ABSTRACT
The production of plastic has dramatically increased in the last 50 y. Because of their stability and durability, plastics are ubiquitously incorporated in both marine and terrestrial ecosystems. Plastic is acted upon by biological, chemical, and physical agents, leading to fragmentation into small pieces [i.e., microplastics (MPs) or nanoplastics (NPs)], classified depending on their size. MPs range from 0.1 to 5000 μm and NPs are fragments between 0.001 to 0.1 μm. MPs and, especially NPs, are easily incorporated into living beings via ingestion. The penetration of MPs and NPs into the food system is an important issue, for both food security and health risk assessment. Ingestion of different MPs and NPs has been associated with different issues in the intestine, such as direct physical damage, increased intestinal permeability, diminished microbiota diversity, and increases in local inflammatory response. However, the potential harmful effects of low-dose dietary plastic are still unclear. Some evidence indicates that intestinal uptake of plastic particles is relatively low and is mostly dependent on the particle's size. However, other evidence highlights that NPs dysregulate key molecular signaling pathways, modify the gut microbiota composition, and may induce important epigenetic changes, including transgenerational effects that might be involved in the onset of many different metabolic disorders. Until now, experiments have been mostly performed on marine organisms, Caenorhabditis elegans, and mouse models, but some research indicates accidental plastic dietary consumption by humans, raising the issue of detrimental health effects of MPs and NPs. This review discusses the impact that MPs and NPs could have on the intestinal tract and the biodistribution and systemic, cellular, and molecular levels. Accumulated evidence of MPs’ effects on the human gut suggests that large exposure to MPs and NPs may have phenotypical untoward effects in humans, calling for urgent research in this field.
Keywords: biological effect, food, microplastics, nanoplastics, microbiota, epigenetic, inflammation, miRNA
Statement of Significance: This review discusses the impact that microplastics (MPs) and nanoplastics (NPs) have on the intestinal tract of animal models at the systemic, cellular, and molecular levels. Accumulated evidence of MPs’ effects on the human gut suggests that large exposure to MPs and NPs may lead to similar phenotypes and harmful effects in humans. Concerning gut health, gut microbiota, and their participation in some inflammatory phenotypes, we review the epigenetic and transgenerational effects of consuming MPs and NPs and their possible role in metabolic disorders, as well as a potential involvement in the dysregulation of some molecular pathways.
Introduction
In the last 50 y, the production of plastics has dramatically increased (1). For instance, in 2019, the worldwide plastic production was approximately 368 million metric tons (MT), with 57.9 million MT produced in Europe alone (www.statista.com). Annual waste production is projected to grow to 3.4 billion MT in the next 30 y (2). Approximately 40% of total plastic production is for packaging, mostly as polyethylene, polypropylene, polyethylene terephthalate, and polystyrene (PS) (3). Plastics help protect food items from damage and contamination, and ensure the freshness and safety of foods for human consumption. When no longer useful, plastics can be reused or recycled (4). Yet, a large percentage of plastic waste is lost and approximately 10 million tons reach the oceans each year (5, 6), a trend that increases steadily (7). Plastics, according to the US National Park Service, last in the environment for approximately 500 y, while small polymers, such as PS foam, last more than 5000 y (1).
Traditionally, plastics have been considered to be inert because of their large molecular size, and concerns about their potential detrimental effects were previously focused on their involvement as vectors of chemical contaminants, which may act as endocrine disruptors (8), the ability to adsorb many toxic compounds (9), or because their accidental intake could produce internal wounds, lesions, or digestive tract blockage—in turn, promoting a feeling of satiation resulting in starvation, weakness, and even death in many animals (particularly marine mammals) (10). However, the exposure of plastics to biological, chemical, and physical conditions (such as solar radiation, heat, winds, waves, and water) leads to their fragmentation into small pieces, termed microplastics (MPs; referring to plastic particles from 0.1 to 5000 μm) or nanoplastics (NPs; particles ranging from 1 to 100 nm). Plastic fragmentation also facilitates the release of chemical molecules attached to their surface [e.g., chemical contaminants and additives (11)]. In addition, antibiotics and pathogen micro-organisms adhere to plastics and the formation of microbial biofilms on the plastic surface may have an impact on human health (12, 13). Of note, plastics can break yet do not degrade, facilitating their uptake by organisms. MPs and NPs are easily incorporated into species (14, 15) via 1) oral exposure (16), 2) direct absorption (i.e., by phytoplankton) (14, 17, 18), 3) uptake by gills (e.g., crabs) (19), 4) adsorption through the epithelial layer (20), or 5) inhalation (21). Also, biomagnification of MPs and NPs in the trophic chain has been reported (15, 22). Until today, experiments have been mostly performed on marine organisms, but there is an increasing number of studies that use different animal models, indicating potential effects of MP and NP intake on human health.
This article reviews the ubiquity of MPs and NPs and their potential consequences on food safety and human health. The potential effects of both MPs and NPs on gut health are also discussed, including actions on the gut microbiota and their implication in the inflammatory framework. In particular, this review outlines that NPs may easily cross cellular barriers and reach different tissues, where they may induce different biological effects. Further, we display the epigenetic and transgenerational effects of consuming MPs and NPs and their implications in the etiology of metabolic disorders, focusing on molecular pathway dysregulation.
Current Status of Knowledge
Ubiquity of plastics and their impact on edible products
There is ample evidence on the ubiquitous presence of MPs and NPs in many environments, such as close to urban centers, terrestrial areas, freshwater environments, deep seafloor (23), and Antarctic (24) and Arctic (25) ice. Likely, atmospheric transport [i.e., through rain (26)] and deposition are relevant to MP and NP dispersion in all ecosystems. For instance, the Mediterranean Sea is one of the largest reservoirs of plastics worldwide, in which the release of MPs has been estimated to be between 70,000 and 130,000 microbeads per year (27). Also, the release of MPs into aquatic habitats has been estimated to be up to 2.3 billion microbeads each year in the United States alone (28). Accordingly, a study conducted by Jambeck et al. (29) calculated that 275 million MT of plastic waste were generated in 192 coastal countries in 2010, with 4.8 to 12.7 million MT entering the ocean. This is worrying, because MPs may break into NPs via biotic and abiotic ways [reviewed by Yee et al. (30)], and their long endurance in the environment may interact with living organisms—in turn, producing biological effects (31).
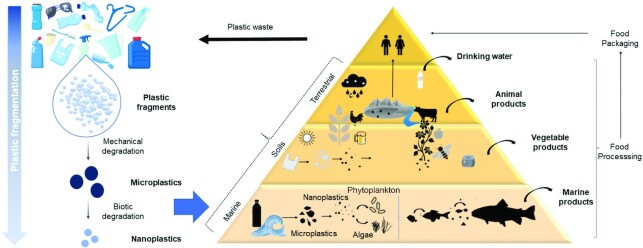
The continuous environmental exposure to plastic pollutants accumulated in the food chains—through agricultural soils or in marine food from water contamination, as well as consumption of products and packaging in plastics—creates new opportunities for plastic to reach the human body (Figure 1). Growing evidence supports the fact that MP pollution probably has negative effects on the marine ecosystem (32, 33) as well as on terrestrial ones (29), as it impacts soil quality and structure, sediments, freshwaters (34, 35), important biotas such as Lumbricus terrestris (36), and plant growth, plant development, and agricultural productivity (37). Indeed, plastic NPs may interact with the rhizodermis of roots and be taken up by plants (38): if they enter into the edible parts, they could enter into the animal and human food chains (39, 40).
FIGURE 1.
Plastic food-chain pyramid. The exposure of plastic debris to both chemical, biological and biotic factors causes mechanical degradation of large pieces of plastics, converting them to microplastics, which , produce nanoplastics. In marine ecosystems, marine organisms may take up microplastics into their organisms, which may remain for a long time in the gastrointestinal tract. The microplastics and nanoplastics were also observed in drinking water, edible fruits and vegetables, as well as in birds and animal farms. Therefore, the exposure of humans to micro- and nanoplastics is imminent, which can be observed at different levels, present in edible foods and in the trophic chain.
Published evidence reports the presence of MPs and NPs in many types of foods such as fruits and vegetables (41), marine products, livestock (e.g., chickens) (42), as well as in honey, beer, table salt (43, 44), and drinking water (45). As an example, Oliveri Conti et al. (41) reported the existence of MPs in different edible vegetables and fruits. In particular, from the analyzed samples, apples were the most contaminated fruits, while carrots were the most contaminated vegetables. In an attempt to explain these data, the authors hypothesized that fruits contain more MPs because of their high vascularization and complexity of the root system, as well as age as compared with vegetables. On the other side, the exposure of the UK population to seafood plastic was estimated to be between 1276 and 5828 particles per year (46).
In addition to the dietary intake, the accidental ingestion of plastic from food containers appears to be much higher than that from food (47, 48), because some oligomers can be released from the polymers that constitute food packages (48). For example, Mason et al. (49) recently demonstrated the presence of MPs in bottled water. Fragments were the most common plastic variety, followed by fibers. Polypropylene is the most frequent polymer, which matches a diffuse plastic used for the manufacture of bottle caps, suggesting that bottled water contamination comes, at least in part, from the packaging and/or the bottling process itself. In addition, Zuccarello et al. (45) estimated the daily average intake of MPs derived from bottled water to be approximately 3,350,208 particles/kg/body weight per day corresponding to 87.8 μg/kg/body weight per day. These data were subsequently validated by other studies, confirming that bottled water [reviewed by Novotna et al. (50)] and food trays (47) are an important source of MPs/NPs in humans. The presence of particles derived from food packages is an important issue that deserves to be addressed because of the increasing use of ready-to-use food products that are often sold or served in plastic containers or manipulated using gloves, increasing human exposure to different particles, namely phthalates and bisphenols (51–53).
MPs and food safety: potential impact on health
The presence of MPs/NPs in food is commonplace, but whether they affect human physiology is still an open question, although evidence collected in other species suggests that plastics do affect health. Plastics have been recovered in human feces. For instance, Schwabl et al. (54) examined 8 human stool samples in a prospective study. They found a median of 20 MPs (50–500 μm in size) per 10 g of stool, both polypropylene and polyethylene being the most abundant types. Another study performed in Beijing in 24 male students found that the abundance of MPs in feces ranged from 1 to 36 particles per gram with sizes between 20 and 800 μm (55).
