Abstract
Aims/Introduction
Metals play an important role in diabetes mellitus. This cross‐sectional study aimed to evaluate the overall, individual and interactive effects of multi‐metal exposure on the prevalence of diabetes mellitus, impaired fasting glucose (IFG) rate and fasting blood glucose (FBG) levels.
Materials and Methods
The FBG levels of a study population from a cadmium (Cd)‐polluted area (n = 250) and an unpolluted area (n = 204), and the metal levels, including magnesium, calcium (Ca), iron (Fe), zinc (Zn), arsenic (As), Cd, copper and lead (Pb) in blood and urine were detected. The study population was divided into a normal fasting glucose group, an IFG group and a diabetes mellitus group on the basis of FBG levels.
Results
The IFG rate and diabetes mellitus prevalence were negatively associated with blood Cd and urine Zn levels (IFG rate: odds ratio [OR] 0.780, 95% confidence interval [CI] 0.655–0.928; OR 0.622, 95% CI 0.465–0.831. Diabetes mellitus prevalence: OR 0.506, 95% CI 0.288–0.888; OR 0.609, 95% CI 0.395–0.939), the IFG rate was positively associated with urine Fe levels (OR 1.876, 95% CI 1.290–2.778), and diabetes mellitus prevalence was positively associated with urine Pb and blood Fe levels (OR 1.185, 95% CI 1.022–1.376; OR 1.008, 95% CI 1.001–1.014). A linear negative correlation was observed between FBG levels and blood Cd, and non‐linear inverted U‐shaped associations were found between FBG levels and Zn, Pb and copper in urine.
Conclusions
This research suggests that multi‐metal exposure, especially Cd, Fe, Zn, copper and Pb, is linked to diabetes mellitus, and the interactive effects of multiple metals require further exploration.
Keywords: Diabetes mellitus, Impaired fasting glucose, Multi‐metal exposure
This cross‐sectional study aimed to evaluate the overall, individual and interactive effects of multi‐metal exposure on the prevalence of diabetes mellitus, impaired fasting glucose rate and fasting blood glucose levels.

INTRODUCTION
Diabetes mellitus is a group of metabolic diseases characterized by chronic hyperglycemia with disorders in insulin secretion and/or utilization caused by multiple etiologies. It is one of the major causes of renal failure, stroke, disability and other cardiovascular diseases 1 , and results in a considerable disease burden and economic loss. In the past 40 years, diabetes mellitus prevalence in China has increased by nearly 17‐fold from 0.67% in 1980 to 11.2% in 2020 2 . Diabetes mellitus has become a major public health problem in China. Fasting blood glucose (FBG) is not only a basic diagnostic index, but also a known independent factor that affects diabetes mellitus development 3 . Research has shown that individuals with impaired fasting glucose (IFG) have a 20–30% chance of developing diabetes mellitus over the next 5–10 years 4 . Compared with other tests (e.g., glycosylated hemoglobin), the FBG test is less expensive and easier to perform; these features are vital for the primary prevention of diabetes, especially in low‐ and middle‐income countries 5 . Therefore, exploring IFG‐ and diabetes mellitus‐related risk factors is important for establishing targeted prevention and treatment strategies.
Traditional risk factors, such as heredity and unhealthy diet patterns, cannot fully explain the etiology and high prevalence of diabetes mellitus. Thus, the interest in non‐traditional and novel risk factors, such as metal exposure, is increasing. Heavy metals can affect mitochondrial mechanisms and increase the production of free radicals, leading to oxidative stress and inflammation, both of which might play a role in metabolic disorders, such as diabetes mellitus or obesity 6 . However, the effects of metal exposure, especially mixed exposure to multiple metals, on diabetes mellitus development have not been fully studied 7 . Most previous studies on the association between metal exposure and elevated FBG or diabetes mellitus risk have focused on single‐metal exposure models 8 , which might ignore the problem of strong correlation or even collinearity between metals and the impact of metal interactions on health 7 . Although several recent studies have explored the relationship between multi‐metal exposure and diabetes mellitus risk, their conclusions are inconsistent. Several population‐based epidemiological surveys from different regions have found that magnesium (Mg), calcium (Ca), iron (Fe), zinc (Zn), arsenic (As), cadmium (Cd), copper (Cu) and lead (Pb) are associated with FBG or diabetes mellitus prevalence 7 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , whereas other studies have found no association 3 , 16 , 17 , 18 , 19 . Furthermore, the interaction of metals presents a challenge for correlation exploration. Thus, further research is required to explore the role of multi‐metal exposure in the onset and development of diabetes mellitus.
Given the common correlation and even collinearity among metals in mixed exposure, complex non‐linear and non‐additive relationships or interactive effects might be exerted on health outcomes due to the mixed exposure, and traditional multivariate regression methods have certain limitations in discovering these associations 20 . Therefore, a new statistical method called Bayesian kernel machine regression (BKMR) was applied in the present study; BKMR has been used to estimate the health effects of mixtures of various pollutants 21 , 22 , 23 . BKMR can effectively reflect the individual effects of each metal on health outcomes under multi‐metal exposure and evaluate the interactive effects of different metals.
Furthermore, we assessed the internal exposure levels to eight metal elements (i.e., Mg, Ca, Fe, Zn, As, Cd, Cu and Pb) of people aged 40–75 years in Cd‐polluted and unpolluted rural areas in southwest China. We examined the correlation between the various metals and diabetes mellitus prevalence, IFG rate, and FBG levels in the case of multi‐metal exposure. The present study is expected to fill the gap in the research on the relationship between multi‐metal exposure and diabetes mellitus in southwest China, and provide new insights into the prevention and treatment of diabetes mellitus.
MATERIALS AND METHODS
Study sites and population
The selected study areas were grouped into a Cd‐polluted area where the Cd levels in rice were >0.2 mg/kg and an unpolluted area where the Cd levels in rice were <0.05 mg/kg 24 . The two groups have similar economic conditions, geographical environments, lifestyles and eating habits. The inclusion and exclusion criteria were as follows:
Inclusion criteria: (i) residential duration ≥15 years; (ii) age 40–75 years; and (iii) living on rice, vegetables and water in the survey areas.
Exclusion criteria: (i) abnormal metal exposure levels (threefold higher than the 99th percentile of each metal); (ii) family genetic history of diabetes mellitus; (iii) suffering from hyperthyroidism liver, chronic gastrointestinal and kidney diseases; (iv) taking drugs that affect glucose metabolism, including oral antidiabetic agents or insulin; and (v) the data on FBG or metal exposure in blood and urine samples are missing.
Data collection
The basic information, including demographic information, health status and living habits, of the research participants was collected through questionnaires. Body mass index was calculated as the ratio of bodyweight divided by the square of height (kg/m2). Smoking status was defined as current smokers who currently smoked at least one cigarette a day and had been smoking for more than a month, former smokers who had quit smoking for >6 months, and non‐smokers who had never smoked.
Blood and urine sample collection
First‐morning mid‐urinary and fasting blood samples were collected in plastic bottles and anticoagulant vacuum blood collection tubes, respectively, and aliquoted into 1.5‐mL cryotubes, then transported to the laboratory under refrigeration. The samples were stored at −80°C until analysis.
Indicator measurement in blood and urine samples
Inductively coupled argon plasma mass spectrometry was carried out with an Agilent 7700 instrument (Agilent Technologies, Santa Clara, CA, USA) to determine the concentrations of Mg, Ca, Fe, Zn, As, Cd, Cu and Pb in the blood and urine samples (the concentrations are referred to as BMg, BCa, BFe, BZn, BAs, BCd, BCu and BPb, as well as UMg, UCa, UFe, UZn, UAs, UCd, UCu and UPb, respectively, in the following text). The limit of quantification for blood and urine metals was ≤0.01 μg/L. Seronorm™ Trace Elements Urine 0511545, Seronorm™ Trace Elements Urine Blank and Seronorm™ Trace Elements Whole Blood Level 1–2 (Nycomed Pharma AS, Oslo, Norway) were used as standard reference materials. Quality control procedures were also carried out. For example, each batch of samples was detected with three blank samples and a repeatedly injected standard material sample at the same time.
The FBG levels and urine creatinine were measured with a Hitachi 7180 automatic biochemical analyzer and the picric acid method using a commercial assay kit (Roche, Basel, Switzerland), respectively. The metal levels in urine were corrected by urinary creatinine levels to minimize the influence of urine amount and expressed as mg/g Cr or μg/g Cr.
