Abstract
Objective: The study investigated the prevalence of mild cognitive impairment (MCI), a common cognitive disorder in late life, among rural older residents in China. The associated risk factors were also analyzed. Methods: Two thousand one hundred forty-six older adults aged 60 or more in a rural town of Zhejiang Province, China, were recruited and analyzed. Demographic characteristics were collected by a self-designed questionnaire. Diagnosis of MCI was made by well-trained primary care physicians according to the Petersen criteria. Results: 23.16% of the analyzed sample were diagnosed with MCI, while the prevalence was significantly higher in women, those never married, not employed, and with older age, lower education, diabetes, coronary heart disease and stroke. Stepwise logistic regression indicated that age, education, diabetes, coronary heart disease, and stroke were significant predictive factors of MCI. Conclusion: The prevalence of MCI in rural older residents in China is high, and those with specific demographic characteristics like women, never married, not employed, and with older age, lower education and chronic physical conditions should be more concerned in primary care management. Integrated care approaches managing MCI and comorbid chronic conditions are recommended in future management practices.
Keywords: mild cognitive impairment, older adults, prevalence, risk factors, rural China
Introduction
Dementia is a major public health concern in the world, including in China. The World Alzheimer Report 2018 indicated that the global number of people living with dementia was estimated at 50 million, which number will triple by 2050 (Patterson, 2018). In China, dementia was estimated to affect about 5% of its aging population (Jia et al., 2020), and the estimated total annual costs of dementia in China increased from 0.9 billion United States dollars (US$) in 1990 to US$47.2 billion in 2010 and were predicted to reach US$ 69.0 billion in 2020 and US$ 114.2 billion in 2030 (Xu et al., 2017). The burden of dementia might become exacerbated with the large internal migration, as fewer young adults available to provide continuing care to the millions of older people with dementia, which is particularly problematic in rural regions (Zhang, 2017). There are no drugs so far to cure dementia or to modify its clinical course, and symptomatic medication has only modest effects (Cummings, 2004; Kaduszkiewicz et al., 2005; Livingston et al., 2020). Approaches for dementia focus on the early detection and treatment, underlining the importance of the prodromal phase of dementia, such as the mild cognitive impairment (MCI).
The concept of mild cognitive impairment represents an intermediate stage between normal aging and dementia. This is especially useful in the early stages of Alzheimer’s disease (AD), where individuals have a cognitive impairment beyond that expected for age and education yet do not have dementia (Petersen, 2011; Petersen et al., 2001, 1999). Several studies indicate that MCI can be regarded as a risk factor for dementia(Burns & Zaudig, 2002; Flicker et al., 1991; Gauthier et al., 2006; Morris et al., 2001; Petersen, 2004), as the conversion rate of MCI to dementia has been estimated to be 12% at 1 year, 20% at 3 years, and 50% at 5 years (Radler et al., 2020; Solfrizzi et al., 2004), and some even find that patients with MCI progress to dementia at a rate of 60% to 100% over 5 to 10 years (Hebert et al., 2003). In the absence of any effective therapy for dementia, identification of risk factors for the development of MCI may hold the best promise for preventing or delaying the progression from early cognitive dysfunction to clinical dementia (Boo et al., 2021; Roberts et al., 2008).
Risk factors related to cognitive impairment have been identified in several studies. A recent review article (Xue et al., 2018) reported that demographic characteristics like age, sex, and region of residence were associated with the incidence of MCI. Clinical characteristics, like depression (Richard et al., 2013), hypertension (Santisteban & Iadecola, 2018), diabetes (You et al., 2021), and other common physical and mental conditions, were also indicated to contribute to the incidence and progression of cognitive impairment. In addition, recent studies have tried to link sleep to the development of neurocognitive disorders, though current evidence for this relationship is conflicting (Rozzini et al., 2018; Stephens et al., 2022; Wams et al., 2017).
Several studies have reported the situation of MCI in China. Ding et al. (2015) reported the prevalence of MCI in Shanghai older residents aged 60 or more was 20.1%; Rao’s group found that 14.2% of elderly individuals aged 65 or more were affected by MCI in Guangzhou (Rao et al., 2018); F. Ma’s et al. (2016) research concluded a prevalence of 11.3% among older adults aged 65 or more in Tianjin. However, most of these studies were conducted in urban areas. In a recent systematical review of 48 studies reporting the prevalence of MCI (Xue et al., 2018), only 17.7% of the analyzed samples were recruited from rural areas, which limited the comprehensive understanding of the disease burden in the left-behind area in China.
