ABSTRACT
The association between dairy consumption and cancer mortality varies among studies and remains unclear. Thus, we conducted a comprehensive meta-analysis of prospective cohort studies to examine the association between dairy consumption and total cancer and cancer-specific mortality. We sought eligible studies in PubMed and Web of Science databases for all publications through March 2021, and pooled RRs and 95% CIs were calculated. We identified 34 prospective cohort studies including 3,171,186 participants and 88,545 deaths. Compared with low milk consumption, high milk consumption was associated with higher cancer mortality in females (RR: 1.10; 95% CI: 1.01, 1.21) and people consuming high/whole-fat milk (fat content ≥3.5%) (RR: 1.17; 95% CI: 1.07, 1.28). Increased risks of cancer-specific mortality were detected for liver (RR: 1.13; 95% CI: 1.02, 1.26), ovarian (RR: 1.32; 95% CI: 1.13, 1.55), and prostate (RR: 1.23; 95% CI: 1.02, 1.48) cancers. Also, females with high consumption of fermented milk had a lower cancer mortality risk (RR: 0.85; 95% CI: 0.77, 0.94). High cheese consumption was not associated with total cancer mortality but rather with higher colorectal cancer mortality (RR: 1.22; 95% CI: 1.02, 1.46). There was no association between butter (RR: 1.06; 95% CI: 0.70, 1.59) or total dairy product consumption (RR: 0.99; 95% CI: 0.95, 1.03) and cancer mortality. Our results imply that high milk consumption, especially high/whole-fat milk, was associated with higher cancer mortality, whereas fermented milk consumption was associated with lower cancer mortality, and this was particularly evident in females. Consequently, further studies are warranted.
Keywords: dairy products, milk, fermented milk, cancer, mortality, meta-analysis
Statement of Significance: This meta-analysis indicated that high-fat milk consumption might be associated with higher cancer mortality, whereas fermented milk consumption is inversely associated with cancer mortality.
Introduction
Cancer is a leading cause of death globally. The WHO reported that cancer accounted for nearly 10 million deaths in 2020 (1). Dairy products are recommended in most dietary guidelines worldwide. Dairy products are a complex blend of bioactive compounds that have both positive and negative impacts on carcinogenesis (2). However, the contents of dairy products differ based on the type of dairy product; therefore, each dairy product may not have the same effect on cancer. The World Cancer Research Foundation International (WCRF) reported that the consumption of dairy products probably protects against colorectal cancer (3). In addition, the WCRF also reported that the consumption of dairy products may reduce the risk of breast cancer and increase the risk of prostate cancer, but the evidence is limited (3). These results imply that dairy products consumption may have varying effects depending on the cancer site.
A few prospective cohort studies have examined the association between dairy products consumption and cancer mortality in the general population, but the association remains inconsistent. Previously, 3 meta-analyses on dairy consumption and cancer mortality showed no significant association between dairy consumption and cancer mortality (4–6). In the analyses, the results of mortality from specific cancers were included for total cancer mortality, such as prostate cancer mortality. One meta-analysis that included studies conducted in the United States only examined total dairy consumption without analyzing other types of dairy products (5). Another meta-analysis assessed total consumption of dairy products and milk without analyzing fermented milk, cheese, or other types of dairy products (6). Although Lu et al. (4) conducted a meta-analysis of several types of dairy products and cancer mortality risk, the subgroup analysis had many limitations due to few studies included in the analysis. In addition, no comprehensive meta-analysis of dairy products consumption on cancer-specific mortality risk has been conducted to date.
Therefore, we conducted a comprehensive meta-analysis to quantitatively assess the association between the consumption of total dairy products and subtypes of dairy products, including milk, fermented milk (which includes yogurt and soured milk), cheese, and butter, and total cancer mortality. Furthermore, we conducted subgroup analyses stratified by sex, cancer site, fat content, geographic region, and adjustment factors.
Methods
Literature search and study selection
We conducted a literature search for all publications through March 2021 as full-length articles and written in English using PubMed and ISI Web of Science. The search terms were as follows: “(dairy OR milk OR cheese OR yogurt OR yoghurt OR butter)” AND “(neoplasia OR neoplasm OR cancer OR carcinoma OR tumor OR tumour)” AND “(mortality OR death OR fatal OR survival).” This meta-analysis included studies that satisfied the following criteria: 1) the study was designed prospectively; 2) the exposure was dairy product consumption, including total dairy, milk, fermented milk, cheese, and butter; 3) the outcomes were all cancer mortality or cancer-specific mortality; and 4) the study reported RRs and CIs. We excluded studies that examined mortality risk in participants with the disease at baseline. Also, if more than 1 publication from the same cohort was available, we selected the publication with a long follow-up period or a high number of cases.
Data extraction
Both authors (SJ and YJ) independently extracted data according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (7). Each study provided the following information: the first author's last name, year of publication, geographical region and cohort name, follow-up period, baseline age, the number of participants, the number of deaths, each category of dairy products consumption, RR and 95% CI for the association between each dairy product consumption category and cancer mortality, and adjustment factors. We adopted the RR that reflected the most adjustment degree for potential confounding factors if the study provided multiple RRs for this association.
Quality assessment
The Newcastle–Ottawa Scale was used to evaluate the quality of studies included in the meta-analysis (8). We examined the quality of each study in the following aspects: the selection of study participants, comparability, and outcome assessment. Studies with scores of 10 or higher (out of 13) were considered high quality, 7–9 as good quality, and 6 or lower as low quality.
Statistical analysis
The DerSimonian and Laird random-effects models (9), which incorporated both within- and between-studies variations, were used to estimate pooled RR of cancer mortality for the highest compared with lowest consumption of total dairy, milk, fermented milk (which includes yogurt and soured milk), cheese, and butter. When a study reported results of specific cancer and other cancers but not total cancer, we used a fixed-effect model to get an overall estimate of total cancer before combining it with other studies (10). If the study reported individual RRs for whole milk, low-fat milk, and skim milk but not total milk, we used a fixed-effect model to get an overall estimate of total milk or low/skimmed milk before merging it with additional studies (11–15). We conducted subgroup analyses stratified by sex, cancer site, fat content (high/whole fat or low/nonfat), and geographic region (United States, Asia, Europe, or Oceania). We classified cancer sites as upper digestive tract (mouth cancer, pharynx cancer, and esophageal cancer), gastrointestinal tract (stomach cancer, colorectal cancer, and anal cancer), hepatobiliary system (liver cancer, gallbladder cancer, pancreatic cancer, and bile duct cancer), respiratory tract (lung cancer, nasopharyngeal cancer, and larynx cancer), women's cancer (breast cancer, cervical cancer, endometrial cancer, and ovarian cancer), men's cancer (prostate cancer), and urological system (kidney cancer and bladder cancer). Furthermore, we examined whether the studies had accounted for crucial confounders such as BMI, total energy intake, alcohol consumption, smoking status, physical activity, and socioeconomic status, conducting stratified analysis by the adjustment factors. The Q statistic assessed heterogeneity among studies included in the meta-analysis (16), and the I2 statistic quantified inconsistency (17). Sensitivity analyses were conducted by excluding each study one at a time.
Dose-response analyses were conducted using the method by Greenland and Longnecker (18–20) to calculate the study-specific slopes across dairy products intake categories. The analysis was conducted for studies that provided RRs with at least 3 exposure categories and the distribution of deaths and person years or subjects in each dairy products intake category. We calculated the midpoint value for each dairy products intake category if a study presented the intake as a range. We adopted the interval of the adjacent category if the highest category was open-ended. If a study reported consumption as a serving, we converted the unit to 177 g for total dairy, 244 g for milk or fermented milk, and 43 g for cheese, as provided in the USDA Food and Nutrient Database for Dietary Studies (21). Begg (22) and Egger (23) tests were used for examining potential publication bias. A 2-tailed P < 0.05 was considered statistically significant. Stata/SE version 14.2 was used for statistical analyses (StataCorp).
Results
Study characteristics
A total of 34 studies (41 publications from 1984 to 2021) (5, 10–15, 24–57) with 3,171,186 participants and 88,545 deaths were included (Figure 1). Table 1 shows the characteristics of prospective cohort studies included in the meta-analysis. Seventeen studies were conducted in the United States (5, 11–15, 24–26, 28–31, 34, 38, 41, 43, 51, 53, 54, 57), 8 studies in Asia (27, 32, 33, 35–37, 39, 40, 44, 47, 48, 56), 8 studies in Europe (10, 45, 46, 49, 50, 52, 55), and 1 study in Oceania (42). Cohort studies included a follow-up period ranging from 4.2 to 41 y with a median follow-up time of 14.15 y, and participants were aged ≥15 y at baseline. All studies adjusted for age, and most studies adjusted for BMI (n = 25) (5, 10, 12–15, 34, 36, 38, 40–49, 51–56), total energy intake (n = 21) (5, 12–15, 31, 38, 41–43, 45, 46, 48, 50–54, 56, 57), alcohol consumption (n = 24) (5, 10, 12–15, 31, 36, 38, 42, 43, 45–57), smoking status (n = 30) (5, 10–15, 28–38, 41–43, 45–57), physical activity (n = 23) (5, 12–15, 36, 38, 40–43, 45–57), and socioeconomic status (n = 22) (5, 10, 12, 14, 26, 30, 34, 36, 40–49, 52, 55–57). In terms of quality assessment, the studies included in the meta-analysis had a mean score of 11.1 out of 13, except for 1 study having a score of 8, which indicates good quality (27), and all studies had a score of >9, indicating high quality.
FIGURE 1.
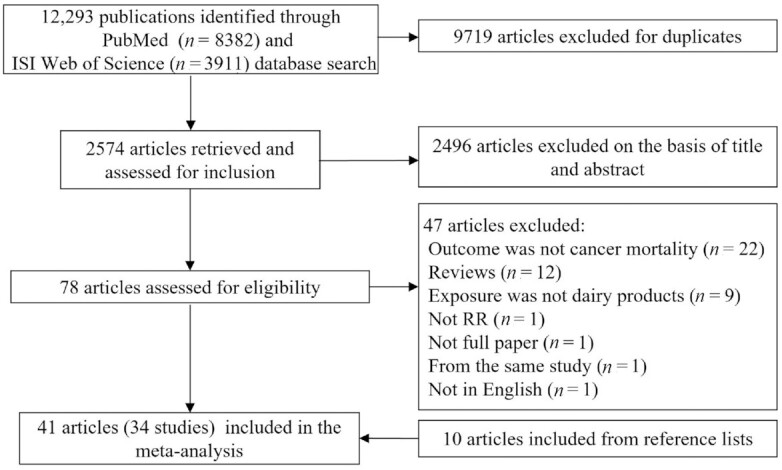
Flow diagram of the study selection.