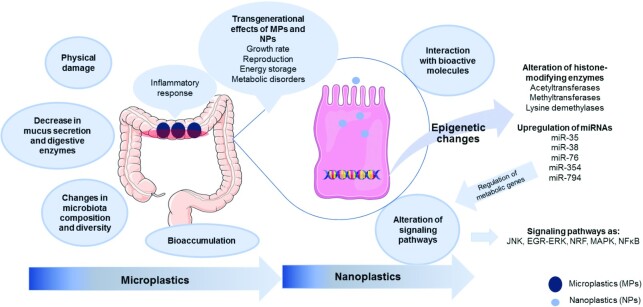
Previous data using a Caco-2 cell in vitro intestinal model suggest that approximately 0.8–7.8% of ingested MPs may remain in the gut (56) and might enter into the systemic circulation. To elucidate the potential effects of MPs/NPs on human health, we discuss their presence and effects on the gastrointestinal tract as described by in vivo studies. Untoward effects encompass the integrity of the gastrointestinal tract, modulation of gut microbiota, and increased inflammatory state. We also discuss the systemic, cellular, and molecular effects of plastics, mediated by MPs and NPs, highlighting their likely repercussions on health (Figure 2).
FIGURE 2.
The effect of MPs and NPs on intestinal health. The exposure of the gastrointestinal tract to MPs produce short-term physical damage at the lumen, but also alter the production of mucus secretion and decrease the digestive enzymatic activity. This issue, in turn, alters the composition of the microbiota, by affecting the diversity of bacteria. Finally, the combination of all these factors produces an inflammatory response, in which a long exposure to MPs may induce a chronic inflammatory state at the gut level. With regard to NPs, these particles enter into the cell through endocytosis, and within the cell they interact with a number of biological molecules, altering many signaling pathways. They also induce changes at epigenetic levels, and such effects are also observed in transgenerational offspring. Abbreviations: EGF-ERK, epidermal growth factor—extracellular signal-regulated kinase 1/2; JNK, c-Jun N-terminal kinase; MAPK, mitogen activated protein kinase; miRNA, microRNA; MP, microplastic; NP, nanoplastic; NRF, nuclear factor E2-related factor.
Plastics in the gastrointestinal tract and their involvement in gut health
Several papers report the presence of MPs in the gastrointestinal content of marine species (57, 58), such as the small-spotted catshark (59), piper gurnard, red mullet, lesser spotted dogfish, brown ray (60), Peter's fish, and silver scabbardfish (61). More specifically, Renzi et al. (62) evaluated the stomach contents of Sardinia pilchardus and Engraulis encrasicolus fished in the Adriatic Sea (located between the Italian peninsula and Balkans, within the Mediterranean Sea), in which such selected species are of great ecological and commercial interest, searching for MPs. In addition, Zitouni and colleagues (63) identified small MPs (1.2 to 3 μm) in the muscle and the gastrointestinal tract of adult benthopelagic fish (Serranus scriba) from the Mediterranean Sea, finding polyethylene-vinyl-acetate, polyethylene, and other plastic fragments as the most abundant plastic types and shapes.
Effects of plastics on gut epithelium
The presence of MPs in the gut has been linked to lacerations, abrasion, perforation, damage to internal tissues, as well as mucosal damage in different species. For instance, in intertidal fish, crypt and villi cell loss due to polystyrene-co-divinylbenzene physical abrasion (64) and cracking of villi and splitting of enterocytes in zebrafish Danio rerio and nematode Caenorhabditis elegans have been reported (65). In this context, the shape and size of MPs (fibers, fragments, or beads) are crucial. In any case, intestinal damage causes malnutrition by limiting nutrient assimilation and absorption, with a negative impact on health that includes increased permeability and intestinal toxicity (58).
Different animal studies indicate that the accumulation of plastics in the gastrointestinal tract leads to local inflammation. For instance, Li et al. (66) found that sustained intake of high doses of polyethylene MPs in C57BL/6 mice increased inflammation and the expression of typical proinflammatory genes, such as Toll-like receptor 4 (TLR4), Jun proto-oncogene, AP-1 transcription factor subunit (AP1), and interferon regulatory factor 5 (IRF5) in the intestine (colon and duodenum). This is because MPs are transported by microfold (M) cells into mucosa-associated lymphoid tissues, and to activate the immune response depends on the molecules carrying the MPs, which initiate humoral and cellular responses. This response also produces local inflammation, releasing typical proinflammatory cytokines and activating several inflammatory pathways. Also, MPs can reduce the intestinal mucus secretion and cause damage to the intestinal barrier of rodents’ gut (67), affect defecation rhythm in C. elegans (68), or can cause leukocyte infiltration, hyperemia, as well as crypt and villi cell loss in fish (64). Further, Jin and collaborators (67) exposed mice to 5 μm pristine and fluorescent PS MPs for 6 wk. This resulted in a reduction in the intestinal mucus secretion and damage of the intestinal barrier function. The reduction in intestinal mucosa secretion can be induced not only by the contact and alteration of the intestinal glands but also by the mechanical damage caused by the MPs to the glands, exerting a “plug effect” that prevents a normal secretion.
Interaction of MPs and NPs with the gut microbiota
Research connecting MPs and NPs with the gut microbiota is still scant and only a few studies performed in animal models have tried to decipher the interplay among these agents. This is probably because of the current low-resolution analytical methods that quantify MPs and NPs in human stools collected in observational and animal studies. The available evidence does not prove that MPs cause gut dysbiosis, but most data show that MPs alter alpha and beta diversity, and some specific linking on the relative abundance of certain phyla (69).
With regard to marine species, Jin et al. analyzed the effect of PS MPs on gut microbiota of zebrafish after 14 d of exposure. The authors found a reduction in the abundance of Bacteroidetes and Proteobacteria, while the abundance of Firmicutes was increased. In general, the richness and diversity of microbiota were changed, suggesting that exposure to MPs can strongly alter the composition of gut microbiota (70). Another study conducted by Yan et al. (71) in marine medaka fish confirmed that MP treatment reduced the diversity and abundance of intestinal microbiota, as shown by changes in Proteobacteria abundance. In addition, Auguste and colleagues (72) exposed Mytilus galloprovincialis to amino-modified nanopolystyrene and found an increase in Arcobacter-like, Psychrobium, and Vibrio, and a decrease in other genuses, such as Shewanella or Mycoplasma.
Studies in mice also found a modulation of the diversity of gut microbiota. For instance, Lu and collaborators (73) exposed mice to 1000 μg/L of 0.5- and 50-μm PS MPs for 5 wk and found decreased body, liver, and lipid weights; decreased relative abundances of Firmicutes and α-Proteobacteria; and significant changes in the richness and diversity of the gut microbiota composition. In addition, Jin et al. (67) reported that the content of Actinobacteria decreased significantly in mice treated with PS MPs. At the genus level, a total of 15 types of bacteria changed significantly after administration. Moreover, Li et al. (66) revealed a significant increase in Staphylococcus abundance alongside a significant decrease in Parabacteroides abundance, as a consequence of polyethylene MP ingestion. Finally, Huang et al. (74) studied the effect of polyethylene MPs on microbial community diversity and structure of human gut microbiota using cultures of human fecal samples. It appears that polyethylene MPs enhance the proportion of Clostridium, Bacteroides, and Escherichia and interfere with the metabolic pathways of gut microbiota.
Mechanisms of MP/NP actions in gastrointestinal inflammatory diseases
Several studies have explored the effects of MPs and NPs in the gastrointestinal tract. As noted, MPs can negatively act at different levels to increase the inflammatory state in the gastrointestinal tract. First, MPs might produce physical damage to the intestine, leading to increased permeability and local inflammation. The induction of cell abrasion may promote the accumulation of oxidative stress, through the release of intracellular reactive oxygen species (ROS) (75). This results in an increase in the inflammatory response at the injury site (67, 69). Chronic inflammation led to an intestinal barrier dysfunction and may increase the incidence of chronic inflammatory conditions (76, 77). In addition, the accumulation of MPs might alter the gut microbiota composition and induce dysbiosis (67, 69). A better explanation for this phenomenon is that MPs and NPs could produce changes in microbial diversity, but also activate the proliferation of harmful bacteria and inhibit the growth of beneficial microorganisms. In addition, MPs trigger the formation of biofilms, in which pathogenic microorganisms find adaptive niches. In general terms, the alteration of gut microbiota composition is closely associated with many physiological processes, such as inflammation (77, 78).
Li et al. (66) evaluated the effect after the administration of polyethylene MPs in a C57BL/6 mouse model. Treatment with a high concentration of MPs increased serum concentrations of IL-1α in treated mice and decreased the percentage of T-helper 17 (Th17) and T-regulatory (Treg) cells among CD4+ cells. The intestine (colon and duodenum) showed higher expression of proinflammatory cytokines, suggesting that MP intake leads to an inflammatory state. At the cellular level, Merkley et al. (79) showed that the phagocytosis of MPs by macrophages induces a metabolic shift toward glycolysis and a reduction in mitochondrial respiration that was associated with an increase in cell surface markers CD80 and CD86 and cytokine gene expression associated with glycolysis, indicating that macrophage phagocytosis of MPs alters cellular metabolism. However, macrophages cannot degrade PS MPs.
Systemic actions of plastics
The biological actions of plastics are closely associated with their size. The large size of MPs makes it difficult to cross the gut barrier and produce cellular internalization (80, 81): only particles <1.5 μm are able to enter capillaries and penetrate deep into organs (82). In this regard, De Jong et al. (83) undertook a kinetic study to elucidate the distribution pattern of plastics in rats using gold nanoparticles. The smaller (10 nm) particles were distributed in various organs, including blood, liver, spleen, kidney, testis, thymus, heart, lung, and brain, whereas the larger (100–250 nm) particles were only detected in blood, liver, and spleen.
