SUMMARY
Stem/progenitor cells exhibit high proliferation rates, elevated nutrient uptake, altered metabolic flux, and stress-induced genome instability. O-GlcNAcylation is an essential post-translational modification mediated by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), which act in a nutrient- and stress-responsive manner. The precise role of O-GlcNAc in adult stem cells and the relationship between O-GlcNAc and the DNA damage response (DDR) is poorly understood. Here, we show that hyper-O-GlcNacylation leads to elevated insulin signaling, hyperproliferation, and DDR activation that mimic the glucose- and oxidative-stress-induced response. We discover a feedback mechanism involving key downstream effectors of DDR, ATM, ATR, and CHK1/2 that regulates OGT stability to promote O-GlcNAcylation and elevate DDR. This O-GlcNAc-dependent regulatory pathway is critical for maintaining gut homeostasis in Drosophila and the DDR in mouse embryonic stem cells (ESCs) and mouse embryonic fibroblasts (MEFs). Our findings reveal a conserved mechanistic link among O-GlcNAc cycling, stem cell self-renewal, and DDR with profound implications for stem-cell-derived diseases including cancer.
In Brief
Na et al. show that stress induces proliferation, DNA damage response (DDR), and O-GlcNAc levels in stem/progenitor cells. Higher O-GlcNAc levels directly induce proliferation and DDR. These data reveal an unexpected conserved autoregulatory loop, wherein the downstream DDR kinases CHK1/2 stabilize OGT, augmenting O-GlcNAcylation and further promoting the DDR pathway.
Graphical Abstract

INTRODUCTION
Turnover of the intestinal epithelium occurs approximately weekly and is controlled by the tight balance of self-renewal and differentiation of stem cells. This balance supports the absorptive, secretory, and barrier functions of the intestine, despite insults from various forms of stress. The tight regulation between self-renewal and differentiation of these adult stem cells is critical, as disruption can cause tissue injury or cancer. One crucial aspect of stem cell homeostasis is a unique metabolic profile that relies heavily on glycolysis to provide the metabolites necessary for growth and reduces the generation of reactive oxygen species and replicative stress. However, how nutrients are utilized to support and maintain the integrity of intestinal stem cells remains a critical unanswered question.
The Drosophila midgut has proven to be an excellent model system to study how adult stem cell proliferation and differentiation are regulated (Lucchetta and Ohlstein, 2012). This conserved high-turnover tissue provides a powerful model system to study adult stem cells under nutrient- and stress-responsive conditions, which allows us to further understand aging-related and stem-cell-involved diseases, including cancer (Jasper, 2015). The Drosophila midgut is maintained by self-renewing intestinal stem cells (ISCs) and consists of ISCs, precursor enteroblasts (EBs), differentiated enterocytes (ECs; absorptive cells), and enteroendocrine cells (EEs; secretory cells) (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). Accumulation of somatic mutations in adult stem cells contributes to cancer initiation and lifespan (Adams et al., 2015), with an increase in the DNA damage response (DDR) in adult stem cells under age- and oxidative-related stress conditions (Park et al., 2012). These characteristics, combined with powerful fly genetics, make the Drosophila midgut an ideal model system for studying stem-cell-driven diseases.
O-GlcNAcylation is a nutrient-driven post-translational modification that is associated with the cellular stress response (Bond and Hanover, 2015; Martinez et al., 2017). The enzyme that adds this monosaccharide onto serine and threonine residues of intracellular proteins, O-GlcNAc transferase (OGT), utilizes the product of the hexosamine biosynthetic pathway UDP-GlcNAc as the sugar donor. The O-GlcNAc modification is hydrolyzed by O-GlcNAcase (OGA), thus creating a dynamic cycle of addition and removal (Figure 1G) (Bond and Hanover, 2015). Evidence suggests that O-GlcNAcylation acts as a nutrient sensor to control many physiological processes, including cell signaling, metabolism, development, and aging (Hart, 2014; Harwood and Hanover, 2014). Interestingly, O-GlcNAc levels are elevated in various cancer types, including breast, prostate, colon, lung, pancreas, and colorectal cancer (Gu et al., 2010; Hanover et al., 2018; Lynch et al., 2012; Ma and Vosseller, 2013; Shi et al., 2010; Yehezkel et al., 2012). In conjunction with its oncogenic role, O-GlcNAc plays a critical role in mammalian stem cell self-renewal by regulating the pluripotency gene network (Jang et al., 2012). However, the role of O-GlcNAc as a nutrient sensor in adult stem cells and how O-GlcNAc promotes oncogenesis remains unclear.
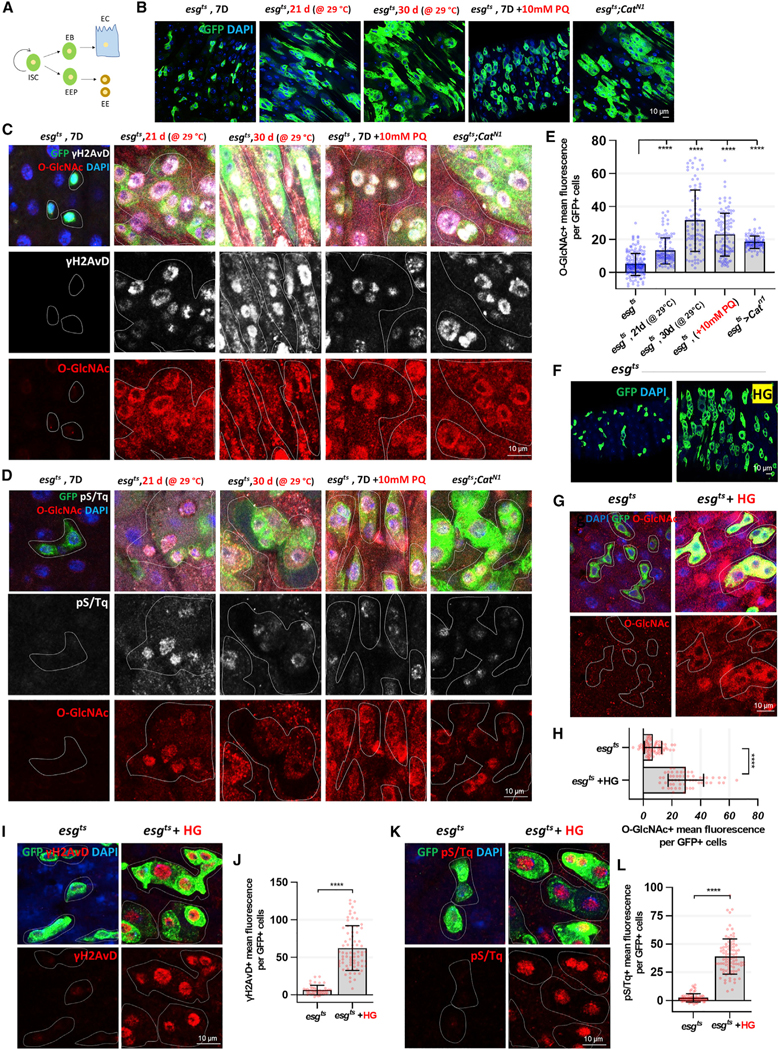
Figure 1. Aging, Oxidative Stress, and High-Glucose Uptake Increase O-GlcNAc, γH2AVD, and pS/Tq in ISCs/EBs.
(A) Model of the ISC regeneration and lineage specification process.
(B) Immunofluorescence staining to analyze esg-GFP (green) in midgut of flies.
(C) Immunofluorescence staining of O-GlcNAc (red) and γH2AvD (white) in esg-GFP-positive cells (green) in midgut of flies.
(D) Immunofluorescence staining of O-GlcNAc (red) and pS/Tq (white), which indicates ATM/ATR activity, in esg-GFP-positive cells (green) from the midgut.
(E) Quantification of O-GlcNAc mean fluorescence per esg-GFP-positive cell.
(F) After 5 days of induction at 29°C, immunofluorescence staining to analyze esg-GFP (green) in midguts of esgts>+ flies fed 5% sucrose or 20% sucrose (HG) for 24 h.
(G) Immunofluorescence staining of O-GlcNAc (red) in midgut of esgts>+ flies fed 5% sucrose or 20% sucrose (HG) for 24 h.
(H) Quantification of O-GlcNAc mean fluorescence per esg-GFP-positive cell.
(I) Immunofluorescence staining of γH2AvD (red) in midgut of esgts>+ flies fed 5% sucrose or 20% sucrose (HG).
(J) Quantification of γH2AvD mean fluorescence per esg-GFP-positive cell.
(K) Immunofluorescence staining of pS/Tq (red) in midgut of esgts>+ flies fed 5% sucrose or 20% sucrose (HG).
(L) Quantification of pS/Tq mean fluorescence per esg-GFP-positive cell.
Outline indicates esg-positive cells. Data are represented as mean ± SD. ****p < 0.0001; see Table S1 for N values.
Dysregulation of O-GlcNAc cycling on cancer-relevant targets, including DNA-damage-repair-related proteins and stress-related pathways, likely contributes to oncogenesis (de Queiroz et al., 2014; Hanover et al., 2018). In order to maintain tissue homeostasis, adult stem cells exquisitely coordinate the action of a network of cellular processes, including DNA replication, DNA repair, and cell-cycle progression, all of which are sources of DNA damage (Hanover et al., 2018; Maréchal and Zou, 2013). The DDR pathway is thought to be regulated by a phosphorylation cascade in which histone H2AX is phosphorylated (γH2AX) by ataxia telangiectasia mutated (ATM), ATM-and Rad3-related (ATR), and DNA-dependent protein kinase (DNA-PK) (Wahl and Carr, 2001). The DDR signal is further propagated downstream to mediator kinases, such as the checkpoint kinases 1 and 2 (CHK1/CHK2) (Wahl and Carr, 2001). The DDR pathway is known to promote differentiation in several cell line-ages and is critical for stem cell development (Oh et al., 2014). Regulation of DDR-related genes is associated with aging and cancer (Li et al., 2016). Interestingly, DDR-related factors are well represented among proteins that accumulate O-GlcNAc under cell stress conditions (Kátai et al., 2016). In addition, in vitro studies suggested that OGT relocates to sites of DNA damage and catalyzes O-GlcNAcylation of histone H2AX, mediator of DNA damage checkpoint 1 (MDC1), and histone H2A. Furthermore, O-GlcNAc has been reported to enhance double-strand break (DSB) repair and promote cancer cell proliferation in vivo and in vitro (Efimova et al., 2019). Thus, O-GlcNAcylation is potentially an important regulator of the DDR and provides a mechanistic link between metabolic reprogramming and genomic instability (Efimova et al., 2019; Hanover et al., 2018). However, how the DDR is linked to O-GlcNAc cycling has not been directly examined.
Here, we used the Drosophila midgut to investigate the role of O-GlcNAc in adult stem cells and the relationship between O-GlcNAc and DDR in ISCs/EBs. We showed that O-GlcNAc levels are increased in age-, oxidative stress-, and high-glucose-diet-induced ISCs/EBs, which become hyperproliferative in response to these sources of stress. Blocking O-GlcNAc cycling in ISCs/EBs by OGA knockdown or deletion increased proliferation. Interestingly, blocked O-GlcNAc cycling induced DDR-related gene markers in ISCs/EBs. The elevated O-GlcNAc levels and hyperproliferation phenotype were rescued by knockdown of DDR-related factors including ATM, ATR, CHK1, and CHK2 in ISCs/EBs. Furthermore, OGT was required for the DDR in ISCs/EBs under oxidative stress conditions. We also identified a positive feedback loop to stabilize OGT protein levels involving CHK1 and CHK2, two key effectors of DDR. Using genetic and pharmacologic tools, we showed that this pathway is conserved in the mouse. Mutants with blocked O-GlcNAc cycling showed dramatically elevated DDR activation in mouse embryonic stem cells (mESCs) and mouse embryonic fibroblasts (MEFs). Our findings reveal that O-GlcNAc acts as an unexpected central regulatory module to control ISC and progenitor cell proliferation and DDR. The results highlight the importance of hexosamine signaling in normal tissue homeostasis and the potential role of O-GlcNAcylation in stress- and nutrient-sensitive stem cell diseases like cancer.