TABLE 1.
Characteristics of the prospective cohort studies included in the meta-analysis1
| First author, year | Country | Cohort name | Follow-up period | Age at baseline, y | Participants | No. of deaths | Exposure category | Cancer type | Adjustment for covariates2 | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Snowdon, 1984 (24) | United States | California Seventh-Day Adventists | 21 y | ≥30 | 6763 | 99 | Milk <1 (ref), 1–2, ≥3 glasses/dCheese <1 (ref), 1–2, ≥3 d/wk | Prostate | 1 | 10 |
| Phillips, 1985 (25) | United States | California Seventh-Day Adventists | 21 y | ≥30 | 25,493 | 182 | Milk <1 (ref), 1–2, ≥3/dCheese <1 (ref), 1–2, ≥3/wk | Colorectal | 1, 2 | 10 |
| Mills, 1988 (26) | United States | California Seventh-Day Adventists | 21 y | 30–85 | 994 | 142 | Milk None/occasional (ref), 1, 2, ≥3 drinks/dCheese None/occasional (ref), 1, 2, ≥3 d/wk | Breast | 9, 13, 14, 15, 16, 17, 18, 19 | 10 |
| Hirayama, 1990 (27) | Japan | Japan 6 Prefectures Cohort Study | 17 y | ≥40 | 265,118 | 14,740 | MilkNondaily (ref), daily | Mouth, pharynx, esophagus, stomach, colon, rectum, liver, gallbladder, pancreas, nasal sinus, larynx, lung, breast, cervix uteri, ovary, prostate, kidney, bladder | 1, 2 | 8 |
| Hsing, 1990 (28) | United States | The Lutheran Brotherhood Cohort | 20 y | ≥35 | 17,633 | 149 | Dairy<26 (ref), 27–51, 52–85, 86–189 times/mo | Prostate | 1, 5 | 12 |
| Kneller, 1991 (29) | United States | The Lutheran Brotherhood Cohort | 20 y | ≥35 | 17,633 | 75 | DairyQ1 (ref), Q2, Q3, Q4Milk<1 (ref), 1, 2–3, ≥4 glasses/d | Stomach | 1, 5 | 12 |
| Chow, 1992 (30) | United States | The Lutheran Brotherhood Cohort | 20 y | ≥35 | 17,633 | 219 | Dairy<46 (ref), 46–95, 96–142, >142 times/mo | Lung | 1, 5, 10 | 12 |
| Hsing, 1998 (31) | United States | The Lutheran Brotherhood Cohort | 20 y | ≥35 | 17,633 | 145 | Dairy<26.0 (ref), 26.0–50.0, 51.0–85.0, >85.0 times/mo | Colorectal | 1, 5, 6, 7 | 12 |
| Breslow, 2000 (11) | United States | The National Health Interview Survey | 8.5 y | 18–87 | 20,195 | 158 | Dairy0–3.0 (ref), 3.0–7.0, 7.0–10.0, >10.0 servings/wkWhole milk0 (ref), 0.02–7, >8.0 servings/wkLow-fat milk0 (ref), 0.02–7, >8.0 servings/wkSkim milk0 (ref), 0.02–5, >5.6 servings/wkCheese0–0.5 (ref), 0.5–1.9, 2.0–3.0, >3.0 servings/wk | Lung | 1, 2, 5 | 11 |
| Ozasa, 2001 (32) | Japan | The Japan Collaborative Cohort | 92 mo | 40–79 | 98,248 | 572 | MilkScarcely any (ref), 1–2/mo to 3–4/wk, almost every dayYogurtScarcely any (ref), 1–2/mo to 1–2/wk, ≥3–4/wkCheeseScarcely any (ref), 1–2/mo to 1–2/wk, ≥3–4/wkButterScarcely any (ref), 1–2/mo to 1–2/wk, ≥3–4/wk | Lung | 1, 5, 12 | 10 |
| Ness, 2001 (10) | United Kingdom | The Collaborative study | 25 y | 35–64 | 5765 | 714 | MilkNone (ref), 1, >1 pint/d | Lung, other cancer | 1, 4, 5, 7, 9, 11, 20, 21, 22, 23, 24 | 10 |
| Rodriguez, 2002 (34) | United States | Cancer Prevention Studies I | 13 y | 52 | 417,018 | 1751 | Dairy<7 (ref), 7–14, 15–28, ≥28 servings/wk | Prostate | 1, 4, 5, 9, 12, 25 | 12 |
| Rodriguez, 2002 (34) | United States | Cancer Prevention Studies II | 14 y | 57 | 447,780 | 3594 | Dairy<7 (ref), 7–14, 15–28, ≥28 servings/wk | Prostate | 1, 4, 5, 9, 12, 25 | 12 |
| Ngoan, 2002 (33) | Japan | 13 y | 15–96 | 13,250 | 116 | Dairy≤2–4 times/mo (ref), 2–4 times/wk, ≥1/dMilk≤2–4 times/mo (ref), 2–4 times/wk, ≥1/d | Stomach | 1, 2, 5, 17, 26, 27, 28, 29 | 12 | |
| Kojima, 2004 (36) | Japan | The Japan Collaborative Cohort | 9.9 y | 40–79 | 107,824 | 457 | MilkSeldom (ref), 0.5–4/wk, every dayYogurtSeldom (ref), 1–2/mo, 1–7/wkCheeseSeldom (ref), 1–2/mo, 1–7/wkButterSeldom (ref), 1–2/mo, 1–7/wk | Colon, rectal | 1, 4, 5, 7, 8, 9, 12, 30 | 10 |
| Khan, 2004 (35) | Japan | Hokkaido Cohort Study | M: 13.8 yF: 14.8 y | 40–97 | 3158 | 244 | MilkNever + several times/y + several times/mo (ref), several times/wk + every dayYogurtNever + several times/y + several times/mo (ref), several times/wk + every dayCheeseNever + several times/y + several times/mo (ref), several times/wk + every day | Total, lung, stomach, colorectal, pancreatic | M: 1, 5F: 1, 5, 31, 32, 33 | 12 |
| Sakauchi, 2004 (37) | Japan | The Japan Collaborative Cohort | 9.8 y | ≥40 | 114,517 | 88 | Milk≤1–2/mo (ref), 1–4/wk, almost every dayYogurtSeldom, 1–2/mo, ≥1–2/wkCheeseSeldom, 1–2/mo, ≥1–2/wkButterSeldom, 1–2/mo, ≥1–2/wk | Urothelial | 1, 2, 5 | 10 |
| Koh, 2006 (38) | United States | The Harvard Alumni Health Study | 11 y | 67 | 10,011 | 99 | Dairy0–<1.25 (ref), 1.25–<2.00, 2.00–<3.25, ≥3.25 servings/d | Prostate | 1, 4, 5, 6, 7, 8, 12, 17, 25 | 12 |
| Sakauchi, 2007 (40) | Japan | The Japan Collaborative Cohort | 13.3 y | 40–79 | 64,327 | 77 | Milk≤1–2 times/mo (ref), 1–4 times/wk, almost every dayYogurtSeldom (ref), 1–2 times/mo, ≥1–2 times/wkCheeseSeldom (ref), 1–2 times/mo, ≥1–2 times/wkButterSeldom (ref), 1–2 times/mo, ≥1–2 times/wk | Ovarian | 1, 4, 8, 9, 34, 35, 36 | 12 |
| Park, 2007 (12) | United States | The NIH-AARP Diet and Health Study | 6 y | 50–71 | 293,888 | 178 | Dairy<0.5 (ref), 0.5–<1, 1–<2, 2–<3, ≥3 servings/dWhole milk0 (ref), >0–<0.5, 0.5–<1, 1–<2, ≥2 servings/dLow-fat milk0 (ref), >0–<0.5, 0.5–<1, 1–<2, ≥2 servings/dSkim milk0 (ref), >0–<0.5, 0.5–<1, 1–<2, ≥2 servings/dYogurt0 (ref), >0–<0.5, ≥0.5 servings/dCheese<0.1 (ref), 0.1–<0.25, 0.25–<0.5, 0.5–<0.75, ≥0.75 servings/d | Prostate | 1, 3, 4, 5, 6, 7, 8, 9, 12, 17, 25, 37, 38, 39, 40, 41, 42 | 10 |
| Matsumoto, 2007 (39) | Japan | The Jichi Medical School Cohort Study | 9.15 y | 18–90 | 11,606 | 255 | MilkNot every day (ref), every dayYogurtNot every day (ref), every dayButterNot every day (ref), every day | Total, colon, stomach, lung, liver, pancreas, bile duct | 1, 2 | 9 |
| Smit, 2007 (41) | United States | The Puerto Rico Heart Health Program | 41 y | 35–79 | 9824 | 167 | Dairy≤2 (ref), 3–4, 5–6, ≥7 servings/d | Prostate | 1, 4, 5, 6, 8, 9, 30 | 12 |
| Bonthuis, 2010 (42) | Australia | 14.4 y | 25–78 | 1529 | 58 | DairyLowest (ref), highest | Total | 1, 2, 4, 5, 6, 7, 8, 9, 43, 44 | 12 | |
| Sharma, 2013 (43) | United States | The Multiethnic Cohort study | 9 y | 45–75 | 146,389 | 3546 | DairyM: 0.5 (ref), 0.6–1.0, 1.1–1.7, >1.7 servings/dF: 0.5 (ref), 0.6–1.0, 1.1–1.6, >1.6 servings/d | Total | 1, 4, 5, 6, 7, 8, 9, 36, 38 | 10 |
| Song, 2013 (13) | United States | The Physicians' Health Study | 28 y | 40–84 | 21,660 | 305 | Dairy≤0.5 (ref), >0.5–1.0, >1.0–1.5, >1.5–2.5, >2.5 servings/dWhole milkRarely (ref), ≤1 serving/wk, 2–6 servings/wk, ≥1 serving/dSkim/low-fat milkRarely (ref), ≤1 serving/wk, 2–6 servings/wk, ≥1 serving/d | Prostate | 1, 3, 4, 5, 6, 7, 8, 17, 18, 44, 45, 46 | 11 |
| Michaelsson, 2014 (45) | Sweden | Swedish Mammography Cohort | 20.1 y | 39–74 | 61,443 | 3283 | Milk<200 (ref), 200–399, 400–599, ≥600 g/dSoured milk and yogurt<1 (ref), 1–199, 200–399, ≥400 g/dCheese<20 (ref), 20–39, 40–59, ≥60 g/d | Total | 1, 4, 5, 6, 7, 8, 9, 36, 37, 41, 43, 47, 48, 49, 50, 51, 52, 53 | 12 |
| Michaelsson, 2014 (45) | Sweden | Cohort of Swedish Men | 11.2 y | 45–79 | 45,339 | 2881 | Milk<200 (ref), 200–399, 400–599, ≥600 g/dSoured milk and yogurt<1 (ref), 1–199, 200–399, ≥400 g/dCheese<20 (ref), 20–39, 40–59, ≥60 g/d | Total | 1, 4, 5, 6, 7, 8, 9, 37, 41, 43, 47, 48, 49, 50,51, 52, 53 | 12 |
