Pancreatic ductal adenocarcinoma (PDAC) patients often present with irresectable or metastasized disease, resulting in an overall 5-year survival rate of 11%.1 To improve current therapeutic modalities, modeling the full complexity of PDAC in a personalized fashion is essential. Organoid technology enables close-to-patient models but currently lacks the typical desmoplastic tumor microenvironment.2,3 Up to 80% of PDAC consists of stromal cells, predominantly cancer-associated fibroblasts (CAFs). CAF populations are heterogenous and crucially involved in tumor growth, chemoresistance, immune evasion, and metastasis.4, 5, 6 To provide basis for improved therapy, it is essential to integrate CAFs and recapitulate desmoplasia in organoid-based models.2,3
In this study, we established a novel human, multicellular mini-tumor (MT) model containing both pancreatic tumor organoids and patient-derived CAFs from tumor resection material and fine-needle biopsies. We induced formation of MTs with heterogenous desmoplastic PDAC characteristics by modulating transforming growth factor β (TGFβ) and platelet-derived growth factor β signaling. MTs contain different archetypical CAF subsets, recapitulating patient CAF heterogeneity of human PDAC. Therefore, our model provides an important novel platform for both basic and preclinical research in a patient-specific fashion and can serve as a gateway for establishment of MTs from other stroma-dense gastrointestinal tumors.
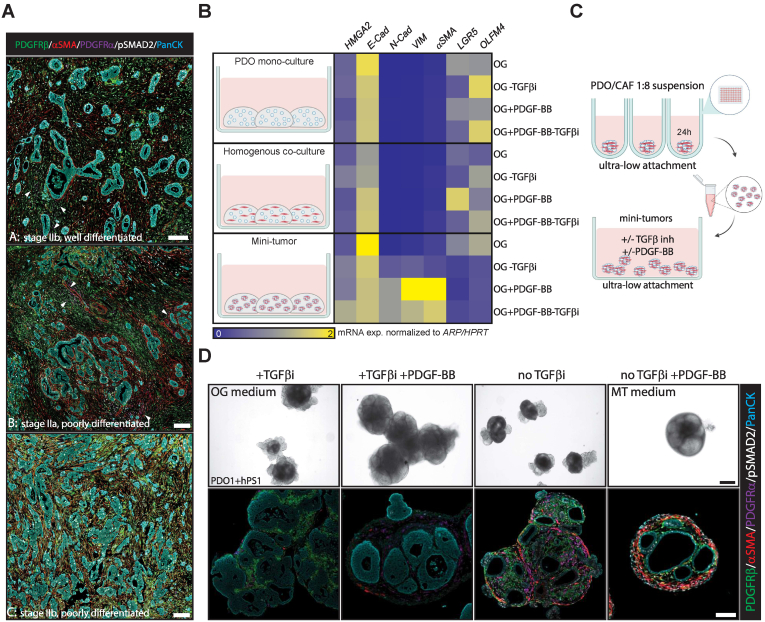
To ensure pathology resemblance of close-to-patient models, we designed a multiplex immunofluorescent panel to identify 2 major CAF subsets.5,6 Myofibroblastic CAFs (MyCAFs) and inflammatory CAFs were defined as being platelet-derived growth factor receptor (PDGFR)-β+/α− smooth muscle actin (αSMA)+/pSMAD2+ and PDGFRα+/αSMA−/pSMAD2−, respectively. The abundant presence of PDGFRβ+ CAFs was confirmed in all specimens investigated (n = 10), while PDGFRα+ CAFs were limited (Figure 1A, Supplemental Methods). To test if desmoplasia with heterogeneous CAF subsets can be recapitulated in an MT model, we cocultured pancreatic tumor-derived organoids (PDOs) and the pancreatic stellate cell line hPS17 that expresses canonical fibroblast and pancreatic stellate cell markers (Figure A1A–C). PDOs and hPS1 cells were mixed in a 1:8 ratio, reflecting the in vivo tumor-stroma ratio and aggregated in 3D Matrigel domes (Figure A1D and E). To stimulate CAF proliferation (PDGFRβ signaling) and differentiation (TGFβ signaling, a central driver of MyCAF differentiation5,6) in MTs, we withdrew the TGFβR1 inhibitor A83-01 from the standard organoid growth medium and added PDGF-BB (MT medium). PDGF-BB induced hPS1 proliferation and invasion into the Matrigel, causing loss of cell-cell contacts between PDO and hPS1 cells (Figure A1E). To avoid this and resemble the physiological juxtaposed CAF localization to PDOs, we induced direct contact through aggregation in ultralow attachment plates. Direct contact cocultures showed higher expression of mesenchymal markers than PDO monoculture and homogenous coculture in Matrigel domes (Figure 1B). Moreover, a direct cell contact in the presence of PDGFRβ and TGFβ activity showed strong induction of mesenchymal genes (N-cadherin, vimentin, αSMA) and repressed stem-cell-related gene expression (LGR5, OLFM4) of the MTs, resembling genetic features of PDAC (Figure 1B and Figure A1F, Table A1).
