corresponding RFS of 89.9% (95% CI, 87.0%–93.0%) and 86.9% (95% CI, 83.4%–90.5%). There were no differences in 5-year OS (97.1% vs. 97.8%) and RFS (89.5% vs. 91.2%) between patients with a histologically confirmed diagnosis (n = 318) and those with a clinical diagnosis (n = 100). No difference in the 5-year RFS by M stage was observed, however, the 5-year OS was lower for M+ patients (90.2% vs. 99.0%, P < .001) (Figure 1). Excessive deaths unrelated to tumor progression in M+ patients explained the reason why no differences in RFS were reported, but significant differences were observed in OS. Nine of 334 M0 patients had died, of which 5 were not related to tumor progression, whereas 7 out of 10 deaths were not related to tumor progression among the 84 M+ patients.
Fig. 1.
Survival outcomes of patients diagnosed with intracranial germinoma. A. Overall survival and B. recurrence-free survival according to metastasis.
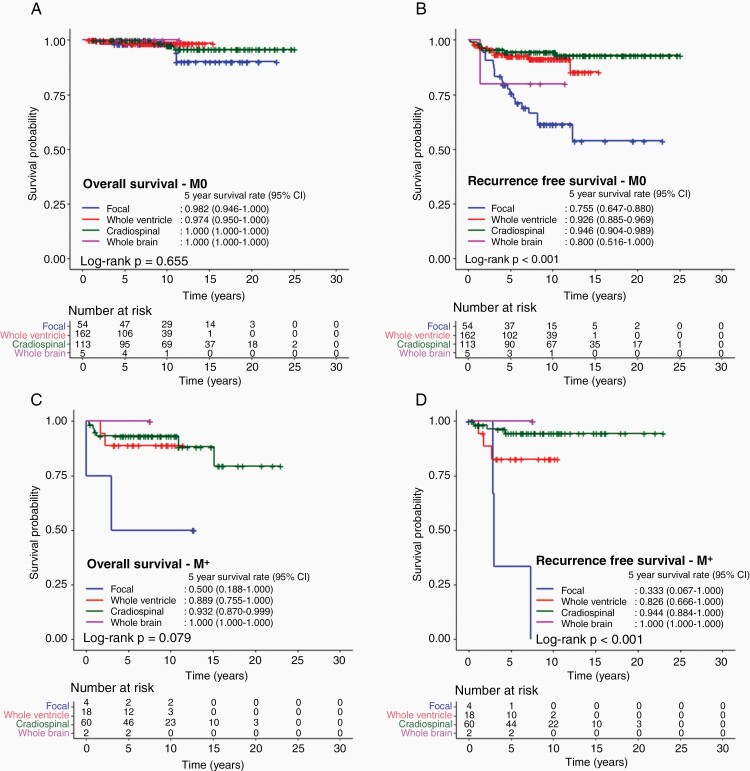
In M0 patients, 5-year RFS and OS did not differ with chemotherapy usage. On the other hand, OS did not differ by the RT field, but RFS was predicted by the RT field, with focal RT having the worst outcome (75.6%; 95% CI, 64.7%–88.0%), followed by WB RT (80.0%; 95% CI, 51.6%–100.0%) and WVI (92.6%; 95% CI, 88.5%–96.9%) and CSI (94.6%; 95% CI, 90.4%–98.9%) (P < .001) (Figure 2A and B).
Fig. 2.
Survival outcomes of patients diagnosed with intracranial germinoma according to radiation volume. A. Overall survival and B. recurrence-free survival of patients without metastasis. C. overall survival and D. recurrence-free survival of patients with metastasis according to radiation volume.
Similarly, in M+ patients, survival did not differ by chemotherapy usage but differed by RT volume. Four M+ patients received only focal RT, with a corresponding lowest RFS of 33.3% (95% CI, 6.7%–100.0%), followed by WVI (82.6%; 95% CI, 66.6%–100.0%), then CSI (94.4%; 95% CI, 88.4%–100.0%), and WB RT (100.0%) (P < .001) (Figure 2C and D).
When M stage and RT fields were considered in multivariable analysis, M+ (hazard ratio [HR] 7.5, P < .001) and CSI (HR 0.3, P= .043) were predictive of OS, while only RT fields were associated with RFS (Table 3).
Table 3.
