Abstract
Introduction:
Nutrition health disparities include differences in incidence, prevalence, morbidity, and mortality of diet-related diseases and conditions. Often race, ethnicity, and the social determinates of health are associated with dietary intake and related health disparities. This report describes nutrition health disparities research supported by NIH over the past decade and offers future research opportunities relevant to NIH’s mission as described in the Strategic Plan for NIH Nutrition Research.
Methods:
Data were extracted from an internal reporting system from 2010 to 2019 using the Research, Condition, and Disease Categorization spending categories for “Nutrition” and “Health Disparities.”
Results:
Over the past decade, NIH-supported nutrition and health disparities research increased, from 860 grants in 2010 to 937 grants in 2019, while total nutrition and health disparities funding remained relatively stable. The top 5 Institutes/Centers that funded nutrition and health disparities research (based on both grant numbers and dollars) were identified. Principal areas of focus included several chronic diseases (e.g., obesity, diabetes, cancer, heart disease) and research disciplines (e.g., clinical research and behavioral and social science). Focus areas related to special populations included pediatrics, minority health, aging, and women’s health.
Conclusions:
The gaps and trends identified in this analysis highlight the need for future nutrition and health disparities research, including a focus on American Indian and Asian populations, and the growing topics of rural health, maternal health, and food insecurity. In alignment with the Strategic Plan for NIH Nutrition Research, health equity may be advanced through innovative research approaches to develop effective targeted interventions to address these disparities.
INTRODUCTION
Disparities in dietary intake and diet-related diseases are prevalent in the U.S.1–3 In an analysis of the burden of disease in the U.S. by age, sex, geography, and year (1990–2016), poor diet was a leading risk factor of attributable disability-adjusted life years4 (1 disability-adjusted life year represents the loss of the equivalent of 1 year of full health). Further, evidence shows that diet-related health disparities vary by race and ethnicity, education, and income level.2,5,6 Systematic reviews and meta-analyses have documented associations between dietary factors and nutrients on chronic diseases such as cardiovascular diseases, diabetes, cancer,7,8 and increased risk of mortality associated with diet-related disease.9,10 Many subgroups experience higher rates of diet-related morbidity and mortality compared with the general population (e.g., across different socioeconomic, racial, and ethnic subpopulations).11 For example, American Indian/Alaska Natives (AI/ANs) have higher prevalence of type 2 diabetes than other groups.12 Those living in rural compared with urban communities are at greater risk of mortality from heart disease and stroke.13 Additionally, there are racial differences in the incidence and mortality for certain cancers.14,15
Nutrition health disparities (NHDs) arise from multiple factors. These include social determinants of health (SDH) that operate within biological, behavioral, and environmental domains, encompassing every socioecological level—individual, interpersonal, community, and societal. Structural drivers (e.g., structural racism and inequitable policies and distribution of resources) are root causes of these inequitable SDH that perpetuate disparities by race and SES.16,17 Factors that contribute to NHDs are complex, and may individually or synergistically affect inter-relationships impacting dietary intake, nutritional status, and health.18 For example, healthy food access and affordability (societal influences) can intersect with cultural norms and practices (individual influences) to affect dietary behaviors and consequent risk for diet-related disease. Synergistic relationships