ABSTRACT
Aims
Assessment for cardiovascular autonomic neuropathy (CAN) in patients with type 1 diabetes mellitus remains time‐consuming in the clinical setting. We aimed to examine the diagnostic performance of a portable point‐of‐care diagnostic tool (POCD) for assessing sural nerve conduction during the screening of CAN.
Methods
Nerve amplitude (AMPPOCD) and conduction velocity (CVPOCD) were measured in a cross‐sectional study including 198 asymptomatic patients with type 1 diabetes. CAN was diagnosed by the Ewing score and power spectral heart rate [low‐frequency (LF) and high‐frequency (HF) activity]. Diagnostic accuracy was determined by ROC curves.
Results
CVPOCD and AMPPOCD showed positive correlations with LF and HF, and a negative correlation with age. Overall, AMPPOCD had an 81.7% accuracy in identifying CAN [AUC = 0.817 (95% CI 0.692–0.942)] with an AMPPOCD ≤6 μV showing 90% sensitivity and 73% specificity. In a stepwise binary logistic regression analysis, the model (R 2: 0.297; P < 0.001) retained the duration of type 1 diabetes [β: 1.131 (95% CI: 1.051–1.216); P = 0.001) and A1c [β: 2.131 (95% CI: 1.060–4.283); P = 0.034) as significant predictors of CAN. The combination of AMPPOCD ≤6 μV + a type 1 diabetes duration of ≥8 years maximized the sensitivity, showing a diagnostic performance of 87% [AUC = 0.867 (95% CI 0.769–0.965)] with 90%, 76%, and 99%, sensitivity, specificity, and NPV, respectively. Adding A1c ≥ 7% to this model maintained accuracy [AUC = 0.867 (95% CI: 0.788–0.963) and NPV (99%), while increasing specificity to 84%.
Conclusions
The combination of AMPPOCD with A1c and the duration of type 1 diabetes mellitus showed a good performance for the detection of asymptomatic CAN, making POCD an easy and rapid test for its routine screening in the clinical setting.
Keywords: Cardiovascular autonomic neuropathy, Diabetic neuropathy, Type 1 diabetes
Given the unfeasibility of a widespread use of CART tests and Fourier‐based method for the spectral analysis of HR variability in the routine clinical screening for cardioautonomic neuropathy (CAN), we addressed the ability of a point‐of‐care nerve conduction device (POCD) to detect asymptomatic CAN in patients with T1D, with the aim of minimizing the proportion of underdiagnosed patients. Thus, we conducted a well‐powered cross‐sectional study in 198 patients with T1D. We found that a novel POCD can be used as a rapid approximation of CAN screening. We have been able to accurately determine specific POCD threshold values that serve to identify abnormal cardioautonomic activity according to the reference standard definition. In conclusion, POCD may provide an easy‐to‐use noninvasive tool to identify a subgroup of patients at risk of CAN.

INTRODUCTION
Cardiovascular autonomic neuropathy (CAN) is a serious complication of type 1 diabetes (T1D) 1 , 2 , 3 that is strongly associated with an increased risk of cardiovascular mortality 4 , 5 . A previous report from our group suggested that asymptomatic CAN was highly prevalent among young adults with T1D even during the early stages of the disease 6 .
Assessment for CAN is hardly affordable in everyday clinical practice. Cardiovascular Autonomic Reflex function Tests (CARTs), as proposed by Ewing et al. 7 in 1970, are considered the gold standard for the diagnosis of CAN. However, CARTs remain a time‐demanding approach, and its accuracy largely depends on the patient’s individual collaboration. Heart rate (HR) variability in short‐ and long‐term electrocardiogram (ECG) recordings analyzed by dedicated software in the frequency domain is also considered a gold‐standard for the diagnosis of CAN. This methodology allows the outpatient diagnosis of CAN, monitoring the progress, and evaluation of patient prognosis. Nonetheless, the use of these tests is frequently restricted to research projects, since the equipment required for their use is only available in highly specialized centers.
In view of these limitations, prior studies have aimed to simplify the diagnosis of CAN 8 , 10 . However, they did not reduce the need for special hardware and qualified staff 4 , 5 . Thus, a simple, noninvasive, and easily available screening test for CAN is still required 4 , 5 , 9 .
In contrast, a novel point‐of‐care nerve conduction device (POCD) has the potential to provide rapid quantification of sensory nerve fiber function, and may serve as a proxy for standard nerve conduction studies 11 . POCD showed a strong diagnostic accuracy for the identification of diabetic polyneuropathy (DPN) in patients with type 1 diabetes mellitus 12 .
Our hypothesis is that the use of a POCD could identify patients with T1D at increased risk of CAN and, by selecting this subgroup of candidates for confirmatory testing with gold standard approaches, could save substantial human and time resources. Hence, the aim of our study was: (i) to examine the diagnostic accuracy of the POCD sural nerve conduction for the detection of subclinical CAN in patients with type 1 diabetes as determined with reference standard tests; and (ii) to assess its performance in combination with clinical variables related to type 1 diabetes.
METHODS
Study design
We conducted a cross‐sectional study including 199 consecutive patients with type 1 diabetes mellitus from an Academic Hospital from Madrid, Spain. This cohort is being recruited for an ample study assessing the presence of sexual dimorphism in the CAN of patients with type 1 diabetes mellitus (clinicaltrials.gov NCT04950634).
Study population
The diagnosis of T1D required a previous episode of ketoacidosis and/or diabetic autoimmunity, and the mandatory use of insulin for survival, following the ADA criteria 13 . Exclusion criteria were: (i) age ≥ 85 years; (ii) inability to understand CAN assessment; (iii) neuropathies different to DPN; (iv) clinical manifestations of CAN; (v) diabetic foot; (vi) end‐stage renal disease; (vii) ongoing pregnancy. Age ≥ 85 years was chosen among exclusion criteria because of age related values of the expiration to inspiration (E/I) ratio assessed during HR variation with deep breathing do not apply for individuals aged ≥85 years 14 , 15 .