Classification of FBG status
The FBG status of the study population was divided into a normal fasting glucose (NFG) group, an IFG group and a diabetes mellitus group. NFG, IFG and diabetes mellitus were defined based on the criteria of the American Diabetes Association; that is, FPG <5.6 mmol/L, 5.6 ≤ FPG < 7.0 mmol/L and FBG ≥7.0 mmol/L or a self‐reported physician diagnosis (with or without FBG ≥7.0 mmol/L), respectively. IFG rate was calculated as the percentage of the number of IFG groups divided by the total population of the study (%), and diabetes mellitus prevalence was calculated as the percentage of the number of diabetes mellitus groups divided by the total population of the study (%).
Statistical analysis
The data were entered using the double‐entry method in Epidata software after rechecking, and statistical packages (including SPSS 21.0 [IBM Corp., Armonk, NY, USA] and R 4.1.0 [The R Foundation for Statistical Computing, Vienna, Austria] software) were used for data analysis.
Normal distribution, non‐normal distribution and categorical data are presented as the mean ± standard deviation, median (25th and 75th percentile) and quantity (percent), respectively. The differences between the two research sites in demographic characteristics, FBG levels, metal exposure levels, IFG rate and diabetes mellitus prevalence were compared through a t‐test, Mann–Whitney U‐test or χ2‐test, and the correlations among the eight metals were analyzed using Spearman’s rank correlation. The associations among metal levels in blood and urine, IFG rate, and diabetes mellitus prevalence were analyzed using unconditional logistic regression. BKMR was used to analyze the overall, individual and interactive effects of the metals on FBG levels. P < 0.05 was considered statistically significant.
RESULTS
Participant characteristics
The demographic characteristics and metal exposure levels of the study population are shown in Tables 1 and 2. A total of 454 participants, including 204 participants from an unpolluted area and 250 participants from a Cd‐polluted area, were recruited. A significant difference in FBG status was observed between the two areas and the FBG levels in the unpolluted area were higher (P < 0.05). Compared with the participants in the unpolluted area, those in the Cd‐polluted area had higher levels of BMg, BAs, BCd and UCd (P < 0.05), but their levels of BCa, BFe, BZn, BCu, UMg, UCa, UFe, UZn and UAs were lower (P < 0.05), which implies that a high Cd‐body burden might adversely affect other beneficial minerals. No significant differences between the two areas were found in terms of age, sex, body mass index and smoking status (P > 0.05).
Table 1.
Characteristics of the study population
| Variables |
Unpolluted area (n = 204) |
Cd‐polluted area (n = 250) |
P‐value |
|---|---|---|---|
| Age (years) | 58.50 ± 8.90 | 57.98 ± 8.68 | 0.521 |
| BMI (kg/m2) | 24.56 ± 3.47 | 24.41 ± 3.22 | 0.639 |
| FBG (mmol/L) | 5.85 ± 1.62 | 4.95 ± 1.61 | 0.000* |
| Sex, n (%) | |||
| Male | 88 (43.14) | 100 (40.00) | 0.500 |
| Female | 116 (56.86) | 150 (60.00) | |
| Smoking status, n (%) | |||
| Non‐smoker | 126 (61.77) | 154 (61.60) | 0.995 |
| Current smoker | 59 (28.92) | 72 (28.80) | |
| Former smoker | 19 (9.31) | 24 (9.60) | |
| FBG status, n (%) | |||
| NFG group | 115 (56.4) | 171 (68.4) | 0.022* |
| IFG group | 69 (33.8) | 57 (22.8) | |
| DM group | 20 (9.80) | 22 (8.80) | |
Total n = 454. *P < 0.05. BMI, body mass index; DM, diabetes mellitus; FBG, fasting blood glucose; IFG, impaired fasting glucose; NFG, normal fasting glucose.
Table 2.
Blood and urinary levels of eight metals/metalloids
| Exposure measure |
Unpolluted area (n = 204) Median (25th, 75th percentile) |
Cd‐polluted area (n = 250) Median (25th, 75th percentile ) |
P‐value |
|---|---|---|---|
| BMg (mg/L) | 25.60 (16.92, 40.27) | 35.60 (32.08, 40.50) | 0.000* |
| BCa (mg/L) | 76.90 (48.23, 92.88) | 65.85 (58.98, 74.08) | 0.028* |
| BFe (mg/L) | 599.00 (440.25, 755.00) | 465.00 (413.50, 538.75) | 0.000* |
| BZn (mg/L) | 5.77 (3.79, 9.51) | 4.98 (4.18, 5.86) | 0.001* |
| BAs (μg/L) | 3.97 (0.50, 8.98) | 6.44 (3.18, 8.73) | 0.000* |
| BCd (μg/L) | 1.90 (1.09, 4.69) | 4.09 (2.88, 6.97) | 0.000* |
| BCu (μg/L) | 933.50 (666.75, 1420.00) | 755.50 (695.75, 900.75) | 0.000* |
| BPb (μg/L) | 28.30 (17.50, 41.03) | 32.30 (19.03, 44.20) | 0.110 |
| UMg (mg/g Cr) | 104.49 (72.96, 175.26) | 86.99 (55.24, 123.00) | 0.000* |
| UCa (mg/g Cr) | 243.55 (164.40, 366.65) | 163.80 (90.78, 263.23) | 0.000* |
| UFe (mg/g Cr) | 2.76 (1.61 ,4.63) | 1.05 (0.53, 2.05) | 0.000* |
| UZn (mg/g Cr) | 0.57 (0.38, 0.89) | 0.42 (0.29, 0.64) | 0.000* |
| UAs (μg/g Cr) | 25.15 (15.92, 42.09) | 15.21 (10.03, 21.77) | 0.000* |
| UCd (μg/g Cr) | 2.17 (1.29, 3.21) | 4.96 (3.43, 7.12) | 0.000* |
| UCu (μg/g Cr) | 12.24 (6.45, 36.91) | 18.02 (9.27, 27.89) | 0.870 |
| UPb (μg/g Cr) | 1.64 (0.74, 3.96) | 1.54 (0.66, 3.73) | 0.544 |
*P < 0.05. BAs, concentration of arsenic in the blood; BCa, concentration of calcium in the blood; BCd, concentration of cadmium in the blood; BCu, concentration of copper in the blood; BFe, concentration of iron in the blood; BMg, concentration of magnesium in the blood; BPb, concentration of lead in the blood; BZn, concentration of zinc in the blood; UAs, concentration of arsenic in the urine; UCa, concentration of calcium in the urine; UCd, concentration of cadmium in the urine; UCu, concentration of copper in the urine; UFe, concentration of iron in the urine; UMg, concentration of magnesium in the urine; UPb, concentration of lead in the urine; UZn, concentration of zinc in the urine.
Spearman’s rank correlation
The correlations between the different metals in the blood and urine samples are shown in Tables 3, 4, 5, 6. Significant associations between most metal levels were found in blood and urine in both areas (P < 0.05 or P < 0.01). For example, UMg and UCa had a positive correlation, whereas BCa and BAs had a negative correlation in both areas. Particularly, BZn and BFe, as well as BZn and BCu, had a strong positive correlation in the unpolluted area (r s = 0.815, P < 0.01; r s = 0.909, P < 0.01).
Table 3.
Spearman’s rank correlation coefficients between blood metals in the unpolluted area
| BMg (mg/L) | BCa (mg/L) | BFe (mg/L) | BZn (mg/L) | BAs (μg/L) | BCd (μg/L) | BCu (μg/L) | BPb (μg/L) | |
|---|---|---|---|---|---|---|---|---|
| BMg (mg/L) | 1.000 | 0.511** | 0.035 | −0.231** | −0.439** | −0.274** | −0.273** | 0.504** |
| BCa (mg/L) | 1.000 | 0.139* | −0.135 | −0.250** | −0.003 | −0.014 | 0.328** | |
| BFe (mg/L) | 1.000 | 0.815** | 0.315** | 0.431** | 0.739** | 0.362** | ||
| BZn (mg/L) | 1.000 | 0.359** | 0.489** | 0.909** | 0.177* | |||
| BAs (μg/L) | 1.000 | 0.319** | 0.337** | 0.013 | ||||
| BCd (μg/L) | 1.000 | 0.425** | 0.190** | |||||
| BCu (μg/L) | 1.000 | 0.091 | ||||||
| BPb (μg/L) | 1.000 |
**P < 0.01; *P < 0.05. BAs, concentration of arsenic in the blood; BCa, concentration of calcium in the blood; BCd, concentration of cadmium in the blood; BCu, concentration of copper in the blood; BFe, concentration of iron in the blood; BMg, concentration of magnesium in the blood; BPb, concentration of lead in the blood; BZn, concentration of zinc in the blood.