Multivariable Analysis with RT Volume and M Stage
| Overall survival | Recurrence-free Survival | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | ||||||||||
| Variables | n | Event | HR (95% CI) | P value | HR (95% CI) | P value | N | Event | HR (95% CI) | P value | HR (95% CI) | P value | |
| M stage | M0 | 334 | 8 | 1 (ref) | 1 (ref) | 334 | 41 | 1 (ref) | 1 (ref) | ||||
| M+ | 84 | 10 | 5.673 (2.235–14.398) |
<0.001 | 7.486 (2.700-20.760) |
<0.001 | 84 | 9 | 0.938 (0.456-1.929) |
0.861 | 1.824 (0.830-4.007) |
0.135 | |
| RT volume | Focal | 58 | 5 | 1 (ref) | 0.550 | 1 (ref) | 0.211 | 58 | 23 | 1 (ref) | <0.001 | 1 (ref) | <0.001 |
| Whole ventricle | 180 | 4 | 0.373 (0.098–1.417) |
0.148 | 0.330 (0.086-1.271) |
0.107 | 180 | 16 | 0.237 (0.125-0.448) |
<0.001 | 0.227 (0.119-0.431) |
<0.001 | |
| Craniospinal | 173 | 9 | 0.620 (0.208–1.851) |
0.392 | 0.294 (0.090-0.964) |
0.043 | 173 | 10 | 0.127 (0.060-0.268) |
<0.001 | 0.104 (0.046-0.233) |
<0.001 | |
| Whole brain | 7 | 0 | - | 0.992 | - | 0.992 | 7 | 1 | 0.371 (0.050-2.751) |
0.332 | 0.302 (0.040-2.294) |
0.247 | |
Abbreviations: RT, radiotherapy; M, metastasis; CI, confidence interval; HR, hazard ratio.
Of the 229 patients who had data on their response to chemotherapy, patients who had complete response (n = 101), very good partial response (n = 27), partial response (n = 78), and stable disease (n = 18) following chemotherapy did not differ in the 5-year RFS (87.7%, 92.1%, 84.5%, and 77.7%, respectively). In contrast, patients with progressive disease (n = 5) after chemotherapy exhibited a trend of inferior RFS (40.0%), but without statistical significance (P = .099). The 5-year OS did not differ according to the response to chemotherapy.
When analyzing the relapse patterns, of 173 patients that had CSI, 12 recurred, of which all were intracranial with one simultaneous intracranial and spinal recurrence. Furthermore, of the other patients without CSI (n = 245), 40 recurred, of which 13 were spinal only and 3 were both intracranial and spinal recurrences.
Furthermore, of 52 recurrences, most patients were salvaged with a 5-year OS of 85.7%. Nine died subsequently; 7 from disease progression, 1 from treatment-related, and 1 unknown cause of death 18 years after relapse. Regarding treatment after relapse, detailed information was available for 34 of 52 relapsed patients. Among them, 21 patients received salvage chemotherapy and radiotherapy (including 8 patients who received high-dose chemotherapy and autologous stem cell transplantation (HDCT/ASCT)), 9 patients received chemotherapy only (including 5 patients who received HSCT/ASCT), and 4 patients received radiotherapy only. As a result, 2 of 25 patients who received radiotherapy as part of their salvage treatment died, whereas 4 of the 9 patients who received salvage chemotherapy without radiotherapy died. Four of five patients who survived the relapse without radiotherapy received HDCT/ASCT. Therefore, the 5-year OS was significantly better for those who received radiotherapy as a component of salvage treatment than those who did not (91.6% vs. 66.7%, P = .026).
Of 418 patients, 399 were alive at the time of this study. Of the 19 deaths, 7 were due to tumor progression after relapse, and 12 were not directly related to tumor progression (5 from treatment toxicities, 1 from electrolyte imbalance, 1 from secondary malignancy, 1 from intracerebral bleeding, 1 from accident, 1 from sudden cardiac arrest, and 2 from an unknown cause). No patient died of primary refractory disease. Four patients also developed secondary malignancies after treatment (4.7, 9.5, 10.9, and unknown years after). Three were intracranial malignancies (2 meningiomas, 1 glioma), and 1 developed diffuse large B-cell lymphoma at the thigh.
