ABSTRACT
The health benefits of nuts reported throughout the literature are extensive and well established for reducing the risk of, and managing several chronic conditions such as cardiovascular disease, type 2 diabetes, nonalcoholic fatty liver disease, and cognition. Despite their comparable nutrient profile to nuts, seeds are often not assessed in clinical and epidemiological studies. Interestingly, dietary guidelines and recommendations often refer to “nuts and seeds” collectively, even though they are not consistently examined together in nutrition research when determining associated health benefits. The purpose of this review is to call for future nutrition research to consider combining nuts and seeds. This review provides justification for this proposal by summarizing current definitions for nuts and seeds and highlighting the similarities or dissimilarities in their nutrient compositions. Following this, we summarize current evidence on the health benefits of nuts and seeds, research gaps that should be addressed, and considerations for future research using both epidemiological and interventional study designs.
Keywords: nuts, seeds, nutrients, health, nutritional sciences, epidemiology, clinical study
Statement of Significance: This article proposes that future nut research should expand to include seeds, in order to support the development of specific recommendations for nuts and seeds as a food group, which is more aligned with the major dietary guidelines.
Introduction
There is substantial evidence demonstrating the health benefits of regularly consuming nuts. For many years nuts have been used as a nutrition management strategy for malnutrition in developing countries (1–3), which has extended to older adults (4). For instance, studies have shown the benefit of nuts, either as an individual dietary component or as part of healthy dietary patterns (e.g. Mediterranean diet), for the prevention and management of various chronic diseases, including cardiovascular disease (CVD), type 2 diabetes, nonalcoholic fatty liver disease, and cognition (5–9). Dietary guidelines often recommend inclusion of nuts and seeds as part of a healthy diet, for example, as meat alternatives (10); however, nuts and seeds have not been consistently researched alongside each other. To date, there has been far less research on the health benefits of seeds alone or in combination with nuts. This is an important consideration as foods containing seeds are becoming increasingly common in our food system such as muesli, granola, linseed sunflower almond (LSA) mix, cereals, and seeds in snack bars.
Seeds being overlooked in nutrition analyses may be at least partly due to the limited ability to sufficiently capture seed intake using dietary assessment methods, or the lack of nutrient composition of seed-containing foods in nutrient databases to support dietary analysis. Whereas nuts can be eaten alone as snacks (11), in many cultures, seeds are common ingredients of foods, which makes it more difficult to assess their independent effects of health. Perhaps more importantly, there is confusion around the definition of both seeds and nuts. The nuts selected and included in research settings has been based on their nutritional properties rather than botanical definitions. Currently, global nut intakes are low; for example, in a study that included 10 European countries, more than a quarter of the almost 400,000 participants did not consume nuts at all while a further quarter consumed <1 g per day (12). One strategy to enhance intake may be by grouping nuts and seeds together in order to increase intakes closer to recommended levels of 2.5 ounces per week (70 g per week) of “nut, seeds, and soy products” by the American Dietary Guidelines for adults, to support optimal nutrition and health (10, 13, 14). Nuts, seeds, and soy products are grouped together in the dietary guidelines based on their protein content; however, only nuts and seeds share very similar nutrient profiles apart from dietary protein content. Thus, nuts and seeds may share similar mechanisms of action for driving health benefits, and therefore complement each other as healthy dietary components. Therefore, this article aims to propose the expansion of nut research to include seeds to better understand the benefits of nuts and seeds collectively as a food group, which is more aligned with the dietary guidelines. However, it is not the intention of this perspective to propose a change to the current dietary recommendations on nuts and seeds until the optimal dose of these foods in meeting dietary requirements and promoting health is more evident. In particular, there are still very limited studies assessing the health effects of seeds. We will compare and discuss the definition, composition, and function of nuts and seeds, summarize the current knowledge and gaps of research in this field, and consider factors to guide future research in examining their health effects.
Definition: A nut or a seed?
The definition of nuts and seeds is confusing and varies depending on which classification system is used: botanical, nutritional, or culinary. Strictly speaking, the botanical classification of a nut is defined as a dry one-seeded fruit with an extremely hard pericarp (outer layer of the ovary wall). This definition includes chestnuts and hazelnuts. Dietary guidelines do not follow botanical definitions and include nuts that are edible and based on their nutrient composition. From a nutrition perspective, not only are true botanical nuts included in research, but also some seeds, legumes, and drupes. The botanical and nutritional definitions of nuts and seeds are provided in Table 1 with a summary of which meet each criterion based on the classification. Culinary definitions, on the other hand, tend to be less precise compared with botanical definitions and often refer to the edible parts of plant foods, for nuts this often means any large oily kernel within a shell that can be eaten (15).
TABLE 1.
Definition of nuts and seeds based on botanical and nutritional classifications (16–19)
| Nuts | Seeds | Legumes | Drupes | |
|---|---|---|---|---|
| Botanical definition | 1-celled, 1-seeded dry fruit with a hard shell (pericarp – the outer layer of the ovary wall) that does not fuse with the ovary wall and does not open at maturity | Embryonic plants enclosed in a nutrient-dense seed coat that works as stored food to nourish the plant as it grows. Some need husks removing before consuming | Have pods (shells) that contain multiple fruit inside. They twist apart to scatter the seeds inside | Drupes include a seed that is contained within a shell and is surrounded by a fleshy fruit. The seed may be discarded (e.g. peach), or eaten |
| Chestnuts and hazelnuts | Brazil nuts, pine nuts, macadamias chia seeds, pumpkin seeds, flaxseeds/linseeds, poppy seeds, sesame seeds, sunflower seeds | Peanuts | Almonds, cashews, coconuts, pistachios, walnuts, pecans | |
| Nutritional research definition | Almonds, Brazil nuts, cashews, hazelnuts, macadamias, peanuts, pecans, pine nuts, pistachios, walnuts.Note: peanuts are included because they have a similar nutrient profile to other tree nuts | Chia seeds, pumpkin seeds, flaxseeds/linseeds, poppy seeds, sesame seeds, sunflower seeds | — | — |
| Excluded from nutritional research definition | Coconut due to high saturated fat content.Chestnuts due to high carbohydrate and water content | — | — | — |
| Culinary definition | No precise cut-offs. All nuts and seeds that are edible and/or for cooking purposes. May also be classified for sensory components such as flavor or texture | — | — | — |
Peanuts, which are legumes, are often included based on the justification that peanuts have very similar nutrient profiles to other tree nuts. Conversely, chestnuts and coconuts are often omitted in the nutritional research definition as chestnuts have a higher carbohydrate content than other nuts and coconuts have a higher amount of saturated fat.
Seeds are defined as a small plant enclosed in a seed coat that works as stored food to nourish the plant as it grows. Some seeds need the exterior husk removed before eating such as sunflower and pumpkin seeds, whereas others, such as sesame and poppy seeds, do not. Examples of commonly consumed seeds are flaxseeds, poppy seeds, pumpkin seeds, sesame seeds, sunflower seeds, and chia seeds. Although the classification systems vary, there are similar nutritional properties between “nuts” and “seeds.”
Nutrient composition of nuts and seeds
As noted above, botanical classifications of nuts and seeds do not appear to be considered for the purpose of analyzing health outcomes. However, from a nutrient composition perspective, it is unclear why nuts and seeds are not considered together. Nuts are often thought to provide health benefits attributable to high protein, monounsaturated and polyunsaturated fats, fiber, micronutrients, as well as their phytosterol composition (4, 20, 21). These nutrients exhibit antioxidant and anti-inflammatory properties (22). In addition, protein and fiber may enhance satiety (23). It has been recently postulated that fiber consumption may improve the gut microbiome (24). The specific macronutrient and micronutrient breakdown per 100 g of nuts and seeds are shown in Figure 1 and Table 2, respectively, which highlight that some “nuts” such as chestnuts and coconuts should be (or in fact were) excluded from the nut category in nutrition research due to their high water and high carbohydrate (chestnuts) or saturated fat content (coconuts). Based on nutrient composition, it is also not clear why seeds such as flaxseed, chia, poppy, pumpkin, sesame, and sunflower seeds are not grouped together with “nuts” in nutrition research. It should be highlighted that there are studies that have investigated the health effects of nut and seed oil (25–28). These studies are outside the context of this article and they are not considered for 2 main reasons: First, oils are grouped very differently from nuts and seeds in the dietary guidelines and the recommendation does not include nut/seed oils; second, oils are a single component of nuts and seeds and they do not have all the properties (e.g. texture) and nutrients (e.g. protein, fiber, many nonfat-soluble vitamins and minerals) that contribute to the known health effects of nuts. Extracting oil from nuts alters the nutrition profile, as most of the antioxidants are located in the pellicle or outer soft shell of the nuts, and removing the skin could result in the loss of >50% of the antioxidants present in nuts (29, 30). Based on the same rationale, nut products such as nut-based milk and flour are not considered in this review.
