Abstract
Purpose of Review
Myocarditis is a disease caused by inflammation of the heart that can progress to dilated cardiomyopathy, heart failure, and eventually death in many patients. Several etiologies are implicated in the development of myocarditis including autoimmune, drug-induced, infectious, and others. All causes lead to inflammation which causes damage to the myocardium followed by remodeling and fibrosis. This review aims to summarize recent findings in biomarkers for myocarditis and highlight the most promising candidates.
Recent Findings
Current methods of diagnosing myocarditis, including imaging and endomyocardial biopsy, are invasive, expensive, and often not done early enough to affect progression. Research is being done to find biomarkers of myocarditis that are cost-effective, accurate, and prognostically informative. These biomarkers would allow for earlier screening for myocarditis, as well as earlier treatment, and a better understanding of the disease course for specific patients.
Summary
Early diagnosis of myocarditis with biomarkers may allow for prompt treatment to improve outcomes in patients.
Keywords: Myocarditis, Inflammatory cardiomyopathy, Biomarkers, Cardio-immunology
Introduction
Myocarditis is defined as inflammation of the myocardium that can progress to inflammatory cardiomyopathy when there are associated cardiac remodeling and dysfunction due to chronic inflammation [1]. Myocarditis can present with a variety of different symptoms and often mimic other common cardiac diseases. As such, it is often difficult to diagnose myocarditis based on clinical symptoms. However, it is very important to make the diagnosis as early as possible as treatment is vastly different and can greatly improve outcomes and prevent disease progression to dilated cardiomyopathy (DCM) or heart failure (HF). Myocarditis is most commonly associated with viral etiologies but can also be caused by other types of microorganisms (bacteria, fungi, protozoa), by systemic immune autoimmune diseases, and by drugs and other substances [2•]. Often, myocarditis is characterized by pathogens or toxins as etiology, as well as by clinical course, which includes the acute myocarditis phase (within 1 month) and the chronic inflammatory cardiomyopathy phase (past 1 month) [1]. Chronic inflammatory cardiomyopathy is a progression of acute myocarditis that includes DCM or some other type of established dysfunction of the myocardium that can lead to HF as the final manifestation of the disease process due to persistent inflammation. However, these stage and subtypes are often ill-defined at the bedside. Mechanistic studies have identified important immune cells and cytokines that are involved with inflammation in myocarditis, with contributions from the innate immune system during the acute phase and from the acquired immune system during the chronic phase [2•, 3]. Several autoantibodies that target different epitopes present on the heart have also been identified as drivers of disease [3].
The clinical spectrum of myocarditis has impacted our understanding of the epidemiology of this condition. The estimated prevalence of myocarditis is estimated to be between 22 per 100,000 people worldwide and has largely been restricted to the subjective clinical determination [4]. Biopsy-proven myocarditis has been shown to be associated with poor prognosis in at least 30% of patients and is one of the most common causes of sudden cardiac death in younger age groups [4, 5]. Recent animal and human data have suggested that men with myocarditis have worse recovery and a greater need for transplantation compared with women, but the reason for the sex difference remains unclear. The true incidence may be higher in part because patients may present in a wide range of clinical manifestations beyond the typical signs and symptoms. Therefore, biomarkers may provide an important insight for diagnostic and therapeutic decision-making. A lot of investigative work has been conducted in the past few decades to identify novel biomarkers of acute myocarditis that may satisfy the following criteria: (1) have good sensitivity and specificity, (2) cost-effective to obtain, (3) ability to determine prognosis, (4) identify phase and etiology specific factors, and finally (5) inform treatment options. In this review, we will highlight several important etiologies, followed by broad categories of biomarkers discussing the up-to-date findings and their implications for contemporary clinical practice.
Current Diagnostic Criteria for Acute Myocarditis
Current clinical definitions for the diagnosis of myocarditis begin with assessment of clinical symptoms, which can include chest pain, dyspnea, fatigue, palpitations, and syncope. Next steps include obtaining an electrocardiogram, which is abnormal yet non-specific in around 85% of cases [1], laboratory tests including complete blood count with differential for clues of leukocyte activation subtypes (e.g., high eosinophilic count), high-sensitivity cardiac troponin assays, creatine kinase-MB, creatine kinase-MM (in the case of myositis associated inflammation), non-specific inflammatory biomarkers such as C reactive protein or erythrocyte sedimentation rate, and peripheral blood serologic/virologic testing in the case that a certain etiology is suspected (albeit not specific). Imaging recommendations include echocardiography, which is used prognostically, and cardiac magnetic resonance imaging, which is used both diagnostically and prognostically, with evolving diagnostic criteria. Using the revised 2018 Lake Louis criteria, cardiac magnetic resonance imaging can document myocarditis with a high sensitivity and specificity if myocardial edema and inflammatory injury are documented both on a T1 and a T2-based criteria. Finally, the gold standard, and most invasive, test is the endomyocardial biopsy, which can be studied for evidence of inflammation by visual inspection of leukocyte infiltration (so-called the Dallas criteria, which traditionally represents the definitive diagnosis of acute myocarditis) and subtyping with immunohistochemistry [1]. Furthermore, endomyocardial biopsy may be subject to sampling bias/error and is invasive. With the exception of endomyocardial biopsy (EMB), these existing diagnostic tests for acute myocarditis are not very sensitivity nor specific and are not able to discriminate between different etiologies nor between the different phases of myocarditis. While EMB and MRI are able to determine prognosis to a certain extent, there is no easily measured biomarker that is able to do so with similar accuracy and low cost.