Risk Factors
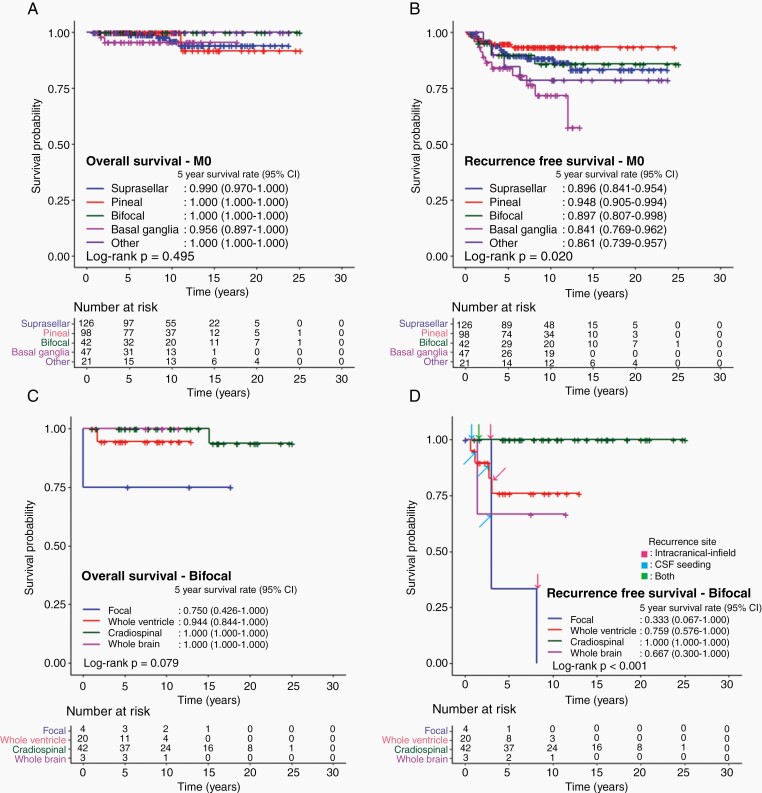
In M0 patients, OS did not differ by primary site, whereas, the 5-year RFS differed by primary site with basal ganglia primary having the worst outcome (84.1%; 95% CI 76.9%–96.2%) (P = .02) (Figure 3A and B).
Fig. 3.
Survival outcomes of patients diagnosed with intracranial germinoma according to primary tumor sites. A. Overall survival and B. recurrence-free survival of patients without metastasis according to primary tumor sites. C. Overall survival and D. recurrence-free survival with an indication of recurrence sites in patients with bifocal tumors according to radiation volume.
Analyzing outcomes of the 53 patients diagnosed with a primary tumor at the basal ganglia, 11 patients relapsed and 7 of them relapsed out of radiation field. Notably, 5 of 12 patients in the focal RT group and 4 of 30 patients in the WVI group relapsed, whereas 2 of 9 patients in the CSI group and none of 2 in the WB group relapsed. Patients who received WB RT or CSI showed a better RFS than those who received focal RT or WVI (P = .006) (Supplementary Table S3).
Of the 69 patients with bifocal primaries, relapse patterns differed by RT types. Specifically, no CSI patient relapsed (n = 42), compared to a higher recurrence rate in reduced field RT (3 of 4 in focal RT, 4 of 20 in WVI, none of 42 in CSI, and 1 of 3 in WB RT) (P < .001) (Figure 3C and D). Furthermore, recurrence sites included 2 intracranial infields and 1 cerebrospinal fluid (CSF) seeding in the focal RT group, 1 intracranial infield, and 3 CSF seeding in the WVI group, and 1 comprising simultaneous intracranial and CSF seedings in the WB RT group (Supplementary Table S4).
βHCG cut-off levels of 10 mIU/mL were associated with both OS and RFS (5-year OS, 97.8% vs. 93.9%, P = .028; 5-year RFS, 91.0% vs. 84.5%, P = .006). In contrast, βHCG cut-off levels of 50 mIU/mL were not associated with RFS but OS (5-year OS 97.7 vs. 90.0%, P = .009; 5-year RFS, 90.3% vs. 88.5%, P = .491) (Supplementary Figure S1).
Other risk factors like age (<10 years) and tumor size (>4 cm) were explored. Larger tumors did not have worse outcomes. Compared by age groups, no significant differences in the 5-year OS (5-year 98.6% vs. 96.9% for younger and older than 10 years, P = .786) and RFS (89.0% vs. 90.1% for younger and older than 10 years, P = .100) was observed according to age at diagnosis (Supplementary Figure S2). Notably, among relapsed patients, younger patients tended to relapse more than 5 years after diagnosis, with a marginal statistical significance (P = .052). That is, 6 out of the 14 relapsed patients (42.9%) younger than 10 years had late relapses, whereas, 6 out of 36 patients (16.7%) in the older patient group had a late relapse.
Discussion
The overall survival of germinomas was high in our study, with a 5- and 10-year OS of 97.2% and 96.2%, consistent with other international reports. A spectrum of practice among the different institutions was also observed with regard to RT treatment fields, use of chemotherapy, biopsy requirements, and βHCG cut-offs.