In general, mild cognitive impairment (MCI) is considered to be a preclinical transitional state of dementia (Petersen et al., 1999) for which targeted interventions may be feasible. Identifying the prevalence of MCI in China is crucial for assessments of potential disease burden and therefore the need for interventions to prevent or slow progression of decline to dementia. However, few studies have reported the prevalence of MCI in rural China. In the present study, we investigated the prevalence of MCI in a rural town of Zhejiang Province, China, and analyzed its associated risk factors.
Methods
Setting and Participants
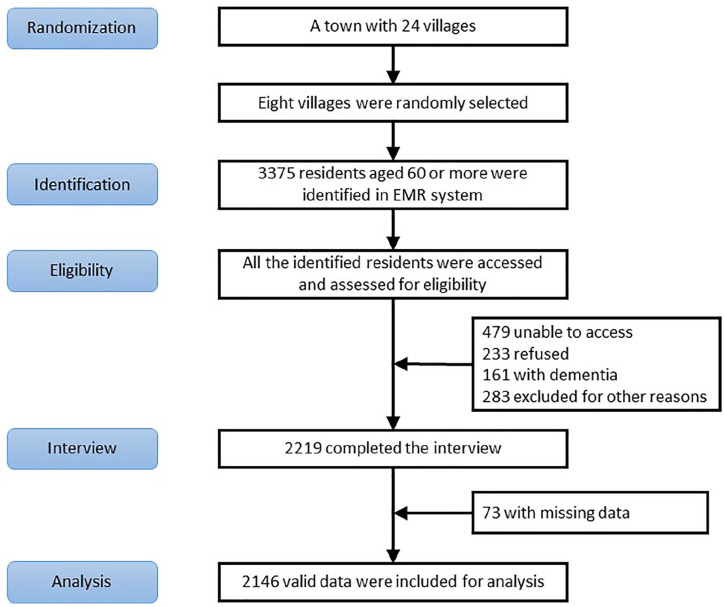
The study was conducted from April to November, 2019, in a rural town of Zhejiang Province, China. Eight out of 24 villages in the town were randomly selected, with population aged 60 or more registered in the electronic medical record (EMR) system was 3375. All these older adults were potential participants of the research, and the participant procedure was shown in Figure 1.
Figure 1.
Participant procedure.
After the randomization and identification, 10 trained primary care physicians (PCPs) employed by the research group accessed these older residents whether in the primary care clinics, senior citizens activity centers, or their homes, introducing the research, inviting them to participate, evaluating for eligibility and making the study interview. The interview content included the participants’ demographic characteristics, comorbidity information and cognitive assessment, and it took about 30 minutes to complete the interview. All participants provided written informed consent before participation.
The inclusion criteria were as follows: (i) community-dwelling residents registered to the village; (ii) aged ≥60 years; (iii) no severe problems with vision, hearing, or speaking reported by the resident or informant (i.e., families) or observed by the PCP; and (iv) willing to give written informed consent.
Among the 3,375 community residents, 1,156 were excluded because of the following: (i) 479 were unable to access; (ii) 233 refused to participate; (iii) 161 were screened with dementia; (iv) 283 were excluded for the other reasons. The total number of the participants completing the interview, therefore, was 2,219. After excluding 73 with missing data, 2,146 pieces of data were available for analyses.
Measurements
Demographic information was collected using a standardized form developed for the study, variables derived included age, sex, education and literacy level, marital status, living conditions, and employment status. Information of chronic diseases, including hypertension, diabetes, hyperlipidemia, coronary heart disease and stroke, was also collected.
The diagnosis of MCI was made according to the Petersen criteria defined as:
(1) Cognitive concern reflecting a change in cognition reported by patient or informant (i.e., families);
(2) Objective evidence of impairment in one or more cognitive domains. Cognitive impairment was evaluated by the Montreal Cognitive Assessment (MoCA), which is a widely used assessment tool for detecting cognitive impairment (Nasreddine et al., 2005) and has been validated in the setting of MCI. The Chinese version of MoCA has been tested with acceptable validity and reliability (H. Li et al., 2009), and the cut-off score adopted in the study was 19/20 (Dong et al., 2013) adjusting for age and educational attainment;
(3) Preservation of independence in functional abilities. It was assessed by the Activities of Daily Living Scale (ADL), which is a commonly used assessment tool for daily functions (Lawton & Brody, 1969) and has been examined with acceptable reliability and validity as a supplement for the diagnosis of dementia and MCI. The cut-off value in the study was 14/15.
(4) Not demented with Clinical Dementia Rating (CDR) < 1 (Morris, 1997).