Specific Etiologies for Myocarditis
Rheumatic or Autoimmune Myocarditis
Autoimmune myocarditis (often considered as cardiac involvement of systemic autoimmune inflammatory disease) is a broad category of myocarditis in which there is an inflammatory response versus self-antigens in the heart [6]. Autoimmune myocarditis can exist either as an independent disease where the primary (or only) organ targeted by the immune system is the heart (as is the case with giant cell myocarditis (GCM) and eosinophilic myocarditis), or it can exist as a sequela of a systemic autoimmune condition such as systemic lupus erythematosus (SLE), Sjӧgren’s syndrome, systemic sclerosis, or certain types of vasculitis. Vaccine-induced myocarditis has also been considered as a form of immune reaction to components of vaccines. Meanwhile, other drug-induced myocarditis, especially recently with the broad use of immune checkpoint inhibitors that augment’s T-cell responses to tumor cells, can cause rare but aggressive forms of myocardial inflammation shortly after their administration. Systemic autoimmune conditions are mostly associated with lymphocytic myocarditis (LM) except for eosinophil involvement in eosinophilic granulomatosis polyarthritis (EGPA), and in aggressive forms with the presence of macrophages. Besides standard diagnostic testing as mentioned above, workup for suspected rheumatic myocarditis may include rheumatologic workup for systemic autoimmune disease to identify potential specific autoimmune etiologies [7].
Giant Cell Myocarditis
GCM is a specific subtype of myocarditis that can either occur due to an autoimmune disorder, from some drug therapy such as immune checkpoint inhibitor (ICI) therapy, or even in otherwise healthy individuals without any clear risk factors. GCM is driven by infiltration of the myocardium by T lymphocytes and macrophages, where they are stimulated to produce several inflammatory cytokines including nitric oxide and tumor necrosis factor (TNF) that are implicated in the pathogenesis of cardiac dysfunction. Studies have demonstrated specific pathological findings including dislocation of plakoglobin, a desmosomal protein, along with increased expression of interleukin-17 (IL-17) and TNF-alpha that can differentiate it from other types of non-inflammatory DCM as well as LM [8]. Currently the gold standard diagnostic tests for GCM are endomyocardial biopsy, especially in rapidly progressive heart failure/cardiac dysfunction or significant ventricular tachyarrhythmia that may lead to cardiogenic shock. Recent studies have demonstrated the utility of certain biomarkers including high sensitivity C-reactive protein (CRP), creatine kinase-MB (CK-MB), and alanine aminotransferase (ALT) for differentiating patients with fulminant GCM vs LM, as well as predicting survival. Specifically, high sensitivity CRP was found to be an independent prognostic factor for fulminant myocarditis patients and could potentially be used to differentiate between GCM and LM [9].
Infectious Myocarditis
Infectious pathogens are a significant cause of myocarditis, especially in younger patients. For the most part, infectious myocarditis is caused by viral pathogens but in some cases can also be due to bacterial infection causing an inflammatory response. The most common causes of infectious myocarditis include coxsackievirus, parvovirus, adenovirus, Epstein-Barr virus (EBV), cytomegalovirus (CMV), human immunodeficiency virus (HIV), herpesvirus, coronavirus (e.g., SARS-CoV2), as well as several bacteria including Borrelia burgdorferi (Lyme disease), and Trypanosoma cruzi (Chagas disease) [4]. The pathogenesis, as well as severity of the disease, is dependent on what the causal pathogen is, and this can also guide therapy. In the case that a viral etiology is suspected, several tests including viral culture of peripheral specimens (stool or urine), viral antibody titers, and viral serology have been used, with minimal success for varying reasons, to identify the specific pathogen. The two methods that have the most sensitivity and specificity are immunohistochemistry and viral genome analysis of the myocardium by PCR [10].
Chagas disease is a particularly severe etiology of myocarditis. It affects approximately 6–7 million people around the world, of which 30% will eventually progress to chagasic myocarditis or cardiomyopathy. Currently there is no cure for the disease, and treatment is mainly supportive. Recent work has sought to gain further understanding into the molecular mechanisms of disease and find useful biomarkers to predict progression as well potential targets for therapeutics. Important cytokines implicated in pathogenesis include TGFβ, connective tissue growth factor, endothelin-1, and platelet-derived growth factor D (PDGF-D). In addition, connective tissue growth factor (CTGF) and PDGF-D have been implicated in the fibrosis process that is a pathognomonic lesion in Chagas cardiomyopathy [11].
Biomarkers of Myocarditis
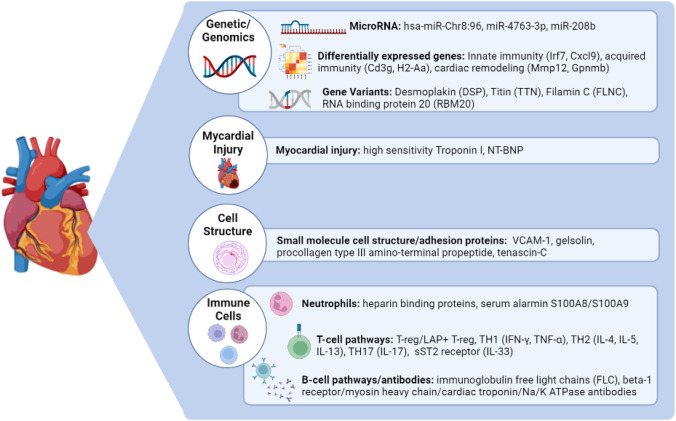
Figure 1 summarizes the broad categories of biomarkers of myocarditis, and Table 1 outlined novel biomarkers that have been investigated for assessing diagnosis and prognosis of myocarditis.
Fig. 1.