In our study, chemotherapy did not affect survival, and its usage differed among the different countries, with the Taiwanese using chemotherapy the least. In addition, a proportion of the patients were successfully treated with a reduced-volume RT without chemotherapy. However, it was difficult to measure the actual impact of additional chemotherapy because this study was a retrospective study, and the radiation field and doses were varied between the two groups. Previously, Lee et al., reported that 18 Gy of WVI was followed by 12.6 Gy of boost RT to the primary tumor after four cycles of induction chemotherapy. The study showed an excellent 5-year PFS of 96.7% and OS of 96.2% outcome in patients with localized germinoma.20 A SIOP CNS GCT II trial was conducted to omit WB and spinal irradiation using combined treatments with standard chemotherapy and ventricular irradiation. Preliminary reports of the trial suggested that patients with localized germinoma and complete responses after initial chemotherapy had an excellent outcome, with results supporting a reduced dose of WVRT of 24 Gy without boost.16 In our study, the median dose of extended RT was 19.8 Gy when combined with chemotherapy. These results suggested that future trials of combining chemotherapy and radiation should adopt a similar dose to 19.8 Gy of WVI for germinoma. In addition, the potential harm of chemotherapy and radiotherapy and the expected benefit from omitting chemotherapy or reducing radiotherapy doses should be carefully weighed based on the adverse effects of both modalities. To prove the definite benefit of neoadjuvant chemotherapy, long-term data, especially from well-executed randomized controlled trials, are needed to show an increment in quality of life, considering cognition, endocrinopathies, secondary malignancies, etc.
Also, in this study, tumor dose in the combined chemotherapy and radiotherapy group was higher than that in the RT-only group, as patients with less than a complete response following chemotherapy received a higher dose of radiotherapy boost. However, our results showed that patients with partial response or stable disease after chemotherapy did not show different RFS from patients with complete responses, and only patients with progressive disease had poor RFS than the rest of the group. Therefore, based on the finding that chemotherapy was not associated with better outcomes and the response after chemotherapy did not influence the outcome, we do not recommend RT dose escalation based on interim chemotherapy responses.
Regarding radiotherapy fields in localized diseases, RT with WVI and CSI resulted in RFS in excess of 90%, with focal RT showing the worst outcome. Furthermore, in M+ diseases, CSI was associated with the best control compared to WVI and focal RT. As a whole, spinal metastasis developed in only 1 of 173 CSI patients, compared to 16 of 245 patients who received less than CSI.
Various salvage regimens were used following a relapse, and most patients were salvaged successfully. This result was shared by other reports on salvage outcomes.21 Our data suggested that radiotherapy should be a component of salvage therapy whenever reirradiation was possible. HDCT/ASCT may be considered for patients who are ineligible for reirradiation. However, the benefits of HDCT/ASCT for relapsed patients who were eligible for re-irradiation were unclear. Thus, its use in recurrent intracranial germinoma should be carefully determined given its toxicity and the overall favorable outcomes of recurrent intracranial germinoma.
To investigate the findings of prior studies showing that some primary sites were associated with worse outcomes, we analyzed outcomes and patterns of failure by tumor location. In M0 patients, the basal ganglia location was associated with the lowest RFS. Notably, patients who received wider field RT showed a better RFS, consistent with previous reports.5,22 This result suggests that at least WB RT should be considered for patients with tumors in the basal ganglia. In bifocal patients, relapse rates were lowest in patients who received CSI. Controversy exists over the classification of bifocal diseases, with most international groups considering it as M0, although some found that CSI was associated with better outcomes.23,24 It thus remains unclear whether the bifocal presentation truly represents a metastatic stage and hence requires CSI, or whether the worse outcome is due to an under-investigation, as up to 50% of bifocal germinomas could be metastatic, and might sometimes be missed without careful imaging or cytology investigation.25
Internationally, βHCG thresholds vary, e.g., <50 mIU/mL in SIOP and <100 mIU/mL in the Children’s Oncology Group. Also, in some patients with presumed germinomas, a high βHCG also served as hints for elements of choriocarcinoma or immature teratoma, thereby necessitating surgical samples to avoid under or over-treatment. In our study, we found that βHCG cut-off levels of 50 mIU/mL did not influence RFS, which is consistent with the findings of other reports.26,27 However, lowering the cut-off levels to 10 mIU/mL, patients with βHCG >/=10 mIU/mL had inferior OS and RFS compared to patients with βHCG <10 mIU/mL. Therefore, the prognostic significance of a minimal elevation in βHCG should be further investigated. In our study as well, we did not find large tumors with worse outcomes, unlike other studies.19
The metastatic stage did not affect RFS, but it affected OS. To explain this contradictory finding, we studied the causes of deaths, which were explained by the excess number of deaths from non-cancer-related causes in M+ patients. The results suggested that the metastatic stage did not add additional risks to tumor-related deaths. In M+ patients, survival outcomes did not also differ according to chemotherapy use, but differed according to RT volumes, with the best being results in the CSI group.