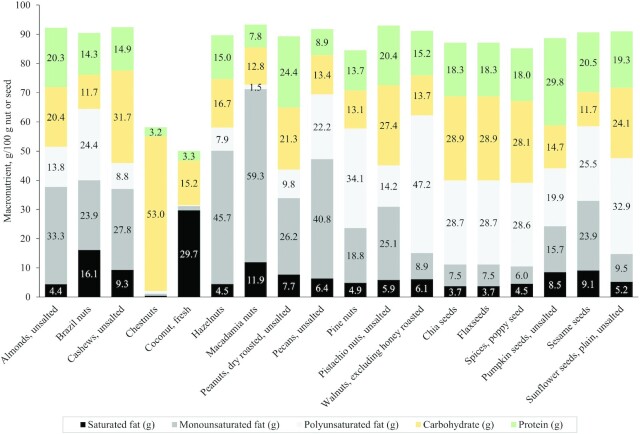
FIGURE 1.
The content of carbohydrate, protein, and fat subtypes in grams (saturated, mono-, and polyunsaturated fats) per 100 g of nuts and seeds. Due to higher water content, both coconut and chestnut have only ∼50–60% of macronutrient content. Unlike other nuts and seeds, chestnuts are high in carbohydrate and coconuts are high in saturated fat. It is noted most nuts are high in monounsaturated fats, whereas walnuts and seeds are higher in polyunsaturated fats. Due to these obvious differences in their nutritional profiles, chestnuts and coconuts should not be included in research that considers nut and seed intake.
TABLE 2.
Nutrient composition of commonly consumed nuts and seeds1
| Nuts | Seeds | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrients,2 unit/100g | Almonds, unsalted | Brazil nuts | Cashews, unsalted | Chestnuts | Coconut, fresh | Hazelnuts | Macadamia nuts | Peanuts, dry roasted, unsalted | Pecans, unsalted | Pine nuts | Pistachio nuts, unsalted | Walnuts, excluding honey roasted | Chia seeds | Flax seeds | Spices, poppy seeds | Pumpkin seeds, unsalted | Sesame seeds | Sunflower seeds, plain, unsalted |
| Energy, kcal (kJ) | 607 (2540) | 659 (2760) | 583 (2440) | 245 (1030) | 354 (1480) | 628 (2630) | 716 (3000) | 587 (2460) | 697 (2920) | 673 (2820) | 581 (2430) | 654 (2740) | 534 (2230) | 534 (2230) | 525 (2200) | 574 (2400) | 631 (2640) | 582 (2440) |
| Protein, g | 20.3 | 14.3 | 14.9 | 3.20 | 3.30 | 15.0 | 7.80 | 24.4 | 8.90 | 13.7 | 20.4 | 15.2 | 18.3 | 18.3 | 18.0 | 29.8 | 20.5 | 19.3 |
| Carbohydrate, g | 20.4 | 11.7 | 31.7 | 53.0 | 15.2 | 16.7 | 12.8 | 21.3 | 13.4 | 13.1 | 27.4 | 13.7 | 28.9 | 28.9 | 28.1 | 14.7 | 11.7 | 24.1 |
| Total sugars, g | 4.70 | 2.30 | 4.90 | 10.6 | 6.20 | 4.30 | 4.10 | 4.90 | 3.90 | 3.60 | 7.50 | 2.60 | 1.60 | 1.60 | 3.00 | 1.30 | 0.50 | 2.70 |
| Total dietary fiber, g | 10.6 | 7.50 | 2.90 | 5.10 | 9.00 | 9.70 | 8.00 | 8.40 | 9.30 | 3.70 | 10.0 | 6.70 | 27.3 | 27.3 | 19.5 | 6.50 | 11.6 | 11.1 |
| Total fat, g | 54.0 | 67.1 | 48.0 | 2.20 | 33.5 | 60.8 | 76.1 | 49.7 | 72.8 | 68.4 | 47.4 | 65.2 | 42.2 | 42.2 | 41.6 | 49.1 | 61.2 | 49.8 |
| Saturated fat, g | 4.40 | 16.1 | 9.30 | 0.400 | 29.7 | 4.50 | 11.9 | 7.70 | 6.40 | 4.90 | 5.90 | 6.10 | 3.70 | 3.70 | 4.50 | 8.50 | 9.10 | 5.20 |
| Monounsaturated fat, g | 33.3 | 23.9 | 27.8 | 0.800 | 1.40 | 45.7 | 59.3 | 26.2 | 40.8 | 18.8 | 25.1 | 8.90 | 7.50 | 7.50 | 6.00 | 15.7 | 23.9 | 9.50 |
| Polyunsaturated fat, g | 13.8 | 24.4 | 8.80 | 0.90 | 0.40 | 7.90 | 1.50 | 9.80 | 22.2 | 34.1 | 14.2 | 47.2 | 28.7 | 28.7 | 28.6 | 19.9 | 25.5 | 32.9 |
| Water, g | 2.34 | 3.42 | 1.65 | 40.5 | 47.0 | 5.31 | 1.61 | 1.81 | 3.41 | 2.28 | 1.79 | 4.07 | 6.96 | 6.96 | 5.95 | 2.03 | 3.75 | 1.20 |
| Vitamin A, RAE (μg_RAE) | 0 | 0 | 0 | 1.00 | 0 | 1.00 | 0 | 0 | 3.00 | 1.00 | 13.0 | 1.00 | 0 | 0 | 0 | 0 | 3.00 | 0 |
| α-Carotene, μg | 0 | 0 | 0 | 0 | 0 | 3.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.00 | 0 | 0 |
| β-Carotene, μg | 1.00 | 0 | 0 | 14.0 | 0 | 11.0 | 0 | 0 | 28.0 | 17.0 | 154 | 12.0 | 0 | 0 | 0 | 5.00 | 40.0 | 5.00 |
| β-Cryptoxanthin, μg | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lutein + zeaxanthin, μg | 1.00 | 0 | 22.0 | 13.0 | 0 | 92.0 | 0 | 0 | 16.0 | 9.00 | 1125 | 9.00 | 651 | 651 | 0 | 30.0 | 0 | 0 |
| Thiamin, mg | 0.0750 | 0.617 | 0.194 | 0.243 | 0.0660 | 0.643 | 0.710 | 0.152 | 0.640 | 0.364 | 0.674 | 0.341 | 1.64 | 1.64 | 0.854 | 0.070 | 0.699 | 0.106 |
| Riboflavin, mg | 1.161 | 0.0350 | 0.194 | 0.175 | 0.0200 | 0.113 | 0.0870 | 0.197 | 0.126 | 0.227 | 0.227 | 0.150 | 0.161 | 0.161 | 0.100 | 0.150 | 0.0900 | 0.246 |
| Niacin, mg | 3.53 | 0.295 | 1.36 | 1.34 | 0.540 | 1.80 | 2.28 | 14.4 | 1.13 | 4.39 | 1.33 | 1.13 | 3.08 | 3.08 | 0.896 | 4.43 | 5.80 | 7.04 |
| Vitamin B-6, mg | 0.132 | 0.101 | 0.248 | 0.497 | 0.0540 | 0.563 | 0.359 | 0.466 | 0.204 | 0.0940 | 1.09 | 0.537 | 0.473 | 0.473 | 0.247 | 0.100 | 0.400 | 0.804 |
| Total folate, μg | 53.0 | 22.0 | 67.0 | 70.0 | 26.0 | 113 | 10.0 | 97.0 | 21.0 | 34.0 | 49.0 | 98.0 | 87.0 | 87.0 | 82.0 | 57.0 | 115 | 237 |
| Total choline, mg | 50.5 | 28.8 | 59.2 | 1.50 | 12.1 | 45.6 | 44.6 | 64.6 | 39.3 | 55.8 | 69.3 | 39.2 | 78.7 | 78.7 | 8.80 | 63.0 | 25.6 | 55.1 |
| Vitamin C, mg | 0 | 0.700 | 0 | 26.0 | 3.30 | 6.30 | 0.700 | 0 | 1.10 | 0.800 | 2.90 | 1.30 | 0.600 | 0.600 | 1.00 | 1.80 | 0 | 1.40 |
| Vitamin E (α-tocopherol), mg | 23.5 | 5.65 | 1.24 | 0.50 | 0.24 | 15.0 | 0.570 | 4.93 | 1.71 | 9.33 | 2.46 | 0.700 | 0.310 | 0.310 | 1.77 | 0.560 | 1.68 | 26.1 |
| Vitamin K, μg | 3.50 | 0 | 37.2 | 7.80 | 0.200 | 14.2 | 0 | 0 | 6.90 | 53.9 | 16.3 | 2.70 | 4.30 | 4.30 | 0 | 4.50 | 0 | 2.70 |
| Calcium, mg | 260 | 160 | 44.0 | 29.0 | 14.0 | 114 | 70.0 | 58.0 | 68.0 | 16.0 | 104 | 98.0 | 255 | 255 | 1440 | 52.0 | 60.0 | 70.0 |
| Phosphorus, mg | 457 | 725 | 475 | 107 | 113 | 290 | 198 | 363 | 269 | 575 | 455 | 346 | 642 | 642 | 870 | 1170 | 667 | 1160 |
| Magnesium, mg | 271 | 376 | 252 | 33.0 | 32.0 | 163 | 118 | 178 | 117 | 251 | 106 | 158 | 392 | 392 | 347 | 550 | 345 | 129 |
| Iron, mg | 3.62 | 2.43 | 5.82 | 0.910 | 2.43 | 4.7 | 2.65 | 1.58 | 2.46 | 5.53 | 3.91 | 2.91 | 5.73 | 5.73 | 9.76 | 8.07 | 6.36 | 3.80 |
| Zinc, mg | 3.21 | 4.06 | 5.43 | 0.57 | 1.10 | 2.45 | 1.29 | 2.77 | 4.39 | 6.45 | 2.27 | 3.09 | 4.34 | 4.34 | 7.90 | 7.64 | 6.73 | 5.29 |
| Copper, mg | 1.07 | 1.74 | 2.15 | 0.51 | 0.435 | 1.73 | 0.570 | 0.428 | 1.16 | 1.32 | 1.25 | 1.59 | 1.22 | 1.22 | 1.63 | 1.28 | 1.40 | 1.83 |
| Selenium, μg | 1.90 | 1920 | 11.3 | 1.20 | 10.1 | 2.40 | 11.7 | 9.30 | 3.70 | 0.700 | 9.70 | 4.90 | 25.4 | 25.4 | 13.5 | 9.40 | 34.4 | 79.3 |
| Potassium, mg | 692 | 659 | 548 | 592 | 356 | 680 | 363 | 634 | 398 | 597 | 977 | 441 | 813 | 813 | 719 | 788 | 370 | 850 |
| Sodium, mg | 3.00 | 3.00 | 16.0 | 2.00 | 20.0 | 0 | 353 | 6.00 | 0 | 2.00 | 6.00 | 2.00 | 30.0 | 30.0 | 26.0 | 18.0 | 47.0 | 3.00 |
| SFAs | ||||||||||||||||||
| 6:0, g | 0 | 0 | 0 | 0 | 0.191 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0370 | 0.00700 | 0 | 0 |
| 8:0, g | 0 | 0 | 0.128 | 0 | 2.35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00200 | 0 | 0 |
| 10:0, g | 0 | 0 | 0.128 | 0 | 1.86 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00200 | 0 | 0 |