can impact nutrition-related health outcomes and widen health disparities throughout the life course. Therefore, research to better understand the complex nutrition-related inter-relationships may lead to the development of tailored interventions that address these dynamics, promote minority health, and reduce health inequities. Moreover, the recent health-related burden caused by the coronavirus disease 2019 (COVID-19) pandemic on racial/ethnic minority populations (e.g., Black, Hispanic, and AI/AN communities), and the diet-related comorbidities (e.g., obesity, hypertension, diabetes, and some cancers) that exacerbate poor outcomes, underscore the importance of exploring multidisciplinary approaches.19,20
Research related to NHDs predominantly includes studies that: (1) characterize nutrition disparities by various demographic factors, (2) examine underlying factors that contribute to NHDs, and (3) implement and test the effectiveness of interventions designed to address observed disparities. In May 2020, NIH released its first Strategic Plan for NIH Nutrition Research (SPNR),21 emphasizing cross-disciplinary and innovative opportunities to advance nutrition research across the research continuum.22 Minority health and health disparities are emphasized as 1 of 5 cross-disciplinary research areas fundamental in nutrition sciences (the complete list of topics is provided in the SPNR). The plan highlights the importance of understanding factors that contribute to NHDs and developing interventions that can improve health. Within this framework, the purpose of the present analysis is to characterize the NIH-funded NHD portfolio from Fiscal Year (FY) 2010 to FY2019 and highlight gaps and potential opportunities for future research. To adequately prevent NHDs and promote research in this area, an understanding of what is currently being federally funded is first needed in order to shape future research directions that could impact practice.22
METHODS
Study Sample and Measures
Data for extramural projects from FY2010 to FY2019 were extracted from NIH internal reporting systems. and included the overlapping grants of 2 official Research, Condition, and Disease Categorization (RCDC) spending categories, “Nutrition” and “Health Disparities.” RCDC was utilized for the “Nutrition” project lists from FY2010 to FY2019 and for the “Health Disparities” project lists from FY2017 to FY2019. The NIH defines health disparities as a health difference that adversely affects disadvantaged populations, based on 1 or more health outcomes and health disparity populations as racial and ethnic minority populations and those less privileged such as low socioeconomic populations, underserved rural populations, sexual and gender minorities, and any subpopulations that can be characterized by 2 or more of these descriptions. Owing to the specific methodology for the development of the “Health Disparities” RCDC category, the NIH RCDC Process Budget Estimating Tool (R-BET) was used to obtain the “Health Disparities” project lists from FY2010 to FY2016. The R-BET system is an NIH internal budgeting tool used to collect a variety of funding data on disease categories and is used primarily for budget officers to generate estimates of those categories. Prior to FY2017, the R-BET system housed the manual categories, (e.g., for minority health and health disparities) that were compiled separately by each individual NIH Institutes and Centers. Once these lists were established, the 2 “Nutrition” and “Health Disparities” categories were extracted and combined, and the overlap of projects were analyzed for each FY. To further characterize the NHD research portfolio, RCDC was also used to identify special populations using pre-defined NIH official spending categories: “Maternal Health,” “Rural Health,” “American Indian or Alaska Native,” “Women’s Health,” “Aging,” “Minority Health,” and “Pediatrics” within the combined nutrition and health disparities project list. Additional keyword searches to identify specific populations (“African American/Black,” “Hispanic/Latino,” and “Asian”) were done within the combined project portfolio. In the search, 291 grants were identified for African Americans/Blacks, 208 for Hispanics/Latinos, and 52 for Asians. A subset of authors (12) reviewed the abstracts and specific aims of each grant for relevance. Each grant was assigned 2 independent reviewers and minor discrepancies were reconciled by the lead authors (AB, TAC).