Apart from the mechanical damage that MPs produce in the gastrointestinal tract and the consequent malnutrition, these particles may enter the lymphatic system, triggering a localized immune response and release of constituent monomers, toxic chemicals added during plastic production, or pollutants adsorbed from the environment (84). These phenomena may induce a localized inflammatory response, which could affect the surrounding tissue and induce important reactions therein. As previously described, MP consumption by mice induces gut dysbiosis and produces various metabolic alterations (67); such results were also confirmed in fish (85–87). Despite growing concern raised by the ingestion of MPs by humans, the true extent of the effect of plastic intake on human physiology is still unclear. Without profound knowledge of retention and egestion rates of MPs by humans, it is difficult to deduce ecological consequences. Yet, De Jong et al. (83) confirmed the presence of MPs in blood, liver, and spleen in rats after they were intravenously injected with these agents in the tail vein. Also, Deng et al. (88) found that MPs (5 μm and 20 μm) may be distributed and accumulate in the liver, kidney, and gut of mice, but such distribution pattern strongly depends on the size of MPs.
In contrast, smaller particles are able to reach different tissues such as blood, liver, spleen, kidney, testis, thymus, heart, lung, and brain, suggesting the wider biodistribution that NPs may have compared with MPs (83). NP particles are able to permeate the gut epithelium and diffuse through cell membranes by endocytosis (86) and induce inflammatory responses in the intestinal walls (see above). In addition, NPs are prone to interact with a number of biomolecules such as proteins (89), carbohydrates, and nucleic acids, due to their surface chemistry and charge of polymers. This interaction might be potentially interesting, because it may alter the secondary, tertiary, and quaternary structures of these biomolecules and, hence, inactivate/activate them in an inadequate way, in turn altering their cellular functions.
Cellular bioavailability
Humans can internalize MPs and NPs through ingestion, inhalation, and to a lesser extent, skin contact. However, whether there is affinity for 1 type of polymer or another remains to be determined. In any case, ingestion of MPs and NPs is considered to be the main pathway of plastics assimilation. The available evidence shows that bioavailability of MPs/NPs depends on their size, shape, density, cellular fate, as well as abundance (90) and other variables, such as the surface charge (electrostatic potential charge), functionalization, hydrophobicity, and protein corona (84). These physico-chemical properties of NPs and MPs are very important, because they determine the kind of interaction at the cellular and molecular levels, once entering the human body. Accordingly, the most accepted mechanisms of cellular internalization are endocytosis, transcytosis, or adsorption (91, 92), but plastic may also enter into the intestinal epithelial cells using a transporter or permeate through the lipid membrane, even if this process is size-dependent (93, 94).
Several pieces of evidence indicate that NPs enter easily into the cell (92) and accumulate into the lysosomes (91). Conversely, larger particles are not easily internalized and may also exit from the lysosomes to intracytoplasmic vacuoles or to the cell cytoplasm. This effect may disrupt lysosomal-induced mitochondrial depolarization and induce apoptosis (95). Of note, the translocation across the mammalian gut into the lymphatic system of various MP sizes is less studied but appears to be most efficient when particles are approximately 0.1 to 150 μm and depends on the species (96). It is likely that MPs are translocated through M cell–rich Peyer's patches in the intestine. The unabsorbed particles could collide with the gut barrier, where they may induce gut dysbiosis and metabolic alterations (58, 67). The proposed model for the absorption of particles in the gastrointestinal tract is passive diffusion. When MPs and NPs reach the intestinal mucosa, they interact with M cells, which are found in the gut- and mucosa-associated lymphoid tissue, and pass through them, towards the lymphatic system and blood vessels, before being excreted in urine. A small percentage of particles is retained in the body (97). The NP–cell interaction is a topic of interest, especially the interaction of NPs with immune cells, such as T cells and macrophages in the mucosa-associated gastrointestinal tract (98, 99).
There is some information on the fate of plastics in different species. For instance, Bayo et al. (100) found (by FTIR (Fourier transform infrared) spectroscopy) MP and fiber particles in gilthead seabream (Sparus aurata L.), both in the intestine and stomach content. In addition to the gastrointestinal tract, MPs were also observed in the bloodstream and in other tissues (i.e., muscle, liver, and kidneys), but to a lesser extent. With regard to mammals, the evidence on tissue biodistribution is still scarce (Table 1), but in general, the smaller the particle size the more biodistribution there is, in a dose-dependent manner. For example, Keinänen et al. (101) used positron emission tomography (PET) to evaluate the biodistribution of PS radioplastics in C57BL/6J mice at 6, 12, 24, and 48 h after ingestion. Their results show that PS particles remain in the gastrointestinal tract and do not accumulate in any organ. In contrast, Walczak et al. (102) administered a high dose and found a 0.38–0.74% uptake of PS particles 6 h after administration to rats. In agreement with this finding, the oral administration of 64 Cu-labeled PS to mice revealed that, after 1 h of administration, PS reaches the liver, heart, and lungs in addition to other organs, attaining maximal bioaccumulation after 6 h of ingestion (103). Further, PS MPs with a 5-μm diameter at a concentration of 0.1 mg/d could accumulate in different tissues (e.g., liver and gut), leading to alterations in different oxidative stress and lipid metabolism parameters (88).
TABLE 1.
Evidence of Biodistribution of Plastics in Mammals.
| Rodent Model | Plastic Type and Size | Dose | Technical Analysis | Biodistribution | Reference |
|---|---|---|---|---|---|
| C57BL/6J mice | PS particles with chelator desferrioxamine radiolabeled–bearing particles with zirconium-89 (89Zr; t1/2 ∼ 3.3 d); size: 20 nm, 220 nm, 1 μm, and 6 μm | Single dose of 0.1 mg ⋅ 0.1 mL−1 and observation after 6, 12, 24, and 48 h | PET | Plastics remain stable in the GI tract; do not accumulate to any substantial degree in any organ | (101) |
| Fischer 344 rats (male) | Amine- and carboxyl-modified PS NPs; size: 50 nm | Single dose of 12.5 mg ⋅ kg body weight−1 | Fluorescent microscopy | ↑↑ Stomach wall, small and large intestinal wall; present in kidney, spleen, testis, heart (0.2 to 1.7% of the administered dose) | (102) |
| ICR mice | Fluorescent and pristine PS MPs at 5 μm and 20 μm | Single and sustained dose of 0.1 mg/d by oral gavage (1.46 × 106 items for 5-μm PS MPs and 2.27 × 104 items for 2-μm PS MPs | Digestion of tissues and determination of concentration by fluorescent spectrophotometer | MPs accumulated in liver, kidney, and gut, with a tissue-accumulation kinetics and distribution pattern dependent on the MP particle size | (88) |
| BALB/c nude mice | PS with [64Cu] Cu-DOTA | Single dose of 57.8 μg PS/100 μL | PET was acquired at 1, 6, 12, 24, and 48 h after oral administration | Stomach, small intestine, large intestine, liver significant | (103) |
| C57BL/6J mice | PS particles (50, 500, 5000 nm) | Single dose of 20 mL · kg body weight−1 monitored at 1, 6, 12, and 24 h at concentration of 2.5–500 mg · kg body weight−1 · d−1 | In vivo Multispectral FX PRO system (Bruker BioSpin) | Single exposure → only detected in the intestine | (99) |
| C57BL/6J mice | PS particles (50, 500, 5000 nm) | 28-d chronic administration by oral gavage, 20 mL · kg body weight−1 · d−1 at a concentration of 2.5–500 mg · kg body weight−1 · d−1 | In vivo Multispectral (MS) FX PRO system (Bruker BioSpin) | Chronic exposure: detected in spleen, kidneys, heart, liver, lungs, blood, testis and epididymis, brain, and thigh bone | (99) |
| Wistar rats (female) | Carboxylated PS NPs (500 nm) | 12.5 mg ⋅ kg body weight−1 · d−1 for 5 d | Fluorescence microscopy | Presence in GI tract, liver, heart, kidney, and spleen | (104) |
| Wistar rats (male) | PS particles labeled with FITC (1 μm) | Single administration of 3.7 ⋅ 109 particles and measure after 6 h of administration | Fluorescence microscopy | Presence in lymph fluid; dependent on dose and age of animal | (105) |
| Sprague-Dawley rats (female) | PS particles (60 nm) | 5-d oral dose by gavage at 14 mg ⋅ kg body weight−1 | Gel permeation chromatography and fluorescence microscopy | Uptake in the small intestine (60%) occurred through the Peyer's patches; large intestine (mostly) lymphoid sections | (106) |
Abbreviations: DOTA, metal-chelating macrocycle 2-(4-aminobenzyl)-1,4,7,10-tetraazacyclododecane-1,4,7, 10-tetraacetic acid; FITC, fluorescein isothiocyanate; GI, gastrointestinal; MP, microplastic; NP, nanoplastic; PET, positron emission tomography; PS, polystyrene; ↑↑, increase.
Interactions of MPs and NPs with biological molecules
The biological effects of MPs and NPs are not fully elucidated, but much research indicates potentially harmful actions on human health. Several studies showed that exposure to MPs and NPs activates several pathways and genes implicated in inflammation, growth inhibition, alterations in reproduction, intestinal damage, oxidative stress, endocrine disruption, and epigenetic alterations (107–110). Indeed, these harmful effects have been linked to several phenotypic diseases, such as dysregulation of gastrointestinal motility, mucin secretion, and chloride ion and water transport in the mid-colon, inducing chronic constipation in ICR mice (111) and intestinal inflammatory diseases in many animal models (87). In humans, the mechanisms of actions responsible for these effects are still unknown. Yet, MPs appear to produce local gut alterations (58), whereas NPs (due to their size) may enter into the cell and alter its biochemistry. Data on the occurrence of NPs in the ecosystem are scant, maybe due to the lack of appropriate laboratory methods to detect and characterize them.
Accordingly, recent studies have indicated that PS MPs easily interact with cell membranes, decrease cell viability, and increase intracellular ROS concentrations (91). In this respect, one of the most investigated plastics is PS. In addition, PS-NP beads of 70 nm become thoroughly distributed in tissues and gut of zebrafish after 7 d of exposure, producing noxious effects such as an inflammatory response, increased oxidative stress, metabolic alterations, and hepatic lipid accumulation (57). Further, PS NPs may also alter cellular machinery by affecting amino acid, bile, and lipid metabolisms and regulate genes involved in stress response, zymogen granules, c-Jun N-terminal kinase (JNK) signaling pathways, sterol transport, or the epidermal growth factor–extracellular signal-regulated kinase 1/2 (EGF-ERK1/2) pathway (112). In addition, PS NPs appear to activate the nuclear factor E2–related factor (Nrf2) signaling pathway (113). Treatment with PS MPs of nondifferentiated Caco-2 cells modulates the expression of genes involved in NF-κB–associated pathways, mitogen-activated protein kinase (MAPK) signaling, cytokine-cytokine receptor interaction, and Toll-like receptors (114).