| Huang, 2014 (44) | Taiwan | The Nutrition and Health Survey in Taiwan | 13.7 y | 19–64 | 3810 | 145 | Dairy0 (ref), 0.1–3.0, 3.1–7.0, >7.0 times/wk | Total | 1, 2, 3, 4, 9, 30, 37, 38, 48 | 10 |
| Wang, 2015 (47) | Japan | Japan Collaborative Cohort study | 19 y | 40–79 | 94,980 | 7688 | MilkNever (ref), 1–2 times/mo, 1–2 times/wk, 3–4 times/wk, almost every day | Total | 1, 4, 5, 7, 8, 9, 25, 31, 38, 54 | 12 |
| Praagman, 2015 (46) | Netherlands | European Prospective Investigation into Cancer and Nutrition-Netherlands cohort | 15 y | 20–70 | 34,409 | 1216 | Yogurt3.8 (ref), 26.6, 62.9, 144.5 g/dCheese15.8 (ref), 26.8, 29.2, 30.2 g/d | Total | 1, 2, 4, 5, 6, 7, 8, 9, 25, 45, 55 | 12 |
| Farvid, 2017 (48) | Iran | The Golestan Cohort Study | 11 y | 36–83 | 42,403 | 859 | Dairy0.4 (ref), 0.8, 1.2, 1.6, 2.4 servings/dHigh-fat dairy0 (ref), 0.05, 0.2, 0.5, 1.1 servings/dLow-fat dairy0.2 (ref), 0.5, 0.8, 1.0, 1.6 servings/dMilk0 (ref), 0.04, 0.1, 0.3, 0.6 servings/dYogurt0.1 (ref), 0.3, 0.4, 0.6, 0.9 servings/dCheese0 (ref), 0.1, 0.3, 0.5, 0.8 servings/d | Total | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 30, 37, 46, 56 | 13 |
| Um, 2017 (14) | United States | Reasons for Geographic and Racial Differences in Stroke study | 10 y | ≥45 | 21,427 | 854 | DairyM: 0–<46.8 (ref), 46.8–<116, 116–<206, 206–<350, 350–1468 g/dF: 0–<37.1 (ref), 37.1–<93.8, 93.8–<177, 177–<319, 319–1368 g/dWhole milkM: 0 (ref), >0–<34.8, 34.8–<112, 112–<246, 246–1216 g/dF: 0 (ref), >0–<20.9, 20.9–<78.0, 78.0–<186, 186–1288 g/dLow-fat milkM: 0 (ref), >0–<39.4, 39.4–<121, 121–<249, 249–1310 g/dF: 0 (ref), >0–<23.7, 23.7–<97.3, 97.3–<230, 230–1351 g/dNonfat milkM: 0 (ref), >0–<109, 109–<239, 239–<383, 383–1220 g/dF: 0 (ref), >0–<105, 105–<208, 208–<382, 382–1175 g/d | Total | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 17, 25, 30, 36, 43, 46, 48, 55 | 12 |
| Bergholdt, 2018 (49) | Denmark | The Copenhagen General Population Study | 7 y | 57 | 74,243 | 1668 | MilkNo milk (ref), 1–3, 4–7, 8–10, ≥11 glasses/wk | Total | 1, 2, 4, 5, 7, 8, 9, 10, 17, 22, 25,37, 40, 46, 55, 57, 58, 59, 60, 61 | 10 |
| Um, 2019 (51) | United States | Iowa Women's Health Study | 27 y | 55–69 | 35,221 | 4665 | Dairy0–7.5 (ref), >7.5–11.8, >11.8–18.0, >18.0–24.5, >24.5–143 servings/wkHigh-fat dairy0–2.5 (ref), >2.5–5.0, >5.0–8.0, >8.0–13.5, >13.5–128 servings/wkLow-fat dairy0 (ref), >0–2.0, >2.0–6.5, >6.5–14.0, >14.0–84.0 servings/wkMilk0–0.5, >0.5–3, >3–6.5, >6.5–14, >14 servings/wkWhole milk0 (ref), >0–0.5, >0.5–1.0, >1.0–5.5, >5.5–42 servings/wkLow-fat/nonfat milk0 (ref), >0–1.0, >1.0–5.5, >5.5–7.0, >7.0–42.0 servings/wk | Total, colorectal | 1, 4, 5, 6, 7, 8, 12, 17, 25, 36, 41, 43, 48, 55 | 12 |
| Ding, 2019 (15) | United States | Nurses’ Health Study, Nurses’ Health Study II, and the Health Professionals Follow-up Study | 32 y | 25–75 | 217,755 | 15,120 | Dairy0.8 (ref), 1.5, 2, 2.8, 4.2 servings/dWhole milk<1/mo (ref), 1–3/mo, 3/mo–2/wk, ≥2/wkSkimmed or low-fat milk<1/wk (ref), 1–4/wk, 4/wk–1.5/d, ≥1.5/dCheese<1/wk (ref), 1–4/wk, 4/wk–1.5/d, ≥1.5/d | Total, colorectal, pancreatic, lung, breast, ovarian, endometrial, prostate | 1, 4, 5, 6, 7, 8, 12, 18, 34, 36, 45, 48, 57 | NHS: 11NHSII:11HPFS: 11 |
| Pala, 2019 (50) | Italy | European Prospective Investigation into Cancer and Nutrition (EPIC)—Italy study | 14.9 y | 50.1 | 45,009 | 1464 | Milk0 (ref), >0–≤50, >50–≤160, >160–≤200, >200 g/dFull-fat milk0 (ref), >0–≤50, >50–≤160, >160–≤200, >200 g/dReduced-fat milk0 (ref), >0–≤50, >50–≤160, >160–≤200, >200 g/dYogurt0 (ref), >0–≤35, >35–≤120, >120 g/d | Total | 1, 2, 5, 6, 7, 8, 18, 30, 47, 48, 62, 63, 64, 65 | 11 |
| Cheese>0–≤28 (ref), >28–≤70, >70–≤100, >100 g/dButter0 (ref), >0–≤1.4, >1.4–100.1 g/d | ||||||||||
| Mazidi, 2019 (5) | United States | National Health and Nutrition Examination Surveys study | 76.4 mo | ≥20 | 24,474 | 827 | Dairy0.25 (ref), 0.79, 1.57, 3.08 cup equivalent servings/dMilk0.10 (ref), 0.15, 0.75, 1.84 cup equivalent servings/dCheeseQ1 (ref), Q2, Q3, Q4YogurtQ1 (ref), Q2, Q3, Q4 | Total | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 37,45, 52, 53, 66, 67 | 9 |
| van den Brandt, 2019 (52) | Netherlands | Netherlands Cohort Study | 10 y | 55–69 | 120,852 | 3917 | Low-fat dairy0 (ref), 85.4, 203.3, 392.9 g/d | Total | 1, 2, 4, 5, 6, 7, 8, 9, 25, 36, 38, 43, 47, 55 | 12 |
| Stasinopoulos, 2020 (55) | United Kingdom | UK Biobank study | 4.2 y | 40–69 | 497,828 | 8297 | DairyNo (ref), yes | Total | 1, 2, 3, 4, 5, 7, 8, 9, 21, 30, 31, 46 | 10 |
| Wang, 2020 (56) | China | The Guangzhou Biobank Cohort Study | 11.5 y | ≥50 | 19,618 | 1209 | Dairy0 (ref), 1, 2, >3 portions/wkMilk0 (ref), 1–3, >3 portions/wk | Total, lung, liver, gastrointestinal, colorectal and anus, esophagus | 1, 2, 4, 5, 6, 7, 8, 9, 10, 11, 31, 45 | 13 |
| Schmid, 2020 (54) | United States | Nurses’ Health Study and Health Professionals Follow-up Study | 32 y | 30–75 | 122,626 | 11,985 | YogurtNever (ref), >0 to ≤1–3 servings/mo, 1 serving/wk, 2–4 servings/wk, >4 servings/wk | Total | 1, 3, 4, 5, 6, 7, 8, 12, 17, 25, 34, 36, 38, 43, 46, 47, 50, 55, 57, 67, 68, 69, 70 | NHS: 11HPFS: 11 |
| Michels, 2020 (53) | United States | Nurses’ Health Study and Health Professionals Follow-up Study | 32 y | 30–75 | 120,052 | 1086 | YogurtNever or <1 serving/mo (ref), 1–3 servings/mo, >1 serving/wk | Colorectal | 1, 4, 5, 6, 7, 8, 12, 14, 15, 17, 34, 36, 41, 43, 46, 47, 50, 57, 67, 68 | NHS: 11HPFS: 11 |
| Sun, 2021 (57) | United States | Women's Health Initiative | 18.1 y | 50–79 | 102,521 | 7516 | DairyQ1 (ref), Q2, Q3, Q4, Q5 | Total | 1, 3, 5, 6, 7, 8, 9, 10, 25, 36, 45, 53, 55, 57, 71, 72, 73 | 12 |
F, female; M, male; NOS, Newcastle–Ottawa Scale; Q, quintile; ref, reference.
Adjustment for covariates: age (1), sex (2), race/ethnicity (3), BMI (4), smoking (5), energy intake (6), alcohol intake (7), physical activity (8), education (9), income (10), occupation (11), family history of cancer (12), age at menarche (13), age at first pregnancy (14), age at menopause (15), percent desirable weight (16), meat (17), subtypes of dairy foods (18), egg (19), FEV1(forced expiratory volume in 1 second) (20), deprivation category (21), household (22), car user (23), bronchitis (24), vegetable (25), liver (26), oil (27), suimono (28), pickled food (29), region (30), health status (31), health education (32), health screening (33), menopausal status (34), number of pregnancies (35), hormone use (36), marital status (37), history of disease (except cancer) (38), screening for prostate cancer by use of prostate-specific antigen (39), fish (40), vitamin (41), α-linolenic acid (42), dietary supplement use (43), β-carotene treatment (44), baseline disease status (except cancer) (45), medication use (46), height (47), dietary pattern (48), Charlson's comorbidity index (49), calcium (50), phosphorus (51), fat (52), protein (53), sleeping duration (54), fruit (55), systolic blood pressure (56), family medical history (57), fast food (58), soda drinks (59), coffee (60), tea (61), weight (62), waist-to-hip ratio (63), relative index of inequality (64), sugar (65), carbohydrates (66), fiber (67), 2-y follow-up cycle (68), glycemic load (69), nuts (70), observational study/clinical trials (71), whole grain (72), and sugar-sweetened beverage (73).
