Abstract
Endocrine therapy (ET) is a pivotal strategy to manage early- and advanced-stage estrogen receptor-positive (ER+) breast cancer. In patients with metastatic breast cancer (MBC), progression of disease inevitably occurs due to the presence of acquired or intrinsic resistance mechanisms. ET resistance can be driven by ligand-independent, ER-mediated signaling that promotes tumor proliferation in the absence of hormone, or ER-independent oncogenic signaling that circumvents endocrine regulated transcription pathways. Estrogen receptor 1 (ESR1) mutations induce constitutive ER activity and upregulate ER-dependent gene transcription, provoking resistance to estrogen deprivation and aromatase inhibitor therapy. The role ESR1 mutations play in regulating response to other therapies, such as the selective estrogen receptor degrader (SERD) fulvestrant and the available CDK4/6 inhibitors, is less clear. Novel oral SERDs and other next-generation ETs are in clinical development for ER+ breast cancer as single agents and in combination with established targeted therapies. Recent results from the phase III EMERALD trial demonstrated improved outcomes with the oral SERD elacestrant compared to standard anti-estrogen therapies in ER+ MBC after prior progression on ET, and other agents have shown promise in both the laboratory and early-phase clinical trials. In this review, we will discuss the emerging data related to oral SERDs and other novel ET in managing ER+ breast cancer. As clinical data continue to mature on these next-generation ETs, important questions will emerge related to the optimal sequence of therapeutic options and the genomic and molecular landscape of resistance to these agents.
Keywords: breast cancer, endocrine therapy, estrogen, metastatic disease, SERD
Introduction: current endocrine therapies and mechanisms of action
Endocrine therapy (ET) is effective in the treatment of estrogen receptor-positive (ER+) breast cancer. 1 These breast tumors express estrogen receptor alpha (ERα) and are dependent on estrogen-mediated growth signaling. Collectively, ET works by depleting circulating estrogens available to bind to the estrogen receptor (ER), targeting the ER directly and antagonizing or degrading, or a combination of the two. Anti-estrogen drugs are part of the standard armamentarium against early- and advanced-stage ER+ tumors, which accounts for close to 80% of newly diagnosed breast cancers and includes ER+/human epidermal growth factor receptor 2 (HER2−) and ER+/HER2+ disease. 2 Approved classes of ET can be broadly categorized into aromatase inhibitors (AIs), selective estrogen receptor modulators (SERMs), and selective estrogen receptor degraders (SERDs), and can be used with or without ovarian suppression.
The AIs (letrozole, anastrozole, and exemestane) block the conversion of androgens to estrogens in non-ovarian tissues and decrease systemic estrogen levels in post-menopausal women. 3 In hormone responsive tumors, adjuvant AIs effectively reduce the risk of recurrence after curative treatment, 1 and they are standard first-line therapy in metastatic disease, often in combination with a CDK4/6 inhibitor (CDK4/6i).4,5 AIs are oral medications with a side effect profile that includes exacerbating menopausal symptoms, vaginal dryness, arthralgias, and accelerated bone loss.
SERMs competitively bind the ER and have tissue-dependent antagonist or agonist properties. 6 They potentiate an anti-estrogenic effect in breast tumors and prevent hormone-dependent proliferation. 6 SERM-bound ER binds estrogen response elements (EREs) and downregulates transcriptional activity in breast tumors by associating with co-repressors. 7 Tamoxifen is the most commonly used SERM in the clinical management of ER+ breast cancer; however, the development of newer agents in this class has demonstrated its exciting potential and is discussed in greater detail below. SERMs are oral and can be advantageous over AIs in terms of less sexual side effects and arthralgias, but can cause undesired adverse events such as endometrial hyperplasia and malignancy from agonist activity and deep venous thrombosis possibly due to their anti-estrogen effect in platelets. 8
SERDs, such as fulvestrant, both antagonize ER transcriptional activity and promote its degradation. 9 These agents bind ER causing immobilization and instability of the ER-SERD complex and facilitate ER degradation by the ubiquitin-proteasome pathway.10,11 More recent research suggests that SERDs exert an effect by slowing ER nuclear translocation, increasing ER turnover as a consequence of limited intracellular mobility. 12 In addition, SERD-bound ER undergoes conformational changes that reduce transcription of ER-modulated genes. 12 Clinical challenges with fulvestrant can be related to pharmacologic limitations, including the absence of oral bioavailability, 13 but this drug can have a favorable side effect profile in terms of arthralgias. SERDs are also advantageous in the clinical setting of estrogen receptor 1 (ESR1) mutation-related ET resistance, discussed in detail below. Several novel oral SERDs are in development and have shown exciting clinical results which are discussed later in this review. 14
In premenopausal women, ovarian suppression with a luteinizing hormone releasing hormone agonist reduces circulating estrogen. Ovarian suppression is generally recommended in pre- or perimenopausal women with distant sites of disease or high-risk early stage breast cancer, where its combination with standard adjuvant therapy improves disease-free survival. 15 Ovarian suppression also enables the use of AIs in high-risk younger women by blocking intrinsic hormonal production when AIs are advantageous over tamoxifen for added risk reduction. 16
In this review, we will discuss the scientific rationale, current data, and upcoming clinical trials related to oral SERDs and other novel ET in management of ER+ breast cancer.
Emerging insights related to endocrine resistance
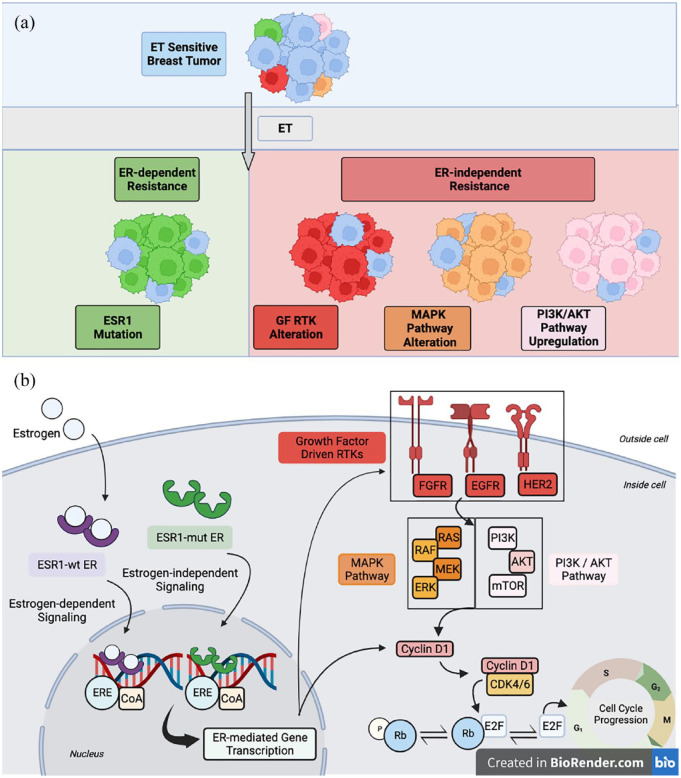
In the metastatic setting, ER+ breast cancer often responds initially to endocrine-directed therapy; however, the development of resistance and disease progression inevitably occur. 4 Response to ET may depend on the presence of intrinsic or acquired drivers of endocrine resistance; acquired resistance emerges following an initial response to therapy (generally six or more months on treatment), whereas intrinsically resistant breast cancers may not respond at all (generally less than 6 months on treatment). 7 Potential drivers of resistance have been elucidated through decades of efforts in the laboratory and clinic, a topic that has been extensively reviewed elsewhere.17,18 Endocrine resistance can be broadly subdivided into two categories including ER-mediated and ER-independent signaling, shown in Figure 1. This review will briefly highlight the mechanisms that have impacted emerging therapeutic strategies.
Figure 1.
Drivers of ET resistance can be broadly subdivided into two categories of (i) ER-dependent and (ii) ER-independent mechanisms. (a) Ligand binding domain Estrogen Receptor 1 (ESR1) mutations mediate ligand-independent ER signaling and promote ET resistance via constitutive ER activity, upregulated coactivator binding, and stability against proteolytic degradation; ER remains a viable therapeutic target in these tumors. ER-independent resistance may be mediated by several mechanisms including mutations or amplifications in growth factor-driven RTKs (HER2, EGFR, and FGFR), alterations in MAPK pathway components including KRAS, BRAF, MAP2K1, and NF1, and upregulation in PI3K/AKT pathway signaling, though notably, PIK3CA and AKT mutations have not been shown to provoke resistance in the clinical setting. These alterations serve to upregulate mitogenic and survival signaling and promote cell cycle progression and drug resistance. (b) At the cellular level, depicted are select pathways implicated in response and resistance to ET in ESR1-mut and ESR1-wt metastatic breast cancer. Note, while ER-dependent and ER-independent pathways are largely depicted separately for conceptualization, there is considerable intracellular crosstalk between these pathways. Purple factors are involved in estrogen-dependent signaling. Green factors facilitate estrogen-independent, ER-mediated signaling. Red, orange, and pink factors engage in ER crosstalk, and dysregulation in mitogenic signaling pathway components can contribute to ER-independent tumor growth and SERD resistance.
AKT, protein kinase B; CDK, cyclin-dependent kinase; CoA, coactivator; EGFR, epidermal growth factor receptor; ER, estrogen receptor; ERE, estrogen response element; ERK, extracellular signal-regulated kinase; ESR1-mut, ESR1 mutant; ESR1-wt, ESR1 wild-type; ET, endocrine therapy; FGFR, fibroblast growth factor receptor; GF RTK, growth factor-driven receptor tyrosine kinase; HER2, human epidermal growth factor receptor 2; MAPK, mitogen-activated protein kinase; MEK, meiotic chromosome-axis-associated kinase; mTOR, mammalian target of rapamycin; p, phosphate; PI3K, phosphoinositide 3-kinase; Rb, retinoblastoma; SERD, selective estrogen receptor degrader.
The ER itself is a ligand-dependent transcription factor that is activated by the steroid hormone estrogen and promotes proliferation through genomic and non-genomic mechanisms. 7 The ligand-bound ER complex associates with DNA at ERE, generally recruiting coactivators in the process, and serves to mediate transcription of ER-controlled genes. 7 Among a myriad of functions, these genes induce production of growth factors such as transforming growth factor alpha (TGFα) and insulin-like growth factor-1 (IGF1), regulate the expression of membrane receptor tyrosine kinases (RTKs) that promote cell survival, and increase cyclin D1 and MYC expression which drive cell cycle progression.18,19 Collectively, these functions can enhance pathologic breast cancer development when dysregulated.