Among eligible participants enrolled in the study, one participant was excluded due to device errors when using the POCD (index test), leaving 198 participants for analysis (Figure 1). CARTs (reference standard) were not performed in five patients due to procedural problems, so the Ewing score could not be calculated (the Ewing score was available for 97.5% of patients) (Figure 1). In four patients, the frequency‐domain of HR variability (reference standard) could not be determined due to technical problems (the frequency‐domain was available for 98% of patients) (Figure 1).
Figure 1.
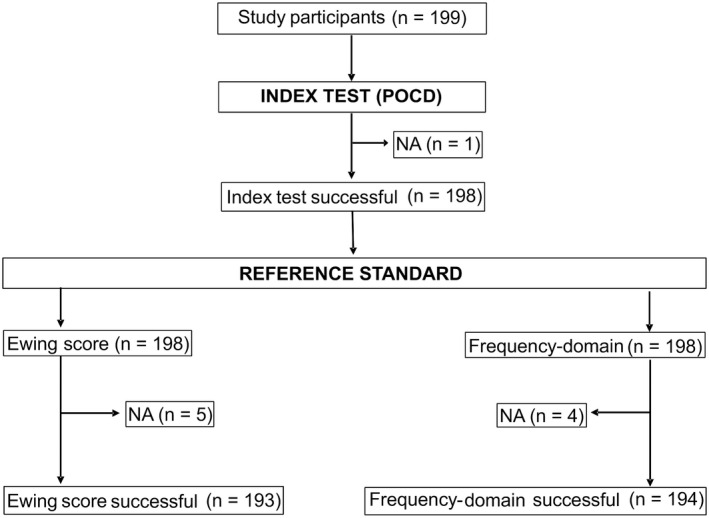
Flow‐chart of study participants. Among 199 study participants included in the study, one study participant was excluded due to an error using the point‐of‐care nerve conduction device (index test), resulting in 198 participants for analysis. Cardioautonomic reflex tests (reference standard) were not performed in five patients due to technical problems, so the Ewing score could not be calculated (number of patients defined by Ewing score: 193). In four patients the frequency‐domain of HR variability (reference standard) could not be determined due to technical problems (total number of patients defined by frequency‐domain: 194).
Clinical, anthropometric, and biochemical variables
We reviewed the medical records of the subjects recording clinical parameters related to type 1 diabetes, medications, smoking status, cardiovascular risk factors, and microvascular complications. Patients underwent a complete physical examination including measurements of waist circumference, height, and weight.
Diagnosis of diabetic nephropathy required an increased urinary albumin‐to‐creatinine ratio (UACR) as measured in a random spot urine collection. All patients were assessed for DPN 16 by means of the Neuropathy Symptoms Score Questionnaire (NSS) and clinical tests for protective sensation, a 128 Hz tuning fork for vibration perception, ankle reflexes, and a 10 g monofilament test 17 . We excluded neuropathies other than DPN by thorough medical records and a review of concomitant medication. In all patients, we analyzed the blood count, serum folic acid, serum B12 vitamin, and thyroid hormones in order to rule out analytical alterations that could indicate symptoms of neuropathy of an etiology other than diabetes.
Assessment of cardiovascular autonomic function: Ewing score and power spectral HRdata (reference standards)
Cardiovascular autonomic neuropathy was diagnosed using the two currently available gold standard methods 4 , 5 , 18 : (i) power spectral HR variability by analyzing beat‐to‐beat intervals from short‐duration ECG recordings; and (ii) the standardized CARTs described by Ewing et al. 7 . We used a modification of the Ewing score 6 to rate CAN, which scored HR variability to deep breathing, Valsalva's maneuver, and orthostatism, as well as the response of blood pressure (BP) to active standing. These responses were categorized as normal (0 points), borderline (0.5 point), or abnormal (1 point). A composite score ≥1 was considered diagnostic of CAN 6 , 7 . We classified CAN as early or mild when the Ewing score was between 1 and 2, or as definite when the score was ≥2 6 .
After resting in the supine position, we assessed HR variability using a VitalScan Medeia®System device (United States, CA). The participants were instructed to avoid particular pharmacological agents (β‐blockers, antidepressants, neuroleptics, nicotine, and caffeine) for the 12 h preceding the examination. Before obtaining cardioautonomic function studies, we assayed serum glucose in all participants to rule out hypoglycemia. No patient had a serum glucose <70 mg/dL, which is the glycemic threshold for epinephrine release 19 .
The HR response to deep breathing was estimated by calculating the ratio of the maximum/minimum HRs during six cycles of paced deep breathing E/I ratio. The HR response to Valsalva's maneuver (VAL ratio) was assessed by calculating the ratio of the longest R‐R interval after the maneuver to the shortest interval during the maneuver. The HR response to orthostatism was calculated as the ratio of the longest R‐R interval (found at about beat 30) to the shortest interval (found at about beat 15) after standing up (30:15 ratio) 6 .
Adrenergic innervation was assessed by the changes in BP and HR 5 min after active standing. Orthostatic hypotension was defined by a fall in response to standing >20 mmHg for systolic BP or > 10 mmHg for diastolic BP 18 . Resting tachycardia was defined by a HR > 100 beats per minute 18 .
We obtained power spectral HR data by analyzing the time series of beat‐to‐beat intervals from ECG recordings (10 min) using specialized frequency‐domain software VitalScan Medeia® (United States, CA) 4 , 14 , 15 . This method uses the Fourier method, which transforms R‐R intervals into wavelets with two basic components: low frequency (LF) and high frequency (HF) bands. Analysis of HR variability in the frequency domain is a widely used tool in the investigation of autonomic cardiovascular function. The oscillatory components are usually differentiated in the spectral profile: (i) the high frequency (HF) band (0.15 to 0.40 Hz), which reflects the effects of respiration on HR, also referred to as respiratory sinus arrhythmia; (ii) the low frequency (LF) band (0.04–0.15 Hz), which represents oscillations related to regulation of BP and vasomotor tone including the so‐called 0.1 Hz fluctuation 20 . Low frequency activity represents the combined effects of sympathetic and parasympathetic influence, whereas HF represents parasympathetic activity 4 , 14 , 15 . The normalization of power components and autonomic balance calculations as the LF/HF ratio are based on the physiological assumption of autonomic reciprocity, which is not supported by the current state of research. Moreover, these mathematical transformations may lead to distortion of data, making questionable any index derived from them. Following these recommendations, in our work we used LF/HF power absolute values20.
