Abstract
Aims/Introduction
Obstructive sleep apnea (OSA) is related to prediabetes and diabetes. Whether patients with OSA have a higher risk of prediabetes/diabetes remains unclear. We aimed to carry out a meta‐analysis of published studies to evaluate the relationships between OSA and prediabetes and diabetes, and the impact of the severity of OSA on diabetes.
Materials and Methods
The PubMed, EMBASE and Cochrane databases were searched from January 2011 to July 2021. The associations between OSA and impaired fasting glucose, impaired glucose tolerance, impaired glucose regulation and diabetes mellitus were analyzed. We estimated the pooled odds ratios using fixed or random effects models. We included 25 studies comprising a total of 154,948 patients with OSA and risk factors for prediabetes/diabetes (20 and 16, respectively) in the analysis.
Results
OSA was associated with a higher risk of impaired fasting glucose, impaired glucose tolerance, impaired glucose regulation and diabetes mellitus in the cohort studies and cross‐sectional studies. The pooled odds ratios were 2.34 (95% confidence interval [CI] 1.16–4.72), 1.58 (95% CI 1.15–2.15), 1.65 (95% CI 1.12–2.42), 2.15 (95% CI 1.68–2.75) and 3.62 (95% CI 2.75–4.75), respectively. Subgroup analyses were based on the proportions of men and women. The results showed that OSA was a risk factor, and there was no significant difference between the two groups. The risk of diabetes increased with the severity of OSA.
Conclusions
The risk of developing prediabetes and diabetes was higher in patients with OSA.
Keywords: Diabetes, Obstructive sleep apnea, Prediabetes
Our study provides further evidence that obstructive sleep apnea is closely correlated with prediabetes and diabetes risk; the prevalence of prediabetes/diabetes in patients with obstructive sleep apnea was higher than that of patients without obstructive sleep apnea.

INTRODUCTION
People spend nearly one‐third of their lives sleeping. Physiological functions and energy are restored through sleep. Therefore, adequate quality sleep is essential for physical and mental health.
Obstructive sleep apnea (OSA), a common and treatable form of sleep‐disordered breathing, is characterized by repetitive episodes of airway closure or partial upper airway collapse during sleep, resulting in chronic intermittent hypoxia and sleep fragmentation 1 . There is increasing evidence that sleep‐disordered breathing is linked to an elevated risk of prediabetes (including impaired fasting glucose [IFG], impaired glucose tolerance [IGT], and IFG plus IGT and diabetes).
Patients with IFG with or without IGT are considered ‘prediabetics.’ A prospective cohort study showed that prediabetes was strongly associated with the development of type 2 diabetes mellitus 2 . Many previous studies have shown an association between OSA and prediabetes. Previous studies have found that the prevalence of prediabetes was significantly higher in patients with OSA, especially in the moderate and severe OSA groups (estimated at 20–59.4%) 3 , 4 , 5 . In contrast, a few studies have shown that not all patients with OSA have a higher prediabetes risk 6 . The results have been inconsistent, warranting further studies.
Currently, the association of OSA and the development of diabetes is gaining increased attention compared with traditional risk factors, such as obesity and family history. Longitudinal studies have shown that the presence of OSA is associated with an increased risk of developing diabetes, even after adjusting for adiposity 7 , 8 . However, contradictory results have been reported on the associations of OSA with prediabetes risk in previous studies, and a meta‐analysis on this topic has not been published yet. Therefore, we carried out a meta‐analysis of all studies that reported a relationship between OSA and prediabetes/diabetes, and compared the prevalence rates among people with and without OSA.
MATERIALS AND METHODS
Study selection
We identified all published studies that evaluated the association between OSA and the incidence and prevalence rates of prediabetes and diabetes. Two investigators independently selected studies published from 2011 to July 2021. Disagreements between the two reviewers were resolved by consensus and discussion with a third person. We searched electronic databases, including PubMed, EMBASE and Cochrane, using the following key terms: ‘sleep apnea’ or ‘obstructive sleep apnea’ or ‘obstructive sleep apnea syndrome’ or ‘OSA’ or ‘sleep disordered breathing’ or ‘SDB’ and ‘diabetes’ or ‘diabetes mellitus’ or ‘DM’ or ‘prediabetes’ or ‘fasting glucose’ or ‘impaired fasting glucose’ or ‘IFG’ or ‘impaired glucose tolerance’ or ‘IGT’ or ‘impaired glucose regulation’ or ‘IGR’ and MeSH terms [Sleep Apnea, Obstructive], [Sleep Apnea Syndromes], [Sleep Wake Disorders], [Respiration], [Glucose Metabolism Disorders], [Diabetes Mellitus, Type 2], [Diabetes Mellitus], [Glucose Intolerance] and [Prediabetic State]. Only studies carried out with human participants were included. No language restriction was imposed.
Inclusion and exclusion criteria
We included studies in this meta‐analysis that met the following inclusion criteria: (i) included patients that were divided into OSA and non‐OSA (as a control) groups, and all patients were aged at least 18 years; (ii) the outcome of interest included the incidence or prevalence of prediabetes with or without type 2 diabetes mellitus; (iii) studies that presented corresponding data for calculations; (iv) the diagnosis of OSA was evaluated by a portable recorder, polysomnography or International Classification of Diseases codes; and (v) the diagnosis of prediabetes and type 2 diabetes mellitus was based either on a 75‐g oral glucose tolerance test or a physician diagnosis with the use of antidiabetic medications.
We excluded articles that: (i) only used self‐reported parameters, such as snoring or Epworth Sleepiness Scale to assess OSA; (ii) only used questionnaires or self‐reported events to define prediabetes or type 2 diabetes mellitus; (iii) lacked data crucial to our analysis; (iv) were reviews, commentaries or letters; and (v) included pregnant or lactating women.
OSA assessment
The presence of OSA was classified according to included studies, except for the self‐reported diagnosis of OSA. The major indicator for OSA severity was the apnea‐hypopnea index (AHI). The AHI was defined as the number of apneas and hypopneas per hour of sleep. Patients were classified as no (AHI <5), mild (AHI of 5–14.9), moderate (AHI of 15–30) or severe (AHI ≥30) OSA 9 . Therefore, we further analyzed the association of OSA with type 2 diabetes mellitus according to AHI severity.
Diabetes mellitus assessment
The presence of prediabetes and diabetes was also based on included studies according to the American Diabetes Association or World Health Organization criteria. Diagnostic methods include 75‐g oral glucose tolerance test, fasting plasma glucose, hospital admissions records and with or without the use of oral medications/insulin.
Data extraction
Data extraction was carried out by two investigators. Disagreement was resolved with a third investigator (Jin Tan). We collected the following factors using a standardized data extraction method: leading authors, publication year, country of origin, sample size, mean age, mean body mass index, number of men and women, assessment of OSA, assessment of diabetes and/or prediabetes, duration of follow up, amount of case/total, quality assessment, and adjustment factors.
Quality assessment
We used the Newcastle–Ottawa Scale 10 for methodological quality assessment of the cohort studies. Two investigators evaluated each study independently and a consensus was reached with an involvement of the third investigator. We appraised three characteristics for the cohort studies: four items for the selection, one item for the comparability of study groups and three items for the outcome of interest. Each numbered item could be awarded a maximum of one star within the selection combined with outcome categories. A maximum of two stars could be given for comparability. A total score greater than six stars was considered a high‐quality paper, with a maximum of nine stars.
Quality assessment of the cross‐sectional and case–control studies was assessed using the Agency for Healthcare Research and Quality 11 . The tool evaluates the risk of bias of individual studies from selection bias, performance bias, attrition bias, detection bias and reporting bias. Each of the bias domains contains different items. Nine of these items apply to the assessment of cross‐sectional and case–control studies. Each item was judged as ‘yes,’ ‘no’ or ‘unclear.’
