Abstract
Objective
To evaluate whether a sandwich technique procedure for large osteochondral lesions (OCL) of the medial femur condyle reduces clinical symptoms and improves activity level as well as to assess repair tissue integration on MRI over 2 years.
Design
Twenty-one patients (median age: 29 years, 18-44 years) who received matrix-associated autologous chondrocyte transplantation (MACT) combined with cancellous bone grafting at the medial femur condyle in a 1-step procedure were prospectively included. Patients were evaluated before surgery (baseline) as well as 3, 6, 12, and 24 months postoperatively, including clinical evaluation, Lysholm score, Tegner Activity Rating Scale, and MRI with Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score and a modified Whole-Organ Magnetic Resonance Imaging Score (WORMS).
Results
Seventeen patients were available for the 24-month (final) follow-up (4 dropouts). Lysholm significantly improved from 48 preoperatively stepwise to 95 at final follow-up (P < 0.05). Tegner improvement from 2.5 at baseline to 4.0 at final follow-up was not significant (P = 1.0). MOCART score improved significantly and stepwise from 65 at 3 months to 90 at 24 months (P < 0.05). Total WORMS improved from 14.5 at surgery to 7.0 after 24 months (P < 0.05). Body mass index and defect size at surgery correlated with total WORMS at final follow-up (P < 0.05) but did not correlate with clinical scores or defect filling.
Conclusion
MACT combined with cancellous bone grafting at the medial femoral condyle reduces symptoms continuously over 2 years. A 1-step procedure may reduce perioperative morbidity. However, despite improvements, patients’ activity levels remain low, even 2 years after surgery.
Keywords: autologous chondrocyte, grafts, cartilage transplantation, grafts, knee, joint involved, MACT, bone grafting
Introduction
Osteochondral lesions (OCL) are defined as full-thickness cartilage lesions with involvement of the adjacent subchondral bone plate. OCL are classified according to the International Cartilage Repair Society (ICRS) with the worst score for cartilage defects, ICRS 4. Comparable to the osteochondritis dissecans, OCL in general can be classified as stable with softened cartilage (ICRS OCD I), stable with partial discontinuity of the cartilage (ICRS OCD II), not yet dislocated but dead in situ (ICRS OCD III), or an empty defect with loose fragment (ICRS OCD IV).1,2
OCL in the knee are caused by osteochondritis dissecans, traumatic lesions, or degenerative changes.3,4 Osteochondritis dissecans itself can be caused by repetitive microtraumata, meniscal instability, or abnormalities in blood supply.5 -10 Treatment options depend on the size and depth of the lesions. Conservative treatment approaches might be possible in younger patients. Large and unstable lesions are challenging to treat with respect to a physiological osteochondral repair in 4 anatomic zones: articular hyaline cartilage, calcified cartilage, subchondral bone plate, and subarticular spongiosa. Conventional osteochondral autograft transfer system (OATS) technique should be performed in cases with defect sizes smaller than 2 cm2. Anatomic reconstruction of OCL larger than 2 cm2 still is difficult. Condyle transfers with allografts or in a Mega-OATS technique have been salvage procedures to maintain knee function in large OCL.1,11 -13 Still, donor-site morbidity, progression of osteoarthritis, or allograft availability is a limiting factor.1,12 As a new procedure accounting for the anatomic zones, a sandwich technique—cancellous bone grafting combined with MACT—has been developed. One- or 2-step combination procedures are possible and are still being discussed controversially. Regarding the osseous reconstruction, it remains controversial whether bone grafting should be performed directly at the time of MACT (1-step procedure) or whether a time-interval of a few weeks in between bone grafting and following MACT may be called for. 14 The 1-step procedure has been favored by most surgeons in the Society for Arthroscopy and Joint-Surgery (AGA). Promising studies have already been published, but prospective studies on this topic are rare.4,15 -18
Individuals with large OCL in the knee usually complain of pain, swelling, and locking sensations in the affected knee as main symptoms as well as of consequently reduced sporting activity. Therefore, the focused treatment of OCL should aim to reduce such symptoms and to allow higher activity levels.
The goal of this prospective study was to assess whether MACT with the bone grafting technique is able to reduce these main symptoms of large OCL ICRS 4, ICRS OCD III, and ICRS OCD IV in the knee and whether it is able to improve the individuals’ activity level over the course of 2 years. An additional aim was to evaluate morphological quality of the repair tissue and defect filling with 3.0 T MRI at different time points and to correlate MR findings with subjective and objective findings.
Materials and Methods
From August 2013 through May 2015, 21 patients (6 women, 15 men) with a median age of 29 years (range: 18-44 years) with large OCL at the medial femur condyle, who received MACT combined with cancellous bone grafting, were prospectively included in this study. Nine patients had previously undergone defect-specific surgery such as fragment removal, Pridie drilling, or microfracturing. Valgization high tibial osteotomy (HTO) was performed as well in 8 patients with a varus malalignement of >2°. In preoperative MR scans, defect size, bony defect depth, and ICRS grade were evaluated. Indication for MACT with cancellous bone grafting was an ICRS OCD lesion III or IV with defect size >2 cm2. Surgery was performed at 2 clinical centers in which any patient receiving this combined procedure at the medial femur condyle only was included. Only patients with combined ligament injuries were excluded.
Surgical Technique
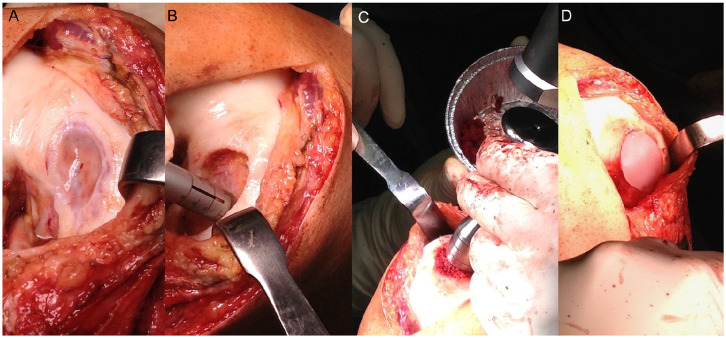
In this study, OCL were filled in a combination of cancellous bone grafting 19 and third-generation chondrocyte implantation, an MACT.20,21 In the first step, chondrocytes were harvested during arthroscopy. For this purpose, a small amount of cylindrical osteochondral tissue was harvested from the notch border with a special chisel. This tissue was stored in a cartilage medium and was sent to TETEC® (Tissue Engineering Technologies AG, Reutlingen, Germany). There, chondrocytes were isolated and proliferated in vitro. Finally, these were seeded on a bioabsorbable, biphasic collagen scaffold (matrix) of bovine origin—NOVOCART® 3D—which is administered as a single matrix unit consisting of a total of 8.25 to 44 million in vitro expanded human autologous articular chondrocytes. The bovine matrix is a type I collagen 3-dimensional (3D) scaffold that contains chondroitin sulfate.22,23 This matrix was sent back for reimplantation in a second step 3 weeks after harvesting. In a second step as seen in Image 1, subchondral bone was restored by local defect (A), debridement (B), and filling with cancellous bone harvested from the iliac crest or the distal femur (C). The impacted cancellous bone was then covered with the prepared chondrocyte matrix (D), which was sutured to the adjacent cartilage for primary stability. After the second step of surgery, weightbearing was restricted for 6 weeks. Then weightbearing was increased step-by-step by 20 kg per week. The range of motion was allowed 48 hours after surgery with flexion/extension of 90/0/0°.