RESULTS
Aging, Oxidative Stress, and High Glucose Exposure Leads to Elevated O-GlcNAc Levels That Correlate with Elevated γH2AVD and pS/Tq in ISCs/EBs
Aberrant O-GlcNAcylation contributes to age-related diseases, including type 2 diabetes, Alzheimer’s disease, and cancer (Banerjee et al., 2016). High proliferation rates and DDR, including DNA replication and DNA repair, are hallmarks of metastatic cancers (Broustas and Lieberman, 2014). Thus, we hypothesized that there may be a mechanistic link between O-GlcNAcylation and DDR that contributes to ISC homeostasis. To test this hypothesis, we used different stress factors such as aging, paraquat (PQ) treatment (an inducer of extrinsic oxidative stress), and Catn1 mutation (a genetic model of intrinsic oxidative stress) using esgGAL4,UASGFP,TubGAL80TS flies (ISC/EB cell-specific inducible GAL4). As shown in Figure 1A, esg-GFP positive precursor EB and EE precursor (EEP) cells arise from ISCs; the cells lose this stem cell marker upon differentiation to EC and EE cells. Our findings indicated that the number of esg-GFP positive cells was significantly increased by the stress-inducing agents we analyzed, including aging, PQ treatment, and esgts;Catn1 mutant midguts, which lead to dysplasia (Figure 1B), similar to previous reports (Choi et al., 2008). We then examined O-GlcNAc, γH2AVD (mammalian phospho H2AX, a double-stranded DNA break marker), and pS/Tq (phospho ATM/ATR marker) levels in age- and oxidative-stress-induced ISCs. H2AX, ATM, and ATR are regulators of DDR, including DSBs (Maréchal and Zou, 2013). Interestingly, O-GlcNAc levels were increased, similar to those of the DDR markers γH2AVD and pS/Tq in ISCs/EBs of the stressed flies (Figures 1C and 1D). The increased O-GlcNAc levels significantly correlated with γH2AVD and pS/Tq signals in esg-positive cells in aged, PQ-treated, and esgts;Catn1 mutant flies (Figures 1C–1E). Therefore, our data revealed that O-GlcNAcylation increased in aged and oxidative-stress-induced ISCs/EBs, suggesting a potential relationship between O-GlcNAc and the DDR in adult stem cells and progenitor cells of the Drosophila midgut.
Next, we assessed O-GlcNAcylation and DDR levels in the midgut ISCs and precursor cells of high-sucrose-fed (increased glucose) flies. O-GlcNAc is regulated, in part, by the nutrient-dependent HBP (hexosamine biosynthetic pathway) pathway; elevated glucose increases flux through the HBP. Elevated glucose metabolism is associated with higher proliferation rates and changes in metabolism and signaling (Hanover et al., 2018). High glucose utilization has been linked to both diabetes and cancer (Joshi et al., 2015). To investigate whether high glucose induced DDR in stem cells and progenitor cells, we fed flies a normal (5% sucrose) diet or high-sugar (20% sucrose) diet. The number of esg-positive cells increased in midguts from high-sucrose-fed flies (Figure 1F). O-GlcNAc, γH2AVD, and pS/Tq levels were also significantly increased in midgut ISCs/EBs from high-sugar-fed flies (Figures 1G–1L).
Because the esg promoter was expressed in both ISCs and EBs, we wanted to confirm the association between stress and O-GlcNAcylation in ISCs and EBs specifically. To this end, we examined O-GlcNAcylation levels and DDR levels in aged, oxidative-stress-induced, and high-glucose uptake using a esgts>GFP,Su-Gal80 (ISC-driven Gal4)/ Suts>GFP (EB-driven Gal4) system. Our data showed O-GlcNAc and γH2AVD levels increased in GFP-positive cells (ISCs) of esgts,Su-Gal80 or GFP-positive cells (EBs) of Suts>GFP in high glucose, oxidative stress, and aging midguts (Figure S1). In the Suts>GFP midguts, increased levels of DDR markers and O-GlcNAc were detected in non-GFP cells, which were likely the ISCs (Figure S1D). These data indicated that stress induces O-GlcNAc and DDR levels in both ISCs and EBs. Therefore, our data indicated that high-sugar uptake induced elevated O-GlcNAc levels and a robust DDR in both ISCs and EBs, suggesting an association between nutrient utilization and DDR in these adult stem and precursor cells.
Blocked O-GlcNAc Cycling in ISCs/EBs Promotes DDR and Hyperproliferation
Our data indicated that O-GlcNAc levels are increased in oxidative-stress-induced ISCs/EBs (Figure 1). Accordingly, we assessed whether blocking O-GlcNAc removal to elevate O-GlcNAcylation in ISCs/EBs would result in intestinal dysplasia in Drosophila. We used flies in which the enzyme that cleaves O-GlcNAc—OGA—was deleted (OGAdel.1 mutant) or knocked down (OGARNAi) in ISCs/EBs. After incubating at 29°C for 7 days, we observed ISC/EB hyperproliferation in esgts;OGAdel.1 and esgts>OGARNAi midguts. There was an increase of the number of esg-positive cells, PH3-positive cells, Delta-positive cells (ISC marker), and EdU-positive cells in esgts;OGAdel.1 and esgts>OGARNAi midguts (Figures 2A, 2B, and S2A–S2D). Loss of OGA in these strains resulted in increased O-GlcNAc (Figures 2C and 2D). O-GlcNAc cycling is known to impact insulin signaling in Drosophila (Sekine et al., 2010). Because insulin signaling has been shown to impact extracellular signal-regulated kinase (ERK) signaling (Slack et al., 2015), we looked at phosphorylation of AKT and ERK and found both significantly increased in esgts;OGAdel.1 and esgts>OGARNAi midgut ISCs/EBs (Figures S2F–S2I). Together, these data suggested that increased O-GlcNAc resulting from loss of OGA led to an activation of insulin signaling and associated ISC hyperproliferation/dysplasia similar to that observed with age- and oxidative-stress-induced Drosophila midguts.
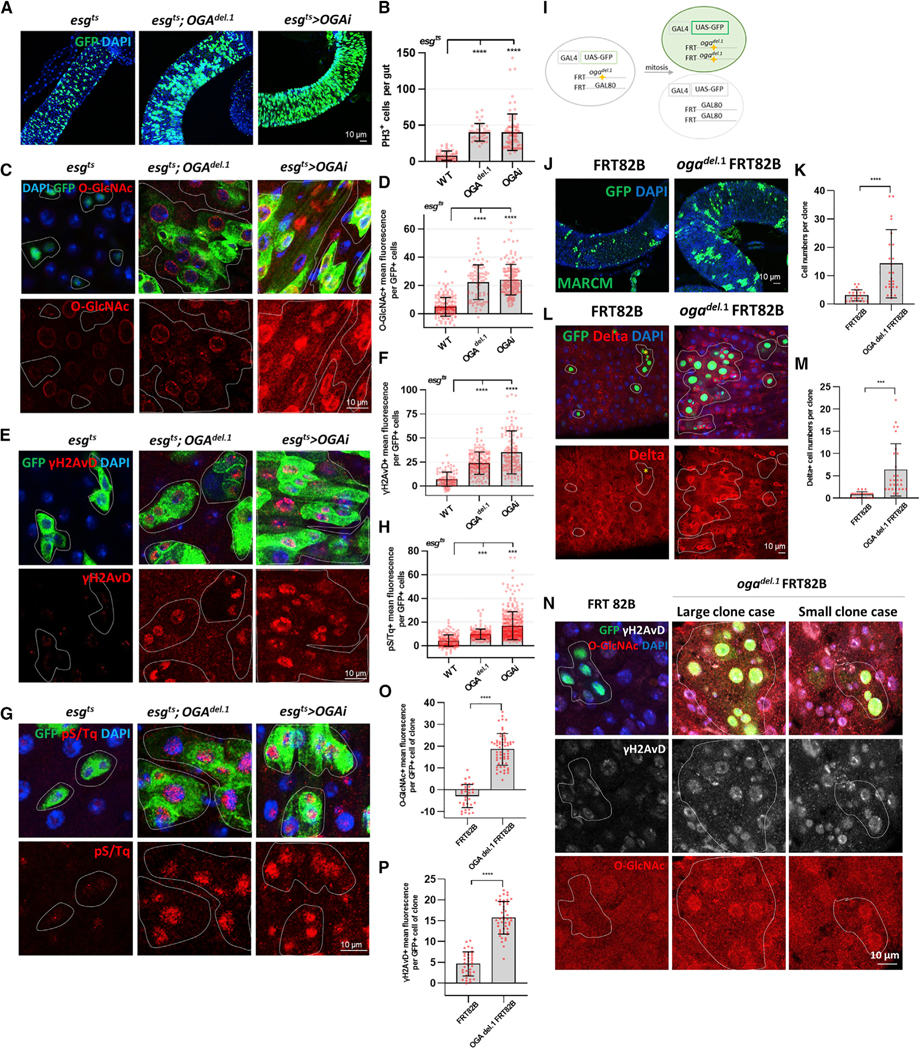
Figure 2. Elevated O-GlcNAc in ISCs/EBs Promoted Proliferation and DDR.
(A) After 7 days of induction at 29°C, immunofluorescence staining of esg-GFP (green) in midgut of flies.
(B) Quantification of PH3-positive cells (mitotic marker) in fly midguts.
(C) Immunofluorescence staining of O-GlcNAc (red) in esg-GFP-positive cells (green) in midgut of flies.
(D) Quantification of O-GlcNAc mean fluorescence per esg-GFP-positive cell.
(E) Immunofluorescence staining of γH2AvD (red) in esg-GFP-positive cells (green) in midgut of flies.
(F) Quantification of γH2AvD mean fluorescence.
(G) Immunofluorescence staining of pS/Tq (red) in esg-GFP-positive cells (green) in midgut of flies.
(H) Quantification of pS/Tq mean fluorescence. Outline indicates esg-positive cells.
(I) The MARCM technique allows one to visually determine, after mitosis and cell division, those cells that maintain one or two copies of Gal80 and are unlabeled,and those cells without Gal80 that are homozygous for the mutation (OGA del.1 FRT82B) and labeled with GFP fluorescence.
(J) Immunofluorescence staining to analyze GFP (green) in midgut of flies.
(K) Quantification of cell numbers per clone in midgut of flies.
(L) Immunofluorescence staining of Delta (red) in midgut.
(M) Quantification of Delta-positive cells per clone.
(N) Immunofluorescence staining of O-GlcNAc (red) and γH2AvD (white) in GFP-positive cells (green) in midgut of flies.
(O) Quantification of O-GlcNAc mean fluorescence per GFP-positive cell.
(P) Quantification of γH2AvD mean fluorescence.
Outline indicates clone (GFP-positive cells). Data are represented as mean ± SD. ****p < 0.0001, ***p < 0.001; see Table S1 for N values.
The observed dysplasia caused by loss of OGA suggested a potential activation of DDR in midguts of these mutant strains. To analyze the DDR, we stained for DDR markers anti-γH2AVD and anti-pS/Tq. Indeed, the levels of γH2AVD and pS/Tq were significantly increased in esgts;OGAdel.1 and esgts>OGARNAi ISCs/EBs compared to control (Figures 2E–2H). Thus, increasing levels of O-GlcNAc without any additional external stressors induced DDR.
In order to examine potential differences between the ISC and EB populations, we examined O-GlcNAcylation and DDR in either ISC- or EB-specific OGA knockdown flies. Our data indicated that O-GlcNAc, DDR, and ISC proliferation increased in ISC-specific and EB-specific OGA knockdown (Figure S3). Therefore, elevated O-GlcNAc promoted proliferation and DDR in both ISCs and EBs.
We next accessed ISC proliferation in OGAdel.1 mutants using the MARCM (mosaic analysis with a repressible cell marker) system (Figure 2I). Our data showed the number of cells per clone (white line) and Delta-positive cells increased in OGAdel.1 mutant midguts (Figures 2J–2M). O-GlcNAc and gH2AvD levels increased not only in large-sized clones, but also in small-sized clones of OGAdel.1 mutant midguts compared to control (Figures 2N–2P). Therefore, O-GlcNAc, DDR level, and ISC proliferation were induced in the OGAdel.1 mutant. These data confirmed the link between O-GlcNAc cycling with DDR and hyperproliferation in midguts.
To explore whether the proliferation and DDR phenotypes were specific to the adult ISCs/EBs, we generated flies that knocked down OGA in ECs using the Myo1Ats,UAS-GFP,Gal4 system (EC-cell-specific inducible GAL4). After incubation at 29°C for 5 days, we observed a significant increase in O-GlcNAc levels in Myo1Ats>OGARNAi GFP-positive cells (ECs) (Figure S4A, white arrows). However, neither γH2AVD nor pS/Tq levels were significantly changed in EC cells or ISCs/ EBs of Myo1Ats>OGARNAi midguts (Figures S4E–S4H). Further, the number of PH3-positive cells was similar to control (Figure S4). Therefore, these data suggested that blocked O-GlcNAc cycling triggers DDR in midgut ISCs/EBs but not closely associated somatic cells. Interestingly, unlike the OGA knockdown, EC-specific OGT knockdown induced apoptosis (as indicated by caspase 3 cleavage; Figure S4D) and caused ISC hyperproliferation (as indicated by Delta staining; Figure S4I) and DNA damage accumulation (Figures S4E–S4H). Similarly, EC-specific DDR knockdown induced apoptosis in these cells, indicating that DDR is required for EC cell survival (Park et al., 2018). This apoptosis caused ISC hyperproliferation and accumulation of DNA damage. Thus, OGT and DDR are required for EC cell maintenance.
