Dear Editor,
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 400 million people globally, resulting in millions of deaths.1 As the virus evolves, a new variant, designated as “Omicron” by the World Health Organization (SARS-CoV-2 variant B.1.1.529), has led to a massive resurgence of COVID-19 cases in many regions.2 The Omicron variant is highly contagious, with a significant transmission advantage compared to prior variants. Currently, Omicron represents ~95% of the new infections in the US, Europe, Brazil, South Africa, and elsewhere.3–5
The remarkable transmissibility of Omicron is likely attributable to its highly mutated spike (S) gene (more than 30 mutations), which promotes S protein binding to the cellular receptor angiotensin-converting enzyme 2 (ACE2) and allows it to escape from both humoral and cellular immunity elicited by prior infection or vaccination.6,7 Several studies show that the Omicron variant significantly affects the protection conferred by previous vaccinations including mRNA vaccines of mRNA-1273 (Moderna) and BNT162b2 (Pfizer BioNTech), inactivated virus vaccine of CoronaVac (Sinovac Life Sciences), viral vector vaccine of Vaxzevria (AstraZeneca), and protein subunit vaccine of ZF2001 (Anhui Zhifei Longcom).8–11 Although a Pfizer booster shot could enhance the titers of neutralizing antibodies (nAbs) in the vaccinees’ sera, the potency of nAbs in neutralizing the Omicron variant was still 20-fold less than that in neutralizing other variants.11 In addition, a recent phase 4 clinical trial in Brazil indicates that about 65% of individuals immunized with three doses of CoronaVac COVID-19 vaccine have very low level of nAbs against Omicron, with some of them barely above the threshold of seropositivity.12
Thus, updated vaccines that can effectively combat the Omicron infection are urgently needed. Here, we discuss our development of an Omicron-specific mRNA vaccine (named SOmicron-6P) based on the variant’s full-length S protein sequence. Specifically, the vaccine was designed with the “hexapro” S protein sequence as the backbone for prefusion conformation stability. In addition, the respective sequences with the Omicron mutations enhance the immune response specificity (Supplementary information, Table S1).13 A robust expression of the Omicron S protein on HEK293T cell surface was detected after transfection (Fig. 1a). Subsequently, high-purity Omicron mRNA was encapsulated into lipid nanoparticles (LNP) with a well-controlled size of 110 nm (Supplementary information, Fig. S1).
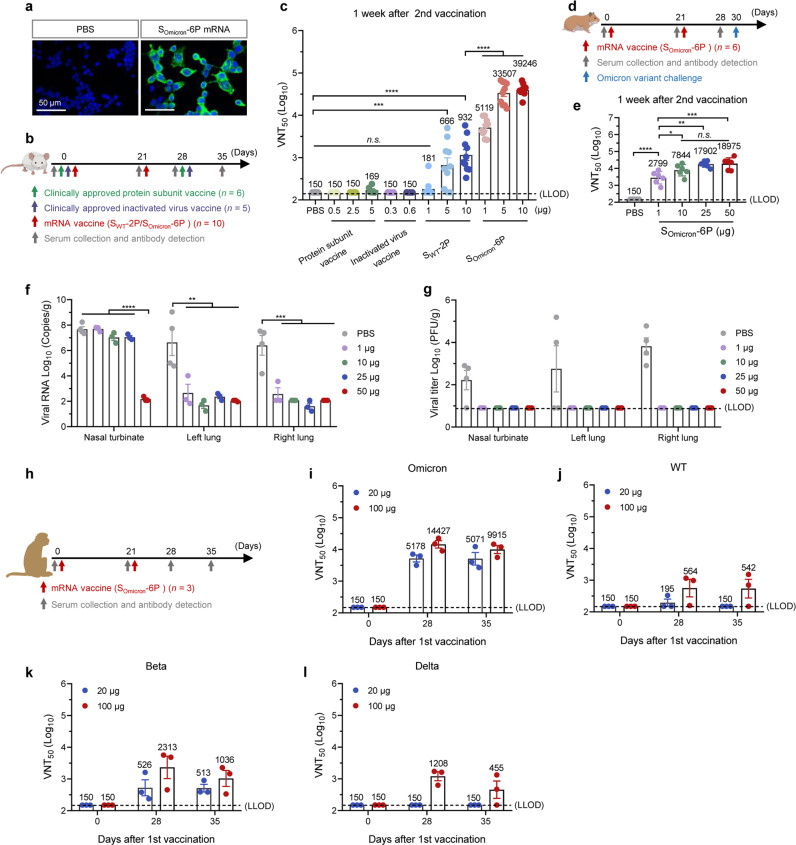
Fig. 1. SOmicron-6P vaccination elicits strong protection against SARS-CoV-2 Omicron in different animal models.