Biomarkers for myocarditis. Graphics created with BioRender.com
Table 1.
Summary of biomarkers for myocarditis
| Biomarker | Sample | Type of biomarker | Metrics/findings | Diagnosis vs. prognosis vs. mechanistic |
|---|---|---|---|---|
| Hsa-miR-Chr8:96/mmu-miR-721 |
5 cohorts Total: 321 patients |
MicroRNA | AUC = 0.927 | Diagnostic |
| miR-4763-3p | 20 patients | MicroRNA | AUC = 0.85 | Diagnostic |
| miR-208b | 8 patients | MicroRNA | Prognostic | |
| Heparin binding protein | 435 HBP genes | Protein | Aggregation coefficient for HBP interaction network in myocarditis = 0.631 | Mechanistic |
| Immunoglobulin free light chain | 111 patients | Protein | AUC = 0.87 | Diagnostic |
| Serum alarmin S100A8/S100A9 | 227 patients | Protein |
Sensitivity = 90.6% Specificity = 92% PPV = 93.5 NPV = 88.5 AUC = 0.949 |
Diagnostic |
| LAP( +) Treg | 15 mice | Immune cell | Diagnostic | |
| Galectin-3 | 115 patients | Lectin |
Correlation between Galectin-3 expression and inflammatory cell count r = 0.56 and − 0.59 in DCM and inflammatory cardiomyopathy respectively Correlation between Galectin and cardiac fibrosis: r = 0.63 in DCM |
Diagnostic, prognostic |
| Soluble VCAM-1 | Protein | Significant increase in expression in EAM model, but not correlated with infiltration of leukocytes | Diagnostic, mechanistic | |
| Gelsolin | 61 patients | Protein | Significantly lower in children with acute rheumatic carditis p = 0.039. Correlation between gelsolin levels and grade of mitral regurgitation (p = 0.03), LVED diameter (p = 0.017), and LVES diameter (p = 0.028) | Diagnostic, prognostic |
| Procollagen type III | 39 patients | Protein | Positive correlation with LV dilation, LVED diameter index (p < 0.0001), and LVED diameter z-score (p = 0.0003). Negative correlation with shortening fraction changes over time (p = 0.001) | Diagnostic, prognostic |
| High-sensitivity cardiac Troponin I |
Study 1: 214 patients on ICIs Study 2: 83 patients with fulminant myocarditis in the hospital |
Protein |
Study 1: PPV = 12.5% for threshold of 55 ng/L NNT = 72 Study 2: Absolute and relative changes within 24 and 48 h were predictive of mortality since survivors had a significant decline in levels p = 0.003 |
Diagnostic, prognostic |
Genetic Biomarkers
One category of biomarkers that a growing number of groups have been studying include genetic and genomic biomarkers including microRNA (miRNA) and other types of non-coding RNA molecules found in the blood. With the advent of efficient and affordable genomic sequencing, it has become easier than ever to perform sequencing on patients relatively quickly to examine their sequencing profile. One group recently discovered a miRNA that has been shown to be a promising biomarker in both mouse models of the disease, and in several human cohorts of patients [12•]. The mouse miRNA mmu-miR-721, which is created by Th17 cells, was found to be highly expressed in both experimental autoimmune myocarditis and coxsackievirus-induced myocarditis in mouse models of both diseases, while simultaneously not being expressed in mice with myocardial infarction. Th17 cells have been shown to be involved in the inflammatory process as well as myocardial remodeling in myocarditis and DCM [13], indicating the biological connection of this miRNA to myocarditis. Its utility as a biomarker for myocarditis is also strengthened by its specificity for myocarditis versus myocardial damage caused by myocardial infarction. Furthermore, the human analogue for this miRNA, hsa-miR-Chr8:96 was found to be elevated in four independent cohorts of patients with myocarditis. Analyses found a Receiver Operative Characteristic curve of 0.927 for distinguishing acute myocarditis versus myocardial infarction using this miRNA. Another group found upregulation of the human miRNA miR-4763-3p in adult fulminant myocarditis, without upregulation in myocardial infarction [14]. Pathway analysis revealed that this miRNA targets, and silences, genes involved in inflammatory and cardiac injury response pathways. In addition, miRNA enrichment was found to be negatively correlated with the severity of fulminant myocarditis, indicating its utility in predicting severity and prognosis of disease, and even as a protective factor against inflammation. Moreover, this biomarker showed high sensitivity and specificity for fulminant myocarditis. In another study, miR-208 was found to be elevated in the acute phase of viral myocarditis in pediatric patients [15]. Additionally, this group found the cardiac associated miR-208b levels during the subacute phase of viral myocarditis to be correlated with systolic left ventricular function recovery during the chronic phase. While more study on this class of biomarkers is required, they are promising as sensitive and specific biomarkers of myocarditis that allow for more accurate diagnosis and prognosis. Furthermore, as microRNA molecules function by silencing gene expression, their utility as biomarkers could not only be for diagnosing and predictive prognosis, but also for novel gene therapies.
Myocarditis is a progressive disease and is split into several phases, as described before, based on pathogenesis and specific features. Studying the temporal characteristics myocarditis can also shed light onto specific pathophysiological processes that drive each stage of disease and may reveal biomarkers that can help determine which stage a patient might be in, and whether they will progress. Recent work looking at a murine model of myocarditis found representative genes at several time-points after initial diagnosis. The early phases were represented by genes involved in innate immunity, including Irf7 and Cxcl9, while later phases were represented by genes implicated in acquired immunity as well as cardiac remodeling, including Cd3g, H2-Aa, Mmp12, and Gpnmb [16].