Even though the potential size effects of plastics are extensively described in the literature (99), some authors downplay the harmful consequences of MP and NP consumption when placed in the context of dietary doses. Some in vitro studies indicated that only a minor fraction of particles are taken up by human cells (80), being the uptake size-dependent (115). In addition, it appears that only excessively (i.e., far beyond realistic dietary exposures) high concentrations induce cytotoxic effects (115). Indeed, chronic exposure of undifferentiated Caco-2 cells to PS at dietary concentrations produced minor changes and no DNA damage and oxidative stress was observed (116). In addition, some in vivo studies did not find any potential deleterious effect of PS. For instance, oral administration of 4.55 × 107 pristine, with different sizes (1, 4, and 10 μm) of PS particles at 10 mL/kg/body weight, to mice did not produce any histological lesions or inflammatory response.
Epigenetic influence of MPs and NPs and their potential transgenerational effects
As mentioned, plastics may have unhealthy effects on gut health and related physiology. Very recently, many authors have reported an epigenetic effect of NP exposure (e.g., the activation of key proteins related to epigenetic processes) (58, 117). However, many of these changes are related to epigenetic alterations that are mainly short-term, such as chromatin accessibility and microRNA (miRNA) modulation. Until now, long-term epigenetic changes (e.g., DNA methylation) have not been reported, suggesting that large exposures in terms of amount and time are necessary to produce long-term epigenetic modifications. The available data on NPs and epigenetics suggest that NPs may indeed modulate the epigenome. However, these data should be interpreted with caution because there are few studies in mammals.
For instance, Yu and colleagues (110) exposed C. elegans to NPs for 72 h. The authors observed that the expression of cell death protein type 3 (Ced3) was increased across generations and was regulated by hypomethylation in the promoter region of Ced3 after maternal NP exposure. NP exposure also reduced the expression of other epigenesis-related genes, such as homoserine O-acetyltransferase (Met2), histone-lysine N-methyltransferase (Set2), and lysine-specific histone demethylase type 1 (Spr5). Transgenerational effects were not observed. This study was also confirmed by other experiments conducted by Wang et al. (118). These authors exposed C. elegans to PS nanoparticles and found decreased expression of Met2. RNA interference (RNAi) knockdown of Met2 suppressed NP toxicity, suggesting that Met2 may elicit a protective response to NP exposure. The authors also elucidated the molecular basis for Met2-mediated methylation regulation, pointing to a close interaction between chromatin remodulation and NPs (118). In addition, Liu et al. (119) found that exposure to PS NPs in C. elegans increased expression of Cbp-1encoding an histone acetyltransferase, suggesting that CBP-1–mediated histone acetylation regulation reflects a protective response to NPs, by modulating functions of insulin and p38 MAPK signaling pathways and Dauer larva development regulatory growth factor daf-7 (DAF-7)/transforming growth factor β (TGF-β) and JNK/MAPK signaling pathways.
The profiles of certain miRNAs might be affected by MP/NP intake. miRNAs regulate gene expression, mostly by inducing mRNA degradation and translational repression (120). miRNAs are involved in the regulation of essential physiological processes via regulation of gene expression at the post-transcriptional level (121). The active secretion of miRNAs by the cells has been suggested to mediate intercellular communication (122). However, the biological significance, the factors that modulate their secretion, as well as the mechanisms by which they reach the target tissue remain elusive. However, miRNAs are present in the systemic circulation and are modulated by dietary components (123, 124). Exogenous miRNAs have also been found in plasma (125, 126).
Evidence of the effects of NPs on miRNA is increasing. However, the mechanisms responsible for NP toxicity on miRNAs are still unclear. Qu and colleagues (127), after exposure of L1-larvae to adult day-3 C. elegans, found that 7 miRNAs, including miR-38, miR-76, or miR-794, were dysregulated by nanopolystyrene in a dose-dependent manner. Overexpression of miR-35, miR-38, or miR-354 induced resistance to nanopolystyrene toxicity, suggesting a protective response to nanopolystyrene triggered by these miRNAs and performed by various biological processes and signaling pathways. An additional study conducted by Yang and collaborators (117) found, in C. elegans, an overexpression of miR-38 caused by exposure to nanopolystyrene. This miRNA works by increasing resistance to NP toxicity and by regulating several epigenetic-related genes such as Nhl2, Ndk1, or Wrt3, among others. Additionally, after exposure to NPs, miR-76 appears to regulate the expression of Glb10: both increase resistance to NP toxicity (128). In addition, Qiu et al. (129) found that C. elegans intestinal overexpression of miR-794 caused susceptibility to nanopolystyrene toxicity. This can be attributed to miR-794–regulated expressions of Daf-16, Skn1, and Mdt15—that is, genes that are linked to insulin and p38 MAPK signaling pathways in nematodes. Therefore, miRNA expression appears to restrain or modulate the toxic effects that NPs produce on the epigenome: miRNAs are emerging as an “immune system” that protects from NP toxicity via alteration of the expression of several genes. However, many more studies are needed to determine the tolerance to NP exposure, the role of miRNAs versus NPs, and the effects of large and prolonged exposures on the epigenome's modulation.
Given that epigenetic changes may be inherited, transgenerational epigenetic inheritance may be affected by MPs/NPs. Transgenerational epigenetic inheritance has been indeed observed in many organisms, including C. elegans (130–132), suggesting a link with the ecological impact of plastics. This issue is still vague in mammals, particularly humans, and additional studies are needed. However, MP fragments of 5–10 μm in size are able to reach the human placenta and, depending on different physiological and genetic conditions, the particles can enter all placental portions (i.e., maternal, fetal, and amniochorial membranes), where they might affect the developing fetus (133). Even though there is still no evidence of a transgenerational effect in humans, maternal transfer of MPs to the developing fetus has been demonstrated in exposed laboratory animals. For example, experiments performed in Daphnia magna described that MP exposure negatively affected several parameters, such as parental mortality, growth, several reproductive indices, and population growth rate (132, 134). Experiments in zebrafish using PS-MP exposure for 21 d resulted in notable MP accumulation in adult fish intestines, but no transgenerational effects were observed (131). Additional analysis in C. elegans showed that PS-MP exposure was related to transgenerational decreased head thrash and body bends (130).
With regard to mouse models, the administration to pregnant mice of PS MPs resulted in altered lipid metabolism—namely, serum triglyceride, total cholesterol, HDL cholesterol, and LDL cholesterol—in the offspring, suggesting a potential relation between MPs and the risk of metabolic disorders in the following generation (135). In addition, Luo et al. (136) evaluated maternal PS-MP exposure in mice during gestation and lactation. The authors observed that maternal MP exposure during gestation and lactation affected glucose and lipid metabolic variables in the 2 subsequent generations, suggesting increased cardiometabolic risk.
With regard to exposure to NPs, transgenerational effects were also observed. Yu et al. (110) exposed C. elegans to NPs over 5 generations. Total litter size was significantly reduced across all offspring generations. The authors also observed chromosomal aberrations in oocytes. In addition, Sun et al. (137) used pristine and amino-modified nanopolystyrene to determine their transgenerational toxicity in C. elegans. Exposure to pristine nanopolystyrene caused a decrease in reproductive capacity and damage on gonad development in successive generations. In contrast, exposure to amino-modified nanopolystyrene caused reproductive capacity and gonad development toxicity in the first generation, demonstrating that a surface modification of NPs elicits a peculiar transgenerational effect. Indeed, long-term exposition to NPs was also related to transgenerational reproduction decline (110).
Many organisms are being exposed to environmental stress and chemical contaminants over several generations. The adaptation to such stress is crucial to survival and maintenance of the protective phenotype over generations. Some of these strategies are related to epigenetic changes that are important to protect against toxic effects. The data presented here highlight the importance of such epigenetic changes to better understand the multigenerational effects of MPs and NPs, to further evaluate long- and short-term exposures, and to understand the mechanisms involved in MP and NP actions on health. The interconnection of MPs and NPs with reproduction and growth suggests malnutrition and, probably, the effect of some endocrine disruptors carried by MPs and NPs.
Potential effects of plastics on chronic disease–related phenotypes
Based on the evidence found in the literature, MPs and also NPs are likely to enter the human body. Experiments in mice have reported that the consequence of MP and NP ingestion might impact a number of health aspects, causing inflammation, genotoxicity, oxidative stress, apoptosis, as well as necrosis. All these effects cause degenerative diseases such as cardiovascular disease, cancer, autoimmune diseases, and intestinal disorders (e.g., inflammatory bowel disease and gut dysbiosis) (138–140). Zheng et al. (141) evaluated the sensitivity to MPs of mice with a chronic disease (i.e., acute colitis). The authors observed that the exposure to PS MPs induced inflammatory effects, affected some hepatic metabolites, and exaggerated the effect of dextran sodium sulfate (an agent used to induce acute colitis), which was accompanied by other metabolic lipid and inflammatory disorders. Therefore, this study suggests that exposure to MPs of populations with chronic diseases might render them more sensitive to the linked phenotypes. In addition, chronic exposure to MPs may induce disruption of the symbiosis between the host and the natural community of the gut microbiota, probably triggering gut dysbiosis [reviewed in (69)]. In addition, some authors suggest a possible link between MP and NP consumption and obesity as modulated by the gut microbiota, alteration of lipid and energy metabolism, oxidative stress, and increased adipocyte differentiation (8). Current knowledge gaps include time, quantity, and quality of exposure; toxicity; and their relationship with adverse health effects.
MPs/NPs as vectors of other diseases
Several studies reported that plastic fragmentation contributes to releasing different hazardous chemical contaminants carried in the plastics, as they have a large capacity to bind to many hydrophobic organic pollutants. For instance, phthalates and bisphenols are widely known as endocrine disruptors, which also may influence multiple endocrine-related pathways (142). For instance, bisphenol A (BPA) is widely used as a building block of polycarbonate plastics (often used for food and beverage storage) and is also a component of epoxy resins that are used to seal food and beverage containers (143). BPA (recognized as an endocrine disruptor) can generate disorders such as low sex-specific neurodevelopment, immune toxicity, and neurotoxicity via interference with cellular pathways (142). The fragmentation of plastics increases the surface area available to adsorption of BPA, thereby increasing the bioavailability and effects of this compound (92). Phthalates are also known by their impact on male fertility (144) and pregnancy loss (145).
