Abstract
The aim of the present study was to improve the properties of soy press cake to be utilized as an ingredient of meat analogues. Soy press cakes were fermented with lactobacillus strains, and separately hydrolyzed by cellulase/xylanase mixture and α-amylase. Meat analogues were produced with 10% fermented or hydrolyzed soy press cakes. The effect of applied processes on protein oxidation, physical and functional properties of soy press cakes were analyzed, as well as sensory and textural properties of meat analogues. The results indicated that soy press cake was a suitable source of fibre and energy with low content of saturated fatty acids, and provided plant-based proteins and essential amino acids. The study demonstrated the potential of lactic acid fermentation, and enzymatic hydrolysis to improve water- and oil-holding capacity and reduce protein oxidation in soy press cakes. L. acidophilus 336 and cellulase/xylanase mixture were recommended for fermentation and hydrolysis of soy press cakes, respectively, regarding reduction of protein oxidation. Fermentation of soy press cakes with L. plantarum P1 improved the texture of meat analogues. Press cakes fermentation reduced bitterness, increased juiciness, and balanced the taste of meat analogues. Fermented soy press cake was recommended for the production of meat analogues.
Keywords: Soy press cake, Fermentation, Enzymatic hydrolysis, Meat analogue
Highlights
-
•
This research was the 1st application of fermented soy press cake in meat analogue.
-
•
Fermentation and hydrolysis improved the functional properties of soy press cakes.
-
•
Protein oxidation in soy press cakes was reduced after fermentation and hydrolysis.
-
•
Fermented soy press cakes improved sensory quality of the meat analogues.
-
•
L. plantarum P1 is recommended for the fermentation of soy press cakes.
1. Introduction
Soy press cake is a by-product of plant-based drink production and is usually applied as feedstocks or directly discarded as waste, leading to the loss of useful nutrients (Li, Qiao, & Lu, 2012), and environmental problems (O'Toole, 1999). Soy press cake contains around 27% protein, 20% fat and 33% dietary fibre on a dry basis (Rashad, Mahmoud, Abdou, & Nooman, 2011). One of the reasons press cakes are not directly used in food production is that they contain large amounts of antinutrients, like phytic acid, tannins, saponins and trypsin inhibitors (Ancuta & Sonia, 2020). Interaction of antinutritional factors with nutrients could reduce nutrient bioavailability (Singh & Jadaun, 2016). One of the strategies to decrease the amount of antinutrients in soy is solid-state fermentation (SSF) with lactic acid bacteria (LAB) (Rui et al., 2016). Solid-state fermentation using numerous microorganisms could improve the flavour of soy press cakes (Chan et al., 2019; Vong, Hua, & Liu, 2018), as well as the antioxidant activity (Chi & Cho, 2016; Guan et al., 2016). However, the effect of LAB on oxidation properties of soy press cakes has not previously been studied. Understanding the impact of fermentation on protein oxidation level in foods could help to develop strategies for minimizing the oxidation processes, as protein oxidation affects sensory and physicochemical properties of food products (Chen, Kong, Sun, Dong, & Liu, 2015).
Interactions of water and oil with proteins are very important in the food systems, due to having effects on flavour and texture of foods. The effect of SSF with LAB on functional properties (water- and oil-holding capacities (WHC/OHC)) of plant materials has been widely studied (Cabuk, Stone, Korber, Tanaka, & Nickerson, 2018; Xing et al., 2020), while it has not been studied in soy press cakes. The composition and structure of proteins and the interactions of proteins with each other and with other substances in the food matrices could affect WHC and OHC. Water holding capacity plays a fundamental role in the food production process affecting sensory quality (perceived juiciness) and other functional properties (Sreerama, Sashikala, Pratape, & Singh, 2012). Solid-state fermentation with LAB improved WHC and OHC in legume proteins (Emkani, Oliete, & Saurel, 2022) and the peanut press cake (Sadh, Chawla, & Duhan, 2018), and has thus shown its potential as a treatment for soy press cake processing.
An alternative strategy to microbial fermentation of soy press cake is enzymatic hydrolysis, which has been widely studied due to its advantages of relatively high efficiency and low energy consumption. Some studies revealed that enzymatic hydrolysis significantly increased WHC and OHC in wheat gluten hydrolyzed with acid protease (Deng et al., 2016), and starch granules hydrolyzed with α-amylase (Jung, Lee, & Sang, 2017). However, these functional properties have not previously been studied in hydrolyzed soy press cakes. Certain enzymes are suitable to improve the nutritional value of foods. For example, xylanase hydrolyzed non-starch polysaccharides, which increased the availability of nutrients in plant-based foods (Zhang et al., 2014). α-Amylase broke down starch to maltose, which in turn was hydrolyzed to glucose, and could easily be absorbed by the body (Kotowaroo, Mahomoodally, Gurib-Fakim, & Subratty, 2006). Cellulase enhanced soluble carbohydrate content, WHC and swelling capacity in coconut cake dietary fibre (Zheng & Li, 2018).
Protein extracts from soybean have often been used to produce meat analogues (Alexander, Brown, Dias, Moran, & Rounsevell, 2019; Osen & Schweiggert-Weisz, 2016). Some studies used soy press cake as an ingredient of meat analogues (Oliveira et al., 2016; Turhan, Temiz, & Sagir, 2007). Turhan et al. (2007) applied 2.5, 5.0, 7.5 and 10% of soy press cake in beef patties and reported that WHC was higher in beef patties containing 10% soy press cake than in beef patties with lower amounts of press cakes. No study has reported the use of fermented or hydrolyzed soy press cakes in meat analogues.
The present study analyzed the impact of fermentation and enzymatic hydrolysis of soy press cakes on their protein oxidation level, physical and functional properties, as well as textural and sensory properties of meat analogues containing 10% fermented or hydrolyzed soy press cakes. The hypothesis was tested that fermentation or enzymatic hydrolysis of soy press cake could improve its properties sufficiently to be used as an ingredient of meat analogues.
2. Materials and methods
2.1. Substrates, microorganisms, and chemicals
Soy press cakes were obtained from a vegetable drink producer (Berief Food GmbH, Oberhausen, Germany) in frozen condition (−18 °C). Lactobacillus plantarum P1, Lactobacillus brevis R, Lactobacillus acidophilus 336, and Lactobacillus acidophilus 308 were obtained from the Kaunas University of Technology, Food Institute microorganisms’ collection, Kaunas, Lithuania. The liquid bacterial α-amylase and the liquid preparation Cellustar XL (containing cellulase and xylanase) were obtained from AB Baltic Enzymes, Vilnius, Lithuania. Hydrogen chloride (HCl) and potassium chloride (KCl) were obtained from JSC EUROCHEMICALS, Vilnius, Lithuania. Guanidine hydrochloride (GuHCl), sodium dodecyl sulphate (SDS), monosodium phosphate (NaH2PO4), ethyl acetate, 2,4-Dinitrophenylhydrazine (DNPH), and trichloroacetic acid (TCA) were obtained from SIGMA-ALDRICH, Darmstadt, Germany. Ethanol was obtained from MV GROUP, Vilnius, Lithuania. Textured wheat proteins, modified starch and maltodextrin were obtained from IMLITEX, Kaunas, Lithuania; oat flakes from Malsena, Vievis, Lithuania; methylcellulose from MOGUNTIJA, Klaipeda, Lithuania; and fibregum B acacia from Orkla Foods Lietuva, Kaunas, Lithuania. Soybean, sunflower, and coconut oils were obtained from the local markets, Kaunas, Lithuania.
2.2. Proximate analysis and amino acid composition measurement
The chemical composition of soy press cake was determined in duplicate (g/100g), in accordance with standard methods. Moisture content was estimated by the oven-dry method at 105 ± 5 °C and weight kinetics was recorded until constant weight was achieved (ISO 712, 2009). Fat content and saturated fatty acids were determined by gas-liquid chromatography (ISO 12966–1, 2014); protein content by the Kjeldahl method (ISO 20483, 2006); and the total fibre by the enzymatic gravimetric method (AOAC 985.29, 1990). Carbohydrate content was estimated by a differential method (Adebiyi, Obadina, Adebo, & Kayitesi, 2017). Amino acid composition of soy press cake was analyzed in duplicate (g/100 g), using an A300 amino acid analyzer (MembraPure GmbH, Berlin, Germany), which involved protein hydrolysis, cation-exchange chromatography of the released amino acids, followed by in-line post-column labelling with ninhydrin (Csapó, Lóki, Csapó-Kiss, & Albert, 2008).
