Abstract
Background
Diffuse Midline Glioma (DMG) with the H3K27M mutation is a lethal childhood brain cancer, with patients rarely surviving 2 years from diagnosis.
Methods
We conducted a multi-site Phase 1 trial of the imipridone ONC201 for children with H3K27M-mutant glioma (NCT03416530). Patients enrolled on Arm D of the trial (n = 24) underwent serial lumbar puncture for cell-free tumor DNA (cf-tDNA) analysis and patients on all arms at the University of Michigan underwent serial plasma collection. We performed digital droplet polymerase chain reaction (ddPCR) analysis of cf-tDNA samples and compared variant allele fraction (VAF) to radiographic change (maximal 2D tumor area on MRI).
Results
Change in H3.3K27M VAF over time (“VAF delta”) correlated with prolonged PFS in both CSF and plasma samples. Nonrecurrent patients that had a decrease in CSF VAF displayed a longer progression free survival (P = .0042). Decrease in plasma VAF displayed a similar trend (P = .085). VAF “spikes” (increase of at least 25%) preceded tumor progression in 8/16 cases (50%) in plasma and 5/11 cases (45.4%) in CSF. In individual cases, early reduction in H3K27M VAF predicted long-term clinical response (>1 year) to ONC201, and did not increase in cases of later-defined pseudo-progression.
Conclusion
Our work demonstrates the feasibility and potential utility of serial cf-tDNA in both plasma and CSF of DMG patients to supplement radiographic monitoring. Patterns of change in H3K27M VAF over time demonstrate clinical utility in terms of predicting progression and sustained response and possible differentiation of pseudo-progression and pseudo-response.
Keywords: circulating tumor DNA, diffuse midline glioma, H3K27M, liquid biopsy, ONC201
Key Points.
Nonrecurrent patients with decreased CSF H3K27M cf-TDNA VAF had prolonged PFS
“Spikes” in cf-tDNA VAF preceded progression in a majority of cases
Serial VAF sampling aids in differentiating pseudo-progression and bevacizumab effect
Importance of the Study.
To our knowledge, we report the first demonstrated feasibility for serial CSF collection in a prospective high-grade glioma clinical trial. Nonrecurrent patients (those enrolled after initial radiation) with a decrease in CSF H3.3K27M variant allele fraction (VAF) displayed a doubling of time to progression. “Spikes” in cf-tDNA VAF (increase of at least 25%) preceded progression in many cases. In individual cases, patterns of change in VAF over time demonstrate additional clinical utility in terms of differentiating pseudo-progression and bevacizumab (“pseudo-response”) effect. These findings represent a new direction for understanding the feasibility and utility of H3K27M cf-tDNA as a clinically relevant prognostic marker.
Diffuse Midline Gliomas (DMGs), including diffuse intrinsic pontine glioma (DIPG), are a subset of brain tumors with associated survival of less than 10% beyond 2 years of diagnosis.1,2 As many as 80% of DIPGs harbor mutations in histone H3, which leads to a lysine-to-methionine substitution (H3K27M) in H3.3A (H3F3A) and H3C2 (HIST1H3B).3,4 Given their location in midline structures, most commonly the brainstem, thalamus or spinal cord, serial tumor biopsy as a means to monitor tumor burden (eg, response to anticancer agents) carries significant risk. Thus, tracking disease burden for patients with DMGs is often restricted to repeat MRI. Increases in tumor area on MRI can be attributed to either true tumor progression or radiation-related swelling or necrosis (pseudo-progression).5
In order to better monitor treatment response on a clinical and molecular level, liquid biopsy technologies have emerged over the last decade to assess plasma and cerebrospinal fluid (CSF) for extracted cell-free tumor DNA (cf-tDNA).6–11 In a recent study with adult glioblastoma (GBM) patients, patient-specific tumor variants were isolated from circulating tumor DNA (ctDNA) in CSF using next-generation sequencing (NGS) in approximately half (42/85) of patients.9 Detection of patient-specific variants was shown to correlate with tumor burden and adverse outcome.9 Serial plasma collection in a cohort of pediatric DMG patients revealed H3K27M ctDNA to correlate with tumor response to radiation in 10 of 12 patients using digital droplet PCR (ddPCR).10 Izquierdo and colleagues used ddPCR to measure ctDNA from point mutations, including H3K27M, derived from isolated plasma and CSF samples.11 The authors found higher detectable levels of ctDNA in samples of cf-tDNA derived from CSF in comparison to other modes of liquid biopsy and presented anecdotal examples of correlation between levels of ctDNA in the plasma and tumor outcomes.11 Finally, our group demonstrated the ability of electronic (Nanopore) sequencing to detect a panel of recurrently mutated genes in DMG and to show correlation with treatment response in individual cases.8
Despite these meaningful advances in the diagnosis and correlation with glioma tumor size on imaging, no studies have demonstrated the ability of cf-tDNA to provide information to predict treatment response or progression, or to address frequent issues with pseudo-progression and or bevacizumab-induced “pseudo-response”. To our knowledge, the feasibility and utility of prospective serial analysis of CSF for cf-tDNA monitoring in a glioma clinical trial has not been employed.