Intestinal Dysplasia Induced by Blocked O-GlcNAc Cycling Was Rescued by DDR-Related Gene Knockdowns in ISCs/EBs
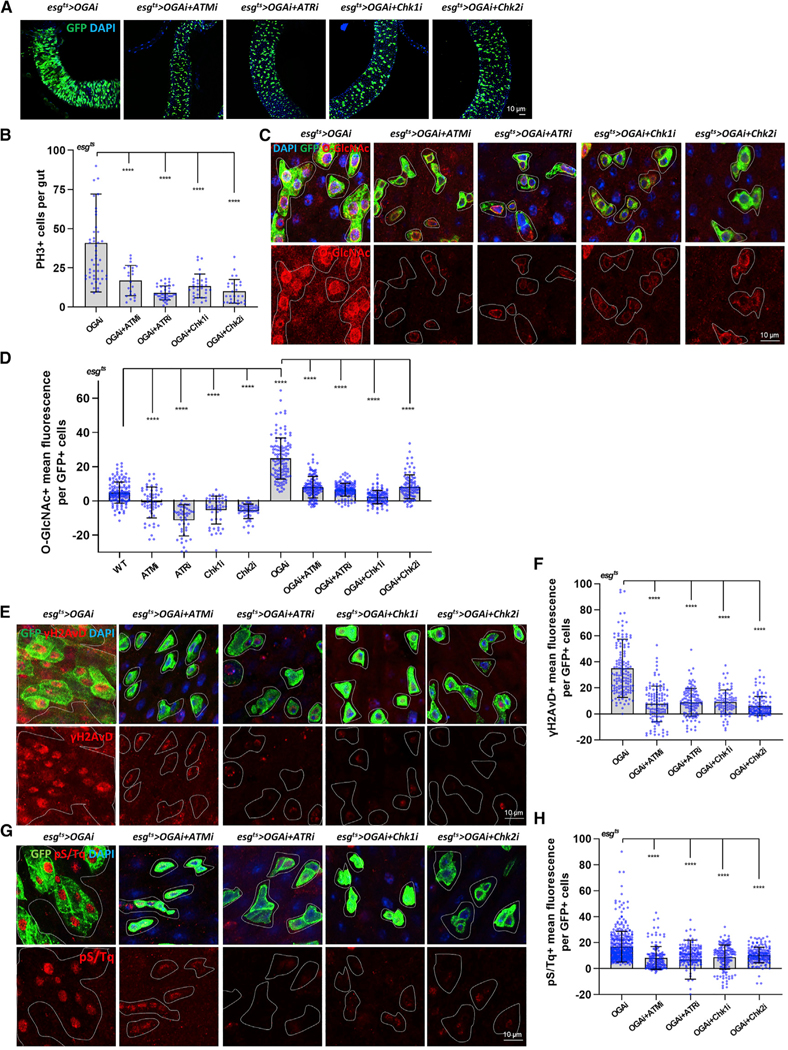
To determine where in the pathway O-GlcNAc may act, we generated double-knockdown flies. We combined the OGA knockdown with knockdown of ATM, ATR (kinases that act as DDR sensors), or CHK1/CHK2 (kinases that act as DDR mediators) strains. We assessed ISC proliferation rate in the midguts (esg-positive cells and PH3-positive cells) and found that the double knockdowns all rescued the hyperproliferation observed in the OGA single-knockdown midgut (Figures 3A and 3B). These findings suggested that ATM, ATR, CHK1, and CHK2 were all required for the hyperproliferation of ISCs observed with loss of OGA.
Figure 3. O-GlcNAc-Induced Intestinal Dysplasia Was Rescued by DDR-Related Gene Knockdowns in ISCs/EBs.
(A) After 7 days of induction at 29°C, immunofluorescence staining of esg-GFP (green) in midgut of flies from the indicated genotype.
(B) The number of PH3-positive cells in midguts from flies of the indicated genotype.
(C) Immunofluorescence staining of O-GlcNAc (red) in esg-GFP-positive cells (green) in midgut of flies from the indicated genotype.
(D) Quantification of O-GlcNAc mean fluorescence.
(E) Immunofluorescence staining of γH2AvD (red) in midgut of flies from the indicated genotypes.
(F) Quantification of gH2AvD fluorescence means per esg-GFP-positive cell.
(G) Immunofluorescence staining of pS/Tq (red) in midgut of flies.
(H) Quantification of pS/Tq fluorescence means per esg-GFP-positive cell.
Outline indicates esg-positive cell. Data are represented as mean ± SD. ****p < 0.0001; see Table S1 for N values.
We assessed O-GlcNAc levels in double-knockdown fly midguts. Interestingly, we found that O-GlcNAc was significantly decreased in ISCs/EBs in all DDR knockdown flies (Figures 3C and 3D), indicating that O-GlcNAc levels were responsive to loss of DDR sensors and mediators. We further analyzed the single knockdown of ATM, ATR, CHK1, and CHK2. As expected, when these factors were knocked down, there were no detectable markers of DDR or change in proliferation (Figures S5D and S5E). Surprisingly, these single mutant knockdown strains had dramatically decreased O-GlcNAcylation compared to wild type (WT) (Figures 3D and S5C).
Next, we assessed DDR accumulation in double-knockdown fly midguts. We found a significant decrease in both γH2AVD and pS/Tq signal in ISCs/EBs of esgts>OGARNAi+ATMRNAi, esgts>OGARNAi+ATRRNAi, esgts>OGARNAi+Chk1RNAi, and esgts> OGARNAi+Chk2RNAi double-knockdown flies compared to esgts>OGARNAi ISCs/EBs (Figures 3E–3H). These findings suggested that OGA was acting upstream of these DDR-related factors since loss of the downstream effectors prevented accumulation of γH2AVD. Therefore, these data indicated that O-GlcNAc may act as both an effector and an inducer of DDR and proliferation to regulate intestinal homeostasis. We therefore designed experiments to understand how DDR signaling might alter O-GlcNAc levels.
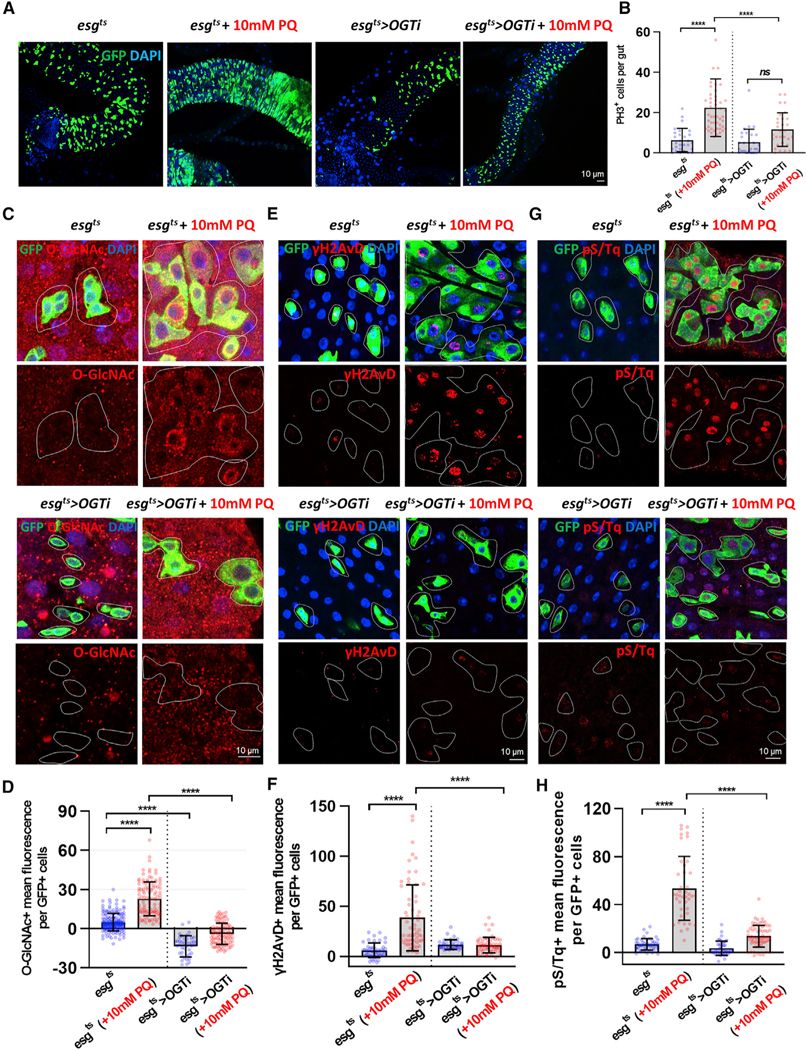
Oxidative-Stress-Induced DDR and Proliferation in ISCs/EBs Required OGT and Was O-GlcNAc Dependent
Our data suggested that hyper-O-GlcNAcylation resulted from PQ treatment (Figure 1) and might contribute to activation of DDR in ISCs/EBs (Figure 2). Next, we investigated whether O-GlcNAc was required for DDR and proliferation in ISCs/EBs under stress conditions. To this end, we examined ISC proliferation and DDR using the strain esgts>OGTRNAi (decreased O-GlcNAc due to OGT reduction) with or without PQ. We investigated whether depletion of OGT prevented PQ-induced hyperproliferation and found that there was a significant decrease in esg-positive cells as well as in PH3-positive ISCs/ EBs in PQ-treated esgts>OGTRNAi midguts versus PQ-treated control midguts (Figures 4A and 4B). Additionally, upon OGT knockdown, these cells had undetectable levels of O-GlcNAc even when treated with PQ (Figures 4C and 4D). Loss of OGT did not affect the number of PH3-positive cells as compared to controls (Figure 4B). Further, there was no change in gH2AVD and pS/Tq in esgts>OGTRNAi midgut esg-positive cells as compared to controls (Figures 4E–4H). Strikingly, loss of OGT resulted in a significant decrease of the DDR markers γH2AVD (Figures 4E and 4F) and pS/Tq (Figures 4G and 4H) in PQ-treated cells compared to PQ-treated control esg-positive cells.
Figure 4. Oxidative-Stress-Induced DDR and Proliferation in ISCs/EBs Required OGT.
(A) After 6 days of induction at 29C, immunofluorescence staining of esg-GFP (green) in midguts of esgts>+ and esgts>OGTRNAi transgene flies followed by treatment with 10 mM PQ in 5% sucrose or 5% sucrose alone for 20 h at 29°C.
(B) The number of PH3-positive cells in midguts from flies.
(C) Immunofluorescence staining images of O-GlcNAc (red) in esg-GFP-positive cells (green) in midgut of flies from the indicated genotype and treatment.
(D) Quantification of O-GlcNAc mean fluorescence per esg-GFP-positive cell.
(E) Immunofluorescence staining of γH2AvD (red) in midgut of flies from the indicated genotype and treatment.
(F) Quantification of γH2AvD mean fluorescence per esg-GFP-positive cell.
(G) Immunofluorescence staining of pS/Tq (red) in midgut of flies from the indicated genotype and treatment.
(H) Quantification of pS/Tq mean fluorescence per esg-GFP-positive cell.
Outline indicates esg-positive cell boundary. Data are represented as mean ± SD. ****p < 0.0001. n.s., not significant; see Table S1 for N values.
Next, we used a Myc-OGT construct to overexpress OGT and increase O-GlcNAc in ISCs/EBs. Since no antibodies are available to reliably detect endogenous Drosophila OGT, we used our previously described Myc-tagged OGT overexpression flies (Sekine et al., 2010) to monitor OGT levels, increase O-GlcNAcylation, and induce DDR. As shown in Figures 5B and 5C, the overexpression of Myc-tagged OGT led to a hyper-proliferation phenotype identical to that induced by stress or loss of OGA. Myc-OGT and O-GlcNAcylation levels were confirmed to be elevated in esgts>Myc-OGT flies ISCs/EBs (Figures 5D–5G). The DDR markers, γH2AVD and pS/Tq signal, were also significantly increased in esgts>Myc-OGT flies ISCs/ EBs (Figures 5H–5K). Thus, our data confirmed that OGT and the resultant O-GlcNAc elevation were required for normal stress-induced DDR and proliferation.