We defined our population according to the Ewing score as having CAN (Ewing score ≥1) or not having CAN. Second, we defined the 5th percentile of LF and HF in our participants with T1D who did not have CAN (1.048 and 0.830, respectively). With this approach, we sought to select our highest‐risk population. Thus, individuals were classified according to their normal (≥5th percentile) or abnormal (<5th percentile) LF and HF values. Lastly, those patients who showed both LF and HF values below the 5th percentile of our healthy population were identified with CAN according to power spectral HR.
Point‐of‐care nerve conduction device (index test)
Participants were examined unilaterally using a portable POCD (DPN‐Check™, Neurometrix Inc., Waltham, MA, USA) 21 , 22 . DPN‐Check™ has been developed to evaluate the sensory nerve conduction velocity (CVPOCD) and amplitude of sensory nerve action potential (AMPPOCD) of the sural nerve 12 , 21 , 22 . The DPN‐Check™ device consisted of a single handheld unit that allowed for placement of a disposable biosensor at a distance of 92.2 mm from the stimulation probes located at the opposite end of the device.
The stimulating probes were coated in a gel to promote the conduction of the impulses generated by the probes. The largest probe was placed on the lateral side of the ankle over the anatomical position of the sural nerve. Once the device was in place, the test was initiated with the start button. If a device error was observed on the display screen, the testing protocol was repeated. The procedure took approximately 2 min per participant.
For the analysis of the baseline characteristics of the patients, we used the cut‐off values obtained in an earlier study 12 : AMPPOCD ≤ 6 μV (cut‐off value that showed 80% sensitivity and 80% specificity for identifying abnormal age‐adjusted NCS values in patients with type 1 diabetes); and/or CVPOCD ≤ 48 m/s (threshold that showed 90% sensitivity and 66% specificity for identifying abnormal age‐adjusted NCS values in patients with type 1 diabetes). For the diagnostic accuracy of POCD in CAN, optimal diagnostic thresholds were calculated in our cohort as described in the following section.
Statistical analysis
Data are shown as the mean, SD, (95% CI), or counts (%) as appropriate. For continuous variables, we checked normality using the Kolmogorov–Smirnov test, ensuring normality by applying logarithmic transformation when necessary. We applied nonparametric tests to variables that did not follow the normal distribution even after transformation. Basal and biochemical characteristics were compared using Student’s t and Mann–Whitney U tests for unpaired comparisons, as appropriate. Comparisons of discrete variables among study subgroups used χ2 or Fisher's exact tests. Associations between AMPPOCD or CVPOCD from DPN‐Check™, and CAN indexes were analyzed using Spearman's correlation.
The overall diagnostic performance of POCD (AMPPOCD, CVPOCD, and AMPPOCD + CVPOCD) for the detection of CAN as defined by frequency‐domain (reference standard definition), was analyzed by receiver operating characteristic (ROC) curves that also provided optimal thresholds for these variables.
We also analyzed if the addition of clinical and biochemical variables to POCD results could improve this accuracy. We used stepwise binary logistic regression analyses introducing the presence of CAN as dependent variable, and age, duration of type 1 diabetes, BMI, A1c, microvascular complications (coded as: absent = 0, present = 1), and glomerular filtration rate as independent variables.
Optimal thresholds were determined by finding the point of the ROC curve closest to the point of the best discrimination using the formula. The second approach's protocol was developed according to the following algorithm: two threshold values were sought for each of significant determinant variables in the regression models, one to maximize sensitivity and the other to maximize specificity, such that the negative likelihood ratio would approach 0.1, while the positive likelihood ratio would approach 10. This model was used by others 12 to test the performance of the POCD in a clinical screening setting.
Our sample size of 198 patients with type 1 diabetes had >99% power to discriminate a conservatively modeled AUC of 0.75 from the null hypothesis in which the diagnostic accuracy is no different than chance alone (AUC = 0.50) 23 . All statistical analyses used IBM SPSS statistical software version 20 (IBM España S. A., Madrid, Spain). A P value <0.05 was considered statistically significant.
RESULTS
Study population characteristics
The mean age of our study population was 40 ± 13 years, and the mean duration of T1D was 18 ± 12 years. Metabolic control, as measured by A1c was good, showing an overall A1c 7.2 ± 0.9%, with 46% of patients meeting target objectives (A1c ≤ 7%). Other demographic and clinical characteristics of the participants are detailed in the Table 1.
Table 1.