2.3. Heat treatment of soy press cakes
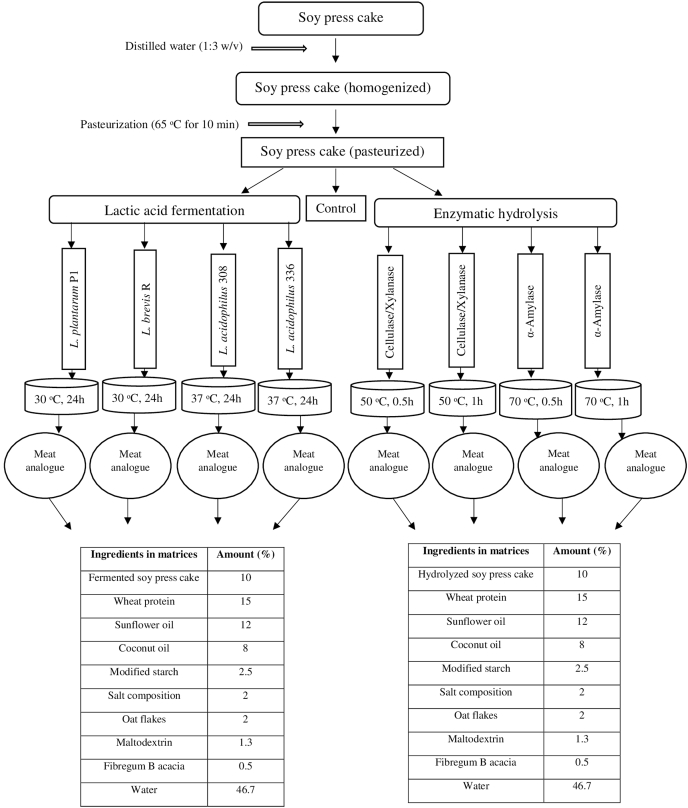
Samples of soy press cake were pasteurized before fermentation and enzymatic hydrolysis. An aqueous emulsion prepared from the mass of soy press cake (in a ratio of 1:3 (w/v)) was distributed into a sterile bag and was heated in a water bath for 10 min at 65 °C, (Saeeduddin et al., 2015), then cooled instantly to room temperature.
2.4. Microbiological analysis of soy press cakes
The total number of microorganisms in homogenized soy press cakes (before and after pasteurization) were determined by incubation for 3 days at 30 °C, according to ISO 4833–1 (2013), and expressed as colony-forming unit per gram (CFU/g).
2.5. Enzymatic hydrolysis of soy press cake
After pasteurization treatment, the enzymatic hydrolysis of soy press cakes was run. The amounts of enzymes added to 100 g of aqueous emulsion were the following: α-amylase 100 AU, and cellulase/xylanase mixture 400 AU. The enzymatic activities are those indicated by the producing companies. The characteristics of these enzymatic treatments were reported in Table 1.
Table 1.
Characteristics of used enzymes.
| Enzyme | Activity, U/g | Organism of Origin | Optimal Temperature, ◦C |
|---|---|---|---|
| α-Amylase | >1400 | Bacillus licheniformis | 70–85 |
| Cellulase/Xylanase mixture | >45,000 cellulase >34,000 xylanase | Trichoderma reesei | 40–60 |
Cellulase/xylanase mixture was added to two press cake samples; one sample was hydrolyzed for 0.5 h at a constant 50 °C, and the other sample for 1 h at 50 °C. Separately, α-amylase was added to two press cake samples; one sample was hydrolyzed for 0.5 h at a constant 70 °C, and the other sample for 1 h at 70 °C (Fig. 1). The hydrolysis process was stopped by rapidly cooling the emulsion samples at about 4 °C. Hydrolyzed samples and control sample (homogenized, pasteurized, non-hydrolyzed sample) were stored at 4 ± 1 °C until usage. Necessary amounts of hydrolyzed soy press cakes and the control sample for chemical analysis were freeze-dried in a microbiological vacuum freeze dryer, Alpha 1–2 LDplus (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany). Freeze-dried samples were stored at – 18 °C until usage.
Fig. 1.
Scheme of experimental design.
2.6. Fermentation of soy press cakes
In a separate experiment from the hydrolysis of soy press cakes, the solid-state fermentation of pasteurized soy press cakes was performed (Fig. 1). The LAB cultures were stored at KTU Food institute in the collection at –72–74 °C in the VIABANK (MWE medical wire) system. Cultures revived in MRS broth (Biolife, Italy): an initial LAB suspension was prepared by seeding of the initial culture on MRS agar (Biolife, Italy) and incubating at 30 °C (L. plantarum P1, L. brevis R) and at 37 °C (L. acidophilus 308, L. acidophilus 336) for 24 h. Each LAB culture (2.5 × 106 CFU/g) was then transformed into the pasteurized sample of soy press cake (homogenized in water with a ratio of 1:3 (w/v)), and incubated at an appropriate temperature for LAB under anaerobic conditions (aerostat with oxygen sorbent). Fermentation process was stopped by rapidly cooling the emulsion samples at about 4 °C. The number of LAB was determined by the method of seeding in petri dishes by incubation on MRS agar under anaerobic conditions for 72 h at 30 or 37 °C. Control sample (homogenized, pasteurized, non-fermented sample) and fermented soy press cakes samples (in emulsion conditions) were stored at 4 ± 1 °C until usage. Necessary amounts of fermented soy press cakes and the control sample were freeze-dried and stored at – 18 °C until usage.
2.7. Protein oxidation measurement
The protein oxidation level of soy press cakes was measured in duplicate, before and after treatment (fermentation or hydrolysis). The carbonyl content of the samples was analyzed (as a marker for protein oxidation measurement) using the methods and equation described by Soglia, Petracci, and Ertbjerg (2016), with some modifications. Briefly, 1 g of freeze-dried sample was homogenized in KCl solution. After adding TCA, the mixture was centrifuged with a Universal High-Speed Centrifuge (Herolab GmbH Laborgeräte, Wiesloch, Germany) at 9000×g (instead of 5000×g, for better separation). After removing the supernatant, SDS was added to the pellet. Two replicates of each sample treated with DNPH in HCl. After adding TCA, the supernatant was separated by centrifugation at 9000×g (instead of 5000×g). The resulting pellets were dissolved in GuHCl in NaH2PO4. The absorbance at 280 nm and 370 nm were measured using a Cary 60 UV-VIS spectrophotometer (Agilent, Santa Clara, USA). The carbonyl content was expressed as nmol/mg of protein.
2.8. Bulk density, viscosity, spreadability, and pH measurements
The physical properties of soy press cakes before and after treatment (fermentation or hydrolysis) were analyzed, in duplicate. Bulk density was measured following the method described by Sadh et al. (2018). Briefly, 1 g of freeze-dried sample was filled in a 10 ml graduated cylinder. Bulk density was determined as mass of sample per unit volume of sample (g/ml). The viscosity was determined through the measurement of shear-thinning factor n, using RM200 rotational rheometer (LAMY RHEOLOGY, Champagne-au-Mont-d'Or, France). A spindle no. 1 was used during the analysis. Thirty gram of emulsion sample was used for the measurement. All measurements were conducted at 22 ± 1 °C and performed in duplicate. Data were evaluated using a Rheometric software (Boisgard et al., 2017). Spreadability was defined as the easiness of sample spread on the surface. It was measured using a Universal Testing Machine Instron 3343 (Instron Engineering Group, High Wycombe, Great Britain) equipped with a 1 kN load cell, by placing the emulsion sample (12 °C) into a special conical container, where the sample rose up the surface at an angle of 45°, while it was pressed at a speed of 5 mm/min (Liutkevičius et al., 2015). The pH was measured using a PP-15 pH electrode (Sartorius, Goettingen, Germany).
2.9. Water- and oil-holding capacity measurement
The WHC and OHC values of soy press cakes before and after treatment (fermentation or hydrolysis) were analyzed in duplicate, following the methods described by Sadh et al. (2018), with some modifications. Briefly, 1 g of freeze-dried sample was mixed with 15 ml of deionized water for WHC measurement, and 15 ml of soybean oil for OHC measurement. Samples were centrifuged at 9000×g (instead of 3000×g, for better separation). WHC and OHC were expressed as grams of water and grams of oil retained per gram of sample, respectively.
2.10. Preparation of meat analogues
Meat analogue produced with non-fermented, non-hydrolyzed soy press cake was used as control. As shown in Fig. 1, the matrices of meat analogues consisted of fermented or hydrolyzed soy press cake (10%), textured wheat protein (25%) with a protein content of 75%, sunflower oil (12%), coconut oil (8.0%), modified starch (2.5%), salt composition (2.0%), oat flakes (2.0%), maltodextrin (1.3%), soluble Fibregum B acacia (0.5%), and methylcellulose (0.4%), remaining part was water (46.7%). No additional spices were added to the matrices with the aim to identify the sensory properties of the basic matrices. Meat analogues were placed in a fan-forced oven (METOS CHEF 240, Kerava, Finland) and cooked at 180 °C for 20 min. The cooked products were cut into quarters and wrapped in aluminum foils. They were put in labeled trays and held at 60 °C until serving for sensory analysis. The cooked samples were used for the analysis of texture parameters as well.
