ABSTRACT
Indoxyl sulfate (IS) and p-cresyl sulfate (PCS), protein-bound uremic toxins, exacerbate the deterioration of renal function and increase the risk of cardiovascular events in chronic kidney disease (CKD) patients. The effects of microbiota-driven therapy (probiotics, prebiotics, or synbiotics) on decreasing circulating IS and PCS concentrations are controversial; thus, we performed the present systematic review and meta-analysis to assess the effects of microbiota-driven therapy on circulating IS and PCS concentrations in CKD patients. PubMed, EMBASE, and Cochrane Library databases were systematically searched from inception to 22 July, 2021, and randomized controlled trials (RCTs) investigating the effects of microbiota-driven therapy on circulating IS and PCS concentrations in CKD patients were included. In all, 14 RCTs with 513 participants were eligible for the meta-analysis. The effects of microbiota-driven therapy on the circulating IS and PCS concentrations were evaluated with weighted mean differences (WMDs) measured by a fixed-effects model or a random-effects model. Compared with placebo, microbiota-driven therapy had no statistically significant effect on the circulating IS concentration (WMD: −1.64 mg/L; 95% CI: −3.46, 0.18 mg/L; P = 0.077) but it decreased the circulating PCS concentration (WMD: −2.42 mg/L; 95% CI: −3.81, −1.04 mg/L; P = 0.001). In the subgroup analyses, prebiotic (n = 6) and synbiotic (n = 3) supplementation significantly decreased the circulating PCS concentration, whereas probiotic (n = 3) supplementation did not. Meta-regression showed that the effects of microbiota-driven therapy were not associated with the supplementation time or the year of publication. Moreover, there was no significant evidence of publication bias. This review found that microbiota-driven therapy decreased the circulating PCS concentration in CKD patients. Additional large, well-designed RCTs with improved methodology and reporting are necessary to assess the effects of microbiota-driven therapy on circulating IS and PCS concentrations in the long term. This systematic review was registered at www.crd.york.ac.uk/prospero/ as CRD42021269146.
Keywords: microbiota-driven therapy, indoxyl sulfate, p-cresyl sulfate, chronic kidney disease, meta-analysis
Statement of Significance: The findings of clinical studies investigating the effects of microbiota-driven therapy on the reduction of indoxyl sulfate (IS) and p-cresyl sulfate (PCS) concentrations in chronic kidney disease (CKD) patients are controversial. To the best of our knowledge, this is the first study to assess systematically the effects of microbiota-driven therapy on circulating IS and PCS concentrations in CKD patients.
Introduction
Chronic kidney disease (CKD) is characterized by the accumulation of protein-bound uremic toxins such as indoxyl sulfate (IS), p-cresyl sulfate (PCS), p-cresyl glucuronide, and indole-3-acetic acid (IAA), which all originate in the gut (1). Protein-bound uremic toxins originate from the intestinal microbial metabolism of the aromatic amino acids tyrosine, phenylalanine, and tryptophan (1). In the distal part of the colon, tyrosine and phenylalanine are converted into p-cresol and tryptophan is converted into indole and IAA (1, 2). Moreover, CKD is accompanied by an inflammatory response and barrier dysfunction in the intestine, which could increase the translocation of gut-derived uremic toxins into the systemic circulation and further contribute to high cardiovascular morbidity and mortality (3–5). IS and PCS are generated from the intestinal microbial breakdown of tryptophan and tyrosine, respectively, and they remain in the blood in CKD patients because of impaired renal clearance (6–8). Among the identified uremic toxins, IS and PCS are closely associated with cardiovascular diseases and mortality (9–11). However, there is currently no effective strategy for eliminating IS and PCS (12).
Considering the important roles of gut microbiota–derived metabolites in regulating CKD progression, new therapies aimed at restoring the synbiotic intestinal environment and improving metabolic disorders are promising strategies to either delay or reverse CKD progression (13). An increasing number of studies over recent years have suggested that microbiota-driven therapy, referring to the intake of probiotics, prebiotics, and/or synbiotics, may decrease the relative quantity of protein-fermenting intestinal flora and consequently reduce the production of uremic toxins (14, 15). Probiotics refer to living microorganisms that can improve the intestinal microbiota profile by increasing the number of beneficial bacteria (16). Prebiotics are nonliving indigestible fibers that may stimulate the growth or activity of beneficial microorganisms in the gut (17). The combination of prebiotics and prebiotics, namely, synbiotics, acts synergistically to improve host gastrointestinal health (18). Therefore, microbiota-driven therapy may be an appealing therapeutic strategy to reduce the IS and PCS concentrations.
However, the findings of clinical studies investigating the effects of microbiota-driven therapy on the reduction of IS and PCS concentrations in CKD patients are controversial. Some studies illustrated that microbiota-driven therapy decreased IS and PCS concentrations, whereas other studies did not observe any beneficial effects of microbiota-driven therapy (17–20). Therefore, this systematic review and meta-analysis was designed to assess the effects of microbiota-driven therapy on circulating IS and PCS concentrations by pooling all available randomized controlled trials (RCTs).
Methods
Protocol and registration
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (21). The protocol for this review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42021269146).
Data sources and searches
One of the authors (LC) conducted a search of several databases—PubMed, EMBASE, and the Cochrane Collaboration Library databases of controlled trials—from inception to 22 July, 2021. The search terms included (“probiotic” OR “prebiotic” OR “synbiotic” OR “VSL” OR “Bifidobacterium” OR “Lactococcus” OR “Enterococcus” OR “Saccharomyces” OR “Lactobacillus” OR “Streptococcus” OR “Bacillus” OR “Clostridium” OR “Escherichia coli” OR “Propionibacterium” OR “GanedenBc” OR “LAFTI B94” OR “Mutaflor” OR “Actimel” OR “Cultura” OR “Camptothecin” OR “Yakult” OR “Proviva” OR “Enterobacteriaceae” OR “Vifit” OR “Verum” OR “Bio-K+”) AND (“indoxyl sulfate” OR “indoxyl” OR “p-cresyl” OR “p-cresyl sulfate”). Supplemental Table 1 presents the search strategy. Moreover, the references of selected studies and relevant review articles were screened to identify eligible studies that were not found through the database searches. There was no restriction on the language or publication year.