Table 4.
Spearman’s rank correlation coefficients between urine metals in the unpolluted area
| UMg (mg/gCr) | UCa (mg/g Cr) | UFe (mg/g Cr) | UZn (mg/g Cr) | UAs (mg/g Cr) | UCd (mg/g Cr) | UCu (mg/g Cr) | UPb (mg/g Cr) | |
|---|---|---|---|---|---|---|---|---|
| UMg (mg/g Cr) | 1.000 | 0.795** | 0.458** | 0.602** | 0.558** | 0.259** | 0.068 | 0.327** |
| UCa (mg/g Cr) | 1.000 | 0.590** | 0.573** | 0.479** | 0.285** | 0.246** | 0.405** | |
| UFe (mg/g Cr) | 1.000 | 0.438** | 0.287** | 0.199** | 0.453** | 0.371 | ||
| UZn (mg/g Cr) | 1.000 | 0.405** | 0.248** | 0.256** | 0.386** | |||
| UAs (μg/g Cr) | 1.000 | 0.281** | 0.124 | 0.390** | ||||
| UCd (μg/g Cr) | 1.000 | 0.295** | 0.261** | |||||
| UCu (μg/g Cr) | 1.000 | 0.430** | ||||||
| UPb (μg/g Cr) | 1.000 |
**P < 0.01; *P < 0.05. UAs, concentration of arsenic in the urine; UCa, concentration of calcium in the urine; UCd, concentration of cadmium in the urine; UCu, concentration of copper in the urine; UFe, concentration of iron in the urine; UMg, concentration of magnesium in the urine; UPb, concentration of lead in the urine; UZn, concentration of zinc in the urine.
Table 5.
Spearman’s rank correlation coefficients between blood metals in the cadmium‐polluted area
| BMg (mg/L) | BCa (mg/L) | BFe (mg/L) | BZn (mg/L) | BAs (μg/L) | BCd (μg/L) | BCu (μg/L) | BPb (μg/L) | ||
|---|---|---|---|---|---|---|---|---|---|
| BMg (mg/L) | 1.000 | 0.163** | 0.348** | 0.449** | 0.129* | 0.108 | 0.292** | 0.075 | |
| BCa (mg/L) | 1.000 | 0.126* | −0.064 | −0.226** | 0.190** | 0.329** | 0.255** | ||
| BFe (mg/L) | 1.000 | 0.643** | 0.050 | 0.262** | 0.201** | 0.508** | |||
| BZn (mg/L) | 1.000 | 0.113 | 0.287** | 0.439** | 0.227** | ||||
| BAs (μg/L) | 1.000 | 0.004 | 0.064 | −0.208** | |||||
| BCd (μg/L) | 1.000 | 0.242** | 0.270** | ||||||
| BCu (μg/L) | 1.000 | 0.040 | |||||||
| BPb (μg/L) | 1.000 | ||||||||
**P < 0.01; *P < 0.05. BAs, concentration of arsenic in the blood; BCa, concentration of calcium in the blood; BCd, concentration of cadmium in the blood; BCu, concentration of copper in the blood; BFe, concentration of iron in the blood; BMg, concentration of magnesium in the blood; BPb, concentration of lead in the blood; BZn, concentration of zinc in the blood.
Table 6.
Spearman’s rank correlation coefficients between urine metals in the cadmium‐polluted area
| UMg (mg/gCr) | Uca (mg/g Cr) | UFe (mg/g Cr) | UZn (mg/g Cr) | UAs (mg/g Cr) | UCd (mg/g Cr) | UCu (mg/g Cr) | UPb (mg/g Cr) | |
|---|---|---|---|---|---|---|---|---|
| UMg (mg/g Cr) | 1.000 | 0.643** | 0.508** | 0.341** | 0.397** | ** | 0.323** | 0.308** |
| Uca (mg/g Cr) | 1.000 | 0.682** | 0.237** | 0.275** | 0.198** | 0.287** | 0.352** | |
| UFe (mg/g Cr) | 1.000 | 0.214** | 0.242** | 0.011 | 0.312** | 0.217** | ||
| UZn (mg/g Cr) | 1.000 | 0.172** | 0.174** | 0.333** | 0.259** | |||
| UAs (μg/g Cr) | 1.000 | 0.297** | 0.424** | 0.257** | ||||
| UCd (μg/g Cr) | 1.000 | 0.301** | 0.187** | |||||
| UCu (μg/g Cr) | 1.000 | 0.349** | ||||||
| UPb (μg/g Cr) | 1.000 |
**P < 0.01; *P < 0.05. UAs, concentration of arsenic in the urine; UCa, concentration of calcium in the urine; UCd, concentration of cadmium in the urine; UCu, concentration of copper in the urine; UFe, concentration of iron in the urine; UMg, concentration of magnesium in the urine; UPb, concentration of lead in the urine; UZn, concentration of zinc in the urine.
Logistic regression analysis
Table 7 presents the logistic regression models for IFG associated with the multiple metals. After adjusting for potential confounding factors, the regression analysis showed a negative association between BCd levels and IFG rate in the unpolluted area (odds ratio [OR] 0.780, 95% confidence interval [CI] 0.655–0.928). A positive association between UFe levels and IFG rate, and a negative association between UZn levels and IFG rate were found in the Cd‐polluted area (OR 1.876, 95% CI 1.290–2.778 for UFe; OR 0.622, 95% CI 0.465–0.831 for UZn).
Table 7.
Logistic regression models for impaired fasting glucose associated with multiple metals
| Unpolluted area (n = 184) | Cd‐polluted area (n = 228) | |||
|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| BMg (mg/L) | 0.960 (0.916, 1.007) | 0.092 | 1.046 (0.985, 1.111) | 0.145 |
| BCa (mg/L) | 0.997 (0.980, 1.015) | 0.770 | 1.002 (0.971, 1.034) | 0.902 |
| BFe (mg/L) | 1.003 (0.999, 1.006) | 0.112 | 1.003 (0.998, 1.008) | 0.255 |
| BZn (mg/L) | 0.876 (0.752, 1.020) | 0.089 | 0.939 (0.597, 1.479) | 0.787 |
| BAs (μg/L) | 1.011 (0.994, 1.028) | 0.195 | 1.031 (0.935, 1.138) | 0.538 |
| BCd (μg/L) | 0.780 (0.655, 0.928) | 0.005* | 0.910 (0.804, 1.031) | 0.140 |
| BCu (μg/L) | 1.000 (0.999, 1.002) | 0.793 | 0.999 (0.997, 1.002) | 0.549 |
| BPb (μg/L) | 1.015 (0.997, 1.034) | 0.103 | 0.994 (0.982, 1.007) | 0.346 |
| UMg (mg/g Cr) | 0.999 (0.995, 1.004) | 0.820 | 1.007 (0.998, 1.016) | 0.127 |
| UCa (mg/g Cr) | 1.001 (0.998, 1.003) | 0.696 | 0.997 (0.993, 1.001) | 0.110 |
| UFe (mg/g Cr) | 1.009 (0.878, 1.161) | 0.895 | 1.876 (1.290, 2.778) | 0.001* |
| UZn (mg/g Cr) | 0.915 (0.643, 1.301) | 0.620 | 0.622 (0.465, 0.831) | 0.001* |
| UAs (μg/g Cr) | 1.005 (0.993, 1.017) | 0.426 | 0.985 (0.963, 1.008) | 0.204 |
| UCd (μg/g Cr) | 0.918 (0.774, 1.090) | 0.329 | 1.047 (0.951, 1.153) | 0.351 |
| UCu (μg/g Cr) | 1.001 (0.997, 1.006) | 0.610 | 0.996 (0.981, 1.012) | 0.655 |
| UPb (μg/g Cr) | 0.982 (0.919, 1.049) | 0.587 | 1.010 (0.983, 1.038) | 0.454 |
All the models have been adjusted for age, sex, body mass index, and smoking status. The normal fasting glucose group is set as the control, and the raw values of all variables are included in models. The total sample for the unpolluted area includes the normal fasting glucose group (n = 115) and the impaired fasting glucose group (n = 69) and that for the cadmium (Cd)‐polluted area includes the normal fasting glucose group (n = 171) and the impaired fasting glucose group (n = 57). *P < 0.05. BAs, concentration of arsenic in the blood; BCa, concentration of calcium in the blood; BCd, concentration of cadmium in the blood; BCu, concentration of copper in the blood; BFe, concentration of iron in the blood; BMg, concentration of magnesium in the blood; BPb, concentration of lead in the blood; BZn, concentration of zinc in the blood; CI, confidence interval; OR, odds ratio; UAs, concentration of arsenic in the urine; UCa, concentration of calcium in the urine; UCd, concentration of cadmium in the urine; UCu, concentration of copper in the urine; UFe, concentration of iron in the urine; UMg, concentration of magnesium in the urine; UPb, concentration of lead in the urine; UZn, concentration of zinc in the urine.