Statistical analysis
We used statistical software (Stata 12.0; StataCorp, College Station, TX, USA) to pool data. We extracted data (events/total) from the OSA group and non‐OSA group, and input into a data matrix. The odds ratio (OR) was calculated to assess the relationship between OSA and the presence of prediabetes/diabetes. A 95% confidence interval (CI) was used to estimate the scope of the overall parameters. Heterogeneity in the included studies was evaluated using the Cochran’s Q and corresponding P‐value, and a substantial level of heterogeneity was evaluated by the I 2 statistic. The studies were homogeneous if I 2 was <50% and P > 0.05, so a fixed effects model was reported. In contrast, if I 2 was ≥50% and P < 0.05, a random effects model was reported 12 .
Sensitivity analysis was carried out by removing each study in turn to re‐estimate the effect size and its contribution. General funnel plots and Egger tests 13 , 14 were used to evaluate publication bias. A P‐value ≤0.05 was considered statistically significant.
RESULTS
Study characteristics
We identified 3,436 studies from the PubMed, EMBASE and Cochrane databases. A total of 1,701 studies were excluded after reviewing titles/abstracts or because they were duplicates. The remaining 1,735 studies were further screened, and 32 were selected for full‐text review. In the full‐text assessment for final inclusion, seven studies were excluded because: (i) data could not be extracted (n = 1) 15 ; (ii) the outcome was accessed only by self‐reports or questionnaires (n = 2) 16 , 17 ; and (iii) different definition criteria (n = 4) 18 , 19 , 20 , 21 . Finally, 25 studies with a total of 154,948 participants met our inclusion criteria (Figure 1). The summary characteristics of all included studies in the present meta‐analysis are listed in Table 1. Eight were cohort studies 6 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 14 were cross‐sectional studies 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 and three were case–control studies 3 , 43 , 44 . A meta‐analysis of each study design was carried out separately. The range of enrollment periods for the included studies was 2011–2021. The sample size ranged widely from 76 to 100,914. Regarding study classifications, most of the studies included both female and male participants, except for three studies that included only men 3 , 22 , 24 and one study that included only women 36 . Eight studies reported OSA and IFG risk 3 , 6 , 31 , 36 , 37 , 40 , 41 , 42 ; 10 reported OSA and IGT risk 3 , 6 , 31 , 32 , 35 , 36 , 37 , 40 , 41 , 43 ; five reported IFG and/or IGT risk 6 , 30 , 33 , 34 , 40 ; and 19 reported OSA and type 2 diabetes mellitus risk 6 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 33 , 34 , 35 , 37 , 38 , 39 , 42 , 44 . Eight studies reported an association of OSA with type 2 diabetes mellitus according to AHI severity 23 , 26 , 28 , 29 , 30 , 33 , 34 , 39 .
Figure 1.
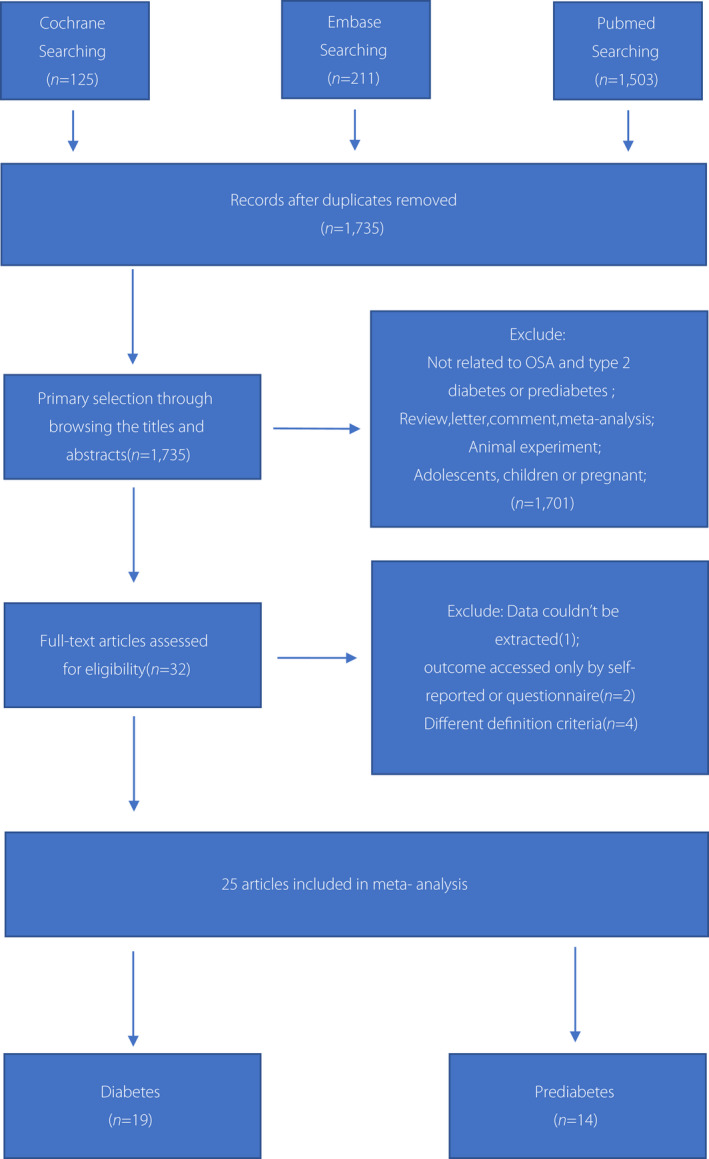
Preferred Reporting Items of Systematic Reviews and Meta‐Analysis (PRISMA) flow diagram showing the process of study selection. The literature search of databases yielded 1,839 records. After title/abstract and full‐text screening, 25 articles were included in the systematic review and meta‐analysis. OSA, obstructive sleep apnea.
Table 1.