Eligibility criteria
We considered studies to be eligible if they met the following criteria: 1) randomized, controlled, parallel, or crossover trial; 2) the intervention group received microbiota-driven therapy, and the control group received placebo; and 3) the outcomes assessed the effects of microbiota-driven therapy on circulating IS and PCS concentrations.
We excluded studies in which 1) the outcomes had not been clearly stated and 2) a dietary or drug co-intervention was not applied in all intervention or placebo groups. If multiple articles reported the same or overlapping data, those articles with a longer duration of the intervention or a larger sample size were included in this study.
Data extraction process
Two authors (LC and HQ) independently abstracted data from the included studies. They recorded the following information (when available): the first author, journal, publication year, study design, intervention duration, number of patients in the groups, country of origin, ages, proportion of men, type of intervention (probiotics, prebiotics, synbiotics), and outcomes. Any discrepancies in opinion were resolved by consulting another author (DZ). We contacted the first or corresponding author of the included studies for specific information if the required data were not available in the published article.
Risk of bias
The methodological quality of the eligible studies was evaluated based on the recommendation of the Cochrane Handbook (22). Two authors (LC and HQ) independently assessed the risk of bias of the included studies by using the Cochrane tool (RoB 2.0). The categorization of “low risk,” “high risk,” or “some concerns” was applied to the included studies according to the following domains: randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results.
Statistical analysis
We calculated the median/mean with the SE from all included studies and unified the units of measurement. Continuous data in the meta-analysis were used to analyze the weighted mean difference (WMD) with the 95% CI. For crossover studies, we planned to use data from the first period, if these were available. Where only combined data for both periods were reported, we treated the study as if it was a parallel study, drawing attention to the potential bias that this confers, and interpreted the results accordingly (23). We assessed the heterogeneity using the I2 test. If significant heterogeneity was not present (I2 < 50%), we used a fixed-effects model to pool the outcomes; we used a random-effects model when significant heterogeneity was present (I2 ≥ 50%). Subgroup analyses were conducted based on the intervention duration or published year. Sensitivity analysis was performed to test the reliability of the results by sequentially eliminating each of the included studies. Publication bias was assessed by funnel plots, Egger regression tests, and Begg–Mazumdar correlation tests. Statistical analysis was performed using Stata version 12 software (StataCorp).
Results
Study selection and characteristics
Figure 1 presents the process of study selection. The reference lists of the published reviews yielded 523 records (225 from PubMed, 241 from EMBASE, and 57 from the Cochrane Library). Of these, 205 were excluded as duplicates, leaving 318 for screening. We excluded 192 during the initial screening phase based on the title and abstract. For the remaining 126 studies, we undertook full-text screening and eliminated 65 studies. Then, 61 full-text articles were assessed for eligibility. Finally, 14 studies (19, 20, 24–35) were included in the qualitative analysis, involving 513 individuals in the quantitative analysis (257 in the microbiota-driven therapy group and 256 in the placebo group). Table 1 shows the characteristics of the individual studies included in the meta-analysis.
FIGURE 1.
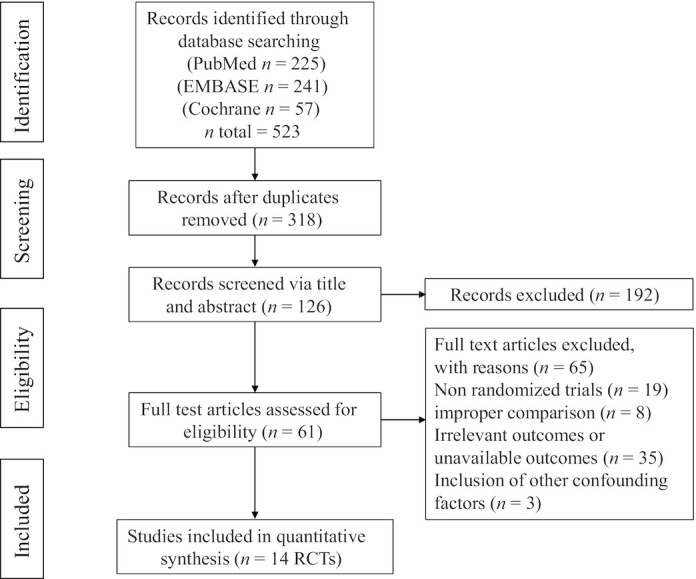
Flow diagram for the study selection process. RCT, randomized controlled trial.
TABLE 1.