Statistical Analysis
Descriptive statistics were used to analyze the research portfolio. These included: total spending by FY (all grants), number of total and new/renewal competitive grants (Type 1 and Type 2)a by FY, and budget mechanisms: Research Projects (R01, other Rs), Cooperative Agreements (Us), Program Projects and Center Grants (P01, Other Ps), and Research Career Programs (Ks) (note: not all training grants are shown as Ks consistently made up 80%–90% of all training grants). The analysis mainly focused on competing Type 1 (new) and Type 2 (renewal) grants. Descriptive statistics are also presented for study type, the special population, and diseases and conditions. To compare shifts in research topics and subtopics between the first 5 years (2010–2014) compared to the latter 5 years (2015–2019), visualizations for each time period were compared using a clustering algorithm that uses words and phrases from funded grant applications (title, abstract, and specific aims fields).
RESULTS
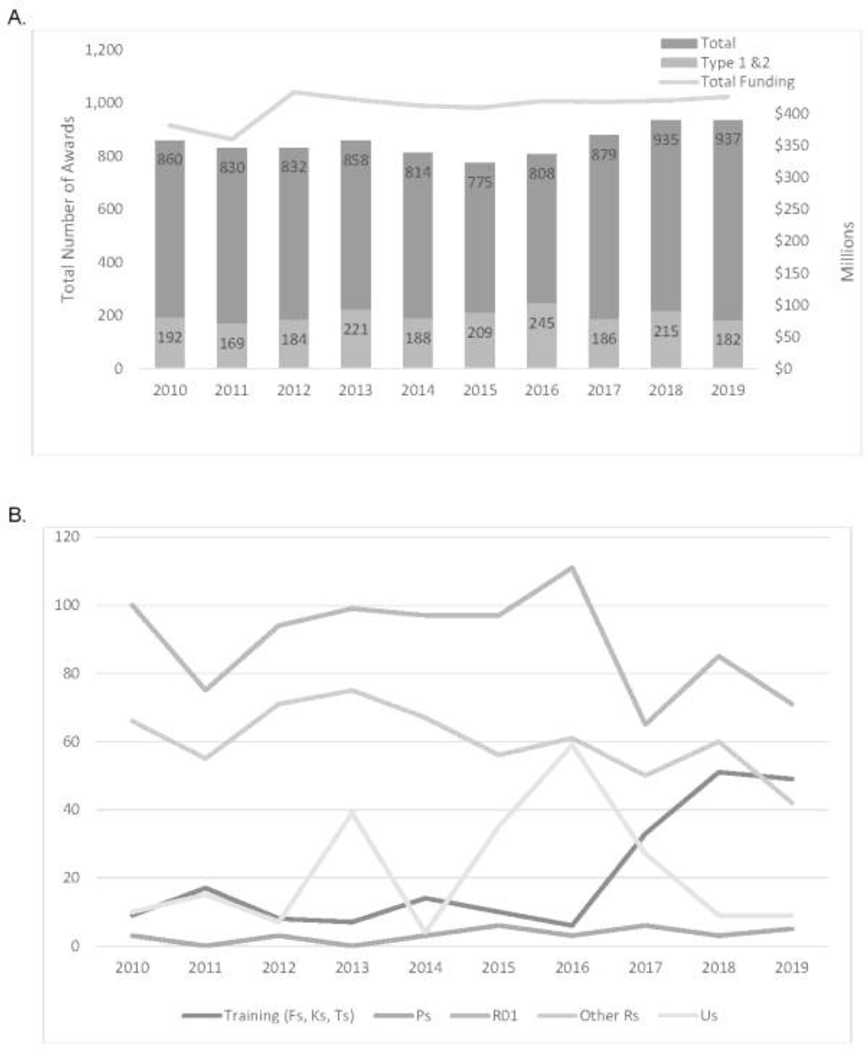
Figure 1A depicts the total number of grants and new grants (Type 1 and Type 2 only) and funding on NHD research for FY2010–FY2019. The total grants on NHD research modestly increased in the past decade, with the greatest increase in the past 5 years (FY2015–FY2019), from the lowest year at 775 to 937 (21% increase) (Figure 1A). New grants in this area ranged from 169 (FY2011) to 245 (FY2016) and averaged about 200 per year (Figure 1A). Funding for this type of research has been relatively stable since FY2012. Comparatively, NIH total research spending steadily increased (26.1%) between FY2013 and FY2019 (data not shown). Nutrition research remained steady at 5% of total NIH research spending, whereas health disparities research fluctuated between 14% and 16% in recent years. Overall, HD research spending was more than double nutrition research spending, so although NHD spending only comprised 8% of health disparities research spending, it ranged from 23% to 25% of nutrition research spending. The leading Institutes and Centers for NHD research during FY2010–FY2019 with respect to both the number of new grants and total cost of funding were the National Institute of Diabetes and Digestive and Kidney Diseases (760 new grants; $1,702 million); National Heart, Lung, and Blood Institute (304 new grants; $835 million), Eunice Kennedy Shriver National Institute of Child Health and Human Development (212 new grants; $356 million), National Cancer Institute (151 new grants; $316 million), and National Institute on Minority Health and Health Disparities (134 new grants; $174 million).
Figure 1.
Total grants, new grants (type 1 and 2), total funding, and funding mechanisms for nutrition and health disparities research, FYs 2010–2019. (A) Total grants, new grants (type 1 and 2) and total funding by FY (2010–2019). (B) Funding mechanisms of new (type 1 and 2) grants by FY (2010–2019). Notes: Fs: F05, F31, F32, F33, F99; Ks: K01, K07, K08, K23, K24, K25, K99; Ps: P01, P20, P42, P50; Ts: T32, T34, T35, T90/R90, T15; Other Rs: R03, R13, R15, R18, R21, R24, R25, R34, R35, R36, R37, R41, R42, R43, R44, R56, R61; Us: U01, U10, U13, U19, U24, U34, U54, UG, UG3, UH2, UH3, UM1. FY, Fiscal Year.
Between FY2016 and FY2019, research on NHDs substantially increased in the training category as shown by the newly funded training grants (Figure 1B), especially for Career (K) awards (data not shown). Meanwhile, there were fluctuations in newly funded R01s and U grants focused on NHD research (Figure 1B).