Several recent genomic studies have identified specific variants in patients with several forms of myocarditis, indicating that some etiologies of myocarditis could be monogenic in nature. Consequently, these variants can be used as diagnostic biomarkers of these specific subtypes of myocarditis. The most commonly identified genes include desmoplakin (DSP), titin (TTN), filamin C (FLNC), and RNA binding protein 20 (RBM20) [17–19, 20•].
Biomarkers of Inflammation and Immunity
Inflammatory pathway dysregulation is the pathophysiological process that drives myocarditis and is mediated by immune cells including macrophages, T lymphocytes, eosinophils, and others that are resident in the heart [21]. The specific pathways and immune cell types involved are unique to myocarditis and have even been shown to be unique to different etiologies of myocarditis [2•, 22]. These unique pathways can be utilized as diagnostic criteria to identify patients with myocarditis based on upregulated inflammatory cytokines, or expression of certain types of immune cells. Neutrophils are important mediators of the innate inflammatory response, and one of the first lines of defense against pathogens. They play a significant role in the inflammation seen in myocarditis by combating pathogens themselves, as well as maintaining inflammation by releasing cytokines, which serve to recruit and activate other immune cells to the myocardium. Heparin binding protein (HBP) is expressed by neutrophils and mediates endothelial rearrangement which allows for leukocyte extravasation from the blood to the site of infection [23]. It also serves to activate other types of immune cells including monocytes and macrophages and amplifies the inflammatory response. Several different HBPs were found to be enriched in myocarditis, myocardial infarction, atherosclerosis, as well as myocardial ischemia, indicating a common link of myocardial inflammation among these diseases [23]. Although not specific for myocarditis, HBPs could be biomarkers for myocardial inflammation and, used in conjunction with other diagnostic tests, can be used to rule in/out myocarditis. Serum alarmin S100A8 and S100A9, proinflammatory molecules also released by neutrophils as well as monocytes, play a role in promoting myocardial inflammation [24]. Serum alarmin levels are elevated in patients with recent onset myocarditis who are still in the acute phase of the disease and found to be a both sensitive and specific biomarker for acute inflammation of the myocardium [25]. Moreover, serum alarmin levels are only slightly elevated in patients in the chronic phase of myocarditis as well as in heart failure patients without inflammation.
Lymphocytes are the key inflammatory cell type that play a prominent pathophysiologic role and likely a primary driver of immune dysregulation in acute myocarditis. T lymphocytes are also an important cell type, as they are responsible for cell-mediated immunity, which causes significant damage to the myocardium due to inflammation. They have been shown to be the primary mediators of inflammation in autoimmune myocarditis [2•, 26]. Several different subsets of T lymphocytes have been implicated in myocarditis including CD4 + , CD8 + , TH17, and Treg cells. TH17 cells have been shown to promote the transition from myocarditis to dilated cardiomyopathy [13, 27, 28], while Treg cells are protective against inflammation and are reduced in patients with myocarditis [13, 29, 30]. While Treg cells are generally involved in myocarditis, those that express latency-associated peptide (LAP), also known as TGF-beta-1, have a greater immunomodulatory effect than normal Treg cells. LAP + Treg cells are also a more stable biomarker for myocarditis with better sensitivity [31]. In some cases of myocarditis, there is a predominance of certain types of CD4 + cells, with TH1 cells producing IFN-gamma and TNF, and TH2 cells producing IL-4, IL-5, and IL-13, or with TH17 cells producing IL-17 [32]. Additionally, even within T cell types, imbalances of expression towards certain types (e.g., TH17 > T-reg and TH2 > TH1) have been associated with progression to a chronic inflammatory phenotype of cardiomyopathy [13, 33, 34]. In contrast, B lymphocytes are the primary mediators of humoral immunity as a part of the adaptive immune response. B lymphocytes, when activated, are responsible for the production of immunoglobulins, which is the primary mechanism by which the adaptive immune system works. In the case of myocarditis, especially autoimmune myocarditis, auto-antibodies are produced against various components of the myocardium including β1-adrenergic receptors, myosin heavy chain, cardiac troponin, Na/K ATPase, and other cardiac proteins, which are implicated in prolonged inflammation and damage to the myocardium [35]. An important activator of B lymphocytes is the NF-kB family of transcription factors, which bind the enhancer of the immunoglobulin lambda light chain gene and promote immunoglobulin lambda light chain production. Elevations of certain types of immunoglobulin light chains could be a biomarker of NF-kB signaling and B lymphocyte activation. Specific patterns of immunoglobulin free light chains (FLC) were observed in patients with heart failure with myocarditis including a significantly lower FLC kappa/lambda ratio. This ratio is an independent prognostic factor for overall survival and showed good diagnostic capability in patient cohorts [36].
Monocytes and macrophages are also important effector cells in the immune system, as well as in myocarditis [2•]. They have been shown to infiltrate the myocardium and, depending on the phenotype, can be either pro-inflammatory or anti-inflammatory. Macrophages contribute to the pro-fibrotic process which leads to adverse cardiac remodeling and eventually, heart failure. Galectin-3 is a lectin produced by activated macrophages and has been shown to mediate this pro-fibrotic process in a mouse model of heart failure [37]. It has more recently been shown to be associated with inflammatory cell count as well as cardiac fibrosis in human patients with myocarditis/inflammatory cardiomyopathy [38].