a Immunofluorescence analysis of the expression of Omicron S protein in HEK293T cells. b Schematic diagram of immunization and sample collection schedule in mice. Female BALB/c mice were intramuscularly (i.m.) immunized on a two-dose schedule with SWT-2P (1, 5, or 10 μg of mRNA, n = 10), SOmicron-6P (1, 5, or 10 μg of mRNA, n = 10), protein subunit vaccine using a dimeric form of the receptor-binding domain of WT SARS-CoV-2 (0.5, 2.5, or 5 μg of RBD-dimer protein, n = 6), or inactivated vaccine of WT SARS-CoV-2 (0.3 or 0.6 μg of SARS-CoV-2 virion, n = 5). c SARS-CoV-2 Omicron 50% virus-neutralization titers (VNT50) were determined by a plaque reduction neutralization test (lower limit of detection (LLOD) = 150) (n = 5–10). d Study design. Female hamsters were prime-vaccinated via the i.m. route on day 0 and boosted on day 21, with 0, 1, 10, 25, or 50 μg of SOmicron-6P (n = 6). On day 30 after the initial immunization, hamsters were intranasally challenged with 1 × 104 PFU of SARS-CoV-2 Omicron. On day 4 after infection, hamsters were euthanized for tissue collection. e VNT50 values were determined by a plaque reduction neutralization test (LLOD = 150) (n = 6). f Viral RNA load in both lungs, and nasal turbinates were determined by qRT-PCR (n = 3–4). g Viral load expressed in PFU per gram of tissue in both lungs, and nasal turbinates at 4 days postinfection (n = 3–4). h Study design. Male macaques were i.m. immunized with 20 or 100 μg SOmicron-6P (n = 3) and boosted with the same dose at a 21-day interval. i–l VNT50 against SARS-CoV-2 Omicron (i), WT (j), Beta (k), and Delta (l) that were determined by a plaque reduction neutralization test (LLOD = 150) (n = 3). Data are shown as means ± SEM. Significance was calculated using one-way ANOVA with multiple comparisons tests (n.s. not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
To study the efficacy of the Omicron-specific mRNA vaccine, three vaccines which were generated to target the WT SARS-CoV-2 strain were used as controls: a clinically approved protein subunit vaccine (ZF2001, provided by Anhui Zhifei Longcom), a clinically approved inactivated virus vaccine (CoronaVac, provided by Sinovac Life Sciences), and an mRNA vaccine (SWT-2P) which has the same sequence as BNT162b2 RNA with only two proline substitutions. SOmicron-6P was developed based on full-length S protein with six proline substitutions, which could have enhanced prefusion conformation stability and higher expression yield.13 First, BALB/c mice were vaccinated twice at various doses of mRNA vaccines (at a 21-day interval), inactivated virus vaccine or protein subunit vaccine (at a 28-day interval), following the instruction for use of the vaccines (Fig. 1b). Mouse sera were collected one week after the second vaccination. The anti-Omicron humoral responses elicited by different vaccines were determined by measuring S-specific IgG geometric mean titers (GMTs) with ELISA (Supplementary information, Fig. S2). Both SOmicron-6P and SWT-2P elicited IgG antibodies in a dose-dependent manner. At 5 µg and 10 µg dose levels, SOmicron-6P induced significantly higher IgG than SWT-2P, by 1.8-and 2.3-fold, respectively. Meanwhile, a pseudovirus neutralization assay was applied to study the capabilities of antibodies in serum to block the viral entry into the host cells. The entry inhibition by serum of immunized mice was measured in a neutralization assay using vesicular stomatitis virus (VSV)-based Omicron pseudovirus. Dramatically but not surprisingly, the SOmicron-6P-vaccinated mice elicited 14.4–27.8-fold higher serum neutralizing activity than those vaccinated with SWT-2P (Supplementary information, Fig. S3). We also investigated the abilities of sera to neutralize authentic Omicron virus by a plaque reduction neutralization test, which reflects the protective efficacies of different vaccines. As expected, 28.3–50.3-fold higher neutralizing titers were observed from mice immunized with SOmicron-6P than those immunized with SWT-2P. In addition, two doses of immunization using inactivated virus vaccine or protein subunit vaccine hardly induced any Omicron nAbs in mice (Fig. 1c). These results suggest that SOmicron-6P is more potent in inducing Omicron-specific antibodies.