In response to estrogen, cytoplasm- and membrane-associated ER also stimulates growth signaling directly, which is considered to be a non-genomic mechanism of regulation. 7 ER interaction with growth factor-dependent RTKs, such as HER2, epidermal growth factor receptor (EGFR) and insulin-like growth factor-1 receptor (IGF1R), as well as other signaling molecules such as SRC kinase, activates downstream mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/AKT oncogenic signal transduction pathways. 20 The MAPK and PI3K/AKT pathways are well characterized in cancer and play a role in tumor proliferation, invasive potential, and drug resistance.21,22
ESR1 mutation as a clinical biomarker of AI resistance and ER pathway dependency
Mutated ER can provoke estrogen-independent ER activity and mediate resistance to estrogen deprivation. 23 Activating mutations in the ligand binding domain (LBD) of ESR1, the gene that codes for ERα, are rare in primary breast cancer but represent a commonly acquired mechanism of endocrine resistance in ER+ metastatic breast cancer (MBC).24–26 ESR1 mutations are expressed in 4–8% of breast cancers that recur after adjuvant AI treatment and less than 1% of de novo metastatic tumors27–29; mechanisms beyond aberrant ESR1 may play a more significant role in resistance to adjuvant ET. 17 Aberrant ESR1 emerges most often following prior AI exposure.27,30 After receiving a line of AI therapy in the metastatic setting, approximately 20–40% of tumors will acquire an ESR1 mutation, with higher prevalence demonstrated on cell-free DNA (cfDNA) analyses than solid tumor biopsies.27,28,30 ESR1 alterations were not readily apparent in The Cancer Genome Atlas due to sequencing of treatment-naïve MBC samples, 31 but subsequent sequencing efforts characterizing ET-resistant tumors revealed ESR1 as an acquired driver of resistance.25,32 The most common mutations seen in the ESR1 gene are Y537S and D538G, with a prevalence of 14–21% and 32–36%, respectively; several other activating mutations with lower rates of incidence have been implicated in resistance, including Y537C, E380Q, S463P, V534E, P535H, L536H, L536P, L536R, L536Q, and Y537N.33–35 Studies characterizing serial cfDNA in MBC demonstrate a relatively frequent polyclonal mutation burden and high levels of genetic heterogeneity in these tumors.28,36,37
There are several implicated mechanisms by which ESR1-mutant (ESR1-mut) ER mediates resistance to ET. Activating ESR1 mutations affect the LBD and stabilize an active receptor conformation.14,38,39 This promotes binding of coactivators and upregulates ER signal transduction in the absence of estrogen.14,38,39 Biochemical changes at the LBD of ESR1-mut confer a decreased affinity for ligands, including SERMs and SERDs, and greater stability against proteolytic degradation.24,25,32,38–40 Activity of ESR1-mut has neomorphic and hypermorphic regulatory effects as well, enhancing the transcription of genes not activated by ESR1 wild-type (ESR1-wt) ER that promote a pro-metastatic phenotype.40–42 Research also demonstrates mutation-specific variability in resistance patterns, where Y537S conveys greater resistance to ET33,40,42 while D538G carries greater metastatic potential.42,43
ESR1 mutations in ER+ breast cancer have been shown to provoke resistance to AIs in both the preclinical and clinical setting.24,25,34,44 In laboratory-based models, higher doses of tamoxifen and fulvestrant are required to inhibit ESR1-mut transcriptional activity, suggesting ESR1 mutations provoke dose-dependent resistance to these agents.24,25,32,38–40 The degree of resistance can be variable and highly mutant specific, ranging from as high as 50-fold dose increase in Y537S mutants, while a more modest 2- to 10-fold dose increase is required to inhibit models of most other ESR1 mutants.24,25,32,38–40 However, the impact of ESR1 mutations to SERD and SERM response remains controversial and likely context dependent.44–48
ESR1 fusions (ESR1-fus) represent rare but notable alterations that eliminate the LBD and drive ET resistance through constitutive ER transcriptional activity.49,50 Since ESR1-fus ER becomes completely independent of the LBD domain, these tumors are likely resistant to most current- and next-generation ETs that target the LBD. Data suggest that ESR1-fus represent about 0.5–1% of all ESR1 alterations,26,51,52 although the frequency of fusion events may be underappreciated as many of the breakpoints are intronic and not captured by conventional next-generation sequencing (NGS) assays. 17 The use of RNA-based fusion detection methods has the potential to increase clinical recognition of ESR1-fus events, especially in post-SERD tumor samples as a mechanism of acquired secondary resistance. 49
Oncogenic signal transduction and ER pathway independence
Mutations and amplifications in oncogenic pathway components can drive ET resistance through upregulated mitogenic and survival pathway signaling. Laboratory and clinical data suggest that alterations in RTKs HER2,53–55 EGFR,26,56 and fibroblast growth factor receptors (FGFRs),57,58 provoke ET resistance by facilitating downstream growth signaling. Similar resistance is seen with loss-of-function mutations in the tumor suppressor NF1, leading to uncontrolled RAS activity.26,59–61 Activating alterations in other MAPK components KRAS, BRAF, and MAP2K1 have been demonstrated in pre- and post-treatment matched tumor samples that acquired ET resistance. 26 Upregulation of the PI3K/AKT pathway provokes resistance to estrogen deprivation in laboratory-based models, 62 and alterations in PIK3CA, AKT, and PTEN are often observed in ER+ MBC, 63 although mutations in these genes have not been shown to promote ET resistance in the clinical setting. Downstream mitogenic signaling increases cyclin D1 expression and facilitates cell cycle progression through the cyclin-dependent kinase 4/6 (CDK4/6)/RB/E2F pathway.64,65
Overall, intracellular communication between these pathways is important in the development of resistance and is exploitable therapeutically. Endocrine resistance can be provoked by loss of ER; however, this mechanisms is only seen in approximately 10% of cases. 66 Other mechanisms, including alterations in gene transcription regulators (MYC, ARID1A, FOXA1, CTCF, others), epigenetic changes, aberrant cofactor activity, and the tumor microenvironment, have been implicated in ET resistance and discussed elsewhere, 17 but they are beyond the scope of this review.
Note, while the major mechanisms such as ER-dependent and ER-independent pathways have been highlighted for conceptual understanding, in reality, intracellular crosstalk is complex. ER signaling is also regulated, in part, by oncogenic signal transduction in a ligand-independent manner. 20 In the absence of estrogen, MAPK and PI3K/AKT pathway activation increases the transcriptional function of ER through phosphorylation of the receptor, 20 and downregulates ER expression. 67 Cyclin D1 can activate ER directly and further promote ER-mediated gene transcription. 64 Thus, continued targeting of the ER will remain the cornerstone of management of ER+ breast cancer despite resistance. This has led to considerable interest in novel endocrine therapies, particularly those that will be active despite ESR1 mutations. There are a number of next-generation ET drugs in clinical development and a list is summarized in Table 1; the mechanisms of action of several current and novel ETs are depicted in Figure 2.
Table 1.
List of next-generation endocrine agents in development for the management of hormone receptor-positive breast cancer.
| Endocrine agent | Developing company | ET class | Mode of delivery | Disease setting | Phase of development |
|---|---|---|---|---|---|
| Elacestrant (RAD1901) | Radius Health | SERD | Oral | Metastatic; neoadjuvant | 3 complete |
| Amcenestrant (SAR439859) | Sanofi | SERD | Oral | Metastatic; adjuvant | 2–3 |
| Camizestrant (AZD9833) | Astra Zeneca | SERD | Oral | Metastatic; neoadjuvant | 2–3 |
| Giredestrant (GDC-9545) | Genentech/Roche | SERD | Oral | Metastatic; adjuvant; neoadjuvant | 2–3 |
| Imlunestrant (LY3484356) | Eli Lilly | SERD | Oral | Metastatic; neoadjuvant | 1 |
| Rintodestrant (G1T48) | G1 Therapeutics | SERD | Oral | Metastatic | 1–2 |
| Borestrant (ZB-716) | Zeno Pharma | SERD | Oral | Metastatic | 1 – 2 |
| ZN-c5 | Zentalis | SERD | Oral | Metastatic | 1–2 |
| D-0502 | Inventisbio | SERD | Oral | Metastatic | 1 |
| Lasofoxifene | Sermonix | SERM | Oral | Metastatic | 2 |
| Bazedoxifene | Pfizer | SERM/SERD Hybrid | Oral | Metastatic; DCIS | 2 |
| H3B-6545 | H3 Biomedicine | SERCA | Oral | Metastatic | 1–2 |
| OP-1250 | Olema Oncology | CERAN | Oral | Metastatic | 1–2 |
| ARV-471 | Arvinas | PROTAC | Oral | Metastatic | 1–2 |
| AC682 | Accutar Biotech | Chimeric ER Degrader | Oral | Metastatic | 1 |
AI, aromatase inhibitors; CERAN, complete estrogen receptor antagonist; ET, endocrine therapy; PROTAC, proteolysis targeting chimer; SERCA, selective estrogen receptor covalent antagonist; SERD, selective estrogen receptor degrader; SERM, selective estrogen receptor modulator.
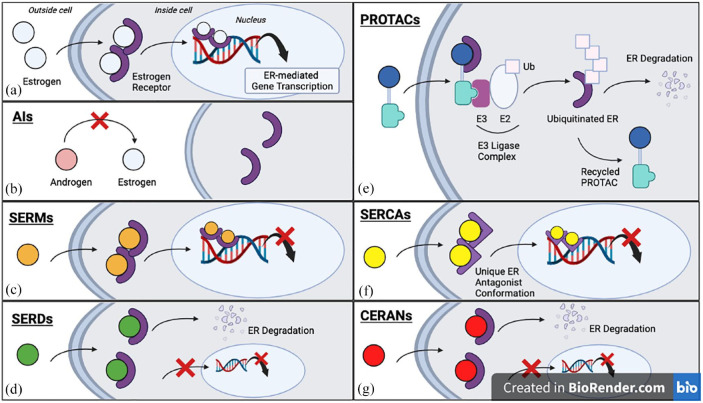
Figure 2.
The mechanisms of action of different ETs, simplified for conceptualization. (a) Estrogen binds the ER, a ligand-dependent transcription factor, promoting ER dimerization and translocation to the nucleus. The estrogen-bound ER dimer regulates gene expression that facilitates cell growth and survival. (b) The AIs block the aromatization of androgens to estrogen. (c) SERMs competitively bind ER and mediate a tissue-dependent anti-estrogen effect. (d) SERDs slow ER nuclear translocation, increase receptor turnover, and reduce transcription of ER-regulated genes. (e) PROTACs mediate an interaction between ER and the E3 ligase complex, facilitating ubiquitination of ER and subsequent proteasomal degradation; the PROTAC molecule is recycled in this process. (f) SERCAs covalently bind the C530 residue in the ER ligand-binding domain and promote a unique antagonist conformation that decreases ER-regulated gene transcription. (g) CERANs bind ER and potentiate their effect by inducing ER degradation and blocking transcriptional activity.
AIs, aromatase inhibitors; CERANs, complete estrogen receptor antagonists; ER, estrogen receptor; ETs, endocrine therapies; PROTACs, proteolysis targeting chimers; SERCAs, selective estrogen receptor covalent antagonists; SERDs, selective estrogen receptor degraders; SERMs, selective estrogen receptor modulators.
Novel hormonal therapies and clinical opportunities
Selective estrogen receptor degraders
Fulvestrant
After the development of resistance to ET, breast cancers frequently continue to depend on estrogen-independent ER-mediated signaling, and the ER remains a viable therapeutic target. 68 Development of an endocrine agent with global ER antagonistic activity in the 1980s–1990s led to the creation of fulvestrant.69,70 As the first clinically impactful SERD, fulvestrant has been incorporated into the standard armamentarium for management of ER+ MBC. Fulvestrant antagonizes ER transcriptional activity by inhibiting nuclear translocation, promoting turnover through the ubiquitin-proteasome pathway, and inducing conformational change that downregulates ER signaling. 12 In MBC exposed to prior ET, a pair of clinical trials demonstrated non-inferiority of a 250 mg dose of monthly fulvestrant compared to anastrozole71,72; the subsequent CONFIRM trial showed improved progression-free survival (PFS) and overall survival (OS) in patients receiving a 500 mg dose versus 250 mg dose of fulvestrant, which is the currently approved dose.73,74 The FALCON trial revealed that in treatment-naïve advanced breast cancer, patients treated with fulvestrant had significantly longer PFS compared to anastrozole (16.6 months versus 13.8 months), leading to its approval in the first-line setting. 75 ESR1 mutations are a recognized mechanism of acquired resistance to AIs, 76 and these patients may retain some clinical sensitivity to SERDs,28,44,46 so the sequencing of ET lines for non-curative breast cancer treatment is an important clinical consideration.