Baseline characteristics of all studies included in the review
| Author (year) | Country | n | OSA cases/total | Non‐OSA cases/total | Mean age (years) | Mean BMI (kg/m2) | Male/female | Sleep assessment | DM assessment | DM criteria | Prediabetes/diabetes | Follow up (years) | NOS | Adjustment factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort studies n = 8 | ||||||||||||||
|
KIM 6 2013 |
Korea | 1,344 | 246/625 | 150/719 | 57.7 | 24.8 | 706/638 | PSG | OGTT | ADA | IFG, IGT, IGR, DM | 4 | 9 |
Age, sex, alcohol use, smoking exercise, HTN CVD, medication for dyslipidemia, WC, VFA |
|
Appleton 22 2015 |
Australia | 736 | 41/374 | 25/362 | 59.7 | 28.4 | 736/0 | PSG | FPG ≥7.0 mmol/L or HbA1c ≥6.5% or physician diagnosis or national Pharmaceutical Benefits Scheme data record | NR | T2DM | 4.7 | 8 | Age, ESS, body fat percentage, education, income, marital status, medication use, shift work, change in WC≥5 cm |
|
Kendzerska 23 2014 |
Canada | 8,678 | 836/6,719 | 166/1,959 |
CG 42.0 Mild 47.0 Mod 50.0 Severe51.0 |
25.8 27.8 28.8 31.1 |
5,377/3,301 | PSG | Ontario Diabetes Database (ODD) | ICD9‐CM250 | DM | 5.58 | 9 | Age, sex, BMI, WC, smoking, comorbidities, income |
| Liu 25 2017 | Taiwan | 100,914 | 653/9,174 | 4,203/91,740 | ≥18 | † | 64,834/36,080 | PSG | Inpatient diagnosis or at least three outpatient diagnoses within 1 year | ICD9‐CM250 | T2DM | 12 | 7 | Age, sex, metabolic factors |
| Naga 26 2016 | USA | 1,453 | 155/688 | 130/765 | 63 | 28.3 | 675/778 | Respiratory monitoring | Physician‐diagnosed diabetes or diabetes medication use | FPG ≥126 mg/dL or non‐FPG of ≥200 mg/dL | DM | 13 | 8 | Age, sex, education, income, occupation, marital status, smoking, alcohol use, exercise, BMI, WC |
|
Strausz 27 2018 FINRISK |
Finland | 28,953 | 250/1,214 | 2,231/27,739 | 48.01 | 26.74 | 13,792/15,161 | ICD codes | Hospital discharges, causes‐of‐deaths register or entitlement to a reimbursed diabetes medication | NR | T2DM | 22 | 9 | Age, sex, geographical area, cohort year, BMI |
|
Strausz 27 2018 H2000 |
Finland | 6,605 | 45/235 | 411/6,370 | 53.8 | 26.9 | 2,940/3,665 | ICD codes | Hospital discharges, causes‐of‐deaths register or entitlement to a reimbursed diabetes medication | NR | T2DM | 14.5 | 9 | Age, sex, geographical area, cohort year, BMI |
|
Xu 28 2019 |
Hong Kong | 1,206 | 136/893 | 16/313 | 51 | 26.9 | 832/374 | PSG | Physician diagnosis or glycemic indices | ADA | T2DM | 7.34 | 9 | Age, sex, BMI, bodyweight change, WC, smoking, alcohol use, family history of T2DM, ESS, comorbidities |
|
Lindberg 24 2012 |
Sweden | 141 | 16/71 | 7/70 | 57.5 | 26.9 | 141/0 | Respiratory monitoring | OGTT | WHO | DM | 11.3 | 8 | Age, BMI, hypertension |
| Author (year) | Country | n | OSA cases/total | Non‐OSA cases/total | Mean age (years) | Mean BMI (kg/m2) | Male/female | Sleep assessment | DM assessment | DM criteria | Prediabetes/diabetes | AHRQ | Adjustment factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross sectional studies n = 14 | |||||||||||||
|
Bikov 29 2020 |
Hungary | 394 | 60/282 | 12/112 | 58 | 32.5 | 288/106 | PSG | Blood samples | NR | DM | 7 | Age, sex, BMI, ESS |
|
Bozkurt 30 2012 |
Turkey | 47 | 23/143 | 1/47 |
CG 42.79 ± 9.55 Mild 47.78 ± 10.35 MOD 49.79 ± 10.62 severe 49.66 ± 10.38 |
29.24 ± 9.55 29.03 ± 4.12 30.76 ± 5.09 33.41 ± 4.64 |
141/96 | PSG | OGTT | ADA | IGR, T2DM | 6 | Sex, AHI |
|
Bulcun 31 2012 |
Turkey | 131 | 16/112 | 0/19 |
CG 45.89 ± 9.6 OSA 48.0 ± 10.1 |
28.8 ± 4.1 30.9 ± 4.6 |
101/30 | PSG | OGTT | WHO |
IFG, IGT, T2DM |
6 | Age, sex, BMI, severity of OSA, arousal index, drowsiness |
|
EI 32 2016 |
Italy | 163 | 83/126 | 17/37 |
CG 41.3 OSA 48.2 |
40 45.4 |
NR | PSG | OGTT | NR | IGT | 5 | NR |
|
Feng 33 2015 |
China | 180 | 78/140 | 12/40 | 45.4 ± 10.5 | 27.5 ± 4.1 | 162/18 | PSG | OGTT | WHO | IGR, T2DM | 7 | Neck circumference |
|
Fredheim 34 2011 |
Norway | 137 | 71/84 | 27/53 | 43 | 46.9 | 36/101 | Portable monitors | OGTT | ADA | IGR, T2DM | 8 | Age, sex, BMI (WC, neck circumference, WHR), HOMA‐IR, hs‐CRP |
|
Gasa 35 2011 |
Spain | 159 | 52/115 | 14/44 | 43 ± 10 | 46.1 ± 5.8 | 44/115 | PSG | OGTT |
DM: PBG ≥11.1 mmol/L IGT: PBG 7.8–11.1 mmol/L |
IGT, T2DM | 8 | Age, sex, smoking, BMI, WC |
|
Gilardini 36 2013 |
Italy | 98 | 7/54 | 7/44 |
PM CG 37.5 ± 9.5 PM OSA 41.7 ± 8.0 M CG 58.5 ± 8.4 M OSA 62.9 ± 6.1 |
36.0 ± 3.4 39.5 ± 4.8 33.5 ± 3.3 36.8 ± 3.7 |
0/98 | PSG | OGTT | ADA | IFG, IGT | 6 |
Age, BMI, WHR, NHR, FM/FFM FPG, 2hPG, ISI |
|
Gu 37 2013 |
China | 179 | 59/120 | 15/59 |
CG 26.25 ± 10.98 mild to MOD35.17 ± 14.68 severe 40.56 ± 13.52 |
34.19 ± 5.65 31.92 ± 5.42 33.89 ± 6.55 |
138/41 | PSG | OGTT | WHO | IFG, IGT, DM | 8 | Age, sex, BMI, smoking, alcohol use |
|
GU 38 2016 |
China | 106 | 13/59 | 2/47 |
CG 23.1 ± 6.2 OSA 26.0 ± 7.1 |
36.0 ± 4.7 37.2 ± 5.3 |
74/32 | PSG | OGTT | WHO | T2DM | 7 | Age, sex, BMI, hypertension |
|
Hasan 39 2012 |
India | 290 | 138/234 | 13/56 |
CG 52 ± 8 OSA 54 ± 11 |
29 ± 4 36 ± 6 |
225/65 | PSG | medical questionnaire or physician | NR | DM | 7 | BMI, hypertension, smoking, alcohol use |
|
Li 40 2020 |
China | 422 | 107/257 | 40/165 | 27.77 ± 7.51 | 34.84 ± 5.69 | 261/161 | PSG | OGTT |
IFG: FPG ≥6.1 mmol/L and <7.0 mmol/L, and 2hPG v<7.8 mmol/L. IGT: FPG <6.1 mmol/L, and 2hPG ≥7.8 mmol/L and <11.1mmol/L. IGR: FPG ≥6.1 mmol/L and <7.0 mmol/L, and 2hPG ≥7.8 mmol/L and <11.1 mmol/L. |
IFG, IGT, IGR | 7 | Age, sex, BMI, neck circumference, smoking |
|
Michalek 41 2021 |
Poland | 102 | 18/85 | 4/17 | 53.02 ± 12.37 | 30.50 ± 6.29 | 71/31 | PSG | Blood samples | NR | IFG, IGT | 6 | Age, AHI, ODI, BMI |
|
Togeiro 42 2013 |
Brazil | 1042 | 173/396 | 139/646 |
CG 37.3 ± 12.3 Mild 48.3 ± 13.6 MOD 53.3 ± 13.3 |
25.3 ± 4.4 28.3 ± 5.2 30.3 ± 6.0 |
466/576 | PSG | Blood samples | DM: FPG ≥126 mg/dL, use of diabetes medications or a previous diagnosis of diabetes. IFG: glucose serum value was≥100 mg/dL | IFG, T2DM | 8 | Age, sex, abdominal obesity (WC ≥88 cm for women and ≥102 cm for men), total sleep time |
| Case–control studies n = 3 | |||||||||||||
|
Bozic 3 2016 |
Croatia | 76 | 24/56 | 3/20 |
CG 52.5 ± 9.0 MOD 54.1 ± 10.9 Severe51.2 ± 11.8 |
28.3 ± 3.1 28.8 ± 2.6 29.8 ± 3.1 |
76/0 | PSG | OGTT | ADA | IFG, IGT | 8 | Subjects in both groups have similar BMI and abdominal circumference. |
|
Papaioannou 43 2011 |
UK | 105 | 37/68 | 12/37 |
CG 45 OSA 49 |
28 30 |
NR | PSG | OGTT | ADA | IGT | 7 | Age, BMI, AHI |
|
Silva 44 2018 |
Brazil | 120 | 12/85 | 2/35 |
CG 36 ± 9 OSA 47 ± 9 |
24 ± 3 28 ± 4 |
59/61 | PSG | Blood samples | FPG ≥126 mg/dL, use of DM medications. | DM | 8 | Age, sex, BMI, WC |
Baseline characteristics of the included studies. †BMI not measured in the original text, obesity‐related cardiometabolic variables were used. 2hPG, 2‐h plasma glucose; ADA, American Diabetes Association; AHI, apnea hypopnea index; BMI, body mass index; CG, control group; CVD, cardiovascular disease; DM, diabetes; ESS, Epworth Sleepiness Scale; FM/FFM, fat mass/fat free mass ratio; FPG, fasting glucose; HbA1c, glycated hemoglobin; HOMA‐IR, homeostasis model assessment of insulin resistance; hs‐CRP, high sensitivity CRP; HTN, hypertension; ICD codes, International Classification of Diseases; IFG, impaired fasting glucose; IGR, impaired glucose regulation; IGT, impaired glucose tolerance; ISI, insulin sensitivity index; M, menopausal women; MOD, moderate; NHR, neck/height ratio; NOS, Newcastle–Ottawa Scale; NR, not reported; ODI, oxygen de‐saturation index; OGTT, oral glucose tolerance test; OSA, obstructive sleep apnea; PBG, post‐load glucose; PM, premenopausal women; PSG, polysomnography; T2DM, type 2 diabetes; VFA, visceral fat area; WC, waist circumference; WHO, World Health Organization; WHR, waist‐to‐hip ratio.