Table 8 presents the logistic regression models for diabetes mellitus associated with multiple metals. After adjusting for potential confounding factors, the regression analysis showed a negative association between BCd levels and diabetes mellitus prevalence in both regions (OR 0.506, 95% CI 0.288–0.888 for the unpolluted area; OR 0.722, 95% CI 0.536–0.972 for the Cd‐polluted area). We also found positive associations of diabetes mellitus prevalence with BFe and UPb levels in the unpolluted area (OR 1.008, 95% CI 1.001–1.014 for BFe; OR 1.185, 95% CI 1.022–1.376 for UPb), and a negative association between diabetes mellitus prevalence and UZn levels in the Cd‐polluted area (OR 0.609, 95% CI 0.395–0.939).
Table 8.
Logistic regression models for diabetes associated with multiple metals
| Unpolluted area (n = 135) | Cd‐polluted area (n = 193) | |||
|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| BMg (mg/L) | 0.968 (0.869, 1.077) | 0.547 | 1.060 (0.957, 1.175) | 0.266 |
| BCa (mg/L) | 1.005 (0.959, 1.052) | 0.848 | 0.961 (0.904, 1.021) | 0.197 |
| BFe (mg/L) | 1.008 (1.001, 1.014) | 0.019* | 1.006 (0.997, 1.014) | 0.185 |
| BZn (mg/L) | 0.943 (0.704, 1.261) | 0.691 | 0.765 (0.379, 1.546) | 0.456 |
| BAs (μg/L) | 0.960 (0.902, 1.023) | 0.209 | 0.959 (0.805, 1.141) | 0.633 |
| BCd (μg/L) | 0.506 (0.288, 0.888) | 0.018* | 0.722 (0.536, 0.972) | 0.032* |
| BCu (μg/L) | 1.000 (0.997, 1.003) | 0.976 | 1.001 (0.997, 1.005) | 0.632 |
| BPb (μg/L) | 1.007 (0.952, 1.064) | 0.818 | 0.986 (0.959, 1.014) | 0.324 |
| UMg (mg/g Cr) | 1.004 (0.995, 1.014) | 0.394 | 1.007 (0.995, 1.020) | 0.260 |
| UCa (mg/g Cr) | 0.999 (0.992, 1.006) | 0.777 | 1.000 (0.996, 1.005) | 0.838 |
| UFe (mg/g Cr) | 0.714 (0.497, 1.028) | 0.070 | 1.471 (0.931, 2.326) | 0.098 |
| UZn (mg/g Cr) | 1.174 (0.830, 1.662) | 0.364 | 0.609 (0.395, 0.939) | 0.025* |
| UAs (μg/g Cr) | 1.005 (0.977, 1.034) | 0.724 | 0.983 (0.951, 1.016) | 0.315 |
| UCd (μg/g Cr) | 0.851 (0.592, 1.225) | 0.387 | 0.984 (0.793, 1.222) | 0.886 |
| UCu (μg/g Cr) | 1.006 (0.997, 1.016) | 0.192 | 1.005 (0.992, 1.019) | 0.442 |
| UPb (μg/g Cr) | 1.185 (1.022, 1.376) | 0.025* | 0.983 (0.871, 1.109) | 0.782 |
All the models have been adjusted for age, sex, body mass index and smoking status. The normal fasting glucose group is set as the control, and the raw values of all variables are included in models. The total sample for the unpolluted area includes the normal fasting glucose group (n = 115) and the diabetes mellitus group (n = 20) and that for the cadmium (Cd)‐polluted area includes the normal fasting glucose group (n = 171) and the diabetes mellitus group (m = 22). *P < 0.05. BAs, concentration of arsenic in the blood; BCa, concentration of calcium in the blood; BCd, concentration of cadmium in the blood; BCu, concentration of copper in the blood; BFe, concentration of iron in the blood; BMg, concentration of magnesium in the blood; BPb, concentration of lead in the blood; BZn, concentration of zinc in the blood; CI, confidence interval; OR, odds ratio; UAs, concentration of arsenic in the urine; UCa, concentration of calcium in the urine; UCd, concentration of cadmium in the urine; UCu, concentration of copper in the urine; UFe, concentration of iron in the urine; UMg, concentration of magnesium in the urine; UPb, concentration of lead in the urine; UZn, concentration of zinc in the urine.
BKMR analyses
The effects of mixed metals, a single metal and metal‐to‐metal interaction on FBG levels under the multi‐metal exposure scenario are shown in Figures 1, 2, 3, 4. For the unpolluted area, we did not find any association between blood metals and FBG (Figure 1a–d). When urine metals were used as the exposure variables, the FBG levels increased when the levels of all metals were higher than their 55th percentile (Figure 2a). A significant non‐linear correlation was found between UZn and UPb, as well as UCu and FBG, which positively correlated with FBG when the metal levels were low, and negatively correlated with FBG when the concentrations were high (Figure 2b and c). The UPb exposure–response curve gradually became steeper with the increase in UZn concentration when UPb was at a high concentration, indicating that a positive interactive effect possibly occurred between UZn and UPb (Figure 2d).
Figure 1.
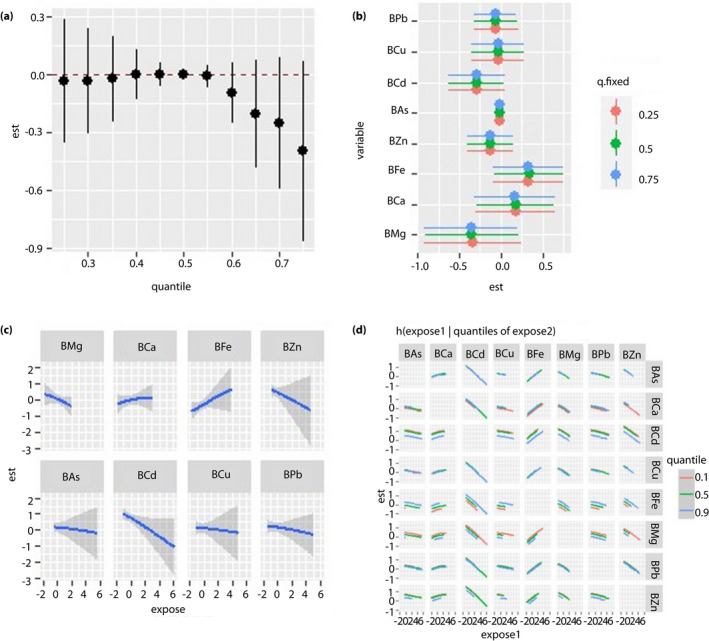
Regression analysis between blood metal and fasting blood glucose (FBG) levels in the unpolluted area. All the models have been adjusted for age, sex, body mass index and smoking status. ‘Est’ stands for ‘estimate,’ which means that the estimates of the effects include the overall, individual and interactive effects of different metals on FBG when the metal levels change. ‘Expose’ stands for metal exposure levels. Here, we use the z‐score for all the exposures to have the same scale. (a) Overall effects of mixed‐metal exposure: the variation of FBG when all the metals are at a particular quantile compared with when all of them are at their median value. (b) Contribution of single‐metal exposure to the overall effects: the variation of FBG when a single metal is at the 75th percentile compared with when it is at its 25th percentile, and all of the remaining metals are fixed at either the 25th, 50th or 75th percentile. (c) Univariate exposure–response function: the univariate relationship between each metal and FBG when all of the other metals are fixed at the 50th percentile. (d) Bivariate exposure–response function: the exposure‐response function of a single metal when the second metal is fixed at either the 10th, 50th or 90th percentile and the remaining metals are fixed to a particular value. BAs, concentration of arsenic in the blood; BCa, concentration of calcium in the blood; BCd, concentration of cadmium in the blood; BCu, concentration of copper in the blood; BFe, concentration of iron in the blood; BMg, concentration of magnesium in the blood; BPb, concentration of lead in the blood; BZn, concentration of zinc in the blood.
Figure 2.