To address this, we designed an arm (Arm D) on the multi-site phase 1 trial with the imipridone ONC201 (NCT03416530) that employed serial CSF collection (baseline, 2 and 6 months) for patients with H3K27M-mutant DMG. ONC201 is a selective dopamine DRD2 antagonist, which results in dual inactivation of Akt and ERK and TRAIL-mediated apoptosis in cancer cells. ONC201 also directly binds and activates the mitochondrial protease ClpP, resulting in impaired respiratory function and mitochondrial toxicity in leukemia12 and breast cancer13 cells. ONC201 demonstrates preclinical activity, brain penetration and safety in the treatment of adult GBM.14–17 While the recently completed phase 2 trial of ONC201 in adult recurrent GBM did not improve PFS,17 multiple patients with H3K27M mutation demonstrated sustained response of multi-focal lesions.17–19 This response prompted phase I trials of ONC201 in H3K27M-mutant pediatric and adult DMG. As this represented a promising trial in a molecularly homogenous group, we employed a clinical trial design for correlate cell-free sample collection in the pediatric phase I.
In the present study, we describe the feasibility of serial CSF cf-tDNA monitoring as well as the clinical utility of longitudinal cf-tDNA H3K27M monitoring. Our findings indicate that patterns of change in serial CSF and plasma collection for cf-tDNA can predict progression, long-term response, and have potential for clarifying “pseudo-progression” and “pseudo-response” during treatment.
Materials and Methods
Patient Samples (CSF) and Tumor Measurements
Informed consent/assent was obtained and studies were conducted according to the multi-institutional phase 1 clinical trial of ONC201 (NCT03416530) in the United States. The University of Michigan, UCSF, Seattle Children’s Hospital, and Miami Cancer Institute participated in Arm D. The study protocol was IRB approved at all participating centers.
Patients on arm D were eligible if they were diagnosed with glioma that was positive for the H3 K27M mutation (positive testing in CLIA laboratory), had completed at least one line of prior therapy, and were willing to undergo serial lumbar puncture to obtain cerebrospinal fluid (CSF) at the time of MRI. Evidence of progression was not required so that ONC201 may be administered to patients in the maintenance setting or to patients with recurrent disease. No more than two prior episodes of recurrence from radiotherapy and/or chemotherapy were allowed. Use of bevacizumab solely for treatment of radiation necrosis, pseudo-progression, or treatment effect was not considered a recurrence. Local anesthesia for lumbar puncture was also allowed. Spinal taps were not to be performed if the treating clinician or proceduralist had concern for signs of elevated intracranial pressure, including recent worsening in headache or somnolence.
Two to 5 ml of CSF were collected for patients <5 years of age and 5–10 ml were collected for patients >5 years of age. CSF samples were centrifuged at 1500 rpm for 5 min to remove cellular contents and/or debris and supernatant was stored at −80 °C until ready for use, in 0.5 ml−1 aliquots were processed depending on initial volume of sample.
Patient Samples (Plasma)
For U of M patients on any arm of the trial (including non-Arm D patients with nonbiopsied DIPG), informed consent/assent was obtained on the IRB-Approved protocol Sample Repository for Pediatric Hematology/Oncology (HUM00123426). Blood samples (6–8 ml) were collected in vacutainer tubes at time of blood draws that were otherwise a part of routine clinical care. The sample was subjected to density gradient separation (Ficoll-Hypaque) via centrifugation at 1500 rpm × 5 min and plasma was then flash frozen at −80 °C until ready for use, in 1 ml aliquots. These aliquots (1 ml) were processed for cf-tDNA isolation.
Magnetic Resonance Imaging (MRI) and Tumor Measurements
Tumor measurements for patients on phase 1 ONC201 (NCT03416530) were provided by investigators using modified RANO Response Criteria. Bi-dimensionally, contrast-enhancing, measurable lesions with clearly defined margins by MRI scan, with a minimal diameter of 1 cm, and visible on 2 axial slices which are at least 5 mm apart with 0 mm skip. If noncontrast enhancing disease is measurable, bi-dimensional T2/FLAIR imaging was used for measurements. Cross-sectional area was determined using the axial image featuring maximal tumor diameter and calculated as the product of this maximal diameter measurement and the diameter perpendicular in the same plane. To determine correlation with tDNA VAF values, MRI images were selected from the imaging study closest to the time of sample collection (plasma or CSF).