Image 1.
Surgical technique: arthrotomy for exploration of OCL (A), defect debridement (B), defect filling with impacted cancellous bone (C), covering with chondrocyte matrix (D).
Clinical Assessment
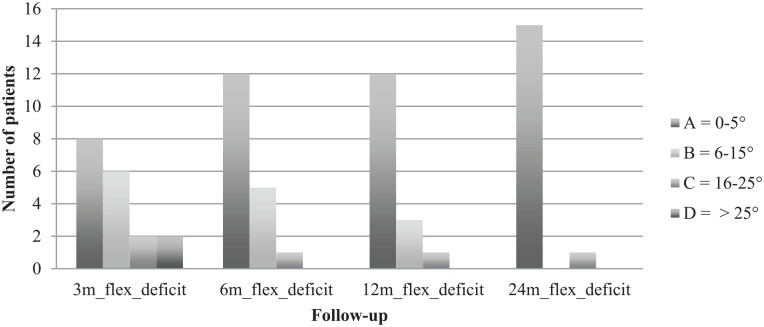
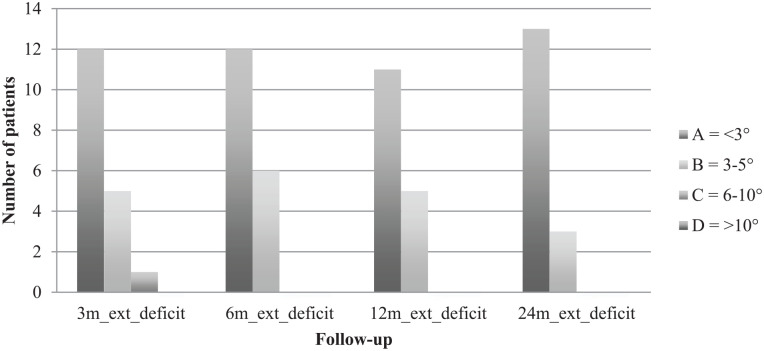
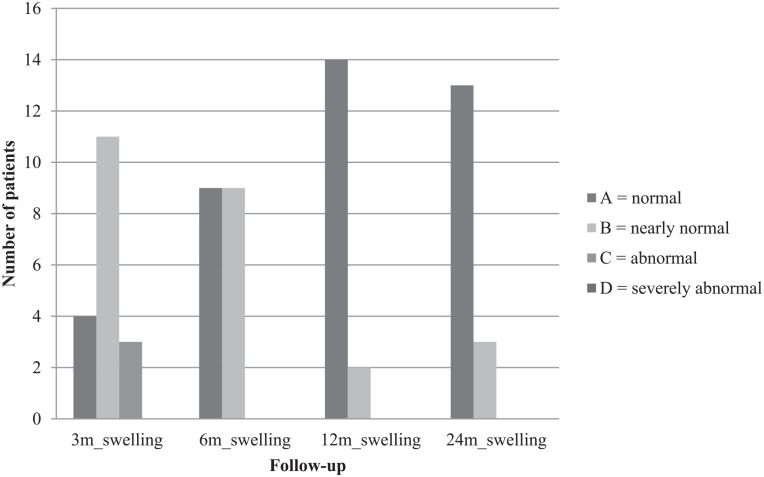
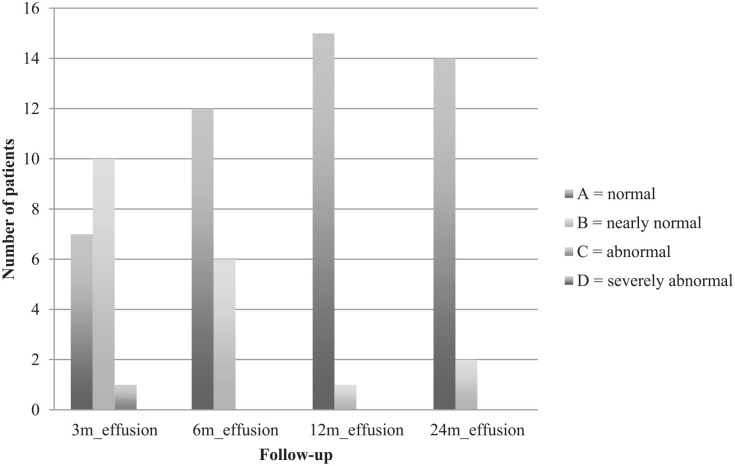
Clinical examination was performed 3, 6, 12, and 24 months postoperatively. Clinical examination was done according to the International Knee Documentation Committee (IKDC) knee examination form 24 and included swelling, effusion, and range of motion. Swelling and effusion were classified as A = normal, B = nearly normal, C = abnormal, and D = severely abnormal. The extension deficit, comparing the operated leg with the other leg, was scaled in A = <3°, B = 3°-5°, C = 6°-10°, and D = >10°. Flexion deficit was scaled in A = 0°-5°, B = 6°-15°, C = 16°-25°, and D = >25°.
Semi-quantitative scoring systems were performed before surgery (baseline) and at 3, 6, 12 and 24 months postoperatively and included the Lysholm and Gillquist 25 score and the Tegner Activity Rating Scale. 26 Lysholm Score includes the parameters limping, walking stairs, feeling of instability, swelling, weightbearing, squatting, pain, and locking symptoms. Validity and reproducibility were confirmed earlier.25,27 The Tegner Activity Rating Scale evaluates sports ability on a scale from 0 = sick note, cause of knee symptoms to 10 = national league soccer. Jogging on even ground, for example, would be represented as “4” on the Tegner Activity Rating Scale. Revision surgery was considered as treatment failure.
MRI and Evaluation
Morphological MRI of affected knees was performed at all postoperative follow-up time points, 3, 6, 12 and 24 months postoperatively, according to a specially designed protocol for 1.5 T and 3.0 T MR scanners ( Table 1 ). Coronal and sagittal orientation of the knees with a slice thickness of 3 mm in T1 and IM fat-saturated (FS) weighting were performed. Morphological features of MR images were assessed for the preoperative time point and for all postoperative time points by 2 radiologists (12 years and 8 years of experience, each blinded for review).
Table 1.
Specially designed MRI protocol for 1.5 T and 3.0 T MR scanners.
| Field strength | 1.5 T | 3.0 T | ||
| Weighting | T1 | IM FS | T1 | IM FS |
| Sequence type | TSE | |||
| Turbo factor | 3 | 7-9 | 3 | 7-9 |
| TR (ms) | 500-650 | >3,000 | 700-800 | >3,500 |
| TE (ms) | 10-15 | 40-45 | 10-15 | 40-45 |
| FOV (mm) | 160 | 140 | ||
| Matrix | 384 | |||
| Slice thickness (mm) | 3 | |||
| Gap (mm) | 0,6 | |||
| Slice orientation | Coronal and sagittal | |||
| Phase | H-F (with oversampling against aliasing) | |||
| Range (Hz/pixel) | 100-150 | 150-200 | ||
| Fat saturation | — | Low | — | High |
| Duration (min) | 3.5 | 4.5 | 3 | 4 |
TSE = turbo spin echo; FS = fat-saturated.