Research has demonstrated conflicting results related to the impact of ESR1 mutations on fulvestrant sensitivity in the laboratory and in the clinic. A robust body of preclinical work showed that ESR1-mut expressing breast cancer models demonstrate relative (dose-related) resistance to fulvestrant, requiring 10- to 50-fold greater concentrations of drug to inhibit ER signaling and tumor growth.24,25,39,40,77 However, clinical studies have demonstrated variable results regarding the efficacy of fulvestrant in the treatment of ESR1-mut MBC. Retrospective analysis of the FERGI, SoFEA, EFECT, and PALOMA-3 trials were performed to assess the impact of ESR1-mut on tumor response to fulvestrant.28,44–46 Combined analysis of the SoFEA and EFECT trials, which compared fulvestrant versus exemestane in MBC after progression on an AI, demonstrated statistically significantly worse PFS (2.4 months versus 4.8 months) and 1-year OS in patients with ESR1 mutations on the exemestane arm. 44 However, ESR1-mut and ESR1-wt MBC treated with fulvestrant had similar PFS (3.9 months versus 4.1 months) and 1-year OS. 44 In the metastatic setting after progression on an AI, the FERGI trial examined fulvestrant versus fulvestrant plus pictilisib 78 and the PALOMA-3 trial examined fulvestrant versus fulvestrant plus palbociclib. 79 Retrospective analysis of the fulvestrant arm in both clinical trials demonstrated the following: (a) no significant difference in PFS in ESR1-mut compared to ESR1-wt tumors; (b) similar prevalence of ESR1 mutations at the start and end of treatment; and (c) that expression of multiple ESR1 alterations or higher variant allele frequency (VAF) burden were not associated with worse outcomes.45,46 Notably, in the PALOMA-3 analysis, there was evidence for increased positive selection for Y537S mutation at time of progression. 45 Although limited by sample size (n = 19), this specific ESR1 mutation trended toward shorter PFS (p = 0.14), highlighting the importance of further investigation into the impact of specific allelic variants on therapeutic resistance. 45
It is important to note that these analyses are retrospective and exploratory. Elucidating the true role of ESR1-mut in mediating response or resistance to SERDs requires large, prospective studies and the use of standardized methods for detecting ESR1 mutations. 80 The importance of this is highlighted by clinical results presented from the plasmaMATCH trial. 47 Trial participants with MBC had cfDNA analyzed for mutations in ESR1, HER2, AKT, and PTEN and were matched to concordant targeted therapies. In this heavily pretreated population, with a median of two prior lines of ET, high-dose fulvestrant monotherapy (500 mg biweekly dosing) was not effective in patients with ESR1-mut, 47 with a PFS of only 2.2 months. However, it is notable that tumors expressing a higher ESR1 VAF (>50%) demonstrated rare responses to fulvestrant [overall response rate (ORR) 12%; n = 44] compared to lower VAF (<50%) (ORR 0%; n = 30), suggesting that mechanisms of resistance in these heavily pre-treated tumors are likely heterogeneous, and stratifying patients based on ESR1 status alone may not be an optimal clinical approach to maximizing benefit. 47 In the first-line setting, the phase III PADA-1 trial is evaluating the clinical benefit of switching from an AI to fulvestrant plus CDK4/6i upon detection of ESR1-mut on serial circulating tumor DNA (ctDNA) analysis prior to disease progression. 81 Presented results demonstrated an improved median PFS of 11.9 months on the fulvestrant-CDK4/6i arm compared to 5.7 months on the AI-CDK4/6i arm; patients with progression on the AI arm could enroll in an optional crossover cohort, and this subgroup had a median second PFS of 3.5 months. 29 Compared with the standard strategy of starting fulvestrant after disease progression, this study highlights the potential benefit of targeting emerging ESR1-mut early in this patient population, although the OS data require more time to mature.
Pharmacologic limitations regarding the bioavailability of fulvestrant have prompted strong interest in an orally bioavailable alternative. 82 A number of novel oral SERDs demonstrating preclinical and clinical efficacy in ER+ breast cancer are in varying stages of clinical development. These drugs could potentially replace fulvestrant, as monotherapy or as the ET backbone of combination treatment, in the management of ESR1-mut and ESR1-wt ER+ breast cancer. 83
Elacestrant
The oral SERD elacestrant (RAD1901) was developed by Radius Health for use in ER+ breast cancer and has demonstrated exciting clinical efficacy. Elacestrant selectively antagonizes ER, promoting ER turnover, and disrupting downstream signaling. 84 Laboratory-based studies demonstrate dose-dependent antitumor activity in ET-resistant breast cancer cells and patient-derived xenograft (PDX) models, including those expressing ESR1 mutations 84,85; efficacy is retained in multiple in vitro models of CDK4/6i resistance as well. 86
Phase I evaluation of elacestrant showed single-agent activity in a heavily pretreated population of postmenopausal women with three median prior lines of therapy and a 50% rate of baseline ESR1 mutation. 87 These encouraging results led to the multicenter phase III EMERALD trial which evaluated elacestrant versus ET SOC (fulvestrant or AI) in ER+ MBC following progression on 1–2 prior lines of ET and prior exposure to a CDK4/6i. 88 Around 477 patients were enrolled, and 228 of them harbored a baseline ESR1 mutation (47.8%). 88 Recently published data from the EMERALD trial demonstrated that, compared with standard of care (SOC), elacestrant prolonged PFS in all patients [hazard ratio (HR) = 0.70] and patients with ESR1-mut tumors (HR = 0.55), which were the primary endpoints of the study. 88 Improved 12-month PFS and median PFS were seen in the overall population (22.3% versus 9.4%; 2.8 months versus 1.9 months) and ESR1-mut cohort (26.8% versus 8.2%; 3.8 month versus 1.9 month). 88 In both arms, the modest difference in median PFS may be explained by an initial drop in the Kaplan–Meier curves, representing a subset of endocrine-resistant tumors in the second-/third-line ET setting, followed by survival curve separation between elacestrant and SOC in the endocrine-sensitive setting. 88 The interim OS analysis showed a trend toward favoring elacestrant in all patients (HR = 0.751) and those with ESR1-mut (HR = 0.592), with expectations for final OS reporting in 2022. 88 The most common treatment-related adverse events were nausea (25.3%), vomiting (11%), and fatigue (11%), primarily grade 1–2. 88
This is the first novel oral SERD in a phase III study to demonstrate a significant and clinically meaningful improvement over fulvestrant or AI in this population. 88 Pretreated patients harboring ESR1 mutations had an even more pronounced clinical benefit with elacestrant and, based on this susceptibility, further efforts to assess the response of ESR1-mut metastatic tumors with novel oral SERDs remain ongoing.
Amcenestrant
Amcenestrant (SAR439859) is another oral SERD in development by Sanofi. Preclinical efficacy was demonstrated in breast cancer cells and PDX tumors, including ESR1-mut models which demonstrated relative (dose related) resistance to amcenestrant. 77 The phase I/II AMEERA-1 trial demonstrated antitumor activity of amcenestrant monotherapy in heavily pretreated ER+ MBC after progression on prior ET. 89 Single-agent amcenestrant conveyed a similar clinical benefit rate (CBR) in tumors expressing ESR1-mut (32.1%) and ESR1-wt (36.7%) (n = 58 for patients with ESR1 status). 89
Based on these monotherapy results, amcenestrant is being evaluated in phase II and III trials characterizing its efficacy compared to SOC ET in MBC after progression on prior ET (AMEERA-3, NCT04059484) and first line in combination with palbociclib for treatment-naïve ER+ MBC (AMEERA-5, NCT04478266). Although the final data have not yet been presented, Sanofi announced that the AMEERA-3 trial did not meet its primary endpoint of improving PFS. 90 The phase I AMEERA-1 trial is ongoing and examining targeted agent combinations of amcenestrant with palbociclib, alpelisib (a PI3K inhibitor), or everolimus [an mammalian target of rapamycin (mTOR) inhibitor] (NCT03284957). In the adjuvant setting, the phase III AMEERA-6 trial is evaluating amcenestrant versus tamoxifen in patients with early-stage ER+ breast cancer who discontinued AI therapy due to treatment-related toxicity (NCT0512877).
Camizestrant
The novel SERD camizestrant (AZD9833) is in development from AstraZeneca. In addition to promoting ER degradation, camizestrant’s novel structure increases potency of the molecule and facilitates a pattern of gene regulation that is distinct when compared to fulvestrant.91,92 Camizestrant inhibits growth in preclinical breast cancer models, and unlike fulvestrant shows no relative (dose dependent) resistance in tumors expressing ESR1-mut. 93 In the phase I SERENA-1 trial, camizestrant showed single-agent activity treating ER+ MBC that had progressed on prior ET.94,95 ESR1 mutations were detected in baseline cfDNA samples in 26/56 (46%) of patients tested; 50% of tumors expressing ESR1-mut had a partial response or stable disease at 24 weeks on therapy, including 5/10 patients harboring Y537S alterations. 95
These encouraging findings have prompted phase II and III clinical trials examining camizestrant in several disease settings: (a) as monotherapy in ER+ MBC after progression on ET (SERENA-2, NCT04214288); (b) in combination with palbociclib as first-line treatment for advanced ER+ breast cancer (SERENA-4, NCT04711252) and ESR1-mut MBC (SERENA-6, NCT04964934); and (c) as neoadjuvant therapy in a window-of-opportunity study for early-stage disease (SERENA-3, NCT04588298). Similar to other oral SERDs, the SERENA-1 trial remains active in evaluating camizestrant in combination with CDK4/6, PI3K, or mTOR inhibitor, and recently presented data demonstrated efficacy and tolerability in combination with palbociclib. 95 Notable side effects of camizestrant documented during the SERENA-1 trial include sinus bradycardia and visual disturbances, neither of which prompted discontinuation of therapy. 95
Giredestrant
Giredestrant (GDC-9545) is under development by Roche/Genentech. This oral SERD induces tumor regression in ESR1-mut PDX models in the laboratory. 96 Evidence of single-agent giredestrant antitumor activity was demonstrated in a phase I clinical trial enrolling patients with ER+ MBC after progression on two or fewer prior lines of ET, including patients harboring baseline ESR1 mutations (NCT03332797).97,98 It is notable that only 21% of patients in this cohort received prior fulvestrant, less than other phase I novel SERD trials reviewed here. 98 As with camizestrant, grade 1–2 sinus bradycardia was seen on therapy, though it appears to be dose dependent with low frequency at 30 mg dose. 98
Giredestrant efficacy data have led to the development of several clinical trials evaluating its use in the both advanced- and early-stage breast cancer at 30 mg dose. In the metastatic setting, first-line giredestrant combined with palbociclib for treatment-naïve MBC is being evaluated in the phase III persevERA trial (NCT04546009). Giredestrant monotherapy for MBC progressed on prior ET was the focus of the phase II acelERA trial (NCT04576455); however, Roche recently announced that the study did not meet its primary endpoint of improving PFS. 99 In early-stage disease, the phase II neoadjuvant window-of-opportunity coopERA study demonstrated superior efficacy of giredestrant over AI in terms of Ki67 suppression in ER+ breast tumors (NCT04436744). 100 The ongoing phase III lidERA trial is examining adjuvant giredestrant versus AI or tamoxifen for stage I–III ER+ breast cancer (NCT04961996).
Imlunestrant
Imlunestrant (LY3484356) is an oral SERD being developed by Eli Lilly and has demonstrated preclinical efficacy against wt- and ESR1-mut breast cancer models, both as a single-agent and in combination with several targeted therapies. 101 The active phase I EMBER trial is characterizing imlunestrant alone or combined with abemaciclib, everolimus, alpelisib, trastuzumab, or AI for ER+ MBC (NCT04188548). Imlunestrant monotherapy data from the EMBER trial showed efficacy in a cohort of pretreated ER+ MBC patients. 102 In the phase III setting, imlunestrant with or without abemaciclib is being compared against fulvestrant or exemestane for management of hormone-sensitive advanced breast cancer after progression on AI (EMBER-3, NCT04975308). In addition, the ongoing window-of-opportunity, phase I EMBER-2 trial is evaluating the pharmacodynamic effect of imlunestrant in the neoadjuvant setting (NCT04647487).
