Abstract
We investigated the effect of aging on the basement membrane (BM) during postinjury muscle recovery. Using a rat model, we found that aging delayed muscle fiber and BM recovery. In addition, expression of BM-related factors peaked 7 days after muscle injury among both young and older rats. Peak expression of collagen IV synthetic factors decreased with age, whereas expression of the degradative factor was unaffected by age. These results suggest that age-related delays in postinjury muscle fiber and BM recovery may be related to the suppression of collagen IV synthetic factors.
Keywords: muscle injury, aging, basement membrane, skeletal muscle, collagen IV, recovery
Skeletal muscle fibers are covered by a basement membrane (BM) that provides mechanical protection [1]. The BM is formed from the action of heat shock protein 47 (HSP47) and secreted protein, acidic and rich in cysteine (SPARC) on collagen IV, which is degraded by matrix metalloproteinase-14 (MMP14) [1–4]. Previous studies on BM-related factors have shown that loss of the collagen IV gene is associated with abnormal BM structure and muscle fiber fragility [1–6]. In addition, collagen IV induction is necessary for recovery from muscle atrophy or injury [7,8]. These results suggest that BM-related factor expression is essential for the maintenance and recovery of skeletal muscles.
In young muscles, reloading after disuse muscle atrophy resulted in smooth recovery with elevated expression of BM-related factors, whereas older muscles showed poor expression of BM-related factors, leading to BM injury and delayed muscle recovery [7]. This suggests that suppressed BM-related factor expression is involved in the delayed recovery of aging-related disuse muscle atrophy. However, aging effects on BM during the recovery of other muscle disorders remain unclear. To prevent muscle disorders in old age, we must clarify how aging influences the BM during recovery from multiple types of muscle problems. Therefore, in this study, we investigated BM response to age during recovery after muscle injury.
Our experiment used two age groups of male Wistar rats: 2- (n = 16) and 23-months-old (n = 14) (Japan SLC, Shizuoka, Japan). Experimental animals were kept in transparent plastic cages with free access to food and water. Breeding room temperature was set at 22 ± 2°C and maintained in a 12/12 h light/dark cycle. This study was approved by the Committee of Animal Care and Use of the Osaka University of Human Sciences (#2). All procedures were in accordance with the institutional guidelines for the use of experimental animals.
Muscle injury was induced with an intramuscular injection of 0.25% dibucaine (0.5 ml) into the right tibialis anterior (TA) muscle, as previously described [9]. Dibucaine is a local anesthetic with myotoxic effects, acting on the sarcoplasmic reticulum to excessively increase intracellular calcium ion concentrations and, thus, damage muscle fibers [10]. After injury of the right TA, rats were placed into separate subgroups, with each experiencing different recovery durations: 2, 7 and 21 days (R2d, R7d and R21d, respectively). Intact TA muscle on the opposite side of R2d was designated as a control (hereafter, Intact muscle). As a superficial muscle that is prone to injury from injection, the TA muscle has been used as a drug-induced muscle injury model [9].
At the end of each experimental period, rats were euthanized via intraperitoneal administration of sodium pentobarbital. Tibialis anterior muscles were harvested and flash-frozen in isopentane cooled on dry ice. Transverse sections of 1-mm thickness were cut from the center of frozen samples and immersed in RNAlater (Thermo Fisher Scientific, Hanover Park, IL).
For morphological analysis, 10-μm transverse sections were sliced using a cryostat (CM1950; Leica, Wetzlar, Germany) from the center of the frozen (−25°C) TA samples. Sections were then stained with hematoxylin and eosin (HE). Staining images were analyzed in ImageJ Fiji to determine necrotic areas on R2d, as well as fiber cross-sectional areas (FCSA) of Intact muscle fibers and central nuclei fibers (CNFs) on R7d and R21d [11]. For the FCSA analysis, 150 muscle fibers were measured per sample, following previous studies [12,13].
For transmission electron microscopy (TEM) analysis, frozen (−25°C) 1-mm tissue sections were prepared from the center of the TA samples. Sections were fixed in 4% paraformaldehyde/2% glutaraldehyde, then treated with osmium tetroxide, dehydrated with ethanol and embedded in Epon. Longitudinal ultrathin sections (90-nm thick) were stained with 4% uranyl acetate and 1% lead citrate for observation under TEM (HT7700; Hitachi, Tokyo, Japan).
Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed using random primers and ReverTra Ace (Toyobo, Osaka, Japan). Target gene expression was measured with quantitative reverse-transcription PCR (StepOnePlus Real-Time PCR System, Applied Biosystems, Carlsbad, CA) using TB Green Premix Ex Taq II (Takara Bio, Shiga, Japan). Endogenous control Rn18s did not change in expression with recovery or age. Variation in target gene expression was calculated as a percentage change from the expression in the Intact muscle of young rats. The primer sets for Col4a1, Hsp47, Sparc, Mmp14 and Rn18s are described in Supplementary Data.
Differences in R2d necrotic areas between young and older rats were examined using t-tests, with means separated using Tukey HSD tests (KaleidaGraph version 4.5.1; Synergy Software, Reading, PA). All data are expressed as the mean ± standard error of the mean. Significance was set at P < 0.05.
To determine age-related differences in recovery, we measured FCSA of CNF, an indicator of muscle regeneration (Fig. 1a–g). We began observing CNFs after 7 days of recovery in both age groups (Fig. 1, arrowheads in b and e). In young rats, the FCSA of CNF increased to Intact muscle size starting from 7 days of recovery and was maintained at 21 days of recovery (Fig. 1c and g). In contrast, older rats had smaller FCSA of CNF than rats with Intact muscle and young rats at 21 days of recovery (Fig. 1f and g). These aging-related characteristics in muscle recovery were consistent with the results from a previous study [14]. Therefore, we conclude that aging delays recovery after muscle injury.
Fig. 1.
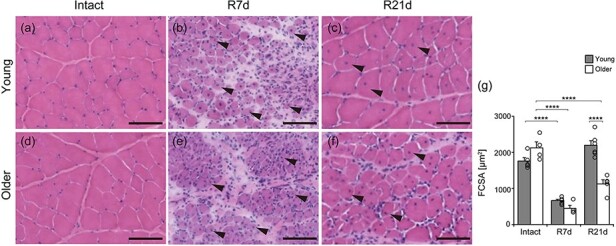
Morphological analysis. Transverse sections of the tibialis anterior (TA) muscle stained with hematoxylin and eosin. Temporal observations from Intact, R7d and R21d in young (a–c) and older rats (d–f). Arrowheads: central nuclei fiber (CNF). Fiber cross-sectional area (FCSA) of CNF on R7d and R21d and of Intact muscle fibers was measured (g). TA samples were from Intact (n = 5), R7d (n = 5) and R21d (n = 6) groups of young rats and Intact (n = 4), R7d (n = 4) and R21d (n = 5) groups of older rats. R2d was the necrotic phase and excluded from measurements of FCSA. ****P < 0.0001. Data are presented as mean ± standard error of the mean. Scale bar is 100 μm.
Next, we used TEM to observe microstructural changes in muscle fibers and BM during recovery (Fig. 2a–h). At 7 days, we observed a pleated BM-like structure (Fig. 2a and black arrow in b) and lamina densa (LD) (Fig. 2b, white arrow) in the CNFs of young rats. However, these structures were not obvious in older rats (Fig. 2c, d and Supplementary Table S1). At 21 days, the BM of CNFs became relatively linear in young rats, and LD continued to be visible (Fig. 2e and white arrow in f), but the earlier pleated BM-like structures were rare. In contrast, pleated BM-like structures (Fig. 2g and black arrow in h) and LD (Fig. 2h, white arrow) only became widely observable among old rats on day 21. Because these pleated structures occur during BM recovery [15,16], our TEM results suggest that aging delays this process.
Fig. 2.
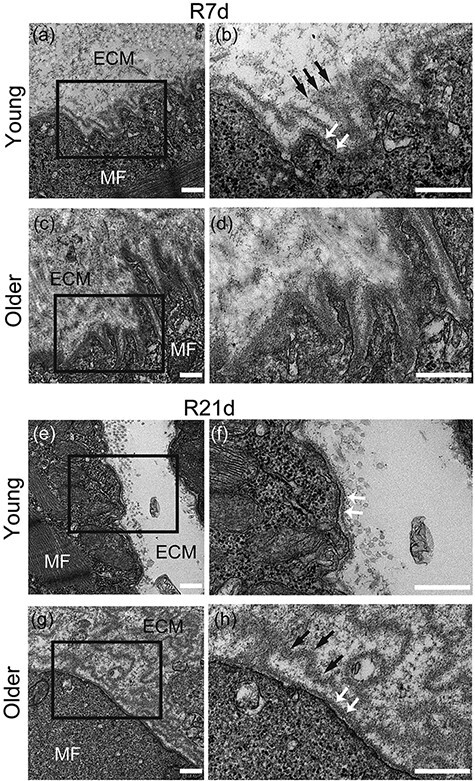
Electron microscopy analysis. Longitudinal sections of tibialis anterior (TA) muscle were observed using transmission electron microscopy (three samples per group). Basement membrane (BM) structures of young rats (a and b) and older rats (c and d) on R7d. BM structures of young (e and f) and older (g and h) rats on R21d. MF, muscle fiber; ECM, extracellular matrix; black arrow, pleated BM-like structure; white arrow, lamina densa. (b), (d), (f) and (h) are enlargements of the black squares in (a), (c), (e) and (g). Scale bar is 500 nm.