Furthermore, although we found that younger patients had a trend toward worse RFS, other studies have been unable to produce the same finding.13 Younger patients showed unique clinical features in our study, such as no male predominance, less metastasis, and a higher rate of teratoma component. Additionally, younger patients were prone to late relapse. These results suggested the possibility that younger patients had unique genomic features or tumor ontogeny.
Four patients developed secondary malignancies, of which three were intracranial. No case of chemotherapy-related myeloid neoplasm in this study was observed. Several studies have shown that the incidence of secondary malignancies was significant.28,29 A large, single-center study with a longer follow-up showed that of 189 patients diagnosed with intracranial germinoma, secondary malignancies developed in ten patients (5.3 %), including 5 patients with glioblastoma with a latency period of 20 years (range, 4–26 years), which caused mortality in 6 of the 10 patients.7 This result emphasizes that the risk of the second malignant neoplasm in the radiation field cannot be overlooked. However, a recent study showed a lower incidence of secondary malignancies due to recent efforts to reduce RT doses.9 Comparing with previous findings, the incidence of secondary malignancies in this study was lower, which may reflect a recent trend to lower RT doses. In addition, the incidence may have been underestimated due to the multicenter, retrospective nature of this study.
As the prognosis of germinoma is excellent, a greater emphasis should be placed on survivorship. In trying to reduce radiotherapy fields, adverse effects of chemotherapy should be considered. Long-term toxicities of RT are also proposed to be mitigated with proton therapy. Thus, dose calculation studies of WVI and CSI have shown increased normal organ sparing with intensity-modulated proton therapy.30,31 Early clinical reports of reduced toxicity with proton therapy for CSI have also been published.32–34 Therefore, in considering future directions of clinical trials, it should be questioned whether there are clinical situations where chemotherapy still plays an essential role in CNS germinoma, or to what extent the radiation dose and volume can be reduced without chemotherapy.
Several limitations were encountered in our study. First, due to its retrospective nature, data collection was not comprehensive. Hence, our study did not provide information on acute toxicities and long-term quality of life. Also, a lot of heterogeneity exists in this study, not just in management, but also in the ethnicity of patients, especially in mixed-race societies like Singapore.35 There are also variations in diagnosis, staging, and follow-up. Non-uniformity of CSF sampling can be seen, as some patients had lumbar, and some ventricular CSF sampling, while some had not done. For this reason, the number of M1 patients might have been underestimated. Furthermore, some patients were not also confirmed with histology. We did not perform a central review of neuroimages. However, the MRIs were interpreted by radiologists subspecializing in neuroradiology or pediatric radiology in tertiary institutions. As relapse and death events were small, the findings from the multivariable analysis should therefore be interpreted with caution. Nevertheless, this study is also not without its strengths. It is the first Asian multinational study, and patients were managed at tertiary institutes with dedicated pediatric neuro-oncology services. Although different institutions had previously reported their outcomes separately, this joint effort resulted in more rigor with bigger numbers.28,36,37 The follow-up duration was also sufficiently long to capture most subsequent relapses.
In conclusion, survival outcomes of patients with germinoma were excellent, with varying outcomes depending on primary tumor locations, metastasis, and radiation volume. Thus, the focus of treating intracranial germinoma should be on survivorship. Additionally, no differences in disease control with or without chemotherapy were observed, which raises questions about additional benefits and the exact role of chemotherapy in intracranial germinoma. However, further prospective studies with a larger number of patients are warranted to confirm these results and find the exact role and the optimal intensity of each treatment modality, considering age, primary tumor location, and extent of disease.
Supplementary Material
Contributor Information
Kyung-Nam Koh, Division of Pediatric Hematology/Oncology, Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Korea.
Ru Xin Wong, Department of Radiation Oncology, National Cancer Centre, Singapore, Singapore.
Dong-Eun Lee, Division of Cancer Epidemiology and Management, Research Institute, National Cancer Center, Goyang, Korea.
