Abstract
Background
Leptomeningeal disease (LMD) is a devastating complication of systemic malignancy, of which there is an unclear etiology. The aim of this study is to determine if surgical or anatomic factors can predict LMD in patients with metastatic melanoma.
Methods
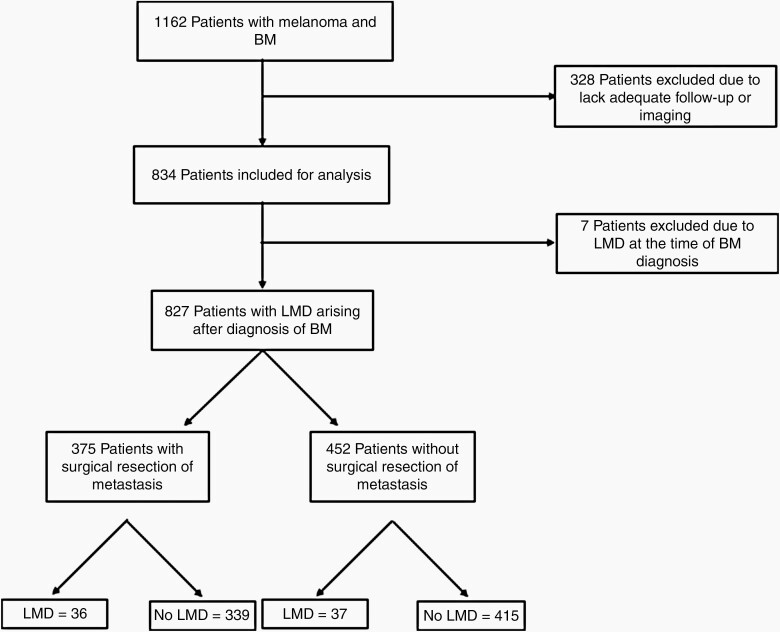
A retrospective chart review was performed of 1162 patients treated at single institution for melanoma brain metastases (MBM). Patients with fewer than 3 months follow-up or lacking appropriate imaging were excluded. Demographic information, surgical, and anatomic data were collected.
Results
Eight hundred and twenty-seven patients were included in the final review. On multivariate analysis for the entire cohort, female gender, dural-based and intraventricular metastasis, and tumor bordering CSF spaces were associated with increased risk of LMD. Surgical resection was not significant for risk of LMD. On multivariate analysis of patients who have undergone surgical resection of a metastatic tumor, dural-based and intraventricular metastasis, ventricular entry during surgery, and metastasis in the infratentorial space were associated with increased risk of LMD. On multivariate analysis of patients who did not undergo surgery, chemotherapy after initial diagnosis and metastasis bordering CSF spaces were associated with increased risk of LMD.
Conclusion
In a single-institution cohort of MBM, we found that surgical resection alone did not result in an increased risk of LMD. Anatomical factors such as dural-based and intraventricular metastasis were significant for developing LMD, as well as entry into a CSF space during surgical resection. These data suggest a strong correlation between anatomic location and tumor cell seeding in relation to the development of LMD.
Keywords: brain metastasis, leptomeningeal disease, melanoma, surgery
Key Points.
Surgical resection alone is not associated with an increased risk developing LMD.
Entrance into the ventricle space during surgical resection results in an increased risk of LMD.
Metastasis near a CSF space is associated with risk of LMD and is facilitated by seeding during surgery.
Importance of the Study.
This is the first study to examine how the relationship between anatomic location of brain metastases and surgical intervention may lead to the development of leptomeningeal disease (LMD) in a large cohort from a single type of malignancy. Our data suggest that, in contrast to previous reports, surgery is not the only risk for developing LMD. We have found that the presence dural-based and intraventricular metastasis, as well metastasis bordering a ventricle or CSF cistern, were associated with an increased risk of developing LMD. We also found in our cohort of patients who underwent surgical resection of their metastasis, that location adjacent to a CSF space as well as entry into the ventricle during surgery was associated with developing LMD. These findings highlight the complex relationship between natural and iatrogenic tumor cell seeding into a CSF space as a direct cause of the development of LMD.
Leptomeningeal disease (LMD) is a devastating complication of systemic malignancy. Prognosis is extremely poor, with a typical overall survival on the order of 4–6 weeks without treatment, and 3–6 months with treatment.1 LMD can occur in several types of solid malignancies, in 10–25% of patients with breast cancer, 5% of patients with melanoma, and 3–5% of patients with non small cell lung cancer.2–4 In particular, LMD from melanoma portends a very poor prognosis, with a reported mean survival of 3.5 months even with treatment.5 The pathogenesis of melanoma LMD development is not fully delineated but is assumed to have mechanisms similar to those in other solid malignancies. These mechanisms include vascular spread to the leptomeninges or choroid plexus, direct extension from known metastases, or perineural invasion.6–8
There is evidence that surgical resection may result in an increased risk of leptomeningeal disease when compared to focal radiation therapy (i.e. stereotactic radiosurgery (SRS)).9 Norris et al. first noted a correlation between surgical resection of posterior fossa metastases and the development of LMD.10,11 This correlation was confirmed by Suki et al., who also suggested that piecemeal resection of metastatic lesions resulted in a higher incidence of LMD compared to en bloc resection.9,12 More recent evidence has suggested that surgical resection may lead to higher rates of nodular-type LMD development, although this risk may be mitigated by use of neoadjuvant SRS.13–16
Given the previous findings relating surgical resection to development of LMD, we hypothesized that surgical and anatomical factors may play a role in the development of LMD in patients with metastatic melanoma. By examining a single institution’s cohort of patients with melanoma brain metastases (MBM) and LMD, we assessed how surgical resection may influence the development of LMD. In particular, we evaluated how tumor seeding in the cerebrospinal fluid (CSF) may result in the development of LMD, by assessing the location of brain metastases and their proximity to a ventricle or CSF cistern, as well as entry into the ventricle during surgery.