Total dairy consumption and cancer mortality
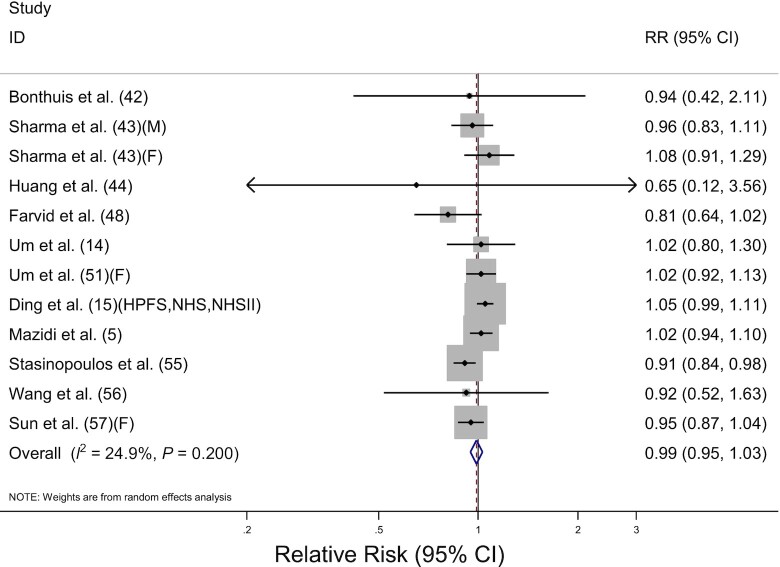
Thirteen prospective cohort studies including 1,112,975 participants and 43,096 deaths investigated the association between total dairy consumption and total cancer mortality. The pooled RR for highest compared with lowest consumption was 0.99 (95% CI: 0.95, 1.03) with no significant heterogeneity among the studies (I2 = 24.9%, P = 0.20) (Table 2, Figure 2). No significant associations were detected when stratified by sex, cancer site, fat content, or adjustment for covariates, and meta-regression analysis revealed no significant differences (P-difference ≥ 0.1 for all comparisons). By the geographic region, pooled RRs were 1.02 in the United States (95% CI: 0.98, 1.06), 0.82 in Asia (95% CI: 0.66, 1.02), 0.94 in Oceania (95% CI: 0.42, 2.11), and 0.91 in Europe (95% CI: 0.84, 0.98). Although the significant inverse association was discovered in Europe, only 1 study was included in the analysis (P-difference for Europe compared with the United States = 0.04). The dose-response analysis for total dairy consumption and total cancer mortality included 10 studies (5, 14, 15, 44, 48, 51, 56, 57) (Table 3). The pooled RR for the 400-g/d increment of total dairy consumption was 1.00 (95% CI: 0.97, 1.04). Although the pooled RR for an increase of 400 g/d in total dairy consumption was 1.07 (95% CI: 1.00, 1.14) in males, only 1 study was included in this dose-response analysis. There was no significant association detected when the analysis was stratified by fat content.
TABLE 2.
Pooled RRs of total cancer and cancer-specific mortality for the highest compared with lowest dairy consumption
| Characteristic | Studies (n) | RR (95% CI) | Heterogeneity | P-difference |
|---|---|---|---|---|
| Total dairy | ||||
| All studies | 13 | 0.99 (0.95, 1.03) | I 2 = 24.9%, P = 0.20 | |
| Sex | ||||
| Male | 2 | 1.03 (0.94, 1.12) | I 2 = 24.8%, P = 0.25 | 0.78 |
| Female | 5 | 1.02 (0.96, 1.08) | I 2 = 23.6%, P = 0.26 | |
| Cancer site | ||||
| Gastrointestinal tract | 7 | 0.89 (0.71, 1.12) | I 2 = 30.9%, P = 0.22 | |
| Stomach | 2 | 1.26 (0.72, 2.23) | I 2 = 0%, P = 0.80 | |
| Colorectal | 5 | 0.83 (0.63, 1.10) | I 2 = 54.6%, P = 0.11 | |
| Pancreatic cancer | 3 | 1.06 (0.86, 1.31) | — | 0.411 |
| Lung cancer | 5 | 0.79 (0.47, 1.32) | I 2 = 79.5%, P = 0.01 | 0.891 |
| Women's cancer | 2 | 1.06 (0.92, 1.23) | I 2 = 0%, P = 0.83 | 0.271 |
| Prostate cancer | 8 | 1.11 (0.99, 1.25) | I 2 = 0%, P = 0.7 | 0.101 |
| Fat content | ||||
| High-fat dairy | 2 | 0.92 (0.69, 1.23) | I 2 = 77.8%, P = 0.03 | 0.99 |
| Low-fat dairy | 3 | 0.96 (0.89, 1.03) | I 2 = 0%, P = 0.61 | |
| Geographic region | ||||
| United States | 8 | 1.02 (0.98, 1.06) | I 2 = 0%, P = 0.61 | |
| Asia | 3 | 0.82 (0.66, 1.02) | I 2 = 0%, P = 0.89 | 0.092 |
| Europe | 1 | 0.91 (0.84, 0.98) | — | 0.042 |
| Oceania | 1 | 0.94 (0.42, 2.11) | — | 0.852 |
| Adjustment for covariates3 | ||||
| Strong adjustment | 6 | 1.00 (0.94, 1.06) | I 2 = 0%, P = 0.60 | 0.88 |
| Weak adjustment | 7 | 0.98 (0.92, 1.05) | I 2 = 60.1%, P = 0.04 | |
| Milk | ||||
| All studies | 17 | 1.04 (0.99, 1.08) | I 2 = 65.8%, P < 0.001 | |
| Sex | ||||
| Male | 5 | 1.00 (0.94, 1.07) | I 2 = 39.1%, P = 0.16 | 0.19 |
| Female | 6 | 1.10 (1.01, 1.21) | I 2 = 71.4%, P = 0.004 | |
| Cancer site | ||||
| Upper digestive tract | 2 | 1.17 (0.71, 1.94) | I 2 = 77.9%, P = 0.004 | |
| Esophageal | 2 | 1.80 (0.53, 6.04) | I 2 = 86.8%, P = 0.006 | |
| Gastrointestinal tract | 12 | 0.97 (0.88, 1.06) | I 2 = 20.7%, P = 0.20 | 0.474 |
| Stomach | 6 | 1.06 (0.80, 1.41) | I 2 = 39.5%, P = 0.13 | |
| Colorectal | 9 | 0.96 (0.87, 1.07) | I 2 = 21.4%, P = 0.23 | |
| Hepatobiliary system | 7 | 1.07 (1.00, 1.14) | I 2 = 0%, P = 0.94 | 0.934 |
| Liver | 3 | 1.13 (1.02, 1.26) | I 2 = 0%, P = 0.82 | |
| Pancreatic | 6 | 1.06 (0.96, 1.18) | I 2 = 0%, P = 0.99 | |
| Respiratory tract | 10 | 1.03 (0.92, 1.15) | I 2 = 27.7%, P = 0.17 | 0.804 |
| Lung | 10 | 1.03 (0.90, 1.17) | I 2 = 34.4%, P = 0.13 | |
| Women's cancer | 5 | 1.13 (1.01, 1.26) | I 2 = 35.5%, P = 0.15 | 0.564 |
| Breast | 4 | 1.02 (0.91, 1.14) | I 2 = 0%, P = 0.52 | |
| Ovarian | 4 | 1.32 (1.13, 1.55) | I 2 = 0%, P = 0.78 | |
| Prostate cancer | 5 | 1.23 (1.02, 1.48) | I 2 = 49.8%, P = 0.09 | 0.274 |
| Urological system | 2 | 1.05 (0.52, 2.11) | I 2 = 90.9%, P < 0.001 | 0.604 |
| Fat content5 | ||||
| High/whole-fat milk | 6 | 1.17 (1.07, 1.28) | I 2 = 45.0%, P = 0.14 | 0.02 |
| Low/skimmed milk | 6 | 1.01 (0.96, 1.05) | I 2 = 0%, P = 0.40 | |
| Geographic region | ||||
| United States | 6 | 1.04 (0.98, 1.09) | I 2 = 64.1%, P = 0.04 | |
| Asia | 6 | 1.02 (0.95, 1.09) | I 2 = 60.2%, P = 0.01 | 0.736 |
| Europe | 5 | 1.06 (0.90, 1.26) | I 2 = 79.9%, P = 0.001 | 0.746 |
| Adjustment for covariates | ||||
| Strong adjustment | 6 | 1.09 (0.97, 1.22) | I 2 = 82.6%, P < 0.001 | 0.25 |
| Weak adjustment | 11 | 1.02 (0.99, 1.06) | I 2 = 37.6%, P = 0.10 | |
| Fermented milk | ||||
| All studies | 10 | 0.95 (0.89, 1.01) | I 2 = 45.9%, P = 0.05 | |
| Sex | ||||
| Male | 3 | 0.96 (0.80, 1.14) | I 2 = 55.8%, P = 0.10 | 0.27 |
| Female | 3 | 0.85 (0.77, 0.94) | I 2 = 0%, P = 0.68 | |
| Cancer site | ||||
| Gastrointestinal tract | 5 | 0.91 (0.79, 1.04) | I 2 = 0%, P = 0.59 | |
| Stomach | 2 | 1.34 (0.67, 2.69) | I 2 = 0%, P = 0.45 | |
| Colorectal | 5 | 0.89 (0.78, 1.03) | I 2 = 0%, P = 0.59 | |
| Hepatobiliary system | 2 | 1.16 (0.50, 2.72) | I 2 = 0%, P = 0.56 | 0.587 |
| Pancreatic | 2 | 0.81 (0.29, 2.22) | I 2 = 0%, P = 0.82 | |
| Lung cancer | 3 | 0.87 (0.65, 1.17) | I 2 = 0%, P = 0.82 | 0.807 |
| Ovarian cancer | 1 | 1.66 (0.71, 3.90) | — | 0.197 |
| Prostate cancer | 1 | 0.78 (0.25, 2.47) | — | 0.807 |
| Urothelial cancer | 1 | 0.72 (0.33, 1.57) | — | 0.577 |
| Geographic region | ||||
| United States | 3 | 0.98 (0.88, 1.08) | I 2 = 71.2%, P = 0.03 | |
| Asia | 3 | 0.85 (0.71, 1.03) | I 2 = 0%, P = 0.60 | 0.508 |
| Europe | 4 | 0.93 (0.85, 1.02) | I 2 = 20.6%, P = 0.29 | 0.278 |
| Adjustment for covariates | ||||
| Strong adjustment | 5 | 0.94 (0.87, 1.02) | I 2 = 54.0%, P = 0.07 | 0.82 |
| Weak adjustment | 5 | 0.95 (0.84, 1.08) | I 2 = 36.8%, P = 0.16 | |
| Cheese | ||||
| All studies | 10 | 0.99 (0.98, 1.01) | I 2 = 0%, P = 0.79 | |
| Sex | ||||
| Male | 3 | 1.00 (0.89, 1.12) | I 2 = 13.9%, P = 0.31 | 0.63 |
| Female | 4 | 1.04 (0.95, 1.14) | I 2 = 0.3%, P = 0.39 | |
| Cancer site | ||||
| Gastrointestinal tract | 6 | 1.22 (1.02, 1.45) | I 2 = 0%, P = 0.85 | |
| Colorectal | 6 | 1.22 (1.02, 1.46) | I 2 = 0%, P = 0.66 | |
| Pancreatic cancer | 4 | 1.25 (0.89, 1.76) | I 2 = 0%, P = 0.69 | 0.899 |
| Lung cancer | 6 | 0.85 (0.63, 1.15) | I 2 = 41.5%, P = 0.13 | 0.039 |
| Women's cancer | 4 | 1.15 (0.93, 1.44) | I 2 = 0%, P = 0.86 | 0.729 |
| Breast | 3 | 1.16 (0.88, 1.53) | I 2 = 0%, P = 0.63 | |
| Ovarian | 3 | 1.24 (0.81, 1.90) | I 2 = 0%, P = 0.50 | |
| Prostate cancer | 3 | 1.17 (0.89, 1.55) | I 2 = 0%, P = 0.53 | 0.849 |
| Urothelial cancer | 1 | 0.91 (0.68, 1.21) | — | 0.119 |
| Geographic region | ||||
| United States | 4 | 0.99 (0.97, 1.01) | I 2 = 0%, P = 0.45 | |
| Asia | 2 | 1.00 (0.80, 1.24) | I 2 = 0%, P = 0.95 | 0.9610 |
| Europe | 4 | 1.04 (0.96, 1.13) | I 2 = 0%, P = 0.47 | 0.2710 |
| Adjustment for covariates | ||||
| Strong adjustment | 5 | 0.99 (0.97, 1.01) | I 2 = 0%, P = 0.49 | 0.35 |
| Weak adjustment | 5 | 1.04 (0.95, 1.14) | I 2 = 0%, P = 0.97 |
P value for difference in RRs of total dairy consumption for hepatobiliary system compared with gastrointestinal tract, lung cancer compared with gastrointestinal tract, women's cancer compared with gastrointestinal tract, and prostate cancer compared with gastrointestinal tract.