Basic characteristics of the included studies1
| Age, y | Men, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors (ref.) | Duration | Inclusion criteria | Patients (T/P) | Country | T | P | T | P | Intervention | Primary outcome |
| Lim et al. (20) | 24 wk | End-stage CKD patients on hemodialysis | 50 (25/25) | China | 61.5 ± 10.3 | 56.28 ± 12.36 | 40 | 40 | Probiotics | Circulating IS and PCS concentrations |
| Borges et al. (24) | 12 wk | CKD patients undergoing hemodialysis | 33 (16/17) | Brazil | 53.6 ± 11.0 | 50.3 ± 8.5 | 68.75 | 58.82 | Probiotics | Circulating IS and PCS concentrations |
| Mafra et al. (25) | 12 wk | Nondialysis CKD patients | 16 (7/9) | Brazil | 64.2 ± 7.2 | 63.4 ± 8.1 | 71.43 | 55.56 | Probiotics | Circulating IS and PCS concentrations |
| Biruete et al. (26) | 4 wk | Hemodialysis patients | 24 (12/12) | USA | 55 ± 10 | 55 ± 10 | 50 | 50 | Prebiotics | Circulating IS and PCS concentrations |
| Esgalhado et al. (19) | 4 wk | Hemodialysis patients | 31 (15/16) | Brazil | 56.0 ± 7.5 | 53.5 ± 11.5 | 46.67 | 68.75 | Prebiotics | Circulating IS and PCS concentrations |
| Armani et al. (27) | 12 wk | Nondialysis CKD patients | 46 (23/23) | Brazil | 61.9 ± 11.4 | 53.4 ± 16.0 | 52 | 52 | Prebiotics | Circulating IS and PCS concentrations |
| de Andrade et al. (28) | 4 wk | CKD patients undergoing peritoneal dialysis | 26 (15/11) | Brazil | 55 ± 12 | 55 ± 12 | 53.8 | 53.8 | Prebiotics | Circulating IS and PCS concentrations |
| Ramos et al. (29) | 12 wk | Non-dialysis-dependent CKD patients | 46 (23/23) | Brazil | 62.2 ± 11.3 | 52.8 ± 16.1 | 54.2 | 53.8 | Prebiotics | Circulating IS and PCS concentrations |
| Li et al. (30) | 12 wk | Patients receiving peritoneal dialysis | 30 (15/15) | China | 30.88 ± 13.94 | 30.88 ± 13.94 | 60 | 60 | Prebiotics | Circulating IS and PCS concentrations |
| Mafra et al. (31) | 4 wk | Hemodialysis patients | 26 (12/14) | Brazil | 54.8 ± 7.9 | 54.2 ± 11.9 | 42 | 71 | Prebiotics | Circulating IS concentration |
| Mirzaeian et al. (32) | 8 wk | Hemodialysis patients (CKD stage 5) | 42 (21/21) | Iran | 58.30 ± 11.3 | 69.74 ± 42.87 | 66.6 | 69.5 | Synbiotics | Circulating IS concentration |
| Lopes et al. (33) | 7 wk | Hemodialysis individuals | 58 (29/29) | Brazil | 63.17 ± 11.16 | 63.03 ± 10.77 | 58.6 | 72.4 | Synbiotics | Circulating IS and PCS concentrations |
| Rossi et al. (34) | 6 wk | Predialysis adults with CKD | 62 (31/31) | Australia | 69 ± 10 | 69 ± 10 | 41 | 70 | Synbiotics | Circulating IS and PCS concentrations |
| Cosola et al. (35) | 8 wk | Stage IIIb–IV CKD patients | 23 (13/10) | Italy | 51.5 ± 2.8 | 51.0 ± 4.3 | 53.8 | 70 | Synbiotics | Circulating IS and PCS concentrations |
1CKD, chronic kidney disease; IS, indoxyl sulfate; P, placebo group; PCS, p-cresyl sulfate, T, treatment group.
Quality assessment
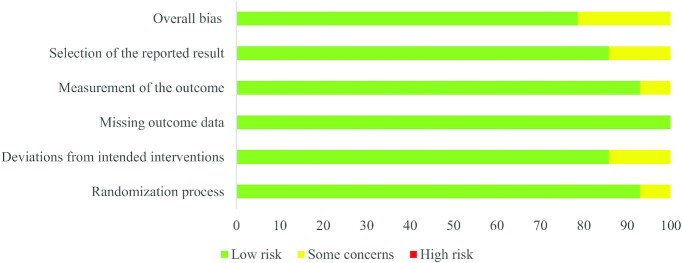
Figures 2 and 3 summarize the risk of bias of the included studies according to the different quality domains using the Cochrane RoB 2.0 tool (22). Among the 14 studies, the randomization process had a low risk of bias in 92.9% of the studies (13 of 14) but there were some concerns in 1 study. Most of the studies had a low risk of bias in the deviations from the intended interventions, whereas 2 had some concerns. The missing outcome data had a low risk of bias for all studies. Moreover, all of the studies except for 1 had a low risk of bias in the measurement of the outcome. The selection of the reported results had a low risk of bias in 85.7% of the studies (12 of 14), and 14.3% (2 of 14) had some concerns.
FIGURE 2.
Risk of bias graph. Values are percentages.
FIGURE 3.
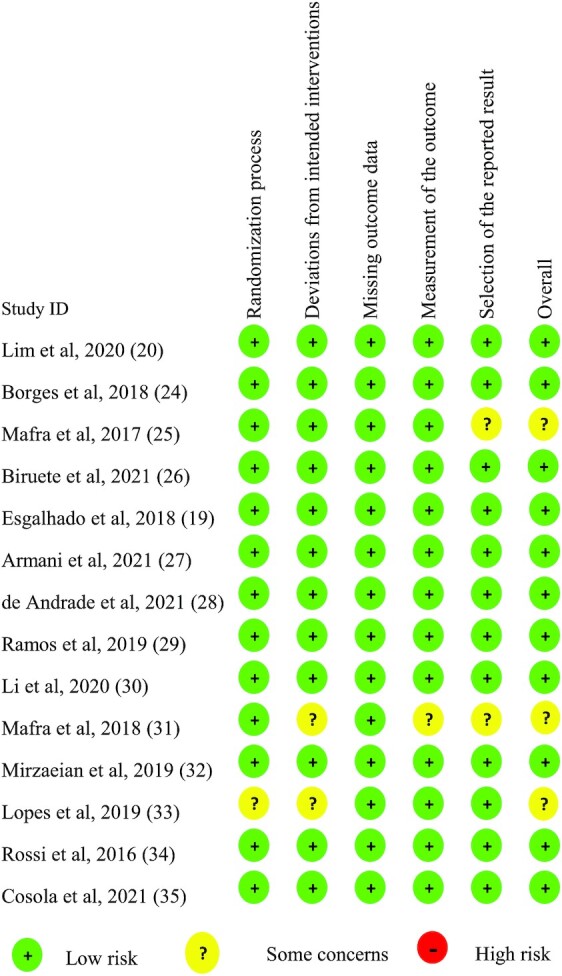
Risk of bias summary.