Methods
Clinical Review
Approval from the institutional review board at H. Lee Moffitt Cancer Center and Research Institute was obtained for a retrospective review of patient clinical data. 1162 patients were identified with a primary diagnosis of melanoma and brain metastases between 1999 and 2020 (Figure 1). Patients included in the study required a minimal follow-up of 3 months from the time of initial diagnosis of brain metastasis, as well as the presence of magnetic resonance imaging (MRI) for review of brain metastases and leptomeningeal disease. Patients with evidence of LMD at the time of their initial MBM diagnosis were excluded. General demographic information, as well as survival data, were obtained. First-line radiation treatment was also obtained, to determine whether whole-brain radiation therapy (WBRT), stereotactic radiosurgery (SRS), or fractionated stereotactic radiation therapy (FSRT), or WBRT with SRS boost was administered. Development of LMD was determined based on MRI or CSF cytology when available.
Fig. 1.
Flowchart demonstrating patient selection.
In patients who underwent multiple resections of intracranial metastases, the initial surgery was used for determination for development of LMD. Surgical data were reviewed with emphasis on determining whether the ventricular space was entered during the resection. This determination was made via a combination of operative report review and evaluation of the postoperative imaging.
Imaging Review
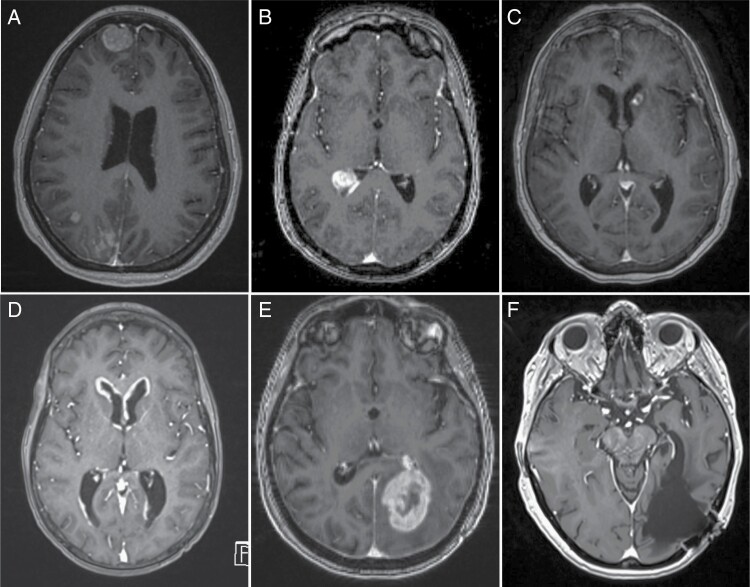
Imaging was reviewed independently by a neurosurgeon (SL) and a neuroradiologist (JA). MRIs were reviewed to determine the presence of dural-based metastases, as well as the presence of metastatic lesions bordering a ventricular or major CSF cistern (e.g. basal cisterns, cisterna magna). Patients whose MRIs were not available for our independent review were excluded from the analysis. Patients without appropriate imaging to ascertain detailed radiographic or anatomic location characteristics (e.g., MRI without contrast) were excluded from the analysis. Patients were described as having a metastasis bordering a ventricle or major CSF cistern if the tumor was located in the parenchyma and noted to be in direct contact with the CSF space or its ependymal lining, without intervening brain parenchyma (Figure 2B). Lesions categorized as intraventricular were required to have an isolated metastatic deposit that was located entirely within the ventricle (Figure 2C). The latter two types of metastases were distinguished from nodular LMD which consisted of multiple enhancing nodular lesions along the ventricular or CSF cistern (Figure 2D). Opening of the ventricle was determined radiographically, if on postoperative imaging, clear ventricular entry was seen and/or the operative report described ventricular entry during the procedure (Figure 2 E,F). If neither of these criteria were satisfied, the patient was not considered to have entry into the ventricle during surgery. LMD was defined by clear radiographic evidence of LMD on imaging as defined by the radiology report, and/or by presence of positive CSF cytology after completion of a lumbar puncture and subsequent clinical diagnosis of LMD by the treating physician.
Fig. 2.
T1 MRI with contrast of various types of brain metastases. A) Dural-based metastatic tumor in the left frontal lobe. B) An intraventricular metastasis in the atrium of the right lateral ventricle. C) A peri-ventricular metastasis bordering the ependyma of the left lateral ventricle. D) Nodular leptomeningeal disease in the bilateral frontal horns of the lateral ventricles. D and E) Pre- and postoperative imaging of a left occipital metastases. Note the entry into the temporal horn of the left lateral ventricle in the postoperative imaging.
Statistical Analysis
Patient characteristics were summarized using descriptive statistics including mean, standard deviation, median, and range for continuous measures and proportions and frequencies for categorical measures.
Univariable and multivariable logistic regressions were also performed to test the association of development of LMD and other variables. The variables with p≤0.1 from univariable logistic regression model analysis were added into initial model, then backward selection was conducted, and the variables with P ≥ .05 were removed from the final model. Odds ratios, with 95% confidence intervals, and P values were calculated. For categorical variables with more than two levels, P values are presented for each level compared to a referent level, and an overall p value using the type-III analysis of variance result. Data analysis was completed using SAS Institute Inc. (2021), version 9.4.