MPs are also able to bind to hydrophilic organic pollutants such as perfluoro-octanesulfonate, perfluoro-octanesulfonamide, and many types of antibiotics (e.g., amoxicillin, tetracycline, or ciprofloxacin), which may have a potential impact on antibiotic resistance [reviewed by Yu et al. (146) and Joo et al. (147)]. Several studies have demonstrated that MPs can interact with and bind heavy metals, then release them. Therefore, the interaction between heavy metals and MPs may lead to serious health risks. Indeed, heavy metals such as chromium, zinc, lead, aluminum, or mercury were also observed on the surface of MPs, which explains the fast adsorption of such metals after a few days of exposure (148). Additionally, it has been reported that marine MPs may harbor diverse microbial species because of the ability of some microorganisms (e.g., bacteria and archaea) to aggregate on different kinds of surfaces. Examples include potentially pathogenic species such as Escherichia coli, Vibrio spp., Campylobacter spp., or fungal pathogens of terrestrial ecosystems (149). Plastics as bacterial vectors may directly contaminate seafood and drinking water, thus spreading enteric diseases.
Concluding Summary and Future Perspectives
The penetration of MPs and NPs into the food system may produce important consequences in terms of food safety and, consequently, health. The available animal model evidence suggests potential harmful short-term effects of MPs, chiefly mediated by transitory damage to the intestine and changes in the microbiota composition to stimulate the inflammatory response. Additional studies (still in animal models) also suggest that NPs deregulate many cellular signaling pathways responsible for cell proliferation and differentiation. Furthermore, they induce epigenetic alterations to be speculatively interpreted as defense reactions. All of these changes are typical phenotypes of metabolic disorders and include an increase in systemic inflammation, alterations in the cell cycle, and important metabolic disorders based on lipid and glucose metabolism alterations. Marked changes in metabolic phenotypes and malnutrition usually lead to noncommunicable diseases, such as cardiovascular or inflammatory bowel disorders, suggesting that long-term exposures to MPs and NPs are risk factors for the aforementioned pathologies. Therefore, the extent of MP and NP ingestion and their metabolic fate must be thoroughly evaluated, which requires detailed knowledge of the many kinds of plastics—that is, their type, size, and shape; exposure levels and quantities; and finally, their dose–response effects in the human body.
In conclusion, the data reviewed here call for urgent research on the human effects of MPs and NPs, starting with the implementation of standardized methods to measure their concentrations in, for example, body fluids and stool. Concomitantly, we need to better understand which phenotypes are associated with MP and NP intake, either in vivo or by using novel available in vitro two- and three-dimensional models, and determine the human effects of both short- and long-term exposures. Future data availability will allow suggesting personalized strategies to avoid accidental ingestion and facilitate their elimination from the body—in turn, lessening the untoward actions of plastics.
Conflict of interest
No potential conflict of interest was reported by the author(s).
ACKNOWLEDGEMENTS
We thank Francesco Visioli for editing the paper. The authors’ responsibilities were as follows—M-CLdlH and AD: conceived the idea, supervised the work, and revised and edited the manuscript; M-CLdlH, HB, and AD: wrote the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by grants from the Spanish “Agencia Estatal de Investigación” and European FEDER Funds to AD (PID2019-109369RB-I00 and AGL2017-90623-REDT) and to M-CLdlH (RTI2018-093873-A-I00 and IJC2020-044353-I/MCIN/AEI/10.13039/501100011033/EU/PRTR ). HB is supported by a predoctoral fellowship (“Plan Propio IBIMA 2020 A.1 Contratos predoctorales,” Ref.: predoc20_002).
Author disclosures: The authors report no conflict of interest.
Abbreviations used: BPA, bisphenol A; Ced3, cell death protein type 3; JNK c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; Met2, homoserine O-acetyltransferase; miRNA, microRNA; MP, microplastic; MT, metric tons; NP, nanoplastic; PS, polystyrene; ROS, reactive oxygen species.
Contributor Information
María-Carmen López de las Hazas, Laboratory of Epigenetics of Lipid Metabolism, Madrid Institute for Advanced Studies (IMDEA)–Food, CEI UAM + CSIC, Madrid, Spain.
Hatim Boughanem, Instituto de Investigación Biomédica de Málaga (IBIMA), Unidad de Gestión Clínica de Endocrinología y Nutrición del Hospital Virgen de la Victoria, Málaga, Spain.
Alberto Dávalos, Laboratory of Epigenetics of Lipid Metabolism, Madrid Institute for Advanced Studies (IMDEA)–Food, CEI UAM + CSIC, Madrid, Spain.
References
- 1. Barnes DKA, Galgani F, Thompson RC, Barlaz M. Accumulation and fragmentation of plastic debris in global environments. Phil Transact R Soc B Biol Sci. 2009;364(1526):1985–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaza S, Yao L, Bhada-Tata P, Van Woerden F. What a waste 2.0: a global snapshot of solid waste management to 2050. The World Bank; 2018. [Internet]. Available from: http://elibrary.worldbank.org/doi/book/10.1596/978-1-4648-1329-0. [Google Scholar]
- 3. Plastics Europe Association of Plastics Manufacturers . Plasticseurope 2016. [Internet]. [Accessed 2021 Nov 24]. Available from: https://www.plasticseurope.org/en/resources/publications/3-plastics-facts-2016. [Google Scholar]
- 4. Plastics Europe . Plastics—the facts 2018: an analysis of European plastics production, demand and waste data. 2018. [Internet]. [Accessed 2021 Nov 24]. Available from: https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics_the_facts_2018_AF_web.pdf.
- 5. Baudrimont M, Arini A, Guégan C, Venel Z, Gigault J, Pedrono B, Prunier J, Maurice L, Ter Halle A, Feurtet-Mazel A. Ecotoxicity of polyethylene nanoplastics from the North Atlantic oceanic gyre on freshwater and marine organisms (microalgae and filter-feeding bivalves). Environ Sci Pollut Res. 2019;24(4):3746–55. [DOI] [PubMed] [Google Scholar]
- 6. ter Halle A, Ladirat L, Gendre X, Goudouneche D, Pusineri C, Routaboul C, Tenailleau C, Duployer B, Perez E. Understanding the fragmentation pattern of marine plastic debris. Environ Sci Technol. 2016;50(11):5668–75. [DOI] [PubMed] [Google Scholar]
- 7. Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL. Marine pollution. Plastic waste inputs from land into the ocean. Science. 2015;347(6223):768–71. [DOI] [PubMed] [Google Scholar]
- 8. Kannan K, Vimalkumar K. A review of human exposure to microplastics and insights into microplastics as obesogens. Front Endocrinol. 2021;12:724989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rist S, Carney Almroth B, Hartmann NB, Karlsson TM. A critical perspective on early communications concerning human health aspects of microplastics. Sci Total Environ. 2018;626:720–6. [DOI] [PubMed] [Google Scholar]
- 10. Jovanović B. Ingestion of microplastics by fish and its potential consequences from a physical perspective. Integr Environ Assess Manag. 2017;13(3):510–5. [DOI] [PubMed] [Google Scholar]
- 11. Teuten EL, Saquing JM, Knappe DRU, Barlaz MA, Jonsson S, Bjrn A, Rowland SJ, Thompson RC, Galloway TS, Yamashita Ret al. . Transport and release of chemicals from plastics to the environment and to wildlife. Phil Transact R Soc B Biol Sci. 2009;364(1526):2027–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Brine T, Thompson RC. Degradation of plastic carrier bags in the marine environment. Mar Pollut Bull. 2010;60(12):2279–83. [DOI] [PubMed] [Google Scholar]
- 13. Zettler ER, Mincer TJ, Amaral-Zettler LA. Life in the “Plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol. 2013;47(13):7137–46. [DOI] [PubMed] [Google Scholar]
- 14. Setälä O, Fleming-Lehtinen V, Lehtiniemi M. Ingestion and transfer of microplastics in the planktonic food web. Environ Pollut. 2014;185:77–83. [DOI] [PubMed] [Google Scholar]
- 15. Farrell P, Nelson K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ Pollut. 2013;177:1–3. [DOI] [PubMed] [Google Scholar]
- 16. Lusher A. Microplastics in the marine environment: distribution, interactions and effects. In: Marine anthropogenic litter. Bergmann M, Gutow L, Klages M.. Cham (Switzerland): Springer Link; 2015. p. 245–307. [Google Scholar]
- 17. Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS. Microplastic ingestion by zooplankton. Environ Sci Technol. 2013;47(12):6646–55. [DOI] [PubMed] [Google Scholar]
- 18. Long M, Moriceau B, Gallinari M, Lambert C, Huvet A, Raffray J, Soudant P. Interactions between microplastics and phytoplankton aggregates: impact on their respective fates. Mar Chem. 2015;175:39–46. [Google Scholar]
- 19. Watts AJR, Lewis C, Goodhead RM, Beckett SJ, Moger J, Tyler CR, Galloway TS. Uptake and retention of microplastics by the shore crab C arcinus maenas. Environ Sci Technol. 2014;48(15):8823–30. [DOI] [PubMed] [Google Scholar]
- 20. van Pomeren M, Brun NR, Peijnenburg W, Vijver MG. Exploring uptake and biodistribution of polystyrene (nano)particles in zebrafish embryos at different developmental stages. Aquatic Toxicol. 2017;190:40–5. [DOI] [PubMed] [Google Scholar]