Interleukin-33 (IL-33) is cytokine that has been shown to play a role in inducing type 2 immune responses by activating several types of cells including T helper 2 cells (Th2), mast cells, as well as T-reg cells, Th1 cells, CD8 + cells, and natural killer cells. It has been shown to play a role in several disease processes including infection, inflammation, cancer, autoimmune diseases, and diseases of the CNS [39]. IL-33 exerts its effects by binding to several receptors, one of which is the ST2 receptor. The ST2 receptor has two important isoforms, the sera soluble ST2 receptor (sST2) and the membrane bound receptor (ST2). Sera sST2 has been shown to be a decoy receptor for IL-33 and inhibits the effect that IL-33 has on type 2 inflammation processes by preventing its binding to the membrane bound isoform, which mediates inflammation. It has been shown to protect against, and even reverse, cardiac hypertrophy in animal models [40]. However, sST2 has also been shown to paradoxically increase autoimmune disease. Sera sST2 is a new clinically available biomarker that has demonstrated prognostic value in chronic heart failure. Elevated circulating sera sST2 levels were associated with poorer prognosis in male patients with acute myocarditis [41]. Interestingly, sST2 levels were also significantly higher in male mice with myocarditis where levels were associated with cardiac inflammation. Gonadectomy with hormone replacement showed that testosterone but not estradiol increased sST2 levels in male mice with myocarditis. These findings indicate that the sera sST2 might underlie the different in disease severity and progression between males and females and could be used as a prognostic biomarker for male patients, as well potential therapeutic target.
Biomarkers of Cardiac Injury or Dysfunction
Traditional markers of cardiac damage include several proteins that make up the myocardium, especially the cardiac isoform of troponin I. The three isoforms of troponin form complexes with actin and myosin filaments in the myocardium and facilitate the contraction of cardiac muscle [42, 43]. In the setting of myocardial damage, these proteins spill out into the blood stream and can be detected in the serum with troponin assays. Troponins can be used to test for cardiac injury due to myocardial infarction, myocarditis, and other myocardial ischemic pathologies. Interestingly, some patients develop heterophile antibodies that may facilitate persistently elevated cardiac troponin levels, while theoretically the presence of troponin auto-antibodies may contribute to “false-negative” results in high-sensitivity cardiac troponin assays. Although troponins are not specific to identifying cardiac injury specific to inflammatory damage, they do have a role in testing patients with suspected myocarditis. Firstly, they can be used for surveillance in patients that have a high likelihood of developing myocarditis, such as those undergoing therapy with immune checkpoint inhibitors (ICI) [44]. High sensitivity troponin I (hs-cTnI) levels, specifically, are greatly elevated in patients with ICI induced myocarditis and prompted early myocarditis evaluation in these patients allowing for timely diagnosis and treatment, thereby improving outcomes [45]. Another role for hs-cTnI is to determine prognosis and mortality in patients with fulminant myocarditis, especially in the acute hospital setting. Studies have found that absolute and relative changes in hs-cTnI, namely a decline in levels in the first 24–48 h, were associated with greater in-hospital mortality [46].
Another important biomarker of cardiac injury is N terminal-pro B-type natriuretic peptide (NT-proBNP). NT-proBNP is a hormone produced by the heart and released into the serum in the setting of excess wall tension due to volume overload and cardiac dysfunction. This hormone affects several other organ systems in the body and produces a concerted effort to reduce volume by inducing natriuresis/diuresis, inhibiting the renin–angiotensin–aldosterone system (RAAS), inhibiting the sympathetic nervous system, and increasing peripheral vascular vasodilation [47]. All of these effects are aimed at reducing intravascular volume to reduce the load on the heart and reduce tension on the myocardial wall. Consequently, when elevated in the serum, NT-proBNP indicates excess tension and ventricular systolic dysfunction [48], for example in the case of heart failure. More recent studies have shown that NT-proBNP is significantly correlated with inflammation levels, as measured by leukocytes and CRP, in patients with acute myocarditis [49]. It is important to highlight that some studies have implied the presence of significantly elevated natriuretic peptide levels that were out of proportion to rise in cardiac troponin may signify more myocardial dysfunction than damage, and thus having a lower likelihood of acute myocarditis as a cause of cardiac dysfunction or cardiogenic shock (compared to other causes such as acute on chronic myocardial dysfunction or Takotsubo cardiomyopathy), but the precise cutoff or ratios have not been established.
Small Molecule Cell Structure/Adhesion Proteins
The endothelium plays an important role in the inflammatory response, allowing for recruitment and extravasation of leukocytes and other immune cells. This process is mediated by specific molecules expressed on the endothelium called cell adhesion molecules (CAMs). One specific type, the soluble form of vascular cell adhesion molecule-1 (VCAM-1), is mostly expressed on the endothelium during inflammation and acts as a ligand for certain integrins which allow for binding of various immune cells. Upregulation of these molecules on the endothelium is associated with myocarditis and virus-induced DCM in comparison to non-inflammatory DCM in a mouse model of experimental autoimmune myocarditis [50]. The acute inflammatory response in myocarditis causes damage to the cells of the myocardium and, when unregulated, can lead to poor healing and dysfunctional remodeling.
On the other hand, gelsolin is a calcium-dependent, actin regulatory protein that normally plays a role in locomotion, phagocytosis as well as the actin scavenging system. In the setting of inflammation, it has shown be protective against damage by allowing for fast severing and removal of actin filaments from dead cells, reducing damage to healthy cells. In diseases such as sepsis, myocarditis, and other systemic autoimmune diseases, where inflammation is dysregulated, there is evidence that plasma levels of gelsolin are reduced. Specifically, in the setting of acute rheumatic carditis, gelsolin levels have been significantly reduced compared to controls, indicating the lack of protection against inflammatory damage in these patients leading to progression of the disease [51].