DNA Isolation
DNA was isolated from fluid samples using the QIAamp Circulating Nucleic Acid kit (Qiagen, 55114) and from tissue using the DNeasy Blood & Tissue kit (Qiagen, 69506). All concentration measurements were performed using the Qubit 3.0 Fluorometer (Invitrogen, Q33216) and Qubit dsDNA HS Assay kit (Invitrogen, Q32851).
ddPCR Primer Design and Assay
Primers for the H3F3A K27M assay were designed using Primer3Plus (https://primer3plus.com/cgi-bin/dev/primer3plus.cgi) and probes were designed by a Scientific Applications Specialist at Integrated DNA Technologies (IDT). Locked nucleic acid (LNA) bases were incorporated into the probe design to increase melting temperature without lengthening probe sequence. Both primers and probes were synthesized by IDT.
Forward primer: 5′-GGTAAAGCACCCAGGAAG-3′
Reverse primer: 5′-CAAGAGAGACTTTGTCCC-3′
WT probe with LNA: 5′-HEX-TC+GC+A+A+GA+GT+GC-IABkFQ-3′, “+” denotes LNA bases, HEX: hexachlorofluorescein, IABkFQ: Iowa Black® FQ quencher
K27M probe with LNA: 5′-6-FAM-TC+GC+A+T+GA+GTGC-IABkFQ-3′, mutant base is bold, “+” denotes LNA bases, 6-FAM: 6-carboxyfluorescein, IABkFQ: Iowa Black® FQ quencher
Reaction mixtures were prepared using ddPCR Supermix for Probes (no dUTP) (Bio-Rad, 1863024). The following PCR protocol was used: 1 cycle at 95 °C for 10 min, 40 cycles at 94 °C for 30 s and 58 °C for 1 min, 1 cycle at 98 °C for 10 min, and 1 cycle at 12 °C infinite, all at a ramp rate of 2 °C/s.
DNA Preamplification
An initial preamplification reaction was run prior to ddPCR using the same primers described above and the Q5 High-Fidelity 2X Master Mix (New England BioLabs, M0492S). The following PCR protocol was used: 1 cycle at 98 °C for 30 s, 12 cycles at 98 °C for 10 s, 58 °C for 30 s, and 72 °C for 30 s, 1 cycle at 72 °C for 2 min, and 1 cycle at 12 °C infinite.
ddPCR Analysis
The Bio-Rad QX200 AutoDG system was used for all ddPCR work. QuantaSoft Analysis Pro (Bio-Rad) was used for initial analysis of results. We applied recommended threshold for “positive samples (per Bio-Rad QX200-Auto DG User Manuel). Briefly, Poisson statistics demonstrates that 3 copies for every measurement are needed in order to detect 1:10 000 mutant events at a 95% confidence level in 10 000 positive droplets, which is the average number of droplets per analysis. We used the threshold of 3 positive mutant droplets (including “double positive” droplets for mutant and wildtype H3.3A). Two or fewer positive droplets were considered negative.
Statistical Analyses
Graphs were plotted and statistical analyses were performed using GraphPad Prism software (version 7.00/8.00, GraphPad, La Jolla, CA) and Microsoft Excel. Unpaired, two-sided t-tests (Mann–Whitney) were performed. Correlation analyses were performed using the Pearson r value. Survival analyses in nonrecurrent patients were performed using Kaplan–Meier analyses with the Log-Rank test, with at least 2 plasma or CSF samples. Data were considered significant if P values were below 0.05 (95% confidence intervals). We set a 25% increase over the baseline level for each patient/sample type to call a spike in cf-tDNA levels based on typical tumor monitoring value for progressive disease of 25%, as well as this being over the average standard deviation for both plasma and CSF triplicate values.