2.11. Texture profile analysis
Measurement of texture parameters of meat analogues was performed in duplicate, using a Universal Testing Machine Instron 3343 equipped with a 1 kN load cell. For the Texture Profile Analysis (TPA) of cooked meat analogues, samples were compressed perpendicularly using a 50 mm diameter cylindrical probe. The TPA test involved two compression cycles with 70% compression and 1 mm/s crosshead speed. The texture variables (hardness and chewiness) were calculated as described by Bourne (1978).
2.12. Sensory analysis
A quantitative descriptive analysis (QDA) was performed (in duplicate) for the evaluation of sensory properties of meat analogues. A total group of twelve trained assessors was applied. The sensory panel orientation training and calibration process consisted of two training sessions for two days. In the first session, the sensory assessors were asked to develop a list of attributes describing the sensory attributes of the tested samples. During the second session, assessors practiced using the scales for each selected attribute. All training and data collection sessions were held in a controlled area at the sensory analysis laboratory established according to ISO 8589 (2007). For sensory assessment, meat analogues were cooked as previously described. Samples were cut into cubes (2.0 × 2.0 × 2.0 cm) and were presented to the assessors in glass containers, coded with three-digit numbers. Panellists quantified the samples attributes with an intensity scale from 1 to 15; where 1 = “attribute not detected” and 15 = “attribute expressed extremely strong”. The following attributes were evaluated: sweet odor, acidic odor, oily odor, juiciness, sweetness, sourness, acidity, and bitterness.
With the aim to identify the main sensory properties of the meat analogues, which can decrease the sensory quality, a preliminary test of acceptability (appearance, odor, taste, texture, and overall acceptability) was performed (in duplicate) by twelve consumers. They were recruited from the local area for testing. Samples were prepared, kept, and presented for analysis in the same way as for descriptive analysis. A 15-point hedonic scale was used for evaluation of the meat analogues (1 = “not acceptable”, 15 = “very acceptable”).
2.13. Statistical analysis
A one-way ANOVA using Fisher's least significant difference (LSD) test was carried out. All determinations were made in duplicate, and the values were averaged and reported along with the standard deviation. Significance of differences was defined at P ≤ 0.05. All statistical analyses were performed using the SPSS software version 22 (Snedecor & Cochran, 1967).
3. Results and discussion
3.1. Proximate composition and amino acids characterization of soy press cake
Results indicated that soy press cake had a significant content of fibre (7.35%) and low content of saturated fatty acids (0.88%), with 7.6% of protein (Table 2). Hence, soy press cake could be applied as a plant-based ingredient in meat analogues, however, other plant-based proteins must be added to the meat analogues matrices to increase the protein content of final products. The textured wheat protein was selected as the major protein source of meat analogues, as it was high in proteins and low in carbohydrates. The foundation of the Impossible Burger (a popular meat analogue) is based mainly on textured wheat protein (Mistry, George, & Thomas, 2020).
Table 2.
Nutritional composition of raw soy press cakes (g/100g).
| Parameter | Composition of soy press cake, (%) |
|---|---|
| Moisture | 77.79 ± 0.15 |
| Fat | 2.8 ± 0.01 |
| Saturated fatty acid | 0.88 ± 0.03 |
| Carbohydrates | 10.87 ± 0.01 |
| Total fibre | 7.35 ± 0.05 |
| Proteins | 7.6 ± 0.1 |
Soy press cake provided all essential amino acids, as well as conditional amino acids (Table 3), with a high amount of tryptophan (0.41 g/100g) compared to its amount in beef (0.22 g/100g) (Kumar, 2016; Nayak, Singh, & Buttar, 2019). Tryptophan is required for normal growth and serves as an in vivo precursor for several bioactive compounds, like nicotinamide (vitamin B6), serotonin, and melatonin. Its role in human health can therefore affect many diseases (Friedman, 2018).
Table 3.
Amino acid composition of soy press cakes.
| Amino acid | Amount (%) |
|---|---|
| Essential amino acidsrowhead | |
| Histidine | 0.16 |
| Threonine | 0.33 |
| aValine | 0.32 |
| aMethionine | 0.17 |
| Lysine | 0.22 |
| aIsoleucine | 0.53 |
| aLeucine | 0.26 |
| aPhenylalanine | 0.39 |
| aTryptophan | 0.41 |
| Conditional amino acidsrowhead | |
| Aspartic acid | 0.84 |
| Glutamic acid | 1.30 |
| Serine | 0.43 |
| aGlycine | 0.29 |
| aAlanine | 0.32 |
| Arginine | 0.42 |
| aProline | 0.00 |
| Cysteine | 0.17 |
| Tyrosine | 0.25 |
Hydrophobic amino acids.
3.2. Properties of soy press cakes
3.2.1. Effect of heat treatment on the safety criteria of soy press cakes
Conventional pasteurization of soy press cakes (at 65 °C for 10 min) made a significant reduction in the total number of microorganisms (reduced from 3.0 × 108 CFU/g before pasteurization to 2.3 × 101 CFU/g after pasteurization). In agreement, Saeeduddin et al. (2015) reported a significant decrease in microbial contamination of pear juice after pasteurization at 65 °C for 10 min.
3.2.2. Bulk density, WHC and OHC
Bulk density of soy press cakes was significantly reduced after fermentation or hydrolysis (P ≤ 0.05), compared with the control, as shown in Table 4. This might be due to the breakdown of starch during fermentation, which reduces starch content and subsequently decreases the bulk density; or the hydrolysis of non-starch polysaccharides (NSP) like fibres, which can decrease the bulk density. Xylanase could perform the hydrolysis of NSP and therefore reduce the bulk density of the press cake samples (Zhang et al., 2014). Likewise, α-amylase contributed to the saccharification process to assist the hydrolysis of starch, which could decrease the bulk density of samples (Shahryari, Mohammad, Younes, Patrik, & Mohammad, 2019). Ogodo, Ugbogu, Onyeagba, Okereke, and Agwaranze (2017) reported that LAB fermentation decreased the bulk density of maize flours from 0.82 ± 0.02 g/ml to 0.79 ± 0.03 g/ml. This was in agreement with the results of the present research, as the bulk density of soy press cakes after fermentation decreased from 1.10 ± 0.01 g/ml to between 1.00 ± 0.01 g/ml and 1.03 ± 0.00 g/ml.
Table 4.
Effect of fermentation or enzymatic hydrolysis on bulk density, Water Holding Capacity (WHC), Oil Holding Capacity (OHC), and protein oxidation level of soy press cakes.
| Treatment conditions | Treatment duration (h) | Bulk density (g/ml) | WHC (g/g) | OHC (g/g) | Carbonyl content (nmol/mg of protein) |
|---|---|---|---|---|---|
| Control | - | 1.10 ± 0.01a | 0.90 ± 0.22c | 1.86 ± 0.01c | 17.93 ± 1.37a |
| Fermentation | |||||
| L. plantarum P1 | 24.0 | 1.03 ± 0.00b | 1.96 ± 0.11a | 2.12 ± 0.05b | 7.42 ± 0.09b |
| L. brevis R | 24.0 | 1.01 ± 0.00c | 1.26 ± 0.04b | 2.26 ± 0.05a | 7.46 ± 0.25b |
| L. acidophilus 308 | 24.0 | 1.02 ± 0.01bc | 1.40 ± 0.02b | 2.32 ± 0.08a | 3.68 ± 0.70c |
| L. acidophilus 336 | 24.0 | 1.00 ± 0.01c | 1.86 ± 0.05a | 2.35 ± 0.02a | 3.15 ± 0.14c |
| Hydrolysis | |||||
| Cellulase/Xylanase | 0.5 | 1.03 ± 0.00bc | 1.50 ± 0.04a | 1.95 ± 0.07bc | 4.50 ± 0.26d |
| 1.0 | 1.04 ± 0.00b | 1.39 ± 0.07ab | 1.91 ± 0.02bc | 4.50 ± 0.01d | |
| α-Amylase | 0.5 | 1.02 ± 0.00c | 1.23 ± 0.04b | 1.97 ± 0.04b | 9.21 ± 0.48c |
| 1.0 | 1.03 ± 0.00bc | 1.54 ± 0.01a | 2.11 ± 0.02a | 10.18 ± 1.25b | |
a,b, c Different letters in the same column for each treatment indicate significant statistical differences, (P ≤ 0.05, LSD test).