Data Analysis
Descriptive statistics (counts and proportions for categorical variables and means [± SD] for continuous outcomes) were used to depict the characteristics of the sample (e.g., age, gender, physical and functional status). We compared basic characteristics (including disease information) between individuals with and without MCI using t-test (for continuous variables) and chi-square examination (for discrete variables), and calculated the prevalence of MCI in the whole sample and in each subgroup.
Stepwise logistic regression model was established to analyze the predictive factors of developing MCI, with basic characteristics included as independent variables and covariates. Age was treated as a continuous variable and the others were treated as categorical variables. For disease information, the answer “yes” was coded as “1” while “no” was coded as “0”, and “male” was coded as “1” while “female” was coded as “2”. Other categorical variables were coded in order as well. Box-Tidwell tests were applied to examine the linearity of age as a continuous variable in the logit fitting processes before establishing the model, and Hosmer and Lemeshow Tests were conducted to test the model goodness-of-fit (Draper & Smith, 1998).
All analyses were performed using the Predictive Analytics Software Statistics, version 18, and a two-sided p value less than .05 were considered statistically significant.
Results
Characteristics of the Participants
As depicted in Figure 1, a total of 2,146 participants were entered into the analysis. Table 1 indicates the demographic and clinical characteristics of the sample. Overall, their mean age (±SD) was 71.14 ± 6.81. Women constituted 61.79% of the sample, while 79.12% were married and 82.71% lived with family or others. 75.30% of the sample had fewer than 6 years of education, and the percentage of those who were not employed was 85.88%. Additionally, the prevalence of MCI in the sample was 23.16%, while 79.82% of individuals investigated had at least one kind of chronic condition.
Table 1.
Characteristics of the Participants (N = 2,146).
| Characteristics | Participants, n (%) |
|---|---|
| Age, mean ± SD | 71.14 ± 6.81 |
| 60–69 | 968 (45.11) |
| 70–79 | 893 (41.61) |
| ≥80 | 285 (13.28) |
| Sex | |
| Female | 1,326 (61.79) |
| Male | 820 (38.21) |
| Education | |
| No schooling | 529 (24.65) |
| 1–5 years | 1,087 (50.65) |
| >6 years | 530 (24.70) |
| Marriage status | |
| Married | 1,698 (79.12) |
| Divorce/widowed | 435 (20.27) |
| Never married | 13 (0.61) |
| Living situation | |
| Alone | 371 (17.29) |
| With family/others | 1,775 (82.71) |
| Employment status | |
| Employed | 303 (14.12) |
| Not employed | 1,843 (85.88) |
| Mild cognitive impairment | 497 (23.16) |
| Chronic disease | 1,713 (79.82) |
| Hypertension | 1,345 (62.67) |
| Diabetes | 451 (21.02) |
| Hyperlipidemia | 440 (20.50) |
| Coronary heart disease | 135 (6.29) |
| Stroke | 70 (3.26) |
| Others | 198 (9.23) |
The Prevalence of MCI in Subgroups
The prevalence of MCI in subgroups was demonstrated in Table 2. 57.74% of those aged 80 or more had MCI, which rate was more than quadruple compared to that in the age group of 60 to 69. In addition, more than half of those affected by MCI had never received education, which proportion for those receiving at least 6 years of education was less than one-tenth. Furthermore, sex (χ2 = 18.56, p < .001), marriage status (χ2 = 62.55, p < .001), living situation (χ2 = 33.98, p < .001) and employment status (χ2 = 11.51, p = .001) were also significantly associated with MCI, and the prevalence in those with chronic conditions, except hypertension and hyperlipidemia, was significantly higher than in those without.
Table 2.
The Prevalence of MCI in the Sample and the Subgroups.