Rintodestrant
Rintodestrant (G1T48) is an oral SERD being developed by G1 therapeutics. Preclinical work showed dose-independent efficacy of this agent in breast cancer cells and ESR1-mut PDX tumors. 103 In a phase I clinical trial, rintodestrant conveyed antitumor activity in ER+ MBC after progression on prior lines of therapy. 104 Trial results demonstrated benefit in patients expressing ESR1 mutations. 104 Evaluation of rintodestrant combined with palbociclib for ET-resistant MBC in the advanced disease setting is ongoing (NCT03455270).
Others
There are several additional next-generation SERDs that have demonstrated preclinical activity and are being evaluated in phase I–II clinical trials, including borestrant [ZB-716; ENZENO trial (NCT04669587)], ZN-c5 (NCT03560531), and D-0502 (NCT03471663).
While many of the current developments in oral SERDs have shown promise, maintaining a perspective on historical challenges is important in this growing field. After the advent of fulvestrant, initial attempts at creating an orally bioavailable SERD proved challenging. 105 Numerous iterations of ER-degrading compounds with chemically modified polarity and solubility were required to overcome bioavailability limitations. 105 Several candidate compounds showing preclinical antitumor activity have also been discontinued during various stages of clinical testing. One example is brilanestrant (GDC0810), an oral SERD that was removed from development by Roche after a phase II clinical trial failed to demonstrate comparable or superior efficacy to fulvestrant (NCT01823835). Another compound, LSZ102, had its phase I testing terminated by trial sponsor Novartis. Prior to discontinuation, interim single-agent results of LSZ102 in heavily pretreated ER+ MBC patients showed an ORR 1.3%, CBR 9.1%, and median PFS 1.8 months. 106 Notably, both of these drugs contain an acrylic acid side chain, whereas many of the novel oral SERDs discussed above were created with a basic amino side chain to optimize ER degradation across multiple breast cancer cell lines. 107 Similarly, clinical development of AZD9496 was discontinued in favor of AZD9833 that was optimized for absorption and activity. Differences in chemical structure, efficacy, and side effects between agents will likely play a significant role in determining which drugs ultimately enter clinical practice.
More broadly, the optimal use of SERDs and sequencing of ET in ER+ MBC remains uncertain. The PARSIFAL trial was conducted to assess the efficacy of fulvestrant compared to letrozole when combined with palbociclib for first-line management of ER+/HER2− MBC. 108 Although significant antitumor activity was demonstrated in the fulvestrant–palbociclib arm, an improvement in PFS over the letrozole–palbociclib regimen was not seen. 108 The results of several ongoing trials examining next-generation SERDs for first-line management of hormone-sensitive MBC will be informative in helping guide their clinical use (Table 3).
Table 3.
Ongoing clinical trials of next-generation endocrine therapies in the metastatic, adjuvant, and neoadjuvant setting.
| Endocrine agent | ET class | Combination therapy (drug class) | Disease setting | Trial description | Phase | Clinical trial identifier |
|---|---|---|---|---|---|---|
| First-line metastatic breast cancer trials | ||||||
| Oral SERDs | ||||||
| Amcenestrant (SAR439859) | SERD | Palbociclib (CDK4/6i) | Advanced/metastatic; first line | Amcenestrant + palbociclib versus letrozole + palbociclib for treatment-naïve ER + MBC | Phase III | NCT04478266 (AMEERA-5) |
| Camizestrant (AZD9833) | SERD | Palbociclib (CDK4/6i) | Advanced/metastatic; first line | Camizestrant + palbociclib versus AI + palbociclib for treatment-naïve ER + MBC | Phase II | NCT04711252 (SERENA-4) |
| Giredestrant (GDC-9545) | SERD | Palbociclib (CDK4/6i) | Advanced/metastatic; first line | Giredestrant + palbociclib versus letrozole + palbociclib for treatment-naïve ER + MBC | Phase III | NCT04546009 (persevERA) |
| Second-line plus metastatic breast cancer trials | ||||||
| Oral SERDs | ||||||
| Amcenestrant (SAR439859) | SERD | None | Advanced/metastatic | Amcenestrant versus AI or fulvestrant or tamoxifen for ER + MBC progressed on ET | Phase II | NCT04059484 (AMEERA-3) |
| Amcenestrant (SAR439859) | SERD | Various | Advanced/metastatic | Amcenestrant + palbociclib, alpelisib, everolimus, or abemaciclib for ER + MBC progressed on ET | Phase I–II | NCT03284957 (AMEERA-1) |
| Camizestrant (AZD9833) | SERD | None | Advanced/metastatic | Camizestrant versus fulvestrant for ER + MBC progressed on ET | Phase II | NCT04214288 (SERENA-2) |
| Camizestrant (AZD9833) | SERD | Various | Advanced/metastatic | Camizestrant alone or in combination with palbociclib, abemaciclib, everolimus, or capivasertib for ER + MBC progressed on ET | Phase I | NCT04214288 (SERENA-1) |
| Camizestrant (AZD9833) | SERD | Abemaciclib/palbociclib (CDK4/6i) | Advanced/metastatic | Camizestrant + palbociclib or abemaciclib versus AI + palbociclib or abemaciclib for ESR1 mutant ER + MBC before progression | Phase III | NCT04964934 (SERENA-6) |
| Giredestrant (GDC-9545) | SERD | None | Advanced/metastatic | Giredestrant versus fulvestrant or AI for ER + MBC progressed on ET | Phase II | NCT04576455 (acelERA) |
| Imlunestrant (LY3484356) | SERD | Various | Advanced/metastatic | Imlunestrant alone or combined with abemaciclib, everolimus, alpelisib, trastuzumab, or AI for ER + MBC | Phase I | NCT04188548 (EMBER) |
| Imlunestrant (LY3484356) | SERD | Abemaciclib (CDK4/6i) | Advanced/metastatic | Imlunestrant with or without abemaciclib versus fulvestrant or exemestane for ER + MBC progressed on ET | Phase III | NCT04975308 (EMBER-3) |
| Rintodestrant (G1T48) | SERD | Palbociclib (CDK4/6i) | Advanced/metastatic | Rintodestrant + palbociclib for ER + MBC progressed on ET | Phase I | NCT03455270 |
| Borestrant (ZB-716) | SERD | Palbociclib (CDK4/6i) | Advanced/metastatic | Borestrant alone or combined with palbociclib for ER + MBC | Phase I–II | NCT04669587 (ENZENO) |
| ZN-c5 | SERD | Abemaciclib (CDK4/6i) | Advanced/metastatic | ZN-c5 + abemaciclib for ER + MBC | Phase I | NCT04514159 |
| ZN-c5 | SERD | Palbociclib (CDK4/6i) | Advanced/metastatic | ZN-c5 alone or combined with palbociclib for ER + MBC | Phase I–II | NCT03560531 |
| D-0502 | SERD | Palbociclib (CDK4/6i) | Advanced/metastatic | D-0502 alone or combined with palbociclib for ER + MBC progressed on ET | Phase I | NCT03471663 |
| SERMs + SERM/SERD hybrids | ||||||
| Lasofoxifene | SERM | Abemaciclib (CDK4/6i) | Advanced/metastatic | Lasofoxifene + abemaciclib for ESR1 mutant ER + MBC progressed on ET | Phase II | NCT04432454 (ELAINE-2) |
| Lasofoxifene | SERM | None | Advanced/metastatic | Lasofoxifene versus fulvestrant for ESR1 mutant ER + MBC progressed on AI + CDK4/6i | Phase II | NCT03781063 (ELAINE) |
| Bazedoxifene | SERM/SERD hybrid | Palbociclib (CDK4/6i) | Advanced/metastatic | Bazedoxifene + palbociclib for ER + MBC progressed on prior ET | Phase II | NCT02448771 |
| Other novel ET | ||||||
| H3B-6545 | SERCA | Palbociclib (CDK4/6i) | Advanced/metastatic | H3B-6545 + palbociclib for ER + MBC progressed on ET | Phase I | NCT04288089 |
| H3B-6545 | SERCA | None | Advanced/metastatic | H3B-6545 for ER + MBC progressed on ET + CDK4/6i | Phase I–II | NCT03250676 |
| OP-1250 | CERAN | None | Advanced/metastatic | OP-1250 for ER + MBC progressed on ET | Phase I–II | NCT04505826 |
| OP-1250 | CERAN | Palbociclib (CDK4/6i) | Advanced/metastatic | OP-1250 + palbociclib for ER + MBC | Phase I | NCT05266105 |
| ARV-471 | PROTAC | Palbociclib (CDK4/6i) | Advanced/metastatic | ARV-471 alone or combined with palbociclib for ER + MBC progressed on ET | Phase I–II | NCT04072952 |
| AC682 | Chimeric ER degrader | None | Advanced/metastatic | AC682 for ER + MBC | Phase II | NCT05080842 |
| Adjuvant breast cancer trials | ||||||
| Oral SERDs | ||||||
| Amcenestrant (SAR439859) | SERD | None | Adjuvant | Amcenestrant versus tamoxifen for early ER + breast cancer after discontinuation of AI | Phase III | NCT0512877 (AMEERA-6) |
| Giredestrant (GDC-9545) | SERD | None | Adjuvant | Giredestrant versus AI or tamoxifen for early ER + breast cancer | Phase III | NCT04961996 (lidERA) |
| Neoadjuvant breast cancer trials | ||||||
| Oral SERDs | ||||||
| Elacestrant (RAD1901) | SERD | None | Neoadjuvant | Elacestrant for ER + early breast cancer prior to surgery | Phase I | NCT04797728 |
| Camizestrant (AZD9833) | SERD | None | Neoadjuvant | Camizestrant for ER + early breast cancer prior to surgery | Phase II | NCT04588298 (SERENA-3) |
| Imlunestrant (LY3484356) | SERD | None | Neoadjuvant | LY3484356 for ER + early breast cancer prior to surgery | Phase I | NCT04647487 (EMBER-2) |
| SERM/SERD hybrids | ||||||
| Bazedoxifene | SERM/SERD Hybrid | None | DCIS | Bazedoxifene + conjugated estrogens for DCIS | Phase II | NCT02694809 (PROMISE) |
AI, aromatase inhibitors; CERAN, complete estrogen receptor antagonist; CDK4/6i, CDK4/6 inhibitor; DCIS, ductal carcinoma in situ; ER+, estrogen receptor positive; ET, endocrine therapy; MBC, metastatic breast cancer; PROTAC, proteolysis targeting chimer; SERCA, selective estrogen receptor covalent antagonist; SERD, selective estrogen receptor degrader; SERM, selective estrogen receptor modulators.
Other novel endocrine agents including SERMs, SERCAs, CERANs, and proteolytic activators
In addition to oral SERDs, several other novel endocrine-based therapies have emerged for the management of ER+ breast cancer.
SERMs and SERM/SERD hybrids
Lasofoxifene is a next-generation SERM with similar characteristics to tamoxifen in terms of ER binding and inhibition of coactivators, but with improved bioavailability. 109 Initially developed by Sermonix for osteoporosis, patients taking lasofoxifene in the PEARL trial were found to have a reduced incidence of breast cancer, and their risk of endometrial hyperplasia or endometrial cancer was not increased. 110 Efficacy of this agent was demonstrated in preclinical breast cancer models, and notably, lasofoxifene was found to retain antagonist activity without evidence of resistance in cancer cells harboring ESR1-mut. 48 Based on laboratory evidence that lasofoxifene is not impacted by ESR1-mut status, clinical evaluation is underway in patients with ESR1-mut MBC. Currently, the ELAINE and ELAINE-2 trials are examining lasofoxifene, as monotherapy or in combination with abemaciclib, respectively, in patients with ESR1-mut advanced breast cancer after progression on prior ET and CDK4/6i (NCT03781063, NCT04432454).
Approved for the management of osteoporosis and postmenopausal hot flashes, bazedoxifene has also been studied for the treatment of breast cancer in preclinical and clinical settings. Developed by Pfizer, this SERM/SERD hybrid functions as a high affinity ER antagonist in breast tumors and promotes ER degradation.13,111,112 Bazedoxifene shows antitumor activity in laboratory-based models of tamoxifen-resistant breast cancer,13,113 and is currently being studied in a phase II clinical trial in MBC after progression on prior ET (NCT02448771).