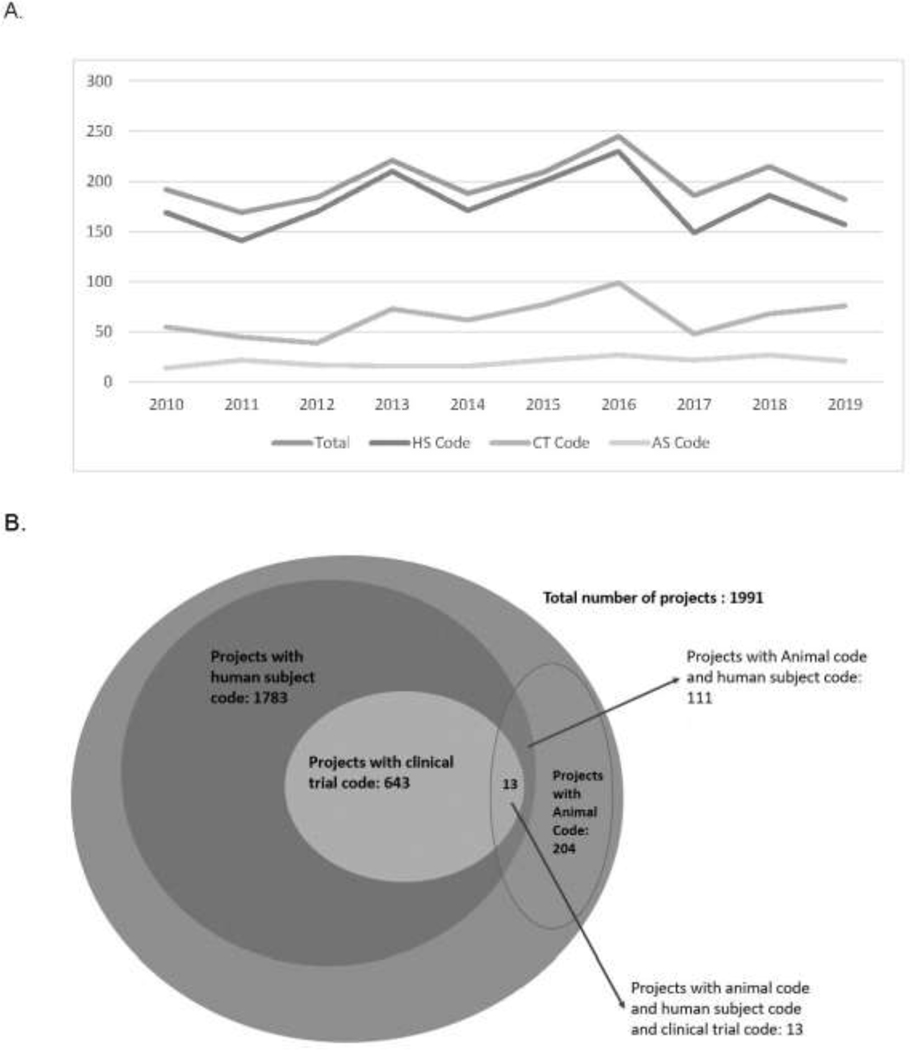
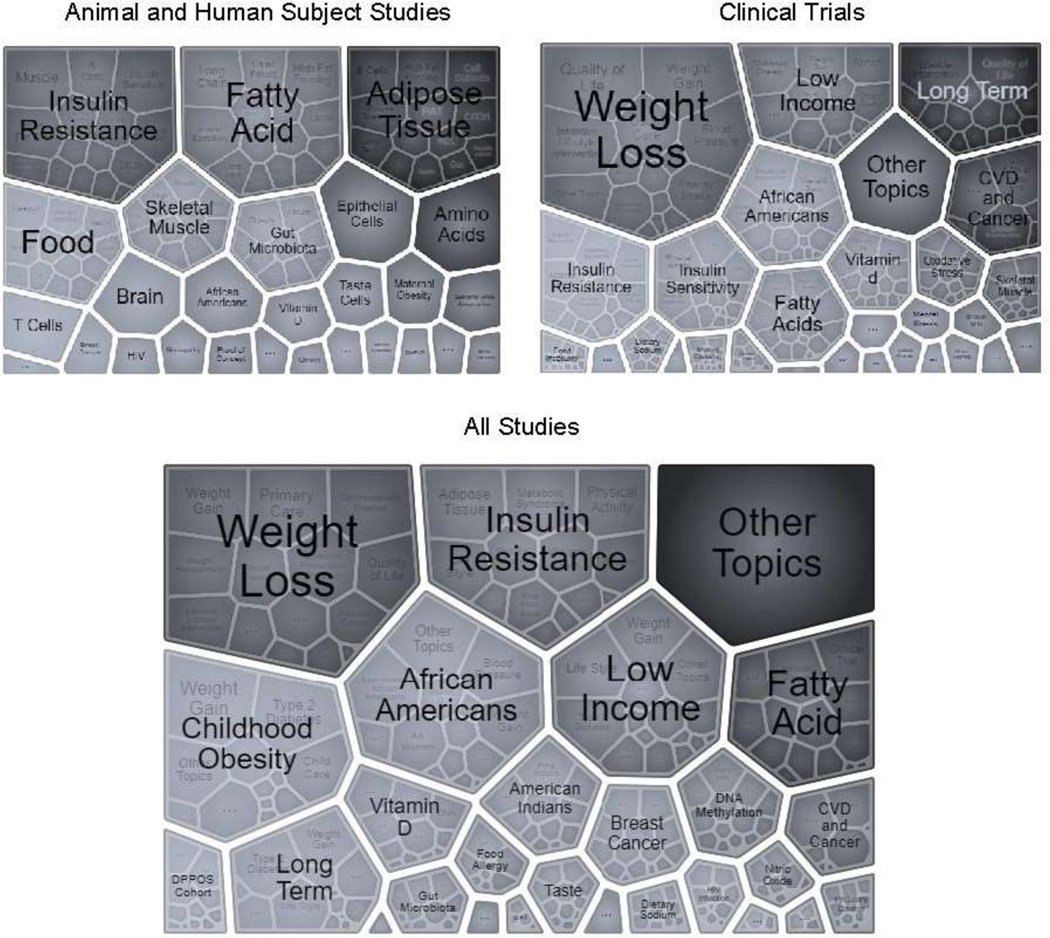
The NIH funds NHD research that involves human participants, animals, or a combination of these study types. From FY2010 to FY2019, a total of 1,991 new awards related to NHDs, of which 1,783 (90%) involved human subject research (Figure 2). Of the awards involving humans, 643 (32%) were clinical trials. Trials were diverse in their focus areas, and as noted in Figure 3, key topics included weight loss, low income, insulin resistance and sensitivity, and African Americans.