Meta-analysis results of the RCTs
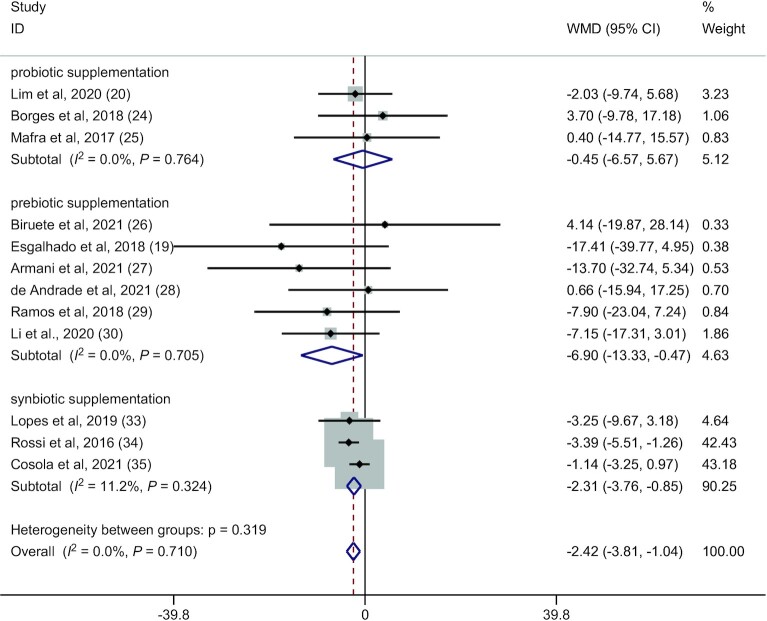
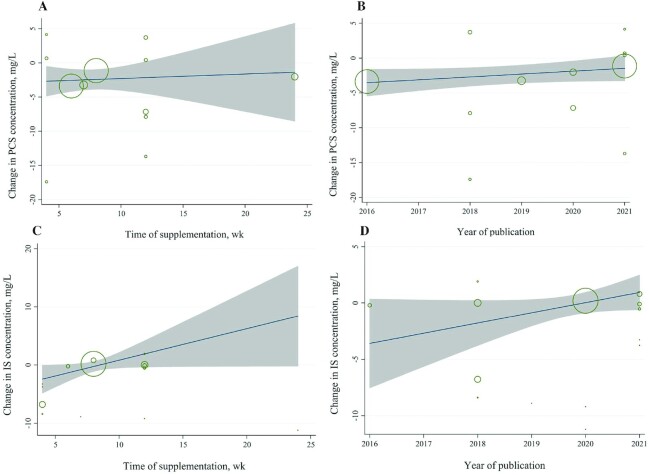
Twelve RCTs (19, 20, 24–30, 33–35) (224 patients in the microbiota-driven therapy group and 221 patients in the placebo group) assessed the effects of microbiota-driven therapy on circulating PCS concentration. Compared with placebo, microbiota-driven therapy decreased the circulating PCS concentration (WMD: −2.42 mg/L; 95% CI: −3.81, −1.04 mg/L; P = 0.001) (Figure 4). There was no obvious heterogeneity for the outcome of circulating PCS concentration (I2 = 0.0%, P = 0.710) (Figure 4). Subgroup analysis showed that the reduction in circulating PCS concentration was greater in participants receiving synbiotics (WMD: −2.31 mg/L; 95% CI: −3.76, −0.85 mg/L; P = 0.002) than in those receiving prebiotics (WMD: −6.90 mg/L; 95% CI: −13.33, −0.47 mg/L; P = 0.036). However, probiotic supplementation had no statistically significant effects on circulating PCS concentration (WMD: −0.45 mg/L; 95% CI: −6.57, 5.67 mg/L; P = 0.885). Meta-regression showed that the effects of microbiota-driven therapy were not associated with the supplementation time (exp.: 1.04; 95% CI: 0.63, 1.71; P = 0.857) or year of publication (exp.: 1.51; 95% CI: 0.78, 2.94; P = 0.196) (Figure 5A, B).
FIGURE 4.
Forest plot for circulating p-cresyl sulfate concentration: microbiota-driven therapy compared with placebo (fixed-effect model). WMD, weighted mean difference.
FIGURE 6.
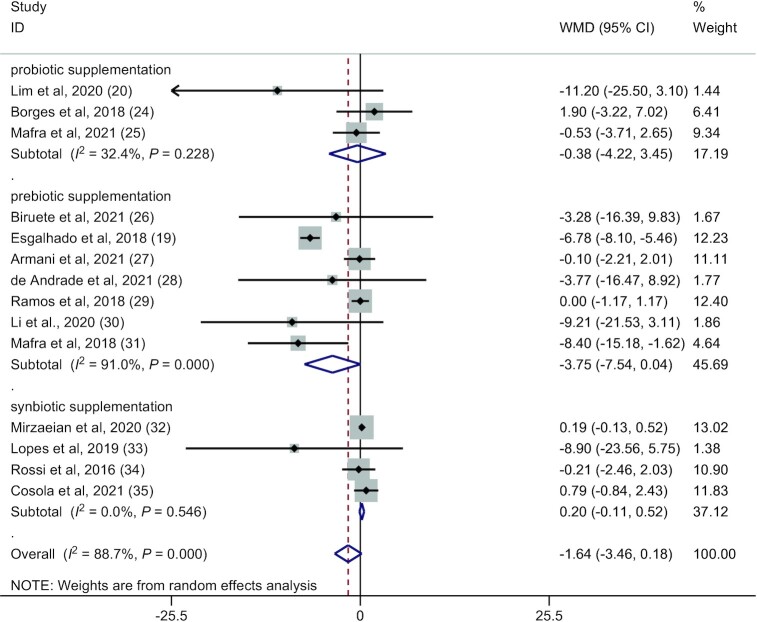
Forest plot for circulating indoxyl sulfate concentration: microbiota-driven therapy compared with placebo (random-effect model). WMD, weighted mean difference.
Fourteen RCTs (19, 20, 24–35) (257 patients in the microbiota-driven therapy group and 256 patients in the placebo group) assessed the effects of microbiota-driven therapy on circulating IS concentration. Compared with the placebo, microbiota-driven therapy did not decrease the circulating IS concentration (WMD: −1.64 mg/L; 95% CI: −3.46, 0.18 mg/L; P = 0.077) (Figure 6). The subgroup analyses demonstrated that probiotic, prebiotic, or synbiotic supplementation did not decrease the circulating IS concentration. Significant heterogeneity for the outcome of circulating IS concentration was observed for prebiotic supplementation (I2 = 91%, P < 0.001) (Figure 6), which had no obvious correlation with the microbiota-driven therapy time (exp.: 1.38; 95% CI: 0.80, 2.39; adjusted R2 = 28.66%; P = 0.22) or the year of publication by meta-regression (exp.: 1.38; 95% CI: 0.39, 4.99; adjusted R2 = −7.52%; P = 0.59) (Figure 5C, D).
FIGURE 5.