| 12:0, g | 0 | 0 | 0.760 | 0 | 14.9 | 0 | 0.0750 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00600 | 0 | 0 |
| 14:0, g | 0.0180 | 0.0460 | 0.337 | 0.0100 | 5.87 | 0 | 0.668 | 0.0160 | 0 | 0 | 0.0120 | 0 | 0.00800 | 0.00800 | 0.0770 | 0.0580 | 0 | 0.051 |
| 16:0, g | 3.54 | 9.63 | 4.51 | 0.373 | 2.84 | 3.10 | 5.93 | 3.98 | 4.53 | 3.21 | 5.14 | 4.40 | 2.17 | 2.17 | 3.58 | 5.19 | 5.273 | 2.808 |
| 18:0, g | 0.779 | 6.24 | 2.98 | 0.0210 | 1.73 | 1.27 | 2.28 | 1.20 | 1.79 | 1.39 | 0.637 | 1.66 | 1.33 | 1.33 | 0.782 | 2.91 | 3.311 | 2.212 |
| MUFAs | ||||||||||||||||||
| 16:1, g | 0.262 | 0.214 | 0.319 | 0.0210 | 0 | 0.116 | 12.7 | 0.0310 | 0.0110 | 0.0170 | 0.461 | 0 | 0.0240 | 0.0240 | 0.0390 | 0.0440 | 0.105 | 0.0490 |
| 18:1, g | 33.0 | 23.6 | 27.2 | 0.728 | 1.43 | 45.4 | 44.4 | 25.4 | 40.6 | 17.9 | 24.4 | 8.80 | 7.36 | 7.36 | 5.86 | 15.6 | 23.6 | 9.40 |
| 20:1, g | 0.0190 | 0.0270 | 0.147 | 0.0100 | 0 | 0.131 | 1.93 | 0.627 | 0.213 | 0.801 | 0.115 | 0.134 | 0.0670 | 0.0670 | 0.0780 | 0.0530 | 0.124 | 0.0480 |
| 22:1, g | 0 | 0 | 0 | 0 | 0 | 0 | 0.237 | 0.0550 | 0 | 0 | 0.00500 | 0 | 0.0130 | 0.0130 | 0 | 0.00100 | 0.0650 | 0 |
| PUFAs | ||||||||||||||||||
| 18:2, g | 13.6 | 24.4 | 8.51 | 0.776 | 0.366 | 7.83 | 1.30 | 9.72 | 21.1 | 33.2 | 13.8 | 38.1 | 5.90 | 5.90 | 28.3 | 19.6 | 25.2 | 32.8 |
| 18:3, g | 0.162 | 0.0360 | 0.308 | 0.0930 | 0 | 0.0870 | 0.196 | 0.0260 | 1.11 | 0.164 | 0.358 | 9.08 | 22.8 | 22.8 | 0.273 | 0.111 | 0.263 | 0.0690 |
| 20:4, g | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0160 | 0 | 0 | 0.00500 | 0 | 0 | 0 | 0 | 0.123 | 0 | 0 |
Nutrient breakdown obtained from the USDA Food and Nutrient Database for Dietary Studies’ (FNDDS) 2017–2018 Food and Nutrient Database for Dietary Studies (31).
Nutrients not found in nuts and seeds, contributing 0 g of nutrition, were omitted from this table these included: cholesterol, retinol, lycopene, folic acid, vitamin B-12, vitamin D, caffeine, theobromine, alcohol, SFA 4:0, PUFA 18:4, 20:5 n–3, 22:5 n–3, 22:6 n–3.
RAE, Retinol Activity Equivalents.
Health benefits of nuts
The regular consumption of nuts has been shown to be associated with multiple health benefits. First, lower CVD risk has been reported with habitual nut consumption (6). The potential mechanisms that explain the protective effects of nuts against CVDs have been reported in detail in a previous review (7), which include lowering blood cholesterol and blood pressure, improving vascular function and oxidative stress in the body; benefits that are proposed to be due to the unsaturated fat, antioxidant, and polyphenolic content of nuts (7). Nuts have also been reported to reduce inflammation (32, 33). Second, nut intake has been shown to play an important role in body weight regulation (34, 35) through the regulation of appetite and food intake (36), increased postprandial energy expenditure (37) and fat oxidation (38), and lower-than-expected fat absorption (39–42). Third, nut-enriched diets have been shown to reduce postprandial glycemic responses to carbohydrate meals (43), and lower acute and long-term fasting insulin and reduce insulin resistance, although no effect was found on fasting glucose or glycated hemoglobin (HbA1c) (44, 45). Fourth, there is emerging evidence that nuts improve cognitive function (46–48) and may lower rates of depression (49). It was proposed that the benefits of nuts on cognition may be via reducing inflammation and improving vascular and endothelial function, which subsequently improves cerebral vascular function (46).
The health benefits of nuts appear to be dose dependent, which is often expressed as benefit per unit increase in the amount of nuts (50–52) or increasing frequency (53, 54) of nut consumption. However, there was also indication that the dose-dependent relation may be curvilinear, where the maximum benefit of nuts is achieved at a certain threshold of nut intake (47). This is an important consideration as understanding the optimal amount that promotes health benefits will guide the development of specific dietary recommendations. Furthermore, having more specific recommendations will avoid the overconsumption of nuts, which may increase the risk of weight gain as nuts are also high in energy density. Besides quantity, a common question raised is whether a specific type of nut is superior to another in reducing the risk of, and management of, chronic diseases. Although different types of nuts are rich sources of specific nutrients, all nuts are generally nutrient dense and have very similar macronutrient profiles (discussed above), hence there are few reasons to support the notion that some nuts are superior to others, or different nuts have differing health benefits. To illustrate this point, almonds have been shown to reduce CVD risk factors (55), but similar benefits have been reported with other nuts such as walnuts (56), peanuts (57), or total nut intake (58). Therefore, although all nuts have their own unique micronutrient composition (e.g. Brazil nuts are high in selenium, almonds and hazelnuts are a good source of vitamin E, hazelnuts and peanuts for folate), mixing different nuts will maximize the completeness of essential nutrients coming from this food group.
In summary, nuts improve several aspects of human health through multiple mechanisms, which are attributed to their nutrient profile. For example, the protein and fiber from nuts may enhance within-meal satiation and between-meals satiety, as well as improving the gut microbiota; unsaturated fats may improve glycemic responses to a carbohydrate meal and improve blood lipid profiles; the amino acid arginine is a precursor of nitric oxide that has been shown to improve vascular function and blood pressure (59), and the antioxidant and polyphenolic compounds in nuts may protect the body and DNA from oxidative damage (60). Furthermore, nuts have been shown to improve overall diet quality, further amplifying their health benefits (4, 61). To date, however, less is known about the health benefits of seeds. The following section will summarize the current state of research on nuts and seeds, and discuss considerations if they are to be grouped together in future research.