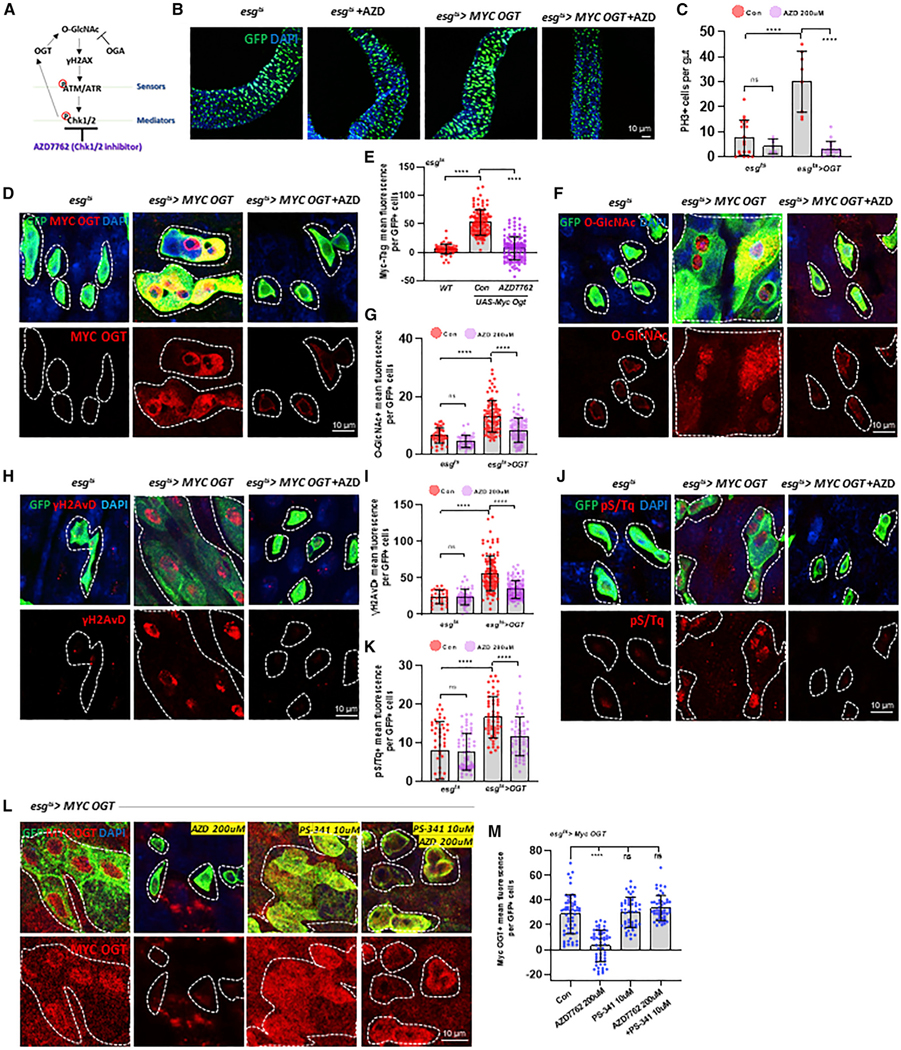
Figure 5. OGT Levels Are Regulated by CHK1/CHK2.
(A) Diagrammatic illustration of the DDR Chk1/2 regulating OGT.
(B) Immunofluorescence staining of esg-GFP (green) in midgut of esgts>+ and esgts>Myc OGT transgene without or with AZD 200-uM treatment for 7 days.
(C) The number of PH3-positive cells in midguts.
(D) Immunofluorescence staining images of Myc-OGT (red) in esg-GFP-positive cells (green) in midgut of flies with or without AZD treatment as indicated.
(E) Quantification of Myc-OGT mean fluorescence per esg-GFP-positive cell in midgut.
(F) Immunofluorescence staining of O-GlcNAc (red) in esg-GFP-positive cells (green) in midgut of flies from the indicated genotype and treatment.
(G) Quantification of O-GlcNAc fluorescence means per esg-GFP-positive cell.
(H) Immunofluorescence staining images of γH2AvD (red) in esg-GFP-positive cells (green) in midgut of flies from the indicated genotype and treatment.
(I) Quantification of γH2AvD mean fluorescence per esg-GFP-positive cell.
(J) Immunofluorescence staining images of pS/Tq (red) in esg-GFP-positive cells (green) in midgut of flies from the indicated genotype and treatments.
(k) Quantification of pS/Tq fluorescence means per esg-GFP-positive cell.
(L) Immunofluorescence staining images of Myc-OGT (red) and GFP (green) in esgts>Myc OGT midguts with AZD 7762 treatment, PS-341 treatment, or both AZD 7762 and PS-341 treatment for 5 days.
(M) Quantification of OGT mean fluorescence.
Outline indicates the boundary of esg-positive cells. Data are represented as mean ± SD. ****p < 0.0001. n.s. not significant; see Table S1 for N values.
OGT Stability, O-GlcNAcylation, and DDR Activation Were Regulated by CHK1/CHK2, Suggesting Feedback Regulation of DDR and ISC Homeostasis
To further understand how O-GlcNAc might be regulated by DDR signaling, we hypothesized that the kinases CHK1 and CHK2 could be involved in the stabilization of OGT. A previous in vitro study in mammalian cell lines demonstrated that CHK1 phosphorylates OGT at serine 20, with apparent stabilization of OGT (Li et al., 2017). Intriguingly, we observed that mammalian OGT ser 20 is in a highly conserved domain in Drosophila (ser49). We then tested whether CHK1 and CHK2 could also be directly regulating OGT abundance in Drosophila ISCs/EBs. First, we used a chemical inhibitor of CHK1/CHK2, AZD7762 (Cahova et al., 2015) (Figure 5A), and examined ISC proliferation in esgts>+ and esgts>Myc-OGT flies treated with AZD compared to non-treated flies. Our data indicated that GFP-positive and PH3-positive cells increased in OGT-overexpressed flies’ midguts (Figures 5B and 5C). In support of our hypothesis, Myc-OGT levels were substantially decreased when esgts>Myc-OGT flies were treated with AZD (Figures 5D and 5E). Concomitant with the loss of OGT was a significant decrease in O-GlcNAc levels in the AZD-treated esgts>MycOGT midgut ISCs/EBs (Figures 5F and 5G). Next, we investigated the impact on DDR in the OGT-overexpression midguts upon AZD treatment. Whereas OGT overexpression induced DDR, these increases in γH2AVD and pS/Tq signal were also rescued by AZD treatment (Figures 5H–5K). Our data revealed that O-GlcNAc-induced DDR in esgts>Myc-OGT flies was dependent on CHK1/CHK2 activity.
To better understand the mechanism of the OGT-DDR interactions, we assessed the levels of Myc-OGT and γH2AVD with OGT overexpression and CHK1 or CHK2 knockdown. We found that Myc-OGT levels significantly decreased when either CHK1 or CHK2 was knocked down (Figures S6A and S6B). In addition, γH2AVD expression levels decreased by knock down of CHK1/CHK2 (Figures S6C and S6D). Because Myc-OGT was expressed from a ubiquitous promoter, it was highly unlikely that OGT levels were increased due to transcriptional regulation. To test if CHK1/2 was acting to stabilize the OGT protein, we treated flies with both AZD and the proteasome inhibitor PS-341. We found that when the proteasome was inhibited, AZD treatment did not impact Myc-OGT levels (Figures 5L and 5M). Taken together, these findings suggested that O-GlcNAc participates in an autoregulatory feedback loop where CHK1/CHK2 stabilizes OGT, contributing to O-GlcNAcylation and further inducing the DDR pathway.
O-GlcNAc Cycling Plays a Conserved Role in DDR in Mammalian Cells
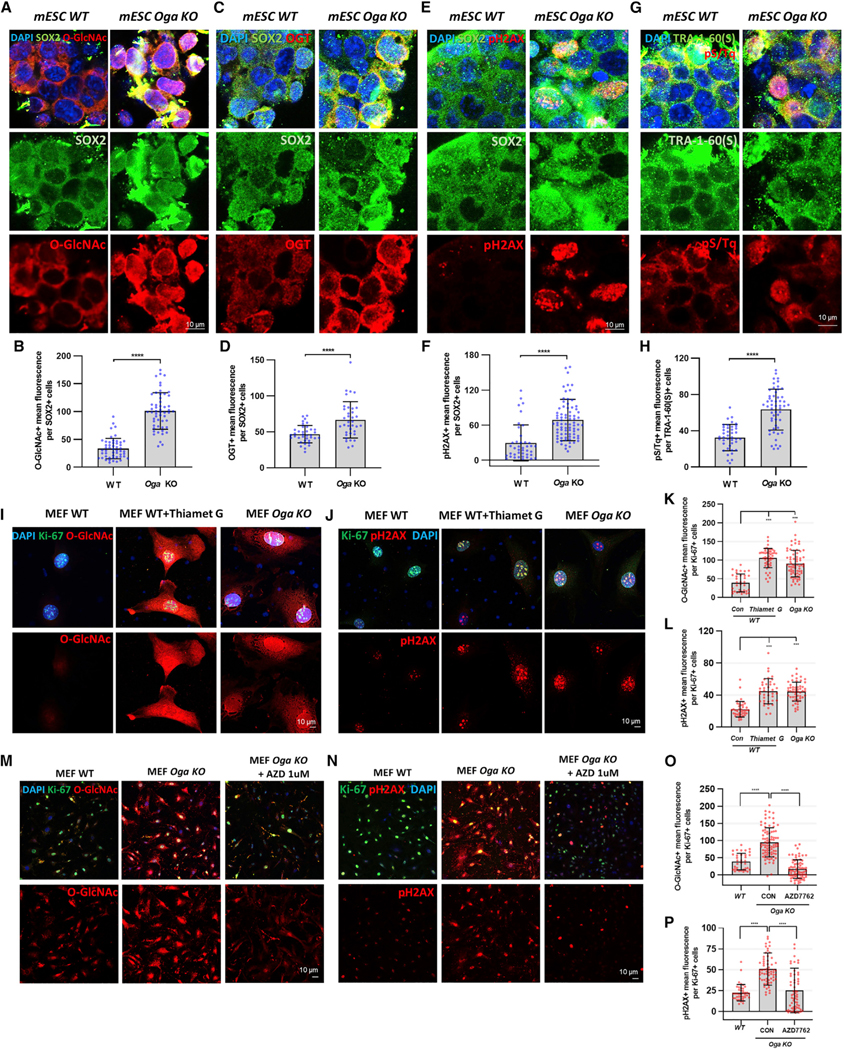
Here, we have presented a mechanism by which O-GlcNAc induced DDR in Drosophila ISCs/EBs. To explore the conservation of this pathway, we assessed the relationship between O-GlcNAc and DDR in mammalian cell lines with defined genetic backgrounds. To investigate the conserved relationship between O-GlcNAc and DDR, we first used Oga knockout (KO) and isogenic WT mESCs previously generated (Olivier-Van Stichelen et al., 2017). The stem cell pluripotency marker SOX2 was monitored in the same cells and showed little variation between mESCs derived from WT and Oga KO animals. We found that Oga KO mESCs had increased levels of O-GlcNAc and OGT compared to WT mESCs. (Figures 6A–6D). We also found that phosphO-H2AX levels and pS/Tq levels were significantly increased in Oga KO mESCs compared to WT cells (Figures 6E–6H). Thus, as we observed in the Drosophila ISCs/EBs, DDR and O-GlcNAc levels were greatly increased in Oga KO mESCs. The resultant increases in OGT in the Oga KO mESCs were also consistent with our findings in the Drosophila ISCs/EBs.
Figure 6. O-GlcNAc Cycling Plays a Conserved Role in DDR in Mammalian Cells.
(A) Immunofluorescence staining images of O-GlcNAc (red) and the pluripotency marker SOX2 (green) in mouse embryonice stem cells (mESCs) derived from wild-type (WT) and Oga knockout (KO) mice.
(B) Quantification of O-GlcNAc mean fluorescence in SOX2-positive cells.
(C) Immunofluorescence staining images of OGT (red) and SOX2 (green) in WT and Oga KO mESCs.
(D) Quantification of OGT mean fluorescence.
(E) Immunofluorescence staining images of pH2AX (red) and SOX2 (green) in WT and Oga KO mESCs.
(F) Quantification of pH2AX mean fluorescence in SOX2-positive cell.
(G) Immunofluorescence staining images of pS/Tq (red) and the pluripotency marker TRA-1–60 (green) in WT and Oga KO mESCs.
(H) Quantification of pS/Tq mean fluorescence in TRA-1–60-positive cells. ****p < 0.0001.
(I) Immunofluorescence staining images of O-GlcNAc (red) and the proliferation marker Ki-67 (green) in WT, Thiamet-G-treated WT, and Oga KO mouse embryonic fibroblasts (MEFs).
(J) Immunofluorescence staining images of pH2AX (red) and Ki-67 (green) in WT, Thiamet-G-treated WT, and Oga KO MEFs.
(K) Quantification of O-GlcNAc mean fluorescence.
(L) Quantification of pH2AX mean fluorescence in Ki-67-positive cell.
(M) Immunofluorescence staining images of O-GlcNAc (red) and Ki-67 (green) in WT and Oga KO MEFs treated with DMSO only or with 1 uM AZD for 7 h.
(N) Immunofluorescence staining images of pH2AX (red) and Ki-67 (green) in WT and Oga KO MEFs treated with DMSO only or with AZD 1 uM for 7 h.