ER antagonists
Optimized by H3 Biomedicine, H3B-6545 is a selective estrogen receptor covalent antagonist (SERCA) which covalently inactivates wt- and ESR1-mut by targeting Cys530 and promoting an antagonist conformation. 39 A precursor drug (H3B-5942) showed dose-dependent growth inhibition of breast cancer cells and PDX models expressing ESR1 mutations, and potency was improved in combination with a CDK4/6 or mTOR inhibitor. 39 Translating these results into the clinical space, H3B-6545 demonstrated single-agent antitumor activity in heavily pretreated patients with ER+ MBC. 114 Sixty-one percent of patients harbored baseline ESR1 mutations detected by ctDNA (n = 94), and among patients with ESR1 Y537S mutations (n = 10), an ORR 40% and median PFS 7.3 months were demonstrated. 114 Notable adverse events included asymptomatic sinus bradycardia and QTc prolongation. 114 Ongoing clinical evaluation in the phase I and II settings includes H3B-6545 as monotherapy (NCT03250676) and combined with palbociclib (NCT04288089) for ER+ MBC progressed on prior ET.
OP-1250 is an orally bioavailable complete estrogen receptor antagonist (CERAN) that completely antagonizes ER, blocks transcriptional activity, and induces ER degradation. 115 This agent is hypothesized to convey superior efficacy to agents with only partial antagonism. 115 In laboratory-based studies, OP-1250 demonstrates antitumor efficacy in both ESR1-mut and ESR1-wt breast cancer models. 115 These findings have prompted an active phase I–II clinical trials that is examining the activity of OP-1250 in ER+ MBC after progression on ET (NCT04505826). 116 Reported phase I data showed benefit in heavily pretreated patients with an ORR 17% and CBR 46% at dose levels within the recommended phase II dose range. 117
Proteolysis targeting chimer
ARV-471 facilitates interactions between ER and the intracellular E3 ligase complex, mediating degradation of ER by the proteasome. 118 This compound, developed by Arvinas, shows tumor growth inhibition in laboratory-based breast cancer models, including those expressing ESR1-mut. 118 An ongoing phase I–II clinical trial is evaluating ARV-471 alone or in combination with palbociclib in ET-resistant MBC (NCT04072952). Presented trial data evaluating ARV-471 monotherapy in a heavily pretreated population demonstrated clinical benefit with ORR 5.2% and CBR 40%. 119
AC682 is an oral chimeric ER degrader in development by Accutar Biotech that engages E3 ligase and ER to induce receptor degradation. 120 Preclinical data showed efficacy of this agent in ER+ breast cancer cells and tumor xenografts, including models harboring ESR1 mutation. 120 A phase I trial examining AC682 activity in ER+ MBC is ongoing (NCT05080842).
The clinical efficacy and adverse effects of the different endocrine agents are summarized in Table 2. Note, Table 2 is meant to be a summary for easy reference and cross-study comparisons among different studies should be performed with caution. Patients enrolled in these studies will have differences related to multiple factors, including prior lines of therapy, baseline genomic alterations, and sensitivity to ET. For example, as mentioned above, phase I trial data from the oral SERDs demonstrates that prior fulvestrant exposure in participants ranges from 21% to 64%.87,89,95,98,102,104 This could have a marked impact on underlying ET resistance and subsequent measures of response to treatment. Active clinical trials designed to further evaluate the efficacy of these agents are summarized in Table 3.
Table 2.
Results from clinical trials of next-generation endocrine therapies in hormone receptor-positive MBC.
| Endocrine agent | ET class | Trial description | Trial identifier | Median prior lines of therapy | Prior SERD exposure (%) | Prior CDK4/6i exposure (%) | Baseline ESR1 mutation rate (%) | ORR (%) and CBR (%) | Progression-free survival | Most frequent (>5%) treatment-related adverse events |
|---|---|---|---|---|---|---|---|---|---|---|
| Oral SERDs | ||||||||||
| Elacestrant (RAD1901) | SERD | Phase III; n = 477 Elacestrant versus fulvestrant or AI for ER+ MBC progressed on 1–2 prior lines of ET and CDK4/6i |
NCT03778931 (EMERALD) | 1 | Not reported | 100% | 48% | Not reported | 12-month PFS rate of 22.3% versus 9.4%; 30% reduction in PD or death, HR 0.697 | Nausea (25.3% G1–2), vomiting (11% G1–2), fatigue (11% G1–2) |
| Elacestrant (RAD1901) | SERD | Phase I; n = 50 Elacestrant for ER+ MBC progressed on ET |
NCT02338349 | 3 | 52% | 52% | 50% | ORR 19.4% CBR 42.6% |
Median 4.5 months | Nausea (33% G1–2), hypophosphatemia (25% G1–3), hypertriglyceridemia (25% G1–2), arthralgia (17% G1–2), fatigue (21% G1–2), diarrhea (12% G1–2), vomiting (17% G1–2), dyspepsia (21% G1–2), constipation (21% G1–2), anemia (12% G1–2), AST increased (12% G1–2), ALT increased (12% G1–2) |
| Amcenestrant (SAR439859) | SERD | Phase I–II; n = 62 Amcenestrant for ER+ MBC progressed on ET |
NCT03284957 (AMEERA-1) | 2 | 47% | 63% | 48% | ORR 8.5% CBR 33.9% |
Not reported | Hot flush (16.1% G1–2); constipation (9.7% G1–2), arthralgia (9.7% G1–2), vomiting (8.1% G1–2), diarrhea (8.1% G1–2), nausea (8.1% G1–2), fatigue (6.5% G1–2) |
| Camizestrant (AZD9833) | SERD | Phase I; n = 98 Camizestrant for ER+ MBC progressed on ET |
NCT04214288 (SERENA-1) | 5 | 58% | 69% | 46% | ORR 10% CBR 35.3% |
Median 5.4 months | Visual disturbances (51% G1–2, 2% G3), sinus bradycardia (45% G1–2), nausea (18% G1–2), fatigue (13% G1–2), dizziness (10% G1–2), vomiting (10% G1–3) |
| Giredestrant (GDC-9545) | SERD | Phase I; n = 111 Giredestrant for ER+ MBC progressed on ET |
NCT03332797 | 1 | 21% | 64% | Not reported | ORR 15% CBR 50% |
Median 7.2 months | Sinus bradycardia (7% G1–2), fatigue (21% G1–2), arthralgia (17% G1–2), nausea (16% G1–2) |
| Imlunestrant (LY3484356) | SERD | Phase I; n = 35 Imlunestrant for ER+ MBC progressed on ET |
NCT04188548 (EMBER) | 2 | 60% | 83% | 37% | ORR 6% CBR 48% |
Not reported | Nausea (32% G1–2), fatigue (25% G1–2), diarrhea (18% G1–2) |
| Rintodestrant (G1T48) | SERD | Phase I; n = 67 Rintodestrant for ER+ MBC progressed on ET |
NCT03455270 | 2 | 64% | 70% | 41% | ORR 5% CBR 30% |
Median 2.6–3.6 months | Hot flush (24% G1–2), fatigue (21% G1–2), nausea (19% G1–2), diarrhea (18% G1–2), vomiting (10% G1–2) |
| Other novel ET | ||||||||||
| H3B-6545 | SERCA | Phase I–II; n = 94 H3B-6545 for ER+ MBC progressed on ET |
NCT03250676 | 3 | 72% | 85% | 61% | ORR 17% CBR 40% |
Median 5.1 months | Sinus bradycardia (34% G1, 5% G2), QTcF prolongation (5% G2–3), anemia (19% G2+), fatigue (16% G2+), nausea (17% G2+), diarrhea (12% G2+), creatinine clearance decrease (38% G2+), hemoglobin decrease (37% G2+), bilirubin increase (12% G2+), ALT increase (14% G2+), AST increase (13% G2+) |
| P-1250 | CERAN | Phase I; n = 41 OP-1250 for ER+ MBC progressed on ET |
NCT04505826 | 3 | 68% | 95% | 49% | ORR 17% CBR 46% |
Not reported | Nausea (49% G1–2), fatigue (34% G1–2), vomiting (22% G1–2), headache (17% G1–2) |
| ARV-471 | PROTAC | Phase I; n = 50 ARV-471 for ER+ MBC progressed on ET |
NCT04072952 | Not reported | 83% | 100% | Not reported | ORR 5.2% CBR 40% |
Not reported | Nausea (24% G1–2), fatigue (12% G1–2), vomiting (10% G1–2) |
AI, aromatase inhibitors; ALT, alanine aminotransferase; AST, aspartate transaminase; CBR, clinical benefit rate; CDK4/6i, CDK4/6 inhibitor; CERAN, complete estrogen receptor antagonist; ER+, estrogen receptor positive; ET, endocrine therapy; G, grade; HR, hazard ratio; MBC, metastatic breast cancer; ORR, overall response rate; PD, progression of disease; PFS, progression-free survival; PROTAC, proteolysis targeting chimer; SERCA, selective estrogen receptor covalent antagonist; SERD, selective estrogen receptor degrader; SERM, selective estrogen receptor modulators; TRAE, treatment-related adverse event.
Combination therapy and future directions
Several next-generation ET agents described above demonstrate efficacy in ER+ advanced breast cancers that can proliferate independent of estrogen. As a drug class, the oral SERDs have shown encouraging results in treating ESR1-mut MBC. ESR1 mutations arise predominately following AI exposure, but often co-occur with other genomic drivers of resistance that upregulate ER-independent growth pathways. 83
Tumors that lose reliance on ER-mediated signaling may be less susceptible to even the most potent SERDs. For example, sensitivity to ET can be diminished by activating alterations in membrane RTKs (HER2, EGFR, FGFR), upregulation of oncogenic transduction pathways (MAPK, PI3K/AKT), and dysregulated cyclin D1/CDK4/6 pathway signaling. 17 These features of ET resistance support a combination approach to therapy that concurrently abrogate non-ER-mediated pathways of resistance. Indeed, therapeutic combinations are standard practice in the management of metastatic ER+ breast cancer, as reviewed below.
(1) Combination with CDK4/6i: Several landmark clinical trials have demonstrated improved PFS and OS when ET is given with a CDK4/6i, leading to the approval of this combination as first-line SOC for ER+ MBC.121–127 Currently, the oral SERDs amcenestrant (AMEERA-5, NCT04478266), camizestrant (SERENA-4, NCT04711252), and giredestrant (persevERA, NCT04546009) are being studied in combination with CDK4/6 blockade in treatment-naïve advanced breast cancer. Many other next-generation ET agents combined with a CDK4/6i are also being evaluated in varying phases of active clinical trials (see Table 3).
(2) Combination with PI3K inhibitors: In the SOLAR-1 trial, patients with PIK3CA alterations demonstrated improved outcomes when receiving the PI3K inhibitor alpelisib, leading to the approval of alpelisib combined with fulvestrant in PIK3CA mutant, ER+ MBC. 128 The AMEERA-1 and EMBER clinical trials are evaluating the efficacy of amcenestrant and imlunestrant, respectively, combined with alpelisib (NCT03284957, NCT04188548).
(3) Combination with AKT inhibitors: The AKT inhibitor capivasertib in combination with fulvestrant showed efficacy in the phase II FAKTION trial, 129 and capivasertib is now being combined with camizestrant in the SERENA-1 trial (NCT04214288). Results from these clinical efforts will inform future combination strategies and could impact the standard treatment of ER+ breast cancer.
(4) Combination with mTOR inhibitors: The BOLERO-2 trial led to approval of the mTOR inhibitor everolimus, which acts downstream of the PI3K/AKT pathway, in combination with exemestane for MBC progressed on prior ET. 130 Antitumor activity of three novel SERDs (amcenestrant, camizestrant, and imlunestrant) are being assessed with everolimus in phase I trials (see Table 3).