Meta-regression plot. (A, B) Change in PCS concentration according to (A) supplementation time of microbiota-driven therapy and (B) year of publication. (C, D) Change in IS concentration according to (C) supplementation time of microbiota-driven therapy and (D) year of publication. IS, indoxyl sulfate; PCS, p-cresyl sulfate.
Sensitivity analysis
To ensure the reliability of the present meta-analysis, we performed a sensitivity analysis to assess the robustness of the pooled WMDs by eliminating each study 1 at a time sequentially, which indicated that the heterogeneity among the studies did not change significantly regarding the outcomes of circulating IS and PCS concentrations. However, subgroup results of the sensitivity analysis for prebiotic supplementation revealed that the studies by Armani et al. (27) and Ramos et al. (29) were influential studies. Removing these 2 studies led to a statistically significant reduction in the circulating IS concentration (n = 6 studies; WMD: −3.10 mg/L; 95% CI: −3.96, −2.24 mg/L; P = 0.000) and PCS concentration (n = 6 studies; WMD: −5.01 mg/L; 95% CI: −6.10, −3.92 mg/L; P = 0.000). Moreover, because of the considerable heterogeneity observed (I2 = 91%) for circulating IS concentration in the prebiotic supplementation groups, meta-analyses were performed with the prebiotic supplementation groups in an attempt to identify the source of heterogeneity. The results showed that the study by Esgalhado et al. (19) was the source of heterogeneity. Removing the study decreased the heterogeneity (I2 = 35.7%).
Publication bias
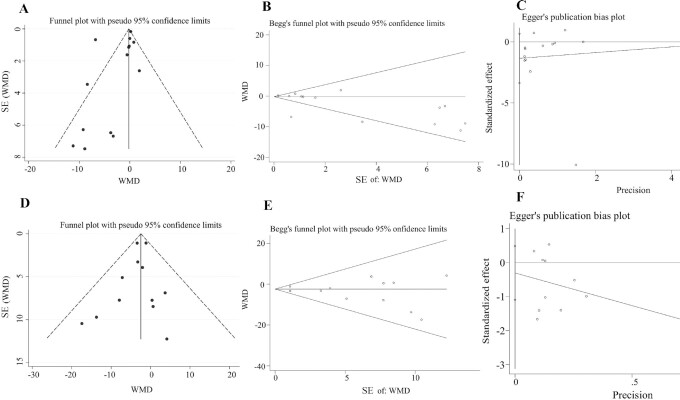
Three methods, including funnel plot, Egger regression test, and the Begg–Mazumdar correlation test, were used to assess publication bias for the effects of microbiota-driven therapy on circulating IS concentration, and no obvious publication bias was found (Begg–Mazumdar correlation test, Kendall's score = −39, continuity-corrected z = −2.08, continuity-corrected P = 0.037; Egger regression test, coefficient: −1.35; 95% CI: −3.37, 0.67; P = 0.17) (Figure 7A–C). For the effects of microbiota-driven therapy on circulating PCS concentration, there was also no evidence of publication bias according to the results of a funnel plot, the Begg–Mazumdar correlation test (Kendall's score = −8, continuity-corrected z = 0.48, continuity-corrected P = 0.63), and the Egger regression test (coefficient: −0.30; 95% CI: −1.09, 0.49; P = 0.42) (Figure 7D–F).
FIGURE 7.
Publication bias. (A) Funnel plot, (B) Begg test, and (C) Egger test for circulating IS concentration. (D) Funnel plot, (E) Begg test, and (F) Egger test for circulating PCS concentration. IS, indoxyl sulfate; PCS, p-cresyl sulfate; WMD, weighted mean difference.
Discussion
Although the intestinal microbiome plays an important role in regulating CKD, few meta-analyses have paid attention to metabolic parameters to assess the effects of microbiota-driven therapy for CKD patients. The present study systematically reviewed and quantitatively synthesized 14 RCTs including 513 CKD patients to elaborate the effects of microbiota-driven therapy on circulating IS and PCS concentrations in CKD patients with or without dialysis. The results showed that microbiota-driven therapy may have no effect on decreasing circulating IS concentration but may significantly decrease the circulating PCS concentration. Moreover, subgroup analyses revealed that probiotic supplementation could not reduce the circulating IS or PCS concentrations.
The results of this meta-analysis demonstrated that microbiota-driven therapy significantly decreased the circulating PCS concentration. There was no significant heterogeneity among the studies. Meta-regression analysis including the duration of the intervention and the year of publication also verified no obvious heterogeneity. Subgroup analysis of the intervention types (probiotics, prebiotics, or synbiotics) showed that prebiotic and synbiotic supplementation were effective in decreasing the circulating PCS concentration, but the reduction was not significant for probiotics. Given the important role of circulating PCS, a reduction in the circulating PCS concentration may be associated with decreased cardiovascular events in individuals with CKD, and this effect will help in the management of CKD-associated cardiovascular diseases. Prebiotics and synbiotics provide nutrition for probiotics and act synergistically to promote healthy gastrointestinal bacteria (36), which may explain the results of the subgroup analyses observed in this meta-analysis. Additional studies should compare the effects of prebiotics and synbiotics on protein-bound uremic toxins to verify their clinical significance. The mechanisms that contribute to a reduction in the circulating PCS concentration may be attributed to prebiotics and synbiotics regulating intestinal tyrosine metabolism. Moreover, prebiotics enter the gut and are selectively utilized, which increases bacterial growth and the functionality of specific genera or species. Bacteria that respond to prebiotic intake can influence the microbiota composition through antimicrobial agents and competitive interactions, possibly reducing the PCS concentration (17). Synbiotics have immunoregulatory effects and antioxidant properties, which may contribute to reducing the PCS concentration.