(O) Quantification of O-GlcNAc mean fluorescence in Ki-67-positive cells.
(P) Quantification of pH2AX mean fluorescence in Ki-67-positive cells.
Data are represented as mean ± SD. ****p < 0.0001, ***p < 0.001; see Table S1 for N values.
We then investigated DDR in Oga KO and WT MEFs established from Oga KO mice (Keembiyehetty et al., 2015). MEFs are phenotypically and functionally similar to mesenchymal stem cells (MSCs) (Yusuf et al., 2013). We have previously shown that MEFs are an excellent system to study the role of O-GlcNAc cycling in mammalian cells (St Amand et al., 2018). We observed increased O-GlcNAcylation upon Thiamet G treatment (OGA inhibitor) and in Oga KO MEFs (Figures 6I and 6K). Here, we used Ki-67 as a marker of proliferative MEFs and found a significant increase in phosphO-H2AX staining in Thiamet-G-treated and Oga KO MEFs as compared to controls (Figures 6J and 6L). We also assessed whether Oga KO-induced DDR could be rescued by AZD treatment. In AZD-treated Oga KO Ki-67 positive MEFs, O-GlcNAc, OGT, and phospho-H2AX expression was significantly decreased (Figures 6M–6P), and levels were similar to WT. As observed in flies, using a proteasome inhibitor (MG-132) rescued OGT protein levels of cells treated with AZD (Figures S7A and S7B), further supporting the model that CHK1/2 acts to stabilize OGT.
To assess whether OGT activity or OGA inhibition was the physiological outcome of stress, we induced stress in our MEFs with PQ and used the OGT inhibitor OSMI1. Our data indicated that O-GlcNAc and OGT levels increased in PQ-treated and Thiamet-G-treated WT Ki-67-positive MEF cells (Figures S7C–S7F). When the OGT inhibitor OSMI1 was used in conjunction with PQ treatment, OGT and phosho-H2AX levels were decreased compared to PQ-treated MEFs in WT MEF Ki-67-positive cells (Figures S7G–S7J). Therefore, OGT activity was required for the oxidative-stress-induced DDR and increased O-GlcNAc.
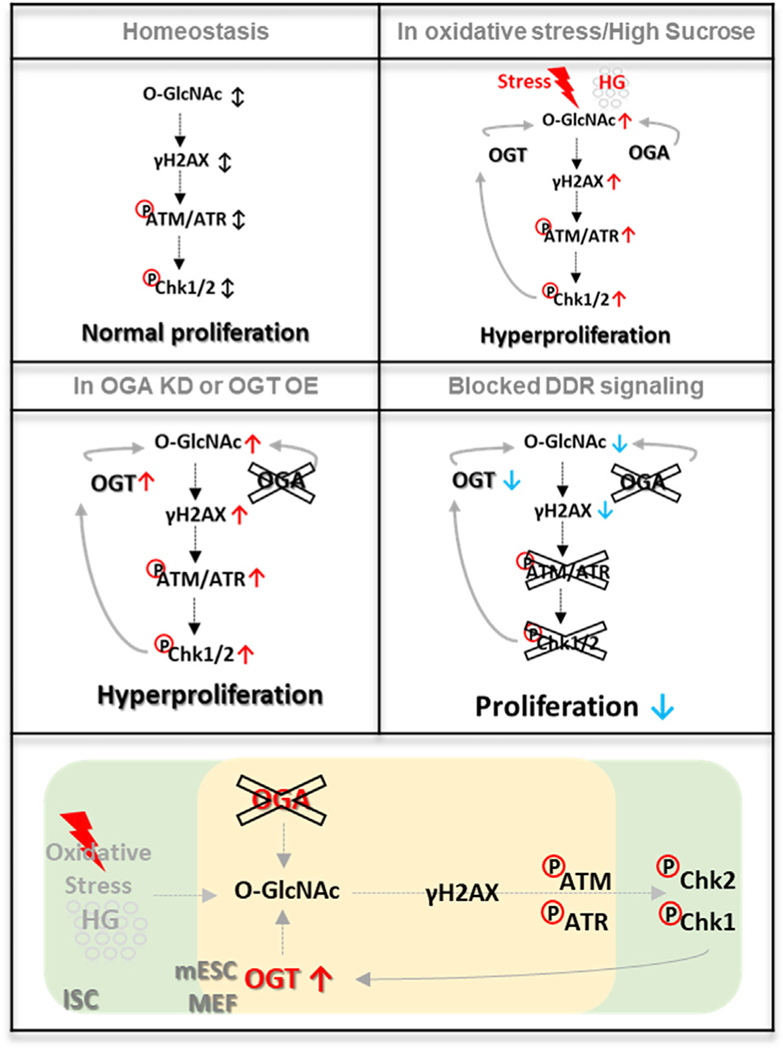
Taken together, O-GlcNAc-induced DDR accumulation was inhibited by CHK1/CHK2 in highly proliferative mammalian cells, as we observed in the Drosophila ISCs/EBs. Our findings suggested that the enzymes of O-GlcNAc cycling each participate in a conserved pathway regulating stem/progenitor cell proliferation and the DDR pathway. A model depicting the roles of O-GlcNAc in intestinal stem cell homeostasis, oxidative stress, altered O-GlcNAc cycling, and altered DDR signaling is shown in Figure 7. Figure 7 also details the conserved features of this pathway including feedforward activation of DDR by stress-induced elevation in O-GlcNAc and feedback regulation of OGT by downstream DDR effectors.
Figure 7. O-GlcNAc Functions as a Key Effector of ISC Proliferation and DDR through Regulation of DDR-Related Factors in Stress-Induced Midguts.
O-GlcNAc expression and DDR increased in high-sucrose diet and oxidative-stress-induced ISCs/EBs, which lead to dysplasia. Knockdown of OGA and overexpression of OGT in ISCs/EBs promoted DDR and hyperproliferation. O-GlcNAc-induced DDR accumulation and hyperproliferation were rescued by DDR-related gene knockdowns such as ATM, ATR, CHK1, and CHK2 in ISCs/ EBs. OGT was regulated by Chk1/Chk2 as a key effector of DDR for ISC/EB homeostasis. Additionally, O-GlcNAc cycling plays a conserved role in DDR in mammalian cells. Therefore, O-GlcNAc is a conserved regulator of ISCs/EBs homeostasis and DNA damage repair, which is critical for maintenance of gut homeostasis.
DISCUSSION
O-GlcNAc-Dependent ISC Dysplasia in Drosophila
The ISC plays an essential role in maintaining tissue and organ function by providing a reservoir of cells for homeostasis and regeneration (Zou et al., 2007). Adult stem cells, like ISCs, must maintain a self-renewing and highly proliferative state supported by elevated nutrient uptake, metabolic reprogramming, and resistance to oxidative damage. How nutrients are utilized to support this task remains largely unknown. Previous reports have shown that pyruvate metabolism mediates Drosophila ISC activation through the HBP pathway leading to UDP-GlcNAc (Mattila et al., 2018). Here, we identified a pathway by which O-GlcNAc regulates ISC homeostasis in vivo (Figure 7). Oxidative stress and a high-sugar diet increased O-GlcNAc in ISCs/EBs, and we showed that elevated O-GlcNAcylation promoted accumulation of DDR and hyperproliferation, triggering intestinal dysplasia. Further, this O-GlcNAc-induced intestinal dysplasia was rescued by knockdown of DDR-related factors in ISCs/EBs. Thus, O-GlcNAc participates in a feedback loop where elevated O-GlcNAcylation triggers DDR, leading to CHK1/CHK2 activation and stabilization of OGT, augmenting O-GlcNAcylation, and further inducing the DDR pathway. Additionally, our data using Oga KO mESCs and proliferating MEFs highlighted the conservation of the pathway. Therefore, we propose that O-GlcNAc is a critical regulator of DDR and proliferation in progenitor cells (Figure 7). Our study underscores the need to define the causative role that O-GlcNAc plays in adult stem cell maintenance and deregulation in disease.
A Conserved Function for O-GlcNAc Cycling in the Initiation and Maintenance of DDR
Our results establish that the general pathway of O-GlcNAc-driven DDR activation is conserved from Drosophila to mammals. Thus, the findings have important implications for stem cell biology and stem-cell-driven diseases such as cancer. Stem cells are often highly exposed to sources of DNA damage, and this can have catastrophic consequences for tissue and organismal homeostasis (Behrens et al., 2014). DDR-related factors, ATM, and DNA-PKcs are themselves tumor suppressors and required for vertebrate development (Maréchal and Zou, 2013). O-GlcNAc has been described to play a protective role during DDR by promoting genome stability via modification of histones (Liu and Li, 2018). Recently, it was also reported that increased O-GlcNAc modification promotes normal functions of DNA damage and repair pathways during irradiation (Efimova et al., 2019). Here, our data indicated that DDR accumulation increased in OGA knockdown ISCs/EBs, mESCs, and MEFs. Furthermore, increased O-GlcNAc correlated with elevated gH2AVD and pS/Tq in age-, oxidative-stress-, and high-glucose-diet-induced ISCs/EBs. This O-GlcNAc-induced intestinal dysplasia could be rescued by knockdown of DDR-related genes and CHK1/2 inhibitor in ISCs/EBs. Interestingly, both OGT levels and O-GlcNAcylation were reduced to very low levels in ATM, ATR, CHK1, and CHK2 knockdown. Therefore, our data suggested that OGT levels are regulated by DDR effectors, and O-GlcNAc acts as both an inducer and a regulatory effector of DDR to regulate ISC/EB homeostasis.
O-GlcNAc has been reported to play multifaceted roles during cell cycle progression, including in DNA replication, mitosis, and cytokinesis (Eustice et al., 2017). Our findings indicated that O-GlcNAcylation is also a key determinant of the DDR. In the case of cancer, our findings could provide a mechanistic link among metabolic reprogramming, genomic instability, and potential therapeutic response. Indeed, O-GlcNAcylation has been shown to be associated with DSB repair, promoting cancer cell proliferation, and preventing therapy-induced senescence in irradiated tumors (Efimova et al., 2019). Unfortunately, the mechanism of these effects had not been studied, despite OGT and O-GlcNAc being implicated in various stress response pathways (Chatham and Marchase, 2010; Zachara et al., 2004). Inhibiting OGT reduced ROS (reactive oxygen species) levels in cells cultured in high glucose (Kim et al., 2017). Our data showed that DHE (dihydroethidium) signal (ROS detection marker) was increased in PQ-treated, high-sucrose-fed (HG), and OGA knockdown ISCs/EBs (Figures S2J and S2K). Furthermore, OGT inhibition ameliorated ER (endoplasmic reticulum) and oxidative stresses and reduced neural tube defects in embryos of diabetic mice (Kim et al., 2017). Increased O-GlcNAc and recruitment of OGT and OGA have been reported at sites of DNA damage. It has been proposed that OGT modifies H2AX on the ATM phosphorylation site S139, decreasing γH2AX formation at sites of DNA damage (Chen and Yu, 2016). This previous study concluded that O-GlcNAcylation of H2AX may serve to limit the expansion of DDR. However, our data indicated that oxidative-stress-induced DDR and proliferation in ISCs/EBs required OGT and that OGT overexpression induced DDR accumulation and proliferation. These phenotypes were rescued by AZD treatment, suggesting a straightforward pharmacological approach to breaking the O-GlcNAc-driven DDR activation process. In vitro, AZD in combination with gemcitabine has been shown to reduce both the percentage and the tumor-initiating capacity of pancreatic cancer stem cells (Venkatesha et al., 2012). Previous work has also shown that CHK1 phosphorylates OGT, stabilizing the protein to regulate the intermediate filament network during cytokinesis (Li et al., 2017). This phosphorylation site is conserved in Drosophila, and we showed that CHK1/CHK2 was required for OGT stabilization. This was an unexpected finding, as OGT has been described to be regulated by O-GlcNAc levels, such that when O-GlcNAc levels increase, OGT expression would decrease (Park et al., 2017). We suggest that the phenotypes described in this manuscript are specific to progenitor cells and are not a feature of fully differentiated cell types. Therefore, we propose that OGT is part of a pathway linking O-GlcNAc metabolism with DDR in Drosophila ISCs/EBs, mESCs, and MEFs. These findings are likely to extend to cancer cells and adult stem cells.