Results (Table 4) demonstrated a significant increase in WHC and OHC values after lactic acid fermentation of soy press cakes, compared with the control. Fermented samples had around 28–54%, and 12–20% higher WHC and OHC than control sample, respectively. Soy press cakes fermented with L. plantarum P1 and L. acidophilus 336 showed a higher increase in WHC (54% and 51%, respectively) than other applied strains. These results were consistent with those reported by Saez, Sabatar, Fara, & Zarate (2021), where the chickpea flour sample was fermented with L. plantarum CRL 2211 for 24h at 37 °C, and WHC and OHC were increased by 6.2% and 20% after fermentation, respectively. The increased WHC of the product was suggested to be due to the decrease in solubility, which meant that the higher contents of insoluble proteins were able to bind more water (Meinlschmidt, Ueberham, Lehmann, Schweiggert-Weisz, & Eisner, 2016). Lactic acid is a highly hygroscopic compound (Wijayasinghe, Vasiljevic, & Chandrapala, 2015) and can absorb and hold water molecules from the surrounding environment. The increased OHC after fermentation could be due to the reduction of pH (Table 5). Cabuk et al. (2018) demonstrated that the reduction of pH after fermentation resulted in decreased protein surface charge and solubility, as well as increased hydrophobicity to allow for increased protein interactions with oil. The increased OHC might also depend on changes in amino acid composition, protein conformation, or a mechanism that was unknown to us.
Table 5.
Effect of fermentation or enzymatic hydrolysis on pH, spreadability, and shear-thinning factor n values of soy press cakes.
| Treatment conditions | Treatment duration, h | Spreadability | Shear-thinning factor n | pH |
|---|---|---|---|---|
| Control | - | 1.21 ± 0.06a | 0.194 ± 0.01b | 7.02 ± 0.04a |
| Fermentation | ||||
| L. plantarum P1 | 24 | 0.87 ± 0.08b | 0.347 ± 0.02d | 4.93 ± 0.03b |
| L. brevis R | 24 | 0.93 ± 0.03b | 0.233 ± 0.02c | 5.17 ± 0.06b |
| L. acidophilus 308 | 24 | 1.01 ± 0.05b | 0.190 ± 0.03b | 4.82 ± 0.03b |
| L. acidophilus 336 | 24 | 0.61 ± 0.06c | 0.173 ± 0.04a | 5.05 ± 0.06b |
| Hydrolysis | ||||
| Cellulase/Xylanase | 0.5 | 1.01 ± 0.04b | 0.400 ± 0.05e | 7.35 ± 0.05a |
| 1 | 0.98 ± 0.07b | 0.298 ± 0.02c | 7.00 ± 0.02a | |
| α-Amylase | 0.5 | 1.03 ± 0.05b | 0.412 ± 0.03e | 7.20 ± 0.00a |
| 1 | 0.85 ± 0.08b | 0.322 ± 0.00d | 7.20 ± 0.01a | |
a,b, c, d, e Different letters in the same column for each treatment indicate significant statistical differences, (P ≤ 0.05, LSD test).
The enzymatic hydrolysis of soy press cakes significantly increased WHC and OHC values of the samples by applying cellulase/xylanase mixture, and α-amylase (Table 4). Deng et al. (2016) showed that the enzymatic hydrolysis of wheat gluten resulted in a substantial increase in WHC and OHC values following hydrolysis for 1.5–2.0 h at 45–55 °C. A similar effect of enzymatic hydrolysis on porous starch granules (PSGs) was found by applying amylase at 40 °C for different periods, indicating that both WHC and OHC values significantly increased after 2–4 h of hydrolysis (Jung et al., 2017). A possible mechanism explaining the impact of hydrolysis on WHC and OHC values could be the effect of heating, as the hydrolysis of press cakes was performed by heating samples at 50 °C and 70 °C using cellulase/xylanase mixture and α-amylase, respectively. Generally, decreased WHC of proteins could be due to the denaturation effect, which reduces the availability of polar amino groups for hydrogen bonding with water molecules. However, heating could also unfold the protein and expose side chains that could bind water and result in improved WHC of fibrous proteins. This mechanism could be considered as a reason for increased WHC after enzymatic hydrolysis of press cakes. Nguyen, Mounir, and Allaf (2015) reported that the major factors affecting WHC were protein denaturation and unfolding, presence of carbohydrates and non-protein components. Therefore, the increased WHC after hydrolysis could be due to several mechanisms. The increased OHC after hydrolysis might be due to the exposing more hydrophobic regions (Deng et al., 2016) or a mechanism that was unknown to us.
3.2.3. Protein oxidation
Fermentation significantly decreased the protein carbonyl content of soy press cakes (Table 4). Fermentation with L. plantarum P1 and L. brevis R strains significantly decreased the protein oxidation level by around 59%, likewise, L. acidophilus 308 and L. acidophilus 336 decreased the protein oxidation level by around 80%, compared with the control sample. In agreement, Ge et al. (2019) revealed that L. plantarum NJAU-01 strain significantly lowered protein carbonyl content in fermented sausage, as fermentation increased the antioxidant activity (Hunaefi et al., 2013). Miri, Hajihosseini, Saedi, Vaseghi, and Rasooli (2019) reported the oxidation stress reduction of fermented soybean meal extract by Lactobacillus plantarum, through the increase in antioxidant activity.
Hydrolysis of soy press cakes using α-amylase significantly reduced protein carbonyl content by around 43%–49%, whereas cellulase/xylanase mixture decreased protein carbonyl content by approximately 75%, compared with the control sample (Table 4). Several studies reported that enzymatic hydrolysis of soy protein enhanced the antioxidant capacity, which was related to the release of bioactive peptides (Zhang et al., 2014; Garcia de Figueiredo et al., 2019). This might be a possible mechanism for reduction of protein oxidation in soy press cakes after enzymatic hydrolysis, as the decrease in carbonyl content correlated with the increased antioxidant activity (Rajesh et al., 2004).
3.2.4. Correlation of protein oxidation stress with WHC and OHC in soy press cakes
Results indicated that WHC and OHC of soy press cakes steadily increased as extent of oxidation of press cakes protein and bulk density decreased (Table 4). There was a strong negative correlation between protein oxidation and WHC in fermented soy press cakes (r = −0.712). In agreement, Wu, Hua, Li, & Xiao (2011) reported that oxidation of soy protein by peroxyl radicals was accompanied by a gradual decrease in WHC. Likewise, Xiong (2000) illustrated that the oxidation of proteins in processed meat products could lead to a decrease in WHC. Oxidation-induced decrease in water-holding was also supported by other studies (Delles & Xiong, 2014; Zakrys-Waliwander, O'Sullivan, O'Neill, & Kerry, 2012). Zayas (1997) reported that OHC was correlated with protein surface hydrophobicity, and insoluble hydrophobic proteins had high OHC values. High amounts of hydrophobic proteins demonstrate an increase in protein oxidation, as oxidized proteins are generally exposing hydrophobic amino acids at their surface (Friguet, 2006). However, our study indicated a reduction in protein oxidation level and an increase in OHC values of soy press cakes after fermentation. There was a very strong negative correlation between protein oxidation and OHC in fermented soy press cakes (r = −0.967). Wu, Hua, Lin, and Xiao (2011) reported that oxidative modification by peroxyl radicals resulted in a decrease in disulphide content of soy protein, which subsequently resulted in a decrease in particle size. Elleuch, Bedigian, Besbes, Blecker, and Attia (2014) reported that a decrease in particle size was associated with an increase in density, and a reduction in WHC and OHC.
3.2.5. Viscosity, spreadability and pH
Obesity is one of the main reasons for avoidable deaths. The promotion of satiety-enhancing food products is a plan to decrease food intake. Food viscosity can affect satiety; therefore, the viscosity is considered important for the development of food products. Ingredients that form strong gels and give high viscosity are preferred for use in meat products and meat analogues, as the higher viscosity of food can influence satiety and reduce hunger and food intake through suppression of appetite (Lamsal, Jung, & Johnson, 2007; Stribiţcaia, Evans, Gibbons, Blundell, & Sarkar, 2020). Hence, the higher viscosity of soy press cake is a preferred factor for using press cakes in the meat analogues matrices. A lower shear-thinning factor n measured by the rheometer implies a higher viscosity of the sample. Shear-thinning is a phenomenon characteristic of some non-Newtonian fluids, in which the fluid viscosity decreases with increasing shear stress (Wilkes, 1981). Results (Table 5) indicated that both fermentation and enzymatic hydrolysis processes reduced the viscosity values of soy press cakes, except for press cakes fermented with L. acidophilus 308, which significantly increased the viscosity. In agreement, Lamsal et al. (2007) reported that the viscosity of soy protein products decreased after enzymatic hydrolysis. In general, lower apparent viscosity was observed in protein, as their molecular mass was reduced by proteolysis. Peptide profiles for the soy substrates were significantly reduced after hydrolysis and could explain the reduction in viscosities. Fermentation using different LAB strains could increase or decrease the viscosity, in comparison to the respective unfermented sample. Fermentative bioprocesses could change the molecular size and inter- and intra-molecular interactions of macromolecules of starch and proteins (Laaksonen et al., 2020). Reduction in viscosity depending on the type of microorganism, could also involve. If the microorganisms produce, for instance, amylases, there could be a possibility of viscosity reduction because of starch hydrolysis (Taylor & Emmambux, 2008).