Baseline characteristics of all patients as a whole and as a function of cardioautonomic neuropathy (CAN)
| All patients (n = 198) | Cardiovascular autonomic status | ||||||
|---|---|---|---|---|---|---|---|
| Ewing score | P | Frequency‐domain | P | ||||
| CAN (n = 52) | Normal (n = 141) | CAN (n = 10) | Normal (n = 184) | ||||
| Clinical characteristics | |||||||
| Female sex, n (%) | 90 (46) | 24 (46) | 62 (44) | 0.787 | 6 (60) | 81 (44) | 0.348 |
| Age, years | 40 ± 13 | 45 ± 14 | 38 ± 13 | 0.003 | 54 ± 11 | 39 ± 13 | <0.001 |
| Duration of diabetes, years | 18 ± 12 | 22 ± 12 | 17 ± 12 | 0.015 | 33 ± 11 | 17 ± 12 | 0.001 |
| Microangiopathy, n (%) | 49 (25) | 23 (44) | 25 (18) | <0.001 | 7 (70) | 41 (22) | 0.003 |
| Retinopathy, n (%) | 22 (11) | 11 (21) | 11 (8) | 0.010 | 4 (40) | 18 (10) | 0.017 |
| Non‐proliferative | 14 (7) | 6 (12) | 8 (6) | 0.209 | 2 (20) | 12 (7) | 0.156 |
| Proliferative | 8 (4) | 5 (10) | 3 (2) | 0.034 | 2 (20) | 6 (3) | 0.057 |
| Nephropathy, n (%) | 16 (8) | 9 (17) | 7 (5) | 0.015 | 1 (10) | 15 (8) | 0.586 |
| Macroangiopathy, n (%) | 7 (4) | 5 (10) | 2 (1) | 0.016 | 2 (20) | 5 (3) | 0.044 |
| Smoking habit, n (%) | 73 (37) | 22 (42) | 49 (35) | 0.334 | 3 (30) | 68 (37) | 0.749 |
| Antiaggregant therapy, n (%) | 22 (11) | 13 (26) | 9 (6) | <0.001 | 5 (50) | 17 (9) | 0.002 |
| Statin therapy, n (%) | 67 (34) | 23 (45) | 41 (29) | 0.038 | 7 (78) | 58 (32) | 0.007 |
| Antihypertensive therapy, n (%) | 27 (14) | 12 (23) | 15 (11) | 0.027 | 4 (40) | 23 (13) | 0.035 |
| Total insulin dose, units/day | 42 ± 19 | 44 ± 18 | 42 ± 20 | 0.569 | 40 ± 16 | 43 ± 20 | 0.644 |
| Daily insulin dose, units/kg/day | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.3 | 0.838 | 0.5 ± 0.2 | 0.6 ± 0.3 | 0.415 |
| Body mass index, kg/m2 | 25 ± 4 | 25 ± 4 | 25 ± 4 | 0.335 | 28 ± 5 | 25 ± 4 | 0.026 |
| Obesity, n (%) | 17 (9) | 8 (15) | 9 (6) | 0.081 | 4 (40) | 13 (7) | 0.006 |
| Waist circumference, cm | 84 ± 12 | 87 ± 14 | 84 ± 11 | 0.159 | 92 ± 17 | 84 ± 12 | 0.053 |
| Fat mass, % | 24 ± 10 | 25 ± 9 | 23 ± 10 | 0.241 | 29 ± 12 | 23 ± 10 | 0.179 |
| Biochemical characteristics | |||||||
| eGFR, mL/min/1.73 m2 | 90 ± 15 | 86 ± 17 | 92 ± 14 | 0.015 | 83 ± 8 | 90 ± 15 | 0.022 |
| UACR, mg/g | 13 ± 32 | 23 ± 58 | 9 ± 10 | 0.006 | 12 ± 17 | 12 ± 32 | 0.963 |
| UACR stages, n (%) | 0.008 | 0.555 | |||||
| Normoalbuminuria, n (%) | 181 (93) | 43 (84) | 134 (96) | 8 (89) | 169 (93) | ||
| Microalbuminuria, n (%) | 13 (7) | 7 (14) | 6 (4) | 1 (11) | 12 (6) | ||
| Macroalbuminuria, n (%) | 1 (1) | 1 (2) | 0 (0) | 0 (0) | 1 (1) | ||
| Hemoglobin A1c, mmol/mol | 55 ± 10 | 57 ± 10 | 54 ± 9 | 0.014 | 62 ± 12 | 54 ± 10 | 0.024 |
| Hemoglobin A1c, % | 7.2 ± 0.9 | 7.4 ± 0.9 | 7.1 ± 1.0 | 0.014 | 7.8 ± 1.1 | 7.1 ± 0.9 | 0.024 |
| Total cholesterol, mg/dL | 174 ± 37 | 176 ± 38 | 174 ± 38 | 0.676 | 170 ± 55 | 175 ± 37 | 0.768 |
| HDL‐cholesterol, mg/dL | 60 ± 16 | 58 ± 18 | 60 ± 15 | 0.409 | 56 ± 16 | 60 ± 16 | 0.554 |
| LDL‐cholesterol, mg/dL | 100 ± 25 | 102 ± 29 | 99 ± 23 | 0.397 | 98 ± 42 | 100 ± 24 | 0.798 |
| Triglycerides, mg/dL | 68 ± 50 | 79 ± 55 | 64 ± 48 | 0.008 | 83 ± 69 | 67 ± 49 | 0.497 |
| B12 vitamin | 524 ± 248 | 517 ± 221 | 518 ± 250 | 0.986 | 570 ± 278 | 517 ± 240 | 0.639 |
| Folic acid | 7.7 ± 3.1 | 7.8 ± 2.9 | 7.7 ± 3.1 | 0.792 | 8.37 ± 3.9 | 7.7 ± 3.1 | 0.670 |
| Cardiovascular autonomic outcomes | |||||||
| Resting SBP, mmHg | 120 ± 13 | 124 ± 15 | 119 ± 12 | 0.009 | 130 ± 16 | 120 ± 13 | 0.027 |
| Resting DBP, mmHg | 77 ± 9 | 79 ± 10 | 76 ± 9 | 0.135 | 81 ± 10 | 76 ± 09 | 0.195 |
| Resting HR, bpm | 71 ± 10 | 72 ± 11 | 71 ± 9 | 0.341 | 75 ± 13 | 71 ± 10 | 0.493 |
| SBP response to orthostatism, mmHg | 1 ± 11 | −3 ± 13 | 3 ± 9 | 0.003 | −7 ± 18 | 2 ± 10 | 0.009 |
| DBP response to orthostatism, mmHg | 5 ± 8 | 3 ± 9 | 6 ± 7 | 0.007 | −4 ± 11 | 6 ± 7 | 0.028 |
| HR response to orthostatism, bpm | 12 ± 7 | 11 ± 8 | 13 ± 6 | 0.113 | 8 ± 4 | 13 ± 7 | 0.011 |
| Orthostatic hypotension, n (%) | 9 (5) | 7 (14) | 2 (1) | 0.002 | 3 (30) | 6 (4) | 0.007 |
| E/I index | 1.4 ± 0.3 | 1.3 ± 0.3 | 1.5 ± 0.3 | <0.001 | 1.2 ± 0.2 | 1.5 ± 0.3 | 0.001 |
| VAL index | 1.4 ± 0.2 | 1.3 ± 0.2 | 1.4 ± 0.2 | <0.001 | 1.2 ± 0.1 | 1.4 ± 0.2 | <0.001 |
| 30:15 index | 1.4 ± 0.3 | 1.3 ± 0.4 | 1.5 ± 0.3 | <0.001 | 1.4 ± 0.4 | 1.1 ± 0.3 | 0.579 |
| Low‐frequency (LF) | 2.4 ± 1.2 | 1.8 ± 1.0 | 2.6 ± 1.3 | <0.001 | 0.7 ± 0.2 | 2.4 ± 1.2 | <0.001 |
| High‐frequency (HF) | 2.5 ± 1.4 | 1.9 ± 1.3 | 2.7 ± 1.5 | <0.001 | 0.6 ± 0.1 | 2.6 ± 1.4 | <0.001 |
Data are mean ± SD, median [IQR], or n (%). CAN was determined by the Ewing score (composite score ≥1). CAN was also defined by power spectral HR data analyzing the frequency domain from short‐duration electrocardiogram recordings. CAN was defined as those patients with low frequency (LF) and high‐frequency (HF) values <5th percentile of patients without CAN according to the Ewing score. CAN, cardiovascular autonomic neuropathy; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; E/I index, expiration/inspiration index; HR, heart rate; SBP, systolic blood pressure; UACR, urinary albumin to creatinine ratio; VAL index, valsalva index. Significant P values are highlighted in bold and italics.