A Vicious Cycle of O-GlcNAc-Driven DDR Activation and Metabolic Reprogramming
Tumorigenesis is dependent on the reprogramming of cellular metabolism as both a direct and an indirect consequence of oncogenic mutations (Hanover et al., 2018; Pavlova and Thompson, 2016). The metabolic reprogramming is associated with elevated glucose uptake (the Warburg effect) contributing to cell growth, altered metabolism, enhanced metastasis, and altered cell surface glycans (Hanover et al., 2018). Dysregulation of O-GlcNAc is associated with tumor growth and metastasis (Singh et al., 2015) and promotes colonic inflammation and tumorigenesis (Yang et al., 2015). Thus, an altered metabolism is associated with cancer cell proliferation and tumorigenesis. Increased metabolite flux can generate a reactive oxygen species that drives activation of DDR. Interestingly, our data indicated that a high-glucose diet induced DDR in nutrient-sensitive adult stem cells. High glucose levels are associated with nutrient-related diseases such as cancer and type 2 diabetes. Thus, the O-GlcNAc-dependent DDR may contribute to the mutagenic effects seen in diseases of aging (Hanover et al., 2018; Liu and Li, 2018). Flux through the HBP terminating in O-GlcNAc has been suggested to play a role in oncogenesis (Flores et al., 2017; Hanover et al., 2018, 2010). The data reported here suggested that activation of the HBP and O-GlcNAcylation are required for activation of DDR downstream of oxidative stress. Moreover, AZD was in phase 1 clinical trials as a cancer therapeutic. Although this inhibitor did not move forward owing to toxic side effects (Sausville et al., 2014), CHK1 and CHK2 remain important therapeutic targets. Our data suggested that targeting CHK1 and CHK2 disrupted the stability of OGT, contributing to the therapeutic potential of targeting these kinases. Taken together, our findings implicate O-GlcNAc as a key regulator of ISCs/EBs homeostasis through regulating the DDR. Of particular significance is the finding that OGT acts to both induce and respond to the DDR pathway. It might be argued that stress first causes DNA damage, inducing the DDR pathway, and then increases O-GlcNAc. However, our data suggest that with just a single genetic mutation in OGA, the DDR was induced, and further, O-GlcNAc was required for stress-mediated DDR. Our study provides a platform for future designs of interventions in which changes in O-GlcNAc can be utilized as a therapeutic for stem-cell-driven diseases like cancer.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, John A. Hanover (jah@helix.nih.gov).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate any unique datasets or code.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Drosophila stocks, culture, and husbandry
Fly stocks were maintained at 25C on standard food under a ~12 h/12 h light/dark cycle. Food consisted of 15.8g yeast, 9g soy flour, 5.2g agar, 67 g cornmeal, and 0.5% propionic acid. To avoid larval overpopulation, < 30 adult flies per vial were transferred to new food vials every 2–3 days.
The following stocks were used in this study: w1118 (BDSC, #3605); esg-Gal4,tub-Gal80ts,UAS-GFP/CyO (esgts>GFP) (gift from B. Edgar) (Jiang et al., 2009); esg-Gal4, UAS-2EYFP/Cyo; Su(H)Gbe-Gal80, tub-Gal80ts/TM3 (esgts;Su-Gal80) (gift from B. Edgar) (Zhang et al., 2019); Su(H)-Gal4-UAS-CD8-GFP/Cyo; tub-Gal80ts/TM6B (Suts>GFP) (gift from B. Edgar) (Xiang et al., 2017); hs-flip, tub-Gal4,UAS-GFP; FRT82B, tub-Gal80/TM6B (gift from B. Edgar) (Xiang et al., 2017); Myo1A-Gal4,tub-Gal80ts,UAS-GFP/ CyO (Myo1Ats>GFP) (gift from B. Edgar) (Jiang et al., 2009); UAS-OGARNAi (VDRC, #106670); UAS-OGARNAi (VDRC, #41822); UAS-OGTRNAi (VDRC, #18610); UAS-OGTRNAi (VDRC, #18611); Cat[n1]/TM3 (BDSC, #4014); UAS-ATMRNAi (BDSC, #31635); UAS-ATRRNAi (BDSC, #35371); UAS-Chk1RNAi (BDSC, #62155); UAS-Chk1RNAi (BDSC, #36685); UAS-Chk2RNAi (BDSC, #64482); and UAS-Chk2RNAi (BDSC, #35152). The UAS-OGT line that contains Myc epitope tag in the N terminus (UAS-Myc-OGT) was made by P-element-mediated transformation and OGAdel.1 mutant was generated by standard P-element excision (Akan et al., 2016).
For transgene expression at specific developmental stages, the Gal80ts technique was used. The flies were set up and maintained at 22 ° C until adulthood. After maintaining the flies at 29 ° C, adult female midguts were dissected and analyzed.
Drosophila stocks and their use in this study
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/+ ; +/+: (Figures 1, 2, 4, 5, and S2).
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP /+ ; Catn1/+: (Figure 1).
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/+ ; OGAdel.1/+: (Figures 2 and S2).
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/OGARNAi ; +/+: (Figures 2, 3, and S2).
hs-flp, tub-Gal4,UAS-GFP; +; FRT82B, tub-Gal80 / FRT82B: (Figure 2)
hs-flp, tub-Gal4,UAS-GFP; +; FRT82B, tub-Gal80 / OGA del.1 FRT82B: (Figure 2)
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/OGARNAi ; ATMRNAi/+: (Figure 3)
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/OGARNAi ; ATRRNAi/+: (Figure 3)
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/OGARNAi ; Chk1RNAi/+: (Figure 3)
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/OGARNAi ; Chk2RNAi/+: (Figure 3)
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/OGTRNAi ; +/+: (Figure 4)
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/+ ; Myc OGT/+: (Figures 5 and S6)
+/+ ; esg-Gal4, UAS-2EYFP/+; Su(H) Gbe-Gal80, tub-Gal80ts/+: (Figures S1 and S3)
+/+ ; Su(H)-Gal4-UAS-CD8-GFP/+; tub-Gal80ts/+: (Figures S1 and S3)
+/+ ; esg-Gal4, UAS-2EYFP/OGARNAi; Su(H) Gbe-Gal80, tub-Gal80ts/+: (Figure S3)
+/+ ; Su(H)-Gal4-UAS-CD8-GFP/ OGARNAi; tub-Gal80ts/+: (Figure S3)
+/+ ; +/+ ; Myo1A-Gal4,tub-Gal80ts,UAS-GFP /+: (Figure S4).
+/+ ; +/ OGARNAi ; Myo1A-Gal4,tub-Gal80ts,UAS-GFP /+: (Figure S4)
+/+ ; +/ OGTRNAi ; Myo1A-Gal4,tub-Gal80ts,UAS-GFP /+: (Figure S4)
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/+ ; ATMRNAi/+: (Figure S5)
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/+; ATRRNAi/+: (Figure S5)
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/+ ; Chk1RNAi/+: (Figure S5)
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/+; Chk2RNAi/+: (Figure S5)
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/Chk1RNAi ; Myc OGT/+: (Figure S6)
+/+ ; esg-Gal4,tub-Gal80ts,UAS-GFP/ Chk2RNAi ; Myc OGT/+: (Figure S6)
mESCs culture
mESCs were derived from wild-type and Oga knockout C57BL/6 mice and cultured as previously published (Olivier-Van Stichelen et al., 2017). Briefly, wild-type and Oga KO mouse embryonic stem cells (mESCs) were cultured in knockout DMEM (GIBCO) supplied with 15% knockout serum replacement (KSR) (GIBCO), 1% penicillin/streptomycin (10,000 U/ml penicillin-streptomycin) (Sigma-Aldrich), 1% L-glutamine (GIBCO), 1% non-essential amino acids (GIBCO), 0.1 mM 2-mercaptoethanol (GIBCO), 1000 units/ml LIF (Milipore), 1 μM PD0325901 (Selleck), and 3 μM CHIR99021 (2i inhibitors) (Selleck). LIF and 2i inhibitors were final concentration of 1 μM. mESCs were analyzed within 20 passages of culture.
MEFs culture
MEFS were derived from wild-type and Oga knockout C56BL/6 mice and cultured as described previously (Keembiyehetty, 2015). Briefly, primary MEFs between passages 3 and 4 were split into 6-well dishes with 2 mL of MEF culture medium and grown in 37°C incubator with 5% CO2. MEFs cells were cultured in DMEM media (GIBCO) added with 15% FBS (GIBCO), 1% P/S (10,000 U/ml penicillin-streptomycin) (Sigma-Aldrich). The MEFs were split every 2– 3 days.
METHOD DETAILS
Paraquat feeding assay – fly
Flies were treated with 10 mM paraquat in 5% sucrose or 5% sucrose only for 20–22 h at 29 °C. After feeding, the midguts were dissected and analyzed.
High sucrose feeding assay – fly
High dietary suger feed method as previously published (May et al., 2019). Five-day-old flies were were feed 5% sucrose (normal) or 20% sucrose (high glucose) for 24–26h at 29 °C. After feeding, the midguts were dissected and analyzed.
AZD 7762 (CHK1/2 inhibitor) and Bortezomib (PS-341) feeding assay – fly
Two-day-old flies were fed 200 uM AZD7762 (Selleckchem, #860352–01-8) or DMSO only mixed in standard food for 7 days at 29°C. Two-day-old flies were fed 10 uM Bortezomib (Selleckchem, #S1013) or with both 10 uM Bortezomib and 200 uM AZD7762 mixed in standard food for 5 days at 29°C. Flies were transferred to new food vials every 2 days.
EdU incorporation – fly
For the EdU feeding assay, a Click-iT® EdU Alexa Fluor® 555 Imaging Kit (Thermo Fisher Scientific C10338) was utilized. Flies were transferred to a vial containing filter paper soaked with 100 μM EdU and 5% sucrose, and were kept for 24 hr. Whole guts were dissected and fixed in 4% para-formaldehyde (PFA) for 30 min. After washing with 3% BSA, EdU incorporation was visualized by Click reaction according to the manufacturer’s instruction. After washing with PBST, guts were stained with 4 μM of Hoechst33342 and then mounted with Vectashield and analyzed using a Zeiss LSM 700 system.
DHE assay – fly
Flies midguts were dissected in Schneider’s medium (HyClone). After incubation in 30 μM DHE (Invitrogen) for 7 min in the dark at room temperature, midguts were washed three times and mounted. Images were captured immediately with a Zeiss LSM700 confocal microscope.
MARCM
For MARCM experiments, flies were maintained at 23C until 3–5 days after eclosion, heat-shocked at 37°C for 60 min, and then maintained back at 23°C before dissection and markers were analyzed at 7 day after induction.
Immunochemistry – fly gut
Intact adult guts were dissected, fixed at room temperature for 1 h in 4% PFA, washed with PBST [0.1% Tween 20 in phosphate-buffered saline (PBS)], and incubated overnight with primary antibody at 4 °C. The primary antibody used in this study include rabbit phospho-Histone H3 (Ser10) (Millpore, Cat# 06–570, 1:500 dilution); mouse anti-Green fluorescent protein (GFP), mouse anti-Delta (DSHB, Cat# C594.9B, 1:10 dilution); rabbit anti-GFP (Thermo Fisher Scientific, Cat# A-11122, 1:500 dilution); mouse anti-O-linked N-acetylglucosamine (O-GlcNAc) (HGAC85) (Thermo Fisher Scientific, Cat# MA1–076, 1:50 dilution); rabbit phospho-ATM/ATR Substrate Motif [(pS/pT) QG] (Cell signaling, Cat# 6966S, 1:100 dilution); rabbit phospho-AKT (Cell signaling, CAT#9271S); rabbit phosphor-p44/42 MAPK (Erk1/2) (Cell signaling, CAT#4370S); rabbit polyclonal anti-Histone H2AvD (pS137) (Rockland, Cat# 600–401-914, 1:100 dilution); mouse anti-Myc tag (abcam, Cat# ab18185, 1:50 dilution). The samples were then incubated for 2 h with secondary antibodies at 25° C. The secondary antibody used in this study include Goat anti-Rabbit Antibody Alexa Fluor 635; Goat anti-Mouse Antibody Alexa Fluor 568; Goat anti-Mouse Antibody Alexa Fluor 488; Goat anti-Rabbit Antibody, Alexa Fluor 568; and Goat anti-Rabbit Antibody, Alexa Fluor 488 (Thermo Fisher Scientific, 1:300 dilution). After washing in PBST, slides were mounted with Vectashield and analyzed using a Zeiss LSM 700 system.
Immunochemistry – mESCs
For pluripotency marker analysis, mESCs were grown on microscope slides precoated with fibronectin, containing MEF feeders. mESCs were fixed with 4% PFA for 20 min, washed in 1% BSA, and incubated overnight with primary antibody at 4 °C. The primary antibody used in this study include mouse anti-phospho-Histone H2A.X (Ser139) (Millpore, Cat# 05–636, 1:100 dilution); mouse anti-O-linked N-acetylglucosamine (O-GlcNAc) (HGAC85) (Thermo Fisher Scientific, Cat# MA1–076, 1:50 dilution); rabbit phospho-ATM/ ATR Substrate Motif [(pS/pT) QG] (Cell signaling, Cat# 6966S, 1:100 dilution); mouse anti-O-GlcNAc transferase (C-10) (Santa Cruz Biotechnology, Cat# sc-376253, 1:100 dilution); rabbit anti-SOX2 (Cell signaling, Cat# 9656S, StemLight Pluripotency Antibody Kit, 1:50 dilution); and rabbit anti-TRA-1–60(S) (Cell signaling, Cat# 9656S, 1:50 dilution). The samples were then incubated for 2 h with secondary antibodies at 25 °C, washed in PBST, mounted with Vectashield and analyzed using a Zeiss LSM 700 system.