Results
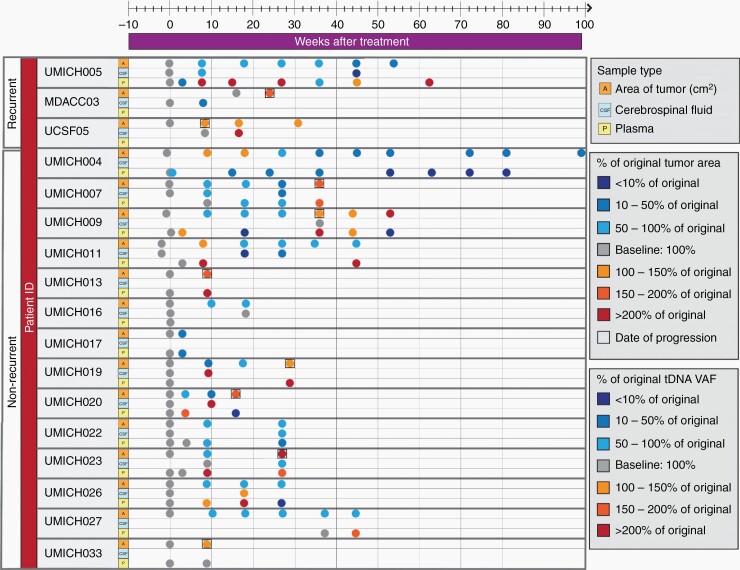
Patients enrolled on Arm D of the ONC201 trial (NCT03416530, n = 24) underwent serial lumbar puncture (LP) for cell-free tumor DNA (cf-tDNA) analysis and patients on all arms at the University of Michigan, including 4 non-Arm D patients, underwent serial plasma collection. Patient disease status and further information are outlined in Table S1. Results in graphical form are in Figure 1 and in numerical form in Table S2, including raw values and percentage change from baseline. Demographic data for all patients (n = 28) is displayed in Table S3. We extracted cf-tDNA from 62 plasma samples (186 replicates) and 29 CSF samples (87 replicates) from 17 patients with sequencing-confirmed H3F3A (H3.3) K27M mutation and samples collected at multiple time points [we excluded patients with HIST1H3B (H3.1) K27M mutation or those with CSF sampling from only 0 or 1 time points due to early progression or logistical/safety constraints (Fig. S1). Collection of CSF proved feasible and safe, with no complications related to LP reported. At a total of 6 (out of 35, 17%) LPs, the patient exhibited concern for increased intracranial pressure (worsening headache or neurologic symptom/neurologic exam in the week prior to procedure) and was waived from LP.
Fig. 1.
Landscape of patient samples and H3K27M variant allele fraction (VAF) by time point.
We performed ddPCR analysis for H3.3 K27M of cf-tDNA extracted according to previous methods Fig. S110. We performed technical replicates (n = 3) for each sample and reported the average value (average standard deviation was 0.032% for plasma and 0.327% for CSF). Six data points were found below our standard deviation threshold (<0.03%), and were included, but changes underneath this level were not considered significant. We were able to detect H3K27M cf-tDNA in 53/62 plasma samples (sensitivity of 85.4%) and 28/29 CSF samples (sensitivity of 96.5%; Figure 1). We detected no H3K27M-positive droplets in nontemplate control wells (n = 70).
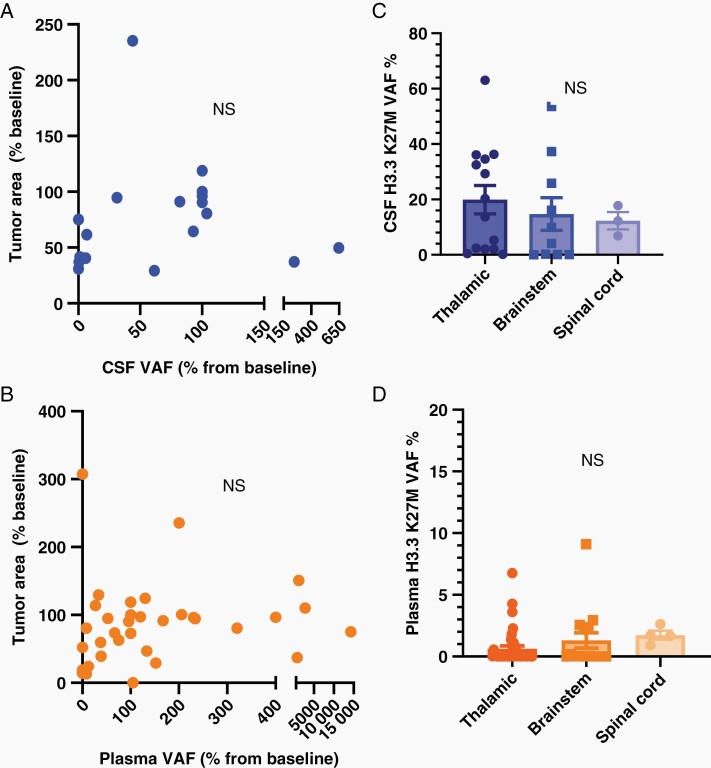
We first assessed the relationship between tumor size by MRI and plasma (n = 47) or CSF (n = 26) H3K27M VAF (% positive H3K27M droplets/total droplets with H3K27 DNA detected). There was no direct correlation between tumor size by MRI (percentage of initial size) and either plasma or CSF VAF (Figure 2A and B), or VAF and tumor location (Figure 2C and D.) Additionally, there was no correlation between VAF and tumor size (Fig. S2a-b) or between CSF or plasma cf-tDNA concentration and H3.3 K27M VAF percentage (Fig. S3a-b.)