Results
Patient Population
A total of 827 patients with melanoma and intracranial metastatic disease with appropriate imaging and follow-up were identified and included for analysis (Table 1). Five hundred and forty-seven (66.1%) of the patients were male. Average age at last follow-up was 60.0 years, and average follow-up time was 22.4 months.
Table 1.
Cohort Demographics
| Variable | Level | N = 827 (%) |
|---|---|---|
| Gender | Female | 280 (33.9) |
| Male | 547 (66.1) | |
| Dural-based metastases | No | 769 (93.0) |
| Yes | 49 (5.9) | |
| Unknown | 9 (1.1) | |
| Intraventricular metastases | No | 804 (97.2) |
| Yes | 23 (2.8) | |
| Tumors bordering ventricle/cistern | 0 | 635 (76.8) |
| 1 | 147 (17.8) | |
| 2 | 28 (3.4) | |
| >2 | 17 (2.1) | |
| Location of brain metastases | Supratentorial | 665 (80.4) |
| Infratentorial | 38 (4.6) | |
| Both | 124 (15.0) | |
| Chemotherapy before initial MBM diagnosis | No | 646 (78.1) |
| Yes | 181 (21.9) | |
| Chemotherapy after initial MBM diagnosis | No | 435 (52.6) |
| Yes | 392 (47.4) | |
| Immune therapy before initial MBM diagnosis | No | 485 (58.6) |
| Yes | 342 (41.4) | |
| Immune therapy after initial MBM diagnosis | No | 437 (52.8) |
| Yes | 390 (47.2) | |
| Craniotomy for tumor resection | No | 452 (54.7) |
| Yes | 375 (45.3) | |
| Ventricular entry during surgery | No | 332 (89.5) |
| Yes | 39 (10.5) | |
| Unknown/Not applicable | 456 | |
| First-line radiation | WBRT | 146 (17.7) |
| SRS/FSRT | 470 (56.8) | |
| WBRT±SRS | 44 (5.3) | |
| None/Unknown | 167 (20.2) | |
| Development of LMD | No | 754 (91.2) |
| Yes | 73 (8.8) | |
| Survival time after LMD diagnosis (months) | Minimum | 0.2 |
| Maximum | 38.5 | |
| Mean | 4.1 | |
| Median | 2.1 | |
| SD | 6.4 | |
| Follow-up time (months) | Minimum | 3.0 |
| Maximum | 217 | |
| Mean | 22.4 | |
| Median | 12.6 | |
| SD | 25.6 |
On imaging review, 49 (5.9%) of patients were recorded as having a dural-based metastasis, 23 (2.8%) of patients had an intraventricular metastatic tumor, and 192 (23.2%) of patients had one or more lesions bordering a ventricle or major CSF cistern. Six hundred and sixty-five (80.4%) patients had only MBM in the supratentorial space, while 38 (4.6%) had only infratentorial tumors, and 124 (15.0%) had both.
The addition of chemotherapy or immunotherapy before or after initial diagnosis of brain metastasis was evaluated. 181 (21.9%) patients received some form of chemotherapy prior to their initial MBM diagnosis, while 392 (47.4%) received some form of chemotherapy following the diagnosis. 342 (41.4%) received some form of immunotherapy prior to the initial diagnosis while 390 (47.2%) received immunotherapy after. First-line radiation after initial MBM diagnosis was evaluated. 146 (17.7%) received only WBRT, 470 (56.8%) received either SRS or FSRT, 44 (5.3%) received a combination of WBRT and SRS.
Three hundred and seventy-five (45.3%) of patients underwent craniotomy for treatment of one or more metastatic tumors. 39 (10.5%) of patients had entry into the ventricular system during surgery. Univariate and multivariate analysis were performed on the entire cohort of patients, as well as on patients who received and did not receive surgery for their initial brain metastasis treatment.
Development of LMD
Seventy-three patients (8.8%) developed LMD after the initial diagnosis of a brain metastasis were included in further analysis. Of these, 21 had positive CSF cytology, 12 had negative CSF cytology (6 of these had atypical cells on CSF analysis), and 40 did not have CSF analysis. Of these 73 patients, 36 (49.3%) had surgical resection of at least one metastatic tumor prior to the development of LMD, and 37 (50.7%) did not. Mean survival after LMD diagnosis was 4.1 months, with the longest-surviving patient expiring 38.5 months after LMD diagnosis.
On univariate logistic regression analysis for the entire cohort, females were more likely to develop LMD than males (OR 1.69, 95% CI 1.04–2.75, P = .033) (Table 2). Patients harboring a dural-based metastasis were more likely to develop LMD than those without (OR 2.52, 95% CI 1.17–5.43, P = .018). Patients with an intraventricular tumor (OR 6.06, 95% CI 2.48–14.84, P < .001) and a tumor bordering a ventricle or major CSF cistern (OR 3.73, 95% CI 2.28–6.10, P < .001) were more likely to develop LMD than those who did not. The presence of a metastasis in the infratentorial space location did not impact LMD rates. Chemotherapy given after initial MBM diagnosis resulted in an increased risk of developing LMD (OR 1.77, 95% CI 1.08–2.89, P = .022).
Table 2.