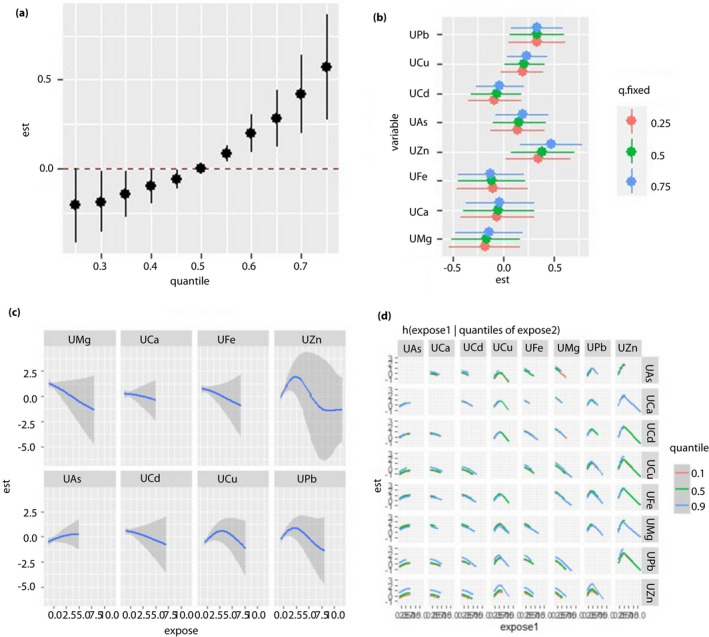
Regression analysis between urine metal and fasting blood glucose (FBG) levels in the unpolluted area. All of the models have been adjusted for age, sex, body mass index and smoking status. ‘Est’ stands for ‘estimate’, which means that the estimates of the effects include the overall, individual and interactive effects of different metals on FBG when the metal levels change. ‘Expose’ stands for metal exposure levels. Here, we use the z‐score for all the exposures to have the same scale. (a) Overall effects of mixed‐metal exposure: the variation of FBG when all the metals are at a particular quantile compared with when all of them are at their median value. (b) Contribution of single‐metal exposure to the overall effects: the variation of FBG when a single metal is at the 75th percentile compared with when it is at its 25th percentile, and all of the remaining metals are fixed at either the 25th, 50th, or 75th percentile. (c) Univariate exposure–response function: the univariate relationship between each metal and FBG when all of the other metals are fixed at the 50th percentile. (d) Bivariate exposure–response function: the exposure‐response function of a single metal when the second metal is fixed at either the 10th, 50th or 90th percentile and the remaining metals are fixed to a particular value. UAs, concentration of arsenic in the urine; UCa, concentration of calcium in the urine; UCd, concentration of cadmium in the urine; UCu, concentration of copper in the urine; UFe, concentration of iron in the urine; UMg, concentration of magnesium in the urine; UPb, concentration of lead in the urine; UZn, concentration of zinc in the urine.
Figure 3.
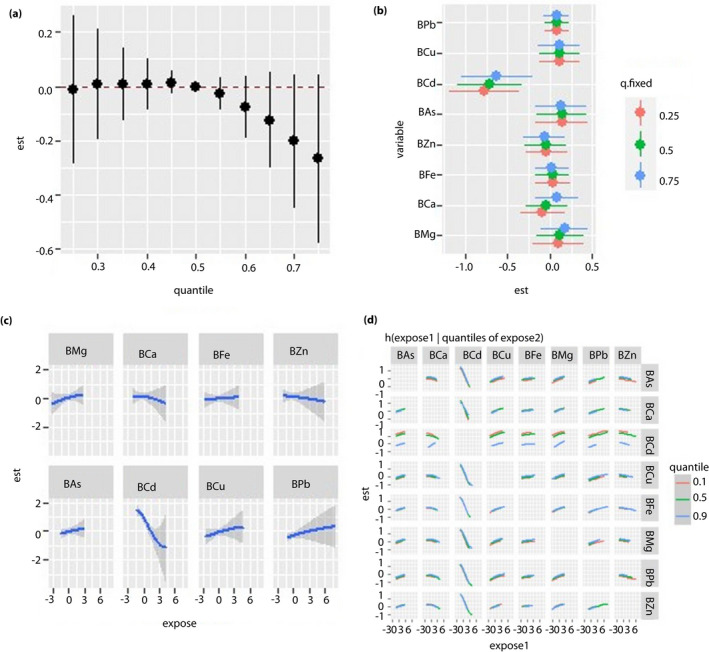
Regression analysis between blood metal and fasting blood glucose (FBG) levels in the cadmium‐polluted area. All the models have been adjusted for age, sex, body mass index and smoking status. ‘Est’ stands for ‘estimate’, which means that the estimates of the effects include the overall, individual and interactive effects of different metals on FBG when the metal levels change. ‘Expose’ stands for metal exposure levels. Here, we use the z‐score for all the exposures to have the same scale. (a) Overall effects of mixed‐metal exposure: the variation of FBG when all the metals are at a particular quantile compared with when all of them are at their median value. (b) Contribution of single‐metal exposure to the overall effects: the variation of FBG when a single metal is at the 75th percentile compared with when it is at its 25th percentile, and all of the remaining metals are fixed at either the 25th, 50th or 75th percentile. (c) Univariate exposure–response function: the univariate relationship between each metal and FBG when all of the other metals are fixed at the 50th percentile. (d) Bivariate exposure–response function: the exposure–response function of a single metal when the second metal is fixed at either the 10th, 50th or 90th percentile and the remaining metals are fixed to a particular value. BAs, concentration of arsenic in the blood; BCa, concentration of calcium in the blood; BCd, concentration of cadmium in the blood; BCu, concentration of copper in the blood; BFe, concentration of iron in the blood; BMg, concentration of magnesium in the blood; BPb, concentration of lead in the blood; BZn, concentration of zinc in the blood.
Figure 4.
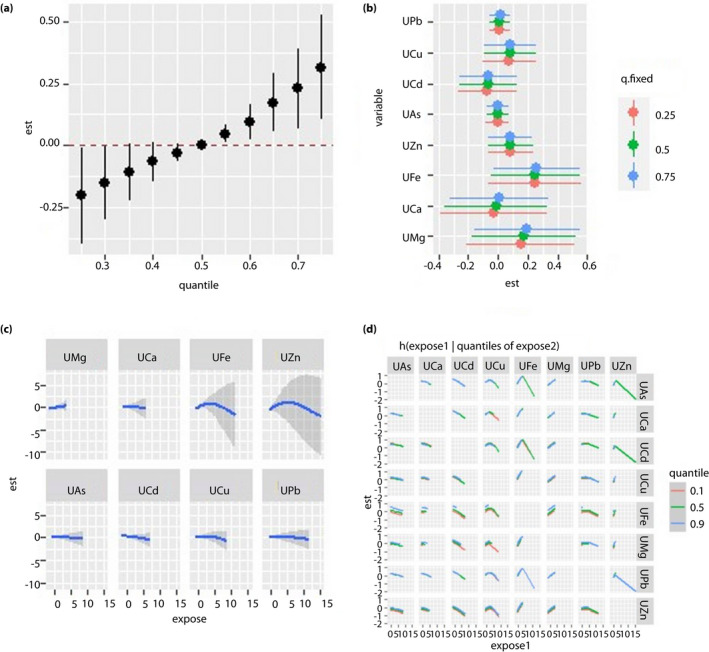
Regression analysis between urine metal and fasting blood glucose (FBG) levels in the cadmium‐polluted area. All the models have been adjusted for age, sex, body mass index and smoking status. ‘Est’ stands for ‘estimate’, which means that the estimates of the effects include the overall, individual and interactive effects of different metals on FBG when the metal levels change. ‘Expose’ stands for metal exposure levels. Here, we use the z‐score for all the exposures to have the same scale. (a) Overall effects of mixed‐metal exposure: the variation of FBG when all the metals are at a particular quantile compared with when all of them are at their median value. (b) Contribution of single‐metal exposure to the overall effects: the variation of FBG when a single metal is at the 75th percentile compared with when it is at its 25th percentile, and all of the remaining metals are fixed at either the 25th, 50th or 75th percentile. (c) Univariate exposure–response function: the univariate relationship between each metal and FBG when all of the other metals are fixed at the 50th percentile. (d) Bivariate exposure‐response function: the exposure‐response function of a single metal when the second metal is fixed at either the 10th, 50th or 90th percentile and the remaining metals are fixed to a particular value. UAs, concentration of arsenic in the urine; UCa, concentration of calcium in the urine; UCd, concentration of cadmium in the urine; UCu, concentration of copper in the urine; UFe, concentration of iron in the urine; UMg, concentration of magnesium in the urine; UPb, concentration of lead in the urine; UZn, concentration of zinc in the urine.