Our results discovering no statistically significant reduction in the circulating IS concentration were consistent with findings from a previous meta-analysis by McFarlane et al. (37), which involved 4 studies of microbiota-driven therapy in CKD and found no difference between intervention and placebo. In addition, Liu et al. (38) also demonstrated that microbiota-driven therapy did not affect serum IS concentration. Moreover, there was significant heterogeneity (I2 = 88.7) among the included studies, and the heterogeneity mainly came from prebiotic supplementation (I2 = 91), which had no obvious correlation with prebiotic supplementation time or the year of publication in the meta-regression analysis. Interestingly, compared with the placebo, prebiotic supplementation in 6 RCTs decreased the circulating IS concentration (WMD: −2.672 mg/L; 95% CI: −3.47, −1.88 mg/L; P = 0.00) according to the results from the fixed-effects model, which was in contrast to the results from the random-effects model (WMD: −3.75 mg/L; 95% CI: −7.54, 0.04 mg/L; P = 0.053). There are some reasons for this result. First, these enrolled studies included different doses and types of prebiotic supplementation. Second, some patients received dialysis treatment, whereas others did not. Dialysis treatment is an important strategy for decreasing uremic toxins in the clinic (39). Third, the small number of studies included in this meta-analysis limited the power of the analysis, and the results should be reassessed when more RCTs are available in the future. There was no reduction in circulating IS concentration after microbiota-driven therapy, indicating that microbiota-driven therapy may not affect the intestinal metabolism of tryptophan. However, the sensitivity analysis of prebiotic supplementation revealed that removing the 2 studies by Armani et al. (27) or Ramos et al. (29) led to a significant reduction in the circulating IS concentration. The reason for this may be that prebiotic supplementation has the same effects as the placebo on increasing or decreasing the IS concentration.Therefore, we currently cannot determine the effects of prebiotic supplementation on IS concentration, and it is necessary to further investigate the roles of prebiotic supplementation on IS concentration in future clinical research.
The mechanisms underlying the effects of microbiota-driven therapy on CKD are diverse. During the progression of CKD, unbalanced microbiota and a dysregulated gut epithelial barrier function accelerate the translocation of noxious luminal contents into the systemic circulation, leading to an inflammatory response, oxidative stress, and dysfunction of lipid metabolism (14, 40). The protective mechanisms of microbiota-driven therapy in CKD mainly include improvements of the metabolic profiles, which are related to regulating bacteria that produce profitable metabolites, and restricting the generation of uremic retention solutes (such as trimethylamine-N-oxide) (41, 42). IS and PCS are separately generated from tryptophan and tyrosine metabolism by intestinal bacteria. Our results showed different effects of microbiota-driven therapy on the circulating IS and PCS concentrations, which may be attributed to their influence on different intestinal bacteria, thus influencing tryptophan and tyrosine metabolism. Moreover, microbiota-driven therapy improves the barrier function of the gut epithelium by upregulating tight junction proteins, improving the transepithelial electrical resistance, and promoting mucus secretion (16). Microbiota-driven therapy has antimicrobial effects by reducing the local pH in the gut lumen and stimulating the production of secretory IgA, has anti-inflammatory effects by regulating inflammatory pathway activity, and improves the host immune system by regulating the expression of immune-related genes, thus regulating the IS and PCS concentrations (43). In particular, the mechanisms of prebiotics in decreasing the circulating PCS concentration may be associated with their promotion of extensive metabolic interactions among bacterial species present in the gastrointestinal microbial community (44). Therefore, there is considerable potential for indirect stimulation of the growth of other microbes within the community through the utilization of by-products of other community members, thus improving the PCS concentration.
Previous studies have described the toxic effects of 2 prototype protein-bound uremic toxins, namely IS and PCS (8–10). The toxic effects include endothelial dysfunction, smooth muscle cell lesions, coagulation disturbances, leukocyte activation, cardiac fibrosis and hypertrophy, insulin resistance, and atherosclerosis, all of which are associated with cardiovascular diseases and increase the propensity for mortality and cardiovascular events in CKD patients (5, 45, 46). Moreover, Bogiatzi et al. (47) found that the plasma concentration of PCS, but not IS, was significantly higher among patients with severe atherosclerosis, which was not explained by traditional risk factors; in linear regression, PCS was a significant predictor of atherosclerosis, but IS was not. Dialysis treatment partially eliminates uremic toxins, but it is difficult for IS and PCS to be entirely excreted from the body (12). Therefore, novel strategies are urgently required to reduce the IS and PCS concentrations. Microbiota-driven therapy has gradually become a hot spot for clinical studies to verify its effects on IS and PCS to lay a foundation for further treatment in the clinic. Given that there are few studies systematically investigating these effects, we conducted a meta-analysis to illustrate the effects of microbiota-driven therapy on circulating IS and PCS concentrations.
Study strengths
There are several strengths of this review. This meta-analysis is the first, to our knowledge, to assess systematically and quantitatively the association between microbiota-driven therapy and circulating IS and PCS concentrations in CKD patients with or without dialysis in RCTs. Moreover, we conducted subgroup analyses and meta-regression for the treatment duration, the type of intervention, and the year of publication on the overall effect sizes.
Study limitations
However, there are some limitations of this analysis that deserve discussion. First, there was no standardization of the microbiota-driven therapy or the route of administration, which caused some challenges in comparing the studies. Most of the published studies were from Brazil, and limited data were available from other countries. Moreover, the studies did not demonstrate any effects of microbiota-driven therapy on IS and PCS concentrations in excrement and urine. Another limitation was the heterogeneity in comparing the effects of microbiota-driven therapy on the circulating IS concentration, which might originate from the individual varieties, treatment duration, different types and dosages of microbiota-driven therapy, and other factors. In addition, the plasma concentration of metabolic products in the intestinal microbiome is closely associated with estimated glomerular filtration rate (eGFR), serum creatinine, and the adequacy of dialysis, and this study did not exclude the influence of eGFR, serum creatinine, or the adequacy of dialysis on the IS and PCS concentrations (48). Additional studies are expected to consider the foregoing limitations and investigate their relation with other clinically significant endpoints, such as the incidence and severity of cardiovascular diseases and CKD, in the general population.
Conclusions
This systematic review and meta-analysis found that microbiota-driven therapy among CKD patients with or without dialysis may have no effect on the circulating IS concentration, but it reduced the circulating PCS concentration. Moreover, larger multicenter trials with standardized interventions and changes in the gut microbial composition are required to investigate the effects of microbiota-driven therapy for decreasing IS and PCS concentrations in CKD patients.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—LC and HQ: initiated the idea of this review; LC: collected the data and wrote the manuscript; JS: researched the data; LC, JS, and XM: analyzed the data; DS and HQ: reviewed the manuscript; DS: contributed to the discussions; and all authors: contributed to the manuscript preparation and read and approved the final manuscript.