Figure 1.
PDGF-BB and TGFβ drive pancreatic cancer MT formation including desmoplasia and MyCAF differentiation. (A) Examples of 3 different PDAC primary tumors (A, B, C) stained for PDGFRβ (green) PDGFRα (magenta), αSMA (red), pSMAD2 (white), and pan-cytokeratin (cyan). Scalebar 100 μm. Arrows indicate PDGFRα+ cells. (B) Relative mRNA expression of HMGA2, E-cadherin N-Cadherin, Vimentin (VIM), αSMA, LGR5 and OLFM4 in PDO1 monocultures, PDO1 + hPS1 homogenous and direct contact MT cocultures grown in organoid growth (OG) medium ± TGFβ inhibitor (TGFβi) or PDGF-BB for 10 days (n = 3). (C) Schematic overview of suspension MT generation. (D) Representative bright-field images (top panel) of PDO1 + hPS1 MTs cultured in OG, OG + PDGF-BB, OG-TGFβ inhibitor, and OG + PDGF-BB-TGFβ inhibitor (MT) medium for 10 days. These were subsequently processed and stained for PDGFRβ (green) PDGFRα (magenta), αSMA (red), pSMAD2 (white), and pan-cytokeratin (cyan) (bottom panel). n = 3; Scalebar 100 μm.
Knowing PDGF-BB induces desmoplasia and cell contact enhances mesenchymal gene expression, we sought to maintain cell contact in the MT model. Continuous MT suspension culture in ultralow-attachment plates prevented loss of cell-cell interaction in culture between PDO and hPS1 over time (Figure 1C). Culture conditions without PDGF-BB led to MTs with no desmoplasia, indicating that exogenous addition of PDGF-BB is crucial to stimulate MT desmoplasia. In accordance, endogenous expression of PDGF-BB in PDOs was negligible (Figure A1G). PDGF-BB stimulation induced desmoplasia but lacked typical CAF marker expression found in vivo when A83-01 was present in the medium (Figure 1D). Differentiation into a pSMAD2+/αSMA+ MyCAF-like state was only observed upon A83-01 removal (Figure 1D). In MT medium, the limited presence of inflammatory CAF-like cells was also observed by costaining of PDGFRα and pSTAT3 (Figure A1H), corresponding to the in vivo prevalence of this cell type compared to MyCAFs.6
Interestingly, in response to PDGF-BB, hPS1 can either encapsulate PDOs (PDO1 & PDO2) or form a desmoplastic core (PDO3; Figures 1D and 2A), hinting at differential PDO-CAF crosstalk in determining spatial self-organization of MTs. In summary, direct cell-cell contact and PDGFRβ/TGFβ signaling resulted in a pathology resembling MTs with regard to desmoplasia and MyCAF differentiation.