Current state of research in nuts, seeds, or the combination
As discussed above, most nutritionally classified nuts and seeds have very similar nutrient profiles. It is hence reasonable to hypothesize that seeds will provide similar health benefits associated with nut consumption. However, there may also be additional health benefits from consuming nuts and seeds together. This is especially important given that global nut intakes are low. Furthermore, all nuts except for walnuts are high in monounsaturated fats, and combining seeds with nuts will increase the proportion of polyunsaturated fats. If similar health benefits are seen with seed consumption, emphasizing both nuts and seeds in guidelines could provide more options for consumers and result in more people consuming amounts aligned with recommendations. A study from the European Prospective Investigation into Cancer and Nutrition (EPIC) study that included almost 37,000 participants reported that 4.4% and 2.3% of participants reported daily intake of tree nuts and peanuts, respectively, and 1.3% reported seed intake in various forms (62). Even among the consumers, the average portion sizes of total nuts and seeds (consumed as whole, from hidden sources and spreads) were 14.6 g/d and 8.5 g/d, respectively, which were below the recommendations (62). In a US representative sample, the proportion of nut and seed consumers were higher than Europe, where over half of the adult population reported daily nut intake and 16.3% of males and 19.9% of females consumed seeds, assessed from two 24-h diet recalls, during the NHANES from 2005 to 2018 (9). Although it is also possible that higher intake in the USA may be due to the 2-d diet recalls, which were more likely to capture nut and seed intake (occasional foods) than the 1-d recall method used in EPIC, there appears to be differences in nut and seed intake between countries. Although the differences between the USA and Europe may be influenced by seed availability and cultural preferences, it should also be noted that intake in the EPIC study was assessed many years ago (in 2006) and intake may have changed since the publication of the findings. Therefore, the established relations between nuts and diseases should be revisited to also include seeds, in order to guide future nutrition research and inform guidelines.
Many observational studies have examined the association between nuts and human health by assessing the intakes of nuts per se (58) whereas others have considered nuts as part of a food group which has included other foods (e.g. together with seeds and legumes) within an overall dietary pattern (63–66). Considering the totality of epidemiological evidence, habitual nut intake has been linked to lower risk of CVD and CVD-related mortality (7, 67), lower fasting insulin and fasting resistance (but no association with fasting glucose or HbA1c) (44, 45), lower prevalence of nonalcoholic fatty liver disease (9, 68, 69), better cognitive performance (70), and lower risk of cancer and cancer-related mortality (71–73). However, the current number of observational studies that considered seeds alone or nuts and seeds together is very limited (74, 75), hence it was unclear if seeds also have similar association with these chronic diseases.
On the other hand, interventional randomized controlled trials (RCTs) mostly used a nut-alone approach, where a specific amount of nuts was added (43, 76) or used to replace energy in a diet (equivalent to nuts) (77, 78) in a habitual diet over a period of time. Some studies also used a whole-diet approach, and nut intake was emphasized [e.g. the Prevención con Dieta Mediterránea (PREDIMED) Study modified the entire diet, and 1 of 2 intervention arms received 30 g/d of mixed nuts (walnuts, almonds, and hazelnuts) (79)]. Nut-alone interventions have demonstrated favorable effects on health outcomes in most studies, although there is some evidence that whole-diet interventions that included nuts may lead to better health outcomes in older adults (48).
Compared with nut studies, fewer interventional studies have been conducted using seeds. Most studies on seeds have focused on CVD risk factors. A randomized controlled crossover study compared the effects of 50 g/d sesame seed powder or 50 g/d rice powder (control) supplement over 5 wk in 24 postmenopausal women. This study observed that sesame seed powder treatment increased the plasma ratio of α- and γ-tocopherol to total cholesterol, and led to significantly greater reductions in blood lipids such as LDL cholesterol, the ratio of LDL to HDL cholesterol, and total cholesterol (80). In CVD research, the most studied seed type has been flaxseed. In a systematic review and meta-analysis of 28 RCTs, flaxseed interventions reduced total cholesterol by 0.10 mmol/L and LDL by 0.08 mmol/L (81). This effect was not observed when flaxseed oil was used, suggesting that the benefits may include a synergistic effect of all nutrients in flaxseeds. In another systematic review and meta-analysis of 11 RCTs, flaxseed interventions reduced systolic and diastolic blood pressure by 1.77 mmHg (P = 0.04) and 1.58 mmHg (P = 0.003) respectively (82). Like the previous systematic review, flaxseeds consumed as whole seeds had greater benefits. The effects of flaxseeds on blood lipids were consistent with findings from 61 interventional studies that used nuts (83). However, nuts did not have a significant effect on blood pressure as observed with flaxseeds, although the reduction with the latter was small and may be of limited clinical importance (82, 83). To our knowledge, only 1 study has directly compared the effects of nuts and seeds on cardiovascular markers (84). In this 3-wk crossover feeding study, involving 22 postmenopausal women with type 2 diabetes, consuming 30 g/d of almonds or sunflower kernels reduced total and LDL cholesterols. However, the sunflower kernel intervention also reduced HDL, triacylglycerol, apoA-1, and B100 more than the almond intervention (84). The findings suggest that both nuts and seeds are beneficial for CVD, although they may have slightly different mechanisms of action.
Other research has been conducted on breast cancer-related outcomes. A case-control study examined the relations between seed intake and the risk of postmenopausal breast cancer (85). In this study, sunflower and pumpkin seed consumption was associated with a lower adjusted OR for breast cancer (OR = 0.91, 95% CI: 0.83–1.00, P = 0.02), and the risk was even lower in those classified as high consumers (those with an intake higher than the population median) when compared with nonconsumers (85). However, sesame and flaxseed consumption did not show similar associations with breast cancer risk in the same study. To summarize, despite the limited number of human studies in seed research, available evidence suggests that seeds may have similar health benefits as nuts. This may be due to the similarity in nutrient profiles between nuts and seeds. However, it is not clear if the benefits can be generalized to all types of seeds, and whether combining nuts and seeds will provide similar health benefits if specific recommendations included nuts and seeds together as a food group. Therefore, more studies are warranted in the future and there are several important issues to be considered when developing practical dietary recommendations, as well as in conducting human research, which we will discuss in the next sections.
Dietary guidelines and recommendations
Plant-rich diets are becoming increasingly popular due to the evidence on their health benefits, as well as their effects on planetary health and sustainability. Many dietary guidelines including the Australian and American dietary guidelines (13, 86) refer to “nuts and seeds” collectively despite the literature, especially that of more robust quality RCTs, being predominantly conducted to include only nuts. Therefore, future studies should include both nuts and seeds because of their similar nutrient profiles. Conversely, reports including the “EAT Lancet report” refer to “nuts” only, a recommendation that may more accurately portray higher quality evidence, but can be limiting with regard to the variety of what can be consumed (87). Furthermore, dietary recommendations and guidelines should consider whether the current nut recommendation (13, 14, 86) should also include seeds if they do in fact provide comparable nutrients and thus, health benefits. Finally, the inclusion of seeds provides more variety and options for individuals with nut allergies, so in addition to providing variety, such recommendations are more inclusive and broadly translatable.
Considerations for future research
Due to the similarities between the nutrient profiles and health benefits (investigated thus far) of nuts and seeds, they should be considered together in future research. Based on the gaps in the literature, more emphasis should be placed on understanding the health benefits of seeds, as well as seeds in combination with nuts. The first step will be to determine what types of nuts and seeds should be considered in nutrition research. We propose that nuts and seeds should at least consider those summarized in Table 3 as they have comparable nutrient profiles. The forms (e.g. whole compared with butter) and level of processing (e.g. raw compared with roasted) should also be considered as these factors can influence nutrient availability (88–92), concentrations of compounds such as phytic acid (93) that can influence the availability and absorption of nutrients (94), and concentrations of additives that may have detrimental health effects (e.g. the addition of sugar, salt, and/or oils/fats), hence altering the nutrient profile and the potential healthiness of nut and seed products. All these factors should be taken into consideration when designing and conducting future studies. Further considerations for epidemiological studies and clinical trials are discussed below.
TABLE 3.
Recommendations for future research in nuts and seeds and health outcomes
| Recommendations | |
|---|---|
| Nut and seed types |
|
| Assessment methods |
|
| Nuts and/or seeds |
|
| Dietary sources of nuts and seeds |
|
| Categorization of intake during analysis |
|
| Nuts and seeds in the context of whole diets |
|
| Clinical trials |
|
Nutrition epidemiology
Assessment of habitual intake
Since both nuts and seeds are episodically consumed, multiple entries of 24-h recalls or an FFQ are ideally needed to capture reliable habitual intake for use in epidemiological analyses. Statistical methods have been developed to help estimate the usual intake of occasional foods, such as the multiple source method (MSM) (95), the National Cancer Institute (NCI) method (96), and the Statistical Program to Assess Dietary Exposure (SPADE) method (97). Although these methods differ in the statistical models used, all methods involve modeling the habitual intake distribution of the food group as a function of age, sex, and other user-defined parameters such as subgroups within a population or consumption frequency. When collecting consumption data, it is important to consider the appropriate dietary assessment method to address the research question(s). For example, although it may be acceptable to use data from a single 24-h recall to estimate the intakes of nuts and seeds from a study sample, using data from an FFQ or multiple 24-h recalls is recommended when examining associations between nut and seed intake and health outcomes, as these methods are more robust and thus reflective of habitual intake. Several considerations for the selection of dietary assessment methods, e.g. validity, measurement errors etc. have been described in detail by the NCI's Dietary Assessment Primer (98).