Preoperative MR images were evaluated for defect size and depth. For evaluation of the osteochondral repair area, the semi-quantitative Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score was used (100, best; 0, worst) as previously described.12,28,29 The modified Whole-Organ Magnetic Resonance Imaging Score (WORMS) was used to grade knee joint degeneration preoperatively and postoperatively (0, best grade; 110, worst).28,30,31 Special attention was given to the WORMS subscores for cartilage, bone marrow edema, and subchondral cysts at the repair site of the medial femoral condyle.32,33
Statistical Analysis
For statistical analysis, the Statistical Package for Social Sciences (IBM®SPSS® Statistic Subscription, version 26) was used. The descriptive statistics were calculated as median with range for each time point and variable. Variables were tested for normal distribution via the Kolmogorov-Smirnov test. Analysis between variables preoperatively (baseline) and after 3, 6, 12, and 24 months was performed using the Friedman test with Bonferroni adaptation and calculation of effect size (r) with a weak effect: 0.10 ≤ r ≤ 0.29; a medium effect: 0.30 ≤ r ≤ 0.49; and a strong effect: 0.50 ≤ r ≤ 1.0. Analysis between groups was calculated via the Mann- Whitney U test. Spearman’s rank correlation coefficient and effect size (r) were used for correlation analyses. Statistical significance was set at P < 0.05.
Funding
The study was funded by the AGA and was granted approval by Ethics Commission School of Medicine Technical University of Munich with reference number 5935/13.
Results
From the initial 21 patients, 17 were available for final MRI (81%) and 16 (76%) for final clinical scoring after 25.4 months (range: 24.0-32.5 months). Of the remaining 5 patients, 1 patient got pregnant during the follow-up period, 1 patient refused to take part in the study, 1 patient was not available for clinical scoring, and 2 patients were lost to follow-up. There were 6 female patients and 15 male patients. The mean age was 29 years (range: 18-44 years). The mean body mass index (BMI) was 24.8 (range: 18-35). Nine patients had previous defect-specific surgery like fragment removal, Pridie drilling, or microfracturing. Valgization HTO was performed as well in 8 patients with a varus malalignement of >2°. In preoperative MR scans, the defect size was 6.0 cm2 (range: 2.7-13.3 cm2), while the bony defect depth was 7.2 mm (range: 2.3-13.6 mm). All patients were graded ICRS 4 and ICRS OCD III or IV before surgery. No patient had revision surgery during follow-up.
Clinical Assessment
Comparing both knees, flexion deficit at the operated knee was seen in 12 patients 3 months after surgery, with the worst being 35° (ID 3). At the final follow-up, only 5 patients still had flexion deficit, with the worst being 20° (ID 3). ID 3 reached a range of motion of flexion/extension 110/5/0° at final follow-up compared with 95/5/0° after 3 months. Respectively, extension deficit at the operated knee was seen in 10 patients 3 months after surgery, with the worst being 7° (ID 15). At the final follow-up, only 6 patients still had extension deficit, with the worst being 5° (ID 3 and ID 15). ID 15 reached a range of motion of flexion/extension 150/0/5° at final follow-up compared with 140/0/3° after 3 months. IKDC groups for flexion and extension deficit are shown in Figs. 1 and 2 . Swelling and effusion became nearly normal over the course of time ( Figs. 3 and 4 ). Median Lysholm scores showed significant and stepwise improvements during longitudinal follow-up ( Table 2 and Fig. 5 ). The Lysholm score improved significantly—from 48.0 (range: 12.0-88.0) before surgery to 95.0 (range: 89.0-100.0) at final follow-up (P < 0.05). Significant Lysholm score changes were observed at 6-month follow-up. From surgery to final follow-up, there was an improvement in all 16 cases available.
Figure 1.
Flexion deficit.
Figure 2.
Extension deficit.
Figure 3.
Swelling.
Figure 4.
Effusion.
Table 2.
Lysholm score over the course of time.
| Lysholm | OP | 3 Months | 6 Months | 12 Months | 24 Months |
|---|---|---|---|---|---|
| Median (range) | 48 (12-88) |
73 (49-93) |
88 (63-100) |
91 (62-100) |
95 (89-100) |
OP = time at surgery.
Figure 5.
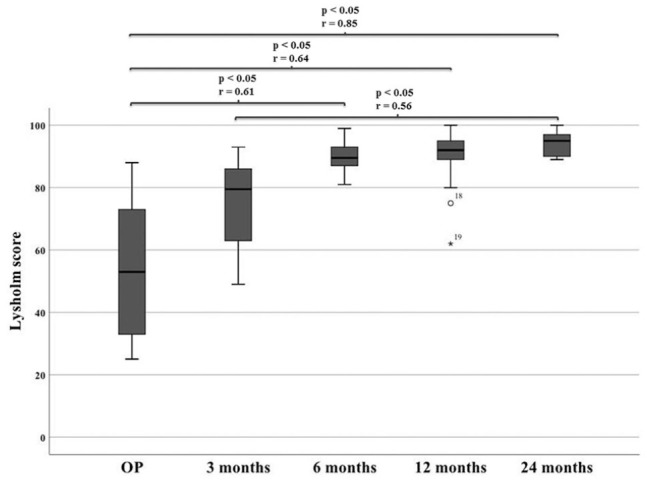
Lysholm score over the course of time. OP = time at surgery.
The pain parameter in the Lysholm score improved from baseline to final follow-up in 11 patients, remained the same in 2 patients, and worsened in only 3 patients, ranging from the absence of pain to occasional pain due to a moderate load. However, no significant difference for the pain parameter in the Lysholm score—comparing baseline with final follow-up using the Friedman test with Bonferroni correction—was detected: P = 0.27, while a moderate improving effect was observed: r = 0.35. The swelling parameter in the Lysholm score improved from baseline to final follow-up in 10 patients, remained the same in 4 patients, and only worsened in 2 patients, ranging from the absence of swelling to swelling due to a heavy load. However, no significant difference for the swelling parameter in the Lysholm score comparing baseline with final follow-up using the Friedman test with Bonferroni correction was detected: P = 0.27, while a moderate improving effect was observed: r = 0.35. The locking parameter in the Lysholm score improved from baseline to final follow-up in 10 patients and remained the same in 6 patients, meaning that 15 patients never experienced locking and only 1 patient occasionally experienced locking at final follow-up. The improvement of the locking symptom in the Lysholm score from baseline to final follow-up was significant: P < 0.05 (P = 0.047) with a moderate effect of r = 0.46. Patients with ID 1, ID 9, and ID 19 worsened in pain parameter as mentioned above, but only ID 1 also worsened in swelling. None of them had locking symptoms. ID 6 worsened in swelling but significantly improved in pain subscore. However, final Lysholm reached 89, 90, 95, and 96, respectively.
The Tegner Activity Rating Scale improved from surgery (median: 2.5, range: 1-7) to final follow-up (median: 4, range: 3-7) in 12 cases, remained the same in 2 cases (ID 1 and ID 20), and worsened in 2 cases (ID 3 and ID 19). No significant differences were observed over the course of time ( Table 3 and Fig. 6 ). Patient ID 3 was playing soccer and had a Tegner level of 7 at surgery. At the final follow-up, he still was able to perform heavy work (Tegner 5). Patient ID 19 was able to jog on uneven ground at least 2 times a week at surgery (Tegner 5) but only performed moderate to heavy work (Tegner 4) at final follow-up. Patient ID 1 and ID 20 remained at Tegner level 4 and 3, respectively. Patient ID 13 only had a Tegner level of 1 at surgery but improved to 4 after 6 months. Unfortunately, patient ID 13 was not available for final follow-up. During the first 6 months after surgery, activity levels remained at Tegner level of 4 or below. After 12 months in 4 of 21 patients, higher activity levels with Tegner scores of ≥5 were possible. At the final follow-up, most patients reached a Tegner level of 4 (9), 1 patient reached level 7, 2 patients reached level 6, 3 patients reached level 5, and only 1 patient remained at level 3.