Univariate and Multivariate Logistic Regression Analysis of LMD for all Patients with MBM
| Univariate | |||||
|---|---|---|---|---|---|
| Covariate | Level | N | Odds Ratio(95% CI) | OR P-value |
Overall P-value |
| Gender | Female | 280 | 1.69 (1.04–2.75) | .033 | .033 |
| Male | 547 | - | - | ||
| Dural metastasis | Yes | 49 | 2.52 (1.17–5.43) | .018 | .018 |
| No | 769 | - | - | ||
| Intraventricular metastasis | Yes | 23 | 6.06 (2.48–14.84) | <.001 | <.001 |
| No | 804 | - | - | ||
| Tumor bordering ventricle/cistern | Yes | 192 | 3.73 (2.28–6.10) | <.001 | <.001 |
| No | 635 | - | - | ||
| Number of lesions bordering ventricle/cistern | >2 | 17 | 4.97 (1.55–16.00) | .007 | <.001 |
| 2 | 28 | 4.41 (1.68–11.53) | .003 | ||
| 1 | 147 | 3.47 (2.03–5.95) | <.001 | ||
| 0 | 635 | - | - | ||
| Location of brain metastasis | Infratentorial/ Both | 162 | 1.39 (0.79–2.43) | .254 | .254 |
| Supratentorial | 665 | - | - | ||
| Chemotherapy before initial MBM diagnosis | Yes | 181 | 0.68 (0.36–1.29) | .241 | .241 |
| No | 646 | - | - | ||
| Chemotherapy after initial MBM diagnosis | Yes | 392 | 1.77 (1.08–2.89) | .022 | .022 |
| No | 435 | - | - | ||
| Immune therapy before initial MBM diagnosis | Yes | 342 | 1.12 (0.69–1.81) | .652 | 0.652 |
| No | 485 | - | - | ||
| Immune therapy after initial MBM diagnosis | Yes | 390 | 0.76 (0.47–1.24) | .278 | .278 |
| No | 437 | - | - | ||
| Craniotomy for tumor resection | Yes | 375 | 1.19 (0.74–1.93) | .476 | .476 |
| No | 452 | - | - | ||
| Radiation for all metastasis | SRS/FSRT | 470 | 0.77 (0.43–1.38) | .382 | .592 |
| WBRT ± SRS | 190 | 0.71 (0.35–1.46) | .349 | ||
| None | 167 | - | - | ||
| Radiation for metastasis bordering ventricle | SRS/FSRT | 95 | 0.61 (0.26–1.45) | .267 | .073 |
| WBRT ± SRS | 59 | 0.28 (0.09–0.83) | .022 | ||
| None | 38 | - | - | ||
| Multivariate | |||||
| Covariate | Level | Odds Ratio(95% CI) |
OR
P-value |
Overall
P-value |
|
| Gender | Female | 1.69 (1.02–2.81) | .042 | .042 | |
| Male | - | - | |||
| Dural metastasis | Yes | 2.33 (1.04–5.23) | .041 | .041 | |
| No | - | - | |||
| Intraventricular metastasis | Yes | 4.48 (1.72–11.70) | .002 | .002 | |
| No | - | - | |||
| Tumor bordering ventricle/cistern | Yes | 3.17 (1.90–5.30) | <.001 | <.001 | |
| No | - | - | |||
Interestingly, surgery for resection of a metastatic tumor was not a statistically significant predictor for the development of LMD (OR 1.19, 95% CI 0.74–1.93, P = .476). The type of first-line radiation also did not have an impact on the development of LMD on univariate analysis. When examining only metastasis that border a ventricle or CSF space, there was a reduced risk of LMD in patients who received WBRT (OR 0.28, 95% CI 0.09–0.83), P = .022).
On multivariate logistic regression analysis, we found that female gender continued to be a risk for development of LMD (OR 1.69, 95% CI 1.05–2.81, P = .042). We also found that anatomic factors were significant drivers of LMD development. The presence of dural-based metastasis (OR 2.33, 95% CI 1.04–5.23, P = .041), intraventricular metastasis (OR 4.48, 95% CI 1.72–11.70, P = .002), and metastasis bordering a ventricle or major cistern (OR 3.17, 95% CI 1.90–5.30, P < .001) were all predictive for development of LMD.
LMD Risk in Surgical Patients
In the cohort of patients who underwent surgery, a similar trend on univariate logistic regression analysis was observed. Dural-based metastasis (OR 2.77, 95% CI 1.04–7.35, P = .041), intraventricular metastasis (OR 6.94, 95% CI 1.86–25.88, P = .004), metastasis bordering a ventricular space (OR 2.97, 95% CI 1.46–6.02, P = .003), and ventricular entry during surgery (OR 4.82, 95% CI 2.15–10.82, P < .001) were all significant predictors of LMD development (Table 3). In contrast to the entire cohort of LMD patients, gender was no longer a predictive variable. Presence of a metastasis in the infratentorial space was a predictor for LMD (OR 2.40, 95% CI 1.02–5.67, P = .045). As with the overall cohort, type of first-line radiation therapy did not impact on the odds of LMD development.
Table 3.