Another important structural protein is collagen. In the setting of cardiac remodeling, the production process of collagen leads to release of procollagen type III amino-terminal pro-peptide, a cleavage product released into the blood. Levels of this molecule in adults with DCM are associated with cardiac remodeling and prognosis, while in pediatric patients with DCM increased levels have been associated with increased extent of left ventricular dilation reduced systolic function [52]. Of the patients with DCM those with myocarditis were found to have higher procollagen type III amino-terminal pro-peptide levels than those with idiopathic DCM.
Tenascin-C is an extracellular matrix glycoprotein that was explored as a biomarker of acute rheumatic carditis. It plays a role in tissue remodeling, an important process in the pathophysiology of myocarditis with remodeling of the myocardium. Tenascin-C levels were shown to be lower in patients with acute rheumatic carditis and were able to distinguish patients from controls with good sensitivity and specificity, as well as provide prognostic information about the degree of valvular insufficiency [53].
Opportunities for Biomarkers as Companion Diagnostics
Current therapy for myocarditis is mainly symptomatic management and supportive care after patients develop cardiac dysfunction and heart failure, as well as immunosuppressive therapy to reduce inflammation [2•, 54]. In addition to biomarkers being important for accurate diagnosis and prognosis of myocarditis in patients, they also open the door to new therapeutics for myocarditis. One of the most promising areas of research on this topic are circulating microRNA biomarkers for acute myocarditis. Several miRNAs have been found to be elevated in the setting of myocarditis, and these miRNAs target genes implicated in inflammatory regulation, cardiac injury response pathways, and differentiation of several important immune cells [12•, 14]. We can utilize this information to develop gene therapies to either mimic these miRNAs or silence them, depending on their function, to mitigate the symptoms of myocarditis as well as control inflammation and disease progression.
Conclusions
Although the pathophysiology and various etiologies of myocarditis are well understood and characterized, there is a lack of biomarkers that are both sensitive and specific, to allow for accurate, timely diagnosis of the condition. Early diagnosis of myocarditis could allow for early treatment which would prevent progression to dilated cardiomyopathy, heart failure, and finally death in patients. In addition to sensitivity and specificity, other important characteristics of a good biomarker include low cost of testing, ease of testing, minimally invasive, and correlation with temporal progression of myocarditis. Recent work in this field has found several promising candidates including specific types of immune cells that are involved in myocarditis, cytokines, and other molecules that are expressed in the disease, as well as the relatively new discover of miRNAs that are highly expressed in myocarditis.
Funding
Dr. Tang is supported by grants from the National Institutes of Health (R01HL146754). Dr. Pieter Martens is supported by a grant from the Belgian American Educational Foundation (BAEF) and by the Frans Van de Werf Fund.
Declarations
Conflict of Interest
Dr. Suresh has no relationships to disclose. Dr. Martens is supported by a grant from the Belgian American Educational Foundation (BAEF) and by the Frans Van de Werf Fund. Dr. Tang is a consultant for Sequana Medical A.V., Cardiol Therapeutics Inc, Genomics plc, Zehna Therapeutics Inc, Renovacor Inc, and has received honorarium from Springer Nature for authorship/editorship and American Board of Internal Medicine for exam writing committee participation.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Biomarkers of Heart Failure
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy. Circulation: Heart Failure. 2020;13:e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maisch B. Cardio-immunology of myocarditis: focus on immune mechanisms and treatment options. Front Cardiovasc Med. 2019;6:48. doi: 10.3389/fcvm.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fung G, Luo H, Qiu Y, Yang D, McManus B. Myocarditis. 2016;118:496–514 (American Heart Association). [DOI] [PubMed]
- 5.Fu M, Kontogeorgos S, Thunström E, Zverkova Sandström T, Kroon C, Bollano E, et al. Trends in myocarditis incidence, complications and mortality in Sweden from 2000 to 2014. Sci Rep. 2022;12:1810. doi: 10.1038/s41598-022-05951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bracamonte-Baran W, Čiháková D. Cardiac autoimmunity: myocarditis. Adv Exp Med Biol. 2017;1003:187–221. doi: 10.1007/978-3-319-57613-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro C, Gourley M. Diagnostic testing and interpretation of tests for autoimmunity. J Allergy Clin Immunol. 2010;125:S238–S247. doi: 10.1016/j.jaci.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bang V, Ganatra S, Shah SP, Dani SS, Neilan TG, Thavendiranathan P, et al. Management of patients with giant cell myocarditis. J Am Coll Cardiol. 2021;77:1122–34. doi: 10.1016/j.jacc.2020.11.074. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y, Ren J, Dong X, Zhang D, Qu Y, Yang C, et al. Fulminant giant cell myocarditis vs. lymphocytic myocarditis: a comparison of their clinical characteristics, treatments, and outcomes. Front Cardiovasc Med. 2021;8:770549. doi: 10.3389/fcvm.2021.770549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Law YM, Lal AK, Chen S, Čiháková D, Cooper LT, Deshpande S, et al. Diagnosis and management of myocarditis in children. Circulation. 