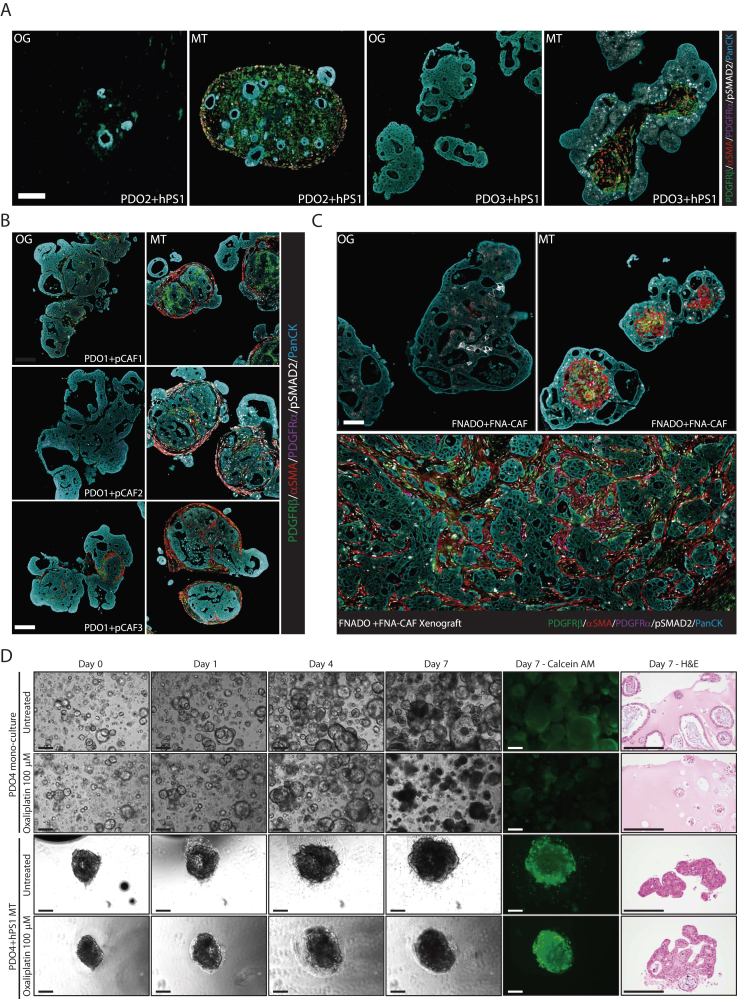
Figure 2.
Characteristics and applicability of the MT model. (A) FFPE PDO2 + hPS1 and PDO3 + hPS1 MTs cultured in OG or MT (OG + PDGF-BB-TGFβ inhibitor) medium for 10 days stained for PDGFRβ (green), PDGFRα (magenta), αSMA (red), pSMAD2 (white), and pan-cytokeratin (cyan). Scalebar 100 μm. (B) Representative FFPE PDO1 + primary CAF1 (pCAF), pCAF2, and pCAF3 MTs cultured in OG or MT medium for 10 days stained for PDGFRβ (green), PDGFRα (magenta), αSMA (red), pSMAD2 (white), and pan-cytokeratin (cyan); n = 2. (C) FFPE FNADO + FNA-CAF MTs cultured in OG or MT medium for 10 days compared to FNADO + FNA-CAF xenograft, stained for PDGFRβ (green), PDGFRα (magenta), αSMA (red), pSMAD2 (white), and pan-cytokeratin (cyan); n = 3, scalebar 100 μm. (D) Monoculture organoids (PDO4) and MTs consisting of PDO4 and hPS1 fibroblasts were treated with oxaliplatin (100 μm) and imaged for 7 days using phase-contrast microscopy. On day 7, viability was assessed using calcein AM staining, and cells were subsequently processed for hematoxylin & eosin (H&E) staining. Scalebar 250 μm.
Having established this model with hPS1 cells, we investigated whether we could generate MTs using primary, patient-derived PDAC fibroblasts. Primary fibroblasts were mixed with PDO1 and cultured either in organoid growth medium (+TGFβi, −PDGF-BB) or MT medium (−TGFβi, +PDGF-BB). In line with our observations, desmoplastic MTs only form when grown in the MT medium (Figure 2B).