| Characteristics | Participants | x2 | p-Value | Prevalence | |
|---|---|---|---|---|---|
| MCI, n (%) | CN, n (%) | ||||
| Age | |||||
| 60–69 | 136 (27.36) | 832 (50.45) | 235.09 | <.001 | 14.05 |
| 70–79 | 197 (39.64) | 696 (42.21) | 22.06 | ||
| ≥80 | 164 (33.00) | 121 (7.34) | 57.54 | ||
| Sex | |||||
| Female | 348 (70.02) | 978 (59.31) | 18.56 | <.001 | 26.24 |
| Male | 149 (29.98) | 671 (40.69) | 18.17 | ||
| Education | |||||
| No schooling | 286 (57.55) | 243 (14.74) | 393.11 | <.001 | 54.06 |
| 1–5 years | 174 (35.01) | 913 (55.37) | 16.01 | ||
| >6 years | 37 (7.44) | 493 (29.90) | 6.98 | ||
| Marriage status | |||||
| Married | 330 (66.40) | 1,368 (82.96) | 62.55 | <.001 | 19.43 |
| Divorce/widowed | 160 (32.19) | 275 (16.68) | 36.78 | ||
| Never married | 7 (1.41) | 6 (0.36) | 46.15 | ||
| Living situation | |||||
| Alone | 129 (25.96) | 242 (14.68) | 33.98 | <.001 | 34.77 |
| With family/others | 368 (74.04) | 1,407 (85.32) | 20.73 | ||
| Employment status | |||||
| Employed | 47 (9.46) | 256 (15.52) | 11.51 | .001 | 15.51 |
| Not employed | 450 (90.54) | 1,393 (84.48) | 24.42 | ||
| Hypertension | |||||
| Yes | 317 (63.78) | 1,028 (62.34) | 0.34 | .560 | 19.89 |
| No | 180 (36.22) | 621 (37.66) | 16.75 | ||
| Diabetes | |||||
| Yes | 131 (26.36) | 320 (19.41) | 11.12 | .001 | 29.05 |
| No | 366 (73.64) | 1,329 (80.59) | 21.59 | ||
| Hyperlipidemia | |||||
| Yes | 115 (23.14) | 325 (19.71) | 2.76 | .097 | 26.14 |
| No | 382 (76.86) | 1,324 (80.29) | 22.39 | ||
| Coronary heart disease | |||||
| Yes | 52 (10.46) | 83 (5.03) | 19.10 | <.001 | 38.52 |
| No | 445 (89.54) | 1,566 (94.97) | 22.13 | ||
| Stroke | |||||
| Yes | 35 (7.04) | 35 (2.12) | 29.29 | <.001 | 50.00 |
| No | 462 (92.96) | 1,614 (97.88) | 22.25 | ||
| Other chronic diseases | |||||
| Yes | 60 (12.07) | 138 (8.37) | 6.26 | .012 | 30.30 |
| No | 437 (87.93) | 1,511 (91.63) | 22.43 | ||
Note. MCI = mild cognitive impairment; CN = cognitive normal.
The Predictive Factors of MCI
Before doing the logistic regression analyses, the Box-Tidwell tests indicated acceptable linearity of age as a continuous variable in the logit fitting processes, which supported the linearity assumption of establishing the logistic regression model.
The Hosmer and Lemeshow Test for the goodness-of-fit of the logistic regression model had a χ2 = 13.30, df = 8, p = .102 > .05, indicating a good fitness of this model. Estimates from the logistic regression were listed in Table 3. Age, education, diabetes, coronary heart disease and stroke were significant predictive factors of MCI in the sample, while the significance of coronary heart disease was marginal. The odds ratios of the variables implied that individuals with diabetes, coronary heart disease and stroke were at 1.35, 1.50, 2.14 greater odds respectively of developing MCI compared to those without.
Table 3.
Estimates From Logistic Regression Modeling Mild Cognitive Impairment.
| Regression | Variable | B | SE | Wals | df | Sig. | Exp (B) | 95% CI for Exp (B) | |
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Step 1 | Sex | 0.14 | 0.14 | 1.03 | 1 | 0.310 | 1.15 | 0.88 | 1.51 |
| Age | 0.10 | 0.01 | 98.65 | 1 | <0.001 | 1.10 | 1.08 | 1.12 | |
| Education | −1.48 | 0.10 | 221.05 | 1 | <0.001 | 0.23 | 0.19 | 0.28 | |
| Marriage status | −0.11 | 0.19 | 0.33 | 1 | 0.564 | 0.89 | 0.61 | 1.31 | |
| Living situation | 0.08 | 0.21 | 0.14 | 1 | 0.705 | 1.08 | 0.71 | 1.64 | |
| Employment status | −0.14 | 0.19 | 0.57 | 1 | 0.452 | 0.87 | 0.59 | 1.26 | |
| Diabetes | 0.32 | 0.14 | 4.94 | 1 | 0.026 | 1.37 | 1.04 | 1.82 | |
| Coronary heart disease | 0.41 | 0.22 | 3.45 | 1 | 0.063 | 1.51 | 0.98 | 2.33 | |
| Stroke | 0.78 | 0.30 | 6.87 | 1 | 0.009 | 2.18 | 1.22 | 3.90 | |
| Other chronic conditions | 0.29 | 0.19 | 2.22 | 1 | 0.136 | 1.33 | 0.91 | 1.94 | |
| Hyperlipidemia | −0.09 | 0.15 | 0.34 | 1 | 0.561 | 0.92 | 0.68 | 1.23 | |
| Constant | −5.45 | 1.00 | 29.61 | 1 | <0.001 | 0.00 | \ | \ | |
| Step 7 | Age | 0.10 | 0.01 | 120.15 | 1 | <0.001 | 1.10 | 1.08 | 1.12 |
| Education | −1.51 | 0.10 | 247.31 | 1 | <0.001 | 0.22 | 0.18 | 0.27 | |
| Diabetes | 0.30 | 0.14 | 4.61 | 1 | 0.032 | 1.35 | 1.03 | 1.77 | |
| Coronary heart disease | 0.40 | 0.22 | 3.37 | 1 | 0.066 | 1.50 | 0.97 | 2.30 | |
| Stroke | 0.76 | 0.29 | 6.86 | 1 | 0.009 | 2.14 | 1.21 | 3.79 | |
| Constant | −5.46 | 0.64 | 72.93 | 1 | <0.001 | 0.00 | \ | \ | |
Discussion