Association of obstructive sleep apnea with prediabetes
A meta‐analysis for each category (IFG, IGT, IFG + IGT) was carried out separately.
Obstructive sleep apnea with impaired fasting glucose
Eight articles 3 , 6 , 31 , 36 , 37 , 40 , 41 , 42 met the required criteria (OSA n = 1,705; non‐OSA n = 1,689). Because there was only one cohort and one case–control study, a meta‐analysis was carried out for the six cross‐sectional studies 31 , 36 , 37 , 40 , 41 , 42 .
The pooled OR of OSA and the prevalence of IFG was 2.34 (95% CI 1.16–4.72, I 2 = 27.7%, P = 0.227). There was low heterogeneity in the meta‐analysis of overall events, suggesting a consistent disease effect (Figure 2). The six studies were further divided into two groups according to the proportions of men and women (male ≤ female groups and male > female groups). The pooled OR of male ≤ female groups (n = 3) and the prevalence of IFG was 3.80 (95% CI 0.99–14.54, I 2 = 50.3%, P = 0.134). The pooled OR of male > female groups (n = 3) and the prevalence of IFG was 1.77 (95% CI 0.45–7.02, I 2 = 29.4%, P = 0.243). Although there were some signs of asymmetry in the funnel plot 45 (Figure S1), the Egger (P = 0.314) tests showed no publication bias 45 (Figure S2).
Figure 2.
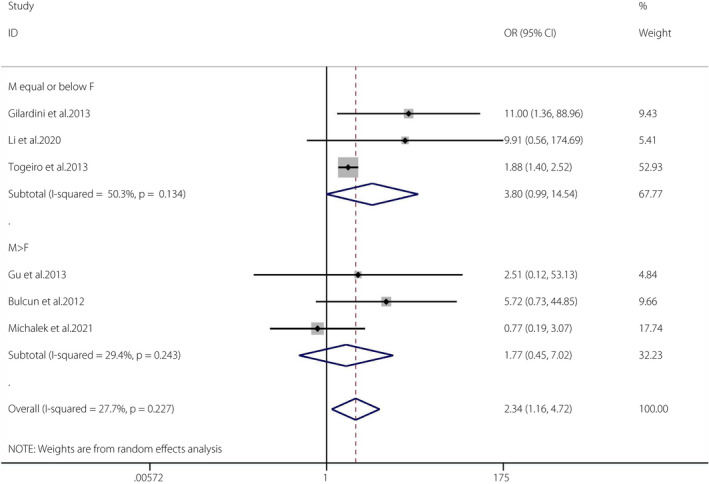
Obstructive sleep apnea and the prevalence of impaired fasting glucose. A forest plot illustrating the meta‐analysis results of the prevalence of impaired fasting glucose in people with obstructive sleep apnea and non‐obstructive sleep apnea. CI, confidence interval; F, female; M, male.
OSA with IGT
A total of 10 articles 3 , 6 , 31 , 32 , 35 , 36 , 37 , 40 , 41 , 43 met the required criteria (OSA n = 1618; non‐OSA n = 1,161). Because there was only one cohort and two case–control studies, a meta‐analysis was carried out for the seven cross‐sectional studies 31 , 32 , 35 , 36 , 37 , 40 , 41 .
The pooled OR of OSA and the prevalence of IGT was 1.58 (95% CI 1.15–2.15, I 2 = 0%, P = 0.805). There was no heterogeneity in the meta‐analysis of overall events, suggesting a consistent disease effect (Figure 3). The seven studies were further divided into two groups according to the proportions of men and women (male ≤ female groups and male > female groups). The pooled OR of male ≤ female groups (n = 3) and the prevalence of IGT was 1.50 (95% CI 1.02–2.23, I 2 = 0%, P = 0.412). The pooled OR of male > female groups (n = 4) and the prevalence of IGT was 1.71 (95% CI 1.02–2.87, I 2 = 0%, P = 0.775). The funnel plot 45 (Figure S3) and the Egger (P = 0.165) tests showed no publication bias 45 (Figure S4).
Figure 3.
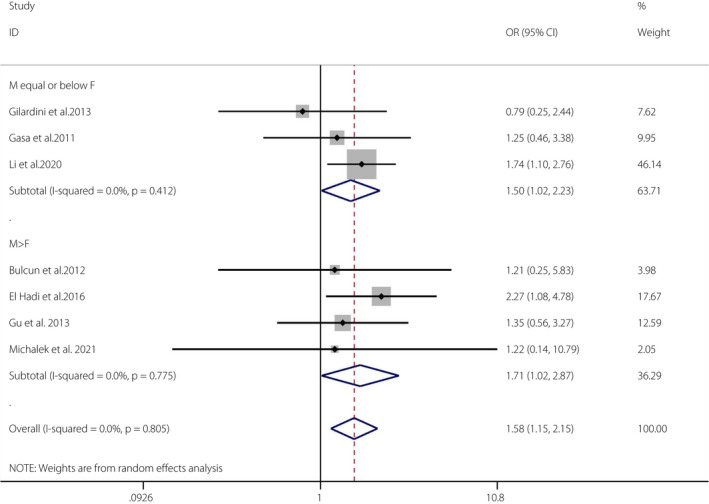
Obstructive sleep apnea and the prevalence of impaired glucose tolerance. A forest plot illustrating the meta‐analysis results of the prevalence of impaired glucose tolerance in people with obstructive sleep apnea and non‐obstructive sleep apnea. CI, confidence interval; F, female; M, male.
OSA with impaired glucose regulation
Five articles 6 , 30 , 33 , 34 , 40 met the required criteria (OSA: n = 1,249; non‐OSA: n = 1,024). Because there was only one cohort, a meta‐analysis was carried out for the four cross‐sectional studies 33 , 34 , 40 .