The protective efficacy of SOmicron-6P was then tested in Syrian hamster, a suitable animal model for SARS-CoV-2 infection14 due to its superiority over protein subunit vaccine, inactivated virus vaccine, and SWT-2P in Omicron neutralizing activity. Five groups of hamsters were vaccinated on day 0 and day 21 with either 1, 10, 25, or 50 µg of SOmicron-6P or PBS. The hamster sera were evaluated for vaccine immunogenicity one week after the second dose (Fig. 1d). A significant amount of IgG antibodies against Omicron S protein was detected on day 14 and 21 after the first immunization, but no apparent dose-dependency was observed. However, the second dose boosts S antibodies over 10 times one week later (on day 28) (Supplementary information, Fig. S4). The pseudovirus neutralization assay showed that high neutralizing antibody titers were elicited even by 1 µg dose of SOmicron-6P (Supplementary information, Fig. S5). In line with this, we observed high levels of neutralizing activity against authentic Omicron in SOmicron-6P-vaccinated animals (Fig. 1e). Moreover, we observed a strong correlation between the pseudovirus neutralization assay and the authentic Omicron neutralization assay, with a correlation co-efficiency of 0.91 (Supplementary information, Fig. S6). On day 30, some hamsters (n = 3–4 per group) were challenged with 1 × 104 plaque-forming units (PFU) of authentic Omicron virus intranasally. Body weight changes were measured during the challenge study (Supplementary information, Fig. S7). The results indicate that Omicron infection caused slight weight loss for the PBS group on day 4. On the contrary, the hamsters immunized with SOmicron-6P showed weight increase due to the low burden of Omicron infection. The hamsters were then sacrificed 4 days postinfection; their lungs and nasal turbinates were analyzed for viral RNA loads and infectious virus titers, and lung sections were histologically evaluated by hematoxylin and eosin (H&E) staining. As shown in Fig. 1f, only a trace amount of viral RNA was detected in the lung tissue of vaccinated animals, with a little more for the 1 μg group, which is a 4–5 magnitude reduction compared to the control group. No infectious virus was detected in both lungs, or nasal turbinates of all vaccinated animals, including the lowest dose group, but marked virus levels were detected in the PBS group (Fig. 1g). The lungs of hamsters were histologically evaluated 4 days after the challenge (Supplementary information, Fig. S8). Control hamsters displayed lung pathology, with immune cell infiltration, airway space thickening, mild alveolar congestion, and interstitial edema. However, animals immunized with SOmicron-6P did not develop lung pathology. These data demonstrate that SOmicron-6P provides robust protection against Omicron infection.
The immunogenicity of SOmicron-6P was also evaluated in non-human primates. Macaques (n = 3 per group) were immunized with 20 µg or 100 µg of SOmicron-6P twice at a 21-day interval (Fig. 1h). Similar to the results in mice and hamsters, two-dose of SOmicron-6P elicited strong humoral responses in macaques and induced high levels of binding antibodies and nAbs against Omicron on day 35 (Fig. 1i; Supplementary information, Figs. S9 and S10). We also tested whether the nAbs elicited by SOmicron-6P could provide cross-protection against wild-type (WT) SARS-CoV-2 and other SARS-CoV-2 variants. SOmicron-6P-vaccinated macaques produced high levels of nAbs against not only the Omicron variants but also against the WT, Beta, or Delta variants (Fig. 1j–l). Given that Omicron currently accounts for most of the new infections, the Omicron-specific mRNA vaccine with high protective efficacy is of great value.
Encouraged by the excellent performance of SOmicron-6P in different naïve animal models, we conducted a study to explore whether boosting WT mRNA vaccines with this Omicron-specific vaccine will increase the protective immune response against the Omicron variant (Supplementary information, Fig. S11). Two-dose SWT-2P-immunized mice were further administrated with SWT-2P or SOmicron-6P as a booster shot after 137 days. We found that the heterologous SOmicron-6P booster elicits 1.7-fold higher IgG and 3.3–6.4-fold higher serum neutralizing activity against Omicron than the homologous SWT-2P booster. These results indicated that SOmicron-6P as a booster shot to WT mRNA vaccine could significantly increase the immune responses against Omicron.