As a proof of concept, we generated fully autologous MTs to study the feasibility of generating treatment-naïve models at the time of diagnosis. PDO (FNADO) and fibroblast (FNA-CAF) cultures were established from a single fine-needle biopsy (Figure A2A). The mutation status of FNADO was consistent with the majority of human PDACs, including KRASp.(Gly12Asp) and TP53p.(Arg196∗) mutations and loss of CDKN2A (Figure A2B). FNADO tumorigenicity was confirmed in mouse xenografts. MTs of FNADO and FNA-CAFs closely resembled a xenografted primary tumor when cultured in the MT medium (Figure 2C).
Finally, we aimed to test the applicability of MTs in comparison to PDO monocultures with regard to drug sensitivity. Stroma-rich tumors often are resistant to chemotherapeutics, including oxaliplatin.8 To this end, we tested oxaliplatin resistance in a treatment-naive PDO (PDO4) and MT model (PD04 + hPS1). Indeed, oxaliplatin induced significant cell death in monoculture conditions but not in MTs (Figure 2D). This indicates that MTs have a potential to faithfully recapitulate drug sensitivity observed in stroma-rich tumors.
Here we report the creation of a human, multicellular MT model that in comparison to earlier efforts9,10 now recapitulates both desmoplastic features of PDAC and differentiation toward the MyCAF subset. Importantly, these pathologies resembling MTs can be generated from both surgical resections as well as endoscopic biopsies, thus, for the first time, facilitating modeling the entire patient spectrum. Especially, endoscopic acquisition of patient material enables MT generation of treatment-naïve tumors and modeling of tumors not amenable to surgical resection. This model is easy to integrate into current organoid-based cell culture models and provides basis to study a plethora of fundamental aspects of PDAC, including the spatial architecture of PDAC tumor cells and associated stroma. For preclinical studies, MT cultures could be implemented in medium-throughput screening platforms. The next essential step to further improve this MT model is to include additional stromal cell types, for example, immune cells, endothelial cells, and adipocytes.2,3 Finally, since the PDGF and TGFβ pathways also play key roles in other stroma-dense gastrointestinal tumors,4 our model is expected to be extendable to, for example, colon, gastric, and esophageal cancers.
Acknowledgments
The authors wish to thank the Hubrecht Organoid Technology (HUB) and CancerGenomicsCenter.nl for providing and funding PDOs and prof. Hemant Kocher (Queen Mary University London) for providing hPS1 cells used in this study. The authors also thank Dr A. Farshadi for providing cells (PDO4) and reagents (oxaliplatin). Illustrations in Figures 1B, 1C and A1D were made with BioRender (www.biorender.com).
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: This work was supported by the CancerGenomicsCenter.nl (NWO Gravitation Program) to P.t.D.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.gastha.2022.04.019.
Contributor Information
L.J.A.C. Hawinkels, Email: L.J.A.C.Hawinkels@LUMC.nl.