The OR of OSA and the prevalence of impaired glucose regulation (IGR) was 1.65 (95% CI 1.12–2.42, I 2 = 0%, P = 0.859). There was no heterogeneity in the meta‐analysis of overall events, suggesting a consistent disease effect (Figure 4). The four studies were further divided into two groups according to the proportions of men and women (male ≤ female groups and male > female groups). The pooled OR of male ≤ female groups (n = 2) and the prevalence of IGR was 1.93 (95% CI 1.09–3.39, I 2 = 0%, P = 0.694). The pooled OR of male > female groups (n = 2) and the prevalence of IGR was 1.44 (95% CI 0.86–2.43, I 2 = 0%, P = 0.795). The funnel plot 45 (Figure S5) and the Egger (P = 0.213) tests showed no publication bias 45 (Figure S6).
Figure 4.
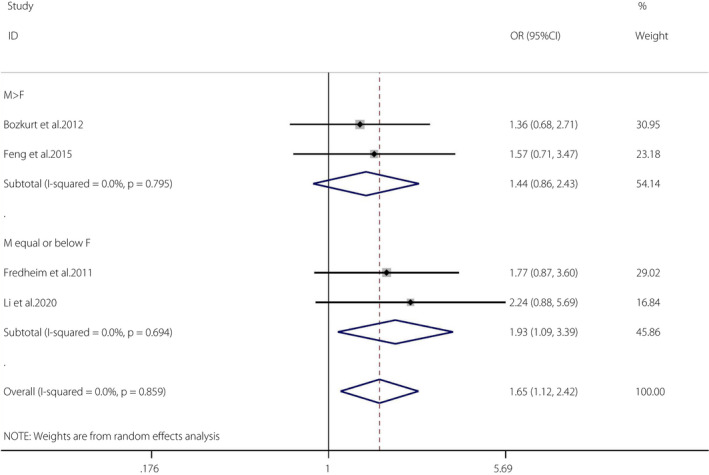
Obstructive sleep apnea and the prevalence of impaired glucose regulation. A forest plot illustrating the meta‐analysis results of the prevalence of impaired glucose regulation in people with obstructive sleep apnea and non‐obstructive sleep apnea. CI, confidence interval; F, female; M, male.
OSA and diabetes mellitus risk
A total of 19 studies 6 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 33 , 34 , 35 , 37 , 38 , 39 , 42 , 44 that evaluated OSA and the incidence of diabetes were included in the present meta‐analyses (OSA n = 49,925; non‐OSA n = 417,175). Because eight were cohort studies 6 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 10 were cross‐sectional studies 29 , 30 , 31 , 33 , 34 , 35 , 37 , 38 , 39 , 42 and one was a case–control 44 study, the meta‐analysis was mainly carried out using the eight cohort and 10 cross‐sectional studies.
The pooled OR of OSA and the incidence of type 2 diabetes mellitus in the cohort studies was 2.15 (95% CI 1.68–2.75, I 2 = 90.4%, P < 0.001; Figure 5). We carried out a sensitivity analysis, and the outcome was unchanged when any study was excluded from the meta‐analysis. Then, we checked the design of each study and found that the assessment of OSA was carried out in participants’ homes rather than at a sleep laboratory in the study by Naga et al. 26 ; patients were selected from a medical center rather than a community in the study by Kend et al. 23 , and individuals matched by sex and year were chosen as a control group in the study by Liu et al. 25 . The pooled results become homogeneous after removing these studies (OR 2.77, 95% CI 2.35–3.27; random effects model). Thus, we considered this the source of heterogeneity. Eight studies were further divided into two groups according to the proportions of men and women (male ≤ female groups and male > female groups). The pooled OR of male ≤ female groups (n = 3) and the incidence of diabetes mellitus was 2.43 (95% CI 1.46–4.06, I 2 = 92.6%, P < 0.001). The pooled OR of male > female groups (n = 6) and the incidence of diabetes mellitus was 1.92 (95% CI 1.55–2.38, I 2 = 74%, P = 0.002). The Egger tests (P = 0.213) showed no publication bias 45 (Figures S7, S8).
Figure 5.
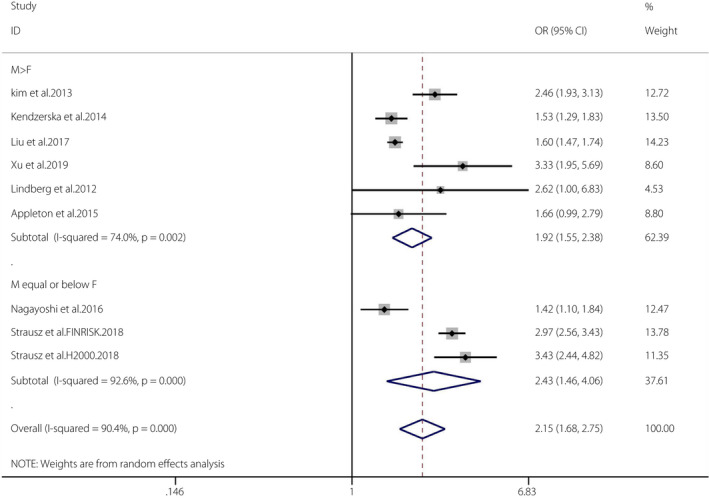
Obstructive sleep apnea and the incidence of diabetes mellitus in cohort studies. A forest plot illustrating the meta‐analysis results of the incidence of diabetes mellitus in people with obstructive sleep apnea and non‐obstructive sleep apnea. CI, confidence interval; F, female; M, male.
The pooled OR of OSA and the prevalence of diabetes mellitus in the cross‐sectional studies was 3.62 (95% CI 2.75–4.75, I 2 = 0%, P = 0.472). There was no heterogeneity in the meta‐analysis of overall events, suggesting a consistent disease effect (Figure 6). A total of 10 studies were further divided into two groups according to the proportions of men and women (male ≤ female groups and male > female groups). The pooled OR of male ≤ female groups (n = 3) and the prevalence of diabetes mellitus was 3.21 (95% CI 1.73–5.96, I 2 = 53.2%, P = 0.118). The pooled OR of male > female groups (n = 7) and the prevalence of DM was 3.65 (95% CI 2.50–5.32, I 2 = 0%, P = 0.627). The Egger tests (P = 0.314) showed no publication bias 45 (Figures S9, S10).
Figure 6.
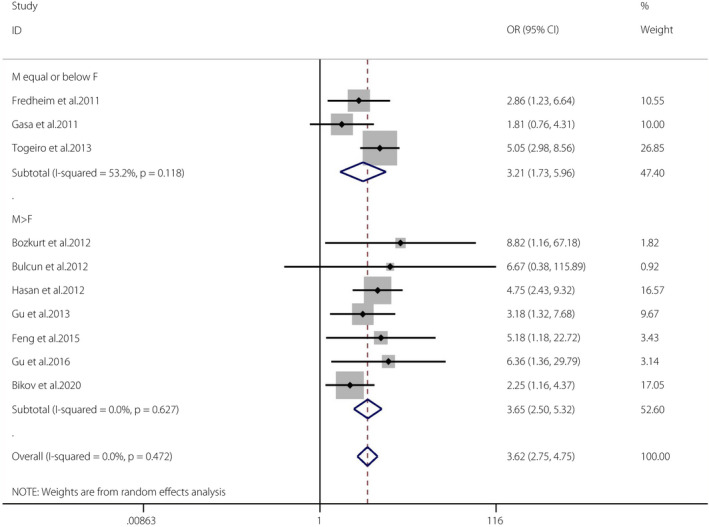
Obstructive sleep apnea and the incidence of diabetes mellitus in cross‐sectional studies. A forest plot illustrating the meta‐analysis results of the prevalence of diabetes mellitus in people with obstructive sleep apnea and non‐obstructive sleep apnea. CI, confidence interval; F, female; M, male.
Subgroup analyses
Subgroup analyses were carried out to assess the relationships between OSA severity and the prevalence or incidence of diabetes mellitus. Eight studies divided OSA participants into mild (AHI of 5–14.9), moderate (AHI of 15–30) and severe (AHI ≥30) groups 23 , 26 , 28 , 29 , 30 , 33 , 34 , 39 . Three were cohort studies 23 , 26 , 28 , and five were cross‐sectional studies 29 , 30 , 33 , 34 , 39 ; a meta‐analysis for each study type was carried out separately.