To investigate the expression of BM-related factors during recovery, we compared changes in Col4a1, Hsp47, Sparc and Mmp14 expression between young and older rats (Fig. 3a–d). In Intact muscle, expression of BM-related factors decreased with age (Supplementary Fig. S4). Among both young and older rats with TA injury, expression of BM-related factors peaked at 7 days of recovery and returned to Intact levels at 21 days (Fig. 3a–d). Col4a1, Hsp47 and Sparc expression tended to be less variable in older rats than in young rats (Fig. 3a–c). In addition, age led to a low peak expression of Hsp47 and Sparc, both of which are collagen IV synthetic factors (Fig. 3b and c). However, no such age difference was observed for Mmp14, the degradative factor (Fig. 3d). These results suggest that aging hampers upregulation of collagen IV synthetic factors after muscle injury but not upregulation of collagen degradative factors.
Fig. 3.
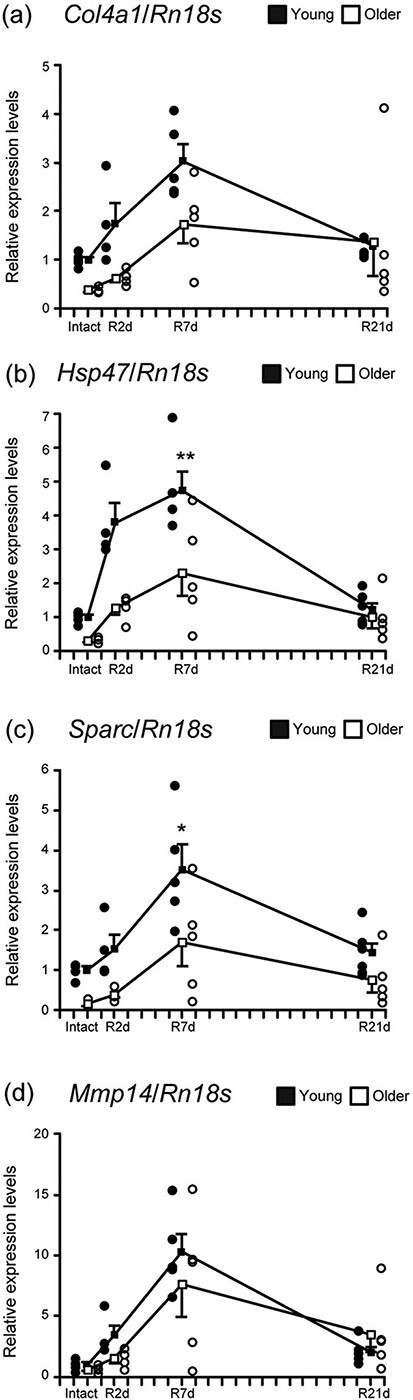
Basement membrane-related factors. Changes in relative mRNA expression of Col4a1 (a), Hsp47 (b), Sparc (c) and Mmp14 (d) among young and older rats. Samples were obtained from TA of young rats in Intact (n = 5), R2d (n = 4), R7d (n = 5) and R21d (n = 6) groups, as well as of old rats in Intact (n = 4), R2d (n = 4), R7d (n = 5) and R21d (n = 5) groups. **P < 0.001, *P < 0.05. Data are presented as mean ± standard error of the mean.
The appearance of pleated BM-like structures may be delayed due to aging-related suppression of collagen IV synthetic factors. This study showed that pleated BM-like structures appeared during recovery in both age groups, but they formed more slowly in older rats than in young rats. Old BMs peel off during recovery and a pleated structure is observed as new BM forms [15,16]. A pleated BM structure also acts as a scaffold, allowing the adherence of activated satellite cells while they differentiate and fuse to form myotubes [16]. Therefore, the delayed appearance of a pleated BM-like structure may indicate slow BM reconstruction. In this study, we found that age is associated with decreased upregulation of collagen IV synthetic factors, major components of BM required for BM reconstruction [1]. These results suggest that decreased expression of collagen IV synthetic factors may be a causal element behind delayed BM reconstruction.