Nuts (± seeds)
In observational studies, the established relations between nuts and health (e.g. body weight, type 2 diabetes mellitus, CVD, cognition) discussed above should be extended to include seeds. For example, epidemiological research should compare the relations between health outcomes and “nuts alone” compared with “seeds alone” compared with “nuts and seeds” as a food group. This will provide crucial understanding and evidence of whether nuts and seeds together are superior to nuts alone, i.e. synergistic effects from a variety of nuts and seeds, in reducing the risk of disease and disease management. In addition, this will help determine the optimal amount of nuts and seeds that provides maximum health benefits. All these will have important implications for dietary recommendations and guidelines in the future.
Dietary sources of nuts and seeds
It is also important to consider how nut and seed intake will be quantified in observational studies. Previous nut studies have used 2 primary approaches in determining nut intake, i.e. apparent nut intake (consumed alone as identified from diet records/recalls and FFQs) or “hidden” nuts (used as an ingredient in foods such as breakfast cereals, snack bars) (99, 100). The main advantage of considering intake of nuts and seeds from hidden sources is that this approach provides a more accurate assessment of actual, total nut intake in the diet as opposed to nuts that are consumed whole and alone only. However, a comprehensive food database is needed in order to accurately quantify nuts and seeds as an ingredient from various foods, meals, and processed foods in the market. For example, in the USA, the Food Commodity Intake Database allows researchers to estimate the proportion of nuts and seeds contained in various foods and mixed meals in the database. Therefore, future studies that quantify nuts and seeds as hidden ingredients, and culture- and country-specific food ingredient databases are needed. On the contrary, an approach that includes hidden nuts and seeds has potential limitations. Although nuts and seeds are known to be nutrient rich and may provide several health benefits, it remains unknown if the total intake of nuts and seeds (apparent intake plus hidden sources) is sufficient, or whether researchers should further consider the sources where nuts and seeds are derived from. For example, should we consider nuts/seeds from desserts or discretionary foods (e.g. pecan pie, peanut brittles) when understanding the relations between intake and disease? Whilst this is an important consideration, the selection of a given research approach should not be rigid but should perhaps be guided by the outcome of interests. For instance, in cases where nutrient intake is more crucial than the food sources (e.g. in malnutrition among older adults or catabolic conditions that increase nutrient demands such as cancer), assessing nuts and seeds from discretionary food choices is warranted, compared to studies where obesity is the primary health outcome.
Categorization of intake during analysis
In nutrition epidemiology, the associations between nut intake and various health outcomes rely on how nut and seed intake is categorized and compared. In previous studies, nut intake has been categorized in a number of ways: 1) binary classification of intake as nonconsumers compared with consumers defined as nut intake of ≥¼ ounce (or 7.1 g/d) (61, 101), 2) based on per unit increments of half (47) or a serving (1 ounce or 28.5 g/d) (102), 3) based on current nut recommendations of 30 g/d (8, 9), 4) per 50 g/d increment (53), and 5) in nut intake quantiles, e.g. tertiles (103) or quartiles (12). Nut intake categorization based on serving sizes is more translatable with regard to guiding recommendations and practice compared to approaches that compare nut intake using tertiles or quartiles. Based on current Australian and American dietary guidelines, the recommendation for nuts, for adults, is ∼30 g (1 serving) on most days during a week (13, 86). The justifications and implications of nut consumption cut-offs described in points 1, 4, and 5 above is unclear. Besides categorization of nut intake based on amount, several studies have reported results based on the frequency of intake over a period of time, often per week (54, 104–107). This frequency approach is equally valid and important as it adds richness to the understanding of the relations between nuts/seeds and health. For example, such analysis can contrast any potential differences between individuals who had frequent intake of small amounts compared with those who consumed nuts/seeds irregularly but in large quantities, while achieving the same total weekly intake.
Nuts and seeds as part of multiple food groups in dietary indices
Nut intake has been associated with better overall diet quality (99, 101, 108). Therefore, in nutrition epidemiology, the examination of the relations between nuts or seeds and health outcomes should account for the overall diet quality in order to ensure the robustness of the relations. Diet quality can be assessed using several indices such as the Healthy Eating Index (HEI) (109) and the Mediterranean Diet Score (MDS) (110). One important consideration when adjusting for diet quality in nuts and seeds research, is the likelihood that they may already be included in the assessment of diet quality within a dietary index. This may partly explain why nut consumption has been associated with better overall diet quality (111–114). In the case of the HEI, intake of “Nuts, seeds, soy products” food group contributes to the “Total protein foods” and “Seafood & plant protein” HEI score components (109). These 2 score components also include several other food groups, hence isolating the diet quality scores contributed by nuts and seeds alone is not possible. The same applies to the MDS (110), hence it is not possible to adjust for diet quality scores from foods other than nuts and seeds. The implications of including nuts and seeds as part of a broader food classification and the implications of modifying the indices or removing a component, for the examination of the relations between nuts and seed intake and disease remains unknown and should be investigated.
Context of nut and seed consumption
To date, the understanding of how nuts and seeds are consumed in habitual diets is limited. For example, little is known about the context of nut and seed intake, which includes cultural influence (what nuts people eat, within meals, cooking techniques etc.), timing of ingestion (e.g. with meals or as snacks), and forms (e.g. peanut butter with toast or nuts and seeds in breakfast cereals). Although this knowledge does not directly contribute to the understanding of the association between nuts or seeds and disease, it will inform the development of practical strategies to promote the consumption of nuts and seeds.
Clinical trials
Although some of the considerations listed for observational studies may apply to clinical trials, the key considerations surround understanding the optimal portion size of nuts and/or seeds that provides the maximum benefits to a range of health outcomes. For example, the optimal amount that provides maximum risk of CVD reduction while minimizing the risk of weight gain from overconsumption. Also, if synergistic effects are seen when nuts and seeds are used in combination, the current recommendation may need to be revised to reflect the optimal amount for maximum health benefits. It is equally important to consider the ratio of nuts and seeds within the prescribed interventional portion size (115). However, based on their similar nutrient profiles, it is likely that they are interchangeable in the diet. Furthermore, a variety of nuts and seeds in line with dietary recommendations may provide the most optimal benefits to maximize the individual nutrients within each type. Providing consumers with more varieties within a whole diet may reduce monotony (115). To enable translation into practice, it is equally important to understand: 1) the availability and affordability of nuts and seeds, 2) the acceptability of the recommended amount, 3) the ease of incorporating nuts and seeds into habitual diets, and 4) the culture and habitual cuisine of study participants. All these factors will, in turn, determine the compliance to the recommendation for maximum health benefits. To summarize, findings from clinical trials that incorporate these considerations will provide evidence to support the development of specific dietary recommendations for nuts and seeds that are best suited to targeted populations.
Conclusion
Nuts are most often classified based on their culinary and nutrition definitions for the purpose of nutrition research. However, based on the comparable composition of nuts and seeds, it appears that research on the health benefits of nuts should expand its scope to also encompass seeds. This call is supported by the fact that nutritionally nuts and seeds are very similar, and there is available, albeit very limited, evidence to support the notion that seeds may provide very similar health benefits to nuts. However, further research is still needed to determine if nuts and seeds provide similar health benefits, and to elucidate the underlying mechanisms that explain the potential role of seeds in improving metabolic health. If the health benefits of seeds are confirmed and are indeed similar to those of nuts, future recommendations should consider nuts and seeds collectively. Since the gobal intake of nuts is generally low, incorporating seeds more broadly into dietary recommendations may provide more variety to consumers and thus may help increase intake for better health.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—ESG and S-YT: conceptualized and designed the research; ESG and S-YT: drafted the manuscript; ESG, RMD, SLT, RB, THTW, and S-YT: critically reviewed and edited the manuscript; S-YT: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This work did not receive any research funding.
Author disclosures: S-YT was previously involved in clinical studies that were funded by the Almond Board of California and the Californian Walnut Commission. S-YT, ESG, and RMD have received funding from the International Nut and Dried Fruit Council (INC). RB and SLT have received funding from the Almond Board of California. THTW reports no conflicts of interest. Funders had no role in the design, analysis, interpretation of data, or writing of articles.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: CVD, cardiovascular disease; EPIC, European Prospective Investigation into Cancer and Nutrition Study; HbA1c, glycated hemoglobin; HEI, Healthy Eating Index; MDS, Mediterranean Diet Score; NCI, National Cancer Institute; RCT, randomized controlled trial.
Contributor Information
Elena S George, Institute for Physical Activity and Nutrition (IPAN), School of Exercise and Nutrition Sciences, Deakin University, Geelong, Victoria, Australia.
Robin M Daly, Institute for Physical Activity and Nutrition (IPAN), School of Exercise and Nutrition Sciences, Deakin University, Geelong, Victoria, Australia.