The spreadability of soy press cakes was significantly reduced after fermentation and hydrolysis. Fermentation reduced the force required to disperse the press cake on certain surfaces, thereby the firmness of the press cake reduced. A possible mechanism for the decreased spreadability in press cakes after hydrolysis could be the reduction in particle size distribution. Kim, Park, and Lim (2008) concluded that enzymatic hydrolysis of waxy rice starch using α-amylase reduced the particle size distribution. Ningtyas, Bhandari, Bansal, and Prakash (2017) reported that reduced particle size distribution resulted in firmer and less spreadable cream cheese.
Results indicated that fermentation significantly reduced pH (Table 5) from 7.02 ± 0.04 in control sample to between 4.82 ± 0.03 and 5.17 ± 0.06 in fermented samples. During fermentation, monosaccharides were firstly fermented by LAB, generating organic acids, which reduced the pH of the substrate (Xiang, Sun-Waterhouse, Waterhouse, Cui, & Ruan, 2019). Fermentation lowers the pH of foods by increasing the level of lactic acids (Karovicova & Kohajdova, 2003).
3.3. Properties of meat analogues
3.3.1. Texture properties
According to the texture profile analysis of the cooked meat analogues (Table 6), there was no significant difference in the degree of hardness and chewiness of meat analogues produced with fermented or hydrolyzed soy press cakes, compared with the control, except for a product containing press cake fermented with L. plantarum P1, which significantly reduced the hardness and chewiness of meat analogue. This strain improved the texture, as the texture acceptability of meat analogue containing press cake fermented with L. plantarum P1 was greater than that of the control sample and other fermented products (Fig. 2, A). In agreement, Hadaegh, Seyyedain Ardabili, Tajabadi Ebrahimi, Chamani, and Azizi Nezhad (2017) reported that fermentation significantly reduced the hardness of toast bread through the application of Lactobacillus plantarum jQ301799. Decreased hardness was assumed to be due to the acidity-induced activation of proteolytic enzymes present in fermented wheat flour (Clarke, Schober, & Arendt, 2002). Khalid and Marth (1990) reported that Lactobacillus plantarum NRRL B-4004 had higher proteolytic activity in milk proteins than other applied strains. Moreover, Arief, Afiyah, Wulandari, and Budiman (2016) indicated that L. plantarum IIA-2C12 had higher proteolytic activity than Lactobacillus acidophilus IIA-2B4 in fermented beef sausages.
Table 6.
Effect of fermentation or enzymatic hydrolysis of soy press cakes on texture parameters of the cooked meat analogues.
| Treatment conditions | Treatment duration,h | Meat analogue properties |
|
|---|---|---|---|
| Hardness, N | Chewiness | ||
| Control | 17.21 ± 0.05a | 7.45 ± 0.02a | |
| Fermentation | |||
| L. plantarum P1 | 24 | 14.2 ± 0.04b | 6.67 ± 0.03b |
| L. brevis R | 24 | 15.22 ± 0.05a | 7.31 ± 0.01a |
| L. acidophilus 308 | 24 | 16.61 ± 0.08a | 8.11 ± 0.05a |
| L. acidophilus 336 | 24 | 16.51 ± 0.02a | 8.12 ± 0.06a |
| Hydrolysis | |||
| Cellulase/Xylanase | 0.5 | 18.1 ± 0.04a | 9.22 ± 0.07a |
| 1 | 18.25 ± 0.01a | 9.45 ± 0.01a | |
| α-Amylase | 0.5 | 19.66 ± 0.00a | 8.35 ± 0.02a |
| 1 | 19.32 ± 0.06a | 8.23 ± 0.03a | |
a,b, c Different letters in the same column for each treatment indicate significant statistical differences, (P ≤ 0.05, LSD test).
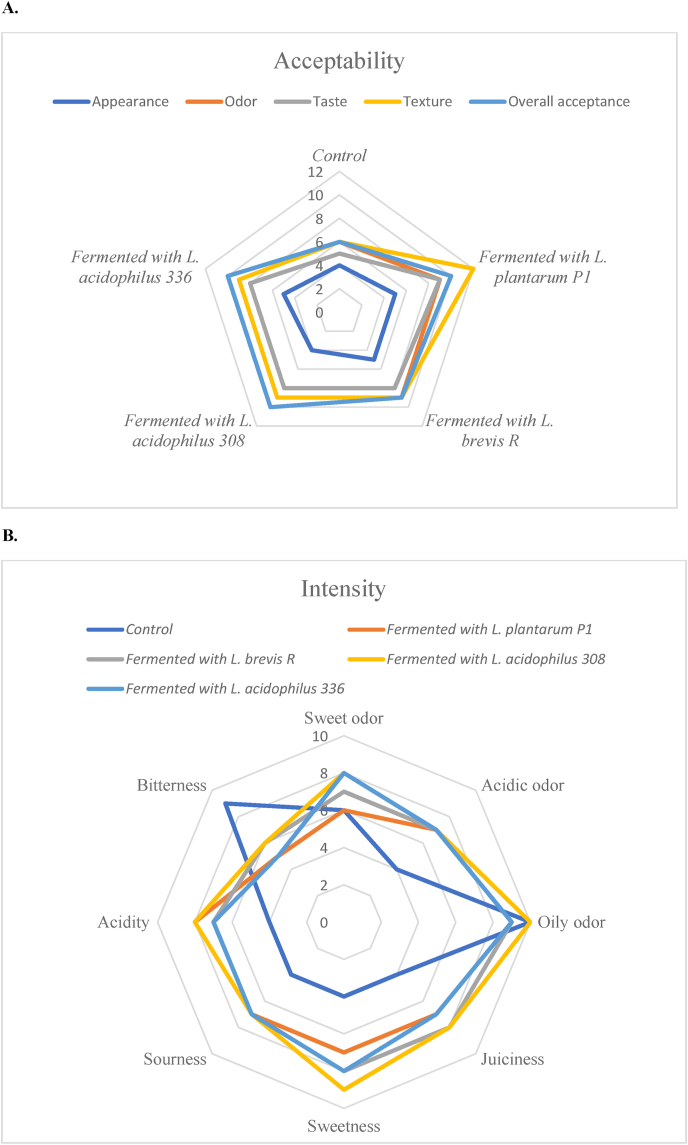
Fig. 2.
Spider plots of sensory evaluation of meat analogues produced with fermented soy press cakes, representing their acceptability scores (A) and intensity scores (B).
3.3.2. Sensory properties
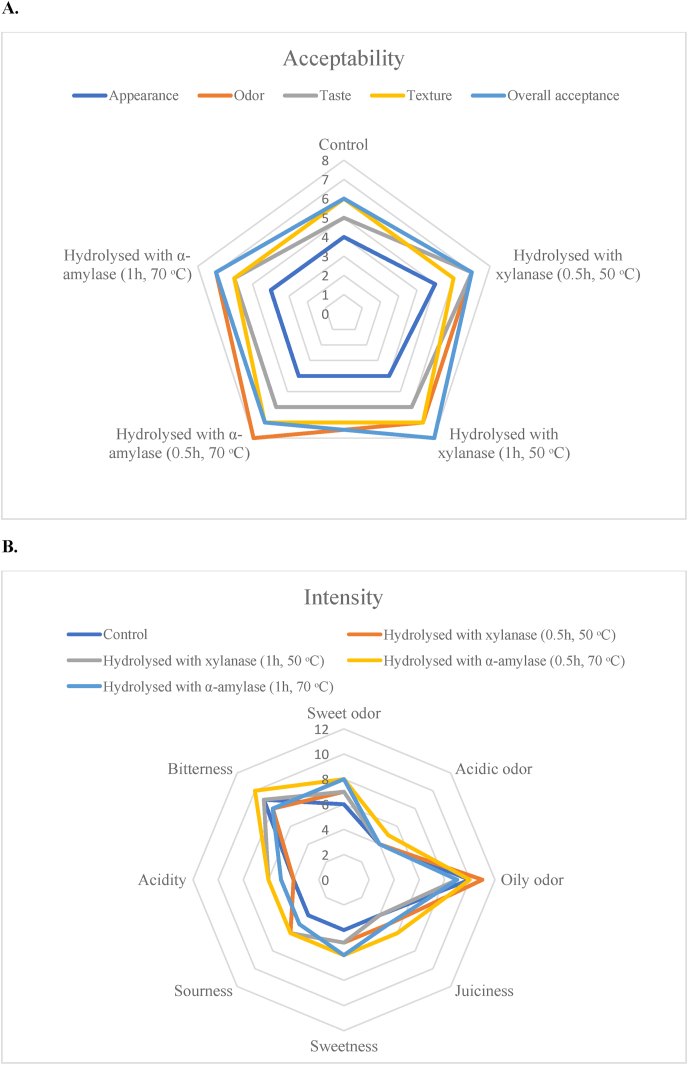
Results of sensory analysis of the cooked meat analogues produced with soy press cakes were presented in Table 7, and spider plots to provide graphical representations of the sensory profiles (Figs. 2 and 3). There were statistically significant differences for 6 of the 8 attributes used to describe sensory intensity of meat analogues containing fermented press cakes (except for sweet odor and oily odor); and for 4 of the 5 attributes used to describe the products’ sensory acceptability (except for the appearance) (Fig. 2). The sensory intensity and acceptability of meat analogues produced with hydrolyzed soy press cakes were not significantly different from the control sample (Fig. 3).