Table 3.
Tegner score over the course of time.
| Tegner | OP | 3 Months | 6 Months | 12 Months | 24 Months |
|---|---|---|---|---|---|
| Median (range) |
2.5 (1-7) |
3 (1-4) |
4 (2-4) |
4 (3-6) |
4 (3-7) |
OP = time at surgery.
Figure 6.
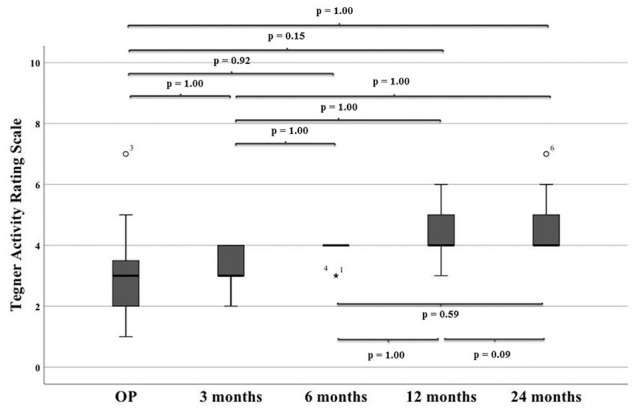
Tegner activity rating scale over the course of time.
OP = time at surgery.
From the clinical point of view, ID 1, ID 9, and ID 19 were seen as clinical failures because of worsened pain at final follow-up.
MR Imaging
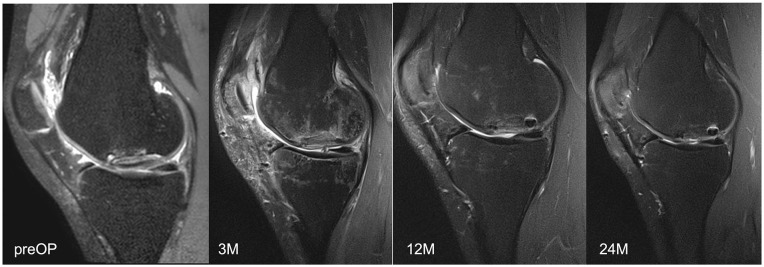
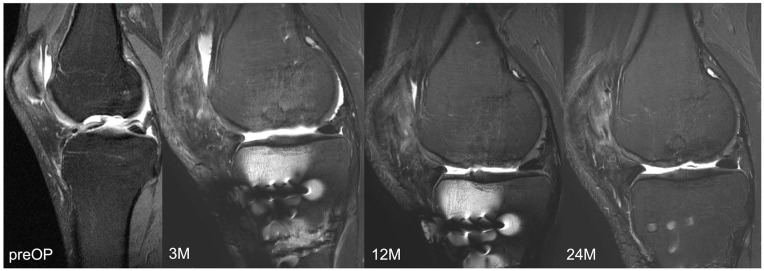
Representative MR images are shown in Images 2 and 3. Image 2 shows sagittal MRI of ID 18 with preoperative scans and after 3, 12 and 24 months (proton density turbo spin echo fat-saturated [PD TSE FS]) with final MOCART of 65. Image 3 shows sagittal MRI of ID 9 with preoperative scans and after 3, 12, and 24 months (PD TSE FS) with final MOCART of 95. The MOCART score improved significantly comparing 3 months with 12 and 24 months of follow-up ( Fig. 7 and Table 4 ). The MOCART score improved in 16 of 17 patients comparing 3 months with final follow-up. The median MOCART score between 12 and 24 months remained stable at 90. Total WORMS significantly improved from 14.5 (range: 0-33.5) at surgery to 7.0 points (range: 0-33.0) after 24 months (P < 0.05), as indicated in Table 5 and Fig. 8 . Qualitative WORMS findings—especially at the medial femur condyle at final follow-up focusing on its cartilage, bone marrow edema, and cysts—were evaluated. Five patients showed normal cartilage sign, 4 patients showed an increased T2 signal, 3 patients had a focal partial defect, and 5 patients had multiple partial thickness defects. Bone marrow edema was detected in 10 of 17 patients. Subchondral cysts were seen in 9 of 17 patients. Bone marrow edema was present in 16 of 18 patients after 3 months at the medial femoral condyle. During further follow-up, this decreased in 10 patients, remained stable in 5, and increased in 1 patient (ID 20, Lysholm 100, Tegner 3 at final follow-up). However, lowest MOCART scores, which were seen as reconstruction failure from the radiological point of view, were noticed in ID 18 (65), ID 19 (40), and ID 21 (45).
Image 2.
Magnetic resonance imaging of ID 18 with final magnetic resonance observation of cartilage repair tissue of 65.
Image 3.
Magnetic resonance imaging of ID 9 with final magnetic resonance observation of cartilage repair tissue of 95.
Figure 7.
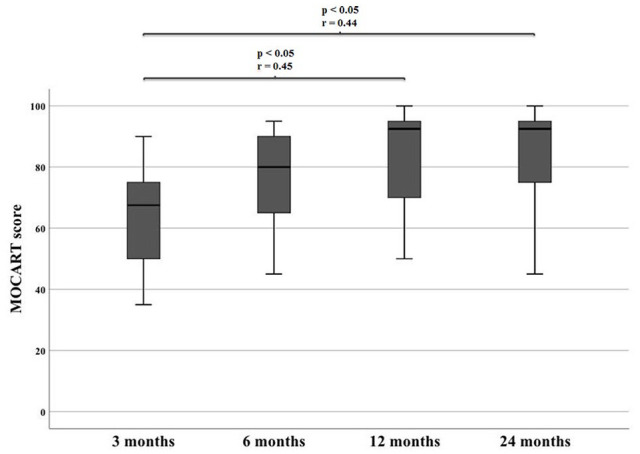
Significant differences of MOCART during follow-up. MOCART = Magnetic Resonance Observation of Cartilage Repair Tissue.
Table 4.
MOCART over the course of time.
| Magnetic Resonance Observation of Cartilage Repair Tissue | 3 Months | 6 Months | 12 Months | 24 Months |
|---|---|---|---|---|
| Median (range) |
65 (30-90) |
77.5 (40-95) |
90 (50-100) |
90 (40-100) |
OP = time at surgery.
Table 5.
WORMS over the course of time.
| Whole-Organ Magnetic Resonance Imaging Score | OP | 3 Months | 6 Months | 12 Months | 24 Months |
|---|---|---|---|---|---|
| Median (range) |
14.5 (0-33.5) |
16.0 (5.0-33.0) |
9.0 (2.0-38.0) |
7.0 (1.0-25.0) |
7.0 (0-33.0) |
OP = time at surgery.
Figure 8.