(5) Other combinations: Other regimens that exploit signaling pathway vulnerabilities could represent valuable areas for future research. MEK and ERK inhibitors are under clinical investigation131–133; these agents have the potential to abrogate ET resistance mediated by activating alterations in MAPK pathway components, such as NF1 or KRAS. Resistance driven by membrane RTK mutations or amplifications could be circumvented by combining ET with a targeted RTK inhibitor. 17 For example, in ER+ MBC harboring HER2 mutations, the combination of neratinib, a HER2 inhibitor, with fulvestrant has demonstrated clinical promise. 134 In the preclinical setting, breast cancer models harboring aberrant FGFR had endocrine resistance reversed with the use of an FGFR inhibitor, 57 and efficacy of this drug class is being studied in the clinic (NCT04024436). In ESR1-mut breast cancer, research demonstrates further genomic dysregulation and downstream signaling modulation that could be leveraged, including aberrant DNA bindings cofactors FOXA1 and CTCF, 41 NOTCH signaling, 41 and Wnt signaling. 135
Ongoing translational research efforts remain focused on the importance of modeling combination therapy resistance. 136 For example, alterations in ESR1 and PIK3CA may convey resistance to ET, but these cancers remain sensitive to a CDK4/6i partner. 136 On the other hand, resistance may require multiple, synergistic driver events to occur. Tumor heterogeneity in pretreated MBC suggests simultaneous genomic and non-genomic resistance drivers can arise on therapy, and future research efforts characterizing their impact on drug sensitivity are important. Serial cfDNA samples collected on patients enrolled in the clinical trials detailed in Tables 2 and 3 could help elucidate high-value targets for translational study.
Conclusions
In the management of ER+ breast cancer, anti-estrogens are a critical component of all early therapeutic regimens. In post-menopausal patients with intact estrogen-ER signaling, AIs decrease systemic estrogen levels and can effectively treat early- and advanced-stage disease. 1 Patients with MBC inevitably progress secondary to acquired or intrinsic mechanisms of resistance. 4 Disease progression on AI therapy is associated with the emergence of ESR1 mutations27,30 that drive resistance via ligand-independent ER-mediated growth signaling and proliferation.38,39 In these tumors, the ER still remains a viable therapeutic target. 68
Ongoing clinical development of next-generation ETs has demonstrated that these agents can effectively target both wt- and ESR1-mut breast cancer, promoting ER degradation and disrupting downstream signaling. These agents have a distinct pharmacologic advantage over fulvestrant in being orally bioavailable. 82 Data from the phase III EMERALD trial demonstrate that, compared to SOC, the oral SERD elacestrant improves 12-month PFS in ER+ MBC following progression on prior ET, and clinical benefit was even more pronounced in patients with ESR1-mut. 88 This exciting result could signal the start of a shifting paradigm toward oral SERDs for ER+ breast cancer.
However, several questions around optimizing ET remain. ESR1 mutation clearly enriches for patients more likely to respond to SERD therapy, possibly due to tumor dependence on ER-mediated signaling, but this association is not perfect or clinically reliable. In the EMERALD trial, about half of the patients demonstrated intrinsic ET resistance regardless of treatment arm, highlighting that some patients are unlikely to derive benefit from ET. Polyclonal resistance also poses a clinical challenge, and tumors with ESR1-mut often have subclones harboring concurrent genomic alterations that could mediate ER-pathway-independent resistance as well. Characterizing metastatic tumors by ER positivity or ESR1 status is not enough, and we need better methodology to select post-CDK4/6i patients that remain endocrine sensitive. Possibilities include genomic profiling panels or novel biomarker identification that could more accurately characterize tumors that are likely to be susceptible. This remains an area of unmet need that future clinical and translation work could significantly impact.
Many of the next-generation ETs under development are being evaluated in ongoing clinical trials as single agents and in combination with targeted therapies (Table 3). Combined therapeutic strategies are important for the management of advanced breast cancer, with CDK4/6 inhibitors, PI3K inhibitors, and mTOR inhibitors already being part of approved combination regimens for MBC. Estrogen- and ER-independent resistance mechanisms can emerge that are not susceptible to Et alone and require one or more drug partners to re-sensitize tumors. Several of these pathways have been implicated, including activating alterations in growth-factor-dependent kinases, upregulation in MAPK and PI3K/AKT signaling, and downstream dysregulation of the cyclin D1/CDK4/6 cell cycle pathway. 17
Next-generation anti-estrogens have the potential to change how ER+ breast cancer is treated. As clinical trial data continue to mature, evaluating the efficacy and tolerability of these agents, as ET backbones in combination regimens and as monotherapy, will be important to determine how they impact outcomes and where they will be adopted into standard practice.
Acknowledgments
None.
Footnotes
ORCID iDs: Maxwell R. Lloyd  https://orcid.org/0000-0001-9810-0437
https://orcid.org/0000-0001-9810-0437
Aditya Bardia  https://orcid.org/0000-0003-4885-1157
https://orcid.org/0000-0003-4885-1157
Contributor Information
Maxwell R. Lloyd, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
Seth A. Wander, Massachusetts General Hospital Cancer Center, Harvard Medical School, Boston, MA, USA
Erika Hamilton, Sarah Cannon Research Institute/Tennessee Oncology, Nashville, TN, USA.
Pedram Razavi, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Aditya Bardia, Massachusetts General Hospital Cancer Center, 10 North Grove Street, Harvard Medical School, Boston, MA 02114-2621, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Maxwell R. Lloyd: Conceptualization; Writing – original draft; Writing – review & editing.
Seth A. Wander: Conceptualization; Writing – original draft; Writing – review & editing.
Erika Hamilton: Conceptualization; Writing – review & editing.
Pedram Razavi: Conceptualization; Writing – review & editing.
Aditya Bardia: Conceptualization; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing Interests: MRL: No disclosures. SAW: Consulting/Advisory Board – Foundation Medicine, Veracyte, Pfizer, Biovica, Hologic, Eli Lilly; Institutional Research Support – Genentech, Nuvation Bio, Regor Therapeutics, Eli Lilly. AB: Consultant/Advisory Board: Pfizer, Novartis, Genentech, Merck, Radius Health, Immunomedics/Gilead, Sanofi, Daiichi Pharma/Astra Zeneca, Phillips, Eli Lilly, Foundation Medicine; Contracted Research/Grant (to institution): Genentech, Novartis, Pfizer, Merck, Sanofi, Radius Health, Immunomedics/Gilead, Daiichi Pharma/Astra Zeneca, Eli Lilly. EH: Contracted Research/Grant (to institution): Abbvie, Acerta Pharma, Accutar Biotechnology, ADC Therapeutics, AKESOBIO Australia, Amgen, Aravive, ArQule, Artios, Arvinas, AstraZeneca, AtlasMedx, Black Diamond, Bliss BioPharmaceuticals, Boehringer Ingelheim, Cascadian Therapeutics, Clovis, Compugen, Cullen-Florentine, Curis, CytomX, Daiichi Sankyo, Dana Farber Cancer Inst, Dantari, Deciphera, Duality Biologics, eFFECTOR Therapeutics, Ellipses Pharma, Elucida Oncology, EMD Serono, Fochon, FujiFilm, G1 Therapeutics, H3 Biomedicine, Harpoon, Hutchinson MediPharma, Immunogen, Immunomedics, Incyte, Infinity Pharmaceuticals, InvestisBio, Jacobio, Karyopharm, Leap Therapeutics, Lilly, Lycera, Mabspace Biosciences, Macrogenics, MedImmune, Merck, Mersana, Merus, Millennium, Molecular Templates, Myriad Genetic Laboratories, Novartis, Nucana, Olema, OncoMed, Onconova Therapeutics, ORIC Pharmaceuticals, Orinove, Pfizer, Pharma Mar, Pieris Pharmaceuticals Pionyr, Immunotherapeutics, Plexxikon, Radius Health, Regeneron, Relay Therapeutics, Repertoire, Immune Medicine, Rgenix, Roche/Genentech, SeaGen, Sermonix Pharmaceuticals, Shattuck Labs, Silverback, StemCentRx, Sutro, Syndax, Syros, Taiho, TapImmune, Tesaro, Tolmar, Torque Therapeutics, Treadwell Therapeutics, Verastem, Vincerx Pharma, Zenith Epigenetics, Zymeworks; Consulting Advisory Role (all to institution only): Arcus, Arvinas, AstraZeneca, Black Diamond, Boehringer Ingelheim, CytomX, Daiichi Sankyo, Dantari, Deciphera Pharmaceuticals, Eisai, Greenwich LifeSciences, H3 Biomedicine iTeosJanssen, Lilly, Loxo, Merck, Mersana, Novartis, Orum Therapeutics, Pfizer, Propella Therapeutics, Puma Biotechnology, Relay Therapeutics, Roche/Genentech, SeaGen, Silverback Therapeutics. PR: Contracted Research/Grant (to institution): Grail/Illumina, Tempus, Novartis, AstraZeneca, Guardant, Epic Sciences, Inivata, Invitae/ArcherDx, Biotheranostics, Biovica, Foundation Medicine; Consultant/Advisory board: Novartis, AstraZeneca, Pfizer, Daiichi, Guardant, Natera, Inivata, Biovica, Epic Sciences, Tempus, Foundation Medicine.
Availability of data and materials: Not applicable.