For the Cd‐polluted area, we found a negative correlation between BCd and FBG, but no overall effect of blood mixed metal on FBG was found (Figure 3a–c). In the bivariate exposure–response function, the exposure–response curve of BCa gradually flattened with the increase in the BCd level. Hence, BCd and BCa might exert an interactive effect on FBG level (Figure 3d). When urine metals were used as the exposure variables, we found a positive correlation between the metal mixture and FBG when the levels of all metals exceeded their 55th percentile (Figure 4a–d).
DISCUSSION
We evaluated the associations among mixed exposure to eight metals, diabetes mellitus prevalence, IFG rate and FBG levels in Cd‐polluted and unpolluted areas in rural areas of southwest China. A separate analysis of the two areas showed that different metal exposure backgrounds might affect the associations between metal mixtures and FBG levels. UZn, UPb and UCu with high posterior inclusion probability in the BKMR analysis played major roles in the overall association between metal exposure and FBG levels in the unpolluted area, whereas BCd with high posterior inclusion probability dominated in the Cd‐polluted area. For the unpolluted area, we found that BCd levels were negatively correlated with IFG rate and diabetes mellitus prevalence, whereas BFe and UPb levels were positively correlated with diabetes mellitus prevalence. Inverted U‐shaped associations were found among UZn, UPb, UCu and FBG levels. For the Cd‐polluted area, we found that BCd levels were negatively correlated with diabetes mellitus prevalence and FBG levels, and UZn levels were negatively correlated with IFG rate and diabetes mellitus prevalence. Meanwhile, UFe levels were positively correlated with IFG rate. We also found possible interactive effects between Zn and Pb, and between Cd and Ca on FBG levels.
A few prospective studies that explored the associations between metal exposure and diabetes mellitus presented the different roles of metals in diabetes mellitus development 15 , 17 , 25 , 26 , 27 , 28 , 29 , 30 . However, no unified conclusion about which metal is harmful, protective or irrelevant for diabetes mellitus has been achieved, partly because of the different metal mixture effects, implications of variable biological samples, times and levels of exposure in these studies.
The present results showed that BCd was negatively correlated with diabetes mellitus prevalence, IFG rate and FBG levels. This finding is consistent with those of Nakamura et al. 31 and Anetor et al. 31 , 32 However, it is inconsistent or even contrary to our initial assumption and the findings of other reports 15 , 33 , 34 , 35 , due to two possible reasons. First, when humans or animals are exposed to low Cd concentrations for a short time, the secretory function of pancreatic islet cells is enhanced, resulting in low blood sugar. In vitro experiments have shown that the insulin release rate increases initially then decreases with the increase in exposure concentration and time when mice pancreatic islet cells are incubated with Cd2+ 36 , 37 . UCd is generally considered to reflect long‐term Cd exposure and accumulation in the body, and BCd reflects recent exposure 38 . The Cd exposure levels of the present study population were lower than the World Health Organization’s standards; that is, 10 μg /L for BCd and 5 μg/g Cr for UCd 39 . These findings suggest that relatively short‐term, low‐level Cd exposure might be related to temporary low FBG levels. Second, co‐exposure to metals might confound the real effects of Cd on blood sugar. For example, we found that the BZn levels among the present study population were positively correlated with BCd levels in Spearman’s rank correlation. Zn and Cd, belonging to the same column in the periodic table, compete for the same biological targets in the pathological process of diabetes due to their shared binding sites and/or ligands 40 , 41 , 42 , 43 . Furthermore, the BKMR models suggested a potential interaction effect between BCd and BCa on FBG levels in the Cd‐polluted area. Ca might interfere with the effects of Cd on FBG by mediating signal transduction during insulin secretion 44 . Another study based on the relationship between multi‐metal exposure and FBG levels showed that the relationship between Cd and FBG might be affected by other co‐exposed metals 45 . Overall, the real effect of Cd on diabetes mellitus and blood sugar still needs to be studied using enlarged samples, and the co‐exposure of other metals should be considered.
Fe overload is a known risk factor for type 2 diabetes mellitus and thought to be involved in the insulin secretion mechanism. High Fe levels can generate free radicals and reactive oxygen species. Increased and continuous exposure to intracellular reactive oxygen species can cause insulin secretion disorders 46 . Many studies have shown that excessive Fe is associated with elevated FBG levels and increased diabetes mellitus prevalence. For example, Blesia et al. 47 showed that pancreatic β‐cells are susceptible to excessive Fe accumulation, leading to decreased cell viability and reduced insulin secretion 47 . A study that used Mendelian randomization found a causal relationship between increased systemic Fe status and increased prevalence of type 2 diabetes mellitus by analyzing serum Fe, ferritin, transferrin and transferrin saturation levels in 74,124 type 2 diabetes mellitus cases and 824,006 controls 48 . Furthermore, many studies have found that the higher the Fe exposure level is in women during pregnancy, the higher the prevalence of gestational diabetes mellitus is 49 , 50 , 51 , 52 , 53 . These findings are consistent with the present results that Fe exposure level is positively correlated with IFG rate and diabetes mellitus prevalence.
Cu and Zn are essential trace elements for humans. Cu can bind to superoxide dismutase or interact with metallothioneins to clear free radicals efficiently, thereby protecting pancreatic β‐cells from damage or apoptosis caused by oxidative stress 54 , 55 . Zn is an anti‐oxidant and can directly participate in the synthesis, storage and secretion of insulin in pancreatic β‐cells 6 . People with Zn deficiency are likely to develop insulin resistance. Zn supplementation can alleviate insulin resistance, which is conducive to blood sugar control in people with and without diabetes mellitus 56 , 57 , 58 . Therefore, Cu and Zn are beneficial for decreasing blood sugar levels, and reducing the IFG rate and diabetes mellitus prevalence to a certain extent, which is consistent with our conclusion that UZn has a negative correlation with IFG rate and diabetes mellitus prevalence. However, Cu and Zn also have pro‐oxidant effects. Cu can regulate electron transfer, and increased Zn levels can inhibit metabolism and the mitochondrial function, thus promoting the production of reactive oxygen species 59 , 60 . The results of a multisite, multiethnic cohort study on women at midlife also showed that women with excess zinc in their urine might face an elevated risk of diabetes 15 . The mechanism might be related to the loss of zinc in pancreatic β‐cells, which leads to a decrease in insulin secretion 15 , 61 . This dual effect might be one of the reasons for the inverted U‐shaped exposure–response relationship between Zn and Cu and FBG levels.
Pb is a common environmental toxic metal 62 . A few studies investigated the relationship between Pb exposure and diabetes mellitus prevalence, and suggested that Pb exposure might promote the occurrence and development of diabetes mellitus 63 . A possible mechanism is that Pb can activate the expression of genes related to glucose metabolism, such as phosphoenolpyruvate carboxykinase, glucose‐6‐phosphatase and glycogen synthase kinase‐3 beta, thereby increasing the activity of hepatic gluconeogenesis enzymes and interfering with the insulin secretory function, and finally leading to increased blood sugar 64 , 65 . However, other studies did not find a relationship between Pb and diabetes mellitus 16 , 17 , 19 . We discovered in the present study that Pb exerted a blood sugar‐lowering effect when it rose to a certain level, which might be related to the co‐exposure of Pb and other metals; however, the specific reason is unclear.
We also found that UZn and UPb exerted a positive interactive effect on FBG. However, many previous studies supported the conclusion that a negative correlation exists between Zn and Pb, and exerts an antagonistic effect on the body 66 . We speculate that this might be due to the influence of other metal elements covering up the real association between Zn and Pb, or our relatively small sample size and low Pb level led to a false connection. Given this limited and conflicting epidemiological evidence, and the high variability and heterogeneity of Zn, Pb and Cu exposure levels in different studies, further research is required to clarify the real individual and interactive effects of Cu, Zn, and Pb on diabetes mellitus and blood sugar.
The present study had the following advantages. First, it analyzed the relationship between metal levels in blood and urine and diabetes mellitus, thereby making up for the shortcomings of using only a kind of biological sample and improving the sensitivity of the research results. Second, we evaluated the effects of metal mixtures, a single metal and metal‐to‐metal interactions on FBG when exposed to multiple metals, and analyzed the exposure–response relationship between each metal and FBG by using the BKMR model. Finally, the present study is the first to explore the relationship between multi‐metal exposure and diabetes mellitus in rural areas in southwest China. It can serve as preliminary evidence for the effects of multi‐metal exposure on diabetes mellitus in the population in this region.