Cardiovascular autonomic function defined by Ewing score and power spectral HRdata (reference standards)
We found a prevalence of CAN, as defined by the Ewing score, of 27% (95% CI: 21–34). CAN was categorized as early/mild in 46 subjects (88%) and definite in 6 (12%) by the Ewing score. As expected, the prevalence was lower, 5% (95% CI: 3–9), according to the dominant spectrum of HF variability, when selecting those patients with LF and HF involvement.
Those patients with CAN according both definitions were older, had a longer duration of the disease, higher A1c and systolic BP, than patients not showing CAN (Table 1). They also presented higher rates of micro and macrovascular complications, and were more likely to use statins, antihypertensive, and antiplatelet medications (Table 1). The stepwise regression model (R 2: 0.130; P < 0.001) retained a previous microvascular complication [β: 3.648 (95% CI: 1.777–7.488); P < 0.0001] and A1c [β: 1.483 (95% CI: 1.051–2.094); P = 0.025] as statistically significant predictors of CAN defined by the Ewing score.
Finally, we found asymptomatic orthostatic hypotension, as defined, in nine individuals (4.5%) and resting tachycardia in only two patients (1%).
Neuropathy outcome and the diagnostic value of POCDfor cardiovascular autonomic dysfunction
Patients with CAN showed higher abnormal NSS scores compared with patients without CAN (Table 2), resulting in a prevalence of symptomatic DPN of 27% (95% CI: 17–40) among those defined by the Ewing score, and a prevalence of 50% (95% CI: 24–76) among those defined by frequency‐domain activity. Compared with patients without CAN, those with CAN had lower AMPPOCD and CVPOCD. The quantitative measures of AMPPOCD and CVPOCD are summarized in Table 2.
Table 2.
Neuropathy and POCD outcomes
| All patients (n = 198) | Cardiovascular autonomic status | ||||||
|---|---|---|---|---|---|---|---|
| Ewing score | P | Frequency‐domain | P | ||||
| CAN (n = 52) | Normal (n = 141) | CAN (n = 10) | Normal (n = 184) | ||||
| Neuropathy outcomes | |||||||
| Abnormal NSS, n (%) | 25 (13) | 14 (27) | 10 (7) | <0.001 | 5 (50) | 19 (10) | 0.003 |
| AMPPOCD, μV | 11 ± 7 | 9 ± 6 | 11 ± 7 | 0.006 | 5 ± 4 | 11 ± 7 | <0.001 |
| Abnormal AMPPOCD, n (%) | 60 (30) | 24 (46) | 35 (25) | 0.004 | 9 (90) | 1 (1) | <0.001 |
| CVPOCD, m/s | 51 ± 11 | 49 ± 12 | 52 ± 10 | 0.111 | 42 ± 17 | 52 ± 10 | 0.036 |
| Abnormal CVPOCD, n (%) | 56 (28) | 21 (40) | 33 (23) | 0.020 | 6 (60) | 49 (27) | 0.032 |
Data are mean ± SD, median [IQR], or n (%). Cardiovascular autonomic status was determined by the Ewing score and power spectral HR data analyzing the frequency domain from electrocardiogram recordings. Combined parasympathetic/sympathetic dysfunction was defined as low frequency (LF) and high frequency (HF) values <5th percentile of healthy patients according to the Ewing score. AMPPOCD, sural nerve amplitude potential; CAN, cardiovascular autonomic neuropathy; CVPOCD, sural nerve conduction velocity; NSS, neuropathy symptoms score; POCD, point‐of‐care nerve conduction device. Significant P values are highlighted in bold and italics.
Considering all patients as a whole, CVPOCD correlated with AMPPOCD (ρ = 0.353, P < 0.001), and with parameters of cardiovascular autonomic dysfunction such as LF, HF (Figure 2, upper panel), and E/I index (ρ = 0.246, P = 0.001). Similarly, the AMPPOCD correlated with LF, HF (Figure 2, lower panel), LF/HF (ρ = −0.145, P = 0.047), and E/I index (ρ = 0.236, P = 0.001).
Figure 2.
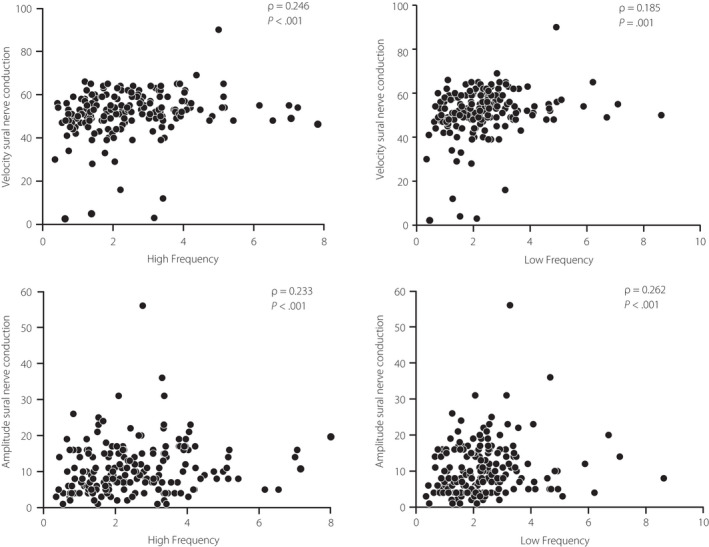
Spearman correlation between velocity and amplitude nerve conduction and high and low frequency values.