P value for difference in RRs of total dairy consumption for Asia compared with United States, Europe compared with United States, and Oceania compared with United States.
Adjustment for at least age, BMI, energy intake, alcohol intake, smoking status, physical activity, and socioeconomic status is considered strong adjustment. Otherwise, it is considered weak adjustment.
P value for difference in RRs of milk consumption for gastrointestinal tract compared with upper digestive tract, hepatobiliary system compared with upper digestive tract, respiratory tract compared with upper digestive tract, women's cancer compared with upper digestive tract, prostate cancer compared with upper digestive tract, and urological system compared with upper digestive tract.
Milk fat content ≥3.5% was defined as high/whole-fat milk, and milk fat content <3.5% was defined as low-fat milk.
P value for difference in RRs of milk consumption for Asia compared with United States and Europe compared with United States.
P value for difference in RRs of fermented milk consumption for hepatobiliary system compared with gastrointestinal tract, lung cancer compared with gastrointestinal tract, ovarian cancer compared with gastrointestinal tract, prostate cancer compared with gastrointestinal tract, and urothelial cancer compared with gastrointestinal tract.
P value for difference in RRs of fermented milk consumption for Asia compared with United States and Europe compared with United States.
P value for difference in RRs of cheese consumption for pancreatic cancer compared with gastrointestinal tract, lung cancer compared with gastrointestinal tract, women's cancer compared with gastrointestinal tract, prostate cancer compared with gastrointestinal tract, and urothelial cancer compared with gastrointestinal tract.
P value for difference in RRs of cheese consumption for Asia compared with United States and Europe compared with United States.
FIGURE 2.
Forest plot of total cancer mortality for the highest compared with lowest categories of total dairy consumption. The sizes of the squares correspond to the inverse of the variance of the natural logarithm of the RR from the individual study, and the diamond indicates the pooled RR. F, female; HPFS, Health Professionals Follow-up Study; M, male; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II.
TABLE 3.
Pooled RRs of dairy consumption and total cancer mortality from dose-response meta-analyses
| Characteristic | Studies (n) | Dose, g/d | RR (95% CI) | Heterogeneity |
|---|---|---|---|---|
| Total dairy | 10 | 400 | 1.00 (0.97, 1.04) | I 2 = 73.8%, P < 0.001 |
| Sex | ||||
| Male | 1 | 400 | 1.07 (1.00, 1.14) | — |
| Female | 4 | 400 | 1.02 (0.98, 1.08) | I 2 = 72.9%, P = 0.01 |
| Fat content | ||||
| High-fat dairy | 2 | 400 | 0.94 (0.74, 1.18) | I 2 = 46.6%, P = 0.17 |
| Low-fat dairy | 3 | 400 | 1.01 (0.98, 1.03) | I 2 = 0%, P = 0.69 |
| Milk | 13 | 200 | 1.01 (0.99, 1.04) | I 2 = 63.0%, P = 0.002 |
| Sex | ||||
| Male | 4 | 200 | 0.99 (0.97, 1.02) | I 2 = 32.4%, P = 0.22 |
| Female | 5 | 200 | 1.04 (1.01–1.06) | I 2 = 49.1%, P = 0.10 |
| Fat content1 | ||||
| High/whole-fat milk | 6 | 200 | 1.12 (1.01, 1.23) | I 2 = 76.9%, P = 0.005 |
| Low-fat milk | 6 | 200 | 1.00 (0.99, 1.02) | I 2 = 0%, P = 0.70 |
| Fermented milk | 7 | 200 | 0.94 (0.90, 0.99) | I 2 = 35.5%, P = 0.16 |
| Sex | ||||
| Male | 2 | 200 | 0.97 (0.93, 1.01) | I 2 = 0%, P = 0.36 |
| Female | 2 | 200 | 0.90 (0.85, 0.94) | I 2 = 0%, P = 0.77 |
| Cheese | 8 | 50 | 1.01 (0.95, 1.07) | I 2 = 36.3%, P = 0.16 |
| Sex | ||||
| Male | 2 | 50 | 0.99 (0.90, 1.08) | I 2 = 34.8%, P = 0.22 |
| Female | 3 | 50 | 1.05 (0.86, 1.29) | I 2 = 83.4%, P = 0.002 |
Milk fat content ≥3.5% was defined as high/whole-fat milk, and milk fat content <3.5% was defined as low-fat milk.
Milk consumption and cancer mortality
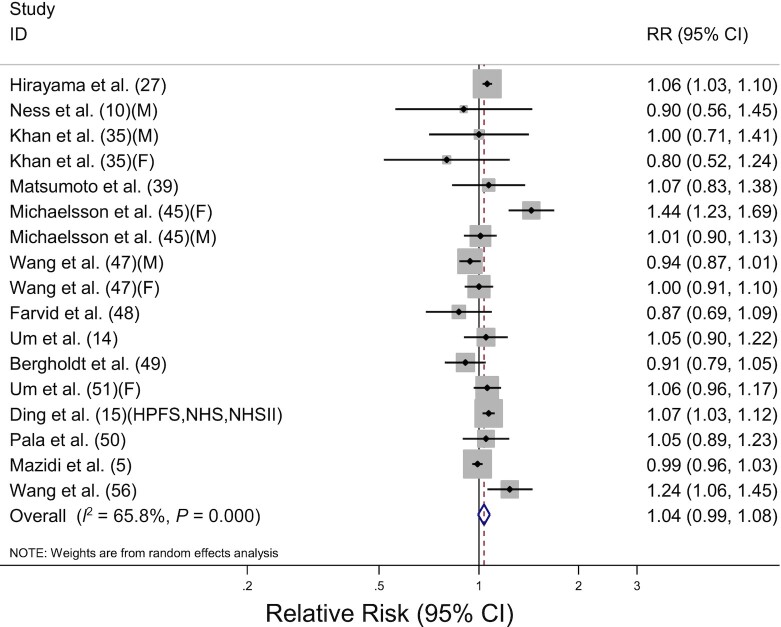
The association between milk consumption and total cancer mortality was evaluated in 17 prospective cohort studies, including 967,559 participants and 56,471 deaths. The pooled RR for highest compared with lowest consumption was 1.04 (95% CI: 0.99, 1.08) with significant heterogeneity among the studies (I2 = 65.8%, P < 0.001) (Table 2, Figure 3). The increased risk of total cancer mortality was observed in females (RR: 1.10; 95% CI: 1.01, 1.21) but not in males (RR: 1.00; 95% CI: 0.94, 1.07; P-difference = 0.19). By cancer site, significant associations were detected in cancers of the hepatobiliary system (RR: 1.07; 95% CI: 1.00, 1.14), women's cancer (RR: 1.13; 95% CI: 1.01, 1.26), and prostate cancer (RR: 1.23; 95% CI: 1.02, 1.48) but not in cancers of the upper digestive tract, gastrointestinal tract, respiratory tract, and urological system. For cancers of the hepatobiliary system, high milk consumption was associated with increased mortality risk from liver cancer (RR: 1.13; 95% CI: 1.02, 1.26), but no significant association was detected in pancreatic cancer (RR: 1.06; 95% CI: 0.96, 1.18). For women's cancer, high milk consumption was associated with increased mortality risk from ovarian cancer (RR: 1.32; 95% CI: 1.13, 1.55), but no significant association was detected in breast cancer (RR: 1.02; 95% CI: 0.91, 1.14). However, pooled RRs were not significantly different with cancer sites in the meta-regression analysis (P-difference > 0.2 for all comparisons). High/whole-fat milk intake was associated with an increased risk of total mortality (RR: 1.17; 95% CI: 1.07, 1.28) but not low-fat milk consumption (RR: 1.01; 95% CI: 0.96, 1.05), and there was a significant difference in fat content (P-difference = 0.02). There was no significant difference in the geographic region or adjustment factors based on meta-regression analyses results (P-difference > 0.2 for all comparisons). The dose-response analysis for milk consumption and total cancer mortality included 13 studies (10, 14, 15, 45, 47–51, 56) ( Table 3). The pooled RR for an increase in milk consumption of 200 g/d was 1.01 (95% CI: 0.99, 1.04). By sex, a 200-g/d increment of milk consumption was associated with a 4% increase in cancer mortality in females (RR: 1.04; 95% CI: 1.01, 1.06), whereas there was no significant association in males (RR: 0.99; 95% CI: 0.97, 1.02). By fat content, a 200-g/d increment of high/whole-fat milk consumption was associated with 12% increase in total cancer mortality (RR: 1.12; 95% CI: 1.01, 1.23) but not low-fat milk consumption (RR: 1.00; 95% CI: 0.99, 1.02).