Univariate and Multivariate Logistic Regression Analysis of LMD for Surgical Patients with MBM
| Univariate | |||||
|---|---|---|---|---|---|
| Covariate | Level | N | Odds Ratio(95% CI) | OR P-value |
Overall P-value |
| Gender | Female | 132 | 1.74 (0.87–3.48) | .116 | .116 |
| Male | 243 | - | - | ||
| Dural metastasis | Yes | 29 | 2.77 (1.04–7.35) | .041 | .041 |
| No | 337 | - | - | ||
| Intraventricular metastasis | Yes | 10 | 6.94 (1.86–25.88) | .004 | .004 |
| No | 365 | - | - | ||
| Tumor bordering ventricle/ cistern | Yes | 88 | 2.97 (1.46–6.02) | .003 | .003 |
| No | 287 | - | - | ||
| Number of lesions bordering ventricle/ cistern | >2 | 4 | 4.45 (0.44–44.76) | .205 | .019 |
| 2 | 7 | 5.34 (0.97–29.28) | .054 | ||
| 1 | 77 | 2.71 (1.28–5.74) | .009 | ||
| 0 | 287 | - | - | ||
| Ventricular entry during surgery | Yes | 39 | 4.82 (2.15–10.82) | <.001 | <.001 |
| No | 332 | - | - | ||
| Location of brain metastases | Infratentorial/ Both | 44 | 2.40 (1.02–5.67) | .045 | .045 |
| Supratentorial | 331 | - | - | ||
| Chemotherapy before initial MBM diagnosis | Yes | 63 | 0.42 (0.13–1.42) | .165 | .165 |
| No | 312 | - | - | ||
| Chemotherapy after initial MBM diagnosis | Yes | 172 | 1.36 (0.68–2.70) | .383 | .383 |
| No | 203 | - | - | ||
| Immune therapy before Initial MBM diagnosis | Yes | 125 | 1.15 (0.56–2.35) | .710 | .710 |
| No | 250 | - | - | ||
| Immune therapy after initial MBM diagnosis | Yes | 175 | 0.70 (0.35–1.42) | .327 | .327 |
| No | 200 | - | - | ||
| Radiation | SRS/FSRT | 206 | 0.83 (0.37–1.84) | .645 | .366 |
| WBRT ± SRS | 86 | 0.45 (0.15–1.38) | .163 | ||
| None | 83 | - | - | ||
| Tumor bordering ventricle w/surgery & ventricular entry | Yes | 30 | 3.12 (1.03–9.48) | .045 | .045 |
| No | 58 | - | - | ||
| Tumor bordering ventricle w/surgery | SRS/FSRT | 51 | 0.29 (0.09–0.98) | .047 | .040 |
| WBRT ± SRS | 19 | 0.09 (0.01–0.81) | .032 | ||
| None | 18 | - | - | ||
| Multivariate | |||||
| Covariate | Level | Odds Ratio(95% CI) |
OR
P-value |
Overall
P-value |
|
| Dural metastasis | Yes | 3.47 (1.22–9.85) | .020 | .020 | |
| No | - | - | |||
| Intraventricular metastasis | Yes | 4.56 (1.01–20.50) | .048 | .048 | |
| No | - | - | |||
| Ventricular entry during surgery | Yes | 4.52 (1.86–11.03) | <.001 | <.001 | |
| No | - | - | |||
| Location of brain metastases | Infratentorial/ Both | 2.89 (1.14–7.35) | .025 | .025 | |
| Supratentorial | - | - | |||
Further analysis of the 88 patients with a metastasis bordering the ventricle and undergoing surgery demonstrated that when the ventricle was entered, there was an increased risk of developing LMD (OR 3.12, 95% CI 1.03–9.48, P = .045). We also found that in this cohort of patients with metastasis bordering the ventricle undergoing surgery, that radiation therapy regardless of modality, SRS (OR = 0.29 (0.09–0.98) P = .047) or WBRT (OR 0.09, 95% CI 0.01–0.81, P = .032), resulted in a decreased risk of developing LMD (overall P = .040).
Multivariate logistic regression model found that dural-based metastasis (OR 3.49, 95% CI 1.20–10.14, P = .022), intraventricular metastasis (OR 5.38, 95% CI 1.21–23.81, P = .027), and ventricular entry during surgery (OR 5.43, 95% CI 2.18–13.52, P < .001) correlated with LMD development. Similar to the univariate analysis, presence of metastasis in the infratentorial space was predictive of LMD (OR 2.89, 95% CI 1.14–7.35, P = .025).
LMD Risk in Nonsurgical Patients
In the cohort of patients who did not undergo surgery, gender (OR 1.63 95% CI 0.83–3.32 P = .159), and presence of dural-based metastasis (OR 2.07, 95% CI 0.58–7.40, P = .265) were no longer associated with development of LMD (Table 4). The presence of intraventricular metastasis (OR 5.47, 95% CI 1.60–18.71, P = .007) and metastasis bordering a ventricular space or cistern (OR 4.64, 95% CI 2.33–9.24, P =< .001) was associated with LMD development. In this cohort, neither the presence of metastasis in the infratentorial space, radiation treatment, nor presence of tumor bordering the ventricle were associated with the development of LMD. Similar to the entire cohort of patients, chemotherapy given after initial MBM diagnosis resulted in an increased risk of developing LMD (OR 2.35, 95% CI 1.15–4.80, P = .019). Radiation treatment in this cohort did not affect the development of LMD.
Table 4.