Jung Woo Han, Division of Pediatric Hematology and Oncology, Department of Pediatrics, Severance Hospital, Yonsei University College of Medicine, Yonsei University Health System, Seoul, Korea.
Hwa Kyung Byun, Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Yonsei University Health System, Seoul, Korea.
Hong In Yoon, Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Yonsei University Health System, Seoul, Korea.
Dong-Seok Kim, Department of Neurosurgery, Yonsei University College of Medicine, Yonsei University Health System, Seoul, Korea.
Chuhl Joo Lyu, Division of Pediatric Hematology and Oncology, Department of Pediatrics, Severance Hospital, Yonsei University College of Medicine, Yonsei University Health System, Seoul, Korea.
Hyoung Jin Kang, Departments of Pediatrics, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Korea.
Kyung Taek Hong, Departments of Pediatrics, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Korea.
Joo Ho Lee, Department of Radiation Oncology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
Il Han Kim, Department of Radiation Oncology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
Ji Hoon Phi, Division of Pediatric Neurosurgery, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Korea.
Seung-Ki Kim, Division of Pediatric Neurosurgery, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Korea.
Tai-Tong Wong, Department of Neurosurgery, Taipei Medical University Hospital, Taipei Medical University, Taipei, Taiwan.
Hsin-Lun Lee, Department of Radiation Oncology, Taipei Medical University Hospital, Taipei Medical University, Taipei, Taiwan.
I-Chun Lai, Department of Neurosurgery, Taipei Medical University Hospital, Taipei Medical University, Taipei, Taiwan.
Yu-Mei Kang, Department of Neurosurgery, Taipei Medical University Hospital, Taipei Medical University, Taipei, Taiwan.
Young-Shin Ra, Division of Pediatric Neurosurgery, Department of Neurosurgery, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Korea.
Seung Do Ahn, Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Ho Joon Im, Division of Pediatric Hematology/Oncology, Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Korea.
Wen Shen Looi, Department of Radiation Oncology, National Cancer Centre, Singapore, Singapore.
Sharon Yin Yee Low, Department of Neurosurgery, National Neuroscience Institute, Singapore, Singapore; Neurosurgical Service, KK Women’s and Children’s Hospital, Singapore, Singapore.
Enrica Ee Kar Tan, Department of Pediatric Subspecialties, Pediatric Hematology/Oncology Service, KK Women’s and Children’s Hospital, Singapore, Singapore.
Hyun Jin Park, Center for Pediatric Oncology, National Cancer Center, Goyang, Korea.
Sang Hoon Shin, Neuro-Oncology Clinic, National Cancer Center, Goyang, Korea.
Hiroshi Fuji, Department of Radiation Oncology, National Center for Child Health and Development, Tokyo, Japan.
Chang-Ok Suh, Department of Radiation Oncology, CHA Bundang Medical Center, CHA University College of Medicine, Seongnam, Korea.
Yi-Wei Chen, Division of Radiation Oncology, Department of Oncology, Taipei Veterans General Hospital, Taipei, Taiwan.
Joo-Young Kim, Department of Radiation Oncology, Research Institute and Hospital, National Cancer Center, Goyang, Korea.
Funding
This study was supported by the National Cancer Center, Korea (Grant No. 2110352-1).
Conflicts of interest statement. The authors declare that they have no conflicts of interest.
Authorship statement. Conceptualization and design: J.Y.K. and Y.W.C. Acquisition of data: K.N.K., R.X.W., J.W.H., H.K.B., H.I.Y., D.S.K., C.J.L., H.J.K., K.T.H., J.H.L., I.H.K., J.H.P., S.K.K., T.T.W., H.L.L, I.C.L., Y.M.K., Y.S.R., S.D.A., H.J.I., W.S.L., S.Y.Y.L., E.E.K.T., H.J.P., S.H.S., H.F., C.O.S., Y.W.C., and J.Y.K. Analysis of data: K.N.K., R.X.W., and D.E.L. Manuscript writing: K.N.K, R.X.W., D.E.L., and J.Y.K. Final editing and approval of the manuscript: K.N.K., R.X.W, D.E.L., J.W.H, H.K.B, H.I.Y, D.S.K, C.J.L, H.J.K, K.T.H., J.H.L, I.H.K., J.H.P., S.K.K, T.T.W, H.L.L, I.C.L, Y.M.K., Y.S.R., S.D.A., H.J.I, W.S.L., S.Y.Y.L, E.E.K.T., H.J.P., S.H.S., H.F., C.O.S., Y.W.C., and J.Y.K.