Figure 2.
New grants (type 1 and 2) for nutrition and health disparities research, FYs 2010–2019: human subject (HS), clinical trials (CT), and animal studies (AS). (A) Total number of new grants (type 1 and 2) with HS, CT or AS code by FY (2010–2019). (B) Total number of new grants with HS, CT, or AS for all years (2010–2019). Note: Grants with HS or AS are not mutually exclusive; for example, a project may involve both animal and human subject. CT is a subset of HS research projects. HS, Human Subject (NIH https://grants.nih.gov/policy/humansubjects/research.htm); AS, Animal Subject; CT, Clinical Trial (NIH https://grants.nih.gov/policy/clinical-trials/definition.htm); FY, Fiscal Year.
Figure 3.
Visualizations of topics for all new grants (types 1 and 2) (bottom), new grants with animal and human subjects studies (upper left), or new grants with clinical trials (upper right), FYs 2010–2019.
Note: Grants with HS or AS are not mutually exclusive; for example, a project may involve both animal and human subject. CT is a subset of HS research projects. BAT, brown adipose tissue; DPPOS, Diabetes Prevention Program Outcomes Study.
Approximately 10% of the total awards (204 awards) involved animal research, of which 93 awards (5%) only used animals, and 111 awards (6%) used both human and animal subjects. Key topic areas for research involving both human and animals included adipose tissue, insulin resistance, fatty acid metabolism, skeletal tissue, and the gut microbiome (Figure 3).
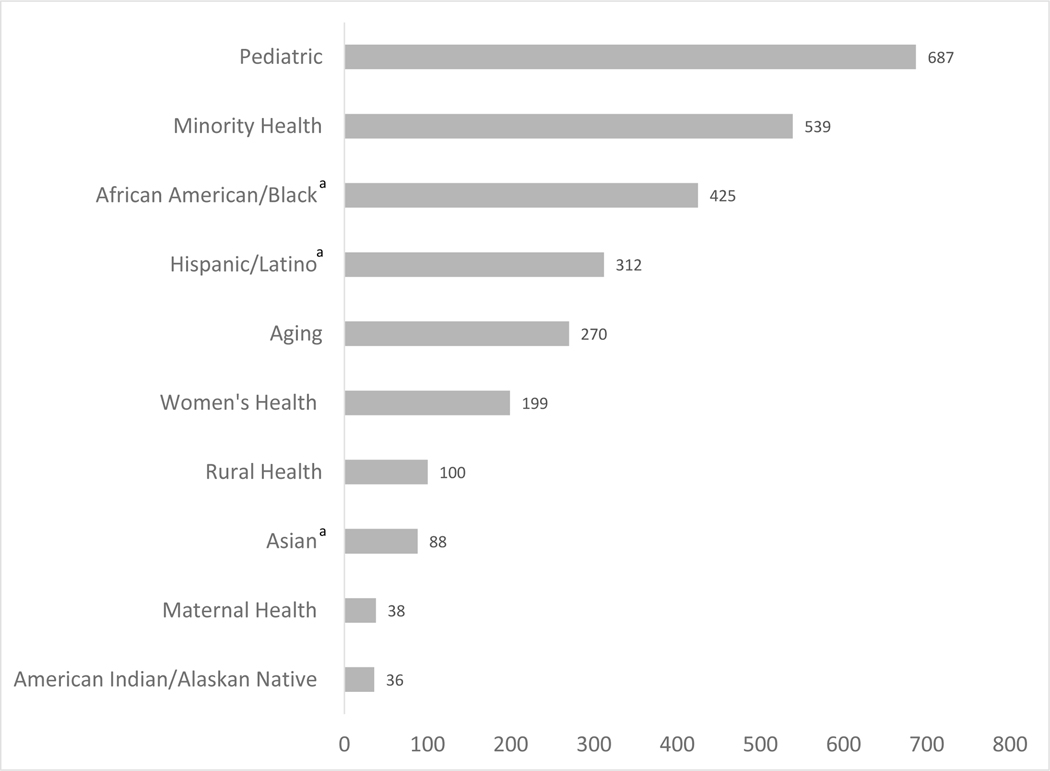
For topics of NHD research at NIH, 7 official special population categories within the RCDC were identified. These included: “Maternal Health,” “Rural Health,” “American Indian or Alaska Native,” “Women’s Health,” “Aging,” “Minority Health,” and “Pediatrics.” As shown in Figure 4, when categorizing all the new grants, the most frequent special populations (based on the number of new grants) were “Pediatric” and “Minority Health,” followed by “Aging” and “Women’s Health.” The top 5 RCDC diseases and conditions categories for NHD grants based on number of grants in each category were obesity, diabetes, digestive diseases, cancer, and heart disease.