In the cohort studies, the pooled OR of the mild OSA group versus the control group and the incidence of type 2 diabetes mellitus was 1.25 (95% CI 1.06–1.48, I 2 = 0%, P = 0.416). The pooled OR of the moderate OSA group versus the control group and the incidence of type 2 diabetes mellitus was 1.76 (95% CI 1.16–2.67, I 2 = 72.3%, P = 0.027). The pooled OR of the severe OSA group versus the control group and the incidence of type 2 diabetes mellitus was 2.73 (95% CI 1.72–4.33, I 2 = 74.2%, P = 0.021; Figure 7). The Egger tests (P = 0.136) showed no publication bias 45 (Figures S11, S12).
Figure 7.
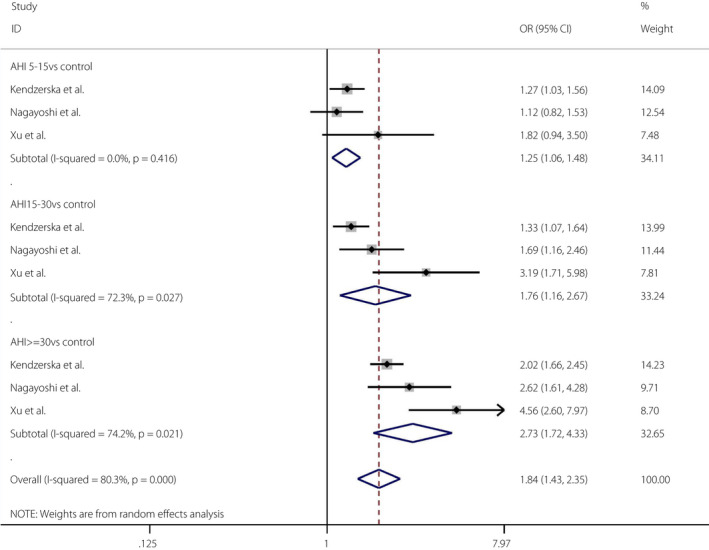
Obstructive sleep apnea severity and the incidence of diabetes mellitus. A forest plot showing the meta‐analysis results of the incidence of diabetes mellitus in people with the severity of obstructive sleep apnea. AHI, apnea‐hypopnea index; CI, confidence interval; F, female; M, male.
In the cross‐sectional studies, the pooled OR of the mild OSA group versus the control group and the prevalence of type 2 diabetes mellitus was 2.88 (95% CI 1.55–5.36, I 2 = 38.3%, P = 0.166). The pooled OR of the moderate OSA group versus the control group and the prevalence of type 2 diabetes mellitus was 3.08 (95% CI 1.90–5.01, I 2 = 0%, P = 0.792). The pooled OR of the severe OSA group versus the control group and the prevalence of type 2 diabetes mellitus was 4.12 (95% CI 2.56–6.65, I 2 = 0%, P = 0.478; Figure 8). The Egger tests (P = 0.241) showed no publication bias 45 (Figures S13, S14).
Figure 8.
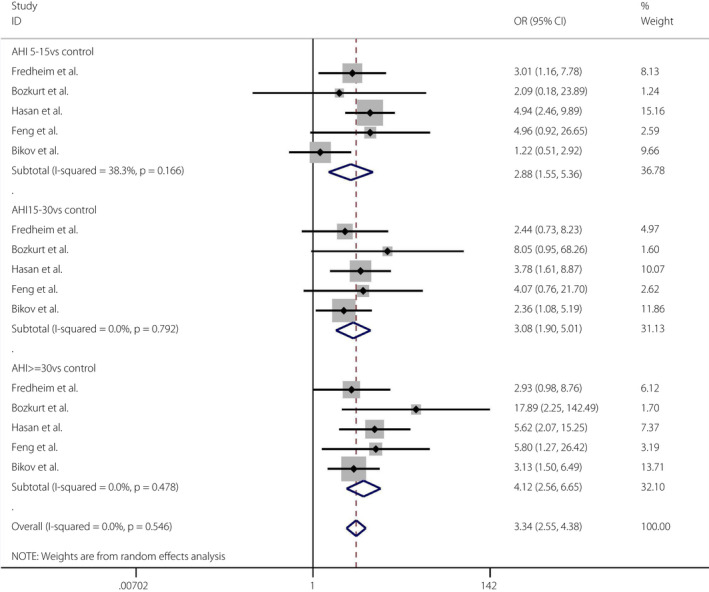
Obstructive sleep apnea severity and the prevalence of diabetes mellitus. A forest plot showing the meta‐analysis results of the prevalence of diabetes mellitus in people with the severity of obstructive sleep apnea. AHI, apnea‐hypopnea index; CI, confidence interval; F, female; M, male.
DISCUSSION
This is the first meta‐analysis of the association between OSA and prediabetes/diabetes risk to date. We collected data from 25 studies, including >100,000 participants. All included studies used gold standards for diagnosing OSA and prediabetes/diabetes. Furthermore, all of the included studies used AHI as the major indicator of OSA severity; therefore, the cut‐off values for the definitions of OSA severity were consistent. For prediabetes, a significant association was identified between OSA and IFG, IGT, and IGR, with low or no heterogeneity. For diabetes, the current meta‐analysis results showed that OSA was associated with increased diabetes risk in the cross‐sectional or cohort studies. Advanced studies that included subgroup analyses showed that differences remained significant when unified by sex. Thus, the main results of the present analysis show that OSA is associated with an increased prevalence of prediabetes/diabetes. Among the patients with OSA, the prevalence of diabetes seemed to increase with increased AHI.
The novelty and strength of the current study were that we focused on the progression of diabetes, emphasized the importance of prediabetes, and first identified the links between OSA and prediabetes. Compared with previous meta‐analyses 46 , the current study analyzed articles from the past 10 years (2011–2021); therefore, the results are relatively new. Furthermore, our inclusion criteria were relatively strict to prevent interference from different diagnostic criteria or methods. For example, the study excluded patients diagnosed by 75‐g oral glucose tolerance test or blood sampling only. OSA had to be diagnosed by polysomnography or International Classification of Diseases codes, and OSA severity had to be measured by AHI, which makes the results more reliable than studies that used only self‐reported diagnostic methods. In addition, sources of heterogeneity were assessed by sensitivity analysis, and the publication bias was also evaluated. Furthermore, all types of observational studies were included, and the meta‐analysis for each study design was carried out separately. Sex was a major confounding factor both in the development of diabetes and evaluation of OSA; therefore, we carried out subgroup analyses. Finally, the association of OSA severity with diabetes risk was further analyzed according to AHI severity.
The limitations of the current meta‐analysis must be considered. First, the cross‐sectional nature of the included studies might have prevented any definitive causal inferences between OSA and prediabetes/diabetes. However, the present results suggest that prediabetes/diabetes are more frequent in patients with OSA, and that these patients should be routinely screened. Second, most of the cross‐sectional studies recruited participants from specialized clinics, which limits the generalizability of their findings. Third, there were not enough cohort studies regarding OSA and different categories of prediabetes to assess whether OSA confers a greater future risk of developing prediabetes. In addition, no studies evaluated whether OSA affected the rate of progression of prediabetes to type 2 diabetes mellitus, which also provides the basis for future research. Fifth, because some articles are grouped by age range, and some use the average value, subgroup analyses were not carried out. Similarly, the weight range of the participants was inconsistent, including those for obese and normal‐weight people, and the distribution standards of obesity were inconsistent. Therefore, we were unable to carry out subgroup analyses of obesity‐related factors. However, most studies adjusted for obesity‐related factors, such as body mass index and waist circumference. The results showed that OSA remained a high‐risk factor for prediabetes/diabetes after adjusting for obesity.