References
- 1. Echevarria ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: a review. Oncologist. 2008;13(6):690–699. [DOI] [PubMed] [Google Scholar]
- 2. Takami H, Perry A, Graffeo CS, et al. . Comparison on epidemiology, tumor location, histology, and prognosis of intracranial germ cell tumors between Mayo Clinic and Japanese consortium cohorts. J Neurosurg. 2020;134(2):446–456. [DOI] [PubMed] [Google Scholar]
- 3. Han J, Koh KN, Kim JE, et al. . Current Trends in Management for Central Nervous System Germ Cell Tumor. Clin Pediatr Hematol Oncol. 2016;23(1):17–27. [Google Scholar]
- 4. Kim JY, Park J. Understanding the treatment strategies of intracranial germ cell tumors: focusing on radiotherapy. J Korean Neurosurg Soc. 2015;57(5):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee DS, Lim DH, Kim IH, et al. . Upfront chemotherapy followed by response adaptive radiotherapy for intracranial germinoma: Prospective multicenter cohort study. Radiother Oncol. 2019;138:180–186. [DOI] [PubMed] [Google Scholar]
- 6. Cho J, Choi JU, Kim DS, Suh CO. Low-dose craniospinal irradiation as a definitive treatment for intracranial germinoma. Radiother Oncol. 2009;91(1):75–79. [DOI] [PubMed] [Google Scholar]
- 7. Lee JH, Eom KY, Phi JH, et al. . Long-term outcomes and sequelae analysis of intracranial germinoma: need to reduce the extended-field radiotherapy volume and dose to minimize late sequelae. Cancer Res Treat. 2021;53(4):983–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen YW, Huang PI, Ho DM, et al. . Change in treatment strategy for intracranial germinoma: long-term follow-up experience at a single institute. Cancer. 2012;118(10):2752–2762. [DOI] [PubMed] [Google Scholar]
- 9. Byun HK, Yoon HI, Cho J, et al. . Optimization of intracranial germinoma treatment: radiotherapy alone with reduced volume and dose. Int J Radiat Oncol Biol Phys. 2020;108(3):657–666. [DOI] [PubMed] [Google Scholar]
- 10. Haas-Kogan DA, Missett BT, Wara WM, et al. . Radiation therapy for intracranial germ cell tumors. Int J Radiat Oncol Biol Phys. 2003;56(2):511–518. [DOI] [PubMed] [Google Scholar]
- 11. Alapetite C, Brisse H, Patte C, et al. . Pattern of relapse and outcome of non-metastatic germinoma patients treated with chemotherapy and limited field radiation: the SFOP experience. Neuro Oncol. 2010;12(12):1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jensen AW, Laack NN, Buckner JC, et al. . Long-term follow-up of dose-adapted and reduced-field radiotherapy with or without chemotherapy for central nervous system germinoma. Int J Radiat Oncol Biol Phys. 2010;77(5):1449–1456. [DOI] [PubMed] [Google Scholar]
- 13. Calaminus G, Kortmann R, Worch J, et al. . SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 2013;15(6):788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joo JH, Park JH, Ra YS, et al. . Treatment outcome of radiation therapy for intracranial germinoma: adaptive radiation field in relation to response to chemotherapy. Anticancer Res. 2014;34(10):5715–5721. [PubMed] [Google Scholar]
- 15. Murray MJ, Bartels U, Nishikawa R, et al. . Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. 2015;16(9):e470–e477. [DOI] [PubMed] [Google Scholar]
- 16. Calaminus G, Bison B, Faure-Conter C, et al. . 24Gy whole ventricular radiotherapy alone is sufficient for disease control in localised germinoma in CR after initial chemotherapy-early results of the SIOP CNS GCT II. Neuro Oncol. 2020;22:iii343–iii344. [Google Scholar]
- 17. Wong TT, Chen YW, Guo WY, et al. . Germinoma involving the basal ganglia in children. Childs Nerv Syst. 2008;24(1):71–78. [DOI] [PubMed] [Google Scholar]
- 18. Shibamoto Y, Sasai K, Oya N, Hiraoka M. Intracranial germinoma: radiation therapy with tumor volume-based dose selection. Radiology. 2001;218(2):452–456. [DOI] [PubMed] [Google Scholar]
- 19. Ogawa K, Yoshii Y, Shikama N, et al. . Spinal recurrence from intracranial germinoma: risk factors and treatment outcome for spinal recurrence. Int J Radiat Oncol Biol Phys. 2008;72(5):1347–1354. [DOI] [PubMed] [Google Scholar]
- 20. Lee JW, Lim DH, Sung KW, et al. . Induction chemotherapy reduces radiation therapy dose and volume in the treatment of intracranial germinoma: results of the SMC-G13 trial. Int J Radiat Oncol Biol Phys. 2020;108(3):649–656. [DOI] [PubMed] [Google Scholar]