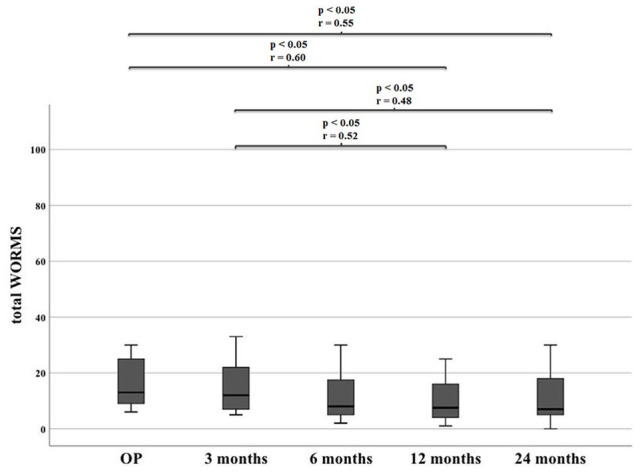
Significant differences of WORMS during follow-up. WORMS = Whole-Organ Magnetic Resonance Imaging Score.
Further Statistical Group Analysis
Significant differences depending on additional performed HTO could not be seen at the final follow-up concerning Lysholm, Tegner, MOCART, or total WORMS ( Table 6 ). Significant differences in outcome for patients with or without previous defect-specific surgery could not be detected for Lysholm, Tegner, MOCART score, or total WORMS at the final follow-up (P < 0.05) ( Table 7 ). There were no significant differences in Lysholm score, pain in Lysholm score, Tegner Activity Rating Scale, and appearance of knee effusion (0 = none, 1 = mild, 2 = moderate, 3 = notable) for patients with intact subchondral bone or subchondral bone not intact in MOCART score at the final follow-up (P > 0.05) ( Table 8 ).
Table 6.
Comparison of patients with and without HTO.
| HTO n = 7 |
Without HTO n = 9 |
Mann-Whitney U Significance | |
|---|---|---|---|
| Lysholm 24 months | 96 (89-100) | 95 (89-100) | P = 0.607 |
| Tegner 24 months | 5.0 (4-6) | 4.0 (3-7) | P = 0.181 |
| MOCART 24 months | 95 (45-100) | 75 (40-95) | P = 0.174 |
| Total WORMS 24 months | 15.5 (0-22) | 7 (1-33) | P = 0.918 |
MOCART = Magnetic Resonance Observation of Cartilage Repair Tissue; WORMS = Whole-Organ Magnetic Resonance Imaging Score; HTO = high tibial osteotomy.
Table 7.
Comparison of patients with and without previous defect specific surgery.
| Defect-Specific Surgery n = 6 |
No Defect-Specific Surgery n = 10 |
Mann-Whitney U Significance | |
|---|---|---|---|
| Lysholm 24 months | 92.5 (89-100) | 95 (90-100) | P = 0.428 |
| Tegner 24 months | 4.5 (4-7) | 4.0 (3-6) | P = 0.313 |
| MOCART 24 months | 87.5 (70-95) | 90 (40-100) | P = 0.660 |
| Total WORMS 24 months | 11.25 (1-32) | 7 (0-33) | P = 0.733 |
MOCART = Magnetic Resonance Observation of Cartilage Repair Tissue; WORMS = Whole-Organ Magnetic Resonance Imaging Score.
Table 8.
Comparison of patients with intact and not intact subchondral bone.
| Subchondral Bone Intact n = 6 |
Subchondral Bone Not Intact n = 10 |
Mann-Whitney U Significance | |
|---|---|---|---|
| Lysholm 24 months | 94 (90-100) | 95 (89-100) | P = 0.713 |
| Pain in Lysholm 24 months | 20 (20-25) | 20 (20-25) | P = 0.875 |
| Tegner 24 months | 4.0 (4-6) | 4.0 (3-7) | P = 0.958 |
| Effusion 24 months | 0 (0-1) | 0 (0-1) | P = 0.875 |
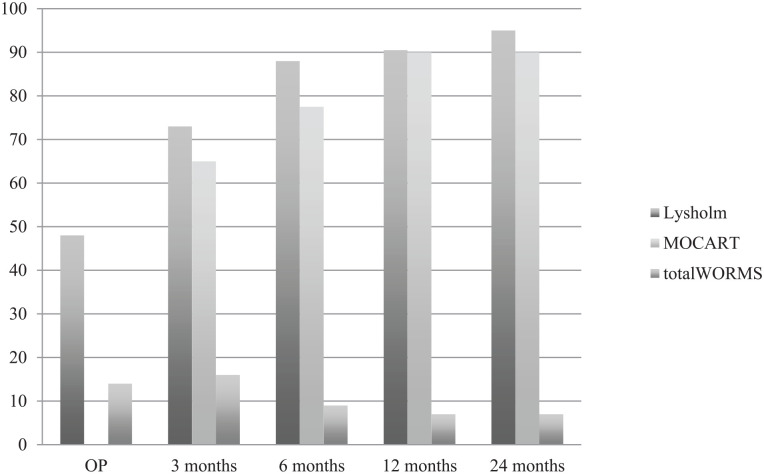
The Lysholm score after 12 months correlated significantly with MOCART score after 12 months (P < 0.05) with a strong effect (r = 0.75), while the Lysholm score after 24 months correlated negatively with total WORMS after 24 months (P < 0.05) (r = 0.56). None of the Lysholm subparameters at the final follow-up correlated significantly with MOCART score after 24 months or with total WORMS after 24 months. The medians of Lysholm, MOCART, and total WORMS over the course of time are visualized in Fig. 9 .
Figure 9.
Lysholm, MOCART, and total WORMS over the course of time. MOCART = Magnetic Resonance Observation of Cartilage Repair Tissue; WORMS = Whole-Organ Magnetic Resonance Imaging Score; OP = time at surgery.
The BMI at surgery and defect size correlated significantly with total WORMS at the final follow-up (P < 0.05) with a strong effect (r = 0.61 and r = 0.72). A higher BMI at surgery was correlated with worse squatting in Lysholm score at the final follow-up (P < 0.05) with a strong effect (r = 0.58). Other significant predictions concerning BMI, defect size, defect depth, and baseline Lysholm could not be seen. After 6-month follow-up, we could not find any significant clinical or radiological improvement.
Discussion
We believe that the sandwich technique described in this report should be performed in symptomatic cases with large and unstable OCL (ICRS 4, ICRS OCD III, and ICRS OCD IV). Our longitudinal study shows promising clinical and MR morphological results after MACT with bone grafting at the medial femoral condyle, as a single locality, over 24 months. In summary, 3 patients of this cohort had a MOCART score <70 and can be seen as reconstruction failures although 2 of them had Lysholm scores of 93 and 90. Interestingly, for all clinical and radiological parameters, there was no further significant improvement between 6- and 24-month follow-ups. Consequently, clinical and radiological control 6 months after surgery using this technique is advised. Higher BMI and larger defect sizes were predictive of inferior total WORMS outcomes after 24 months. Therefore, weight reduction in case of overweight should be advised. The 3 patients with supposed anatomic reconstruction failure (MOCART <70 at final follow-up) all had OCL exceeding 8 cm2 of defect size (8.1, 10.5 and 13 cm2). Hence, defect sizes larger than 8 to 10 cm2 should be considered as a critical size using the sandwich technique. The combination of cancellous bone grafting and MACT in a 1-step procedure is possible and patient-friendly because weightbearing is restricted for a 6-week period only. In cases of leg malalignement, HTO is advised and can be performed additionally.