Table 7.
Effect of fermentation or enzymatic hydrolysis of soy press cakes on sensory attributes of the cooked meat analogues.
| Control |
Fermentation |
Hydrolysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| L. plantarum P1 | L. brevis R | L. acidophilus 308 | L. acidophilus 336 | Cellulase/Xylanase | α-Amylase | ||||
| Treatment duration, h | |||||||||
| – | 24 | 24 | 24 | 24 | 0.5 | 1 | 0.5 | 1 | |
| Intensity | |||||||||
| Sweet odor | 6a | 6a | 7a | 8a | 8a | 7a | 7a | 8a | 8a |
| Oily odor | 10a | 9a | 9a | 10a | 9a | 11a | 9a | 10a | 9a |
| Acidic odor | 4a | 7b | 7b | 7b | 7b | 4a | 4a | 5a | 4a |
| Juiciness | 4a | 7b | 8b | 8b | 7b | 5a | 4a | 6a | 5a |
| Sweetness | 4a | 7b | 8b | 9b | 8b | 5a | 5b | 6a | 6a |
| Sourness | 4a | 7b | 7b | 7b | 7b | 6a | 6a | 6a | 5a |
| Acidity | 4a | 8b | 7b | 8b | 7b | 4a | 6a | 6a | 5a |
| Bitterness | 9b | 5a | 6a | 6a | 5a | 8b | 9b | 10b | 8b |
| Acceptability | |||||||||
| Appearance | 4a | 5a | 5a | 4a | 5a | 5a | 4a | 4a | 4a |
| Odor | 6a | 9b | 9b | 9b | 9b | 7a | 7a | 8a | 7a |
| Taste | 5a | 9b | 8b | 8b | 8b | 7a | 6a | 6a | 6a |
| Texture | 6a | 12c | 9b | 9b | 9b | 6a | 7a | 7a | 6a |
| Overall | 6a | 10b | 9b | 10b | 10b | 7a | 8a | 7a | 7a |
a, b, c Different letters in the same row indicate significant statistical differences, (P ≤ 0.05, LSD test).
Fig. 3.
Spider plots of sensory evaluation of meat analogues produced with hydrolyzed soy press cakes, representing their acceptability scores (A) and intensity scores (B).
Results indicated that the addition of fermented soy press cakes to the meat analogues, significantly reduced bitterness, increased juiciness, and improved odor, taste, texture, and overall acceptability of the meat analogues. Increased juiciness of the meat analogues was in agreement with the increased WHC in fermented soy press cakes (Table 4).
A major comment on the appearance of meat analogues (control and processed) was regarding the particles of vegetable press cakes with uneven size, shape and color, which were clearly recognizable in meat analogues and had a negative effect on the appearance acceptability of meat analogues (Fig. 2, A and Fig. 3, A), as all samples (control and processed) received very low scores (4 or 5 from 15) on appearance acceptability from the consumers. A general comment regarding odor properties was a perceived oiliness of all products. The taste properties of fermented products were identified with higher sweetness, sourness and acidity, and lower bitterness, compared with the control sample. Thus, fermented products were described as fresher with a balanced taste, which was confirmed with the higher taste acceptability of these products. The preliminary test for acceptability analysis of the products revealed that the meat analogues produced with fermented soy press cakes had significantly higher overall acceptance score than the control.
4. Conclusion
In conclusion, to increase the utilization of soy press cake for human nutrition, new strategies are needed, like its incorporation into novel and healthy foods (plant-based protein foods), such as meat analogues. This study recommends the utilization of soy press cake as an ingredient of meat analogues, as it contains protein, fibre, essential amino acids, and energy with a low content of saturated fatty acids. Fermentation with selected LAB and enzymatic hydrolysis with selected enzymes improve functional properties and reduce protein oxidation level in soy press cakes. L. acidophilus 336 and L. plantarum P1 are recommended for fermentation of soy press cakes to reduce protein oxidation and increase viscosity. Likewise, hydrolysis of soy press cakes by cellulase/xylanase mixture is recommended regarding protein oxidation reduction in press cakes. When used as an ingredient of meat analogues, 10% of soy press cakes fermented with selected LAB strains improve the sensory quality of meat analogues. L. plantarum P1 is also recommended for soy press cake fermentation, as it improves the texture properties of meat analogues. Therefore, fermented soy press cake is a promising and convenient novel ingredient of meat analogues.
Further studies are required to analyze the effect of fermented and hydrolyzed soy press cakes on the proximate and amino acid compositions of press cakes, as well as protein oxidation level and functional properties of the meat analogues.
Declaration of competing interest
The authors declare no conflict of interest.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Authors' contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript.
CRediT authorship contribution statement
Seyedmahmood Razavizadeh: Conceptualization, Methodology, Validation, Visualization, Formal analysis, Writing – original draft, Investigation, Writing – review & editing. Gitana Alencikiene: Conceptualization, Methodology, Investigation, Resources, Visualization, Writing – review & editing, Supervision. Lina Vaiciulyte-Funk: Methodology, Investigation, Resources. Per Ertbjerg: Methodology, Writing – review & editing. Alvija Salaseviciene: Conceptualization, Investigation, Supervision, Resources, Visualization, Funding acquisition.
Acknowledgments and Funding
This research is a part of project, “Disaggregation of Conventional Vegetable Press Cakes by Novel Techniques to Receive New Products and to Increase the Yield” (DISCOVERY) H2020 activity ERA-NET SUSFOOD2. Research was funded by national/regional sources LR Ministry of Agriculture TM-18 1/2, and co-funding by the European Union's Horizon 2020 research and innovation programme.