- 21. Callec L, Lardy-Cleaud A, Guerrini-Rousseau L, et al. . Relapsing intracranial germ cell tumours warrant retreatment. Eur J Cancer. 2020;136:186–194. [DOI] [PubMed] [Google Scholar]
- 22. Li B, Feng J, Chen L, et al. . Relapse pattern and quality of life in patients with localized basal ganglia germinoma receiving focal radiotherapy, whole-brain radiotherapy, or craniospinal irradiation. Radiother Oncol. 2021;158:90–96. [DOI] [PubMed] [Google Scholar]
- 23. Esfahani DR, Alden T, DiPatri A, et al. . Pediatric suprasellar germ cell tumors: a clinical and radiographic review of solitary vs. bifocal tumors and its therapeutic implications. Cancers (Basel). 2020;12(9): 2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chung SY, Han JW, Kim DS, Yoon HI, Suh CO. Treatment outcomes based on radiation therapy fields for bifocal germinoma: synchronous or disseminated disease? PLoS One. 2019;14(10):e0223481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phi JH, Kim SK, Lee J, et al. . The enigma of bifocal germ cell tumors in the suprasellar and pineal regions: synchronous lesions or metastasis? J Neurosurg Pediatr. 2013;11(2):107–114. [DOI] [PubMed] [Google Scholar]
- 26. Shirato H, Aoyama H, Ikeda J, et al. . Impact of margin for target volume in low-dose involved field radiotherapy after induction chemotherapy for intracranial germinoma. Int J Radiat Oncol Biol Phys. 2004;60(1):214–217. [DOI] [PubMed] [Google Scholar]
- 27. Ogino H, Shibamoto Y, Takanaka T, et al. . CNS germinoma with elevated serum human chorionic gonadotropin level: clinical characteristics and treatment outcome. Int J Radiat Oncol Biol Phys. 2005;62(3):803–808. [DOI] [PubMed] [Google Scholar]
- 28. Hong KT, Lee DH, Kim BK, et al. . Treatment outcome and long-term follow-up of central nervous system germ cell tumor using upfront chemotherapy with subsequent photon or proton radiation therapy: a single tertiary center experience of 127 patients. BMC Cancer. 2020;20(1):979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Acharya S, DeWees T, Shinohara ET, Perkins SM. Long-term outcomes and late effects for childhood and young adulthood intracranial germinomas. Neuro Oncol. 2015;17(5):741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Correia D, Terribilini D, Zepter S, et al. . Whole-ventricular irradiation for intracranial germ cell tumors: dosimetric comparison of pencil beam scanned protons, intensity-modulated radiotherapy and volumetric-modulated arc therapy. Clin Transl Radiat Oncol. 2019;15:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park J, Park Y, Lee SU, et al. . Differential dosimetric benefit of proton beam therapy over intensity modulated radiotherapy for a variety of targets in patients with intracranial germ cell tumors. Radiat Oncol. 2015;10:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hashimoto T, Shimizu S, Takao S, et al. . Clinical experience of craniospinal intensity-modulated spot-scanning proton therapy using large fields for central nervous system medulloblastomas and germ cell tumors in children, adolescents, and young adults. J Radiat Res. 2019;60(4):527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mahajan A. Proton craniospinal radiation therapy: rationale and clinical evidence. Int J Part Ther. 2014;1(2):399–407. [Google Scholar]
- 34. Song S, Park HJ, Yoon JH, et al. . Proton beam therapy reduces the incidence of acute haematological and gastrointestinal toxicities associated with craniospinal irradiation in pediatric brain tumors. Acta Oncol. 2014;53(9):1158–1164. [DOI] [PubMed] [Google Scholar]
- 35. Low SYY, Cheng H, Zou R, et al. . Molecular exploration of paediatric intracranial germinomas from multi-ethnic Singapore. BMC Neurol. 2020;20(1):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Foo AS, Lim C, Chong DQ, Tan DY, Tham CK. Primary intracranial germ cell tumours: experience of a single South-East Asian institution. J Clin Neurosci. 2014;21(10):1761–1766. [DOI] [PubMed] [Google Scholar]
- 37. Wong LC, Yang TL, Gao F, et al. . Intracranial germ cell tumour: experience of a Singaporean institution over 11-year period. Singapore Med J. 2002;43(4):182–188. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.