The ROC curves served as measures of the diagnostic performance of POCD results for the prediction of CAN. Firstly, we calculated the ROC curve AUC separately for the two measures obtained by the POCD: CVPOCD and AMPPOCD. The AMPPOCD AUC was 0.815 (95% CI: 0.693–0.937); and the CVPOCD AUC was 0.697 (95% CI: 0.524–0.870). Subsequently, we calculated the AUC with both these measurements combined (CVPOCD + AMPPOCD): AUC = 0.817 (95% CI: 0.692–0.942). Since CVPOCD contributed a low diagnostic yield to the model, we excluded this variable in the following models, using AMPPOCD alone in combination with clinical variables (Figure 3).
Figure 3.
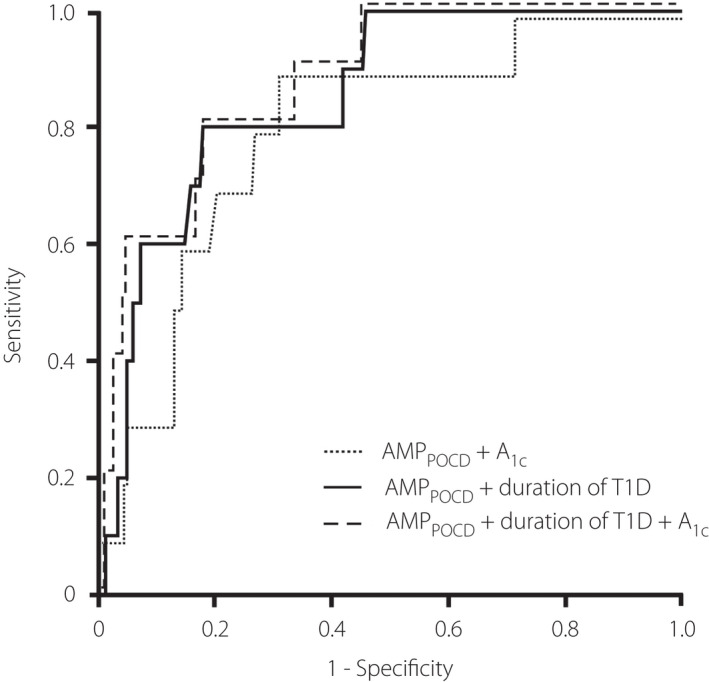
ROC curve displaying the diagnostic validity of the POCD for identification of parasympathetic and sympathetic dysfunction as defined by standard diagnostic test.
We generated predictive models combining AMPPOCD with the main significant predictors of CAN as detailed previously. Firstly, the clinical stepwise regression model (R 2: 0.297; P < 0.001) retained duration of T1D [β: 1.131 (95% CI: 1.051–1.216); P = 0.0001] and A1c [β: 2.131 (95% CI: 1.060–4.283); P = 0.034] as statistically significant predictors of CAN. Then, we added AMPPOCD to duration of T1D and/or A1c levels, because CVPOCD was less accurate than AMPPOCD for the diagnosis of CAN, and its addition resulted in a very small increase in diagnostic performance compared with the use of AMPPOCD alone. Hence, we generated the following models:
-
•
Model 1: AMPPOCD + A1c [AUC = 0.812 (95% CI: 0.668–0.957)].
-
•
Model 2: AMPPOCD + duration of T1D [AUC = 0.867 (95% CI: 0.769–0.965)].
-
•
Model 3: AMPPOCD + duration of T1D + A1c [AUC = 0.875 (95% CI: 0.788–0.963)].
Optimal thresholds were determined by finding the point of the ROC curve closest to the point of the best discrimination as described above: ≤6 μV for AMPPOCD, ≥8.5% for A1c, and ≥8 years for T1D duration. Abnormal values for both AMPPOCD and A1c had a sensitivity of 22% and specificity of 96%, with a negative predictive value (NPV) of 96% for diagnosing CAN, while abnormal values in AMPPOCD and duration of T1D had a sensitivity of 90% and specificity of 76%, with a NPV of 99%. Abnormal values in AMPPOCD, A1c, and duration of T1D improved marginally the diagnostic performance of CAN, with a sensitivity of 22%, specificity of 98%, and NPV of 96% (Table 3).
Table 3.
Diagnostic performance of POCD tools and clinical variables for the diagnosis of CAN in patients with type 1 diabetes mellitus
| Model | Threshold | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Best discrimination |
A1c ≥ 8.5% + AMPPOCD ≤6 μV |
22 | 96 | 33 | 96 |
|
Duration of T1D ≥ 8 years + AMPPOCD ≤6 μV |
90 | 76 | 17 | 99 | |
|
A1c ≥ 8.5% + Duration of T1D ≥ 8 years + AMPPOCD ≤6 μV |
22 | 98 | 40 | 96 | |
| Favors sensitivity |
Duration of T1D ≥ 5 years + AMPPOCD ≤6 μV |
90 | 75 | 9 | 99 |
|
A1c ≥ 7% + AMPPOCD ≤6 μV |
78 | 83 | 18 | 99 | |
|
A1c ≥ 7% + Duration of T1D ≥ 5 years + AMPPOCD ≤6 μV |
78 | 84 | 20 | 99 | |
| Favors specificity |
A1c ≥ 8% + Duration of T1D ≥ 35 years + AMPPOCD ≤3 μV |
22 | 100 | 100 | 96 |
|
A1c ≥ 8% + AMP≤3 μV |
33 | 99 | 40 | 96 | |
|
Duration of T1D ≥ 35 years + AMPPOCD ≤3 μV |
20 | 99 | 50 | 99 |
AMPPOCD, sural nerve amplitude potential; CAN, cardiovascular autonomic neuropathy; NPV, negative predictive value; POCD, point‐of‐care nerve conduction device; PPV, positive predictive value; T1D, type 1 diabetes.