Inhibitor treatments – MEFs
For AZD 77662 cells were treated with 1uM AZD 7762 or DMSO alone in culture media for 7 hours at 37 °C. For Thiamet G treatment, cells were treated with 100uM Thiamet G (Cayman Chemical) dissolved in DMSO or DMSO alone in culture media for 18 hours at 37 °C. For AZD7762 and MG132 treatment, cells were treated with 1uM AZD 7762 or 10 uM MG132 or with both 1uM AZD 7762 and 10 uM MG132 or DMSO alone in culture media for 18 hours at 37 °C. For AZD7762 and OSMI-1 treatment, cells were treated with 100 uM PQ or 20 uM OSMI-1 or with both 100 uM PQ and 20 uM OSMI-1 or DMSO alone in culture media for 18 hours at 37 °C. After treatments, cells were fixed.
Immunochemistry – MEFs
MEFs were grown on microscope slides precoated with fibronectin. MEFs were fixed with 4% PFA for 20 min, washed in 1% BSA, and incubated overnight with primary antibody at 4 C. The primary antibody used in this study include mouse anti-phospho-Histone H2A.X (Ser139) (Millpore, Cat# 05–636, 1:100 dilution); mouse anti-O-linked N-acetylglucosamine (O-GlcNAc) (HGAC85) (Thermo Fisher Scientific, Cat# MA1–076, 1:50 dilution); mouse anti-O-GlcNAc transferase (C-10) (Santa Cruz Biotechnology, Cat# sc376253, 1:100 dilution); rabbit anti-Ki-67 (abcam, Cat# ab15580, 1:100 dilution); and mouse anti-Ki-67 (SP6) (Thermo Fisher Scientific, Cat# MA5–14520, 1:50 dilution). The samples were then incubated for 2 h with secondary antibodies at 25 °C, washed in PBST, mounted with vectashield and analyzed using a Zeiss LSM 700 system.
QUANTIFICATION AND STATISTICAL ANALYSIS
Quantitative of PH3 positive cell – fly gut
To quantitatively analyze PH3-positive cells, the number of PH3-positive cells in the whole gut was counted. N represents the number of guts.
Measurement of O-GlcNAc, gH2AvD, Myc-OGT, pS/Tq, pAKT, and pERK fluorescence
The fluorescence images of O-GlcNAc, γH2AvD, Myc-OGT, pS/Tq, pAKT, and pERK staining were captured at the same exposure time in each experiment and was measured by quantifying the level of fluorescence (IHC staining) in individual cells normalized to nearby background in FIJI (ImageJ) software. The mean fluorescence was analyzed after exclusion of the mean of the background region (from two spots, excluding the nuclear portion in the posterior midgut). At least 10 cells were quantified in each image, and > 10 images (> 1 image per fly) were used to calculate the average intensity of fluorescence of each fly. N represents the number of guts. n represents the number of cells.
Measurement of O-GlcNAc, pH2AX, OGT, and pS/Tq fluorescence in mESC or MEF
The fluorescence images of O-GlcNAc, pH2AX, OGT, and pS/Tq staining were captured at the same exposure time in each experiment and was measured by quantifying the level of fluorescence (IHC staining) in individual mESC (pluripotency positive cell) or MEF (Ki-67 positive cell) normalized to nearby background in FIJI (ImageJ) software. The mean fluorescence was analyzed after exclusion of the mean of the background region (from two spots). n represents the number of cells.
Statistical analysis
Data representation and statistical analysis were performed using GraphPad Prism software. Statistical analysis was performed using a t test and multiple comparisons were performed with a One-Way ANOVA. All experiments were replicated independently 2–3 times. N represents the number of guts. n represents the number of cells.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT OR RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
|
| ||
| Rabbit polyclonal anti-phosphO-Histone H3 (Ser10) | Millpore | Cat# 06-570; RRID: AB_310177 |
| Mouse monoclonal anti-Green fluorescent protein (GFP) | DSHB | Cat# DSHB-GFP-4C9; RRID: AB_2617422 |
| Mouse monoclonal anti-Delta | DSHB | Cat# C594.9B; RRID: AB_528194 |
| Mouse monoclonal anti-O-linked N-acetylglucosamine (O-GlcNAc) (HGAC85) | Thermo Fisher Scientific | Cat# MA1-076; RRID: AB_326365 |
| Rabbit monoclonal PhosphO-ATM/ATR Substrate Motif [(pS/pT) QG] | Cell signaling | Cat# 6966S; RRID: AB_10889739 |
| Rabbit polyclonal anti-Histone H2AvD (pS137) | Rockland | Cat# 600-401-914; RRID: AB_828383 |
| Rabbit polyclonal anti-GFP | Thermo Fisher Scientific | Cat# A-11122; RRID: AB_221569 |
| Mouse-monoclonal anti-Myc tag | abcam | Cat# ab18185; RRID: AB_331783 |
| Rabbit polyclonal anti-PhosphO-Akt (Ser473) | Cell signaling | Cat# 9271S; RRID: AB_329825 |
| Rabbit monoclonal anti-PhosphO-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) | Cell signaling | Cat# 4370S; RRID: AB_2315112 |
| Mouse monoclonal anti-phosphO-Histone H2A.X (Ser139) | Millpore | Cat# 05-636; RRID: AB_2755003 |
| Rabbit polyclonal anti-Ki-67 | abcam | Cat# ab15580; RRID: AB_443209 |
| Mouse monoclonal anti-Ki-67 (SP6) | Thermo Fisher Scientific | Cat# MA5-14520; RRID: AB_10979488 |
| Mouse monoclonal anti-O-GlcNAc transferase (C-10) | Santa Cruz Biotechnology | Cat# sc-376253; RRID: AB_10987682 |
| Rabbit anti-SOX2 (StemLight Pluripotency Antibody Kit) | Cell signaling | Cat# 9656S; RRID: AB_1658242 |
| Rabbit anti-TRA-1-60(S) (StemLight Pluripotency Antibody Kit) | Cell signaling | Cat# 9656S; RRID: AB_1658242 |
| Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 635 | Thermo Fisher Scientific | Cat# A-31576; RRID:AB_2536186 |
| Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 568 | Thermo Fisher Scientific | Cat# A-11004; RRID: AB_2534072 |
| Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-11001; RRID: AB_2534069 |
| Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 568 | Thermo Fisher Scientific | Cat# A-11011; RRID:AB_143157 |
| Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-11008; RRID:AB_143165 |
|
| ||
| Chemicals, Peptides, and Recombinant Proteins | ||
|
| ||
| 16% Paraformaldehyde (formaldehyde) aqueous solution | Electron Microscopy Sciences | Cat# 15710 |
| 4’,6-DiamidinO-2-phenylindole dihydrochloride (DAPI) | Thermo Fisher Scientific | Cat# D1306 |
| ProLong Diamond Antifade Mountant with DAPI | Thermo Fisher Scientific | Cat# P36971 |
| ProLong Diamond Antifade Mountant | Thermo Fisher Scientific | Cat# P36970 |
| Click-iT EdU Alexa Fluor 555 Imaging Kit | Thermo Fisher Scientific | Cat# C10338 |
| MG-132 | Selleck Chemicals | Cat# S2619 |
| PS-341 | Selleck Chemicals | Cat# S1013 |
| AZD7762 (10mM/1mL In DMSO) | Selleck Chemicals | Cat# S1532 |
| OSMI-1 | Sigma-Aldrich | Cat# SML1621-5MG |
| Paraquat dichloride hydrate | Sigma-Aldrich | Cat# 36541-100MG |
| Dihydroethidium (Hydroethidine) | Thermo Fisher Scientific | Cat# D11347 |
| Thiamet G | Cayman Chemical | Cat# 13237 |
|
| ||
| Experimental Models: Organisms/Strains | ||
|
| ||
| Experimental Models: D. melanogaster | ||
|
| ||
| w[1118] | BDSC | RRID:BDSC_3605 |
| esg-Gal4,tub-Gal80ts,UAS-GFP/CyO (esg ts >GFP) | Bruce A. Edgar (HCI, USA) | N/A (Jiang et al., 2009) |
| Myo1A-Gal4,tub-Gal80ts,UAS-GFP/CyO (Myo1Ats > GFP) | Bruce A. Edgar (HCI, USA) | N/A (Jiang et al., 2009) |
| FRT82B | John A. Hanover (NIH,USA) | N/A |
| OGA del.1 FRT82B | John A. Hanover (NIH,USA) | N/A |
| esg-Gal4, UAS-2EYFP/Cyo; Su(H) Gbe-Gal80, tub-Gal80ts/TM3 | Bruce A. Edgar (HCI, USA) | N/A (Zhang et al., 2019) |
| Su(H)-Gal4-UAS-CD8-GFP/Cyo; tub-Gal80ts/TM6B | Bruce A. Edgar (HCI, USA) | N/A (Xiang et al., 2017) |
| hs-flip, tub-Gal4,UAS-GFP; +; FRT82B, tub-Gal80 / TM6B | Bruce A. Edgar (HCI, USA) | N/A (Xiang et al., 2017) |
| UAS-Myc OGT/TM6 | John A. Hanover (NIH,USA) | N/A |
| UAS-Myc OGT/TM3 | John A. Hanover (NIH,USA) | N/A |
| OGA del.1 /TM6 | John A. Hanover (NIH,USA) | N/A |
| UAS-OGA RNAi | VDRC | 106670 ; RRID: FlyBase_FBgn0038870 |
| UAS-OGA RNAi | VDRC | 41822 ; RRID: FlyBase_FBgn0038870 |
| UAS-OGT RNAi | VDRC | 18610 ; RRID: FlyBase_FBgn0027591 |
| UAS-OGT RNAi | VDRC | 18611 ; RRID: FlyBase_FBgn0027591 |
| Cat[n1]/TM3, Sb[1] Ser[1] | BDSC | RRID:BDSC_4014 |
| w[*]; Kr[If-1]/CyO; D[1]/TM3, Ser[1] | BDSC | RRID:BDSC_7198 |
| y[1] v[1]; P{y[+t7.7] v[+t1.8] = TRiP.JF01422}attP2 | BDSC | RRID:BDSC_31635 ATM(3) |
| y[1] sc[*] v[1]; P{y[+t7.7] v[+t1.8] = TRiP.HMS02790}attP40 | BDSC | RRID:BDSC_44073 ATM(2) |
| y[1] v[1]; P{y[+t7.7] v[+t1.8] = TRiP.HMS02331}attP40 | BDSC | RRID:BDSC_41934 ATR(2) |
| y[1] sc[*] v[1]; P{y[+t7.7] v[+t1.8] = TRiP.GL00284}attP2 | BDSC | RRID:BDSC_35371 ATR(3) |
| y[1] sc[*] v[1]; P{y[+t7.7] v[+t1.8] = TRiP.HMC05162}attP40 | BDSC | RRID:BDSC_62155 CHK1(2) |
| y[1] sc[*] v[1]; P{y[+t7.7] v[+t1.8] = TRiP.HMS01573}attP2 | BDSC | RRID:BDSC_36685 CHK1(3) |
| y[1] v[1]; P{y[+t7.7] v[+t1.8] = TRiP.HMC05499}attP40 | BDSC | RRID:BDSC_64482 CHK2(2) |
| y[1] sc[*] v[1]; P{y[+t7.7] v[+t1.8] = TRiP.GL00020}attP2/TM3, Sb[1] | BDSC | RRID:BDSC_35152 CHK2(3) |
|
| ||
| Experimental Models: Cell lines | ||
|
| ||
| WT MEFs | John A. Hanover (NIH,USA) | N/A |
| Oga KO MEFs | John A. Hanover (NIH,USA) | N/A |
| WT mouse ESCs | John A. Hanover (NIH,USA) | N/A |
| Oga KO mouse ESCs | John A. Hanover (NIH,USA) | N/A |
|
| ||
| Software and Algorithms | ||
|
| ||
| Fiji | NIH | https://fiji.sc/ |
| Prism 6 | GraphPad | RRID:SCR_002798 |
Highlights.
Stress induces proliferation, O-GlcNAcylation, and DDR in ISCs/EBs of Drosophila
Elevating O-GlcNAcylation promotes proliferation and DDR in the absence of stress
OGT depletion prevents stress-induced proliferation and DDR in ISCs/EBs
OGT is stabilized by DDR effector CHK1/2 in ISC/EB, a pathway conserved in mammals
ACKNOWLEDGMENTS
We thank B. Edgar, Bloomington Stock Center, and the Drosophila Knockout Consortium for fly stocks. We would also like to thank the Developmental Studies Hybridoma Bank for the antibodies and the Bloomington Stock Center for the Drosophila stocks. This work was supported by intramural NIDDK, National Institutes of Health. We also acknowledge Rachel Vanjoske, Jeff Reece, and Marcy Comly for their contributions to this work.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2020.107632.