Combination procedures with different bone grafting and MACT with comparable purpose have already been described earlier but failed to provide the same degree of homogeneity. Bone grafting techniques using cancellous bone, corticospongious bone, or bone blocks are inconsistent. In previous studies, frequently different lesion localities are mixed in the statistical analyses. In addition, objective analyses of MR images by use of MR scores such as MOCART or WORMS are missing: Konst et al. reported the outcome of a small sample size of 9 patients after bone grafting at lesions of different locations, the medial or lateral femur condyle, combined with gel-derived autologous chondrocyte implantation (Chondron®). Clinical scores like the IKDC improved significantly after 1 year although there was one single failure in that study. MRI 3 months after surgery showed a demarcation of the bone graft and complete defect filling with repair tissue. However, qualitative or quantitative MR scoring using MOCART or WORMS was not assessed. 16
Ochs et al. used another technique using corticospongious bone grafting combined with MACT (Novocart 3D) for defect repair for lesions at the medial or lateral femur condyle. Results of 22 patients also showed improvements of the Lysholm score and Tegner activity level, reaching 84.5 and 4.1, respectively, after a mean follow-up of 16 months. In that study, MR findings have also been reported with good defect filling at follow-up, but MOCART or WORMS scoring was not assessed. 17
In a mid-term follow-up with a very heterogeneous collective, Maus et al. 18 reported significant improvements of subjective and objective IKDC scores 36 months after defect filling of OCL of the knee at any locality, femur or tibia, with different bone grafting (cancellous bone and/or corticospongious bone) in combination with another MACT (CaRes®; ArthroKinetics, Esslingen, Germany) in 13 patients.
In an even longer follow-up, Vijayan et al. also found significant improvements of clinical scores (Cincinnati Score and Visual Analogue Pain Score) after a mean follow-up of 5.2 years in 14 patients after defect filling of OCL at different localities (medial or lateral femur condyle) with cancellous bone augmented with even 2 MACTs (bilayer collagen matrix; Genzyme®, Naarden, The Netherlands). Only 1 failure after 6 years was reported. The strength of this study was that 7 patients received arthroscopic controls with valid graft biopsies showing mixed hyaline and fibrocartilage (3), fibrocartilage (2), and fibrous tissue (2). 15
Zellner et al. reported results of the largest study cohort. They compared bone grafting in combination with MACT (Novocart 3D) depending on defect depth. Shallow lesions (<10 mm) of 16 patients filled with cancellous bone and MACT and deep lesions (> 10 mm) of 31 patients filled with bone blocks and MACT both showed significant improvements of IKDC and Cincinnati Score at 2-year follow-up. They have been the first to report good MR findings with a mean MOCART score of 82.6 at 1-year follow-up. No significant differences between both groups could be found. 4
In the sandwich technique described in the present work, we used cancellous bone to optimize shaping at the defect as an advantage. However, based on the results, use of more stable bone grafting with bone blocks similar to those described by Zellner et al. 4 especially for large lesions exceeding 8 cm2 should be considered.
The sandwich technique used in this study should be performed for large OCL ICRS 4, ICRS OCD III, and ICRS OCD IV between 2 and 8 cm2. In earlier stages and skeletally immature patients, a conservative treatment with weightbearing restriction may represent one strategy. 34 As ICRS OCD I and ICRS OCD II lesions still may have vital activity, a refixation still can be successful as primary surgical intervention.35-37 However, in such cases and after refixation of unstable OCL (ICRS OCD III), the reoperation rate tends to be high due to loose bodies.38,39 Another strategy is to refix an unstable meniscus without reconstruction of the lesion itself similar to that described by Camathias et al. 10 and later on by Blanke et al., 7 suggesting a “door stop phenomenon” as the reason for the OCL. However, this technique should only be considered if regeneration of the lesion is still possible. For ICRS OCD IV, different kinds of surgical procedures have been described. Fragment removal seems to be a fast option to reduce locking and pain, but joint degeneration will progress.40,41 Smaller OCL <2 cm2 can be treated with autologous osteochondral transplantation (OATS) with up to 72% successful outcomes. 42 Although there are several reports of good clinical outcomes for OATS in OCL up to 4 cm2, treatment gets more challenging with increasing defect size and depth.43,44 Condyle transfers with allografts or in a Mega-OATS technique have been salvage procedures to maintain the knee function in large OCL.1,11-13 Hohmann and Tetsworth 13 described 8 of 9 good osseous integration in MRI and improving Lysholm from 40 to 90 two years after fresh-frozen femur condyle allograft transplantation.
However, a reported long-term reoperation rate of 47% is rather high. 45 In addition, tissue may not be available in all countries. Moreover, an adequate infrastructure is required for maintaining the viability of cartilage until transplantation. 46 Autologous condyle transfer (Mega-OATS) could achieve a mean Lysholm of 78.9 and a mean MOCART of 61.5 in a retrospective 9-year follow-up. However, higher T2 relaxation times were found for ipsilateral knees, indicating early osteoarthritic changes. 12
The advantage of the sandwich technique compared with allografts and Mega-OATS is the possibility to reconstruct the joint surface with autologous tissue without local donor-site morbidity. A disadvantage of this technique may be the need for 2 surgical procedures and the time-consuming process during rehabilitation. Also, further long-term studies are needed to assess whether the sandwich technique can stop or slow down early osteoarthritic changes.
Limitations
Despite good values in final Lysholm score, 3 patients claimed to have worsened pain compared with baseline score. One patient (ID 19) also showed a worse final MOCART score with 40 points and worsened final Tegner score. Two more patients (ID 18 and ID 21) only reached 65 and 45 points in the final MOCART score. So overall in 5 patients, the main aim of the study, to reconstruct the OCL and to reduce main symptoms, could not be achieved.
The appearance of bone marrow edema, subchondral cysts, and partial thickness defects of cartilage repair tissue will likely compound 3-dimensional defect filling with the 2 separate layers, cancellous bone grafting and chondrocyte matrix. Several factors might play a role in insufficient bone defect filling. Bone defect filling with cancellous bone chips, although impacted, might provide less primary stability and could be better restored with cylindric bone block transplantation as described in Zellner et al. 4 Primary stability—especially at the defect border—appears to be the clue to bone defect filling. Shear forces caused by uneven borders might play a role in inflammation of adjacent cartilage and bone that causes bone marrow edema, cysts, and resorption of the transplanted bone. Similar findings have been seen in a surface-replacing model by Manda et al. 47
Although a significant difference could not be seen between patients with or without HTO, or in patients with or without previous defect-specific surgery, these differences may cause a bias in the cohort. But even for further studies, it may be difficult to collect data from more homogeneous patient cohort because most patients with this morbidity will have any defect-specific surgery or will need an additional HTO.
As the appearance of bone marrow edema in MRI after chondrocyte transplantation is very common, clinical correlation is questioned. However, the reported correlations between clinical scores and MR findings are inconsistent. Although the Lysholm score after 12 months was found to correlate with the MOCART score at this time point, no correlation could be seen at the final follow-up. However, a better Lysholm score was found to correlate with a lower total WORMS score at the final follow-up. Other studies also show inconsistent correlations between MR scans and clinical findings.48,49
Despite a prospective approach, 4 patients were not available for final MRI at 24 months. Nevertheless, they are mentioned to underscore the intention to provide treatment.
The period 24 months has been chosen as the final follow-up, but even longer follow-up studies may be needed to determine whether the medial compartment can be preserved or whether a replacement is required after several years.