2021;144:e123–e135. doi: 10.1161/CIR.0000000000001001. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman KA, Reynolds C, Bottazzi ME, Hotez P, Jones K. Improved biomarker and imaging analysis for characterizing progressive cardiac fibrosis in a mouse model of chronic chagasic cardiomyopathy. J Am Heart Assoc. 2019;8:e013365. doi: 10.1161/JAHA.119.013365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanco-Domínguez R, Sánchez-Díaz R, de la Fuente H, Jiménez-Borreguero LJ, Matesanz-Marín A, Relaño M, et al. A novel circulating MicroRNA for the detection of acute myocarditis. N Engl J Med. 2021;384:2014–27. doi: 10.1056/NEJMoa2003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers JM, Cooper LT, Kem DC, Stavrakis S, Kosanke SD, Shevach EM, et al. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight. 2016;1:e85851. doi: 10.1172/jci.insight.85851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nie X, He M, Wang J, Chen P, Wang F, Lai J, et al. Circulating miR-4763-3p is a novel potential biomarker candidate for human adult fulminant myocarditis. Mol Ther - Methods Clin Dev. 2020;17:1079–1087. doi: 10.1016/j.omtm.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg L, Tirosh-Wagner T, Vardi A, Abbas H, Pillar N, Shomron N, et al. Circulating MicroRNAs: a potential biomarker for cardiac damage, inflammatory response, and left ventricular function recovery in pediatric viral myocarditis. J Cardiovasc Transl Res. 2018;11:319–328. doi: 10.1007/s12265-018-9814-0. [DOI] [PubMed] [Google Scholar]
- 16.Omura S, Kawai E, Sato F, Martinez NE, Chaitanya GV, Rollyson PA, et al. Bioinformatics multivariate analysis determined a set of phase-specific biomarker candidates in a novel mouse model for viral myocarditis. Circ Cardiovasc Genet. 2014;7:444–454. doi: 10.1161/CIRCGENETICS.114.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Artico J, Merlo M, Delcaro G, Cannatà A, Gentile P, De Angelis G, et al. Lymphocytic myocarditis: a genetically predisposed disease? J Am Coll Cardiol. 2020;75:3098–3100. doi: 10.1016/j.jacc.2020.04.048. [DOI] [PubMed] [Google Scholar]
- 18.Poller W, Haas J, Klingel K, Kühnisch J, Gast M, Kaya Z, et al. Familial recurrent myocarditis triggered by exercise in patients with a truncating variant of the desmoplakin gene. J Am Heart Assoc. 2020;9:e015289. doi: 10.1161/JAHA.119.015289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichl K, Kreykes SE, Martin CM, Shenoy C. Desmoplakin variant-associated arrhythmogenic cardiomyopathy presenting as acute myocarditis. Circ Genom Precis Med. 2018;11:e002373. doi: 10.1161/CIRCGEN.118.002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kontorovich AR, Patel N, Moscati A, Richter F, Peter I, Purevjav E, et al. Myopathic cardiac genotypes increase risk for myocarditis. JACC: Basic Transl Sci. 2021;6:584–92. doi: 10.1016/j.jacbts.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hua X, Hu G, Hu Q, Chang Y, Hu Y, Gao L, et al. Single-Cell RNA sequencing to dissect the immunological network of autoimmune myocarditis. Circulation. 2020;142:384–400. doi: 10.1161/CIRCULATIONAHA.119.043545. [DOI] [PubMed] [Google Scholar]
- 22.Rose NR. Critical cytokine pathways to cardiac inflammation. J Interferon Cytokine Res. 2011;31:705–710. doi: 10.1089/jir.2011.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai Y, Zhang X, Shen J, Jiang B, Hu D, Zhao M. Heparin-binding protein: a novel biomarker linking four different cardiovascular diseases. Cardiol Res Pract. 2020;2020:9575373. doi: 10.1155/2020/9575373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller I, Vogl T, Pappritz K, Miteva K, Savvatis K, Rohde D, et al. Pathogenic role of the damage-associated molecular patterns S100A8 and S100A9 in Coxsackievirus B3-induced myocarditis. Circ Heart Fail. 2017;10:e004125. doi: 10.1161/CIRCHEARTFAILURE.117.004125. [DOI] [PubMed] [Google Scholar]
- 25.Müller I, Vogl T, Kühl U, Krannich A, Banks A, Trippel T, et al. Serum alarmin S100A8/S100A9 levels and its potential role as biomarker in myocarditis. ESC Heart Fail. 2020;7:1442–1451. doi: 10.1002/ehf2.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anzai A, Mindur JE, Halle L, Sano S, Choi JL, He S, et al. Self-reactive CD4+ IL-3+ T cells amplify autoimmune inflammation in myocarditis by inciting monocyte chemotaxis. J Exp Med. 2019;216:369–383. doi: 10.1084/jem.20180722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nindl V, Maier R, Ratering D, De Giuli R, Züst R, Thiel V, et al. Cooperation of Th1 and Th17 cells determines transition from autoimmune myocarditis to dilated cardiomyopathy. Eur J Immunol. 2012;42:2311–2321. doi: 10.1002/eji.201142209. [DOI] [PubMed] [Google Scholar]
- 28.Interleukin-17A Is Dispensable for myocarditis but essential for the progression to dilated cardiomyopathy | Circulation Research [Internet]. [cited 2022 Mar 1]. Available from: https://www.ahajournals.org/doi/10.1161/circresaha.109.213157 [DOI] [PubMed]
- 29.Vdovenko D, Eriksson U. Regulatory role of CD4+ T cells in myocarditis. J Immunol Res. 2018;2018:4396351. doi: 10.1155/2018/4396351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Han B. Dysregulated CD4+ T cells and microRNAs in myocarditis. Front Immunol. 2020;11:539. doi: 10.3389/fimmu.2020.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Ge J, Chen R. LAP+ Treg is a better biomarker than total Treg in viral myocarditis. J Med Virol. 2019;91:886–889. doi: 10.1002/jmv.25378. [DOI] [PubMed] [Google Scholar]