As the health of the rural population improved and life expectancy lengthened, the burden of mental health increased. Nearly 5% of rural residents aged over 60 in China had a diagnosis of depression (Zhong et al., 2020), and the lifetime prevalence of suicidal ideation and attempt in the population was found to be 28.9% and 5.3%, respectively (Chiu et al., 2012). Moreover, the disability rate in rural older population was 10.9%, more than two times higher of 4.7% found in urban area (L. Ma et al., 2017). However, very few studies have been conducted on this left-behind area.
The purposes of the present study were to investigate the prevalence of MCI in older adults aged over 60 in rural China, and identify its associated risk factors. The principal finding of the study is that 23.16% of the investigated group had MCI. This rate is significantly higher than that reported in urban area of about 13% to 14% (Deng et al., 2021; Xue et al., 2018). Although the situation in rural area is serious, medical resources here is insufficient and the health care system is not adequately prepared to screen, identify and manage the cognitive disorder. More studies focusing on the cognitive conditions in this population are recommended and efficient approaches should be provided to bridge the gap.
In addition to the high prevalence, we also found that women, those with older age, lower education, never married, living alone, and not employed were more likely to develop MCI. These characteristics highlighted were consistent with what reported in previous studies (X. Li et al., 2013; Ren et al., 2018), and the finding would be helpful to deliver targeted services for individuals at risk of neurodegenerative diseases.
Although nearly all the demographic and clinical characteristics collected were significantly associated with MCI, results of the stepwise logistic regression model indicated that only age, education as well as the diabetes, coronary heart disease, and stroke were significant predictive factors of MCI in the sample, while the significance of coronary heart disease was marginal. Hypertension, which was traditional believed as an important associated factor of cognitive disorders, was tested with no significant association with MCI. Benefit from the reform on chronic disease management in China, the cardiovascular effect of hypertension on neurodegenerative disorders is supposed to be narrowed as most older adults with hypertension currently are under standard management and regularly taking anti-hypertension drugs.
Several studies have suggested chronic medical conditions as crucial risk factors of cognitive impairment (CI). Actually, estimated more than 80% of older adults aged over 65 have at least one chronic condition, and almost 80% of deaths in China in people aged 60 years are from chronic non-communicable diseases (Yang et al., 2013). A previous study demonstrated that chronic kidney disease is associated with elevated risk for dementia in elderly people with poor executive function, cognitive function, memory and language ability (Kurella et al., 2005). Singh and colleagues’ study found that a diagnosis of chronic obstructive pulmonary disease is associated with an increased risk for MCI, particularly non-amnestic MCI (Singh et al., 2014). In the present study, we found that rural older adults with chronic medical conditions are at 1.35~2.14 greater odds of developing MCI compared to those without, after controlling for sex, age, and other associated variables.
As a prevalent mental illness in the elderly, MCI commonly co-occurs with chronic medical illnesses and would interact in complex ways to increase the severity, impede treatment, and worsen the outcomes of both disorders. Faced with the challenge, integrated care approaches to manage MCI and comorbid medical conditions simultaneously in primary care settings are recommended. Evidence suggests that patients may be more engaged when mental illness is integrated into care for physical health than other forms of care provision (Katon et al., 2010), and effectiveness of such approaches have been extensively tested in Western countries (Katon et al., 2006; Smith et al., 2007), but rare studies were conducted in low and middle-income countries, including China, to examine the validity and feasibility of the integrated care. The outcomes of our study support the need to integrate the management of MCI into chronic disease management in rural China.