Univariate and Multivariate Logistic Regression Analysis of LMD for Nonsurgical Patients with MBM
| Univariate | |||||
|---|---|---|---|---|---|
| Covariate | Level | N | Odds Ratio(95% CI) | OR P-value |
Overall P-value |
| Gender | Female | 148 | 1.63 (0.83–3.23) | .159 | .159 |
| Male | 304 | - | - | ||
| Dural metastasis | Yes | 20 | 2.07 (0.58–7.40) | .265 | .265 |
| No | 432 | - | - | ||
| Intraventricular metastasis | Yes | 13 | 5.47 (1.60–18.71) | .007 | .007 |
| No | 439 | - | - | ||
| Tumor bordering ventricle/ cistern | Yes | 104 | 4.64 (2.33–9.24) | <.001 | <.001 |
| No | 348 | - | - | ||
| Number of lesions bordering ventricle/ cistern | >2 | 13 | 5.84 (1.47–23.20) | .012 | <.001 |
| 2 | 21 | 4.58 (1.39–15.11) | .012 | ||
| 1 | 70 | 4.44 (2.05–9.64) | <.001 | ||
| 0 | 348 | - | - | ||
| Location of brain metastasis | Infratentorial/ Both | 118 | 1.05 (0.49–2.25) | .894 | .894 |
| Supratentorial | 334 | - | - | ||
| Chemotherapy before initial MBM diagnosis | Yes | 118 | 0.90 (0.41–1.97) | .797 | .797 |
| No | 334 | - | - | ||
| Chemotherapy after initial MBM diagnosis | Yes | 220 | 2.35 (1.15–4.80) | .019 | .019 |
| No | 232 | - | - | ||
| Immune therapy before initial MBMdiagnosis | Yes | 217 | 1.16 (0.59–2.27) | .671 | .671 |
| No | 235 | - | - | ||
| Immune therapy after initial MBM diagnosis | Yes | 215 | 0.83 (0.42–1.63) | .583 | .583 |
| No | 237 | - | - | ||
| Radiation | SRS/FSRT | 264 | 0.74 (0.31–1.75) | .489 | .663 |
| WBRT ± SRS | 104 | 1.01 (0.38–2.69) | .983 | ||
| None | 84 | - | - | ||
| Tumor bordering ventricle w/o surgery | SRS/FSRT | 44 | 1.33 (0.37–4.85) | .662 | .358 |
| WBRT ± SRS | 40 | 0.57 (0.14–2.42) | .447 | ||
| None | 20 | - | - | ||
| Multivariate | |||||
| Covariate | Level | Odds Ratio(95% CI) |
OR
P-value |
Overall
P-value |
|
| Chemotherapy after initial BM diagnosis | Yes | 2.12 (1.02–4.40) | .044 | .044 | |
| No | - | - | |||
| Tumor bordering ventricle/ cistern | Yes | 4.38 (2.18–8.77) | <.001 | <.001 | |
| No | - | - | |||
On multivariate analysis, treatment with chemotherapy following initial MBM diagnosis (OR=2.12 95% CI 1.02–4.40 P = .044) and the presence of metastasis bordering a ventricle or cistern were associated with the development of LMD (OR 4.38, 95% CI 2.18–8.77, P < .001).
Discussion
Standard therapy for intracranial metastatic disease has been driven by the Patchell et al. study which demonstrated that addition of surgery to WBRT results in improved local control and overall survival compared to WBRT alone.17 This study has been supplemented over time with additional data supporting the use of SRS as a useful adjunct in various circumstances.18 RTOG 9508 demonstrated the addition of SRS to WBRT also can result in local control and survival advantages, and now is commonly used as standard practice in place of WBRT for many indications.18,19
Controversy exists on the best methods for achieving local control and long-term prevention of LMD, and there is a relative paucity of data examining which factors are predictive of LMD development. Several studies have suggested that surgery for brain metastases confers a higher risk of developing LMD than SRS alone.12,20,21 Other studies dispute this conclusion, and note that surgery in and of itself is not an independent predictor of LMD development.22 Of note, some of the prior studies included LMD resulting from several different types of malignancies.
In regards to surgery as a risk factor for LMD, prior studies have focused on surgical technique of en bloc versus piecemeal resection as a risk factor for developing LMD, indicating that piecemeal resection resulted in higher risk of LMD development compared to en bloc resection or SRS.9,12 It is important to note that even though these cohorts were not homogenous with regard to the primary malignancy, these studies noted that patients with melanoma had the most pronounced effect with regard to technique of resection. Conversely, a phase 3 trial comparing postoperative SRS to observation did not demonstrate a difference between piecemeal and en bloc resection.23,24 In our cohort of patients harboring MBM, there was no association between surgical intervention alone and LMD development. Due to the retrospective nature of the data collection over a long period of time and potential inconsistencies in reporting operative techniques, we suggest that one cannot make an accurate assessment on how impactful resection technique is on LMD development.
In the current study, however, amongst patients who underwent craniotomy for resection of a metastatic tumor, entry into the ventricles was strongly associated with LMD development. This finding suggests that LMD may develop through a mechanism of direct CSF seeding of tumor cells at the time of surgery. This hypothesis is further supported by the analysis which demonstrates that entry into the ventricle during removal of a metastasis that borders a ventricle or CSF cistern results in an increased risk of LMD. It would be interesting to understand whether en bloc or piecemeal resection techniques may impact LMD development in this subset of tumors.
A strength of this study is the combined analysis of 2 cohorts of patients with the same pathology (melanoma brain metastasis); one that underwent surgical resection and one that did not. This design element allowed us to separate out the risks of LMD inherent to the disease from those related to surgery. The primary finding in our study was that surgery alone did not increase the risk of LMD development. Instead, in the combined cohort, anatomic factors dominated as the most significant factors that resulted in LMD on multivariate analysis, such as the presence of dural-based and intraventricular metastasis, and metastasis bordering a ventricle or cistern. Within the cohort of patients who had surgery, dural-based and intraventricular metastasis, ventricular entry, and presence of metastasis in the infratentorial space were all found to be predictive of LMD development on multivariate analysis. These findings support that surgical seeding of tumor cells may play an important role in the development of LMD. In the nonsurgical cohort, the only anatomic variable that remained significant on multivariate analysis was metastasis bordering a ventricle or cistern. This supports the hypothesis that seeding relating to proximity to a CSF space is a key element underlying the development of LMD. We speculate that the lack of significance of dural-based or intraventricular metastasis on multivariate analysis of nonsurgical patients may indicated that tumors in those locations are well encapsulated and that seeding occurs secondary to surgical manipulation or local migration of tumor cells within the brain parenchyma.