Siew Ling Tey, Department of Human Nutrition, University of Otago, Dunedin, New Zealand.
Rachel Brown, Department of Human Nutrition, University of Otago, Dunedin, New Zealand.
Tommy Hon Ting Wong, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Pok Fu Lam, Hong Kong Special Administrative Region, People's Republic of China.
Sze-Yen Tan, Institute for Physical Activity and Nutrition (IPAN), School of Exercise and Nutrition Sciences, Deakin University, Geelong, Victoria, Australia.
References
- 1. Saaka M, Osman SM, Amponsem A, Ziem JB, Abdul-Mumin A, Akanbong Pet al. Treatment outcome of severe acute malnutrition cases at the tamale teaching hospital. Journal of Nutrition and Metabolism. 2015;2015:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Defourny I, Minetti A, Harczi G, Doyon S, Shepherd S, Tectonidis Met al. A large-scale distribution of milk-based fortified spreads: evidence for a new approach in regions with high burden of acute malnutrition. PLoS One. 2009;4(5):e5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yebyo HG, Kendall C, Nigusse D, Lemma W. Outpatient therapeutic feeding program outcomes and determinants in treatment of severe acute malnutrition in Tigray, northern Ethiopia: a retrospective cohort study. PLoS One. 2013;8(6):e65840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan S-Y, Tey SL, Brown R. Can nuts mitigate malnutrition in older adults? A conceptual framework. Nutrients. 2018;10(10):1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Souza RGM, Schincaglia RM, Pimentel GD, Mota JF. Nuts and human health outcomes: a systematic review. Nutrients. 2017;9(12):1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim Y, Keogh J, Clifton PM. Nuts and cardio-metabolic disease: a review of meta-analyses. Nutrients. 2018;10(12):1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coates AM, Hill AM, Tan SY. Nuts and cardiovascular disease prevention. Curr Atheroscler Rep. 2018;20(10):48. [DOI] [PubMed] [Google Scholar]
- 8. Tan S-Y, Georgousopoulou EN, Cardoso BR, Daly RM, George ES. Associations between nut intake, cognitive function and non-alcoholic fatty liver disease (NAFLD) in older adults in the United States: NHANES 2011–14. BMC Geriatrics. 2021;21(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cardoso BR, Tan S-Y, Daly RM, Dalla Via J, Georgousopoulou EN, George ES. Intake of nuts and seeds is associated with a lower prevalence of nonalcoholic fatty liver disease in US adults: findings from 2005–2018 NHANES. J Nutr. 2021;151(11):3507–15. [DOI] [PubMed] [Google Scholar]
- 10. U.S. Department of Health and Human Services/U.S. Department of Agriculture . Dietary Guidelines for Americans, 2020–2025. Washington (DC): 2020; 164. [Google Scholar]
- 11. Rehm CD, Drewnowski A. Replacing American snacks with tree nuts increases consumption of key nutrients among US children and adults: results of an NHANES modeling study. Nutrition Journal. 2017;16(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freisling H, Noh H, Slimani N, Chajes V, May AM, Peeters PHet al. Nut intake and 5-year changes in body weight and obesity risk in adults: results from the EPIC-PANACEA study. Eur J Nutr. 2018;57(7):2399–408. [DOI] [PubMed] [Google Scholar]
- 13. National Health and Medical Research Council . Australian Dietary Guidelines Summary. In: Department of Health and Ageing, (ed.). Canberra (Australia); National Health and Medical Research Council; 2013. [Google Scholar]
- 14. Ministry of Health . Eating and Activity Guidelines for New Zealand adults (updated 2020). Wellington (New Zealand); 2015. [Google Scholar]
- 15. Vainio H, Weiderpass E. Fruit and vegetables in cancer prevention. Nutr Cancer. 2006;54(1):111–42. [DOI] [PubMed] [Google Scholar]
- 16. Kendall CW, Josse AR, Esfahani A, Jenkins DJ. Nuts, metabolic syndrome and diabetes. Br J Nutr. 2010;104(4):465–73. [DOI] [PubMed] [Google Scholar]
- 17. Roberts TA, Cordier J-L, Gram L, Tompkin RB, Pitt JI, Gorris KMJ, Nuts, oilseeds, and dried legumes. In: Micro-Organisms in Foods 6. Boston, MA, USA: Springer;2005; 10.1007/0-387-28801-5_9. [DOI] [Google Scholar]
- 18. Rosengarten F Jr. The book of edible nuts. Mineola (NY): Dover Publications; 2004. [Google Scholar]
- 19. Boyd C. Nuts and Seeds, London: Foods Matter;2009:10–1., https://search.proquest.com/openview/96e7a91da433b00d0da15353c0a4255d/1?pq-origsite=gscholar&cbl=39578. [Google Scholar]
- 20. Phillips KM, Ruggio DM, Ashraf-Khorassani M. Phytosterol composition of nuts and seeds commonly consumed in the United States. J Agric Food Chem. 2005;53(24):9436–45. [DOI] [PubMed] [Google Scholar]
- 21. Vecka M, Staňková B, Kutová S, Tomášová P, Tvrzická E, Žák A. Comprehensive sterol and fatty acid analysis in nineteen nuts, seeds, and kernel. SN Applied Sciences. 2019;1(12):1–12. [Google Scholar]
- 22. Alasalvar C, Bolling BW. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br J Nutr. 2015;113(S2):S68–78. [DOI] [PubMed] [Google Scholar]
- 23. Clark MJ, Slavin JL. The effect of fiber on satiety and food intake: a systematic review. J Am Coll Nutr. 2013;32(3):200–11. [DOI] [PubMed] [Google Scholar]
- 24. Frame LA, Costa E, Jackson SA. Current explorations of nutrition and the gut microbiome: a comprehensive evaluation of the review literature. Nutr Rev. 2020;78(10):798–812. [DOI] [PubMed] [Google Scholar]
- 25. Coelho SB, de Sales RL, Iyer SS, Bressan J, Costa NM, Lokko Pet al. Effects of peanut oil load on energy expenditure, body composition, lipid profile, and appetite in lean and overweight adults. Nutrition. 2006;22(6):585–92. [DOI] [PubMed] [Google Scholar]
- 26. Iyer SS, Boateng LA, Sales RL, Coelho SB, Lokko P, Monteiro JBet al. Effects of peanut oil consumption on appetite and food choice. Int J Obes. 2006;30(4):704–10. [DOI] [PubMed] [Google Scholar]
- 27. Valsta LM, Jauhiainen M, Aro A, Katan MB, Mutanen M. Effects of a monounsaturated rapeseed oil and a polyunsaturated sunflower oil diet on lipoprotein levels in humans. Arteriosclerosis and Thrombosis: a Journal of Vascular Biology. 1992;12(1):50–7. [DOI] [PubMed] [Google Scholar]
- 28. Karatzi K, Stamatelopoulos K, Lykka M, Mantzouratou P, Skalidi S, Manios Eet al. Acute and long-term hemodynamic effects of sesame oil consumption in hypertensive men. The Journal of Clinical Hypertension. 2012;14(9):630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kris-Etherton PM, Hu FB, Ros E, Sabate J. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr. 2008;138(9):1746S–51S. [DOI] [PubMed] [Google Scholar]
- 30. Bullo M, Lamuela-Raventos R, Salas-Salvado J. Mediterranean diet and oxidation: nuts and olive oil as important sources of fat and antioxidants. Curr Top Med Chem. 2011;11(14):1797–810. [DOI] [PubMed] [Google Scholar]
- 31. U. S. Department of Agrigulture . USDA Food and Nutrient Database for Dietary Studies 2017–2018. Food Surveys Research Group Home Page. [Internet]. 2018. [Accessed 2021 Oct 4]. Available from: www.ars.usda.gov/nea/bhnrc/fsrg. [Google Scholar]
- 32. Neale EP, Tapsell LC, Guan V, Batterham MJ. The effect of nut consumption on markers of inflammation and endothelial function: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2017;7(11):e016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao Y, Xia J, Ke Y, Cheng J, Yuan J, Wu Set al. Effects of nut consumption on selected inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Nutrition. 2018;54:129–43. [DOI] [PubMed] [Google Scholar]
- 34. Tan SY, Dhillon J, Mattes RD. A review of the effects of nuts on appetite, food intake, metabolism, and body weight. Am J Clin Nutr. 2014;100(suppl_1):412S–22S. [DOI] [PubMed] [Google Scholar]
- 35. Guarneiri LL, Cooper JA. Intake of nuts or nut products does not lead to weight gain, independent of dietary substitution instructions: a systematic review and meta-analysis of randomized trials. Adv Nutr. 2021;12(2):384–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Akhlaghi M, Ghobadi S, Zare M, Foshati S. Effect of nuts on energy intake, hunger, and fullness, a systematic review and meta-analysis of randomized clinical trials. Crit Rev Food Sci Nutr. 2020;60(1):84–93. [DOI] [PubMed] [Google Scholar]
- 37. Casas-Agustench P, Lopez-Urirte P, Bullo M, Ros E, Gomez-Flores A, Salas-Salvado J. Acute effects of 3 high-fat meals with different saturation on energy expenditure, substrate oxidation and satiety. Clin Nutr. 2009;28:1, 39–45. [DOI] [PubMed] [Google Scholar]