REFERENCES
- Adams PD, Jasper H, and Rudolph KL (2015). Aging-Induced Stem Cell Mutations as Drivers for Disease and Cancer. Cell Stem Cell 16, 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akan I, Love DC, Harwood KR, Bond MR, and Hanover JA (2016). Drosophila O-GlcNAcase Deletion Globally Perturbs Chromatin O-GlcNAcylation. J. Biol. Chem. 291, 9906–9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee PS, Lagerlöf O, and Hart GW (2016). Roles of O-GlcNAc in chronic diseases of aging. Mol. Aspects Med. 51, 1–15. [DOI] [PubMed] [Google Scholar]
- Behrens A, van Deursen JM, Rudolph KL, and Schumacher B. (2014). Impact of genomic damage and ageing on stem cell function. Nat. Cell Biol. 16, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond MR, and Hanover JA (2015). A little sugar goes a long way: the cell biology of O-GlcNAc. J. Cell Biol. 208, 869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broustas CG, and Lieberman HB (2014). DNA damage response genes and the development of cancer metastasis. Radiat. Res. 181, 111–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahova M, Palenickova E, Dankova H, Sticova E, Burian M, Drahota Z, Cervinkova Z, Kucera O, Gladkova C, Stopka P, et al. (2015). Metformin prevents ischemia reperfusion-induced oxidative stress in the fatty liver by attenuation of reactive oxygen species formation. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G100–G111. [DOI] [PubMed] [Google Scholar]
- Chatham JC, and Marchase RB (2010). Protein O-GlcNAcylation: A critical regulator of the cellular response to stress. Curr. Signal Transduct. Ther. 5, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, and Yu X. (2016). OGT restrains the expansion of DNA damage signaling. Nucleic Acids Res. 44, 9266–9278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi NH, Kim JG, Yang DJ, Kim YS, and Yoo MA (2008). Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell 7, 318–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Queiroz RM, Carvalho E, and Dias WB (2014). O-GlcNAcylation: The Sweet Side of the Cancer. Front. Oncol. 4, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova EV, Appelbe OK, Ricco N, Lee SS, Liu Y, Wolfgeher DJ, Collins TN, Flor AC, Ramamurthy A, Warrington S, et al. (2019). O-GlcNAcylation Enhances Double-Strand Break Repair, Promotes Cancer Cell Proliferation, and Prevents Therapy-Induced Senescence in Irradiated Tumors. Mol. Cancer Res. 17, 1338–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustice M, Bond MR, and Hanover JA (2017). O-GlcNAc cycling and the regulation of nucleocytoplasmic dynamics. Biochem. Soc. Trans. 45, 427–436. [DOI] [PubMed] [Google Scholar]
- Flores A, Schell J, Krall AS, Jelinek D, Miranda M, Grigorian M, Braas D, White AC, Zhou JL, Graham NA, et al. (2017). Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat. Cell Biol. 19, 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C, Yang J, Han F, Lu X, and Yu W. (2010). GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res. 70, 6344–6351. [DOI] [PubMed] [Google Scholar]
- Hanover JA, Krause MW, and Love DC (2010). The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim. Biophys. Acta 1800, 80–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JA, Chen W, and Bond MR (2018). O-GlcNAc in cancer: An On-cometabolism-fueled vicious cycle. J. Bioenerg. Biomembr. 50, 155–173. [DOI] [PubMed] [Google Scholar]
- Hart GW (2014). Three Decades of Research on O-GlcNAcylation - A Major Nutrient Sensor That Regulates Signaling, Transcription and Cellular Metabolism. Front. Endocrinol. (Lausanne) 5, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood KR, and Hanover JA (2014). Nutrient-driven O-GlcNAc cycling think globally but act locally. J. Cell Sci. 127, 1857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Kim TW, Yoon S, Choi SY, Kang TW, Kim SY, Kwon YW, Cho EJ, and Youn HD (2012). O-GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell Stem Cell 11, 62–74. [DOI] [PubMed] [Google Scholar]
- Jasper H. (2015). Exploring the physiology and pathology of aging in the intestine of Drosophila melanogaster. Invertebr. Reprod. Dev. 59 (sup1), 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, and Edgar BA (2009). Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Liu M, and Turner N. (2015). Diabetes and its link with cancer: providing the fuel and spark to launch an aggressive growth regime. BioMed Res. Int. 2015, 390863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kátai E, Pá l J, Poó r VS, Purewal R, Miseta A, and Nagy T. (2016). Oxidative stress induces transient O-GlcNAc elevation and tau dephosphory-lation in SH-SY5Y cells. J. Cell. Mol. Med. 20, 2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keembiyehetty C. (2015). Disruption of O-GlcNAc cycling by deletion of O-GlcNAcase (Oga/Mgea5) changed gene expression pattern in mouse embryonic fibroblast (MEF) cells. Genom. Data 5, 30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keembiyehetty C, Love DC, Harwood KR, Gavrilova O, Comly ME, and Hanover JA (2015). Conditional knock-out reveals a requirement for O-linked N-Acetylglucosaminase (O-GlcNAcase) in metabolic homeostasis. J. Biol. Chem. 290, 7097–7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Cao L, Reece EA, and Zhao Z. (2017). Impact of protein O-GlcNAcylation on neural tube malformation in diabetic embryopathy. Sci. Rep. 7, 11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Zhou ZW, Ju Z, and Wang ZQ (2016). DNA Damage Response in Hematopoietic Stem Cell Ageing. Genomics Proteomics Bioinformatics 14, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Li X, Nai S, Geng Q, Liao J, Xu X, and Li J. (2017). Checkpoint kinase 1-induced phosphorylation of O-linked b-N-acetylglucosamine transferase regulates the intermediate filament network during cytokinesis. J. Biol. Chem. 292, 19548–19555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, and Li J. (2018). O-GlcNAc: A Sweetheart of the Cell Cycle and DNA Damage Response. Front. Endocrinol. (Lausanne) 9, 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchetta EM, and Ohlstein B. (2012). The Drosophila midgut: a model for stem cell driven tissue regeneration. Wiley Interdiscip. Rev. Dev. Biol. 1, 781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch TP, Ferrer CM, Jackson SR, Shahriari KS, Vosseller K, and Reginato MJ (2012). Critical role of O-Linked b-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J. Biol. Chem. 287, 11070–11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, and Vosseller K. (2013). O-GlcNAc in cancer biology. Amino Acids 45, 719–733. [DOI] [PubMed] [Google Scholar]
- Maréchal A, and Zou L. (2013). DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 5, a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez MR, Dias TB, Natov PS, and Zachara NE (2017). Stress-induced O-GlcNAcylation: an adaptive process of injured cells. Biochem. Soc. Trans. 45, 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila J, Kokki K, Hietakangas V, and Boutros M. (2018). Stem Cell Intrinsic Hexosamine Metabolism Regulates Intestinal Adaptation to Nutrient Content. Dev. Cell 47, 112–121.e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May CE, Vaziri A, Lin YQ, Grushko O, Khabiri M, Wang QP, Holme KJ, Pletcher SD, Freddolino PL, Neely GG, et al. (2019). High Dietary Sugar Reshapes Sweet Taste to Promote Feeding Behavior in Drosophila melanogaster. Cell Rep. 27, 1675–1685.e1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli CA, and Perrimon N. (2006). Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479. [DOI] [PubMed] [Google Scholar]
- Oh J, Lee YD, and Wagers AJ (2014). Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat. Med. 20, 870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, and Spradling A. (2006). The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470–474. [DOI] [PubMed] [Google Scholar]
- Olivier-Van Stichelen S, Wang P, Comly M, Love DC, and Hanover JA (2017). Nutrient-driven O-linked N-acetylglucosamine (O-GlcNAc) cycling impacts neurodevelopmental timing and metabolism. J. Biol. Chem. 292, 6076–6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Lee SH, Na HJ, Pyo JH, Kim YS, and Yoo MA (2012). Age-and oxidative stress-induced DNA damage in Drosophila intestinal stem cells as marked by Gamma-H2AX. Exp. Gerontol. 47, 401–405. [DOI] [PubMed] [Google Scholar]
- Park SK, Zhou X, Pendleton KE, Hunter OV, Kohler JJ, O’Donnell KA, and Conrad NK (2017). A Conserved Splicing Silencer Dynamically Regulates O-GlcNAc Transferase Intron Retention and O-GlcNAc Homeostasis. Cell Rep. 20, 1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Jeon HJ, Pyo JH, Kim YS, and Yoo MA (2018). Deficiency in DNA damage response of enterocytes accelerates intestinal stem cell aging in Drosophila. Aging (Albany NY) 10, 322–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova NN, and Thompson CB (2016). The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 23, 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausville E, Lorusso P, Carducci M, Carter J, Quinn MF, Malburg L, Azad N, Cosgrove D, Knight R, Barker P, et al. (2014). Phase I dose-escalation study of AZD7762, a checkpoint kinase inhibitor, in combination with gemcitabine in US patients with advanced solid tumors. Cancer Chemother. Pharmacol. 73, 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine O, Love DC, Rubenstein DS, and Hanover JA (2010). Blocking O-linked GlcNAc cycling in Drosophila insulin-producing cells perturbs glucose-insulin homeostasis. J. Biol. Chem. 285, 38684–38691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Tomic J, Wen F, Shaha S, Bahlo A, Harrison R, Dennis JW, Williams R, Gross BJ, Walker S, et al. (2010). Aberrant O-GlcNAcylation characterizes chronic lymphocytic leukemia. Leukemia 24, 1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JP, Zhang K, Wu J, and Yang X. (2015). O-GlcNAc signaling in cancer metabolism and epigenetics. Cancer Lett. 356, 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C, Alic N, Foley A, Cabecinha M, Hoddinott MP, and Partridge L. (2015). The Ras-Erk-ETS-Signaling Pathway Is a Drug Target for Longevity. Cell 162, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Amand MM, Bond MR, Riedy J, Comly M, Shiloach J, and Hanover JA (2018). A genetic model to study O-GlcNAc cycling in immortalized mouse embryonic fibroblasts. J. Biol. Chem. 293, 13673–13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesha VA, Parsels LA, Parsels JD, Zhao L, Zabludoff SD, Simeone DM, Maybaum J, Lawrence TS, and Morgan MA (2012). ). Sensitization of pancreatic cancer stem cells to gemcitabine by Chk1 inhibition. Neoplasia 14, 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl GM, and Carr AM (2001). The evolution of diverse biological responses to DNA damage: insights from yeast and p53. Nat. Cell Biol. 3, E277–E286. [DOI] [PubMed] [Google Scholar]
- Xiang J, Bandura J, Zhang P, Jin Y, Reuter H, and Edgar BA (2017). EGFR-dependent TOR-independent endocycles support Drosophila gut epithelial regeneration. Nat. Commun. 8, 15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YR, Kim DH, Seo YK, Park D, Jang HJ, Choi SY, Lee YH, Lee GH, Nakajima K, Taniguchi N, et al. (2015). Elevated O-GlcNAcylation promotes colonic inflammation and tumorigenesis by modulating NF-kB signaling. Oncotarget 6, 12529–12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehezkel G, Cohen L, Kliger A, Manor E, and Khalaila I. (2012). O-linked b-N-acetylglucosaminylation (O-GlcNAcylation) in primary and metastatic colorectal cancer clones and effect of N-acetyl-b-D-glucosaminidase silencing on cell phenotype and transcriptome. J. Biol. Chem. 287, 28755–28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf B, Gopurappilly R, Dadheech N, Gupta S, Bhonde R, and Pal R. (2013). Embryonic fibroblasts represent a connecting link between mesenchymal and embryonic stem cells. Dev. Growth Differ. 55, 330–340. [DOI] [PubMed] [Google Scholar]
- Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, and Hart GW (2004). Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J. Biol. Chem. 279, 30133–30142. [DOI] [PubMed] [Google Scholar]
- Zhang P, Holowatyj AN, Roy T, Pronovost SM, Marchetti M, Liu H, Ulrich CM, and Edgar BA (2019). An SH3PX1-Dependent Endocytosis-Autophagy Network Restrains Intestinal Stem Cell Proliferation by Counteracting EGFR-ERK Signaling. Dev. Cell 49, 574–589.e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Yang S, Hu S, Chaudry IH, Marchase RB, and Chatham JC (2007). The protective effects of PUGNAc on cardiac function after traumahemorrhage are mediated via increased protein O-GlcNAc levels. Shock 27, 402–408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate any unique datasets or code.