Notes
Supported by China Association for Science and Technology Youth Talent Promotion Project of Chinese Society 539 grant 2020-QNRCI-02 (to HQ) and National Natural Science Foundation of China project grant 8210143377 (to HQ).
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; IAA, indole-3-acetic acid; IS, indoxyl sulfate; PCS, p-cresyl sulfate; RCT, randomized controlled trial; WMD, weighted mean difference.
Contributor Information
Li Chen, Xi yuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China; Peking University Traditional Chinese Medicine Clinical Medical School (Xi yuan), Beijing, China; National Clinical Research Center for Chinese Medicine Cardiology, Beijing, China.
Junhe Shi, Xi yuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China.
Xiaojuan Ma, Xi yuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China; National Clinical Research Center for Chinese Medicine Cardiology, Beijing, China.
Dazhuo Shi, Xi yuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China; Peking University Traditional Chinese Medicine Clinical Medical School (Xi yuan), Beijing, China; National Clinical Research Center for Chinese Medicine Cardiology, Beijing, China.
Hua Qu, Xi yuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China; National Clinical Research Center for Chinese Medicine Cardiology, Beijing, China; National Medical Products Administration Key Laboratory for Clinical Research and Evaluation of Traditional Chinese Medicine, Beijing, China.
References
- 1. Gryp T, De Paepe K, Vanholder R, Kerckhof F-M, Van Biesen W, Van de Wiele T, Verbeke F, Speeckaert M, Joossens M, Couttenye MMet al. Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease. Kidney Int. 2020;97(6):1230–42. [DOI] [PubMed] [Google Scholar]
- 2. Meijers B, Evenepoel P, Anders HJ. Intestinal microbiome and fitness in kidney disease. Nat Rev Nephrol. 2019;15(9):531–45. [DOI] [PubMed] [Google Scholar]
- 3. Tanaka K, Watanabe T, Takeuchi A, Ohashi Y, Nitta K, Akizawa T, Matsuo S, Imai E, Makino H, Hishida Aet al. Cardiovascular events and death in Japanese patients with chronic kidney disease. Kidney Int. 2017;91(1):227–34. [DOI] [PubMed] [Google Scholar]
- 4. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–52. [DOI] [PubMed] [Google Scholar]
- 5. Wang Z, Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. 2018;9(5):416–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis. 2016;67(3):483–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwiatkowska I, Hermanowicz JM, Mysliwiec M, Pawlak D. Oxidative storm induced by tryptophan metabolites: missing link between atherosclerosis and chronic kidney disease. Oxid Med Cell Longev. 2020;2020:6656033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gryp T, Vanholder R, Vaneechoutte M, Glorieux G. p-Cresyl sulfate. Toxins. 2017;9(2):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Opdebeeck B, Maudsley S, Azmi A, De Maré A, De Leger W, Meijers B, Verhulst A, Evenepoel P, D'Haese PC, Neven E. Indoxyl sulfate and p-cresyl sulfate promote vascular calcification and associate with glucose intolerance. J Am Soc Nephrol. 2019;30(5):751–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakano T, Katsuki S, Chen M, Decano JL, Halu A, Lee LH, Pestana DVS, Kum AST, Kuromoto RK, Golden WSet al. Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activation via the interplay of OATP2B1 and Dll4-notch signaling. Circulation. 2019;139(1):78–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meijers BK, Claes K, Bammens B, de Loor H, Viaene L, Verbeke K, Kuypers D, Vanrenterghem Y, Evenepoel P. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol. 2010;5(7):1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dobre M, Meyer TW, Hostetter TH. Searching for uremic toxins. Clin J Am Soc Nephrol. 2013;8(2):322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang T, Richards EM, Pepine CJ, Raizada MK. The gut microbiota and the brain–gut–kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14(7):442–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ondrussek-Sekac M, Navas-Carrillo D, Orenes-Piñero E. Intestinal microbiota alterations in chronic kidney disease and the influence of dietary components. Crit Rev Food Sci Nutr. 2021;61(9):1490–502. [DOI] [PubMed] [Google Scholar]
- 15. Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25(4):657–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25(5):716–29. [DOI] [PubMed] [Google Scholar]
- 17. Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16(10):605–16. [DOI] [PubMed] [Google Scholar]
- 18. Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NMet al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17(11):687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Esgalhado M, Kemp JA, Azevedo R, Paiva BR, Stockler-Pinto MB, Dolenga CJ, Borges NA, Nakao LS, Mafra D. Could resistant starch supplementation improve inflammatory and oxidative stress biomarkers and uremic toxins levels in hemodialysis patients? A pilot randomized controlled trial. Food Funct. 2018;9(12):6508–16. [DOI] [PubMed] [Google Scholar]
- 20. Lim PS, Wang HF, Lee MC, Chiu L-S, Wu M-Y, Chang W-C, Wu TK. The efficacy of Lactobacillus-containing probiotic supplementation in hemodialysis patients: a randomized, double-blind, placebo-controlled trial. J Ren Nutr. 2021;31(2):189–98. [DOI] [PubMed] [Google Scholar]
- 21. Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SEet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SMet al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 23. Stedman MR, Curtin F, Elbourne DR, Kesselheim AS, Brookhart MA. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2011;40(6):1732–4. [DOI] [PubMed] [Google Scholar]
- 24. Borges NA, Carmo FL, Stockler-Pinto MB, de Brito JS, Dolenga CJ, Ferreira DC, Nakao LS, Rosado A, Fouque D, Mafra D. Probiotic supplementation in chronic kidney disease: a double-blind, randomized, placebo-controlled trial. J Ren Nutr. 2018;28(1):28–36. [DOI] [PubMed] [Google Scholar]