Fig. 2.
(A-B) Correlation of tumor area change from baseline with CSF and plasma VAF percentage change from baseline (P value by Pearson correlation.) (C-D) H3K27M VAF by location in CSF (all time points) (C) and plasma (D, t-test.).
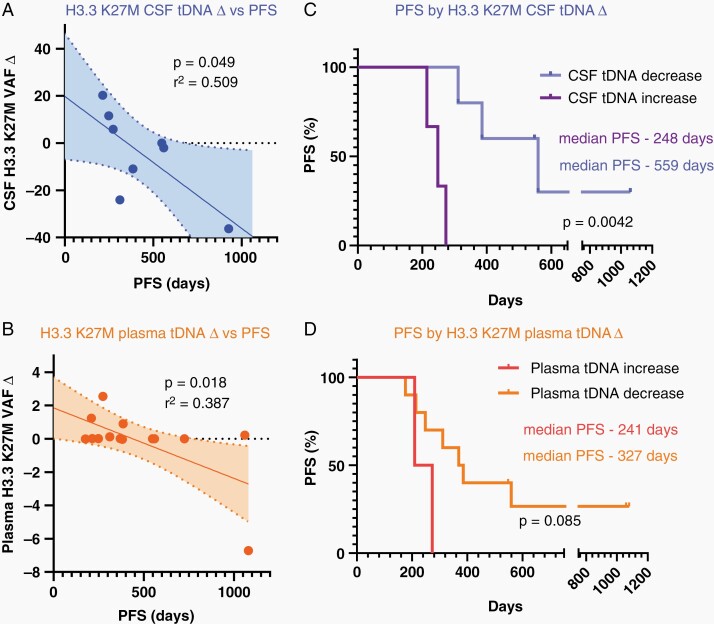
We then assessed the relationship between change in cf-tDNA VAF while on ONC201 therapy and progression free survival (PFS). “VAF delta”, defined as the H3K27M VAF at baseline minus the last serial VAF assessed in plasma or CSF (within sample type), correlated with prolonged PFS (Figure 3A and B). Of note, the correlation slope in plasma was driven most heavily by one outlier sample from UMICH04 which showed a reduction in plasma H3.3 K27M VAF of 6.72 %, thus potentially reducing the strength of this correlation in our dataset. Nonrecurrent patients (patients who enrolled after initial radiation but before progression, n = 13) that showed a decrease in CSF tDNA over time displayed a longer PFS (559 days vs. 248 days, P = .0042, Figure 3C). Decrease in plasma VAF displayed a similar, albeit nonsignificant, trend (P = .06, Figure 3D).
Fig. 3.
(A-B) Correlation of VAF change (delta: baseline-final time point VAF) to PFS (days of treatment) for CSF (A) and plasma (B, Pearson correlation) for nonrecurrent patients. (C-D) Progression free survival (PFS) (days of treatment) for nonrecurrent patients when patients are separated by VAF delta (increase or decrease from first to last value) for CSF (C) and plasma (D).
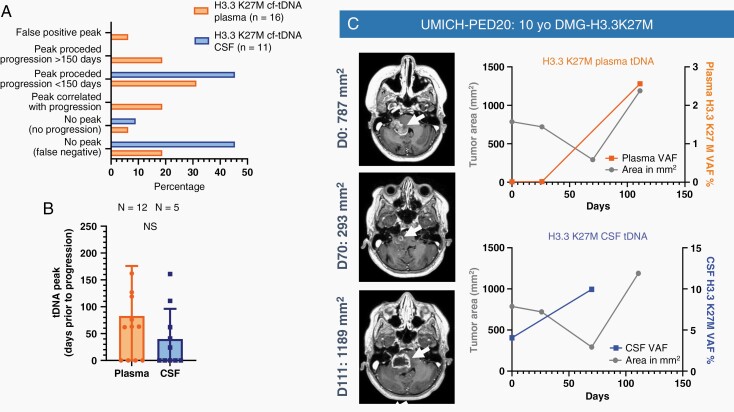
Some patients demonstrated a “spike” from baseline in cf-tDNA VAF (defined as an increase of at least 25%) that preceded tumor progression. False positive peaks (no progression for 6 months after peak) were only seen in one plasma case. Plasma VAF spikes preceded progression in 8/16 cases (50%) (Figure 4A and B) and cooccurred with progression in 3/16 (18.75%) patients. CSF VAF spikes preceded progression in 5/11 cases (45.4%). As an example, a 10-year-old female with pontine DMG enrolled after initial radiation and demonstrated an initial reduction in tumor area (T1+ contrast area) at day 70 that was felt to represent response to radiation; followed by clinical and radiographic progression at day 111. For this patient, H3K27M VAF “spike” preceded progression in CSF but correlated with progression in plasma (Figure 4C).