Figure 4.
Special populations of nutrition and health disparities grants (type 1 and 2), FY2010–2019.
aGrants for the African American/Black, Hispanic/Latino, and Asian categories were determined by a keyword search review process and the other categories were based on existing RCDC categories. The special population categories are not mutually exclusive; e.g. a project may be categorized under Minority Health, African American/Black, Hispanic/Latino and Maternal Health. For example, the AMPLIFI study above is categorized in the Minority Health, African American/Black, Aging, and Rural Health categories.
FY, Fiscal Year.
From FY2010 to FY2014, a total of 954 new grants (48%) were identified, compared with 1,037 new grants (52%) from FY2015 to FY2019. Based on the NIH iSearch visualization tool, topics common between both time periods included weight loss, weight gain, insulin resistance, African Americans, long term, skeletal muscle, breast cancer, and vitamin D (data not shown). Prevalent NHD areas that emerged from FY2015 to FY2019 included gut microbiota, food insecurity, AI/ANs, sodium intake, sugar-sweetened beverage consumption, and food allergies (data not shown).
DISCUSSION
This analysis explored trends for NIH NHD research from FY2010 to FY2019. To the authors’ knowledge, this analysis is unique as no prior NIH-wide portfolio analysis focused on NHDs. Over the past decade, there have been minimal changes in the number of new awards (i.e., Type 1 and Type 2) and in total funding for NHD research. Since FY2012, total NHD research funding remained stable, yet there was a 21% rise in the number of NHD awards. This pattern can be explained, in part, by a shift in funding mechanisms over this time period, with a considerable increase in the number of newly funded training grants such as increased K award funding. Comparatively, K awards typically have smaller budgets than other funding mechanisms (e.g., R01s, U grants). These increases in funding for trainees and early-stage investigators beginning in 2016 could be driven by the release of program announcements specific to stimulating mentored research scientist development awards (K01s) (e.g., PA-16–190). These findings signal the potential for increased NHD research in the coming years as early-stage investigators advance in their research careers. Fluctuations in cooperative agreement (U mechanism) funding during the past decade have been driven by specific program announcements such as RFA-DK-14–501. There have been fluctuations in R01 funding, particularly from FY2016 to FY2017, as well as variations in the other R mechanisms. These fluctuations are largely due to the changes in the health disparities category for FY2017. Overall, these findings suggest that NHD research may be sensitive to the release of program announcements and researchers are actively responsive to funding opportunities related to this research area. Although NIH funding increased between FY2010 and FY2019, it is not possible to definitively determine the precise factor in any given year that influence the number of relevant grants funded. The factors that influence the number of grants funded vary and include (but are not limited to) Institute and Center budgets and funding pay lines, funding opportunity announcements, Institute and Center priorities, national priorities, and overall NIH funding.
This analysis also highlights the types of NHD research funded within the last decade, which has largely involved human subjects (90%), including nearly a third focused on clinical trials among groups that experience health disparities (e.g., racial/ethnic groups, those of lower SES). Some research indicates that health disparities are mainly driven by SDH and structural factors affecting interpersonal, community, and society rather than genetic and biological factors alone,23 which explains the large percentage of human funded grants instead of animal-based studies. Also, foundational mechanistic studies examining the interplay between the various SDH and genetics may lead to a better understanding of the inter-relationships between these driving factors to address these disparities. Related are the increases in funded research on topics such as the gut microbiome, sodium intake, sugar-sweetened beverage consumption, and food allergies. Additionally, food insecurity and AI/ANs are additional opportunities for future research. AI/ANs are at increased risk for food insecurity and numerous diet-related diseases such as obesity, diabetes, and heart disease, warranting further research among this group.24 Although Hispanic/Latino and African American/Black populations experience diet-related disparities at disproportionally higher rates than other racial/ethnic groups, the limited number of studies targeting the Asian population suggest the potential for more research, particularly with the growing Asian population in the U.S. and the increased risk of type 2 diabetes among South Asians.25
Analyses of the research focus areas, based on the RCDC categories and keyword searches, revealed additional scientific gaps and areas opportunity for research in the areas of Rural Health and Maternal Health. Accelerating research to prevent disparities in maternal morbidity and mortality is also of particular interest at NIH and researchers are encouraged to utilize resources provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and NIH’s Office of Research on Women’s Health. Diet- and nutrition-related conditions related to pregnancy such as pre-eclampsia and gestational diabetes are worth further investigation. The National Heart, Lung, and Blood Institute–funded RURAL (Risk Underlying Rural Areas Longitudinal) Cohort Study, which will include dietary measures and several health-related outcomes, could also be leveraged by NHD researchers for future ancillary studies or