Given that the incidence of prediabetes is increasing at an alarming rate 47 , it is crucial to halt the progression from prediabetes to diabetes and focus on patients with prediabetes as the key to preventing diabetes. Early screening and interventions can significantly reduce the incidence of diabetes. Insulin resistance and impaired pancreatic β‐cell function are the two main features involved in the pathogenesis of type 2 diabetes mellitus 48 . As the first‐line treatment for symptomatic OSA, continuous positive airway pressure treatment could help improve this question 49 , as it has been showed that OSA has a causal relationship with abnormal glucose tolerance. Furthermore, OSA is an independent risk factor for cardiovascular disease 50 . Patients with multiple sleepiness‐related symptoms and very high Epworth Sleepiness Scale scores are more likely to have cardiovascular consequences because of their OSA 50 . The present findings support an association of OSA with the presence of prediabetes/diabetes, and suggest that healthcare providers working in the fields of diabetes and OSA should screen patients presenting with one condition for the presence of the other. Early intervention can prevent both diabetic and cardiovascular events.
In conclusion, the present study provides further evidence that OSA is closely correlated with prediabetes and diabetes risk; the prevalence of prediabetes/diabetes in patients with OSA was higher than that of patients without OSA. Further studies are required to evaluate whether an early diagnosis of OSA in populations with prediabetes/diabetes and dual‐disease management reduces morbidity in this growing segment of the population.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: N/A.
Informed consent: N/A.
Approval date of registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Supporting information
Figure S1 | Funnel plots of obstructive sleep apnea and impaired fasting glucose risk.
Figure S2 | Egger’s test of obstructive sleep apnea and impaired fasting glucose risk.
Figure S3 | Funnel plots of obstructive sleep apnea and impaired glucose tolerance risk.
Figure S4 | Egger’s test of obstructive sleep apnea and impaired glucose tolerance risk.
Figure S5 | Funnel plots of obstructive sleep apnea and impaired glucose regulation risk.
Figure S6 | Egger’s test of obstructive sleep apnea and impaired glucose regulation risk.
Figure S7 | Funnel plots of obstructive sleep apnea and diabetes mellitus risk.
Figure S8 | Egger’s test of obstructive sleep apnea and diabetes mellitus risk.
Figure S9 | Funnel plots of obstructive sleep apnea and diabetes mellitus risk.
Figure S10 | Egger’s test of obstructive sleep apnea and impaired fasting glucose risk.
Figure S11 | Funnel plots of obstructive sleep apnea severity and the incidence of diabetes mellitus.
Figure S12 | Egger’s test of obstructive sleep apnea severity and the incidence of diabetes mellitus.
Figure S13 | Funnel plots of obstructive sleep apnea severity and the prevalence of diabetes mellitus.
Figure S14 | Egger’s test of obstructive sleep apnea severity and the prevalence of diabetes mellitus.
Table S1 | Methodological quality of the selected cohort studies according to the Newcastle–Ottawa Scale.
Table S2 | Methodological quality of the selected cross‐sectional and case control studies according to the Agency for Healthcare Research and Quality.
ACKNOWLEDGMENTS
This work was supported by grants from National Natural Science Foundation of China (No.9216320033, 81970085), the major special project for the prevention and control of chronic diseases in Tianjin (No.17ZXMFSY00080), and Tianjin Key Medical Discipline (Specialty) Construction Project.
J Diabetes Investig. 2022; 13: 1396–1411
REFERENCES
- 1. Kapur VK. Obstructive sleep apnea: diagnosis, epidemiology, and economics. Respir Care 2010; 55: 1155–1167. [PubMed] [Google Scholar]
- 2. Brunisholz KD, Joy EA, Hashibe M, et al. Incidental risk of type 2 diabetes mellitus among patients with confirmed and unconfirmed prediabetes. PLoS One 2016; 11: e0157729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bozic J, Galic T, Supe‐Domic D, et al. Morning cortisol levels and glucose metabolism parameters in moderate and severe obstructive sleep apnea patients. Endocrine 2016; 53: 730–739. [DOI] [PubMed] [Google Scholar]
- 4. Khani S, Tayek JA. Cortisol increases gluconeogenesis in humans: its role in the metabolic syndrome. Clin Sci 2001; 101: 739–747. [DOI] [PubMed] [Google Scholar]
- 5. Wang X, Bi Y, Zhang Q, et al. Obstructive sleep apnoea and the risk of type 2 diabetes: a meta‐analysis of prospective cohort studies. Respirology 2013; 18: 140–146. [DOI] [PubMed] [Google Scholar]
- 6. Kim NH, Cho NH, Yun CH, et al. Association of obstructive sleep apnea and glucose metabolism in subjects with or without obesity. Diabetes Care 2013; 36: 3909–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reichmuth KJ, Austin D, Skatrud JB, et al. Association of sleep apnea and type II diabetes: a population‐based study. Am J Respir Crit Care Med 2005; 172: 1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muraki I, Tanikawa T, Yamagishi K, et al. Nocturnal intermittent hypoxia and the development of type 2 diabetes: the Circulatory Risk in Communities Study (CIRCS). Diabetologia 2010; 53: 481–488. [DOI] [PubMed] [Google Scholar]
- 9. Fleetham J, Ayas N, Bradley D, et al. Canadian Thoracic Society guidelines: diagnosis and treatment of sleep disordered breathing in adults. Can Respir J 2006; 13: 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 11. Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta‐analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015; 8: 2–10. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 13. Egger M, Davey Smith G, Schneider M, et al. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research ed.) 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 15. Cizza G, Piaggi P, Lucassen EA, et al. Obstructive sleep apnea is a predictor of abnormal glucose metabolism in chronically sleep deprived obese adults. PLoS One 2013; 8: e65400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pinto JA, Ribeiro DK, Cavallini AF, et al. Comorbidities associated with obstructive sleep apnea: a retrospective study. Int Arc Otorhinolaryngol 2016; 20: 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tveit RL, Lehmann S, Bjorvatn B. Prevalence of several somatic diseases depends on the presence and severity of obstructive sleep apnea. PLoS One 2018; 13: e0192671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kent BD, Grote L, Ryan S, et al. Diabetes mellitus prevalence and control in sleep‐disordered breathing: the European Sleep Apnea Cohort (ESADA) study. Chest 2014; 146: 982–990. [DOI] [PubMed] [Google Scholar]
- 19. Matsumoto T, Murase K, Tabara Y, et al. Impact of sleep characteristics and obesity on diabetes and hypertension across genders and menopausal status: the Nagahama study. Sleep 2018; 41: 1–10. 10.1093/sleep/zsy071 [DOI] [PubMed] [Google Scholar]
- 20. Breuer H‐WM. Hypertension, metabolic syndrome, prediabetes, and diabetes associated with obstructive sleep apnea syndrome. Somnologie ‐ Schlafforschung und Schlafmedizin 2011; 15: 14–23. 10.1007/s11818-011-0503-3 [DOI] [Google Scholar]