The strengths of the study include the large sample investigated and standard procedure and criteria of diagnosis. However, the study has limitations. The sample was from one town in rural Zhejiang Province, so our findings may not be generalizable to urban or other rural areas. The data in this study were cross-sectional, which limited the analysis of the long-term associations between demographics, comorbidities and MCI. Longitudinal studies would help to sort out the relationship between these conditions. Finally, we did not have data about prescribed treatment or adherence to treatment for diseases, and cognitive performance could be influenced by the use of those drugs. We acknowledge that medication use can potentially confound the results.
In conclusion, our investigation adds new evidence to the literature on the burden and risk factors of MCI in rural China. The prevalence of MCI in this group is high, and the prevalence of MCI was significantly higher in women, those with older age, lower education, never married, living alone and not employed. Age, education and chronic diseases were significant predictive factors of MCI in the sample, while the significance of hypertension was marginal.
Acknowledgments
We thank all the participants in the study, and we are also grateful to primary care physicians and research assistants who participated in the study for their assistance in research coordination and data collection.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project was supported by the China Postdoctoral Science Foundation, grant number: 2020TQ0265, and the Natural Science Foundation of Zhejiang Province, China, grant number: LGF21C090002.
Ethical Statement: The study was approved by the Academic Review Board of Zhejiang University Department of Psychology, approval number: [2021]027.
ORCID iD: Jiang Xue  https://orcid.org/0000-0002-3237-2330
https://orcid.org/0000-0002-3237-2330
References
- Boo Y. Y., Jutila O.-E., Cupp M. A., Manikam L., Cho S.-I. (2021). The identification of established modifiable mid-life risk factors for cardiovascular disease which contribute to cognitive decline: Korean Longitudinal Study of Aging (KLoSA). Aging Clinical and Experimental Research, 33(9), 2573–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns A., Zaudig M. (2002). Mild cognitive impairment in older people. Lancet, 360(9349), 1963–1965. [DOI] [PubMed] [Google Scholar]
- Chiu H., Dai J., Xiang Y., Chan S., Leung T., Yu X., Hou Z. J., Caine E. (2012). Suicidal thoughts and behaviors in older adults in rural China: A preliminary study. International Journal of Geriatric Psychiatry, 27(11), 1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. L. (2004). Treatment of Alzheimer’s disease: Current and future therapeutic approaches. Reviews in Neurological Diseases, 1(2), 60–69. [PubMed] [Google Scholar]
- Deng Y., Zhao S., Cheng G., Yang J., Li B., Xu K., Xiao P., Li W., Rong S. (2021). The prevalence of mild cognitive impairment among Chinese people: A meta-analysis. Neuroepidemiology, 55(2), 79–91. [DOI] [PubMed] [Google Scholar]
- Ding D., Zhao Q., Guo Q., Meng H., Wang B., Luo J., Mortimer J. A., Borenstein A. R., Hong Z. (2015). Prevalence of mild cognitive impairment in an urban community in China: A cross-sectional analysis of the Shanghai Aging Study. Alzheimer’s & Dementia, 11(3), 300–309. e302. [DOI] [PubMed] [Google Scholar]
- Dong Y., Lee W. Y., Hilal S., Saini M., Wong T. Y., Chen C. L.-H., Venketasubramanian N., Ikram M. K. (2013). Comparison of the montreal cognitive assessment and the mini-mental state examination in detecting multi-domain mild cognitive impairment in a Chinese sub-sample drawn from a population-based study. International Psychogeriatrics, 25(11), 1831–1838. [DOI] [PubMed] [Google Scholar]
- Draper N. R., Smith H. (1998). Applied regression analysis (Vol. 326). John Wiley & Sons. [Google Scholar]
- Flicker C., Ferris S. H., Reisberg B. (1991). Mild cognitive impairment in the elderly Predictors of dementia. Neurology, 41(7), 1006–1009. [DOI] [PubMed] [Google Scholar]
- Gauthier S., Reisberg B., Zaudig M., Petersen R. C., Ritchie K., Broich K., Belleville S., Brodaty H., Bennett D., Chertkow H. (2006). Mild cognitive impairment. Lancet, 367(9518), 1262–1270. [DOI] [PubMed] [Google Scholar]
- Hebert L. E., Scherr P. A., Bienias J. L., Bennett D. A., Evans D. A. (2003). Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Archives of Neurology, 60(8), 1119–1122. http://www.ncbi.nlm.nih.gov/pubmed/12925369 10.1001/archneur.60.8.1119 [DOI] [PubMed] [Google Scholar]