Fig. 4.
(A) Patients with tDNA peak (25% increase) and relation to tumor progression on tumor imaging (B) and days prior to progression by sample type. (C) Increase in VAF predicts progression in CSF and correlates with progression in a 10-year-old female with DIPG treated with ONC201.
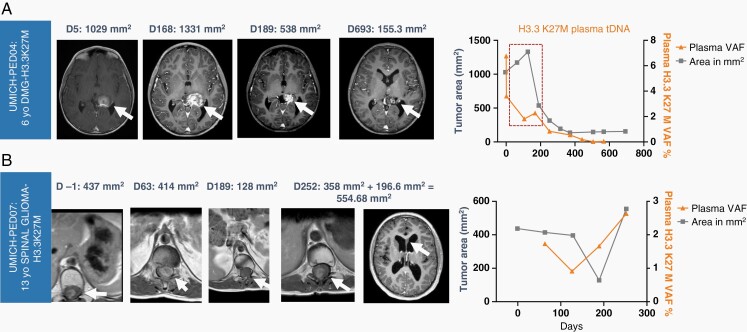
VAF change over time showed distinct patterns from tumor area changes (MRI) and in some cases offered potential clinical utility of distinguishing pseudo-progression and pseudo-response. Regarding pseudo-progression, a 6-year-old female with thalamic DMG treated with ONC201 following initial radiation demonstrated an initial increase in tumor area on MRI of nearly 25%, followed by a near complete response (85% regression by RANO) that has continues after 32 months of treatment (Figure 5A). Importantly, her plasma VAF showed a brisk reduction in plasma cf-tDNA VAF during the tumor imaging pseudo-progression, which more accurately reflected treatment response. Regarding pseudo-response, a 14-year-old male with spinal cord DMG received concurrent bevacizumab with ONC201, which resulted in a decrease in spinal tumor area on T1 postcontrast, as expected with bevacizumab. However, the patient showed continued increase in plasma VAF, which accurately predicted radiologic progression 8 weeks later (Figure 5B). We found no correlation between tumor size and VAF (Figure S2a-b) or overall cell-free DNA concentration and VAF (Figure S3a-b.)
Fig. 5.
(A) Pseudo-progression on tumor area by MRI (red dotted box) is not reflected in cf-tDNA H3K27M VAF reduction in a 6-year-old DMG patient with long-term response to ONC201. (B) A 13-year-old male with spinal H3K27M-mutant glioma demonstrates plasma VAF increase prior to radiologic progression. The drop in tumor area (gray plot) occurred during administration of bevacizumab, which caused reduction in tumor edema/area and contrast enhancement.
Discussion
Our work demonstrates the feasibility and clinical utility of serial cf-tDNA in both plasma and CSF of DMG patients to supplement radiographic monitoring. Patterns of change in VAF over time demonstrate clinical utility in terms of correlating with sustained response, predicting progression, and possibly identifying pseudo-progression and pseudo-response. In our cohort, CSF cf-tDNA displayed a greater sensitivity for H3K27M detection than plasma (96.5% vs. 85.4%). We found higher H3K27M VAF values in cf-tDNA derived from CSF compared to plasma, consistent with previous work.9,15–17 Most importantly, our data show that CSF H3K27M “responses” (reduction in VAF over time) is a stronger biomarker of clinical response (prolonged PFS). Interestingly, individual VAF values did not correlate with tumor area in either CSF or plasma. This is perhaps best explained by the individual time points when VAF diverged from tumor area on MRI in a way that added potential clinical utility (eg pseudo-progression, pseudo-response, prediction of tumor progression). Based on the enrichment of CSF with glioma tumor DNA, CSF may allow for the assessment of other tumor biomarkers that may be enriched in spinal fluid, such as cell-free methylation sequencing,20 exosomal RNA,21 and mitochondrial DNA.22 Overall these benefits favor the serial monitoring of CSF over plasma for glioma monitoring. However, CSF collection is more logistically complex and carries more procedural risk than a blood draw, thus encouraging further research to optimize plasma diagnostics for glioma to complement or potentially replace CSF diagnostics.