- 25. Mafra D, Borges NA, Nakau L, Dolenga C, Bergman P, Stenvinkel P. Effects of probiotic supplementation on uremic toxins levels in non-dialysis CKD patients. Nephrol Dial Transplant. 2017;32(suppl_3):iii587–8. [Google Scholar]
- 26. Biruete A, Cross TL, Allen JM, Kistler BM, de Loor H, Evenepoel P, Fahey GC Jr, Bauer L, Swanson KS, Wilund KR. Effect of dietary inulin supplementation on the gut microbiota composition and derived metabolites of individuals undergoing hemodialysis: a pilot study. J Ren Nutr. 2021;31(5):512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Armani RG, Carvalho AB, Ramos CI, Hong V, Bortolotto LA, Cassiolato JL, Oliveira NF, Cieslarova Z, do Lago CL, Klassen Aet al. Effect of fructooligosaccharide on endothelial function in CKD patients: a randomized controlled trial. Nephrol Dial Transplant. 2021;2021:gfaa335. [DOI] [PubMed] [Google Scholar]
- 28. de Andrade LS, Sardá FAH, Pereira NBF, Teixeira RR, Rodrigues SD, de Lima JD, Dalboni MA, Aoike DT, Nakao LS, Cuppari L. Effect of unripe banana flour on gut-derived uremic toxins in individuals undergoing peritoneal dialysis: a randomized, double-blind, placebo-controlled, crossover trial. Nutrients. 2021;13(2):646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramos CI, Armani RG, Canziani MEF, Dalboni MA, Dolenga CJR, Nakao LS, Campbell KL, Cuppari L. Effect of prebiotic (fructooligosaccharide) on uremic toxins of chronic kidney disease patients: a randomized controlled trial. Nephrol Dial Transplant. 2019;34(11):1876–84. [DOI] [PubMed] [Google Scholar]
- 30. Li L, Xiong Q, Zhao J, Lin X, He S, Wu N, Yao Y, Liang W, Zuo X, Ying C. Inulin-type fructan intervention restricts the increase in gut microbiome–generated indole in patients with peritoneal dialysis: a randomized crossover study. Am J Clin Nutr. 2020;111(5):1087–99. [DOI] [PubMed] [Google Scholar]
- 31. Mafra D, Esgalhado M, Macedo RD, Kemp JA, Paiva B, Nakao LS, Cunha DB, Borges NA. Resistant starch supplementation reduces indoxyl sulfate levels in hemodialysis patients: a randomized, double-blind, crossover, placebo-controlled study. J Am Soc Nephrol. 2018;29:72. [Google Scholar]
- 32. Mirzaeian S, Saraf-Bank S, Entezari MH, Hekmatdoost A, Feizi A, Atapour A. Effects of synbiotic supplementation on microbiota-derived protein-bound uremic toxins, systemic inflammation, and biochemical parameters in patients on hemodialysis: a double-blind, placebo-controlled, randomized clinical trial. Nutrition. 2020;73:110713. [DOI] [PubMed] [Google Scholar]
- 33. Lopes R, Theodoro JMV, da Silva BP, Queiroz VAV, de Castro Moreira ME, Mantovani HC, Hermsdorff HH, Martino HSD. Synbiotic meal decreases uremic toxins in hemodialysis individuals: a placebo-controlled trial. Food Res Int. 2019;116:241–8. [DOI] [PubMed] [Google Scholar]
- 34. Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM, Szeto C-C, McWhinney BC, Ungerer JPJ, Campbell KL. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol. 2016;11(2):223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cosola C, Rocchetti MT, di Bari I, Acquaviva PM, Maranzano V, Corciulo S, Di Ciaula A, Di Palo DM, La Forgia FM, Fontana Set al. An innovative synbiotic formulation decreases free serum indoxyl sulfate, small intestine permeability and ameliorates gastrointestinal symptoms in a randomized pilot trial in stage IIIb-IV CKD patients. Toxins. 2021;13(5):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Moayyedi P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547–61.; quiz 1546, 1562. [DOI] [PubMed] [Google Scholar]
- 37. McFarlane C, Ramos CI, Johnson DW, Campbell KL. Prebiotic, probiotic, and synbiotic supplementation in chronic kidney disease: a systematic review and meta-analysis. J Ren Nutr. 2019;29(3):209–20. [DOI] [PubMed] [Google Scholar]
- 38. Liu T, Wang X, Li R, Zhang ZY, Fang J, Zhang X. Effects of probiotic preparations on inflammatory cytokines in chronic kidney disease patients: a systematic review and meta-analysis. Curr Pharm Biotechnol. 2021;22(10):1338–49. [DOI] [PubMed] [Google Scholar]
- 39. Niwa T. Update of uremic toxin research by mass spectrometry. Mass Spectrom Rev. 2011;30(3):510–21. [DOI] [PubMed] [Google Scholar]
- 40. Meijers B, Jouret F, Evenepoel P. Linking gut microbiota to cardiovascular disease and hypertension: lessons from chronic kidney disease. Pharmacol Res. 2018;133:101–7. [DOI] [PubMed] [Google Scholar]
- 41. Tsai Y-L, Lin T-L, Chang C-J, Wu T-R, Lai W-F, Lu C-C, Lai H-C. Probiotics, prebiotics and amelioration of diseases. J Biomed Sci. 2019;26(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Felizardo RJF, Watanabe IKM, Dardi P, Rossoni LV, Câmara NOS. The interplay among gut microbiota, hypertension and kidney diseases: the role of short-chain fatty acids. Pharmacol Res. 2019;141:366–77. [DOI] [PubMed] [Google Scholar]
- 43. Schluter J, Peled JU, Taylor BP, Markey KA, Smith M, Taur Y, Niehus R, Staffas A, Dai A, Fontana Eet al. The gut microbiota is associated with immune cell dynamics in humans. Nature. 2020;588(7837):303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014;25(9):1897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Holmar J, de la Puente-Secades S, Floege J, Noels H, Jankowski J, Orth-Alampour S. Uremic toxins affecting cardiovascular calcification: a systematic review. Cells. 2020;9(11):2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bogiatzi C, Gloor G, Allen-Vercoe E, Reid G, Wong RG, Urquhart BL, Dinculescu V, Ruetz KN, Velenosi TJ, Pignanelli Met al. Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis. 2018;273:91–7. [DOI] [PubMed] [Google Scholar]
- 48. Pignanelli M, Bogiatzi C, Gloor G, Allen-Vercoe E, Reid G, Urquhart BL, Ruetz KN, Velenosi TJ, Spence JD. Moderate renal impairment and toxic metabolites produced by the intestinal microbiome: dietary implications. J Ren Nutr. 2019;29(1):55–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.