- 21. Abbasi S, Keshavarzi B, Moore F, Turner A, Kelly FJ, Dominguez AO, Jaafarzadeh N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Environ Pollut. 2019;244:153–64. [DOI] [PubMed] [Google Scholar]
- 22. Carbery M, O'Connor W, Palanisami T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ Int. 2018;115:400–9. [DOI] [PubMed] [Google Scholar]
- 23. Bergmann M, Tekman MB, Gutow L. Sea change for plastic pollution. Nature. 2017;544(7650):297–7. [DOI] [PubMed] [Google Scholar]
- 24. Lacerda ALdF, Rodrigues L dos S, van Sebille E, Rodrigues FL, Ribeiro L, Secchi ER, Kessler F, Proietti MC. Plastics in sea surface waters around the Antarctic peninsula. Sci Rep. 2019;9(1):3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergmann M, Mützel S, Primpke S, Tekman MB, Trachsel J, Gerdts G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci Adv. 2019;5(8):eaax1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wetherbee GA, Baldwin AK, Ranville JF. It is raining plastic. USGS Numbered Series. Open-File Report. 2019. U.S. Geological Survey. 10.3133/ofr20191048 [DOI] [Google Scholar]
- 27. Compa M, Alomar C, Mourre B, March D, Tintoré J, Deudero S. Nearshore spatio-temporal sea surface trawls of plastic debris in the Balearic Islands. Mar Environ Res. 2020;158:104945. [DOI] [PubMed] [Google Scholar]
- 28. Rochman CM, Kross SM, Armstrong JB, Bogan MT, Darling ES, Green SJ, Smyth AR, Veríssimo D. Scientific evidence supports a ban on microbeads. Environ Sci Technol. 2015;49(18):10759–61. [DOI] [PubMed] [Google Scholar]
- 29. Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL. Plastic waste inputs from land into the ocean. Science. 2015;347(6223):768–71. [DOI] [PubMed] [Google Scholar]
- 30. Yee MS-L, Hii L-W, Looi CK, Lim W-M, Wong S-F, Kok Y-Y, Tan B-K, Wong C-Y, Leong C-O. Impact of microplastics and nanoplastics on human health. Nanomaterials. 2021;11(2):496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Y-L, Lee Y-H, Chiu I-J, Lin Y-F, Chiu H-W. Potent impact of plastic nanomaterials and micromaterials on the food chain and human health. Int J Mol Sci. 2020; 21(5):1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pereao O, Opeolu B, Fatoki O. Microplastics in aquatic environment: characterization, ecotoxicological effect, implications for ecosystems and developments in South Africa. Environ Sci Pollut Res. 2020;27(18):22271–91. [DOI] [PubMed] [Google Scholar]
- 33. Kelly A, Lannuzel D, Rodemann T, Meiners KM, Auman HJ. Microplastic contamination in east Antarctic sea ice. Mar Pollut Bull. 2020;154:111130. [DOI] [PubMed] [Google Scholar]
- 34. de Souza Machado AA, Kloas W, Zarfl C, Hempel S, Rillig MC. Microplastics as an emerging threat to terrestrial ecosystems. Global Change Biol. 2018;24(4):1405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bläsing M, Amelung W. Plastics in soil: analytical methods and possible sources. Sci Total Environ. 2018;612:422–35. [DOI] [PubMed] [Google Scholar]
- 36. Lwanga EH, Gertsen H, Gooren H, Peters P, Salánki T, der P M, Besseling E, Koelmans AA, Geissen V. Microplastics in the terrestrial ecosystem: implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ Sci Technol. 2016;50(5):2685–91. [DOI] [PubMed] [Google Scholar]
- 37. Ullah R, Tsui MTK, Chen H, Chow A, Williams C, Ligaba-Osena A. Microplastics interaction with terrestrial plants and their impacts on agriculture. J Environ Qual. 2021;50(5):1024–41. [DOI] [PubMed] [Google Scholar]
- 38. Yang J, Cao W, Rui Y. Interactions between nanoparticles and plants: phytotoxicity and defense mechanisms. J Plant Interact. 2017;12(1):158–69. [Google Scholar]
- 39. Tympa L-E, Katsara K, Moschou PN, Kenanakis G, Papadakis VM. Do microplastics enter our food chain via root vegetables? A raman based spectroscopic study on Raphanus sativus. Materials (Basel). 2021;14(9):2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bouwmeester H, Hollman PCH, Peters RJB. Potential health impact of environmentally released micro- and nanoplastics in the human food production chain: experiences from nanotoxicology. Environ Sci Technol. 2015;49(15):8932–47. [DOI] [PubMed] [Google Scholar]
- 41. Oliveri Conti G, Ferrante M, Banni M, Favara C, Nicolosi I, Cristaldi A, Fiore M, Zuccarello P. Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ Res. 2020;187. [DOI] [PubMed] [Google Scholar]
- 42. Huerta Lwanga E, Mendoza Vega J, Ku Quej V, Chi J de los A, Sanchez del Cid L, Chi C, Escalona Segura G, Gertsen H, Salánki T, van der Ploeg Met al. . Field evidence for transfer of plastic debris along a terrestrial food chain. Sci Rep. 2017;7(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Toussaint B, Raffael B, Angers-Loustau A, Gilliland D, Kestens V, Petrillo M, Rio-Echevarria IM, Van den Eede G. Review of micro- and nanoplastic contamination in the food chain. Food Addit Contam A. 2019;36(5):639–73. [DOI] [PubMed] [Google Scholar]
- 44. European Food Safety Authority . Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016;14:e04501. [Google Scholar]
- 45. Zuccarello P, Ferrante M, Cristaldi A, Copat C, Grasso A, Sangregorio D, Fiore M, Oliveri Conti G. Exposure to microplastics (<10 μm) associated to plastic bottles mineral water consumption: the first quantitative study. Water Res. 2019;157:365–71. [DOI] [PubMed] [Google Scholar]
- 46. Akoueson F, Sheldon LM, Danopoulos E, Morris S, Hotten J, Chapman E, Li J, Rotchell JM. A preliminary analysis of microplastics in edible versus non-edible tissues from seafood samples. Environ Pollut. 2020;263:114452. [DOI] [PubMed] [Google Scholar]
- 47. Kedzierski M, Lechat B, Sire O, Le Maguer G, Le Tilly V, Bruzaud S. Microplastic contamination of packaged meat: occurrence and associated risks. Food Packag Shelf Life. 2020;24:100489. [Google Scholar]
- 48. Gelbke HP, Banton M, Block C, Dawkins G, Eisert R, Leibold E, Pemberton M, Puijk IM, Sakoda A, Yasukawa A. Risk assessment for migration of styrene oligomers into food from polystyrene food containers. Food Chem Toxicol. 2019;124:151–67. [DOI] [PubMed] [Google Scholar]
- 49. Mason SA, Welch VG, Neratko J. Synthetic polymer contamination in bottled water. Front Chem. 2018;6:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Novotna K, Cermakova L, Pivokonska L, Cajthaml T, Pivokonsky M. Microplastics in drinking water treatment—current knowledge and research needs. Sci Total Environ. 2019;667:730–40. [DOI] [PubMed] [Google Scholar]
- 51. Steele EM, Khandpur N, Louzada ML da C, Monteiro CA. Association between dietary contribution of ultra-processed foods and urinary concentrations of phthalates and bisphenol in a nationally representative sample of the US population aged 6 years and older. PLoS One. 2020;15(7):e0236738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Buckley JP, Kim H, Wong E, Rebholz CM. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ Int. 2019;131:105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Edwards L, McCray NL, VanNoy BN, Yau A, Geller RJ, Adamkiewicz G, Zota AR. Phthalate and novel plasticizer concentrations in food items from U.S. fast food chains: a preliminary analysis. J Expo Sci Environ Epidemiol. 2021. 10.1038/s41370-021-00392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schwabl P, Köppel S, Königshofer P, Bucsics T, Trauner M, Reiberger T, Liebmann B. Detection of various microplastics in human stool: a prospective case series. Ann Intern Med. 2019;171(7):453–57. [DOI] [PubMed] [Google Scholar]
- 55. Zhang N, Li Y B, He HR, Zhang JF, Ma GS. You are what you eat: microplastics in the feces of young men living in Beijing. Sci Total Environ. 2021;767:144345. [DOI] [PubMed] [Google Scholar]
- 56. Walczak AP, Kramer E, Hendriksen PJM, Tromp P, Helsper J, Van Der Zande M, Rietjens I, Bouwmeester H. Translocation of differently sized and charged polystyrene nanoparticles in in vitro intestinal cell models of increasing complexity. Nanotoxicology. 2015;9(4):453–61. [DOI] [PubMed] [Google Scholar]
- 57. Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, Ding L, Ren H. Uptake and accumulation of polystyrene microplastics in zebrafish (D anio rerio) and toxic effects in liver. Environ Sci Technol. 2016;50(7):4054–60. [DOI] [PubMed] [Google Scholar]
- 58. Qiao R, Deng Y, Zhang S, Wolosker MB, Zhu Q, Ren H, Zhang Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere. 2019;236:124334. [DOI] [PubMed] [Google Scholar]
- 59. Mancia A, Chenet T, Bono G, Geraci ML, Vaccaro C, Munari C, Mistri M, Cavazzini A, Pasti L. Adverse effects of plastic ingestion on the Mediterranean small-spotted catshark (Scyliorhinus canicula). Mar Environ Res. 2020;155:104876. [DOI] [PubMed] [Google Scholar]
- 60. de Haan WP, Sanchez-Vidal A, Canals M. Floating microplastics and aggregate formation in the Western Mediterranean Sea. Mar Pollut Bull. 2019;140:523–35. [DOI] [PubMed] [Google Scholar]
- 61. Bottari T, Savoca S, Mancuso M, Capillo G, GiuseppePanarello G, MartinaBonsignore M, Crupi R, Sanfilippo M, D'Urso L, Compagnini Get al. . Plastics occurrence in the gastrointestinal tract of Zeus faber and Lepidopus caudatus from the Tyrrhenian Sea. Mar Pollut Bull. 2019;146:408–16. [DOI] [PubMed] [Google Scholar]
- 62. Renzi M, Specchiulli A, Blašković A, Manzo C, Mancinelli G, Cilenti L. Marine litter in stomach content of small pelagic fishes from the Adriatic Sea: sardines (Sardina pilchardus) and anchovies (Engraulis encrasicolus). Environ Sci Pollut Res. 2019;26(3):2771–81. [DOI] [PubMed] [Google Scholar]