References
- Adebiyi J.A., Obadina A.O., Adebo O.A., Kayitesi E. Comparison of nutritional quality and sensory acceptability of biscuits obtained from native, fermented, and malted pearl millet (Pennisetum glaucum) flour. Food Chemistry. 2017;232:210–217. doi: 10.1016/j.foodchem.2017.04.020. http://10.1016/j.foodchem.2017.04.020 [DOI] [PubMed] [Google Scholar]
- Alexander P., Brown C., Dias C., Moran D., Rounsevell M. In: Proteins: Sustainable source, processing, and applications. Galanakis C., editor. Elsevier; 2019. Sustainable proteins production; pp. 1–39. [Google Scholar]
- Ancuta P., Sonia A. Oil press-cakes and meals valorization through circular economy approaches: A review. Applied Sciences. 2020;10(21):7432. doi: 10.3390/app10217432. [DOI] [Google Scholar]
- Arief I.I., Afiyah D.N., Wulandari Z., Budiman C. Physicochemical properties, fatty acid profiles, and sensory characteristics of fermented beef sausage by probiotics Lactobacillus plantarum IIA-2C12 or Lactobacillus acidophilus IIA-2B4. Journal of Food Science. 2016;81(11):2761–2769. doi: 10.1111/1750-3841.13509. http://10.1111/1750-3841.13509 [DOI] [PubMed] [Google Scholar]
- Boisgard A.-S., Lamrayah M., Dzikowski M., Salmon D., Kirilov P., Primard C., et al. Innovative drug vehicle for local treatment of inflammatory skin diseases: Ex vivo and in vivo screening of five topical formulations containing poly (lactic acid) (PLA) nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics. 2017;116:51–60. doi: 10.1016/j.ejpb.2016.09.021. http://10.1016/j.ejpb.2016.09.021 [DOI] [PubMed] [Google Scholar]
- Bourne M.C. Texture profile analysis. Food Technology. 1978;32:62–66. 72. [Google Scholar]
- Cabuk B., Stone A.K., Korber D.R., Tanaka T., Nickerson M.T. Effect of Lactobacillus plantarum fermentation on the surface and functional properties of pea protein-enriched flour. Food Technology and Biotechnology. 2018;56(3):411–420. doi: 10.17113/ftb.56.03.18.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L.Y., Takahashi M., Lim P.J., Aoyama S., Makino S., Ferdinandus F., et al. Eurotium Cristatum fermented okara as a potential food ingredient to combat diabetes. Scientific Reports. 2019;9 doi: 10.1038/s41598-019-54021-4. Article 17536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Kong B., Sun Q., Dong F., Liu Q. Antioxidant potential of a unique LAB culture isolated from Harbin dry sausage: In vitro and in a sausage mode. Meat Science. 2015;110:185–188. doi: 10.1016/j.meatsci.2015.07.021. http://10.1016/j.meatsci.2015.07.021 [DOI] [PubMed] [Google Scholar]
- Chi C.H., Cho S.J. Improvement of bioactivity of soybean meal by solid-state fermentation with Bacillus amyloliquefaciens versus Lactobacillus spp. and Saccharomyces cerevisiae. LWT - Food Science and Technology. 2016;68:619–625. doi: 10.1016/j.lwt.2015.12.002. [DOI] [Google Scholar]
- Clarke C., Schober T.J., Arendt E. The effect of single strain and traditional mixed strain starter culture on rheological properties of wheat dough and bread quality. Cereal Chemistry. 2002;79:640–647. doi: 10.1094/CCHEM.2002.79.5.640. [DOI] [Google Scholar]
- Csapó J., Lóki K., Csapó-Kiss Z.S., Albert C.S. Separation and determination of the amino acids by ion exchange column chromatography applying post-column derivatization. Acta Universitatis Sapientiae - Alimentaria. 2008;1:5–29. https://core.ac.uk/download/pdf/233610052.pdf Retrieved from. [Google Scholar]
- Delles R.M., Xiong Y.L. The effect of protein oxidation on hydration and water-binding in pork packaged in an oxygen-enriched atmosphere. Meat Science. 2014;97:181–188. doi: 10.1016/j.meatsci.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Deng L., Wang Z., Yang S., Song J., Que F., Zhang H., et al. Improvement of functional properties of wheat gluten using acid protease from Aspergillus usamii. PLoS One. 2016;11(7):1–13. doi: 10.1371/journal.pone.0160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleuch M., Bedigian D., Besbes S., Blecker C., Attia H. Dietary fibre characteristics and antioxidant activity of sesame seed coats (testae) International Journal of Food Properties. 2014;15:25–37. http://10.1080/10942911003687231 [Google Scholar]
- Emkani M., Oliete B., Saurel R. Effect of lactic acid fermentation on legume protein properties, a review. Fermentation. 2022;8:244. doi: 10.3390/fermentation8060244. [DOI] [Google Scholar]
- Friedman M. Analysis, nutrition, and health benefits of tryptophan. International Journal of Tryptophan Research. 2018;11:1–12. doi: 10.1177/1178646918802282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friguet B. Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Letters. 2006;580(12):2910–2916. doi: 10.1016/j.febslet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Garcia de Figueiredo V.R., Justus A., Garcia Pereira D., Regina Georgetti S., Iouko Ida1 E., Kurozawa L.E. Production of hydrolysate of okara protein concentrate with high antioxidant capacity and aglycone isoflavone content. Brazilian Archives of Biology and Technology. 2019;62 doi: 10.1590/1678-4324-2019180478. [DOI] [Google Scholar]
- Ge Q., Chen S., Liu R., Chen L., Yang B., Yu H., et al. Effects of Lactobacillus plantarum NJAU-01 on the protein oxidation of fermented sausage. Food Chemistry. 2019;295:361–367. doi: 10.1016/j.foodchem.2019.05.154. [DOI] [PubMed] [Google Scholar]
- Guan Y., Wang J., Wu J., Wang L., Rui X., Xing G., et al. Enhancing the functional properties of soymilk residues (okara) by solid-state fermentation with Actinomucor elegans. CyTA - Journal of Food. 2016;15(1):1–9. doi: 10.1080/19476337.2016.1226955. [DOI] [Google Scholar]
- Hadaegh H., Seyyedain Ardabili S.M., Tajabadi Ebrahimi M., Chamani M., Azizi Nezhad R. The impact of different lactic acid bacteria sourdoughs on the quality characteristics of toast bread. Journal of Food Quality. 2017 doi: 10.1155/2017/7825203. Article 7825203. [DOI] [Google Scholar]
- Hunaefi D., Gruda N., Riedel H., Divine N.A., Thaw-Saw N.M.M., Smetanska I. Improvement of antioxidant activities in red cabbage sprouts by lactic acid bacterial fermentation. Food Biotechnology. 2013;27(4):279–302. doi: 10.1080/08905436.2013.836709. [DOI] [Google Scholar]
- Jung Y.S., Lee B.H., Sang H.Y. Physical structure and absorption properties of tailor-made porous starch granules produced by selected amylolytic enzymes. PLoS One. 2017;12(7):1–14. doi: 10.1371/journal.pone.0181372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karovicova J., Kohajdova Z. Lactic acid fermented vegetable juices. Horticultural Science. 2003;30(4):152–158. http://10.17221/3878-HORTSCI [Google Scholar]
- Khalid N.M., Marth E.H. Proteolytic activity by strains of Lactobacillus plantarum and. Lactobacillus case. Journal of Dairy Science. 1990;73(11):3068–3076. http://10.3168/jds.s0022-0302(90)78994-1 [Google Scholar]
- Kim J.Y., Park D.J., Lim S.T. Fragmentation of waxy rice starch granules by enzymatic hydrolysis. Cereal Chemistry Journal. 2008;85(2):182–187. http://10.1094/cchem-85-2-0182 [Google Scholar]
- Kotowaroo M.I., Mahomoodally M.F., Gurib-Fakim A., Subratty A.H. Screening of traditional antidiabetic medicinal plants of Mauritius for possible α-amylase inhibitory effects in vitro. Phytotherapy Research. 2006;20:228–231. doi: 10.1002/ptr.1839. [DOI] [PubMed] [Google Scholar]
- Kumar S. Meat analogues: Plant based alternatives to meat products- a review. International Journal of Food and Fermentation Technology. 2016;5:107–119. http://10.5958/2277-9396.2016.00001.5 [Google Scholar]
- Laaksonen O., Kahala M., Marsol-Vall A., Blasco L., Jarvenpaa E., Rosenvald S., et al. Impact of lactic acid fermentation on sensory and chemical quality of dairy analogues prepared from lupine (Lupinus angustifolius L.) seeds. Food Chemistry. 2020;1(346) doi: 10.1016/j.foodchem.2020.128852. http://10.1016/j.foodchem.2020.128852 Article 128852. [DOI] [PubMed] [Google Scholar]
- Lamsal B.P., Jung S., Johnson L.A. Rheological properties of soy protein hydrolysates obtained from limited enzymatic hydrolysis. LWT - Food Science And Technology. 2007;40:1215–1223. doi: 10.1016/j.lwt.2006.08.021. [DOI] [Google Scholar]
- Li B., Qiao M., Lu F. Composition, nutrition, and utilization of okara (soybean residue) Food Reviews International. 2012;28(3):231–252. doi: 10.1080/87559129.2011.595023. [DOI] [Google Scholar]
- Liutkevičius A., Speičienė V., Alenčikienė G., Sekmokienė D., Žvirdauskienė R., Mieželienė A. Influence of chitosan on microbiological data and quality charecteristics of spreadable curd cheese and mayonnaise. Veterinarija ir Zootechnika. 2015;69(91):38–47. [Google Scholar]
- Meinlschmidt P., Ueberham E., Lehmann J., Schweiggert-Weisz U., Eisner P. Immunoreactivity, sensory and physicochemical properties of fermented soy protein isolate. Food Chemistry. 2016;205:229–238. doi: 10.1016/j.foodchem.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Miri S., Hajihosseini R., Saedi H., Vaseghi M., Rasooli A. Fermented soybean meal extract improves oxidative stress factors in the lung of inflammation/infection animal model. Annals of Microbiology. 2019;69:1507–1515. doi: 10.1007/s13213-019-01534-y. [DOI] [Google Scholar]