However, the present research also had limitations. First, due to the limits of cross‐sectional studies, we could not establish a causal relationship between metal exposure and diabetes mellitus or FBG. Second, we adopted only FBG as a diagnostic indicator of diabetes mellitus, making the determination of diabetes mellitus cases not accurate enough. Other indicators, such as glycosylated hemoglobin and oral glucose tolerance test, should be included in the future. Third, the occurrence and development of diabetes are affected by many factors, including genetics, lifestyle, personal characteristics and environment. We did not adjust for all possible potential confounding factors. Fourth, excluding individuals taking medications might create selection bias, and the sample size of the present study was smaller than calculated. More samples would make the results more reliable.
In summary, the present findings suggest that multi‐metal exposure, especially Cd, Fe, Zn, Cu and Pb, is linked to diabetes mellitus. For instance, diabetes mellitus prevalence and IFG rate were negatively correlated with Cd and Zn levels, but positively correlated with Fe levels. Non‐linear exposure–response relationships were also observed between Zn, Pb, and Cu and FBG levels. Furthermore, interactive effects existed between Zn and Pb, and between Cd and Ca on FBG levels. The underlying mechanisms regarding such an interaction are perhaps related to similar biotransportation ways, signaling pathways or other competitive/synergistic action of metals. Future research should explore the effects and mechanisms of metal‐to‐metal interactions on the etiology of diabetes mellitus.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The protocol for this study was reviewed and approved by the Ethical Committee of West China School of Public Health and West China Fourth Hospital, Sichuan University (Approval No. Gwll2013052).
Informed consent: All survey participants signed informed consent.
Approval date of Registry and the Registration No. of the study/trial: N/A.
Animal studies: N/A.
ACKNOWLEDGMENTS
This study was supported by the National Key Research and Development Program of China (Grant No. 2017YFC1600200 and 2017YFC1600500) and the Special Fund for Scientific Research of the Public Welfare Projects of China (Grant No. 201302005). We also thank all staff and students in the Public Health and Preventive Medicine Experiment Teaching Center and Food Safety Monitoring and Risk Assessment Key Laboratory of Sichuan Province.
J Diabetes Investig. 2022; 13: 1412–1425
REFERENCES
- 1. Roglic G. WHO Global report on diabetes: a summary. Int J Noncommun Dis 2016; 1: 3–8. [Google Scholar]
- 2. Wang R. Interpretation of updating key points of the new Chinese Diabetes Prevention guide. Jiangsu Health Care 2021; 05: 50–51. [Google Scholar]
- 3. Yang A, Liu S, Cheng Z, et al. Dose‐response analysis of environmental exposure to multiple metals and their joint effects with fasting plasma glucose among occupational workers. Chemosphere 2017; 186: 314–321. [DOI] [PubMed] [Google Scholar]
- 4. Ali A, Taj A, Ahmed MU, et al. Frequency of impaired fasting glucose in first degree relatives of Type‐II diabetic patients and its association with Body Mass Index. Pak J Med Sci 2020; 36: 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inoue K, Inoue M, Matsumoto M, et al. Persistent fasting hyperglycaemia is more predictive of type 2 diabetes than transient fasting hyperglycaemia. Diabet Med 2012; 29: e75–81. [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez‐Villalva A, Colin‐Barenque L, Bizarro‐Nevares P, et al. Pollution by metals: is there a relationship in glycemic control? Environ Toxicol Pharmacol 2016; 46: 337–343. [DOI] [PubMed] [Google Scholar]
- 7. Ge X, Yang A, Huang S, et al. Sex‐specific associations of plasma metals and metal mixtures with glucose metabolism: an occupational population‐based study in China. Sci Total Environ 2021; 760: 143906. [DOI] [PubMed] [Google Scholar]
- 8. Mousavi SN, Faghihi A, Motaghinejad M, et al. Zinc and selenium co‐supplementation reduces some lipid peroxidation and angiogenesis markers in a rat model of NAFLD‐fed high fat diet. Biol Trace Elem Res 2018; 181: 288–295. [DOI] [PubMed] [Google Scholar]
- 9. Yang A, Liu S, Cheng N, et al. Multiple metals exposure, elevated blood glucose and dysglycemia among Chinese occupational workers. J Diabetes Complications 2017; 31: 101–107. [DOI] [PubMed] [Google Scholar]
- 10. Lv Y, Xie L, Dong C, et al. Co‐exposure of serum calcium, selenium and vanadium is nonlinearly associated with increased risk of type 2 diabetes mellitus in a Chinese population. Chemosphere 2021; 263: 128021. [DOI] [PubMed] [Google Scholar]
- 11. Forte G, Bocca B, Peruzzu A, et al. Blood metals concentration in type 1 and type 2 diabetics. Biol Trace Elem Res 2013; 156: 79–90. [DOI] [PubMed] [Google Scholar]
- 12. Liu B, Feng W, Wang J, et al. Association of urinary metals levels with type 2 diabetes risk in coke oven workers. Environ Pollut 2016; 210: 1–8. [DOI] [PubMed] [Google Scholar]
- 13. Afridi HI, Kazi TG, Kazi N, et al. Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res Clin Pract 2008; 80: 280–288. [DOI] [PubMed] [Google Scholar]
- 14. Swaddiwudhipong W, Limpatanachote P, Mahasakpan P, et al. Progress in cadmium‐related health effects in persons with high environmental exposure in northwestern Thailand: a five‐year follow‐up. Environ Res 2012; 112: 194–198. [DOI] [PubMed] [Google Scholar]
- 15. Wang X, Karvonen‐Gutierrez CA, Herman WH, et al. Urinary metals and incident diabetes in midlife women: study of Women’s Health Across the Nation (SWAN). BMJ Open Diabetes Res Care 2020; 8: e001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji JH, Jin MH, Kang JH, et al. Relationship between heavy metal exposure and type 2 diabetes: a large‐scale retrospective cohort study using occupational health examinations. BMJ Open 2021; 11: e039541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Menke A, Guallar E, Cowie CC. Metals in urine and diabetes in U.S. Adults. Diabetes 2016; 65: 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li XT, Yu PF, Gao Y, et al. Association between plasma metal levels and diabetes risk: a case‐control study in China. Biomed Environ Sci 2017; 30: 482–491. [DOI] [PubMed] [Google Scholar]
- 19. Moon SS. Association of lead, mercury and cadmium with diabetes in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Diabet Med 2013; 30: e143–148. [DOI] [PubMed] [Google Scholar]
- 20. Bobb JF, Claus Henn B, Valeri L, et al. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 2018; 17: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Dong T, Hu W, et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: comparison of three statistical models. Environ Int 2019; 123: 325–336. [DOI] [PubMed] [Google Scholar]
- 22. Lim JT, Tan YQ, Valeri L, et al. Association between serum heavy metals and prostate cancer risk ‐ A multiple metal analysis. Environ Int 2019; 132: 105109. [DOI] [PubMed] [Google Scholar]
- 23. Souter I, Bellavia A, Williams PL, et al. Urinary concentrations of phthalate metabolite mixtures in relation to serum biomarkers of thyroid function and autoimmunity among women from a fertility center. Environ Health Perspect 2020; 128: 67007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huo J, Huang Z, Li R, et al. Dietary cadmium exposure assessment in rural areas of Southwest China. PLoS One 2018; 13: e0201454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eshak ES, Iso H, Maruyama K, et al. Associations between dietary intakes of iron, copper and zinc with risk of type 2 diabetes mellitus: A large population‐based prospective cohort study. Clin Nutr (Edinburgh, Scotland) 2018; 37: 667–674. [DOI] [PubMed] [Google Scholar]
- 26. Yuan Y, Xiao Y, Yu Y, et al. Associations of multiple plasma metals with incident type 2 diabetes in Chinese adults: the Dongfeng‐Tongji Cohort. Environ Pollut (Barking, Essex 1987; 2018: 917–925. [DOI] [PubMed] [Google Scholar]
- 27. Soomro MH, Baiz N, Huel G, et al. Exposure to heavy metals during pregnancy related to gestational diabetes mellitus in diabetes‐free mothers. Sci Total Environ 2019; 656: 870–876. [DOI] [PubMed] [Google Scholar]
- 28. Hendryx M, Luo J, Chojenta C, et al. Exposure to heavy metals from point pollution sources and risk of incident type 2 diabetes among women: a prospective cohort analysis. Int J Environ Health Res 2021; 31: 453–464. [DOI] [PubMed] [Google Scholar]