References
- 1. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015; 386: 1341–1352. [DOI] [PubMed] [Google Scholar]
- 2. DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019; 69: 438–451. [DOI] [PubMed] [Google Scholar]
- 3. Brueggemeier RW. Aromatase inhibitors mechanisms of steroidal inhibitors. Breast Cancer Res Treat 1994; 30: 31–42. [DOI] [PubMed] [Google Scholar]
- 4. Mauri D, Pavlidis N, Polyzos NP, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst 2006; 98: 1285–1291. [DOI] [PubMed] [Google Scholar]
- 5. Gao JJ, Cheng J, Bloomquist E, et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol 2020; 21: 250–260. [DOI] [PubMed] [Google Scholar]
- 6. Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer 2007; 7: 46–53. [DOI] [PubMed] [Google Scholar]
- 7. Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer 2004; 11: 643–658. [DOI] [PubMed] [Google Scholar]
- 8. Pather K, Augustine TN. Tamoxifen induces hypercoagulation and alterations in ERα and ERβ dependent on breast cancer sub-phenotype ex vivo. Sci Rep 2020; 10: 19256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wardell SE, Marks JR, McDonnell DP. The turnover of estrogen receptor α by the selective estrogen receptor degrader (SERD) fulvestrant is a saturable process that is not required for antagonist efficacy. Biochem Pharmacol 2011; 82: 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer 2004; 90 Suppl 1: S2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Long X, Nephew KP. Fulvestrant (ICI 182,780)-dependent interacting proteins mediate immobilization and degradation of estrogen receptor-alpha. J Biol Chem 2006; 281: 9607–9615. [DOI] [PubMed] [Google Scholar]
- 12. Guan J, Zhou W, Hafner M, et al. Therapeutic ligands antagonize estrogen receptor function by impairing its mobility. Cell 2019; 178: 949–963.e18. [DOI] [PubMed] [Google Scholar]
- 13. Wardell SE, Nelson ER, Chao CA, et al. Bazedoxifene exhibits antiestrogenic activity in animal models of tamoxifen-resistant breast cancer: implications for treatment of advanced disease. Clin Cancer Res 2013; 19: 2420–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fanning SW, Greene GL. Next-generation ERα inhibitors for endocrine-resistant ER+ breast cancer. Endocrinology 2019; 160: 759–769. [DOI] [PubMed] [Google Scholar]
- 15. Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: American Society of Clinical Oncology clinical practice guideline update on ovarian suppression. J Clin Oncol 2016; 34: 1689–1701. [DOI] [PubMed] [Google Scholar]
- 16. Francis PA, Pagani O, Fleming GF. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med 2018; 379: 122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell 2020; 37: 496–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011; 62: 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prall OW, Rogan EM, Musgrove EA, et al. c-Myc or cyclin D1 mimics estrogen effects on cyclin E-Cdk2 activation and cell cycle reentry. Mol Cell Biol 1998; 18: 4499–4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schiff R, Massarweh SA, Shou J, et al. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res 2004; 10: 331s–336s. [DOI] [PubMed] [Google Scholar]
- 21. Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 2005; 4: 988–1004. [DOI] [PubMed] [Google Scholar]
- 22. Braicu C, Buse M, Busuioc C, et al. A comprehensive review on MAPK: a promising therapeutic target in cancer. Cancers 2019; 11: 1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma CX, Reinert T, Chmielewska I, et al. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer 2015; 15: 261–275. [DOI] [PubMed] [Google Scholar]
- 24. Jeselsohn R, Yelensky R, Buchwalter G. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor positive breast cancer. Clin Cancer Res 2014; 20: 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet 2013; 45: 1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Razavi P, Chang MT, Xu G. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 2018; 34: 427–438.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schiavon G, Hrebien S, Garcia-Murillas I, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med 2015; 7: 313ra182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fribbens C, O’Leary B, Kilburn L, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol 2016; 34: 2961–2968. [DOI] [PubMed] [Google Scholar]
- 29. Bidard F-C, Hardy-Bessard A-C, Bachelot T, et al. Abstract GS3-05: fulvestrant-palbociclib vs continuing aromatase inhibitor-palbociclib upon detection of circulating ESR1 mutation in HR+ HER2. Cancer Res 2022; 82: GS3-05. [Google Scholar]
- 30. Hermida-Prado F, Jeselsohn R. The ESR1 mutations: from bedside to bench to bedside. Cancer Res 2021; 81: 537–538. [DOI] [PubMed] [Google Scholar]
- 31. The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robinson DR, Wu YM, Vats P, et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet 2013; 45: 1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toy W, Weir H, Razavi P, et al. Activating ESR1 mutations differentially affect the efficacy of ER antagonists. Cancer Discov 2017; 7: 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Najim O, Seghers S, Sergoynne L, et al. The association between type of endocrine therapy and development of estrogen receptor-1 mutation(s) in patients with hormone-sensitive advanced breast cancer: a systematic review and meta-analysis of randomized and non-randomized trials. Biochim Biophys Acta Rev Cancer 2019; 1872: 188315. [DOI] [PubMed] [Google Scholar]
- 35. Jeselsohn R, De Angelis C, Brown M, et al. The evolving role of the estrogen receptor mutations in endocrine therapy-resistant breast cancer. Curr Oncol Rep 2017; 19: 35. [DOI] [PubMed] [Google Scholar]
- 36. Fribbens C, Garcia Murillas I, Beaney M, et al. Tracking evolution of aromatase inhibitor resistance with circulating tumour DNA analysis in metastatic breast cancer. Ann Oncol 2018; 29: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chandarlapaty S, Chen D, He W, et al. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol 2016; 2: 1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fanning SW, Mayne CG, Dharmarajan V, et al. Estrogen receptor alpha somatic mutations Y537S and D538G confer breast cancer endocrine resistance by stabilizing the activating function-2 binding conformation. eLife 2016; 5: e12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Puyang X, Furman C, Zheng GZ. Discovery of selective estrogen receptor covalent antagonists for the treatment of ERαWT and ERαMUT breast cancer. Cancer Discov 2018; 8: 1176–1193. [DOI] [PubMed] [Google Scholar]
- 40. Bahreini A, Li Z, Wang P, et al. Mutation site and context dependent effects of ESR1 mutation in genome-edited breast cancer cell models. Breast Cancer Res 2017; 19: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arnesen S, Blanchard Z, Williams MM, et al. Estrogen receptor alpha mutations in breast cancer cells cause gene expression changes through constant activity and secondary effects. Cancer Res 2021; 81: 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jeselsohn R, Bergholz JS, Pun M, et al. Allele-specific chromatin recruitment and therapeutic vulnerabilities of ESR1 activating mutations. Cancer Cell 2018; 33: 173–186.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Merenbakh-Lamin K, Ben-Baruch N, Yeheskel A, et al. D538G mutation in estrogen receptor-α: a novel mechanism for acquired endocrine resistance in breast cancer. Cancer Res 2013; 73: 6856–6864. [DOI] [PubMed] [Google Scholar]
- 44. Turner NC, Swift C, Kilburn L, et al. ESR1 mutations and overall survival on fulvestrant versus exemestane in advanced hormone receptor-positive breast cancer: a combined analysis of the phase III SoFEA and EFECT trials. Clin Cancer Res 2020; 26: 5172–5177. [DOI] [PubMed] [Google Scholar]
- 45. O’Leary B, Cutts RJ, Liu Y, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov 2018; 8: 1390–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spoerke JM, Gendreau S, Walter K, et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun 2016; 7: 11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Turner NC, Kingston B, Kilburn LS, et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol 2020; 21: 1296–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Andreano KJ, Baker JG, Park S, et al. The dysregulated pharmacology of clinically relevant ESR1 mutants is normalized by ligand-activated WT receptor. Mol Cancer Ther 2020; 19: 1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hartmaier RJ, Trabucco SE, Priedigkeit N, et al. Recurrent hyperactive ESR1 fusion proteins in endocrine therapy-resistant breast cancer. Ann Oncol 2018; 29: 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lei JT, Gou X, Ellis MJ. ESR1 fusions drive endocrine therapy resistance and metastasis in breast cancer. Mol Cell Oncol 2018; 5: e1526005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Veeraraghavan J, Tan Y, Cao X-X, et al. Recurrent ESR1–CCDC170 rearrangements in an aggressive subset of oestrogen receptor-positive breast cancers. Nat Commun 2014; 5: 4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lei JT, Gou X, Seker S, et al. ESR1 alterations and metastasis in estrogen receptor positive breast cancer. J Cancer Metastasis Treat 2019; 5: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nayar U, Cohen O, Kapstad C, et al. Acquired HER2 mutations in ER+ metastatic breast cancer confer resistance to estrogen receptor-directed therapies. Nat Genet 2019; 51: 207–216. [DOI] [PubMed] [Google Scholar]
- 54. Gutierrez MC, Detre S, Johnston S, et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol 2005; 23: 2469–2476. [DOI] [PubMed] [Google Scholar]
- 55. Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 2009; 27: 5538–5546. [DOI] [PubMed] [Google Scholar]
- 56. Massarweh S, Osborne CK, Creighton CJ, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res 2008; 68: 826–833. [DOI] [PubMed] [Google Scholar]
- 57. Mao P, Cohen O, Kowalski KJ, et al. Acquired FGFR and FGF alterations confer resistance to estrogen receptor (ER) targeted therapy in ER+ metastatic breast cancer. Clin Cancer Res 2020; 26: 5974–5989. [DOI] [PubMed] [Google Scholar]
- 58. Shee K, Yang W, Hinds JW, et al. Therapeutically targeting tumor microenvironment-mediated drug resistance in estrogen receptor-positive breast cancer. J Exp Med 2018; 215: 895–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zheng Z-Y, Anurag M, Lei JT, et al. Neurofibromin is an estrogen receptor-α transcriptional co-repressor in breast cancer. Cancer Cell 2020; 37: 387–402.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sokol ES, Feng YX, Jin DX, et al. Loss of function of NF1 is a mechanism of acquired resistance to endocrine therapy in lobular breast cancer. Ann Oncol 2019; 30: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dischinger PS, Tovar EA, Essenburg CJ, et al. NF1 deficiency correlates with estrogen receptor signaling and diminished survival in breast cancer. NPJ Breast Cancer 2018; 4: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Miller TW, Hennessy BT, González-Angulo AM, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Investig 2010; 120: 2406–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Angus L, Smid M, Wilting SM, et al. Genomic landscape of metastatic breast cancer and its clinical implications. Nat Genet 2019; 51: 1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res 2016; 18: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Miller TW, Balko JM, Fox EM, et al. ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov 2011; 1: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shiino S, Kinoshita T, Yoshida M, et al. Prognostic impact of discordance in hormone receptor status between primary and recurrent sites in patients with recurrent breast cancer. Clin Breast Cancer 2016; 16: e133–e140. [DOI] [PubMed] [Google Scholar]
- 67. Creighton CJ, Fu X, Hennessy BT, et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res 2010; 12: R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nardone A, De Angelis C, Trivedi MV, et al. The changing role of ER in endocrine resistance. Breast 2015; 24 Suppl 2: S60–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wakeling AE, Bowler J. Novel antioestrogens without partial agonist activity. J Steroid Biochem 1988; 31: 645–653. [DOI] [PubMed] [Google Scholar]
- 70. Wakeling AE, Bowler J. ICI 182,780, a new antioestrogen with clinical potential. J Steroid Biochem Mol Biol 1992; 43: 173–177. [DOI] [PubMed] [Google Scholar]
- 71. Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol 2002; 20: 3386–3395. [DOI] [PubMed] [Google Scholar]
- 72. Howell A, Robertson JF, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol 2002; 20: 3396–3403. [DOI] [PubMed] [Google Scholar]
- 73. Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM Phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 2010; 28: 4594–4600. [DOI] [PubMed] [Google Scholar]
- 74. Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst 2014; 106: djt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 2016; 388: 2997–3005. [DOI] [PubMed] [Google Scholar]
- 76. Jeselsohn R, Buchwalter G, De Angelis C, et al. ESR1 mutations—a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol 2015; 12: 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shomali M, Cheng J, Sun F, et al. SAR439859, a novel selective estrogen receptor degrader (SERD), demonstrates effective and broad antitumor activity in wild-type and mutant ER-positive breast cancer models. Mol Cancer Ther 2021; 20: 250–262. [DOI] [PubMed] [Google Scholar]
- 78. Krop IE, Mayer IA, Ganju V, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016; 17: 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. New Engl J Med 2018; 379: 1926–1936. [DOI] [PubMed] [Google Scholar]
- 80. Patel HK, Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther 2018; 186: 1–24. [DOI] [PubMed] [Google Scholar]
- 81. Berger F, Marce M, Delaloge S, et al. Randomised, open-label, multicentric phase III trial to evaluate the safety and efficacy of palbociclib in combination with endocrine therapy, guided by ESR1 mutation monitoring in oestrogen receptor-positive, HER2-negative metastatic breast cancer patients: study design of PADA-1. BMJ Open 2022; 12: e055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang L, Sharma A. The quest for orally available selective estrogen receptor degraders (SERDs). ChemMedChem 2020; 15: 2072–2097. [DOI] [PubMed] [Google Scholar]
- 83. Brett JO, Spring LM, Bardia A, et al. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res 2021; 23: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bihani T, Patel HK, Arlt H, et al. Elacestrant (RAD1901), a selective estrogen receptor degrader (SERD), has antitumor activity in multiple ER+ breast cancer patient-derived xenograft models. Clin Cancer Res 2017; 23: 4793–4804. [DOI] [PubMed] [Google Scholar]
- 85. Garner F, Shomali M, Paquin D, et al. RAD1901: a novel, orally bioavailable selective estrogen receptor degrader that demonstrates antitumor activity in breast cancer xenograft models. Anticancer Drugs 2015; 26: 948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Patel HK, Tao N, Lee K-M, et al. Elacestrant (RAD1901) exhibits anti-tumor activity in multiple ER+ breast cancer models resistant to CDK4/6 inhibitors. Breast Cancer Res 2019; 21: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bardia A, Kaklamani V, Wilks S, et al. Phase I study of elacestrant (RAD1901), a novel selective estrogen receptor degrader, in ER-Positive, HER2-Negative advanced breast cancer. J Clin Oncol 2021; 39: 1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bidard F-C, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. Epub ahead of print May 2022. DOI: 10.1200/JCO.22.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Linden HM, Campone M, Bardia A, et al. Abstract PD8-08: a phase 1/2 study of SAR439859, an oral selective estrogen receptor (ER) degrader (SERD), as monotherapy and in combination with other anti-cancer therapies in postmenopausal women with ER-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (mBC): AMEERA-1. Cancer Res 2021; 81: PD8-08. [Google Scholar]
- 90. Sanofi. Press Releases, Monday, 14 March 2022, https://ml-eu.globenewswire.com/Resource/Download/ca5fc4ef-adc6-4995-a4f5-24af50d67d61 (accessed 13 May 2022).