- 38. Tapsell LC, Batterham MJ, Tan S-Y, Warensjo E. The effects of a calorie controlled diet containing walnuts on substrate oxidation during 8 hours in a room calorimeter. J Am Coll Nutr. 2009;28(5):611–7. [DOI] [PubMed] [Google Scholar]
- 39. Baer DJ, Gebauer SK, Novotny JA. Measured energy value of pistachios in the human diet. Br J Nutr. 2012;107(1):120–5. [DOI] [PubMed] [Google Scholar]
- 40. Baer DJ, Gebauer SK, Novotny JA. Walnuts consumed by healthy adults provide less available energy than predicted by the Atwater factors. J Nutr. 2016;146(1):9–13. [DOI] [PubMed] [Google Scholar]
- 41. Novotny JA, Gebauer SK, Baer DJ. Discrepancy between the Atwater factor predicted and empirically measured energy values of almonds in human diets. Am J Clin Nutr. 2012;96(2):296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Traoret C, Lokko P, Cruz A, Oliveira C, Costa N, Bressan Jet al. Peanut digestion and energy balance. Int J Obes. 2008;32(2):322–8. [DOI] [PubMed] [Google Scholar]
- 43. Tan S-Y, Mattes RD. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: a randomized, controlled trial. Eur J Clin Nutr. 2013;67(11):1205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tindall AM, Johnston EA, Kris-Etherton PM, Petersen KS. The effect of nuts on markers of glycemic control: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2019;109(2):297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim Y, Keogh JB, Clifton PM. Benefits of nut consumption on insulin resistance and cardiovascular risk factors: multiple potential mechanisms of actions. Nutrients. 2017;9(11):1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barbour JA, Howe PR, Buckley JD, Bryan J, Coates AM. Nut consumption for vascular health and cognitive function. Nutr Res Rev. 2014;27(1):131–58. [DOI] [PubMed] [Google Scholar]
- 47. Tan S-Y, Georgousopoulou EN, Cardoso BR, Daly RM, George ES. Associations between nut intake, cognitive function and non-alcoholic fatty liver disease (NAFLD) in older adults in the United States: NHANES 2011–14. BMC Geriatrics. 2021;21(1):313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tan S-Y, Tey SL, Brown R. Nuts and older adults’ health: a narrative review. Int J Environ Res Public Health. 2021;18(4):1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arab L, Ang A. A cross sectional study of the association between walnut consumption and cognitive function among adult US populations represented in NHANES. J Nutr Health Aging. 2015;19(3):284–90. [DOI] [PubMed] [Google Scholar]
- 50. Jackson CL, Hu FB. Long-term associations of nut consumption with body weight and obesity. Am J Clin Nutr. 2014;100(suppl_1):408S–11S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shao C, Tang H, Zhao W, He J. Nut intake and stroke risk: a dose-response meta-analysis of prospective cohort studies. Sci Rep. 2016;6(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sabate J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170(9):821–7. [DOI] [PubMed] [Google Scholar]
- 53. Bes-Rastrollo M, Sabate J, Gomez-Gracia E, Alonso A, Martinez JA, Martinez-Gonzalez MA. Nut consumption and weight gain in a Mediterranean cohort: the SUN study. Obes. 2007;15(1):107–16. [DOI] [PubMed] [Google Scholar]
- 54. Albert CM, Gaziano JM, Willett WC, Manson JE. Nut consumption and decreased risk of sudden cardiac death in the physicians' health study. Arch Intern Med. 2002;162(12):1382–7. [DOI] [PubMed] [Google Scholar]
- 55. Lee-Bravatti MA, Wang J, Avendano EE, King L, Johnson EJ, Raman G. Almond consumption and risk factors for cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10(6):1076–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kris-Etherton PM. Walnuts decrease risk of cardiovascular disease: a summary of efficacy and biologic mechanisms. J Nutr. 2014;144(4):547S–54S. [DOI] [PubMed] [Google Scholar]
- 57. Jafari Azad B, Daneshzad E, Azadbakht L. Peanut and cardiovascular disease risk factors: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020;60(7):1123–40. [DOI] [PubMed] [Google Scholar]
- 58. Becerra-Tomás N, Paz-Graniel I, WC Kendall C, Kahleova H, Rahelić D, Sievenpiper JLet al. Nut consumption and incidence of cardiovascular diseases and cardiovascular disease mortality: a meta-analysis of prospective cohort studies. Nutr Rev. 2019;77(10):691–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cooke JP, Tsao P, Singer A, Wang B-y, Kosek J, Drexler H. Anti-atherogenic effect of nuts: is the answer NO?. Arch Intern Med. 1993;153(7):896–9. [PubMed] [Google Scholar]
- 60. Guaraldi F, Deon V, Del Bo C, Vendrame S, Porrini M, Riso Pet al. Effect of short-term hazelnut consumption on DNA damage and oxidized LDL in children and adolescents with primary hyperlipidemia: a randomized controlled trial. J Nutr Biochem. 2018;57:206–11. [DOI] [PubMed] [Google Scholar]
- 61. Dikariyanto V, Berry SE, Pot GK, Francis L, Smith L, Hall WL. Tree nut snack consumption is associated with better diet quality and CVD risk in the UK adult population: National Diet and Nutrition Survey (NDNS) 2008–2014. Public Health Nutr. 2020;23(17):3160–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jenab M, Sabaté J, Slimani N, Ferrari P, Mazuir M, Casagrande Cet al. Consumption and portion sizes of tree nuts, peanuts and seeds in the European Prospective Investigation into Cancer and nutrition (EPIC) cohorts from 10 European countries. Br J Nutr. 2006;96(S2):S12–23. [DOI] [PubMed] [Google Scholar]
- 63. Wengreen H, Munger RG, Cutler A, Quach A, Bowles A, Corcoran Cet al. Prospective study of Dietary Approaches to Stop Hypertension–and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on Memory, Health and Aging. Am J Clin Nutr. 2013;98(5):1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Castro JM, Bellisle F, Dalix AM. Palatability and intake relationships in free-living humans: measurement and characterization in the French. Physiol Behav. 2000;68(3):271–7. [DOI] [PubMed] [Google Scholar]
- 65. Karimi B, Nabizadeh R, Yunesian M, Mehdipour P, Rastkari N, Aghaie A. Foods, dietary patterns and occupational class and leukocyte telomere length in the male population. American Journal of Men's Health. 2018;12(2):479–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ballesteros J-M, Struijk EA, Rodríguez-Artalejo F, López-García E. Mediterranean diet and risk of falling in community-dwelling older adults. Clin Nutr. 2020;39(1):276–81. [DOI] [PubMed] [Google Scholar]
- 67. Kim Y, Keogh JB, Clifton PM. Does nut consumption reduce mortality and/or risk of cardiometabolic disease? An updated review based on meta-analyses. Int J Environ Res Public Health. 2019;16(24):4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen BB, Han Y, Pan X, Yan J, Liu W, Li Yet al. Association between nut intake and non-alcoholic fatty liver disease risk: a retrospective case-control study in a sample of Chinese Han adults. BMJ Open. 2019;9(9):e028961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang S, Fu J, Zhang Q, Liu L, Meng G, Yao Zet al. Association between nut consumption and non-alcoholic fatty liver disease in adults. Liver Int. 2019;39(9):1732–41. [DOI] [PubMed] [Google Scholar]
- 70. Theodore LE, Kellow NJ, McNeil EA, Close EO, Coad EG, Cardoso BR. Nut consumption for cognitive performance: a systematic review. Adv Nutr. 2021;12(3):777–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Naghshi S, Sadeghian M, Nasiri M, Mobarak S, Asadi M, Sadeghi O. Association of total nut, tree nut, peanut, and peanut butter consumption with cancer incidence and mortality: a comprehensive systematic review and dose-response meta-analysis of observational studies. Adv Nutr. 2021;12(3):793–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dai Zhang CD, Zhou L, Li Y, Liu K, Deng Y-J, Li Net al. Meta-analysis of the association between nut consumption and the risks of cancer incidence and cancer-specific mortality. Aging. 2020;12(11):10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Long J, Ji Z, Yuan P, Long T, Liu K, Li J, Cheng L. Nut consumption and risk of cancer: a meta-analysis of prospective studies. Cancer Epidemiol Biomarkers Prev. 2020;29(3):565–73. [DOI] [PubMed] [Google Scholar]
- 74. Tucker LA. Consumption of nuts and seeds and telomere length in 5,582 men and women of the National Health and Nutrition Examination Survey (NHANES). J Nutr Health Aging. 2017;21(3):233–40. [DOI] [PubMed] [Google Scholar]
- 75. Jiang R, Jacobs DR Jr, Mayer-Davis E, Szklo M, Herrington D, Jenny NSet al. Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163(3):222–31. [DOI] [PubMed] [Google Scholar]