- 63. Zitouni N, Bousserrhine N, Belbekhouche S, Missawi O, Alphonse V, Boughatass I, Banni M. First report on the presence of small microplastics (≤3 μm) in tissue of the commercial fish Serranus scriba (Linnaeus. 1758) from Tunisian coasts and associated cellular alterations. Environ Pollut. 2020;263:114576. [DOI] [PubMed] [Google Scholar]
- 64. Ahrendt C, Perez-Venegas DJ, Urbina M, Gonzalez C, Echeveste P, Aldana M, Pulgar J, Galbán-Malagón C. Microplastic ingestion cause intestinal lesions in the intertidal fish Girella laevifrons. Mar Pollut Bull. 2020;151:110795. [DOI] [PubMed] [Google Scholar]
- 65. Lei L, Wu S, Lu S, Liu M, Song Y, Fu Z, Shi H, Raley-Susman KM, He D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci Total Environ. 2018;619–620:1–8. [DOI] [PubMed] [Google Scholar]
- 66. Li B, Ding Y, Cheng X, Sheng D, Xu Z, Rong Q, Wu Y, Zhao H, Ji X, Zhang Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere. 2020;244:125492. [DOI] [PubMed] [Google Scholar]
- 67. Jin Y, Lu L, Tu W, Luo T, Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci Total Environ. 2019;649:308–17. [DOI] [PubMed] [Google Scholar]
- 68. Shang X, Lu J, Feng C, Ying Y, He Y, Fang S, Lin Y, Dahlgren R, Ju J. Microplastic (1 and 5 μm) exposure disturbs lifespan and intestine function in the nematode Caenorhabditis elegans. Sci Total Environ. 2020;705:135837. [DOI] [PubMed] [Google Scholar]
- 69. Fackelmann G, Sommer S. Microplastics and the gut microbiome: how chronically exposed species may suffer from gut dysbiosis. Mar Pollut Bull. 2019;143:193–203. [DOI] [PubMed] [Google Scholar]
- 70. Jin Y, Xia J, Pan Z, Yang J, Wang W, Fu Z. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ Pollut. 2018;235:322–9. [DOI] [PubMed] [Google Scholar]
- 71. Yan W, Hamid N, Deng S, Jia P-P, Pei D-S. Individual and combined toxicogenetic effects of microplastics and heavy metals (Cd, Pb, and Zn) perturb gut microbiota homeostasis and gonadal development in marine medaka (Oryzias melastigma). J Hazard Mater. 2020;397:122795. [DOI] [PubMed] [Google Scholar]
- 72. Auguste M, Lasa A, Balbi T, Pallavicini A, Vezzulli L, Canesi L. Impact of nanoplastics on hemolymph immune parameters and microbiota composition in Mytilus galloprovincialis. Mar Environ Res. 2020;159:105017. [DOI] [PubMed] [Google Scholar]
- 73. Lu L, Wan Z, Luo T, Fu Z, Jin Y.. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci Total Environ. 2018;631–632:449–58. [DOI] [PubMed] [Google Scholar]
- 74. Huang W, Yin H, Yang Y, Jin L, Lu G, Dang Z. Influence of the co-exposure of microplastics and tetrabromobisphenol A on human gut: simulation in vitro with human cell Caco-2 and gut microbiota. Sci Total Environ. 2021;778:146264. [DOI] [PubMed] [Google Scholar]
- 75. Banerjee A, Shelver WL. Micro- and nanoplastic induced cellular toxicity in mammals: a review. Sci Total Environ. 2021;755:142518. [DOI] [PubMed] [Google Scholar]
- 76. Miner-Williams WM, Moughan PJ. Intestinal barrier dysfunction: implications for chronic inflammatory conditions of the bowel. Nutr Res Rev. 2016;29(1):40–59. [DOI] [PubMed] [Google Scholar]
- 77. Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Doré J, Franceschi C, Lehtinen MJ, Recker T, Salvioli Set al. . Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev. 2017;40:95–119. [DOI] [PubMed] [Google Scholar]
- 78. Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15(2):111–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Merkley SD, Moss HC, Goodfellow SM, Ling CL, Meyer-Hagen JL, Weaver J, Campen MJ, Castillo EF. Polystyrene microplastics induce an immunometabolic active state in macrophages. Cell Biol Toxicol. 2021. doi: 10.1007/s10565-021-09616-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stock V, Böhmert L, Lisicki E, Block R, Cara-Carmona J, Pack LK, Selb R, Lichtenstein D, Voss L, Henderson CJet al. . Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch Toxicol. 2019;93(7):1817–33. [DOI] [PubMed] [Google Scholar]
- 81. Rafiee M, Dargahi L, Eslami A, Beirami E, Jahangiri-rad M, Sabour S, Amereh F. Neurobehavioral assessment of rats exposed to pristine polystyrene nanoplastics upon oral exposure. Chemosphere. 2018;193:745–53. [DOI] [PubMed] [Google Scholar]
- 82. EFSA Panel on Contaminants in the Food Chain (CONTAM) . Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016;14(6):e04501. 10.2903/j.efsa.2016.4501 [DOI] [Google Scholar]
- 83. De Jong WH, Hagens WI, Krystek P, Burger MC, Sips A, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29(12):1912–9. [DOI] [PubMed] [Google Scholar]
- 84. Wright SL, Kelly FJ. Plastic and human health: a micro issue?. Environ Sci Technol. 2017;51(12):6634–47. [DOI] [PubMed] [Google Scholar]
- 85. Collard F, Gilbert B, Compère P, Eppe G, Das K, Jauniaux T, Parmentier E. Microplastics in livers of European anchovies (Engraulis encrasicolus, L.). Environ Pollut. 2017;229:1000–5. [DOI] [PubMed] [Google Scholar]
- 86. Al-Sid-Cheikh M, Rowland SJ, Stevenson K, Rouleau C, Henry TB, Thompson RC. Uptake, whole-body distribution, and depuration of nanoplastics by the scallop P ecten maximus at environmentally realistic concentrations. Environ Sci Technol. 2018;52(24):14480–6. [DOI] [PubMed] [Google Scholar]
- 87. Qiao R, Sheng C, Lu Y, Zhang Y, Ren H, Lemos B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci Total Environ. 2019;662:246–53. [DOI] [PubMed] [Google Scholar]
- 88. Deng Y, Zhang Y, Lemos B, Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci Rep. 2017;7(1):46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hollóczki O, Gehrke S. Nanoplastics can change the secondary structure of proteins. Sci Rep. 2019;9(1):16013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wright SL, Thompson RC, Galloway TS. The physical impacts of microplastics on marine organisms: a review. Environ Pollut. 2013;178:483–92. [DOI] [PubMed] [Google Scholar]
- 91. Wu B, Wu X, Liu S, Wang Z, Chen L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere. 2019;221:333–41. [DOI] [PubMed] [Google Scholar]
- 92. Wang Q, Bai J, Ning B, Fan L, Sun T, Fang Y, Wu J, Li S, Duan C, Zhang Yet al. . Effects of bisphenol A and nanoscale and microscale polystyrene plastic exposure on particle uptake and toxicity in human Caco-2 cells. Chemosphere. 2020;254:126788. [DOI] [PubMed] [Google Scholar]
- 93. Jeong CB, Kang HM, Lee MC, Kim DH, Han J, Hwang DS, Souissi S, Lee SJ, Shin KH, Park HGet al. . Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci Rep. 2017;7:41323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Von Moos N, Burkhardt-Holm P, Köhler A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ Sci Technol. 2012;46(20):11327–35. [DOI] [PubMed] [Google Scholar]
- 95. Li Y, Hu Q, Miao G, Zhang Q, Yuan B, Zhu Y, Fu X, Chen X, Mao C. Size-dependent mechanism of intracellular localization and cytotoxicity of mono-disperse spherical mesoporous nano-and micron-bioactive glass particles. J Biomed Nanotechnol. 2016;12(5):863–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hussain N, Jaitley V, Florence AT. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Deliv Rev. 2001;50(1–2):107–42. [DOI] [PubMed] [Google Scholar]
- 97. Galloway TS. Micro- and nano-plastics and human health. In: Bergmann M, Gutow L, Klages Meditors. Marine anthropogenic litter. Cham (Switzerland): Springer Link; 2015. p. 343–66. [Google Scholar]
- 98. Hirt N, Body-Malapel M. Immunotoxicity and intestinal effects of nano- and microplastics: a review of the literature. Part Fibre Toxicol. 2020;17(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liang B, Zhong Y, Huang Y, Lin X, Liu J, Lin L, Hu M, Jiang J, Dai M, Wang Bet al. . Underestimated health risks: polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part Fibre Toxicol. 2021;18(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bayo J, Rojo D, Martínez-Baños P, López-Castellanos J, Olmos S. Commercial gilthead seabream (Sparus aurata L.) from the Mar Menor Coastal Lagoon as hotspots of microplastic accumulation in the digestive system. Int J Environ Res Public Health. 2021;18(13):6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Keinänen O, Dayts EJ, Rodriguez C, Sarrett SM, Brennan JM, Sarparanta M, Zeglis BM. Harnessing PET to track micro- and nanoplastics in vivo. Sci Rep. 2021;11:11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Walczak AP, Hendriksen PJM, Woutersen RA, van der Zande M, Undas AK, Helsdingen R, van den Berg HHJ, Rietjens I, Bouwmeester H. Bioavailability and biodistribution of differently charged polystyrene nanoparticles upon oral exposure in rats. J Nanopart Res. 2015;17(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Im C, Kim H, Zaheer J, Kim JY, Lee YJ, Kang CM, Kim JS. PET tracing of biodistribution for orally administered 64 Cu-labeled polystyrene in mice. J Nucl Med. 2021. doi: jnumed.120.256982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hussain N, Jani PU, Florence AT. Enhanced oral uptake of tomato lectin-conjugated nanoparticles in the rat. Pharm Res. 1997;14(5):613–8. [DOI] [PubMed] [Google Scholar]
- 105. Seifert J, Haraszti B, Sass W. The influence of age and particle number on absorption of polystyrene particles from the rat gut. J Anat. 1996;189:483. [PMC free article] [PubMed] [Google Scholar]
- 106. Hillery AM, Jani PU, Florence AT. Comparative, quantitative study of lymphoid and non-lymphoid uptake of 60 nm polystyrene particles. J Drug Target. 1994;2:151–6. [DOI] [PubMed] [Google Scholar]