To evaluate the performance of the device in a clinical model, we sought two additional thresholds – one that maximized sensitivity and other that maximized specificity – for each of AMPPOCD, A1c, and duration of T1D (Table 3).
Most favorable thresholds were determined by finding the point of the ROC curve closest to the point that maximized specificity (≤3 μV for AMPPOCD, ≥8% for A1c, and ≥ 35 years for T1D duration) or sensitivity (≤6 μV for AMPPOCD, ≥7% for A1c, and ≥5 years for T1D duration). AMPPOCD ≤3 μV, A1c ≥ 8%, and T1D duration ≥35 years showed a sensitivity of 22%, specificity of 100%, PPV of 100%, and NPV of 99%, whereas AMPPOCD ≤6 μV and T1D duration ≥5 years had a sensitivity of 90%, specificity of 75%, and NPV of 99% (Table 3).
DISCUSSION
In this cross‐sectional analysis of 198 adults with T1D, we found that POCD can be used as a rapid approximation for CAN screening. We have been able to accurately determine specific POCD threshold values that serve to identify CAN. In addition, we confirmed that the device was able to accurately identify patients at risk of CAN using a combination of these specific thresholds of AMPPOCD, and clinical parameters such as metabolic control or duration of T1D. In fact, an AMPPOCD ≤ 6 μV in subjects with a T1D duration ≥8 years had a sensitivity of 90%, with a specificity of 76%, and NPV of 99%, making this POCD reliable for the screening of asymptomatic CAN in subjects with T1D.
Earlier research has also attempted to simplify the diagnosis of CAN by using DPN diagnostic tools, even though these studies primarily focused on the evaluation of diagnostic performance of sudomotor function 24 , 25 , 26 . In a population including 45 individuals with T1D and 25 healthy volunteers, the sensitivity and specificity of another non‐invasive medical device (Sudoscan®, Impeto Medical, Paris, France) for CAN (defined as ≥1 abnormal out of the five CARTs) was 65% and 85%, respectively 27 . Recently, Sudoscan® showed a sensitivity and specificity of 83% and 67%, respectively, in detecting the diagnosis of CAN among a population of 102 individuals with diabetes 24 . Our findings show that the POCD assessed, by using the combination of AMPPOCD and duration of T1D for diagnosis of CAN, has a higher sensitivity and specificity than Sudoscan® methodology, with the advantage of being a much simpler, and cheaper technique than sudomotor function assessment.
In type 2 diabetes mellitus, Pafili et al. 8 evaluated a variety of simple available DPN tools to define their diagnostic performances for CAN. The assessment of small nerve fiber function (pinprick sensation and temperature perception) yielded a very high NPV (97%), with a sensitivity of 89% and moderate specificity (73%). However, these diagnostic methods are somehow subjective and require the full cooperation of patients. In the same study 8 , the authors also analyzed POCD performance in diagnosing CAN, showing a low sensitivity (50%), and moderate specificity (76%). Unlike us, they did not use absolute values of AMPPOCD and/or nerve conduction velocities 8 . The POCD examination was considered abnormal when AMPPOCD was <4 μV and/or when CVPOCD was <40 m/s in at least one of the two lower extremities. Such thresholds were predefined, and these authors did not consider the addition of clinical variables that could improve the diagnostic performance of POCD results.
Given the unfeasibility of a widespread use of CART tests and Fourier‐based method for the spectral analysis of HR variability in the routine clinical screening for CAN, we addressed the ability of POCD to detect CAN in patients with type 1 diabetes mellitus, with the aim of minimizing the proportion of undiagnosed patients. After selecting the best clinical and POCD‐specific threshold values for the identification of CAN, we defined models combining POCD results with those clinical variables, with the goal of improving sensitivity or specificity as desired. In those models including AMPPOCD, metabolic control, and duration of T1D, we used two different sets of diagnostic thresholds, one that maximizes sensitivity (and the negative likelihood ratio), and another one that maximizes specificity (and positive likelihood ratio). Furthermore, in order to simplify our diagnostic approach, we left out CVPOCD recordings that improved diagnostic performance only marginally. Our results strongly suggest that triage based on these models is effective for the screening of asymptomatic CAN in subjects with T1D. However, these models and diagnostic cutoffs will require standardization and validation in other populations and clinical settings.
The practical implications of these findings should be highlighted. A simple and extendable test such as the one proposed here would have the potential to fill a gap in clinical care. CAN have a long and latent subclinical phase, in which it is estimated that most of the cases are asymptomatic. However, these patients are associated with increased subclinical cardiovascular morbidity 5 , 15 . The best strategy for intervention would be to identify early those asymptomatic cases of CAN, in order to implement a successful disease‐modifying therapy for preventing the onset of cardiovascular manifestations.
Nevertheless, we are aware that our study has several limitations: (i) our cross‐sectional design precluded any conclusions about causality; (ii) We did not perform any specific CAN screening questionnaire for patients; (iii) most of our patients were young, a fact that may decrease prevalence figures of CAN in our population; (iv) in an academic setting such as ours, patients with T1D might be managed better than in a general medicine setting, where the prevalence of CAN might be higher; and (v) the pathogenesis of large fibers (larger myelinated Aβ fibers) and small fibers (C fibers) damage is different. Hence, the hypothesis that POCD testing can be used as a CAN screening should be interpreted with caution.
In summary, our findings indicate that a combination of PCOD recordings and a few clinical variables is accurate enough to effectively rule out asymptomatic CAN in patients with T1D in the clinical setting. Of paramount importance for clinical practice, such an approach would save time and resources by restricting the more demanding and expensive diagnostic tests to patients showing positive results in these screening tests.
FUNDING
This study was funded by grants from Fondo de Investigación Sanitaria (PIE1600050 & PI1801122) of Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation Economy and an unrestricted grant from Laboratorios Menarini SA. The funding organizations played no role in the study design, collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the report for publication.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The study protocol was approved by Ramón y Cajal ethics committee (Date: 25/09/2017; Protocol ID: 189–17). All procedures performed were in accordance with the 1964 Helsinki declaration and its later amendments.
Informed consent: Informed consent was obtained from all participants.