We are not aware of a prior study that demonstrates that anatomic factors, such as a metastasis bordering, or residing within, a CSF space results in an increased risk of developing LMD. While it is unsurprising to imagine the contribution of direct access to and dissemination through the cerebrospinal fluid pathways as a pathogenetic driver of LMD, previous work on the issue generally lacks direct data to support this observation. Hence, we have demonstrated that patients with tumors in contact with the CSF space are at higher risk of developing LMD than those who do not have peri-ventricular brain metastases.
Previous reports have demonstrated that posterior fossa metastases results in an increased risk of LMD compared to supratentorial metastases.11,25,26 Although the presence of infratentorial tumors was not a significant contributor of LMD in the entire cohort of patients, there was a significant difference in LMD rates for the surgical cohort on multivariate analysis. The significance of this finding in the surgical cohort suggests that CSF seeding is more likely to occur when operating in the posterior fossa, a finding that has been documented by others.9,20 In line with the hypothesis of surgical seeding as a source of LMD, our data also indicates that dural-based tumors are associated with a higher risk of LMD development in surgical patients, which indicate that these malignant cells may spread via direct extension through the subdural space at the time of surgery.
There is recent literature suggesting that the development of postsurgical LMD is higher in patients receiving postoperative SRS as compared to SRS alone, WBRT, or preoperative SRS.13,16,27,28 Although we did not find that addition of any type of radiation therapy to the first-line treatment of MBM reduced the risk of LMD on univariate or multivariate analysis in any of the cohorts, we did find that radiation reduced the risk of LMD overall. In the entire cohort of patients, univariate analysis demonstrated a decrease risk of LMD in patients with metastasis bordering a CSF space who have received WBRT. We also found in patients with metastases bordering a CSF space who underwent surgical resection (N = 88), the addition of either SRS/FSRT or WBRT resulted in a decreased risk of LMD on univariate analysis. For patients who have peri-ventricular metastases that did not undergo surgery (N = 104), radiation therapy did not affect LMD. These results indicate the importance of adjuvant radiation therapy for patients who underwent surgical resection of a metastasis bordering a CSF space, further supporting the idea that seeding during surgery occurs and radiation may help to reduce the development of LMD. Unfortunately, due to the wide variety in radiation treatments performed over the timeframe (including some treatments performed at outside institutions), a more definitive statement on the impact of specific radiotherapy regimens, timing strategies, or dose/fractionation strategies in this cohort cannot be made at this time.
Two variables that indicated a risk for development of LMD on multivariate analysis that are unexplained are gender and chemotherapy after diagnosis of MBM. Female gender was significantly associated with risk of developing LMD. There is no previously published evidence suggesting that gender impacts on the risk of LMD in melanoma or outcome from brain metastasis/LMD. Recent data from patients with primary malignant brain tumors have shown that there is a difference in peripheral immune responses between males and females with respect to polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs).29 These cells also have been shown to affect tumor cell dissemination and metastases in animal models, but their role in the pathophysiology of brain metastasis or LMD in patients with melanoma remains unknown.30 The significance of the association between treatment with chemotherapy after initial MBM diagnosis and LMD may be an indication of performance status, in which patients with higher performance status received chemotherapy and had a greater chance to develop LMD.
The limitations of this study include the retrospective nature of the data collection. Due to the time span in which patient records were reviewed, detailed radiation treatment data were not available to assess the impact of specific radiotherapy regimens on the development of LMD. Additionally, surgical reports did not consistently describe the technique used for us to determine use of an en bloc versus piecemeal resection approach. Our analysis also did not distinguish postsurgical nodular versus classic LMD. Although recent literature has established the a nodular characteristic associated with postsurgical LMD,15 we did not make this determination in our analysis, but since we were able to separate the surgical from the nonsurgical cohort of patients, we can accurately assess surgery itself as a factor in the development of LMD.
Conclusions
The data presented demonstrate a correlation between anatomic location of brain metastases and the risk for development of LMD. Although our data do not demonstrate that surgical resection alone is a risk factor for LMD, we have demonstrated that surgery for dural-based, intraventricular, or infratentorial metastasis, or entry into a ventricle during surgery, did result in an increased risk of developing LMD.
Contributor Information
Stephen R Lowe, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
Christopher P Wang, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Amanda Brisco, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Junmin Whiting, Department of Biostatistics & Bioinformatics, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
John Arrington, Department of Radiology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
Kamran Ahmed, Department of Radiation Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
Michael Yu, Department of Radiation Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
Timothy Robinson, Department of Radiation Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
Daniel Oliver, Department of Radiation Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
Arnold Etame, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
Nam Tran, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
Andre Beer Furlan, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
Solmaz Sahebjam, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
Sepideh Mokhtari, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
Yolanda Piña, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
Robert Macaulay, Department of Pathology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
Peter Forsyth, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
Michael A Vogelbaum, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA.
James K C Liu, Department of Neuro-Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA; University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Funding
No external funding supported this research.
Conflict of interest statement. There are no author conflict of interest.
Authorship statement. Contribution to manuscript: Conception and Design: SRL, CPW, AB, JKCL. Data collection: SRL, CPW, AB, JA, JW, JKL. Imaging Review: SRL, JA, MAV, JKCL. Statistical Analysis: SRL, JW, MAV, JCKL. Manuscript drafting: SRL, PF, MAV, JKCL. Interpretation of data, conceptual refinement and critical manuscript review: all authors.