Our analyses of VAF patterns over time support that “spikes” in cf-tDNA VAF can support the diagnosis of clinical or radiographic disease progression. Importantly, VAF spikes in plasma and CSF occurred, on average, 1-3 months before radiographic progression in our cohort. Future use of cf-tDNA monitoring in patients with DMG may allow clinicians to respond to spikes in VAF by performing closer interval follow-up or to adjust treatment sooner. As the spike was a median of ~3 months and ~2 months (CSF) prior to progression, a 1–2 month frequency of collection should be adequate for predicting progression. Future use of Ommaya collection of CSF could improve safety/feasibility of frequent CSF collection.
In addition to providing a minimally invasive method for molecular profiling and longitudinal monitoring of tumor progression, serial monitoring of cf-tDNA derived from plasma or CSF may be useful in clarifying pseudo-progression and bevacizumab (“pseudo-response”) effect. In individual cases, patterns of change in VAF over time demonstrated that cf-tDNA more tightly correlated with the subsequent treatment course than fluctuations on MRI found later to represent radiation or bevacizumab effect. Our cohort was too heterogeneous to provide statistical analysis to these clinical situations that frequently complicate tumor imaging interpretation. Future studies may employ liquid cf-tDNA monitoring for selected cohorts of patients on bevacizumab or postirradiation to help clarify the full utility.
Finally, our cohort offers further evidence of the potential efficacy of ONC201 in H3K27M-mutant DMG. While our arm of the trial was primarily designed to study the utility of correlate CSF monitoring, the individual long-term responses seen in some patients (Figure 5A) are compelling, and the median PFS seen in patients with CSF or plasma tDNA reduction (559 and 327 days, respectively) compare very favorably to historic survival in H3K27M patients.23 Current work is underway to determine the patient populations most likely to respond to this therapy and the mechanism of response in H3K27M-mutant cells.
In summary, our work demonstrates the feasibility and potential utility of serial cf-tDNA in both plasma and CSF of DMG patients to supplement radiographic monitoring. Optimization of the above methods and transition of these tests to a CLIA-certified setting will allow for improved decision making in clinical management of DMG patients, and potentially broader glioma patient populations.
Supplementary Material
Acknowledgments
The authors thank the patients and their families for participation in this study.
Contributor Information
Evan Cantor, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Kyle Wierzbicki, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Rohinton S Tarapore, Chimerix, Durham, North Carolina, USA.
Karthik Ravi, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Chase Thomas, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Rodrigo Cartaxo, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Viveka Nand Yadav, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Ramya Ravindran, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Amy K Bruzek, Department of Neurosurgery, Michigan Medicine, Ann Arbor, Michigan, USA.
Jack Wadden, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Vishal John, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Clarissa May Babila, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Jessica R Cummings, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Abed Rahman Kawakibi, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Sunjong Ji, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Johanna Ramos, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Alyssa Paul, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Dustin Walling, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Marcia Leonard, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Patricia Robertson, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Andrea Franson, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Rajen Mody, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Hugh J L Garton, Department of Neurosurgery, Michigan Medicine, Ann Arbor, Michigan, USA.
Sriram Venneti, Department of Pathology, Michigan Medicine, Ann Arbor, Michigan, USA.
Yazmin Odia, Department of Neuro-Oncology, Miami Cancer Institute, Baptist Health South Florida, Miami, Florida, USA.
Cassie Kline, Division of Oncology, Department of Pediatrics, Children’s Hospital of Philadelphia, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA.
Nicholas A Vitanza, Department of Neurology, The Ben Towne Center for Childhood Cancer Research, Seattle Children’s Research Institute, Seattle, Washington, USA; Division of Pediatric Hematology/Oncology, Department of Pediatrics, Seattle Children’s Hospital, University of Washington, Seattle, Washington, USA.
Soumen Khatua, Department of Pediatric and Adolescent Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Sabine Mueller, Department of Neurology, Neurosurgery, and Pediatrics, University of California, San Francisco, San Francisco, California, USA.
Joshua E Allen, Chimerix, Durham, North Carolina, USA.
Sharon L Gardner, Department of Pediatrics, NYU Langone Health, New York, New York, USA.
Carl Koschmann, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Funding
This work was supported by NIH/NINDS [grant numbers K08-NS099427 (CK), R01-NS119231 (CK), R01NS124607 (CK), and R01NS110572 (SV)] and Department of Defense [grant number CA201129P1]; the University of Michigan Chad Carr Pediatric Brain Tumor Center; the ChadTough Defeat DIPG Foundation; the DIPG Collaborative; Catching Up With Jack; The Pediatric Brain Tumor Foundation; Prayers from Maria; Yuvaan Tiwari Foundation; The Morgan Behen Golf Classic; and the Michael Miller Memorial Foundation. Clinical Trial was supported by Chimerix.