In this study, we generated an Omicron-specific mRNA vaccine and provided a comprehensive analysis of its efficacy using different animal models. Our data showed that SOmicron-6P mRNA vaccine could induce high titers of nAbs against Omicron and protect animals from Omicron infection. Compared to the prior vaccines designed based on WT SARS-CoV-2 (including CoronaVac, ZF2001 and mRNA vaccines), the Omicron-specific mRNA vaccine demonstrated superiority in protection against Omicron infection. In addition, compared to homologous boosting, heterologous boosting with SOmicron-6P following initial vaccination with WT mRNA vaccines elicited a more robust protective immune response against the Omicron variant. However, there are some limitations to the current study. First, a considerable fraction of the world population has been vaccinated with protein subunit vaccine (e.g., ZF2001), inactivated virus vaccine (e.g., CoronaVac), or recombinant adenovirus-vectored COVID-19 vaccine (e.g., Ad5-nCoV), and it is still unclear whether boosting these prior vaccinations using SOmicron-6P will be more immunogenic than other vaccines. Second, this Omicron-specific vaccine elicits considerable cross-protection against Beta variants but lower protection against WT and Delta variants. The low cross-protection potency of the Omicron-specific vaccine highlights the necessity of developing a multivalent or pan-coronavirus vaccine to fight against the evolution of SARS-CoV-2.
Supplementary information
Acknowledgements
This work was supported by the National Key R&D Program of China (2020YFA0710700), the National Natural Science Foundation of China (52025036, 51961145109), the Fundamental Research Fund for the Central Universities (WK9100000014, WK2480000006, and WK9110000061), and the project of collaborative innovation for colleges of Anhui province (GXXT-2021-070). This work was partially carried out at the USTC Center for Micro and Nanoscale Research and Fabrication. We thank Jia Wu, Jun Liu and Hao Tang from Wuhan Institute of Virology for managing of BSL-3 facility, where all the authentic SARS-CoV-2 experiments were conducted. We also thank National Virus Resource Center for providing the Omicron variant (CCPM-B-V-049-2112-18). We thank Weiheng Chen from the Animal Facility of USTC, where the mice, hamsters and macaques were vaccinated. We thank the Joint Laboratory of Innovation in Life Sciences University of Science and Technology of China (USTC) and Changchun Zhuoyi Biological Co. Ltd.
Author contributions
C.W., N.-N.X., Y.-C.W., and S.C. supervised the project. Yi W., Y.-Q.S., N.-M.W., Y.-C.W., and S.C. conceived the experiments. Yi W., Y.-Q.S., N.-M.W., X.-H.Z., S.-H.C., C.Y., J.-H.Z., Yan W., D.C., L.W. conducted the experiments and analyzed the data. Yi W., Y.-Q.S., N.-M.W., N.-N.X., and Y.-C.W. wrote and revised the manuscript. C.W., H.-J.Z., and S.C. revised the manuscript. All authors read and approved the manuscript.
Competing interests
N.-N.X., Y.-C.W. are co-inventors on pending patent applications related to the Omicron mRNA vaccine. The other authors declare no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
These authors contributed equally: Yi Wu, Yanqiong Shen, Namei Wu, Xinghai Zhang.
Contributor Information
Huajun Zhang, Email: hjzhang@wh.iov.cn.
Ninuo Xia, Email: nxia007@ustc.edu.cn.
Sandra Chiu, Email: qiux@ustc.edu.cn.
Yucai Wang, Email: yucaiwang@ustc.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41422-022-00706-x.
References
- 1.Koh HK, et al. JAMA. 2021;325:133–134. doi: 10.1001/jama.2020.25381. [DOI] [PubMed] [Google Scholar]
- 2.Karim SSA, et al. Lancet. 2021;398:2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki R, et al. Nature. 2022;603:700–705. doi: 10.1038/s41586-022-04462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, et al. medRxiv. 2022 doi: 10.1101/2022.02.21.22271300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Accorsi EK, et al. JAMA. 2022;327:639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, et al. Signal Transduct. Target. Ther. 2022;7:118. doi: 10.1038/s41392-022-00965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y, et al. Nature. 2021;602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flemming A. Nat. Rev. Immunol. 2022;22:75. doi: 10.1038/s41577-022-00676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cele S, et al. Nature. 2022;602:654–656. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J, et al. Cell Mol. Immunol. 2022;19:293–295. doi: 10.1038/s41423-021-00836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Planas D, et al. Nature. 2022;602:671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 12.Clemens SAC, et al. Lancet. 2022;399:521–529. doi: 10.1016/S0140-6736(22)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh CL, et al. Science. 2020;369:1501–1505. doi: 10.1126/science.abd0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munoz-Fontela C, et al. Nature. 2020;586:509–515. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.