Fibroblast senescence is potentially involved in age-related downregulation of collagen IV synthetic factors. Our data suggest that aging suppresses collagen IV synthetic factor expression after muscle injury. Previously, we linked age-related delays in disuse atrophy recovery with inhibition of collagen IV synthetic factor upregulation [7]. Taken together, our current and prior research indicate that age-related suppression of collagen IV synthetic factors is common across different muscle disorders (e.g. injury and atrophy), as well as their recovery processes. The common causative link may be fibroblast senescence. One study showed that inhibiting the function of intramuscular connective tissue fibroblasts during muscle recovery resulted in residual immature myofibers and inadequate recovery [17]. In addition, senescent fibroblasts appear to have reduced collagen production capacity [18]. Therefore, we hypothesize that senescent fibroblasts lack the capacity to adequately express collagen IV, thereby causing delayed muscle recovery.
In contrast to that seen for collagen IV synthetic factors, induction of the MMP14 BM-degrading protein during BM reconstruction may be less sensitive to aging. MMPs are induced by tissue necrosis due to post-ischemia reflux [19]. MMP14 is involved in BM reconstruction. In this study, we observed injury-induced extensive necrosis, similar to our previous study where we observed dibucaine-induced necrosis [9]. Our results indicate that severe necrosis can induce Mmp14 expression independent of age. This is consistent with the results of another study that reported that aging does not affect MMP expression [20]. As senescent fibroblasts can still produce MMPs [20], we hypothesize that after severe muscle injury fibroblasts produce MMP14 in the initial step of BM reconstruction. However, our previous study showed that Mmp14 is not expressed during recovery from disuse atrophy [7] which indicates the differences in BM reconstruction processes and emphasizes the importance of extending studies to different muscle disorders.
In this study, we found that aging delays BM recovery after muscle injury. Aging also suppressed the upregulation of collagen IV synthetic factors. Together, these results imply that the inhibition of collagen IV synthetic factor expression is an underlying mechanism of aging-related delays in postinjury BM recovery.
Supplementary Material
Acknowledgement
We thank the Life Science Research Institute of Kindai University for their technical support.
Contributor Information
Yuji Kanazawa, Department of Medical Technology and Clinical Engineering, Hokuriku University, Kanazawa 920-1180, Japan; Department of Anatomy and Neurobiology, Graduate School of Medical Sciences, Kindai University, Ohnohigashi, Osakasayama 589-8511, Japan.
Mamoru Nagano, Department of Anatomy and Neurobiology, Graduate School of Medical Sciences, Kindai University, Ohnohigashi, Osakasayama 589-8511, Japan.
Satoshi Koinuma, Department of Anatomy and Neurobiology, Graduate School of Medical Sciences, Kindai University, Ohnohigashi, Osakasayama 589-8511, Japan.
Shinichi Sugiyo, Department of Physical Therapy, Osaka University of Human Sciences, Shojyaku, Settsu 566-8501, Japan.
Yasufumi Shigeyoshi, Department of Anatomy and Neurobiology, Graduate School of Medical Sciences, Kindai University, Ohnohigashi, Osakasayama 589-8511, Japan.
Funding
Hokuriku University Special Research Grant and JSPS KAKENHI Grant Number 22K11300.
Supplementary data
Supplementary data are available at Microscopy online.