- Jia L., Quan M., Fu Y., Zhao T., Li Y., Wei C., Tang Y., Qin Q., Wang F., Qiao Y., Shi S. (2020). Dementia in China: Epidemiology, clinical management, and research advances. The Lancet Neurology, 19(1), 81–92. [DOI] [PubMed] [Google Scholar]
- Kaduszkiewicz H., Zimmermann T., Beck-Bornholdt H.-P., van den Bussche H. (2005). Cholinesterase inhibitors for patients with Alzheimer’s disease: Systematic review of randomised clinical trials. BMJ, 331(7512), 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon W., Lin E. H., Von Korff M., Ciechanowski P., Ludman E., Young B., Rutter C., Oliver M., McGregor M. (2010). Integrating depression and chronic disease care among patients with diabetes and/or coronary heart disease: The design of the TEAMcare study. Contemporary Clinical Trials, 31(4), 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon W., Unützer J., Fan M.-Y., Williams J. W., Schoenbaum M., Lin E. H., Hunkeler E. M. (2006). Cost-effectiveness and net benefit of enhanced treatment of depression for older adults with diabetes and depression. Diabetes Care, 29(2), 265–270. [DOI] [PubMed] [Google Scholar]
- Kurella M., Chertow G. M., Fried L. F., Cummings S. R., Harris T., Simonsick E., Satterfield S., Ayonayon H., Yaffe K. (2005). Chronic kidney disease and cognitive impairment in the elderly: The health, aging, and body composition study. Journal of the American Society of Nephrology, 16(7), 2127–2133. [DOI] [PubMed] [Google Scholar]
- Lawton M. P., Brody E. M. (1969). Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist, 9(3_Part_1), 179–186. [PubMed] [Google Scholar]
- Li H., Wang Y., Huang S., Yang S., Chen S., Deng Y. (2009). Application of Montreal Cognitive assessment in screening mild cognitive impairment in elderly patients. Zhonghua Shen Jing Yi Xue Za Zhi, 8(4), 376–379. [Google Scholar]
- Li X., Ma C., Zhang J., Liang Y., Chen Y., Chen K., Wang J., Zhang Z., Wang Y., & Beijing Ageing Brain Rejuvenation Initiative. (2013). Prevalence of and potential risk factors for mild cognitive impairment in community-dwelling residents of Beijing. Journal of the American Geriatrics Society, 61(12), 2111–2119. [DOI] [PubMed] [Google Scholar]
- Livingston G., Huntley J., Sommerlad A., Ames D., Ballard C., Banerjee S., Brayne C., Burns A., Cohen-Mansfield J., Cooper C., Costafreda S. G. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet, 396(10248), 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F., Wu T., Zhao J., Ji L., Song A., Zhang M., Huang G. (2016). Prevalence of mild cognitive impairment and its subtypes among Chinese older adults: Role of vascular risk factors. Dementia and Geriatric Cognitive Disorders, 41(5–6), 261–272. [DOI] [PubMed] [Google Scholar]
- Ma L., Li Z., Tang Z., Sun F., Diao L., Li J., He Y., Dong B., Li Y. (2017). Prevalence and socio-demographic characteristics of disability in older adults in China: Findings from China Comprehensive Geriatric Assessment Study. Archives of Gerontology and Geriatrics, 73, 199–203. [DOI] [PubMed] [Google Scholar]
- Morris J. C. (1997). Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. International Psychogeriatrics, 9(S1), 173–176. [DOI] [PubMed] [Google Scholar]
- Morris J. C., Storandt M., Miller J. P., Mckeel D. W., Price J. L., Rubin E. H., Berg L. (2001). Mild cognitive impairment represents early-stage Alzheimer disease. Archives of Neurology, 58(3), 397–405. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J. L., Chertkow H. (2005). The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. [DOI] [PubMed] [Google Scholar]
- Patterson C. (2018). World alzheimer report 2018. Alzheimer Disease International. [Google Scholar]
- Petersen R. C. (2004). Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine, 256(3), 183–194. <Go to ISI>://WOS:000223470900003. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- Petersen R. C. (2011). Mild cognitive impairment. New England Journal of Medicine, 364(23), 2227–2234. [DOI] [PubMed] [Google Scholar]
- Petersen R. C., Doody R., Kurz A., Mohs R. C., Morris J. C., Rabins P. V., Ritchie K., Rossor M., Thal L., Winblad B. (2001). Current concepts in mild cognitive impairment. Archives of Neurology, 58(12), 1985–1992. [DOI] [PubMed] [Google Scholar]
- Petersen R. C., Smith G. E., Waring S. C., Ivnik R. J., Tangalos E. G., Kokmen E. (1999). Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology, 56(3), 303–308. [DOI] [PubMed] [Google Scholar]