- 91. De Savi C, Bradbury RH, Rabow AA, et al. Optimization of a novel binding motif to (E)-3-(3,5-Difluoro-4-((1R,3R)-2-(2-fluoro-2-methylpropyl)-3-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-1-yl)phenyl)acrylic acid (AZD9496), a potent and orally bioavailable selective estrogen receptor downregulator and antagonist. J Med Chem 2015; 58: 8128–8140. [DOI] [PubMed] [Google Scholar]
- 92. Weir HM, Bradbury RH, Lawson M, et al. AZD9496: an oral estrogen receptor inhibitor that blocks the growth of ER-positive and ESR1-mutant breast tumors in preclinical models. Cancer Res 2016; 76: 3307–3318. [DOI] [PubMed] [Google Scholar]
- 93. Scott JS, Moss TA, Balazs A. Discovery of AZD9833, a potent and orally bioavailable selective estrogen receptor degrader and antagonist. J Med Chem 2020; 63: 14530–14559. [DOI] [PubMed] [Google Scholar]
- 94. Hamilton EP, Oliveira M, Banerji U, et al. A phase I dose escalation and expansion study of the next generation oral SERD AZD9833 in women with ER-positive, HER2-negative advanced breast cancer. J Clin Oncol 2020; 38: 1024–1024. [Google Scholar]
- 95. Baird R, Oliveira M, Gil EMC, et al. Abstract PS11-05: updated data from SERENA-1: a phase 1 dose escalation and expansion study of the next generation oral SERD AZD9833 as a monotherapy and in combination with palbociclib, in women with ER-positive, HER2-negative advanced breast cancer. Cancer Res 2021; 81: PS11-05. [Google Scholar]
- 96. Liang J, Zbieg JR, Blake RA, et al. GDC-9545 (giredestrant): a potent and orally bioavailable selective estrogen receptor antagonist and degrader with an exceptional preclinical profile for ER+ breast cancer. J Med Chem 2021; 64: 11841–11856. [DOI] [PubMed] [Google Scholar]
- 97. Jhaveri K, Winer EP, Lim E, et al. Abstract PD7-05: a first-in-human phase I study to evaluate the oral selective estrogen receptor degrader (SERD), GDC-9545, in postmenopausal women with estrogen receptor-positive (ER+) HER2-negative (HER2−) metastatic breast cancer. Cancer Res 2020; 80: PD7-05. [Google Scholar]
- 98. Jhaveri K, Boni V, Sohn J. Safety and activity of single-agent giredestrant (GDC-9545) from a phase Ia/b study in patients (pts) with estrogen receptor-positive (ER+), HER2-negative locally advanced/metastatic breast cancer (LA/mBC). J Clin Oncol 2021; 39: 1017–1017. [Google Scholar]
- 99. Taylor P. Roche’s oral SERD giredestrant fails breast cancer trial, https://pharmaphorum.com/news/roches-oral-serd-giredestrant-fails-breast-cancer-trial/ (2022, accessed 13 May 2022).
- 100. Hurvitz SA, Park YH, Bardia A, et al. LBA14 neoadjuvant giredestrant (GDC-9545) + palbociclib (palbo) vs anastrozole (A) + palbo in post-menopausal women with oestrogen receptor-positive, HER2-negative, untreated early breast cancer (ER+/HER2– eBC): interim analysis of the randomised, open-label, phase II coopERA BC study. Ann Oncol 2021; 32: S1285–S1286. [Google Scholar]
- 101. Bhagwat SV, Zhao B, Shen W, et al. Abstract 1236: preclinical characterization of LY3484356, a novel, potent and orally bioavailable selective estrogen receptor degrader (SERD). Cancer Res 2021; 81: 1236–1236. [Google Scholar]
- 102. Jhaveri KL, Lim E, Hamilton EP, et al. A first-in-human phase 1a/b trial of LY3484356, an oral selective estrogen receptor (ER) degrader (SERD) in ER+ advanced breast cancer (aBC) and endometrial endometrioid cancer (EEC): results from the EMBER study. J Clin Oncol 2021; 39: 1050–1050. [Google Scholar]
- 103. Andreano KJ, Wardell SE, Baker JG, et al. G1T48, an oral selective estrogen receptor degrader, and the CDK4/6 inhibitor lerociclib inhibit tumor growth in animal models of endocrine-resistant breast cancer. Breast Cancer Res Treat 2020; 180: 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Aftimos P, Neven P, Pegram M. Abstract PS12-04: rintodestrant (G1T48), an oral selective estrogen receptor degrader in ER+/HER2− locally advanced or metastatic breast cancer: updated phase 1 results and dose selection. Cancer Res 2021; 81: PS12-04. [Google Scholar]
- 105. Shagufta Ahmad I, Mathew S, et al. Recent progress in selective estrogen receptor downregulators (SERDs) for the treatment of breast cancer. RSC Med Chem 2020; 11: 438–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jhaveri K, Juric D, Yap Y-S, et al. LBA1 interim results of a phase I/Ib study of LSZ102, an oral selective estrogen receptor degrader (SERD), in combination with ribociclib (RIB) or alpelisib (ALP) in patients with ER+ breast cancer (BC) who had progressed after endocrine therapy (ET). Ann Oncol 2020; 31: S62. [Google Scholar]
- 107. Hernando C, Ortega-Morillo B, Tapia M, et al. Oral selective estrogen receptor degraders (SERDs) as a novel breast cancer therapy: present and future from a clinical perspective. Int J Mol Sci 2021; 22: 7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Llombart-Cussac A, Pérez-García JM, Bellet M, et al. Fulvestrant-palbociclib vs letrozole-palbociclib as initial therapy for endocrine-sensitive, hormone receptor-positive, ERBB2-negative advanced breast cancer: a randomized clinical trial. JAMA Oncol 2021; 7: 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Vajdos FF, Hoth LR, Geoghegan KF. The 2.0 Å crystal structure of the ERα ligand-binding domain complexed with lasofoxifene. Protein Sci 2007; 16: 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. LaCroix AZ, Powles T, Osborne CK, et al. Breast cancer incidence in the randomized PEARL trial of lasofoxifene in postmenopausal osteoporotic women. J Natl Cancer Inst 2010; 102: 1706–1715. [DOI] [PubMed] [Google Scholar]
- 111. Wardell SE, Ellis MJ, Alley HM, et al. Efficacy of SERD/SERM Hybrid-CDK4/6 inhibitor combinations in models of endocrine therapy-resistant breast cancer. Clin Cancer Res 2015; 21: 5121–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lewis-Wambi JS, Kim H, Curpan R, et al. The selective estrogen receptor modulator bazedoxifene inhibits hormone-independent breast cancer cell growth and down-regulates estrogen receptor α and cyclin D1. Mol Pharmacol 2011; 80: 610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fanning SW, Jeselsohn R, Dharmarajan V, et al. The SERM/SERD bazedoxifene disrupts ESR1 helix 12 to overcome acquired hormone resistance in breast cancer cells. eLife 2018; 7: e37161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hamilton EP, Wang JS, Pluard TJ, et al. Phase I/II study of H3B-6545, a novel selective estrogen receptor covalent antagonist (SERCA), in estrogen receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2). J Clin Oncol 2021; 39: 1018–1018. [Google Scholar]
- 115. Hodges-Gallagher L, Sun R, Myles D, et al. OP-1250: A potent orally available complete antagonist of estrogen receptor-mediated signaling that shrinks wild type and mutant breast tumors. Eur J Cancer 2020; 138: S55. [Google Scholar]
- 116. Hamilton E, Alemany C, Lin NU, et al. Abstract OT-09-10: a phase I/II open-label, first-in-human, multicenter, dose escalation and dose expansion study of OP-1250 monotherapy in adult subjects with advanced and/or metastatic hormone receptor (HR)-positive, HER2-negative breast cancer. Cancer Res 2021; 81: OT-09-10. [Google Scholar]
- 117. Patel M, Alemany C, Mitri Z, et al. Abstract P1-17-12: Preliminary data from a phase I/II, multicenter, dose escalation study of OP-1250, an oral CERAN/SERD, in subjects with advanced and/or metastatic estrogen receptor (ER)-positive, HER2-negative breast cancer. Cancer Res 2022; 82: P1-17-12. [Google Scholar]
- 118. Snyder LB, Flanagan JJ, Qian Y, et al. Abstract 44: the discovery of ARV-471, an orally bioavailable estrogen receptor degrading PROTAC for the treatment of patients with breast cancer. Cancer Res 2021; 81: 44–44. [Google Scholar]
- 119. Hamilton E, Vahdat L, Han HS, et al. Abstract PD13-08: first-in-human safety and activity of ARV-471, a novel PROTAC® estrogen receptor degrader, in ER+/HER. Cancer Res 2022; 82: PD13-08. [Google Scholar]
- 120. He W, Zhang H, Perkins L, et al. Abstract PS18-09: novel chimeric small molecule AC682 potently degrades estrogen receptor with oral anti-tumor efficacy superior to fulvestrant. Cancer Res 2021; 81: PS18-09. [Google Scholar]
- 121. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. New Engl J Med 2016; 375: 1925–1936. [DOI] [PubMed] [Google Scholar]
- 122. Hortobagyi GN, Stemmer SM, Burris HA. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 123. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35: 3638–3646. [DOI] [PubMed] [Google Scholar]
- 124. Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. New Engl J Med 2015; 373: 209–219. [DOI] [PubMed] [Google Scholar]
- 125. Sledge GW, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017; 35: 2875–2884. [DOI] [PubMed] [Google Scholar]
- 126. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. J Clin Oncol 2018; 36: 2465–2472. [DOI] [PubMed] [Google Scholar]
- 127. Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018; 19: 904–915. [DOI] [PubMed] [Google Scholar]
- 128. André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. New Engl J Med 2019; 380: 1929–1940. [DOI] [PubMed] [Google Scholar]
- 129. Jones RH, Casbard A, Carucci M, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2020; 21: 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. New Engl J Med 2012; 366: 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lian T, Li C, Wang H. Trametinib in the treatment of multiple malignancies harboring MEK1 mutations. Cancer Treat Rev 2019; 81: 101907. [DOI] [PubMed] [Google Scholar]
- 132. Sullivan RJ, Infante JR, Janku F, et al. First-in-class ERK1/2 inhibitor ulixertinib (BVD-523) in patients with MAPK mutant advanced solid tumors: results of a phase I dose-escalation and expansion study. Cancer Discov 2018; 8: 184–195. [DOI] [PubMed] [Google Scholar]
- 133. Memorial Sloan Kettering Cancer Center. A phase 1b/2a, open-label platform study to evaluate mirdametinib as monotherapy or in combination with other anticancer agents in patients with advanced solid cancers harboring MAPK-activating mutations. Clinical Trial Registration NCT05054374, clinicaltrials.gov, https://clinicaltrials.gov/ct2/show/NCT05054374 (2021, accessed 23 May 2022).
- 134. Smyth LM, Piha-Paul SA, Won HH. Efficacy and determinants of response to HER kinase inhibition in HER2-mutant metastatic breast cancer. Cancer Discov 2020; 10: 198–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Li Z, Wu Y, Yates ME, et al. Hotspot ESR1 mutations are multimodal and contextual modulators of breast cancer metastasis. Cancer Res 2022; 82: 1321–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wander SA, Cohen O, Gong X. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov 2020; 10: 1174–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]