Conflict of interest statement. JEA and RST are employees and shareholders of Chimerix Inc that is developing ONC201.
Authorship statement. All authors have contributed in meaningful ways and reviewed the manuscript.
References
- 1. Karremann M, Gielen GH, Hoffmann M, et al. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol. 2018; 20(1):123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012; 22(4):425–437. [DOI] [PubMed] [Google Scholar]
- 3. Schroeder KM, Hoeman CM, Becher OJ. Children are not just little adults: recent advances in understanding of diffuse intrinsic pontine glioma biology. Pediatr Res. 2014; 75(1-2):205–209. [DOI] [PubMed] [Google Scholar]
- 4. Castel D, Philippe C, Calmon R, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015; 130(6):815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brandes AA, Tosoni A, Spagnolli F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol. 2008; 10(3):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Springer S, Zhang M, et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci USA. 2015; 112(31):9704–9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stallard S, Savelieff MG, Wierzbicki K, et al. CSF H3F3A K27M circulating tumor DNA copy number quantifies tumor growth and in vitro treatment response. Acta Neuropathol Commun. 2018; 6(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruzek AK, Ravi K, Muruganand A, et al. Electronic DNA analysis of CSF cell-free tumor DNA to quantify multi-gene molecular response in pediatric high-grade glioma. Clin Cancer Res. 2020; 26(23):6266–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller AM, Shah RH, Pentsova EI, et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019; 565(7741):654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panditharatna E, Kilburn LB, Aboian MS, et al. Clinically relevant and minimally invasive tumor surveillance of pediatric diffuse midline gliomas using patient-derived liquid biopsy. Clin Cancer Res. 2018; 24(23):5850–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Izquierdo E, Proszek P, Pericoli G, et al. Droplet digital PCR-based detection of circulating tumor DNA from pediatric high grade and diffuse midline glioma patients. Neurooncol Adv. 2021; 3(1):vdab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishizawa J, Zarabi SF, Davis RE, et al. Mitochondrial ClpP-Mediated proteolysis induces selective cancer cell lethality. Cancer Cell. 2019; 35(5):721–737.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greer YE, Porat-Shliom N, Nagashima K, et al. ONC201 kills breast cancer cells in vitro by targeting mitochondria. Oncotarget. 2018; 9(26):18454–18479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prabhu VV, Allen JE, Dicker DT, El-Deiry WS. Small-molecule ONC201/TIC10 targets chemotherapy-resistant colorectal cancer stem-like cells in an Akt/Foxo3a/TRAIL-dependent manner. Cancer Res. 2015; 75(7):1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allen JE, Krigsfeld G, Mayes PA, et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med. 2013; 5(171):171ra–17117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prabhu VV, Madhukar NS, Gilvary C, et al. Dopamine receptor D5 is a modulator of tumor response to dopamine receptor D2 antagonism. Clin Cancer Res. 2019; 25(7):2305–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arrillaga-Romany I, Odia Y, Prabhu VV, et al. Biological activity of weekly ONC201 in adult recurrent glioblastoma patients. Neuro Oncol. 2020; 22(1):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hall MD, Odia Y, Allen JE, et al. First clinical experience with DRD2/3 antagonist ONC201 in H3 K27M-mutant pediatric diffuse intrinsic pontine glioma: a case report. J Neurosurg Pediatr. 2019; 23(6):1–7. [DOI] [PubMed] [Google Scholar]
- 19. Chi AS, Tarapore RS, Hall MD, et al. Pediatric and adult H3 K27M-mutant diffuse midline glioma treated with the selective DRD2 antagonist ONC201. J Neurooncol. 2019; 145(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li J, Zhao S, Lee M, et al. Reliable tumor detection by whole-genome methylation sequencing of cell-free DNA in cerebrospinal fluid of pediatric medulloblastoma. Sci Adv. 2020; 6(42):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Welton JL, Loveless S, Stone T, von Ruhland C, Robertson NP, Clayton A. Cerebrospinal fluid extracellular vesicle enrichment for protein biomarker discovery in neurological disease; multiple sclerosis. J Extracell Vesicles. 2017; 6(1):1369805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peng Y, Zheng D, Zhang X, et al. Cell-free mitochondrial DNA in the CSF: a potential prognostic biomarker of anti-NMDAR encephalitis. Front Immunol. 2019; 10(6):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mackay A, Burford A, Carvalho D, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017; 32(4):520–537 e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.