- 76. Tey SL, Brown RC, Chisholm AW, Delahunty CM, Gray AR, Williams SM. Effects of different forms of hazelnuts on blood lipids and alpha-tocopherol concentrations in mildly hypercholesterolemic individuals. Eur J Clin Nutr. 2011;65(1):117–24. [DOI] [PubMed] [Google Scholar]
- 77. Dhillon J, Tan SY, Mattes RD. Almond consumption during energy restriction lowers truncal fat and blood pressure in compliant overweight or obese adults. J Nutr. 2016;146(12):2513–9. [DOI] [PubMed] [Google Scholar]
- 78. Rock CL, Flatt SW, Barkai H-S, Pakiz B, Heath DD. Walnut consumption in a weight reduction intervention: effects on body weight, biological measures, blood pressure and satiety. Nutrition Journal. 2017;16(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Martínez-González MÁ, Corella D, Salas-Salvadó J, Ros E, Covas MI, Fiol Met al. Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol. 2012;41(2):377–85. [DOI] [PubMed] [Google Scholar]
- 80. Wu W-H, Kang Y-P, Wang N-H, Jou H-J, Wang T-A. Sesame ingestion affects sex hormones, antioxidant status, and blood lipids in postmenopausal women. J Nutr. 2006;136(5):1270–5. [DOI] [PubMed] [Google Scholar]
- 81. Pan A, Yu D, Demark-Wahnefried W, Franco OH, Lin X. Meta-analysis of the effects of flaxseed interventions on blood lipids. Am J Clin Nutr. 2009;90(2):288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Khalesi S, Irwin C, Schubert M. Flaxseed consumption may reduce blood pressure: a systematic review and meta-analysis of controlled trials. J Nutr. 2015;145(4):758–65. [DOI] [PubMed] [Google Scholar]
- 83. Del Gobbo LC, Falk MC, Feldman R, Lewis K, Mozaffarian D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am J Clin Nutr. 2015;102(6):1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Richmond K, Williams S, Mann J, Brown R, Chisholm A. Markers of cardiovascular risk in postmenopausal women with type 2 diabetes are improved by the daily consumption of almonds or sunflower kernels: a feeding study. ISRN Nutr. 2012;2013::626414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zaineddin AK, Buck K, Vrieling A, Heinz J, Flesch-Janys D, Linseisen J, Chang-Claude J. The association between dietary lignans, phytoestrogen-rich foods, and fiber intake and postmenopausal breast cancer risk: a German case-control study. Nutr Cancer. 2012;64(5):652–65. [DOI] [PubMed] [Google Scholar]
- 86. US Department of Health and Human Services, US Department of Agriculture . Dietary Guidelins for Americans 2020–2025. 8th Edition. [Internet]. [Accessed 2021 Feb 18]. Available from: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf. [Google Scholar]
- 87. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen Set al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet North Am Ed. 2019;393(10170):447–92. [DOI] [PubMed] [Google Scholar]
- 88. Levine AS, Silvis SE. Absorption of whole peanuts, peanut oil, and peanut butter. N Engl J Med. 1980;303(16):917–8. [DOI] [PubMed] [Google Scholar]
- 89. Grassby T, Picout DR, Mandalari G, Faulks RM, Kendall CW, Rich GTet al. Modelling of nutrient bioaccessibility in almond seeds based on the fracture properties of their cell walls. Food Funct. 2014;5(12):3096–106. [DOI] [PubMed] [Google Scholar]
- 90. Mandalari G, Faulks RM, Rich GT, Lo Turco V, Picout DR, Lo Curto RBet al. Release of protein, lipid, and vitamin E from almond seeds during digestion. J Agric Food Chem. 2008;56(9):3409–16. [DOI] [PubMed] [Google Scholar]
- 91. Kong F, Oztop MH, Singh RP, McCarthy MJ. Effects of boiling, roasting and frying on disintegration of peanuts in simulated gastric environment. LWT-Food Sci Technol. 2013:32–8. [Google Scholar]
- 92. Capuano E, Pellegrini N, Ntone E, Nikiforidis CV. In vitro lipid digestion in raw and roasted hazelnut particles and oil bodies. Food & Function. 2018;9(4):2508–16. [DOI] [PubMed] [Google Scholar]
- 93. Kumari S, Gray AR, Webster K, Bailey K, Reid M, Kelvin KAHet al. Does “activating’ nuts affect nutrient bioavailability?. Food Chem. 2020;319:126529. [DOI] [PubMed] [Google Scholar]
- 94. Gupta RK, Gangoliya SS, Singh NK. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol. 2015;52(2):676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Harttig U, Haubrock J, Knüppel S, Boeing H, on behalf of the EC. The MSM program: web-based statistics package for estimating usual dietary intake using the multiple source method. Eur J Clin Nutr. 2011;65(S1):S87–91. [DOI] [PubMed] [Google Scholar]
- 96. Tooze JA, Kipnis V, Buckman DW, Carroll RJ, Freedman LS, Guenther PMet al. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: the NCI method. Stat Med. 2010;29(27):2857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dekkers ALM, Verkaik-Kloosterman J, van Rossum CTM, Ocké MC. SPADE, a new statistical program to estimate habitual dietary intake from multiple food sources and dietary supplements. J Nutr. 2014;144(12):2083–91. [DOI] [PubMed] [Google Scholar]
- 98. National Cancer Institute . [Internet]. [Accessed 2021 Sep 30]. Available from: https://dietassessmentprimer.cancer.gov/. [Google Scholar]
- 99. Brown RC, Tey SL, Gray AR, Chisholm A, Smith C, Fleming Eet al. Nut consumption is associated with better nutrient intakes: results from the 2008/09 New Zealand Adult Nutrition Survey. Br J Nutr. 2016;115(1):105–12. [DOI] [PubMed] [Google Scholar]
- 100. O'Neil CE, Keast DR, Fulgoni VL, Nicklas TA. Tree nut consumption improves nutrient intake and diet quality in US adults: an analysis of National Health and Nutrition Examination Survey (NHANES) 1999–2004. Asia Pac J Clin Nutr. 2010;19(1):142–50. [PubMed] [Google Scholar]
- 101. O'Neil C, Nicklas T. Tree nut consumption is associated with better nutrient adequacy and diet quality in adults: National Health and Nutrition Examination Survey 2005–2010. Nutrients. 2015;7(1):595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hosseinpour-Niazi S, Hosseini S, Mirmiran P, Azizi F. Prospective study of nut consumption and incidence of metabolic syndrome: Tehran Lipid and Glucose Study. Nutrients. 2017;9(10):1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med. 1992;152(7):1416–24. [PubMed] [Google Scholar]
- 105. Fraser GE. Nut consumption, lipids, and risk of a coronary event. Clin Cardiol. 1999;22(S3):11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ellsworth JL, Kushi LH, Folsom AR. Frequent nut intake and risk of death from coronary heart disease and all causes in postmenopausal women: the Iowa Women's Health Study. Nutr Metab Cardiovasc Dis. 2001;11(6):372–7. [PubMed] [Google Scholar]
- 107. Hu FB, Stampfer MJ, Manson JE, Rimm EB, Colditz GA, Rosner BAet al. Frequent nut consumption and risk of coronary heart disease in women: prospective cohort study. BMJ. 1998;317(7169):1341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. King JC, Blumberg J, Ingwersen L, Jenab M, Tucker KL. Tree nuts and peanuts as components of a healthy diet. J Nutr. 2008;138(9):1736S–S40. [DOI] [PubMed] [Google Scholar]
- 109. Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JAet al. Update of the healthy eating index: HEI-2015. Journal of the Academy of Nutrition and Dietetics. 2018;118(9):1591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Panagiotakos DB, Pitsavos C, Stefanadis C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis. 2006;16(8):559–68. [DOI] [PubMed] [Google Scholar]
- 111. Griel AE, Eissenstat B, Juturu V, Hsieh G, Kris-Etherton PM. Improved diet quality with peanut consumption. J Am Coll Nutr. 2004;23(6):660–8. [DOI] [PubMed] [Google Scholar]
- 112. Bitok E, Jaceldo-Siegl K, Rajaram S, Serra-Mir M, Roth I, Feitas-Simoes Tet al. Favourable nutrient intake and displacement with long-term walnut supplementation among elderly: results of a randomised trial. Br J Nutr. 2017;118(3):201–9. [DOI] [PubMed] [Google Scholar]
- 113. Jaceldo-Siegl K, Sabaté J, Rajaram S, Fraser GE. Long-term almond supplementation without advice on food replacement induces favourable nutrient modifications to the habitual diets of free-living individuals. Br J Nutr. 2004;92(3):533–40. [DOI] [PubMed] [Google Scholar]
- 114. Tey SL, Brown R, Gray A, Chisholm A, Delahunty C. Nuts improve diet quality compared to other energy-dense snacks while maintaining body weight. Journal of Nutrition and Metabolism. 2011;2011:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tey SL, Brown RC, Gray AR, Chisholm AW, Delahunty CM. Long-term consumption of high energy-dense snack foods on sensory-specific satiety and intake. Am J Clin Nutr. 2012;95(5):1038–47. [DOI] [PubMed] [Google Scholar]