- 21. Byun JI, Cha KS, Jun JE, et al. Dynamic changes in nocturnal blood glucose levels are associated with sleep‐related features in patients with obstructive sleep apnea. Sci Rep 2020; 10: 17877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Appleton SL, Vakulin A, McEvoy RD, et al. Nocturnal hypoxemia and severe obstructive sleep apnea are associated with incident type 2 diabetes in a population cohort of men. J Clin Sleep Med 2015; 11: 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kendzerska T, Gershon AS, Hawker G, et al. Obstructive sleep apnea and incident diabetes. a historical cohort study. Am J Respir Crit Care Med 2014; 190: 218–225. [DOI] [PubMed] [Google Scholar]
- 24. Lindberg E, Theorell‐Haglöw J, Svensson M, et al. Sleep apnea and glucose metabolism: a long‐term follow‐up in a community‐based sample. Chest 2012; 142: 935–942. [DOI] [PubMed] [Google Scholar]
- 25. Liu CL, Wu CS. Assessing whether the association between sleep apnea and diabetes is bidirectional. Can J Diabetes 2017; 41: 197–203. [DOI] [PubMed] [Google Scholar]
- 26. Nagayoshi M, Punjabi NM, Selvin E, et al. Obstructive sleep apnea and incident type 2 diabetes. Sleep Med 2016; 25: 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strausz S, Havulinna AS, Tuomi T, et al. Obstructive sleep apnoea and the risk for coronary heart disease and type 2 diabetes: a longitudinal population‐based study in Finland. BMJ Open 2018; 8: e022752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu PH, Hui CKM, Lui MMS, et al. Incident type 2 diabetes in OSA and effect of CPAP treatment: a retrospective clinic cohort study. Chest 2019; 156: 743–753. [DOI] [PubMed] [Google Scholar]
- 29. Bikov A, Mészáros M, Kunos L. Characteristics of Hungarian patients with obstructive sleep apnoea. Orv Hetil 2020; 161: 2117–2123. [DOI] [PubMed] [Google Scholar]
- 30. Bozkurt NC, Cakal E, Sahin M, et al. The relation of serum 25‐hydroxyvitamin‐D levels with severity of obstructive sleep apnea and glucose metabolism abnormalities. Endocrine 2012; 41: 518–525. [DOI] [PubMed] [Google Scholar]
- 31. Bulcun E, Ekici M, Ekici A. Disorders of glucose metabolism and insulin resistance in patients with obstructive sleep apnoea syndrome. Int J Clin Pract 2012; 66: 91–97. [DOI] [PubMed] [Google Scholar]
- 32. European Obesity Summit (EOS) ‐ Joint Congress of EASO and IFSO‐EC, Gothenburg, Sweden, June 1 ‐ 4, 2016: Abstracts. Obesity Facts 2016; 9: 1–376. 10.1159/000446744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng Y, Guo D, Peng H, et al. The impact of obstructive sleep apnea hypopnea syndrome on glucose metabolism. Zhonghua Nei Ke Za Zhi 2015; 54: 691–694. [PubMed] [Google Scholar]
- 34. Fredheim JM, Rollheim J, Omland T, et al. Type 2 diabetes and pre‐diabetes are associated with obstructive sleep apnea in extremely obese subjects: a cross‐sectional study. Cardiovasc Diabetol 2011; 10: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gasa M, Salord N, Fortuna AM, et al. Obstructive sleep apnoea and metabolic impairment in severe obesity. Eur Respir J 2011; 38: 1089–1097. [DOI] [PubMed] [Google Scholar]
- 36. Gilardini L, Lombardi C, Redaelli G, et al. Glucose tolerance and weight loss in obese women with obstructive sleep apnea. PLoS One 2013; 8: e61382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gu CJ, Li M, Li QY, et al. Obstructive sleep apnea is associated with impaired glucose metabolism in Han Chinese subjects. Chin Med J 2013; 126: 5–10. [PubMed] [Google Scholar]
- 38. Gu CJ, Li QY, Li M, et al. Factors influencing glucose metabolism in young obese subjects with obstructive sleep apnea hypopnea syndrome. Zhonghua Yi Xue Za Zhi 2016; 96: 1405–1409. [DOI] [PubMed] [Google Scholar]
- 39. Hasan A, Uzma N, Swamy TL, et al. Correlation of clinical profiles with obstructive sleep apnea and metabolic syndrome. Sleep Breath = Schlaf Atmung 2012; 16: 111–116. [DOI] [PubMed] [Google Scholar]
- 40. Li N, Fan Y, Zhou JP, et al. Obstructive sleep apnea exacerbates glucose dysmetabolism and pancreatic β‐cell dysfunction in overweight and obese nondiabetic young adults. Diabetes, Metab Syndr Obes: Targets Ther 2020; 13: 2465–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Michalek‐Zrabkowska M, Macek P, Martynowicz H, et al. Obstructive sleep apnea as a risk factor of insulin resistance in nondiabetic adults. Life 2021; 11: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Togeiro SM, Carneiro G, Ribeiro Filho FF, et al. Consequences of obstructive sleep apnea on metabolic profile: a Population‐Based Survey. Obesity (Silver Spring, Md.) 2013; 21: 847‐851. [DOI] [PubMed] [Google Scholar]
- 43. Papaioannou I, Patterson M, Twigg GL, et al. Lack of association between impaired glucose tolerance and appetite regulating hormones in patients with obstructive sleep apnea. J Clin Sleep Med 2011; 7: 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Silva LOE, Guimarães TM, Luz GP, et al. Metabolic profile in patients with mild obstructive sleep apnea. Metab Syndr Related Disord 2018; 16: 6–12. [DOI] [PubMed] [Google Scholar]
- 45. Cong W, Jin T, Yuyang M & Qiang Z Data from: Obstructive sleep apnea, prediabetes, and progression of type 2 diabetes: a systematic review and meta‐analysis. Figshare. Deposited 29 December 2021. 10.6084/m9.figshare.17701727 [DOI]
- 46. Qie R, Zhang D, Liu L, et al. Obstructive sleep apnea and risk of type 2 diabetes mellitus: a systematic review and dose‐response meta‐analysis of cohort studies. J Diabetes 2020; 12: 455–464. [DOI] [PubMed] [Google Scholar]
- 47. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention . National Diabetes Statistics Report 2020 estimates of diabetes and its burden in the United States, 2020. Accessed 26 July 2020. Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national‐diabetes‐statistics‐report.pdf [Google Scholar]
- 48. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840–846. [DOI] [PubMed] [Google Scholar]
- 49. Shang W, Zhang Y, Wang G, et al. Benefits of continuous positive airway pressure on glycaemic control and insulin resistance in patients with type 2 diabetes and obstructive sleep apnoea: a meta‐analysis. Diabetes Obes Metab 2021; 23: 540–548. [DOI] [PubMed] [Google Scholar]
- 50. Mazzotti DR, Keenan BT, Lim DC, et al. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med 2019; 200: 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Funnel plots of obstructive sleep apnea and impaired fasting glucose risk.
Figure S2 | Egger’s test of obstructive sleep apnea and impaired fasting glucose risk.
Figure S3 | Funnel plots of obstructive sleep apnea and impaired glucose tolerance risk.
Figure S4 | Egger’s test of obstructive sleep apnea and impaired glucose tolerance risk.
Figure S5 | Funnel plots of obstructive sleep apnea and impaired glucose regulation risk.
Figure S6 | Egger’s test of obstructive sleep apnea and impaired glucose regulation risk.
Figure S7 | Funnel plots of obstructive sleep apnea and diabetes mellitus risk.
Figure S8 | Egger’s test of obstructive sleep apnea and diabetes mellitus risk.
Figure S9 | Funnel plots of obstructive sleep apnea and diabetes mellitus risk.
Figure S10 | Egger’s test of obstructive sleep apnea and impaired fasting glucose risk.
Figure S11 | Funnel plots of obstructive sleep apnea severity and the incidence of diabetes mellitus.
Figure S12 | Egger’s test of obstructive sleep apnea severity and the incidence of diabetes mellitus.
Figure S13 | Funnel plots of obstructive sleep apnea severity and the prevalence of diabetes mellitus.
Figure S14 | Egger’s test of obstructive sleep apnea severity and the prevalence of diabetes mellitus.
Table S1 | Methodological quality of the selected cohort studies according to the Newcastle–Ottawa Scale.
Table S2 | Methodological quality of the selected cross‐sectional and case control studies according to the Agency for Healthcare Research and Quality.