- 29. Cabral M, Kuxhaus O, Eichelmann F, et al. Trace element profile and incidence of type 2 diabetes, cardiovascular disease and colorectal cancer: results from the EPIC‐Potsdam cohort study. Eur J Nutr 2021; 60: 3267–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodríguez‐Pérez C, Gómez‐Peña C, Pérez‐Carrascosa FM, et al. Trace elements concentration in adipose tissue and the risk of incident type 2 diabetes in a prospective adult cohort. Environ Pollut (Barking, Essex) 1987; 2021: 117496. [DOI] [PubMed] [Google Scholar]
- 31. Nakamura K, Nishiyama S, Takata T, et al. Effects of zinc on cadmium‐induced alterations in hepatic functions and blood glucose of rats. Environ Res 1983; 30: 175–181. [DOI] [PubMed] [Google Scholar]
- 32. Anetor JI, Uche CZ, Ayita EB, et al. Cadmium level, glycemic control, and indices of renal function in treated type ii diabetics: implications for polluted environments. Front Public Health 2016; 4: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cai J, Li Y, Liu S, et al. Associations between multiple heavy metals exposure and glycated hemoglobin in a Chinese population. Chemosphere 2022; 287(Pt 2): 132159. [DOI] [PubMed] [Google Scholar]
- 34. Hong H, Xu Y, Xu J, et al. Cadmium exposure impairs pancreatic β‐cell function and exaggerates diabetes by disrupting lipid metabolism. Environ Int 2021; 149: 106406. [DOI] [PubMed] [Google Scholar]
- 35. Peng S, Liu L, Zhang X, et al. A nested case‐control study indicating heavy metal residues in meconium associate with maternal gestational diabetes mellitus risk. Environ Health 2015; 14: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nilsson T, Rorsman F, Berggren P‐O, et al. Accumulation of cadmium in pancreatic β cells is similar to that of calcium in being stimulated by both glucose and high potassium. Biochimica Et Biophysica Acta (BBA) ‐ Molecular Cell Res 1986; 888: 270–277. [DOI] [PubMed] [Google Scholar]
- 37. Deng W, Chen KY, Liu WY, et al. Effects of vitamin D on pancreatic islet β cell injury induced by low concentration cadmium in mice. J Biol Regul Homeost Agents 2019; 33: 245–250. [PubMed] [Google Scholar]
- 38. Li X, Li R, Yan J, et al. Co‐exposure of cadmium and lead on bone health in a southwestern Chinese population aged 40–75 years. J Appl Toxicol 2020; 40: 352–362. [DOI] [PubMed] [Google Scholar]
- 39. Guo FF, Hu ZY, Li BY, et al. Evaluation of the association between urinary cadmium levels below threshold limits and the risk of diabetes mellitus: a dose‐response meta‐analysis. Environ Sci Pollut Res Int 2019; 26: 19272–19281. [DOI] [PubMed] [Google Scholar]
- 40. Buha A, Dukic‐Cosic D, Curcic M, et al. Emerging links between cadmium exposure and insulin resistance: human, animal, and cell study data. Toxics 2020; 8: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bulat Z, Dukic‐Cosic D, Antonijevic B, et al. Can zinc supplementation ameliorate cadmium‐induced alterations in the bioelement content in rabbits? Arh Hig Rada Toksikol 2017; 68: 38–45. [DOI] [PubMed] [Google Scholar]
- 42. Matovic V, Buha A, Bulat Z, et al. Cadmium toxicity revisited: focus on oxidative stress induction and interactions with zinc and magnesium. Arh Hig Rada Toksikol 2011; 62: 65–76. [DOI] [PubMed] [Google Scholar]
- 43. Moulis JM. Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals 2010; 23: 877–896. [DOI] [PubMed] [Google Scholar]
- 44. Zhou X, Hao W, Shi H, et al. Calcium homeostasis disruption ‐ a bridge connecting cadmium‐induced apoptosis, autophagy and tumorigenesis. Oncol Res Treat 2015; 38: 311–315. [DOI] [PubMed] [Google Scholar]
- 45. Li Z, Xu Y, Huang Z, et al. Association between exposure to arsenic, nickel, cadmium, selenium, and zinc and fasting blood glucose levels. Environ Pollut (Barking, Essex) 1987; 255: 113325. [DOI] [PubMed] [Google Scholar]
- 46. Kasuga M. Insulin resistance and pancreatic beta cell failure. J Clin Invest 2006; 116: 1756–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blesia V, Patel VB, Al‐Obaidi H, et al. Excessive iron induces oxidative stress promoting cellular perturbations and insulin secretory dysfunction in MIN6 beta cells. Cells‐Basel 2021; 10: 1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang X, Fang X, Zheng W, et al. Genetic support of a causal relationship between iron status and type 2 diabetes: a Mendelian randomization study. J Clin Endocrinol Metab 2021; 106: e4641‐e4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rawal S, Hinkle SN, Bao W, et al. A longitudinal study of iron status during pregnancy and the risk of gestational diabetes: findings from a prospective, multiracial cohort. Diabetologia 2017; 60: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang X, Wu M, Zhong C, et al. Association between maternal plasma ferritin concentration, iron supplement use, and the risk of gestational diabetes: a prospective cohort study. Am J Clin Nutr 2021; 114: 1100–1106. [DOI] [PubMed] [Google Scholar]
- 51. Zhang Y, Xu S, Zhong C, et al. Periconceptional iron supplementation and risk of gestational diabetes mellitus: a prospective cohort study. Diabetes Res Clin Pract 2021; 176: 108853. [DOI] [PubMed] [Google Scholar]
- 52. Khambalia AZ, Aimone A, Nagubandi P, et al. High maternal iron status, dietary iron intake and iron supplement use in pregnancy and risk of gestational diabetes mellitus: a prospective study and systematic review. Diabet Med 2016; 33: 1211–1221. [DOI] [PubMed] [Google Scholar]
- 53. Si S, Shen Y, Xin X, et al. Hemoglobin concentration and iron supplement during pregnancy were associated with an increased risk of gestational diabetes mellitus. J Diabetes 2021; 13: 211–221. [DOI] [PubMed] [Google Scholar]
- 54. Martín Giménez VM, Bergam I, Reiter RJ, et al. Metal ion homeostasis with emphasis on zinc and copper: potential crucial link to explain the non‐classical antioxidative properties of vitamin D and melatonin. Life Sci 2021; 281: 119770. [DOI] [PubMed] [Google Scholar]
- 55. Darenskaya MA, Kolesnikova LI, Kolesnikov SI. Oxidative stress: pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull Exp Biol Med 2021; 171: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Islam MR, Arslan I, Attia J, et al. Is serum zinc level associated with prediabetes and diabetes? A cross‐sectional study from Bangladesh. PLoS One 2013; 8: e61776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Farooq DM, Alamri AF, Alwhahabi BK, et al. The status of zinc in type 2 diabetic patients and its association with glycemic control. J Fam Commun Med 2020; 27: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pompano LM, Boy E. Effects of dose and duration of zinc interventions on risk factors for type 2 diabetes and cardiovascular disease: a systematic review and meta‐analysis. Adv Nutr 2021; 12: 141–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee SR. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid Med Cell Longev 2018; 2018: 9156285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lian S, Zhang T, Yu Y, et al. Relationship of circulating copper level with gestational diabetes mellitus: a meta‐analysis and systemic review. Biol Trace Elem Res 2021; 199: 4396–4409. [DOI] [PubMed] [Google Scholar]
- 61. Jansen J, Karges W, Rink L. Zinc and diabetes–clinical links and molecular mechanisms. J Nutr Biochemist 2009; 20: 399–417. [DOI] [PubMed] [Google Scholar]
- 62. Liao LM, Friesen MC, Xiang YB, et al. Occupational lead exposure and associations with selected cancers: the shanghai men's and women's health study cohorts. Environ Health Perspect 2016; 124: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Leff T, Stemmer P, Tyrrell J, et al. Diabetes and exposure to environmental lead (Pb). Toxics 2018; 6: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mostafalou S, Baeeri M, Bahadar H, et al. Molecular mechanisms involved in lead induced disruption of hepatic and pancreatic glucose metabolism. Environ Toxicol Pharmacol 2015; 39: 16–26. [DOI] [PubMed] [Google Scholar]
- 65. Tyrrell JB, Hafida S, Stemmer P, et al. Lead (Pb) exposure promotes diabetes in obese rodents. J Trace Elem Med Biol 2017; 39: 221–226. [DOI] [PubMed] [Google Scholar]
- 66. Wani AL, Hammad Ahmad Shadab GG, Afzal M. Lead and zinc interactions – An influence of zinc over lead related toxic manifestations. J Trace Elem Med Biol 2021; 64: 126702. [DOI] [PubMed] [Google Scholar]