mini-tumor workgroup:
A. Inderson, J.E. van Hooft, P. Micke, J.S. Johansen, T. van Wezel, S. Crobach, V.M. Baart, B.S. Quist, J.E.G. Roet, and M.E. Kop
Supplementary Materials
(A) Representative brightfield image of PDO1 and hPS1. Scalebar 100 μm. (B) Relative mRNA expression of CYCG, FABP4, FAP, PLIN2, PDPN, VIM, CCL2, CXCL1, IL1α, IL6, PDGFRα, PDGFRβ, αSMA, and COL1A1 in hPS1. (C) PDGFRB and GAPDH protein levels in hPS1 cells treated with TGFβ receptor inhibitor A83-01 and TGFβ3 ligand for 24 hours; N = 3. (D) Schematic overview of MT 3D matrigel culture generation. (E) Representative brightfield images of PDO1 + hPS1 and PDO1-H2B-mCerulean3 (Cyan) + hPS1 MTs 3D matrigel cultures grown for 10 days in OG, OG + PDGF-BB, OG-TGFβ inhibitor, or OG + PDGF-BB-TGFβ inhibitor (MT) medium; n = 3, scalebar 100 μm. (F) Relative mRNA expression of FABP4, FAP, PLIN2, PDPN, VIM, CCL2, CXCL1, CTGF, IL6, PDGFRα, PDGFRβ, αSMA, and COL1A1 in hPS1 cultured in 2D or as aggregates in 3D Matrigel domes in medium ± TGFβ inhibitor an PDGF-BB for 5 days (n = 3). (G) Relative expression (2ˆ−ΔCT) of PDGFB in PDO1 and PDO3. The endothelial cell line ECRF was used as a positive control. (H) Multiplex staining of patient-derived PDAC resection material and MTs (PDO1 + hPS1) for PDGFRα and pSTAT3
(A) Representative brightfield image of FNAPDO after 7 days culture in matrigel; scalebar 100 μm. (B) Overview table of cancer-related mutations in FNAPDO based on AmpliSeq Cancer Hotspot Panel V2 genome sequencing
References
- 1.Siegel R.L., et al. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Tuveson D., et al. Science. 2019;364:952–955. doi: 10.1126/science.aaw6985. [DOI] [PubMed] [Google Scholar]
- 3.Lau H.C.H., et al. Nat Rev Gastroenterol Hepatol. 2020;17:203–222. doi: 10.1038/s41575-019-0255-2. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi H., et al. Nat Rev Gastroenterol Hepatol. 2019;16:282–295. doi: 10.1038/s41575-019-0115-0. [DOI] [PubMed] [Google Scholar]
- 5.Helms E., et al. Cancer Discov. 2020;10:648–656. doi: 10.1158/2159-8290.CD-19-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biffi G., et al. Physiol Rev. 2021;101:147–176. doi: 10.1152/physrev.00048.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Froeling F.E.M., et al. Am J Pathol. 2009;175:636–648. doi: 10.2353/ajpath.2009.090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ham I.H., et al. Cancers (Basel) 2021;13:1172. doi: 10.3390/cancers13051172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seino T., et al. Cell Stem Cell. 2018;22:454–467.e6. doi: 10.1016/j.stem.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Tsai S., et al. BMC Cancer. 2018;18:335. doi: 10.1186/s12885-018-4238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Representative brightfield image of PDO1 and hPS1. Scalebar 100 μm. (B) Relative mRNA expression of CYCG, FABP4, FAP, PLIN2, PDPN, VIM, CCL2, CXCL1, IL1α, IL6, PDGFRα, PDGFRβ, αSMA, and COL1A1 in hPS1. (C) PDGFRB and GAPDH protein levels in hPS1 cells treated with TGFβ receptor inhibitor A83-01 and TGFβ3 ligand for 24 hours; N = 3. (D) Schematic overview of MT 3D matrigel culture generation. (E) Representative brightfield images of PDO1 + hPS1 and PDO1-H2B-mCerulean3 (Cyan) + hPS1 MTs 3D matrigel cultures grown for 10 days in OG, OG + PDGF-BB, OG-TGFβ inhibitor, or OG + PDGF-BB-TGFβ inhibitor (MT) medium; n = 3, scalebar 100 μm. (F) Relative mRNA expression of FABP4, FAP, PLIN2, PDPN, VIM, CCL2, CXCL1, CTGF, IL6, PDGFRα, PDGFRβ, αSMA, and COL1A1 in hPS1 cultured in 2D or as aggregates in 3D Matrigel domes in medium ± TGFβ inhibitor an PDGF-BB for 5 days (n = 3). (G) Relative expression (2ˆ−ΔCT) of PDGFB in PDO1 and PDO3. The endothelial cell line ECRF was used as a positive control. (H) Multiplex staining of patient-derived PDAC resection material and MTs (PDO1 + hPS1) for PDGFRα and pSTAT3
(A) Representative brightfield image of FNAPDO after 7 days culture in matrigel; scalebar 100 μm. (B) Overview table of cancer-related mutations in FNAPDO based on AmpliSeq Cancer Hotspot Panel V2 genome sequencing