Conclusion
Symptoms of large and unstable OCL (ICRS OCD III and ICRS OCD IV) of the medial femur condyle can be reduced by defect reconstruction using cancellous bone grafting in combination with MACT. This combination can contribute significantly to improvements in patients’ activity levels. Although there was no need for reoperation during follow-up, reconstruction failure was observed in 1 patient from the clinical and radiological point of view, 2 patients from the clinical point of view, and 2 patients from the radiological view; however, clinical results and MRI findings again had little overlap. Based on our results, the 1-step sandwich technique may be recommended for surgical treatment of OCL at the medial femoral condyle, although more stable subchondral bone transplants such as cylindrical bone transplantation may be suggested with increasing size and depth of the OCL. Between 6 and 24 months of follow-ups, we could not find any significant clinical or radiological improvement. Hence, MRI control 6 and 12 months postoperatively is useful for failure detection. Long-term follow-up studies are needed for this technique, especially to clarify early osteoarthritic changes and the correlation between MR findings and clinical outcome.
Footnotes
Authors’ Note: Institutions: Klinikum rechts der Isar der Technischen Universität München, Abteilung und Poliklinik für Sportorthopädie, Ismaningerstraße 22, 81675 München, Germany; Universitätsklinikum Regensburg, Klinik für Unfallchirurgie, Franz-Josef-Strauß-Allee 11, 93053 Regensburg, Germany.
Acknowledgments and Funding: We would like to thank the AGA—Society for Arthroscopy and Joint-Surgery for funding this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was granted approval by Ethics Commission School of Medicine Technical University of Munich with reference number 5935/13.
ORCID iD: C. Holwein  https://orcid.org/0000-0002-1655-1435
https://orcid.org/0000-0002-1655-1435
References
- 1. Minzlaff P, Braun S, Haller B, Wortler K, Imhoff AB. [Autologous transfer of the posterior femoral condyle for large osteochondral lesions of the knee: 5-year results of the Mega-OATS technique]. Orthopade. 2010;39(6):631-6. doi: 10.1007/s00132-010-1608-2. [DOI] [PubMed] [Google Scholar]
- 2. Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):58-69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 3. Edmonds EW, Polousky J. A review of knowledge in osteochondritis dissecans: 123 years of minimal evolution from Konig to the ROCK study group. Clin Orthop Relat Res. 2013;471(4):1118-26. doi: 10.1007/s11999-012-2290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zellner J, Grechenig S, Pfeifer CG, Krutsch W, Koch M, Welsch G, et al. Clinical and radiological regeneration of large and deep osteochondral defects of the knee by bone augmentation combined with matrix-guided autologous chondrocyte transplantation. Am J Sports Med. 2017;45(13):3069-80. doi: 10.1177/0363546517717679. [DOI] [PubMed] [Google Scholar]
- 5. Clanton TO, DeLee JC. Osteochondritis dissecans. History, pathophysiology and current treatment concepts. Clin Orthop Relat Res. 1982;167:50-64. Available from: https://www.ncbi.nlm.nih.gov/pubmed/6807595. [PubMed] [Google Scholar]
- 6. Hughes JA, Cook JV, Churchill MA, Warren ME. Juvenile osteochondritis dissecans: a 5-year review of the natural history using clinical and MRI evaluation. Pediatr Radiol. 2003;33(6):410-7. doi: 10.1007/s00247-003-0876-y. [DOI] [PubMed] [Google Scholar]
- 7. Blanke F, Feitenhansl A, Haenle M, Vogt S. Arthroscopic meniscopexy for the treatment of nontraumatic osteochondritis dissecans in the knee joint of adult patients. Cartilage. 2020;11(4):441-6. doi: 10.1177/1947603518800541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laor T, Zbojniewicz AM, Eismann EA, Wall EJ. Juvenile osteochondritis dissecans: is it a growth disturbance of the secondary physis of the epiphysis? AJR Am J Roentgenol. 2012;199(5):1121-8. doi: 10.2214/AJR.11.8085. [DOI] [PubMed] [Google Scholar]
- 9. Petrie PW. Aetiology of osteochondritis dissecans. Failure to establish a familial background. J Bone Joint Surg Br. 1977;59(3):366-7. doi: 10.1302/0301-620X.59B3.893517. [DOI] [PubMed] [Google Scholar]
- 10. Camathias C, Hirschmann MT, Vavken P, Rutz E, Brunner R, Gaston MS. Meniscal suturing versus screw fixation for treatment of osteochondritis dissecans: clinical and magnetic resonance imaging results. Arthroscopy. 2014;30(10):1269-79. doi: 10.1016/j.arthro.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 11. Agneskirchner JD, Brucker P, Burkart A, Imhoff AB. Large osteochondral defects of the femoral condyle: press-fit transplantation of the posterior femoral condyle (MEGA-OATS). Knee Surg Sports Traumatol Arthrosc. 2002;10(3):160-8. doi: 10.1007/s00167-001-0259-6. [DOI] [PubMed] [Google Scholar]
- 12. Jungmann PM, Brucker PU, Baum T, Link TM, Foerschner F, Minzlaff P, et al. Bilateral cartilage T2 mapping 9 years after Mega-OATS implantation at the knee: a quantitative 3T MRI study. Osteoarthritis Cartilage. 2015;23(12):2119-28. doi: 10.1016/j.joca.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 13. Hohmann E, Tetsworth K. Large osteochondral lesions of the femoral condyles: treatment with fresh frozen and irradiated allograft using the Mega OATS technique. Knee. 2016;23(3):436-41. doi: 10.1016/j.knee.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 14. Perkins CA, Willimon SC. Management of the failed OCD. Curr Rev Musculoskelet Med. 2020;13(2):173-9. doi: 10.1007/s12178-020-09611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vijayan S, Bartlett W, Bentley G, Carrington RW, Skinner JA, Pollock RC, et al. Autologous chondrocyte implantation for osteochondral lesions in the knee using a bilayer collagen membrane and bone graft: a two- to eight-year follow-up study. J Bone Joint Surg Br. 2012;94(4):488-92. doi: 10.1302/0301-620X.94B4.27117. [DOI] [PubMed] [Google Scholar]
- 16. Konst YE, Benink RJ, Veldstra R, van der Krieke TJ, Helder MN, van Royen BJ. Treatment of severe osteochondral defects of the knee by combined autologous bone grafting and autologous chondrocyte implantation using fibrin gel. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2263-9. doi: 10.1007/s00167-012-1891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ochs BG, Muller-Horvat C, Rolauffs B, Fritz J, Weise K, Schewe B. [Treatment of osteochondritis dissecans of the knee: one-step procedure with bone grafting and matrix-supported autologous chondrocyte transplantation]. Z Orthop Unfall. 2007;145(2):146-51. doi: 10.1055/s-2007-965167. [DOI] [PubMed] [Google Scholar]
- 18. Maus U, Schneider U, Gravius S, Müller-Rath R, Mumme T, Miltner O, et al. [Clinical results after three years use of matrix-associated ACT for the treatment of osteochondral defects of the knee]. Z Orthop Unfall. 2008;146(1):31-7. doi: 10.1055/s-2007-989353. [DOI] [PubMed] [Google Scholar]
- 19. van Dyk GE, Dejardin LM, Flo G, Johnson LL. Cancellous bone grafting of large osteochondral defects: an experimental study in dogs. Arthroscopy. 1998;14(3):311-20. doi: 10.1016/s0749-8063(98)70148-3. [DOI] [PubMed] [Google Scholar]