- Radler K. H., Zdrodowska M. A., Dowd H., Cersonsky T. E., Huey E. D., Cosentino S., Louis E. D. (2020). Rate of progression from mild cognitive impairment to dementia in an essential tremor cohort: A prospective, longitudinal study. Parkinsonism & Related Disorders, 74, 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D., Luo X., Tang M., Shen Y., Huang R., Yu J., Ren J., Cheng X., Lin K. (2018). Prevalence of mild cognitive impairment and its subtypes in community-dwelling residents aged 65 years or older in Guangzhou, China. Archives of Gerontology and Geriatrics, 75, 70–75. [DOI] [PubMed] [Google Scholar]
- Ren L., Zheng Y., Wu L., Gu Y., He Y., Jiang B., Zhang J., Zhang L., Li J. (2018). Investigation of the prevalence of cognitive impairment and its risk factors within the elderly population in Shanghai, China. Scientific Reports, 8(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard E., Reitz C., Honig L. H., Schupf N., Tang M. X., Manly J. J., Mayeux R., Devanand D., Luchsinger J. A. (2013). Late-life depression, mild cognitive impairment, and dementia. JAMA Neurology, 70(3), 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. O., Geda Y. E., Knopman D. S., Cha R. H., Pankratz V. S., Boeve B. F., Ivnik R. J., Tangalos E. G., Petersen R. C., Rocca W. A. (2008). The Mayo Clinic Study of Aging: Design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology, 30(1), 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzini L., Conti M. Z., Riva M., Ceraso A., Caratozzolo S., Zanetti M., Padovani A. (2018). Non-amnestic mild cognitive impairment and sleep complaints: A bidirectional relationship? Aging Clinical and Experimental Research, 30(6), 661–668. [DOI] [PubMed] [Google Scholar]
- Santisteban M. M., Iadecola C. (2018). Hypertension, dietary salt and cognitive impairment. Journal of Cerebral Blood Flow & Metabolism, 38(12), 2112–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Mielke M. M., Parsaik A. K., Cha R. H., Roberts R. O., Scanlon P. D., Geda Y. E., Christianson T. J., Pankratz V. S., Petersen R. C. (2014). A prospective study of chronic obstructive pulmonary disease and the risk for mild cognitive impairment. JAMA Neurology, 71(5), 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Allwright S., O’Dowd T. (2007). Effectiveness of shared care across the interface between primary and specialty care in chronic disease management. Cochrane Database of Systematic Reviews, 3. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V., Panza F., Colacicco A., D’introno A., Capurso C., Torres F., Grigoletto F., Maggi S., Del Parigi A., Reiman E. M., Reiman E. (2004). Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology, 63(10), 1882–1891. [DOI] [PubMed] [Google Scholar]
- Stephens E. R., Sarangi A., Gude J. (2022). Short sleep duration and dementia: A narrative review [Paper presentation]. Baylor University Medical Center Proceedings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wams E. J., Wilcock G. K., Foster R. G., Wulff K. (2017). Sleep-wake patterns and cognition of older adults with amnestic mild cognitive impairment (aMCI): A comparison with cognitively healthy adults and moderate alzheimer’s disease patients. Current Alzheimer Research, 14(10), 1030–1041. [DOI] [PubMed] [Google Scholar]
- Xu J., Wang J., Wimo A., Fratiglioni L., Qiu C. (2017). The economic burden of dementia in China, 1990–2030: Implications for health policy. Bulletin of the World Health Organization, 95(1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J., Li J., Liang J., Chen S. (2018). The prevalence of mild cognitive impairment in China: A systematic review. Aging and Disease, 9(4), 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Wang Y., Zeng Y., Gao G. F., Liang X., Zhou M., Wan X., Yu S., Jiang Y., Naghavi M., Naghavi M. (2013). Rapid health transition in China, 1990–2010: Findings from the Global Burden of Disease Study 2010. The Lancet, 381(9882), 1987–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y., Liu Z., Chen Y., Xu Y., Qin J., Guo S., Huang J., Tao J. (2021). The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Acta Diabetologica, 58(6), 671–685. [DOI] [PubMed] [Google Scholar]
- Zhang H. (2017). Opportunity or new poverty trap: Rural-urban education disparity and internal migration in China. China Economic Review, 44, 112–124. [Google Scholar]
- Zhong B.-L., Ruan Y.-F., Xu Y.-M., Chen W.-C., Liu L.-F. (2020). Prevalence and recognition of depressive disorders among Chinese older adults receiving primary care: A multi-center cross-sectional study. Journal of Affective Disorders, 260, 26–31. [DOI] [PubMed] [Google Scholar]