Approval date of registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
J Diabetes Investig. 2022; 13: 1347–1356
DATA AVAILABILITY STATEMENT
All data and materials as well as software application comply with field standards.
REFERENCES
- 1. Poulsen PL, Ebbehøj E, Hansen KW, et al. 24‐h Blood pressure and autonomic function is related to albumin excretion within the normoalbuminuric range in IDDM patients. Diabetologia 1997; 40: 718–725. [DOI] [PubMed] [Google Scholar]
- 2. Duvnjak L, Vucković S, Car N, et al. Relationship between autonomic function, 24‐h blood pressure, and albuminuria in normotensive, normoalbuminuric patients with type 1 diabetes. J Diabetes Complications 2001; 15: 314–319. [DOI] [PubMed] [Google Scholar]
- 3. Afsar B. Disruption of circadian blood pressure, heart rate and the impact on glycemic control in type 1 diabetes. Diabetes Metab Syndr 2015; 9: 359–363. [DOI] [PubMed] [Google Scholar]
- 4. Serhiyenko VA, Serhiyenko AA. Cardiac autonomic neuropathy: risk factors, diagnosis and treatment. World J Diabetes 2018; 9: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duque A, Mediano MFF, De Lorenzo A, et al. Cardiovascular autonomic neuropathy in diabetes: Pathophysiology, clinical assessment and implications. World J Diabetes 2021;12: 855–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nattero‐Chávez L, Redondo López S, Alonso Díaz S, et al. Association of cardiovascular autonomic dysfunction with peripheral arterial stiffness in patients with type 1 diabetes. J Clin Endocrinol Metab 2019; 104: 2675–2684. [DOI] [PubMed] [Google Scholar]
- 7. Ewing DJ, Martyn CN, Young RJ, et al. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985; 8: 491–498. [DOI] [PubMed] [Google Scholar]
- 8. Pafili K, Trypsianis G, Papazoglou D, et al. Clinical tools for peripheral neuropathy to exclude cardiovascular autonomic neuropathy in type 2 diabetes mellitus. Diabetes Ther 2020; 11: 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stranieri A, Abawajy J, Kelarev A, et al. An approach for Ewing test selection to support the clinical assessment of cardiac autonomic neuropathy. Artif Intell Med 2013; 58: 185–193. [DOI] [PubMed] [Google Scholar]
- 10. Bönhof GJ, Herder C, Strom A, et al. Emerging biomarkers, tools, and treatments for diabetic polyneuropathy. Endocr Rev 2019; 40: 153–192. [DOI] [PubMed] [Google Scholar]
- 11. Shibata Y, Himeno T, Kamiya T, et al. Validity and reliability of a point‐of‐care nerve conduction device in diabetes patients. J Diabetes Investig 2019; 10: 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scarr D, Lovblom LE, Cardinez N, et al. Validity of a point‐of‐care nerve conduction device for polyneuropathy identification in older adults with diabetes: Results from the Canadian study of longevity in type 1 diabetes. PLoS One 2018; 13: e0196647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes – 2021. Diabetes Care 2021; 44(): S15–S33. [DOI] [PubMed] [Google Scholar]
- 14. Spallone V, Bellavere F, Scionti L, et al. Recommendations for the use of cardiovascular tests in diagnosing diabetic autonomic neuropathy. Nutr Metab Cardiovasc Dis 2011; 21: 69–78. [DOI] [PubMed] [Google Scholar]
- 15. Spallone V, Ziegler D, Freeman R, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011; 27: 639–653. [DOI] [PubMed] [Google Scholar]
- 16. Nattero‐Chávez L, Redondo López S, Alonso Díaz S, et al. The peripheral atherosclerotic profile in patients with type 1 diabetes warrants a thorough vascular assessment of asymptomatic patients. Diabetes Metab Res Rev 2019; 35: e3088. [DOI] [PubMed] [Google Scholar]
- 17. 11. Microvascular complications and foot care: Standards of medical Care in Diabetes‐2021. Diabetes Care 2021; 44(): S151–S167. [DOI] [PubMed] [Google Scholar]
- 18. Pop‐Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017; 40: 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silva TP, Rolim LC, Sallum Filho C, et al. Association between severity of hypoglycemia and loss of heart rate variability in patients with type 1 diabetes mellitus. Diabetes Metab Res Rev 2017; 33: 1. [DOI] [PubMed] [Google Scholar]
- 20. Reyes del Paso GA, Langewitz W, Mulder LJ, et al. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology 2013; 50: 477–487. [DOI] [PubMed] [Google Scholar]
- 21. Pafili K, Maltezos E, Papanas N. NC‐stat for the diagnosis of diabetic polyneuropathy. Expert Rev Med Devices 2017; 14: 251–254. [DOI] [PubMed] [Google Scholar]
- 22. Chatzikosma G, Pafili K, Demetriou M, et al. Evaluation of sural nerve automated nerve conduction study in the diagnosis of peripheral neuropathy in patients with type 2 diabetes mellitus. Arch Med Sci 2016; 12: 390–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Obuchowski NA, Lieber ML, Wians FH Jr. ROC curves in clinical chemistry: uses, misuses, and possible solutions. Clin Chem 2004; 50: 1118–1125. [DOI] [PubMed] [Google Scholar]
- 24. D'Amato C, Greco C, Lombardo G, et al. The diagnostic usefulness of the combined COMPASS 31 questionnaire and electrochemical skin conductance for diabetic cardiovascular autonomic neuropathy and diabetic polyneuropathy. J Peripher Nerv Syst 2020; 25: 44–53. [DOI] [PubMed] [Google Scholar]
- 25. Casellini CM, Parson HK, Richardson MS, et al. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther 2013; 15: 948–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yajnik CS, Kantikar V, Pande A, et al. Screening of cardiovascular autonomic neuropathy in patients with diabetes using non‐invasive quick and simple assessment of sudomotor function. Diabetes Metab 2013; 39: 126–131. [DOI] [PubMed] [Google Scholar]
- 27. Selvarajah D, Cash T, Davies J, et al. SUDOSCAN: a simple, rapid, and objective method with potential for screening for diabetic peripheral neuropathy. PLoS One 2015; 10: e0138224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials as well as software application comply with field standards.