secondary data analysis. Additionally, the NCI Cancer Control Research in Persistent Poverty Areas (U54) provides an opportunity to examine or modify diet behaviors and outcomes among populations (e.g., rural, racial/ethnic) living in persistent poverty census tracts in partnerships with local communities, community-based organizations, and primary/local clinics/hospitals. Overall, the findings from this analysis provide an informative overview of NIH funding trends in NHD research. This analysis outlines NIH’s continued commitment to advancing NHD extramural research over the last decade. The report may inform the design of future programmatic efforts to advance nutrition equity research. The increase in training grants also signals the rise in early-stage investigators’ interest in NHD research and an opportunity to further understand how to reduce NHDs and advance health equity. The launch of NIH’s UNITE Initiative with the goal to identify and address structural racism within the scientific community will provide an opportunity to stimulate NHD research. NIH is also currently supporting related Common Fund programs that could be leveraged to support NHD research, including Transformative Research to Address Health Disparities and Advance Health Equity and Nutrition for Precision Health powered by the All of Us Research Program. Institutions that are funded under the Faculty Institutional Recruitment for Sustainable Transformation (FIRST), which seeks to enhance and maintain cultures of inclusive excellence in the biomedical research community, could also support researchers interested in pursuing NHD research. The recent release of the SPNR and NIH’s UNITE Initiative provide a unique opportunity to nurture NHD research given the cross-cutting theme of minority health and health disparities and emphasis on addressing structural factors that drive disparities.22,26 As diet and nutrition play a key role in most chronic diseases and conditions, NHD research has great potential to further contribute to the reduction of chronic disease morbidity and mortality rates, and ultimately enhance population health.
Limitations
Although this analysis includes many strengths, the results must be interpreted in the context of several limitations. First, the study data sources have had a variety of categorical definition and methodological changes in data collection over the years; however, the search categories provided an overall interpretation of year-to-year comparisons and funding trends. Second, this analysis is targeting the external research community and therefore excludes intramural projects, Research and Development contracts, and certain types of grants (e.g., SC1, SC2 mechanisms). Finally, this study was not designed to evaluate the cause of differences in funding (e.g., appropriateness of applications, review process, and award policies). Future analyses could examine unfunded NHD applications and the success rates of these applications. Analyses of funded grants from other federal agencies could also identify the spectrum of gaps and opportunities in NHD research.
CONCLUSIONS
This characterization and analysis of the NIH NHD research portfolio from FY2010 to FY2019 highlights research gaps and potential opportunities to advance NHD research. The higher mortality rates of COVID-19 among racial/ethnic minorities, people with lower SES, the aging population, and individuals with chronic conditions could be related to the higher prevalence of nutrition-related comorbidities, less optimal or constrained food access, and poor food quality among these groups, and further underscores the timeliness of this research. In alignment with the SPNR’s cross-cutting theme and ongoing NIH efforts to address health disparities, health equity can be advanced through innovative research examining the influence of biological, behavioral, social, and structural factors on NHDs and developing effective targeted interventions to address these disparities.
ACKNOWLEDGMENTS
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; Office of Nutrition Research; National Institute of Environmental Health Sciences; National Institute on Minority Health and Health Disparities; National Cancer Institute; National Institute on Aging; NIH; or HHS. All authors were employees of NIH when they were actively engaged in work related to this paper. NIH is the sole source of support for the reported work. No financial disclosures were reported by the authors of this paper.
Footnotes
CRediT AUTHORS STATEMENT
• Alison G.M. Brown: Conceptualization, Writing-Original draft preparation, Visualization, Project administration, Formal analysis
• Scarlet Shi: Writing-Original draft preparation, Visualization, Data Curation, Formal analysis
• Samantha Adas: Conceptualization, Writing - Review & Editing, Data Curation
• Josephine E. A. Boyington: Conceptualization, Writing - Review & Editing, Data Curation
• Paul A. Cotton: Conceptualization, Writing - Review & Editing, Data Curation
• Bill Jirles: Conceptualization, Writing - Review & Editing, Data Curation
• Nishadi Rajapakse: Conceptualization, Writing - Review & Editing, Data Curation
• Jill Reedy: Conceptualization, Writing - Review & Editing, Data Curation
• Karen Regan: Conceptualization, Writing - Review & Editing, Data Curation, Formal analysis
• Dan Xi: Conceptualization, Writing - Review & Editing, Data Curation
• Giovanna Zappalà: Conceptualization, Writing - Review & Editing, Data Curation
• Tanya Agurs-Collins: Writing-Original draft preparation, Supervision, Data Curation
Josephine Boyington was staff at the NHLBI during the preparation of this manuscript but has since left NHLBI.
Giovanna Zappalà was staff at the NIA during the preparation of this manuscript but has since left NIA.
Type 1 (New Grant): initial request for support of a project that has not yet been funded.