- 107. Rehse S, Kloas W, Zarfl C. Short-term exposure with high concentrations of pristine microplastic particles leads to immobilisation of Daphnia magna. Chemosphere. 2016;153:91–9. [DOI] [PubMed] [Google Scholar]
- 108. Besseling E, Wang B, Lürling M, Koelmans AA. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ Sci Technol. 2014;48(20):12336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rochman CM, Kurobe T, Flores I, Teh SJ. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci Total Environ. 2014;493:656–61. [DOI] [PubMed] [Google Scholar]
- 110. Yu C-W, Luk TC, Liao VH-C. Long-term nanoplastics exposure results in multi and trans-generational reproduction decline associated with germline toxicity and epigenetic regulation in Caenorhabditis elegans. J Hazard Mater. 2021;412:125173. [DOI] [PubMed] [Google Scholar]
- 111. Choi YJ, Park JW, Kim JE, Lee SJ, Gong JE, Jung Y-S, Seo S, Hwang DY. Novel characterization of constipation phenotypes in ICR mice orally administrated with polystyrene microplastics. Int J Mol Sci. 2021,22(11):5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tang J, Ni X, Zhou Z, Wang L, Lin S. Acute microplastic exposure raises stress response and suppresses detoxification and immune capacities in the scleractinian coral Pocillopora damicornis. Environ Pollut. 2018;243:66–74. [DOI] [PubMed] [Google Scholar]
- 113. Qu M, Xu K, Li Y, Wong G, Wang D. Using acs-22 mutant Caenorhabditis elegans to detect the toxicity of nanopolystyrene particles. Sci Total Environ. 2018;643:119–26. [DOI] [PubMed] [Google Scholar]
- 114. Wu S, Wu M, Tian D, Qiu L, Li T. Effects of polystyrene microbeads on cytotoxicity and transcriptomic profiles in human Caco-2 cells. Environ Toxicol. 2020; 35(4):495–506. [DOI] [PubMed] [Google Scholar]
- 115. Stock V, Laurisch C, Franke J, Dönmez MH, Voss L, Böhmert L, Braeuning A, Sieg H. Uptake and cellular effects of PE, PP, PET and PVC microplastic particles. Toxicol In Vitro. 2021;70:105021. [DOI] [PubMed] [Google Scholar]
- 116. Domenech J, de Britto M, Velázquez A, Pastor S, Hernández A, Marcos R, Cortés C. Long-term effects of polystyrene nanoplastics in human intestinal Caco-2 cells. Biomolecules. 2021;11(10):1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yang Y, Wu Q, Wang D. Epigenetic response to nanopolystyrene in germline of nematode Caenorhabditis elegans. Ecotoxicol Environ Saf. 2020;206:111404. [DOI] [PubMed] [Google Scholar]
- 118. Wang S, Zhang R, Wang D. Induction of protective response to polystyrene nanoparticles associated with methylation regulation in Caenorhabditis elegans. Chemosphere. 2021;271:129589. [DOI] [PubMed] [Google Scholar]
- 119. Liu H, Tian L, Qu M, Wang D. Acetylation regulation associated with the induction of protective response to polystyrene nanoparticles in Caenorhabditis elegans. J Hazard Mater. 2021;411:125035. [DOI] [PubMed] [Google Scholar]
- 120. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. [DOI] [PubMed] [Google Scholar]
- 121. Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Fehlmann T, Ludwig N, Backes C, Meese E, Keller A. Distribution of microRNA biomarker candidates in solid tissues and body fluids. RNA Biol. 2016;13(11):1084–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mantilla-Escalante DC, López de las Hazas M-C, Crespo MC, Martín-Hernández R, Tomé-Carneiro J, del Pozo-Acebo L, Salas-Salvadó J, Bulló M, Dávalos A. Mediterranean diet enriched in extra-virgin olive oil or nuts modulates circulating exosomal non-coding RNAs. Eur J Nutr. 2021;60: 4279–93. [DOI] [PubMed] [Google Scholar]
- 124. López de las Hazas MC, Gil-Zamorano J, Cofán M, Mantilla-Escalante DC, Garcia-Ruiz A, del Pozo-Acebo L, Pastor O, Yañez-Mo M, Mazzeo C, Serra-Mir Met al. . One-year dietary supplementation with walnuts modifies exosomal miRNA in elderly subjects. Eur J Nutr. 2020;60(4):1999–2011.. doi: 10.1007/s00394-020-02390-2. [DOI] [PubMed] [Google Scholar]
- 125. Zhang L, Hou D, Chen X, Li D, Zhu L, Zhang Y, Li J, Bian Z, Liang X, Cai Xet al. . Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2012;22(1):107–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pozo-Acebo L, López de las Hazas M, Margollés A, Dávalos A, García-Ruiz A. Eating microRNAs: pharmacological opportunities for cross-kingdom regulation and implications in host gene and gut microbiota modulation. Br J Pharmacol. 2021;178(11):2218–45. [DOI] [PubMed] [Google Scholar]
- 127. Qu M, Luo L, Yang Y, Kong Y, Wang D. Nanopolystyrene-induced microRNAs response in Caenorhabditis elegans after long-term and lose-dose exposure. Sci Total Environ. 2019;697:134131. [DOI] [PubMed] [Google Scholar]
- 128. Liu H, Zhao Y, Bi K, Rui Q, Wang D. Dysregulated mir-76 mediated a protective response to nanopolystyrene by modulating heme homeostasis related molecular signaling in nematode Caenorhabditis elegans. Ecotoxicol Environ Saf. 2021;212:112018. [DOI] [PubMed] [Google Scholar]
- 129. Qiu Y, Liu Y, Li Y, Wang D. Intestinal mir-794 responds to nanopolystyrene by linking insulin and p38 MAPK signaling pathways in nematode Caenorhabditis elegans. Ecotoxicol Environ Saf. 2020;201:110857. [DOI] [PubMed] [Google Scholar]
- 130. Chen H, Hua X, Li H, Wang C, Dang Y, Ding P, Yu Y. Transgenerational neurotoxicity of polystyrene microplastics induced by oxidative stress in Caenorhabditis elegans. Chemosphere. 2021;272:129642. [DOI] [PubMed] [Google Scholar]
- 131. Qiang L, Lo LSH, Gao Y, Cheng J. Parental exposure to polystyrene microplastics at environmentally relevant concentrations has negligible transgenerational effects on zebrafish (Danio rerio). Ecotoxicol Environ Saf. 2020;206:111382. [DOI] [PubMed] [Google Scholar]
- 132. Martins A, Guilhermino L. Transgenerational effects and recovery of microplastics exposure in model populations of the freshwater cladoceran Daphnia magna Straus. Sci Total Environ. 2018;631–632:421–8. [DOI] [PubMed] [Google Scholar]
- 133. Ragusa A, Svelato A, Santacroce C, Catalano P, Notarstefano V, Carnevali O, Papa F, Rongioletti MCA, Baiocco F, Draghi Set al. . Plasticenta: first evidence of microplastics in human placenta. Environ Int. 2021;146:106274. [DOI] [PubMed] [Google Scholar]
- 134. Zhang C, Jeong C-B, Lee J-S, Wang D, Wang M. Transgenerational proteome plasticity in resilience of a marine copepod in response to environmentally relevant concentrations of microplastics. Environ Sci Technol. 2019;53(14):8426–36. [DOI] [PubMed] [Google Scholar]
- 135. Luo T, Zhang Y, Wang C, Wang X, Zhou J, Shen M, Zhao Y, Fu Z, Jin Y. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ Pollut. 2019;255:113122. [DOI] [PubMed] [Google Scholar]
- 136. Luo T, Wang C, Pan Z, Jin C, Fu Z, Jin Y. Maternal polystyrene microplastic exposure during gestation and lactation altered metabolic homeostasis in the dams and their F1 and F2 offspring. Environ Sci Technol. 2019;53(18):10978–92.;acs.est.9b03191. [DOI] [PubMed] [Google Scholar]
- 137. Sun L, Liao K, Wang D. Comparison of transgenerational reproductive toxicity induced by pristine and amino modified nanoplastics in Caenorhabditis elegans. Sci Total Environ. 2021;768:144362. [DOI] [PubMed] [Google Scholar]
- 138. Rahman A, Sarkar A, Yadav OP, Achari G, Slobodnik J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: a scoping review. Sci Total Environ. 2021;757:143872. [DOI] [PubMed] [Google Scholar]
- 139. Prata JC. Airborne microplastics: consequences to human health?. Environ Pollut. 2018;234:115–26. [DOI] [PubMed] [Google Scholar]
- 140. Schmidt C, Lautenschlaeger C, Collnot EM, Schumann M, Bojarski C, Schulzke JD, Lehr CM, Stallmach A. Nano- and microscaled particles for drug targeting to inflamed intestinal mucosa—a first in vivo study in human patients. J Control Release. 2013;165(2):139–45. [DOI] [PubMed] [Google Scholar]
- 141. Zheng H, Wang J, Wei X, Chang L, Liu S. Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci Total Environ. 2021;750:143085. [DOI] [PubMed] [Google Scholar]
- 142. Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8(1):e55387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127(1–2):27–34. [DOI] [PubMed] [Google Scholar]
- 144. Amjad S, Rahman MS, Pang WK, Ryu DY, Adegoke EO, Park YJ, Pang MG. Effects of phthalates on the functions and fertility of mouse spermatozoa. Toxicology. 2021;454:152746. [DOI] [PubMed] [Google Scholar]
- 145. Liao KW, Kuo PL, HB, Chang JW, Chiang HC, Huang PC. Increased risk of phthalates exposure for recurrent pregnancy loss in reproductive-aged women. Environ Pollut. 2018;241:969–77. [DOI] [PubMed] [Google Scholar]
- 146. Yu F, Yang C, Zhu Z, Bai X, Ma J. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci Total Environ. 2019;694:133643. [DOI] [PubMed] [Google Scholar]
- 147. Joo SH, Liang Y, Kim M, Byun J, Choi H. Microplastics with adsorbed contaminants: mechanisms and treatment. Environ Challenge. 2021;3:100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Godoy V, Blázquez G, Calero M, Quesada L, Martín-Lara MA. The potential of microplastics as carriers of metals. Environ Pollut. 2019;255:113363. [DOI] [PubMed] [Google Scholar]
- 149. Fournier E, Etienne-Mesmin L, Grootaert C, Jelsbak L, Syberg K, Blanquet-Diot S, Mercier-Bonin M. Microplastics in the human digestive environment: a focus on the potential and challenges facing in vitro gut model development. J Hazard Mater. 2021;415:125632. [DOI] [PubMed] [Google Scholar]