- 20. Zak L, Albrecht C, Wondrasch B, Widhalm H, Vekszler G, Trattnig S, et al. Results 2 years after matrix-associated autologous chondrocyte transplantation using the Novocart 3D scaffold: an analysis of clinical and radiological data. Am J Sports Med. 2014;42(7):1618-27. doi: 10.1177/0363546514532337. [DOI] [PubMed] [Google Scholar]
- 21. Marlovits S, Zeller P, Singer P, Resinger C, Vecsei V. Cartilage repair: generations of autologous chondrocyte transplantation. Eur J Radiol. 2006;57(1):24-31. doi: 10.1016/j.ejrad.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 22. Nuernberger S, Cyran N, Albrecht C, Redl H, Vecsei V, Marlovits S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials. 2011;32(4):1032-40. doi: 10.1016/j.biomaterials.2010.08.100. [DOI] [PubMed] [Google Scholar]
- 23. Foldager CB, Gomoll AH, Lind M, Spector M. Cell seeding densities in autologous chondrocyte implantation techniques for cartilage repair. Cartilage. 2012;3(2):108-17. doi: 10.1177/1947603511435522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226-34. doi: 10.1007/BF01560215. [DOI] [PubMed] [Google Scholar]
- 25. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-4. doi: 10.1177/036354658201000306. [DOI] [PubMed] [Google Scholar]
- 26. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/4028566. [PubMed] [Google Scholar]
- 27. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-7. doi: 10.1177/0363546508330143. [DOI] [PubMed] [Google Scholar]
- 28. Gersing AS, Feuerriegel G, Holwein C, Suchowierski J, Karampinos DC, Haller B, et al. T2-relaxation time of cartilage repair tissue is associated with bone remodeling after spongiosa-augmented matrix-associated autologous chondrocyte implantation. Osteoarthritis Cartilage. 2019;27(1):90-8. doi: 10.1016/j.joca.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 29. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16-23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 30. Jungmann PM, Tham SC, Liebl H, Nevitt MC, McCulloch CE, Lynch J, et al. Association of trochlear dysplasia with degenerative abnormalities in the knee: data from the Osteoarthritis Initiative. Skeletal Radiol. 2013;42(10):1383-92. doi: 10.1007/s00256-013-1664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jungmann PM, Nevitt MC, Baum T, Liebl H, Nardo L, Liu F, et al. Relationship of unilateral total hip arthroplasty (THA) to contralateral and ipsilateral knee joint degeneration—a longitudinal 3T MRI study from the Osteoarthritis Initiative (OAI). Osteoarthritis Cartilage. 2015;23(7):1144-53. doi: 10.1016/j.joca.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177-90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 33. Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2012;64(2):248-55. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andriolo L, Candrian C, Papio T, Cavicchioli A, Perdisa F, Filardo G. Osteochondritis dissecans of the knee—conservative treatment strategies: a systematic review. Cartilage. 2019;10(3):267-77. doi: 10.1177/1947603518758435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barrett I, King AH, Riester S, van Wijnen A, Levy BA, Stuart MJ, et al. Internal fixation of unstable osteochondritis dissecans in the skeletally mature knee with metal screws. Cartilage. 2016;7(2):157-62. doi: 10.1177/1947603515622662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Millington KL, Shah JP, Dahm DL, Levy BA, Stuart MJ. Bioabsorbable fixation of unstable osteochondritis dissecans lesions. Am J Sports Med. 2010;38(10):2065-70. doi: 10.1177/0363546510371369. [DOI] [PubMed] [Google Scholar]
- 37. Weckstrom M, Parviainen M, Kiuru MJ, Mattila VM, Pihlajamaki HK. Comparison of bioabsorbable pins and nails in the fixation of adult osteochondritis dissecans fragments of the knee: an outcome of 30 knees. Am J Sports Med. 2007;35(9):1467-76. doi: 10.1177/0363546507300692. [DOI] [PubMed] [Google Scholar]
- 38. Leland DP, Bernard CD, Camp CL, Nakamura N, Saris DBF, Krych AJ. Does internal fixation for unstable osteochondritis dissecans of the skeletally mature knee work? a systematic review. Arthroscopy. 2019;35(8):2512-22. doi: 10.1016/j.arthro.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 39. Scioscia TN, Giffin JR, Allen CR, Harner CD. Potential complication of bioabsorbable screw fixation for osteochondritis dissecans of the knee. Arthroscopy. 2001;17(2):E7. doi: 10.1053/jars.2001.17995. [DOI] [PubMed] [Google Scholar]
- 40. Aglietti P, Ciardullo A, Giron F, Ponteggia F. Results of arthroscopic excision of the fragment in the treatment of osteochondritis dissecans of the knee. Arthroscopy. 2001;17(7):741-6. doi: 10.1053/jars.2001.25336. [DOI] [PubMed] [Google Scholar]
- 41. Michael JW, Wurth A, Eysel P, Konig DP. Long-term results after operative treatment of osteochondritis dissecans of the knee joint-30 year results. Int Orthop. 2008;32(2):217-21. doi: 10.1007/s00264-006-0292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pareek A, Reardon PJ, Maak TG, Levy BA, Stuart MJ, Krych AJ. Long-term outcomes after osteochondral autograft transfer: a systematic review at mean follow-up of 10.2 years. Arthroscopy. 2016;32(6):1174-84. doi: 10.1016/j.arthro.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 43. Dekker TJ, Aman ZS, DePhillipo NN, Dickens JF, Anz AW, LaPrade RF. Chondral lesions of the knee: an evidence-based approach. J Bone Joint Surg Am. 2021;103(7):629-45. doi: 10.2106/JBJS.20.01161. [DOI] [PubMed] [Google Scholar]
- 44. Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):434-47. doi: 10.1007/s00167-010-1072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Levy YD, Gortz S, Pulido PA, McCauley JC, Bugbee WD. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471(1):231-7. doi: 10.1007/s11999-012-2556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Filardo G, Andriolo L, Soler F, Berruto M, Ferrua P, Verdonk P, et al. Treatment of unstable knee osteochondritis dissecans in the young adult: results and limitations of surgical strategies-the advantages of allografts to address an osteochondral challenge. Knee Surg Sports Traumatol Arthrosc. 2019;27(6):1726-38. doi: 10.1007/s00167-018-5316-5. [DOI] [PubMed] [Google Scholar]
- 47. Manda K, Ryd L, Eriksson A. Finite element simulations of a focal knee resurfacing implant applied to localized cartilage defects in a sheep model. J Biom. 2011;44(5):794-801. doi: 10.1016/j.jbiomech.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 48. Weigelt L, Hartmann R, Pfirrmann C, Espinosa N, Wirth SH. Autologous matrix- induced chondrogenesis for osteochondral lesions of the talus: a clinical and radiological 2- to 8-year follow-up study. Am J Sports Med. 2019;47(7):1679-86. doi: 10.1177/0363546519841574. [DOI] [PubMed] [Google Scholar]
- 49. Kreuz PC, Kalkreuth RH, Niemeyer P, Uhl M, Erggelet C. Long-term clinical and MRI results of matrix-assisted autologous chondrocyte implantation for articular cartilage defects of the knee. Cartilage. 2019;10(3):305-13. doi: 10.1177/1947603518756463. [DOI] [PMC free article] [PubMed] [Google Scholar]