Type 2 (Renewal Grant): initial request for additional funding for a period subsequent to that provided by a current award.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Satia JA. Diet-related disparities: understanding the problem and accelerating solutions. J Acad Nutr Diet. 2009;109(4):610–615. 10.1016/j.jada.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkpatrick SI, Dodd KW, Reedy J, Krebs-Smith SM. Income and race/ethnicity are associated with adherence to food-based dietary guidance among US adults and children. J Acad Nutr Diet. 2012;112(5):624–635.e6. 10.1016/j.jand.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Chen X. How much of racial/ethnic disparities in dietary intakes, exercise, and weight status can be explained by nutrition-and health-related psychosocial factors and socioeconomic status among US adults? J Acad Nutr Diet. 2011;111(12):1904–1911. 10.1016/j.jada.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mokdad AH, Ballestros K, Echko M, et al. The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319(14):1444–1472. 10.1001/jama.2018.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rehm CD, Peñalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999–2012. JAMA. 2016;315(23):2542–2553. 10.1001/jama.2016.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang FF, Liu J, Rehm CD, Wilde P, Mande JR, Mozaffarian D. Trends and disparities in diet quality among US adults by Supplemental Nutrition Assistance Program participation status. JAMA Netw Open. 2018;1(2):e180237. 10.1001/jamanetworkopen.2018.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micha R, Shulkin ML, Penalvo JL, et al. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PloS One. 2017;12(4):e0175149. 10.1371/journal.pone.0175149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang FF, Cudhea F, Shan Z, et al. Preventable cancer burden associated with poor diet in the United States. JNCI Cancer Spectr. 2019;3(2):pkz034. 10.1093/jncics/pkz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. 2017;317(9):912–924. 10.1001/jama.2017.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afshin A, Sur PJ, Fay KA, et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393(10184):1958–1972. 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson JG, Russomanno J, Tree JMJ. Sexual orientation disparities in food insecurity and food assistance use in US adult women: National Health and Nutrition Examination Survey, 2005–2014. BMC Public Health. 2020;20:1155. 10.1186/s12889-020-09261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National diabetes statistics report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, HHS; 2017. [Google Scholar]
- 13.Cross SH, Mehra MR, Bhatt DL, et al. Rural-urban differences in cardiovascular mortality in the US, 1999–2017. JAMA. 2020;323(18):1852–1854. 10.1001/jama.2020.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211–233. 10.3322/caac.21555. [DOI] [PubMed] [Google Scholar]
- 15.Howlader N, Noone A, Krapcho Me, et al. SEER cancer statistics review, 1975–2016. Bethesda, MD: National Cancer Institute; 2019. https://seer.cancer.gov/archive/csr/1975_2016/#citation. Accessed February 15, 2022. [Google Scholar]
- 16.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–1463. 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. A conceptual framework for action on the social determinants of health. https://www.who.int/publications/i/item/9789241500852. Published 2010. Accessed February 15, 2022.
- 18.Seligman HK, Berkowitz SA. Aligning programs and policies to support food security and public health goals in the United States. Annu Rev Public Health. 2019;40:319–337. 10.1146/annurev-publhealth-040218-044132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belanger MJ, Hill MA, Angelidi AM, Dalamaga M, Sowers JR, Mantzoros CS. Covid-19 and disparities in nutrition and obesity. N Engl J Med. 2020;383(11):e69. 10.1056/NEJMp2021264. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs L, Minnelli N, Larrivee J, Sahu KK, Siddiqui AD. Oncology dietitians sound alarm in key nutrition needs of cancer patients during COVID-19 pandemic. JCO Oncol Pract. 2020;16(10):621–623. 10.1200/OP.20.00349. [DOI] [PubMed] [Google Scholar]
- 21.NIH Nutrition Research Task Force. 2020–2030 Strategic Plan for NIH Nutrition Research. NIH; 2020. https://dpcpsi.nih.gov/sites/default/files/2020NutritionStrategicPlan_508.pdf. Accessed February 15, 2022. [Google Scholar]
- 22.Cockerham WC, Hamby BW, Oates GR. The social determinants of chronic disease. Am J Prev Med. 2017;52(1 suppl 1):S5–S12. 10.1016/j.amepre.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jernigan VBB, Huyser KR, Valdes J, Simonds VW. Food insecurity among American Indians and Alaska Natives: a national profile using the Current Population Survey-Food Security Supplement. J Hunger Environ Nutr. 2017;12(1):1–10. 10.1080/19320248.2016.1227750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah A, Kanaya AM. Diabetes and associated complications in the South Asian population. Curr Cardiol Rep. 2014;16(5):476. 10.1007/s11886-014-0476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodgers GP, Collins FS. Precision nutrition-the answer to “what to eat to stay healthy”. JAMA. 2020;324(8):735–736. 10.1001/jama.2020.13601. [DOI] [PubMed] [Google Scholar]