FIGURE 3.
Forest plot of total cancer mortality for the highest compared with lowest categories of milk consumption. The sizes of the squares correspond to the inverse of the variance of the natural logarithm of the RR from the individual study, and the diamond indicates the pooled RR. F, female; HPFS, Health Professionals Follow-up Study; M, male; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II.
Fermented milk consumption and cancer mortality
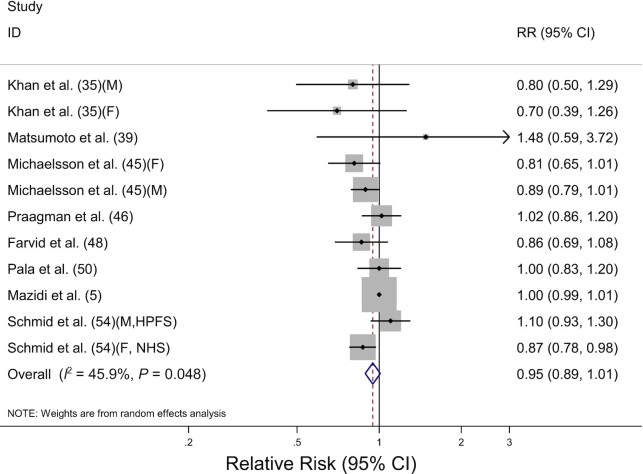
The association between fermented milk consumption and total cancer mortality was evaluated in 10 prospective cohort studies, including 390,467 participants and 23,014 deaths. The pooled RR for highest compared with lowest consumption was 0.95 (95% CI: 0.89, 1.01) with evidence of moderate heterogeneity (I2 = 45.9%, P = 0.05) (Table 2, Figure 4). By sex, high fermented milk consumption was inversely associated with total cancer mortality in females (RR: 0.85; 95% CI: 0.77, 0.94) but not in males (RR: 0.96; 95% CI: 0.80, 1.14). The pooled RRs, however, were not significantly different by sex (P-difference = 0.27). We discovered no significant difference in cancer mortality by cancer site, geographic region, or adjustment factors. The dose-response analysis for fermented milk consumption and total cancer mortality included 7 studies (45, 46, 48, 50, 54) (Table 3). A 200-g/d increment of fermented milk consumption was associated with a 6% decrease in total cancer mortality (RR: 0.94; 95% CI: 0.90, 0.99) with no significant heterogeneity (I2 = 35.5%, P = 0.16). Similarly, we discovered that increasing fermented milk consumption by 200 g/d was associated with a 10% decrease in total cancer mortality in females (RR: 0.90; 95% CI: 0.85, 0.94), whereas no association was found in males (RR: 0.97; 95% CI: 0.93, 1.01).
FIGURE 4.
Forest plot of total cancer mortality for the highest compared with lowest categories of fermented milk consumption. The sizes of the squares correspond to the inverse of the variance of the natural logarithm of the RR from the individual study, and the diamond indicates the pooled RR. F, female; HPFS, Health Professionals Follow-up Study; M, male; NHS, Nurses’ Health Study.
Cheese consumption and cancer mortality
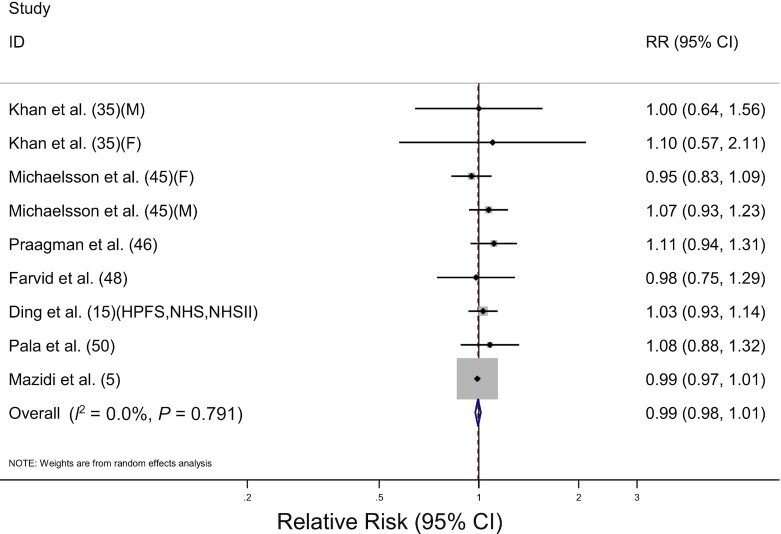
The association between cheese consumption and total cancer mortality was evaluated in 10 prospective cohort studies including 473,990 participants and 25,894 deaths. The pooled RR for highest compared with lowest consumption was 0.99 (95% CI: 0.98, 1.01) with no heterogeneity among the studies (I2 = 0%, P = 0.79) (Table 2, Figure 5). By the cancer site, high cheese consumption was associated with an increased risk of death from colorectal cancer (RR: 1.22; 95% CI: 1.02, 1.46), but no other cancer sites showed a significant association. For lung cancer mortality, the pooled RR was 0.85 (95% CI: 0.63, 1.15), which was significantly different from colorectal cancer mortality (P-difference = 0.03). There was no significant difference in cancer mortality when stratified by sex, geographic region, or adjustment factors. Eight studies (15, 45, 46, 48, 50) were included in the dose-response analysis for cheese consumption and total cancer mortality (Table 3). The pooled RR for 50 g/d increment of cheese consumption was 1.01 (95% CI: 0.95, 1.07), showing no significant association in males and females.
FIGURE 5.
Forest plot of total cancer mortality for the highest compared with lowest categories of cheese consumption. The sizes of the squares correspond to the inverse of the variance of the natural logarithm of the RR from the individual study, and the diamond indicates the pooled RR. F, female; HPFS, Health Professionals Follow-up Study; M, male; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II.
Butter consumption and cancer mortality
The association between butter consumption and total cancer mortality was evaluated in 2 studies including 56,615 participants and 1719 deaths. The pooled RR for all studies was 1.06 (95% CI: 0.70, 1.59) with evidence of moderate heterogeneity (I2 = 65.8%, P = 0.09) (data not shown). Similarly, by cancer site, we detected no significant association in cancer of the gastrointestinal tract (RR: 1.06; 95% CI: 0.81, 1.39), hepatobiliary system (RR: 1.47; 95% CI, 0.34, 6.31), lung cancer (RR: 0.97; 95% CI: 0.73, 1.30), ovarian cancer (RR: 1.35; 95% CI: 0.56, 3.25), or urothelial cancer (RR: 0.66; 95% CI: 0.30, 1.45), and the meta-regression analysis result showed no significant differences (P-difference > 0.2 for all comparisons).
Publication bias
Begg (P > 0.5 in all analyses) and Egger tests (P > 0.09 in all analyses) of cancer mortality for total dairy, milk, fermented milk, and cheese consumption indicated no evidence of publication bias.
Discussion
The potential associations between different types of dairy products consumption and total cancer mortality or cancer-specific mortality were examined in the current meta-analysis of 34 prospective cohort studies. Compared with low consumption, high milk consumption was associated with higher total cancer mortality in females. Conversely, females in the highest category of fermented milk intake had decreased risk of cancer mortality. The dose-response meta-analysis also supports the associations. There was no significant association between cheese consumption and total cancer mortality, but we discovered an increased risk of colorectal cancer mortality in people with high cheese consumption. There was no significant association between butter consumption or total dairy consumption and cancer mortality.
Three previous meta-analyses have been conducted on the association of total cancer mortality for highest compared with lowest consumption of dairy products (4–6), and a meta-analyses only included studies reported in the United States (5). There was no significant association between total dairy consumption and total cancer mortality in the meta-analyses, which were similar to the results of total dairy consumption in this study. For milk consumption, 2 previous meta-analyses on the association between milk consumption and total cancer mortality showed no significant association (4, 6). However, in the current meta-analysis, we discovered a significant positive association between milk consumption and total cancer mortality in females. In addition, we discovered that the consumption of high/whole-fat milk but not low-fat milk was associated with an increased cancer mortality risk, and our findings were consistent with the results of Naghshi et al. (6). Only 1 previous meta-analysis was conducted to examine the association between fermented milk consumption and total cancer mortality, and it reported no association (4). In this study, we discovered a significant inverse association between fermented milk consumption and cancer mortality. Moreover, all of the previous meta-analyses included the results of all cancer mortality and cancer-specific mortality from individual studies to calculate a pooled RR of total cancer mortality, whereas we included studies that only reported all cancer mortality for the main analysis.
In this study, we discovered that milk consumption was associated with increased cancer mortality in females. The milk consumption elevated circulating concentrations of insulin-like growth factor 1 (IGF-1) (58), and previous studies indicated high IGF-1 concentrations were associated with increased risk of cancer, particularly breast and prostate cancer (59–61). Another previous study reported that IGF-1 could enhance the growth and metastasis of hepatocellular carcinoma (62). Furthermore, multiple large prospective cohort studies revealed that dairy products consumption was associated with a higher risk of hepatocellular carcinoma (63, 64). Milk consumption was significantly associated with an increased risk of prostate and liver cancer mortality. In addition, this positive association has also been found in women's cancer. A previous meta-analysis of ovarian cancer risk indicated that high milk and lactose intake was associated with increased ovarian cancer risk (65). In the current meta-analysis, we observed a similar positive association between milk consumption and ovarian cancer mortality. Milk contained 4.5–5.5% lactose and 227 mg free galactose per 100 mL (66). Several studies have supported the relation between lactose and free galactose consumption and increased risk of ovarian cancer (65, 66). The accumulation of galactose and galactose metabolites, such as galactose-1-phosphate and galactitol, may interfere with gonadotropin signaling and ovarian apoptosis, resulting in galactose-induced ovarian toxicity (66, 67). However, there was no significant association between women's cancer with fermented milk or cheese consumption in this study. This result could be attributed to the fact that fermented milk and cheese have significantly less lactose and total galactose than milk (67, 68). Furthermore, we discovered high/whole-fat milk consumption was associated with a higher risk of total cancer mortality. The previous meta-analysis indicated that diets high in saturated fat were associated with higher cancer mortality (69), and another study showed that whole milk consumption was associated with higher ovarian cancer risk but not for low-fat milk, yogurt, and cheese intake (67). Moreover, although a meta-analysis of milk consumption and total cancer mortality indicated evidence of heterogeneity among studies, the observed heterogeneity tended to disappear when stratified by cancer site.