References
- 1. Wang N, Bertalan MS, Brastianos PK. Leptomeningeal metastasis from systemic cancer: review and update on management. Cancer 2018;124(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19(1):e43–e55. [DOI] [PubMed] [Google Scholar]
- 3. Smalley KS, Fedorenko IV, Kenchappa RS, Sahebjam S, Forsyth PA. Managing leptomeningeal melanoma metastases in the era of immune and targeted therapy. Int J Cancer. 2016;139(6):1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Figura NB, Rizk VT, Armaghani AJ, et al. Breast leptomeningeal disease: a review of current practices and updates on management. Breast Cancer Res Treat. 2019;177(2):277–294. [DOI] [PubMed] [Google Scholar]
- 5. Ferguson SD, Bindal S, BassettRL, Jr., et al. Predictors of survival in metastatic melanoma patients with leptomeningeal disease (LMD). J Neurooncol. 2019;142(3):499–509. [DOI] [PubMed] [Google Scholar]
- 6. Kokkoris CP. Leptomeningeal carcinomatosis. How does cancer reach the pia-arachnoid? Cancer 1983;51(1):154–160. [DOI] [PubMed] [Google Scholar]
- 7. Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int 2013;4(Suppl 4):S265–S288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang PC, Fischbein NJ, McCalmont TH, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradiol. 2004;25(1):5–11. [PMC free article] [PubMed] [Google Scholar]
- 9. Suki D, Abouassi H, Patel AJ, et al. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg. 2008;108(2):248–257. [DOI] [PubMed] [Google Scholar]
- 10. Ojerholm E, Lee JY, Thawani JP, et al. Stereotactic radiosurgery to the resection bed for intracranial metastases and risk of leptomeningeal carcinomatosis. J Neurosurg. 2014;121(Suppl):75–83. [DOI] [PubMed] [Google Scholar]
- 11. Norris LK, Grossman SA, Olivi A. Neoplastic meningitis following surgical resection of isolated cerebellar metastasis: a potentially preventable complication. J Neurooncol. 1997;32(3):215–223. [DOI] [PubMed] [Google Scholar]
- 12. Suki D, Hatiboglu MA, Patel AJ, et al. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery 2009;64(4):664–74; discussion 674. [DOI] [PubMed] [Google Scholar]
- 13. Turner BE, Prabhu RS, Burri SH, et al. Nodular leptomeningeal disease-a distinct pattern of recurrence after postresection stereotactic radiosurgery for brain metastases: a multi-institutional study of interobserver reliability. Int J Radiat Oncol Biol Phys. 2020;106(3):579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prabhu RS, Patel KR, Press RH, et al. Preoperative vs postoperative radiosurgery for resected brain metastases: a review. Neurosurgery 2019;84(1):19–29. [DOI] [PubMed] [Google Scholar]
- 15. Vogelbaum MA, Yu HM. Nodular leptomeningeal disease after surgery for a brain metastasis-should we be concerned? Neuro Oncol 2019;21(8):959–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prabhu RS, Turner BE, Asher AL, et al. A multi-institutional analysis of presentation and outcomes for leptomeningeal disease recurrence after surgical resection and radiosurgery for brain metastases. Neuro Oncol 2019;21(8):1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500. [DOI] [PubMed] [Google Scholar]
- 18. Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363(9422):1665–1672. [DOI] [PubMed] [Google Scholar]
- 20. Siomin VE, Vogelbaum MA, Kanner AA, et al. Posterior fossa metastases: risk of leptomeningeal disease when treated with stereotactic radiosurgery compared to surgery. J Neurooncol. 2004;67(1-2):115–121. [DOI] [PubMed] [Google Scholar]
- 21. Johnson MD, Avkshtol V, Baschnagel AM, et al. Surgical resection of brain metastases and the risk of leptomeningeal recurrence in patients treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2016;94(3):537–543. [DOI] [PubMed] [Google Scholar]
- 22. Huang AJ, Huang KE, Page BR, et al. Risk factors for leptomeningeal carcinomatosis in patients with brain metastases who have previously undergone stereotactic radiosurgery. J Neurooncol. 2014;120(1):163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahajan A, Ahmed S, Li J, et al. Postoperative stereotactic radiosurgery versus observation for completely resected brain metastases: results of a prospective randomized study. Int J Radiat Oncol 2016;96(2):S2–S2. [Google Scholar]
- 25. Seute T, Leffers P, ten Velde GP, Twijnstra A. Leptomeningeal metastases from small cell lung carcinoma. Cancer 2005;104(8):1700–1705. [DOI] [PubMed] [Google Scholar]
- 26. van der Ree TC, Dippel DW, Avezaat CJ, et al. Leptomeningeal metastasis after surgical resection of brain metastases. J Neurol Neurosurg Psychiatry. 1999;66(2):225–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prabhu RS, Turner BE, Asher AL, et al. Leptomeningeal disease and neurologic death after surgical resection and radiosurgery for brain metastases: a multi-institutional analysis. Adv Radiat Oncol 2021;6(2):100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Teyateeti A, Brown PD, Mahajan A, Laack NN, Pollock BE. Outcome comparison of patients who develop leptomeningeal disease or distant brain recurrence after brain metastases resection cavity radiosurgery. Neurooncol Adv 2021;3(1):vdab036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bayik D, Zhou Y, Park C, et al. Myeloid-derived suppressor cell subsets drive glioblastoma growth in a sex-specific manner. Cancer Discov 2020;10(8):1210–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ouzounova M, Lee E, Piranlioglu R, et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat Commun. 2017;8:14979. [DOI] [PMC free article] [PubMed] [Google Scholar]