References
- 1. Sanes J R (2003) The basement membrane/basal lamina of skeletal muscle. J. Biol. Chem. 278: 12601–12604. [DOI] [PubMed] [Google Scholar]
- 2. Kuo D S, Labelle-Dumais C, and Gould D B (2012) COL4A1 and COL4A2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets. Hum. Mol. Genet. 21: 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chioran A, Duncan S, Catalano A, Brown T J, and Ringuettea M J (2017) Collagen IV trafficking: the inside-out and beyond story. Dev. Biol. 431: 124–133. [DOI] [PubMed] [Google Scholar]
- 4. Yana I, Sagara H, Takaki S, Takatsu K, Nakamura K, Nakao K, Katsuki M, Taniguchi S, Aoki T, Sato H, Weiss S J, and Seiki M (2007) Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J. Cell Sci. 120: 1607–1614. [DOI] [PubMed] [Google Scholar]
- 5. Labelle-Dumais C, Dilworth D J, Harrington E P, de Leau M, Lyons D, Kabaeva D, Manzini M C, Dobyns W B, Walsh C A, Michele D E, and Gould D B (2011) COL4A1 mutations cause ocular dysgenesis, neuronal localization defects, and myopathy in mice and Walker-Warburg syndrome in humans. PLoS Genet. 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelemen-Valkony I, Kiss M, Csiha J, Kiss A, Bircher U, Szidonya J, Maróy P, Juhász G, Komonyi O, Csiszár K, and Mink M (2012) Drosophila basement membrane collagen col4a1 mutations cause severe myopathy. Matrix Biol. 31: 29–37. [DOI] [PubMed] [Google Scholar]
- 7. Kanazawa Y, Ikegami K, Sujino M, Koinuma S, Nagano M, Oi Y, Onishi T, Sugiyo S, Takeda I, Kaji H, and Shigeyoshi Y (2017) Effects of aging on basement membrane of the soleus muscle during recovery following disuse atrophy in rats. Exp. Gerontol. 98: 153–161. [DOI] [PubMed] [Google Scholar]
- 8. Kanazawa Y, Nagano M, Koinuma S, Sujino M, Minami Y, Sugiyo S, Takeda I, and Shigeyoshi Y (2020) Basement membrane recovery process in rat soleus muscle after exercise-induced muscle injury. Connect. Tissue Res. 22: 1–12. [DOI] [PubMed] [Google Scholar]
- 9. Foster A H and Carlson B M (1980) Myotoxicity of local anesthetics and regeneration of the damaged muscle fibers. Anesth. Analg. 59: 727–736. [PubMed] [Google Scholar]
- 10. Hussain N, McCartney C J L, Neal J M, Chippor J, Banfield L, and Abdallah F W (2018) Local anaesthetic-induced myotoxicity in regional anaesthesia: a systematic review and empirical analysis. Br. J. Anaesth. 121: 8228–8241. [DOI] [PubMed] [Google Scholar]
- 11. Schindelin J, Carreras I A, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White D J, Hartenstein V, Eliceiri K, Tomancak P, and Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat. Methods 9: 6766–6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beitzel F, Gregorevic P, Ryall J G, Plant D R, Sillence M N, and Lynch G S (2004) β2-Adrenoceptor agonist fenoterol enhances functional repair of regenerating rat skeletal muscle after injury. J. Appl. Physiol. 96: 1385–1392. [DOI] [PubMed] [Google Scholar]
- 13. David R P, Fiona E C, and Gordon S L (2006) Notexin causes greater myotoxic damage and slower functional repair in mouse skeletal muscles than bupivacaine. Muscle Nerve 34: 5775–5785. [DOI] [PubMed] [Google Scholar]
- 14. Rahman F A, Angus S A, Stokes K, Karpowicz P, and Krause M P (2020) Impaired ECM remodeling and macrophage activity define necrosis and regeneration following damage in aged skeletal muscle. Int. J. Mol. Sci. 21: 12–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carraro U, Boncompagni S, Gobbo V, Rossini K, Zampieri S, Mosole S, Ravara B, Nori A, Stramare R, Ambrosio F, Piccione F, Masiero S, Vindigni V, Gargiulo P, Protasi F, Kern H, Pond A, and Marcante A (2015) Persistent muscle fiber regeneration in long term denervation. Past, present, future. Eur J. Transl. Myol. 25: 4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mackey A L and Kjaer M (2017) The breaking and making of healthy adult human skeletal muscle in vivo. Skelet. Muscle 7: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mathew S J, Hansen J M, Merrell A J, Murphy M M, Lawson J A, Hutcheson D A, Hansen M S, Angus-Hill M, and Kardon G (2011) Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development 138: 3713–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furth J J (1991) The steady-state levels of type I collagen mRNA are reduced in senescent fibroblasts. J. Gerontol. 46: 1221–1224. [DOI] [PubMed] [Google Scholar]
- 19. Roach D M, Fitridge R A, Laws P E, Millard S H, Varelias A, and Cowled P A (2002) Up-regulation of MMP-2 and MMP-9 leads to degradation of type IV collagen during skeletal muscle reperfusion injury; protection by the MMP inhibitor, doxycycline. Eur. J. Vasc. Endovasc. Surg. 23: 2602–2669. [DOI] [PubMed] [Google Scholar]
- 20. Freitas-Rodríguez S, Folgueras A R, and López-Otín C (2017) The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochim. Biophys. Acta Mol. Cell Res. 1864: 2015–2025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