We observed that fermented milk consumption was associated with a decreased risk of total cancer mortality. Fermented milk differs from other dairy products in that it has a high concentration of probiotic bacteria. Yogurt can increase IgA, T cells, and macrophages activities, which inhibit pathogenic microbiota growth, reducing infection and improving anticarcinogenic effects (54, 70). Schmid et al. (54) indicated that yogurt consumption might have different effects on the intestinal microbiota of males and females, and the commensal microbial community may alter sex hormone concentration. For cheese consumption, we discovered that cheese consumption was associated with a higher risk of colorectal cancer mortality. Our previous meta-analysis found no significant association between cheese consumption and colorectal cancer mortality, but the meta-analysis included only 3 prospective studies on cheese consumption (71). The recent analysis included 6 prospective studies, which were supplemented with more current data.
There are several advantages to this meta-analysis. First, to the best of our knowledge, this is the most comprehensive meta-analysis to examine the association between dairy consumption and overall cancer-specific mortality. Although previous meta-analyses of dairy consumption and cancer-specific mortality have been performed, few studies have conducted this analysis and analyzed only one cancer type, such as prostate cancer (72), lung cancer (73), and colorectal cancer (71) mortality. Second, to calculate the pooled RR for total cancer mortality, we only considered studies that reported mortality from all cancers. To calculate the pooled RR, the previous meta-analyses included studies reporting total cancer or cancer-specific mortality (4–6). Third, we added more recent studies and included the largest number of participants and cancer deaths. Due to numerous studies included, we observed several associations between each type of dairy product consumption and cancer mortality, which were not observed in the previous meta-analyses. Fourth, we assessed several dairy products that were individually stratified by sex, cancer site, fat content, and geographical region, especially its first subgroup analysis for the fermented milk and cheese consumption. Fifth, we conducted a linear dose-response meta-analysis between total dairy and each type of dairy product consumption and cancer mortality, with further stratified analysis by sex and fat content.
Despite these advantages, this meta-analysis has several limitations. First, this study was based on observational studies, and the unmeasured or residual confounding cannot be completely solved. However, most of the studies included in the meta-analysis provided estimates that were adjusted for various cancer mortality risk factors, and we further performed a stratified analysis to examine the effects of potential confounding factors. Second, measurement errors could occur when recording information during the assessment because most of the studies included in this meta-analysis employed self-reported questionnaires to estimate the dairy product intake. However, some nondifferential misclassification of dairy products intake categories may attenuate the association and likely biased the results toward the null. Third, the highest and lowest categories of dairy products consumption varied among the studies. Most studies had comparable lowest categories, such as only 1 serving size per day, but the highest category varied each study, and the highest category of most studies was open-ended. To address this limitation, we performed a dose-response analysis. Last, due to limited data, it was difficult to examine the association of dairy product consumption with cancer mortality when stratified by geographic region.
Conclusively, high milk consumption, particularly high/whole-fat milk, was associated with increased cancer mortality compared with low milk consumption, whereas high fermented milk consumption was associated with decreased cancer mortality, and this association was especially noticeable in females. Furthermore, high milk consumption was associated with increased mortality of liver cancer, ovarian cancer, and prostate cancer. Future well-designed large prospective cohort studies that investigate the association between each type of dairy product and cancer mortality in different cancer sites and populations are warranted.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—SJ: data collection, statistical analysis, and writing—original draft; YJ: writing—review and editing and study supervision; SJ and YJ: study concept and design, interpretation of the data, critical revision of the paper for important intellectual content, and approval of the final paper for submission; and both authors: read and approved the final manuscript.
Notes
Supported by the National Research Foundation of Korea (NRF–2021R1F1A1050847). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: IGF-1, insulin-like growth factor 1; WCRF, World Cancer Research Foundation International.
Contributor Information
Shaoyue Jin, Department of Food and Nutrition, Kyung Hee University, Seoul, South Korea.
Youjin Je, Department of Food and Nutrition, Kyung Hee University, Seoul, South Korea.
References
- 1. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global cancer observatory: cancer today. [Internet]. Lyon (France): International Agency for Research on Cancer; 2020; [accessed Apr 15, 2021]. Available from: https://gco.iarc.fr/today. [Google Scholar]
- 2. Thorning TK, Raben A, Tholstrup T, Soedamah-Muthu SS, Givens I, Astrup A. Milk and dairy products: good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr Res. 2016;60:32527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Export Report . Meat, fish and dairy products and the risk of cancer. [Internet]. London (UK): World Cancer Research Fund International; American Institute for Cancer Research; 2018 [cited Apr 14, 2021]. Available from:https://www.wcrf.org/dietandcancer/exposures/meat-fish-dairy. [Google Scholar]
- 4. Lu W, Chen H, Niu Y, Wu H, Xia D, Wu Y. Dairy products intake and cancer mortality risk: a meta-analysis of 11 population-based cohort studies. Nutr J. 2016;15(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mazidi M, Mikhailidis DP, Sattar N, Howard G, Graham I, Banach M; Lipid and Blood Pressure Meta-analysis Collaboration Group . Consumption of dairy product and its association with total and cause specific mortality—a population-based cohort study and meta-analysis. Clin Nutr. 2019;38(6):2833–45. [DOI] [PubMed] [Google Scholar]
- 6. Naghshi S, Sadeghi O, Larijani B, Esmaillzadeh A. High vs. low-fat dairy and milk differently affects the risk of all-cause, CVD, and cancer death: a systematic review and dose-response meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. [epub ahead of print 5 Jan 2021]. In press. [DOI] [PubMed] [Google Scholar]
- 7. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SEet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Internet]. [cited Mar 30, 2021]. Available from:http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 9. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 10. Ness AR, Smith GD, Hart C. Milk, coronary heart disease and mortality. J Epidemiol Community Health. 2001;55(6):379–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breslow RA, Graubard BI, Sinha R, Subar AF. Diet and lung cancer mortality: a 1987 National Health Interview Survey cohort study. Cancer Causes Control. 2000;11(5):419–31. [DOI] [PubMed] [Google Scholar]
- 12. Park Y, Mitrou PN, Kipnis V, Hollenbeck A, Schatzkin A, Leitzmann MF. Calcium, dairy foods, and risk of incident and fatal prostate cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2007;166(11):1270–9. [DOI] [PubMed] [Google Scholar]
- 13. Song Y, Chavarro JE, Cao Y, Qiu W, Mucci L, Sesso HD, Stampfer MJ, Giovannucci E, Pollak M, Liu Set al. Whole milk intake is associated with prostate cancer-specific mortality among U.S. male physicians. J Nutr. 2013;143(2):189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Um CY, Judd SE, Flanders WD, Fedirko V, Bostick RM. Associations of calcium and dairy products with all-cause and cause-specific mortality in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) prospective cohort study. Nutr Cancer. 2017;69(8):1185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ding M, Li J, Qi L, Ellervik C, Zhang X, Manson JE, Stampfer M, Chavarro JE, Rexrode KM, Kraft Pet al. Associations of dairy intake with risk of mortality in women and men: three prospective cohort studies. BMJ. 2019;367:l6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–29. [Google Scholar]
- 17. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. [DOI] [PubMed] [Google Scholar]
- 19. Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4(3):218–28. [DOI] [PubMed] [Google Scholar]
- 20. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6(1):40–57. [Google Scholar]
- 21. Bodner-Montville J, Ahuja JKC, Ingwersen LA, Haggerty ES, Enns CW, Perloff BP. USDA Food and Nutrient Database for Dietary Studies: released on the web. J Food Compos Anal. 2006;19:S100–S7. [Google Scholar]
- 22. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 23. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snowdon DA, Phillips RL, Choi W. Diet, obesity, and risk of fatal prostate cancer. Am J Epidemiol. 1984;120(2):244–50. [DOI] [PubMed] [Google Scholar]
- 25. Phillips RL, Snowdon DA. Dietary relationships with fatal colorectal cancer among Seventh-Day Adventists. J Natl Cancer Inst. 1985;74(2):307–17. [PubMed] [Google Scholar]
- 26. Mills PK, Annegers JF, Phillips RL. Animal product consumption and subsequent fatal breast cancer risk among Seventh-Day Adventists. Am J Epidemiol. 1988;127(3):440–53. [DOI] [PubMed] [Google Scholar]
- 27. Hirayama T. Contribution of a long-term prospective cohort study to the issue of nutrition and cancer with special reference to the role of alcohol drinking. Prog Clin Biol Res. 1990;346:179–87. [PubMed] [Google Scholar]
- 28. Hsing AW, McLaughlin JK, Schuman LM, Bjelke E, Gridley G, Wacholder S, Chien HT, Blot WJ. Diet, tobacco use, and fatal prostate cancer: results from the Lutheran Brotherhood Cohort Study. Cancer Res. 1990;50(21):6836–40. [PubMed] [Google Scholar]
- 29. Kneller RW, McLaughlin JK, Bjelke E, Schuman LM, Blot WJ, Wacholder S, Gridley G, CoChien HT, Fraumeni JF Jr. A cohort study of stomach cancer in a high-risk American population. Cancer. 1991;68(3):672–8. [DOI] [PubMed] [Google Scholar]