- 32.Song J, Chen X, Cheng L, Rao M, Chen K, Zhang N, et al. Vitamin D receptor restricts T helper 2-biased inflammation in the heart. Cardiovasc Res. 2018;114:870–879. doi: 10.1093/cvr/cvy034. [DOI] [PubMed] [Google Scholar]
- 33.Gil-Cruz C, Perez-Shibayama C, De Martin A, Ronchi F, van der Borght K, Niederer R, et al. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science. 2019;366:881–886. doi: 10.1126/science.aav3487. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Zeng X-H, Wang M, Chen L, Zhang N, Rao M, et al. Bcl2-like protein 12 is required for the aberrant T helper-2 polarization in the heart by enhancing interleukin-4 expression and compromising apoptotic machinery in CD4+ T cells. Circulation. 2018;138:2559–2568. doi: 10.1161/CIRCULATIONAHA.118.033890. [DOI] [PubMed] [Google Scholar]
- 35.Kaya Z, Leib C, Katus HA. Autoantibodies in heart failure and cardiac dysfunction. Circ Res. 2012;110:145–158. doi: 10.1161/CIRCRESAHA.111.243360. [DOI] [PubMed] [Google Scholar]
- 36.Matsumori A, Shimada T, Nakatani E, Shimada M, Tracy S, Chapman NM, et al. Immunoglobulin free light chains as an inflammatory biomarker of heart failure with myocarditis. Clin Immunol. 2020;217:108455. doi: 10.1016/j.clim.2020.108455. [DOI] [PubMed] [Google Scholar]
- 37.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JPM, Schroen B, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 38.Besler C, Lang D, Urban D, Rommel K-P, von Roeder M, Fengler K, et al. Plasma and cardiac galectin-3 in patients with heart failure reflects both inflammation and fibrosis: implications for its use as a biomarker. Circ Heart Fail. 2017;10:e003804. doi: 10.1161/CIRCHEARTFAILURE.116.003804. [DOI] [PubMed] [Google Scholar]
- 39.Liew FY, Girard J-P, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 40.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coronado MJ, Bruno KA, Blauwet LA, Tschöpe C, Cunningham MW, Pankuweit S, et al. Elevated sera sST2 is associated with heart failure in men ≤50 years old with myocarditis. J Am Heart Assoc. 2019;8:e008968. doi: 10.1161/JAHA.118.008968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babuin L, Jaffe AS. Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ. 2005;173:1191–1202. doi: 10.1503/cmaj/051291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mair J, Genser N, Morandell D, Maier J, Mair P, Lechleitner P, et al. Cardiac troponin I in the diagnosis of myocardial injury and infarction. Clin Chim Acta. 1996;245:19–38. doi: 10.1016/0009-8981(95)06168-1. [DOI] [PubMed] [Google Scholar]
- 44.Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9:e013757. doi: 10.1161/JAHA.119.013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waliany S, Neal JW, Reddy S, Wakelee H, Shah SA, Srinivas S, et al. Myocarditis surveillance with high-sensitivity troponin I during cancer treatment with immune checkpoint inhibitors. JACC CardioOncol. 2021;3:137–139. doi: 10.1016/j.jaccao.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu C, Wang Z, Chen K, Cui G, Chen C, Wang L, et al. The absolute and relative changes in high-sensitivity cardiac troponin I are associated with the in-hospital mortality of patients with fulminant myocarditis. BMC Cardiovasc Disord. 2021;21:571. doi: 10.1186/s12872-021-02386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber M, Hamm C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart. 2006;92:843–849. doi: 10.1136/hrt.2005.071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bay M, Kirk V, Parner J, Hassager C, Nielsen H, Krogsgaard K, et al. NT-proBNP: a new diagnostic screening tool to differentiate between patients with normal and reduced left ventricular systolic function. Heart. 2003;89:150–154. doi: 10.1136/heart.89.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sara B, Monteiro J, Carvalho P, Ribeiro Carvalho C, Chemba J, Ferreira C, et al. Are high NT-proBNP levels more related to inflammation than to left ventricular systolic dysfunction in acute myocarditis? Eur Heart J Acute Cardiovasc Care. 2021;10:zuab020.189. doi: 10.1093/ehjacc/zuab020.189. [DOI] [Google Scholar]
- 50.Grabmaier U, Kania G, Kreiner J, Grabmeier J, Uhl A, Huber BC, et al. Soluble vascular cell adhesion molecule-1 (VCAM-1) as a biomarker in the mouse model of experimental autoimmune myocarditis (EAM) PLoS ONE. 2016;11:e0158299. doi: 10.1371/journal.pone.0158299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Argun M, Baykan A, Narin F, Özyurt A, Pamukçu Ö, Elmalı F, et al. Plasma gelsolin as a biomarker of acute rheumatic carditis. Cardiol Young. 2015;25:1276–1280. doi: 10.1017/S1047951114002327. [DOI] [PubMed] [Google Scholar]
- 52.Kaufman BD, Videon N, Zhang X, Harris MA, Shaddy RE, Goldmuntz E. Procollagen type III amino-terminal propeptide: a serum biomarker of left ventricular remodelling in paediatric dilated cardiomyopathy. Cardiol Young. 2015;25:228–236. doi: 10.1017/S1047951113001820. [DOI] [PubMed] [Google Scholar]
- 53.Karatas Z, Baysal T, Alp H, Toker A. Serum tenascin-C: a novel biomarker for diagnosis and predicting prognosis of rheumatic carditis? J Trop Pediatr. 2013;59:476–482. doi: 10.1093/tropej/fmt058. [DOI] [PubMed] [Google Scholar]
- 54.Frustaci A, Chimenti C. Immunosuppressive therapy in myocarditis. Circ J. 2015;79:4–7. doi: 10.1253/circj.CJ-14-1192. [DOI] [PubMed] [Google Scholar]