- Mistry M., George A., Thomas S. Alternatives to meat for halting the stable to table continuum – an update. Arab Journal of Basic and Applied Sciences. 2020;27(1):324–334. doi: 10.1080/25765299.2020.1807084. [DOI] [Google Scholar]
- Nayak B.N., Singh R.B., Buttar H.S. Role of tryptophan in health and disease: Systematic review of the antioxidant, anti-inflammation, and nutritional aspects of tryptophan and its metabolites. World Heart Journal. 2019;11(2):161–178. https://www.researchgate.net/publication/336104734 Retrieved from. Accessed June 20, 2022. [Google Scholar]
- Nguyen D.Q., Mounir S., Allaf K. Functional properties of water holding capacity, oil holding capacity, wettability, and sedimentation of swell-dried soybean powder. Scholars Journal of Engineering and Technology. 2015;3(4B):402–412. http://saspublisher.com/wp-content/uploads/2015/06/SJET34B402-412.pdf Retrieved from. Accessed June 20, 2022. [Google Scholar]
- Ningtyas D.W., Bhandari B., Bansal N., Prakash S. Effect of homogenisation of cheese milk and high-shear mixing of the curd during cream cheese manufacture. International Journal of Dairy Technology. 2017;71:417–431. doi: 10.1111/1471-0307.12482. [DOI] [Google Scholar]
- Ogodo A.C., Ugbogu O.C., Onyeagba R.A., Okereke H.C., Agwaranze D.I. Dynamics of functional properties of sorghum flours fermented with lactic acid bacteria (LAB)-consortium isolated from cereals. International Food Research Journal. 2017;24(6):2666–2671. https://www.researchgate.net/publication/322330992 Retrieved from. [Google Scholar]
- Oliveira R.B.S., Lucia F.D., Ferreira E.B., Oliveira R.M.E., Pimenta C.J., Pimenta M.E.S.G. Quality of beef burger with addition of wet okara along the storage. Ciencia E Agrotecnologia. 2016;40(6):706–717. doi: 10.1590/1413-70542016406005816. [DOI] [Google Scholar]
- Osen R., Schweiggert-Weisz U. In: Reference module in food science. Smithers G.W., editor. Elsevier; 2016. High-moisture extrusion: Meat analogues; pp. 1–7. [Google Scholar]
- O'Toole D.K. Characteristics and use of okara, the soybean residue from soy milk production-a review. Journal of Agricultural and Food Chemistry. 1999;47:363–371. doi: 10.1021/jf980754l. [DOI] [PubMed] [Google Scholar]
- Rajesh M., Sulochana K.N., Coral K., Punitham R., Biswas J., Babu K., et al. Determination of carbonyl group content in plasma proteins as a useful marker to assess impairment in antioxidant defense in patients with Eales' disease. Indian Journal of Ophthalmology. 2004;52(2):139–144. https://journals.lww.com/ijo/Fulltext/2004/52020/Determination_of_Carbonyl_Group_Content_in_Plasma.7.aspx Retrieved from. [PubMed] [Google Scholar]
- Rashad M.M., Mahmoud A.E., Abdou H.M., Nooman M.U. Improvement of nutritional quality and antioxidant activities of yeast fermented soybean curd residue. African Journal of Biotechnology. 2011;10(28):5504–5513. [Google Scholar]
- Rui X., Wang M., Zhang Y., Chen X., Li L., Liu Y., et al. Optimization of soy solid-state fermentation with selected lactic acid bacteria and the effect on the anti-nutritional components. Journal of Food Processing and Preservation. 2016;41(6) http://10.1111/jfpp.13290 [Google Scholar]
- Sadh P.K., Chawla P., Duhan J.S. Fermentation approach on phenolic, antioxidants and functional properties of peanut press cake. Food Bioscience. 2018;22:113–120. doi: 10.1016/j.fbio.2018.01.011. [DOI] [Google Scholar]
- Saeeduddin M., Abid M., Jabbar S., Wu T., Hashim M.M., Awad F.N., et al. Quality assessment of pear juice under ultrasound and commercial pasteurization processing conditions. LWT - Food Science and Technology. 2015;64:452–458. doi: 10.1016/j.lwt.2015.05.005. [DOI] [Google Scholar]
- Saez G.D., Sabater C., Fara A., Zarate G. Fermentation of chickpea flour with selected lactic acid bacteria for improving its nutritional and functional properties. Journal of Applied Microbiology. 2021:1–19. doi: 10.1111/jam.15401. 00. [DOI] [PubMed] [Google Scholar]
- Shahryari Z., Mohammad H.F., Younes G., Patrik R.L., Mohammad J.T. Amylase and xylanase from edible fungus Neurospora intermedia: Production and characterization. Molecules. 2019;24(4):721. doi: 10.3390/molecules24040721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh E., Jadaun S. Potential of millets: Nutrients composition and health benefits. Journal of Scientific and Innovative Research. 2016;5(2):46–50. http://10.31254/jsir.2016.5204 [Google Scholar]
- Snedecor G.W., Cochran W.G. 6th ed. Iowa State University Press; 1967. Statistical methods. [Google Scholar]
- Soglia F., Petracci M., Ertbjerg P. Novel DNPH-based method for determination of protein carbonylation in muscle and meat. Food Chemistry. 2016;197:670–675. doi: 10.1016/j.foodchem.2015.11.038. [DOI] [PubMed] [Google Scholar]
- Sreerama Y.N., Sashikala V.B., Pratape V.M., Singh V. Nutrients and antinutrients in cowpea and horse gram flours in comparison to chickpea flour: Evaluation of their flour functionality. Food Chemistry. 2012;131:462–468. doi: 10.1016/j.foodchem.2011.09.008. [DOI] [Google Scholar]
- Stribiţcaia E., Evans C., Gibbons C., Blundell J., Sarkar A. Food texture influences on satiety: Systematic review and meta-analysis. Scientific Reports. 2020;10 doi: 10.1038/s41598-020-69504-y. http://10.1038/s41598-020-69504-y Article 12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.R.N., Emmambux M.N. In: Technology of functional cereal products. Hamaker B.R., editor. Woodhead Publishing Series in Food Science, Technology and Nutrition; 2008. Products containing other speciality grains: Sorghum, the millets and pseudocereals; pp. 281–355. [Google Scholar]
- Turhan S., Temiz H., Sagir I. Utilization of wet okara in low-fat beef patties. Journal of Muscle Foods. 2007;18:226–235. http://10.1111/j.1745-4573.2007.00081.x [Google Scholar]
- Vong W.C., Hua X.Y., Liu S.Q. Solid-state fermentation with Rhizopus oligosporus and Yarrowia lipolytica improved nutritional and flavour properties of okara. LWT - Food Science and Technology. 2018;90:316–322. doi: 10.1016/j.lwt.2017.12.050. [DOI] [Google Scholar]
- Wijayasinghe R., Vasiljevic T., Chandrapala J. Water-lactose behavior as a function of concentration and presence of lactic acid in lactose model systems. Journal of Dairy Science. 2015;98(12):8505–8514. doi: 10.3168/jds.2015-9959. [DOI] [PubMed] [Google Scholar]
- Wilkes G.L. An overview of the basic rheological behavior of polymer fluids with an emphasis on polymer melts. Journal of Chemical Education. 1981;58:880–892. doi: 10.1021/ed058p880. [DOI] [Google Scholar]
- Wu W., Hua Y., Lin Q., Xiao H. Effects of oxidative modification on thermal aggregation and gel properties of soy protein by peroxyl radicals. International Journal of Food Science and Technology. 2011;46:1891–1897. doi: 10.1007/s13197-011-0533-7. http://10.1111/j.1365-2621.2011.02698.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H., Sun‐Waterhouse D., Waterhouse G.I., Cui C., Ruan Z. Fermentation‐enabled wellness foods: A fresh perspective. Food Science and Human Wellness. 2019;8:203–243. doi: 10.1016/j.fshw.2019.08.003. [DOI] [Google Scholar]
- Xing Q., Dekker S., Kyriakopoulou K., Boom R.M., Smid E.J., Schutyser M.A. Enhanced nutritional value of chickpea protein concentrate by dry separation and solid-state fermentation. Innovative Food Science & Emerging Technologies. 2020;59 doi: 10.1016/j.ifset.2019.102269. Article 102269. [DOI] [Google Scholar]
- Xiong Y. In: Antioxidants in muscle foods. Faustman C., Lopez-Bote C.J., editors. John Wiley & Sons Inc; 2000. Protein oxidation and implications for muscle foods quality; pp. 85–111. [Google Scholar]
- Zakrys-Waliwander P.I., O'Sullivan M.G., O'Neill E.E., Kerry J.P. The effects of high oxygen modified atmosphere packaging on protein oxidation of bovine M. longissimus dorsi muscle during chilled storage. Food Chemistry. 2012;131:527–532. doi: 10.1016/j.foodchem.2011.09.017. [DOI] [Google Scholar]
- Zayas J.F. 1st ed. Springer; 1997. Functionality of proteins in food. (Chapter 4)) [Google Scholar]
- Zhang M., Chekan J.R., Dodd D., Hong P.Y., Radlinski L., Revindran V., et al. Xylan utilization in human gut commensal bacteria is orchestrated by unique modular organization of polysaccharide-degrading enzymes. Proceedings of the National Academy of Sciences. 2014;111:E3708–E3717. doi: 10.1073/pnas.1406156111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Li Y. Physicochemical and functional properties of coconut (Cocos nucifera L) cake dietary fibres: Effects of cellulase hydrolysis, acid treatment and particle size distribution. Food Chemistry. 2018;257:135–142. doi: 10.1016/j.foodchem.2018.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.