- 30. Chow WH, Schuman LM, McLaughlin JK, Bjelke E, Gridley G, Wacholder S, Chien HT, Blot WJ. A cohort study of tobacco use, diet, occupation, and lung cancer mortality. Cancer Causes Control. 1992;3(3):247–54. [DOI] [PubMed] [Google Scholar]
- 31. Hsing AW, McLaughlin JK, Chow WH, Schuman LM, Co Chien HT, Gridley G, Bjelke E, Wacholder S, Blot WJ. Risk factors for colorectal cancer in a prospective study among U.S. white men. Int J Cancer. 1998;77(4):549–53. [DOI] [PubMed] [Google Scholar]
- 32. Ozasa K, Watanabe Y, Ito Y, Suzuki K, Tamakoshi A, Seki N, Nishino Y, Kondo T, Wakai K, Ando Met al. Dietary habits and risk of lung cancer death in a large-scale cohort study (JACC study) in Japan by sex and smoking habit. Jpn J Cancer Res. 2001;92(12):1259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ngoan LT, Mizoue T, Fujino Y, Tokui N, Yoshimura T. Dietary factors and stomach cancer mortality. Br J Cancer. 2002;87(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodriguez C, Jacobs EJ, Patel AV, Calle EE, Feigelson HS, Fakhrabadi-Shokoohi D, Thun MJ. Jewish ethnicity and prostate cancer mortality in two large US cohorts. Cancer Causes Control. 2002;13(3):271–7. [DOI] [PubMed] [Google Scholar]
- 35. Khan MM, Goto R, Kobayashi K, Suzumura S, Nagata Y, Sonoda T, Sakauchi F, Washio M, Mori M. Dietary habits and cancer mortality among middle aged and older Japanese living in Hokkaido, Japan by cancer site and sex. Asian Pac J Cancer Prev. 2004;5(1):58–65. [PubMed] [Google Scholar]
- 36. Kojima M, Wakai K, Tamakoshi K, Tokudome S, Toyoshima H, Watanabe Y, Hayakawa N, Suzuki K, Hashimoto S, Ito Yet al. Diet and colorectal cancer mortality: results from the Japan Collaborative Cohort Study. Nutr Cancer. 2004;50(1):23–32. [DOI] [PubMed] [Google Scholar]
- 37. Sakauchi F, Mori M, Washio M, Watanabe Y, Ozasa K, Hayashi K, Miki T, Nakao M, Mikami K, Ito Yet al. Dietary habits and risk of urothelial cancer death in a large-scale cohort study (JACC study) in Japan. Nutr Cancer. 2004;50(1):33–9. [DOI] [PubMed] [Google Scholar]
- 38. Koh KA, Sesso HD, Paffenbarger RS Jr, Lee IM. Dairy products, calcium and prostate cancer risk. Br J Cancer. 2006;95(11):1582–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matsumoto M, Ishikawa S, Nakamura Y, Kayaba K, Kajii E. Consumption of dairy products and cancer risks. J Epidemiol. 2007;17(2):38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sakauchi F, Khan MM, Mori M, Kubo T, Fujino Y, Suzuki S, Tokudome S, Tamakoshi A. Dietary habits and risk of ovarian cancer death in a large-scale cohort study (JACC study) in Japan. Nutr Cancer. 2007;57(2):138–45. [DOI] [PubMed] [Google Scholar]
- 41. Smit E, Garcia-Palmieri MR, Figueroa NR, McGee DL, Messina M, Freudenheim JL, Crespo CJ. Protein and legume intake and prostate cancer mortality in Puerto Rican men. Nutr Cancer. 2007;58(2):146–52. [DOI] [PubMed] [Google Scholar]
- 42. Bonthuis M, Hughes MC, Ibiebele TI, Green AC, van der Pols JC. Dairy consumption and patterns of mortality of Australian adults. Eur J Clin Nutr. 2010;64(6):569–77. [DOI] [PubMed] [Google Scholar]
- 43. Sharma S, Vik S, Pakseresht M, Shen L, Kolonel LN. Diet impacts mortality from cancer: results from the multiethnic cohort study. Cancer Causes Control. 2013;24(4):685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang LY, Wahlqvist ML, Huang YC, Lee MS. Optimal dairy intake is predicated on total, cardiovascular, and stroke mortalities in a Taiwanese cohort. J Am Coll Nutr. 2014;33(6):426–36. [DOI] [PubMed] [Google Scholar]
- 45. Michaelsson K, Wolk A, Langenskiold S, Basu S, Warensjo Lemming E, Melhus H, Byberg L. Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ. 2014;349:g6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Praagman J, Dalmeijer GW, van der Schouw YT, Soedamah-Muthu SS, Monique Verschuren WM, Bas Bueno-de-Mesquita H, Geleijnse JM, Beulens JW. The relationship between fermented food intake and mortality risk in the European Prospective Investigation into Cancer and Nutrition–Netherlands cohort. Br J Nutr. 2015;113(3):498–506. [DOI] [PubMed] [Google Scholar]
- 47. Wang C, Yatsuya H, Tamakoshi K, Iso H, Tamakoshi A. Milk drinking and mortality: findings from the Japan Collaborative Cohort Study. J Epidemiol. 2015;25(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Farvid MS, Malekshah AF, Pourshams A, Poustchi H, Sepanlou SG, Sharafkhah M, Khoshnia M, Farvid M, Abnet CC, Kamangar Fet al. Dairy food intake and all-cause, cardiovascular disease, and cancer mortality: the Golestan Cohort Study. Am J Epidemiol. 2017;185(8):697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bergholdt HKM, Nordestgaard BG, Varbo A, Ellervik C. Lactase persistence, milk intake, and mortality in the Danish general population: a Mendelian randomization study. Eur J Epidemiol. 2018;33(2):171–81. [DOI] [PubMed] [Google Scholar]
- 50. Pala V, Sieri S, Chiodini P, Masala G, Palli D, Mattiello A, Panico S, Tumino R, Frasca G, Fasanelli Fet al. Associations of dairy product consumption with mortality in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Italy cohort. Am J Clin Nutr. 2019;110(5):1220–30. [DOI] [PubMed] [Google Scholar]
- 51. Um CY, Prizment A, Hong CP, Lazovich D, Bostick RM. Associations of calcium and dairy product intakes with all-cause, all-cancer, colorectal cancer and CHD mortality among older women in the Iowa Women's Health Study. Br J Nutr. 2019;121(10):1188–200. [DOI] [PubMed] [Google Scholar]
- 52. van den Brandt PA. Red meat, processed meat, and other dietary protein sources and risk of overall and cause-specific mortality in the Netherlands Cohort Study. Eur J Epidemiol. 2019;34(4):351–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Michels KB, Willett WC, Vaidya R, Zhang X, Giovannucci E. Yogurt consumption and colorectal cancer incidence and mortality in the Nurses' Health Study and the Health Professionals Follow-up Study. Am J Clin Nutr. 2020;112(6):1566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schmid D, Song M, Zhang X, Willett WC, Vaidya R, Giovannucci EL, Michels KB. Yogurt consumption in relation to mortality from cardiovascular disease, cancer, and all causes: a prospective investigation in 2 cohorts of US women and men. Am J Clin Nutr. 2020;111(3):689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stasinopoulos LC, Zhou A, Hyppönen E. Association of supplemental calcium and dairy milk intake with all-cause and cause-specific mortality in the UK Biobank: a prospective cohort study. Br J Nutr. 2020;123(5):574–82. [DOI] [PubMed] [Google Scholar]
- 56. Wang XJ, Jiang CQ, Zhang WS, Zhu F, Jin YL, Woo J, Cheng KK, Lam TH, Xu L. Milk consumption and risk of mortality from all-cause, cardiovascular disease and cancer in older people. Clin Nutr. 2020;39(11):3442–51. [DOI] [PubMed] [Google Scholar]
- 57. Sun Y, Liu B, Snetselaar LG, Wallace RB, Shadyab AH, Kroenke CH, Haring B, Howard BV, Shikany JM, Valdiviezo Cet al. Association of major dietary protein sources with all-cause and cause-specific mortality: prospective cohort study. J Am Heart Assoc. 2021;10(5):e015553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Qin LQ, He K, Xu JY. Milk consumption and circulating insulin-like growth factor-I level: a systematic literature review. Int J Food Sci Nutr. 2009;60(Suppl 7):330–40. [DOI] [PubMed] [Google Scholar]
- 59. Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet North Am Ed. 2004;363(9418):1346–53. [DOI] [PubMed] [Google Scholar]
- 60. Shi R, Yu H, McLarty J, Glass J. IGF-I and breast cancer: a meta-analysis. Int J Cancer. 2004;111(3):418–23. [DOI] [PubMed] [Google Scholar]
- 61. Harrison S, Lennon R, Holly J, Higgins JPT, Gardner M, Perks C, Gaunt T, Tan V, Borwick C, Emmet Pet al. Does milk intake promote prostate cancer initiation or progression via effects on insulin-like growth factors (IGFs)? A systematic review and meta-analysis. Cancer Causes Control. 2017;28(6):497–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lei T, Ling X. IGF-1 promotes the growth and metastasis of hepatocellular carcinoma via the inhibition of proteasome-mediated cathepsin B degradation. World J Gastroenterol. 2015;21(35):10137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Duarte-Salles T, Fedirko V, Stepien M, Trichopoulou A, Bamia C, Lagiou P, Lukanova A, Trepo E, Overvad K, Tjønneland Aet al. Dairy products and risk of hepatocellular carcinoma: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2014;135(7):1662–72. [DOI] [PubMed] [Google Scholar]
- 64. Yang W, Sui J, Ma Y, Simon TG, Chong D, Meyerhardt JA, Willett WC, Giovannucci EL, Chan AT, Zhang X. A prospective study of dairy product intake and the risk of hepatocellular carcinoma in U.S. men and women. Int J Cancer. 2020;146(5):1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Larsson SC, Orsini N, Wolk A. Milk, milk products and lactose intake and ovarian cancer risk: a meta-analysis of epidemiological studies. Int J Cancer. 2006;118(2):431–41. [DOI] [PubMed] [Google Scholar]
- 66. Liu G, Hale GE, Hughes CL. Galactose metabolism and ovarian toxicity. Reprod Toxicol. 2000;14(5):377–84. [DOI] [PubMed] [Google Scholar]
- 67. Qin B, Moorman PG, Alberg AJ, Barnholtz-Sloan JS, Bondy M, Cote ML, Funkhouser E, Peters ES, Schwartz AG, Terry Pet al. Dairy, calcium, vitamin D and ovarian cancer risk in African-American women. Br J Cancer. 2016;115(9):1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ohlsson JA, Johansson M, Hansson H, Abrahamson A, Byberg L, Smedman A, Lindmark-Månsson H, Lundh Å. Lactose, glucose and galactose content in milk, fermented milk and lactose-free milk products. Int Dairy J. 2017;73:151–4. [Google Scholar]
- 69. Kim Y, Je Y, Giovannucci EL. Association between dietary fat intake and mortality from all-causes, cardiovascular disease, and cancer: a systematic review and meta-analysis of prospective cohort studies. Clin Nutr. 2021;40(3):1060–70. [DOI] [PubMed] [Google Scholar]
- 70. Perdigon G, Alvarez S, Rachid M, Agüero G, Gobbato N. Immune system stimulation by probiotics. J Dairy Sci. 1995;78(7):1597–606. [DOI] [PubMed] [Google Scholar]
- 71. Jin S, Kim Y, Je Y. Dairy consumption and risks of colorectal cancer incidence and mortality: a meta-analysis of prospective cohort studies. Cancer Epidemiol Biomarkers Prev. 2020;29(11):2309–22. [DOI] [PubMed] [Google Scholar]
- 72. Aune D, Navarro Rosenblatt DA, Chan DS, Vieira AR, Vieira R, Greenwood DC, Vatten LJ, Norat T. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr. 2015;101(1):87–117. [DOI] [PubMed] [Google Scholar]
- 73. Yang Y, Wang X, Yao Q, Qin L, Xu C. Dairy product, calcium intake and lung cancer risk: a systematic review with. meta-analysis. Sci Rep. 2016;6:20624. [DOI] [PMC free article] [PubMed] [Google Scholar]