ABSTRACT
Blueberries have been extensively studied for the health benefits associated with their high phenolic content. The positive impact of blueberry consumption on human health is associated in part with modulation of proinflammatory molecular pathways and oxidative stress. Here, we review in vitro studies examining the anti-inflammatory and antioxidant effects of blueberry phytochemicals, discuss the results in terms of relevance to disease and health, and consider how different blueberry components modulate cellular mechanisms. The dampening effects of blueberry-derived molecules on inflammation and oxidative stress in cell models have been demonstrated through downregulation of the NF-κB pathway and reduction of reactive oxygen species (ROS) and lipid peroxidation. The modulatory effects of blueberry phytochemicals on the mitogen-activated protein kinase (MAPK) pathway and antioxidant system are not as well described, with inconsistent observations reported on immune cells and between models of endothelial, dermal, and ocular inflammation. Although anthocyanins are often reported as being the main bioactive compound in blueberries, no individual phytochemical has emerged as the primary compound when different fractions are compared; rather, an effect of whole blueberry extracts or synergy between different phenolic and nonphenolic extracts seems apparent. The major molecular mechanisms of blueberry phytochemicals are increasingly defined in cell models, but their relevance in more complex human systems needs further investigation using well-controlled clinical trials, in which systemic exposures to blueberry-associated molecules are measured concurrently with physiologic indices of inflammation and oxidative stress.
Keywords: blueberry, inflammation, in vitro, NF-κB, oxidative stress, phytochemicals, polyphenols
Statement of Significance: Blueberries have been extensively studied for their health benefits using in vitro models of inflammation and oxidative stress, however a comprehensive review of the in vitro literature is lacking. This review provides the first in depth overview of in vitro models of inflammation and oxidative stress using blueberry polyphenols.
Introduction
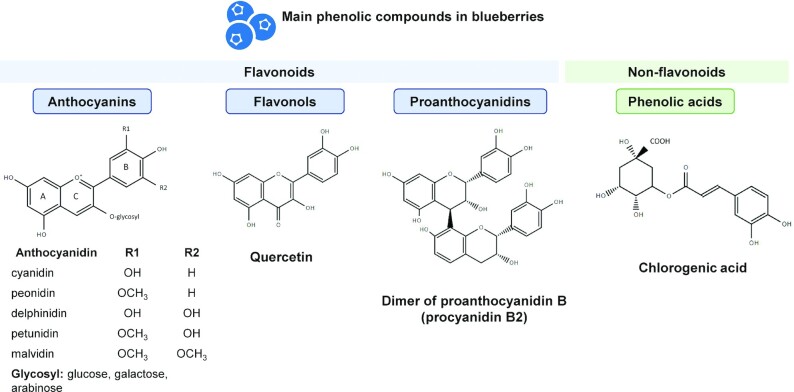
Blueberries (Vaccinium genus) have been widely studied for their high phytonutrient content, particularly phenolic compounds. Dietary polyphenols found in blueberries consist of flavonoids (anthocyanins, flavonols, proanthocyanidins) and phenolic acids (Figure 1). Anthocyanins are the pigments responsible for the color of berries and blueberries have one of the highest anthocyanin contents among foods (1). The individual anthocyanin profile of blueberries is complex and contains 5 of the 6 anthocyanidins commonly present in food: malvidin, cyanidin, delphinidin, petunidin, and peonidin (2, 3). The glycoside moieties attached to the anthocyanidin are predominantly galactose, arabinose, and glucose (2, 4), with all combinations of the 5 anthocyanidins and 3 sugars found across blueberry cultivars. Blueberries are also rich in flavonols, with a predominance of quercetin derivatives (5, 6), and proanthocyanidins, formed by polymerization of catechin and/or epicatechin units (7). Nonflavonoid phenolic acids are mainly represented by chlorogenic acid, which results from the esterification of caffeic acid with a quinic acid molecule (2, 7). Blueberry phytochemicals used in the treatment of cells in vitro often consist of a whole extract, delivered as a reconstituted powder, juice, or pomace (residue after the juice extraction), concentrated or not. Specific classes of phytochemicals such as polyphenol-rich extracts and phenolic fractions, including anthocyanins, phenolic acids, and proanthocyanidins are prepared using solvent extraction and purified through solid phase extraction.
FIGURE 1.
Classes and structures of the main phenolic compounds found in blueberries.
Polyphenols found in blueberries have been shown to contribute to their health benefits (8). A number of reviews discuss the association between blueberry consumption and cardiovascular health (8, 9), inflammatory markers (10), type 2 diabetes, neuroprotection, and ocular health (8). These claims on the health benefits of blueberry consumption are supported by epidemiological studies (11, 12), animal studies (13, 14), and diverse cell culture models (15, 16). Randomized controlled trials have investigated antioxidant and anti-inflammatory effects of blueberry in the context of hypertension, cardiovascular diseases, arthritis, insulin resistance, and metabolic syndrome and supplemented with doses between 20 and 50 g of wild blueberry powder, equivalent to 1 to 2 cups of fresh blueberries daily for 6 to 16 wk (17–21). However, few reported direct modulation of molecular markers via blueberry supplementation, including circulating inflammatory cytokines and adhesion molecules.
To complete and extend the body of literature covering the in vivo physiological effects of blueberry feeding, the current review considers mechanisms of action by focusing on in vitro responses to blueberry components. Evaluation of the bioactive potential of berry phytochemicals or extracts often uses cell models (e.g., immortalized cell lines induced with LPS, proinflammatory cytokines, oxidant species, or physical stimulus), which serve as controlled, simplified systems (22, 23). Numerous limitations exist regarding cell culture conditions and the artificial environment in which the cells are maintained, because they are not completely representative of the body's physiology (24). In general, cells are treated with parent compounds, disregarding potential host and microbial metabolism between consumption and the moment compounds reach the target organ (25, 26). Cells are also not always treated with amounts representative of physiological concentrations in the body, which can be low due to the limited absorption of polyphenols (27, 28). They are relatively simple to access and maintain, and provide insights into the cellular mechanisms of the studied compounds (29). Although blueberry phytochemicals may impact a multitude of health-related mechanisms, we focused on 2 intrinsically related systems (inflammation and oxidative stress), the regulation of which are central to health, and when dysregulated, underlie many disease outcomes. The objective of this narrative review is to discuss observations related to the modulatory role of blueberry phytochemicals on key pathways implicated in systems, and to consider the results from a physiological perspective.
Search strategy
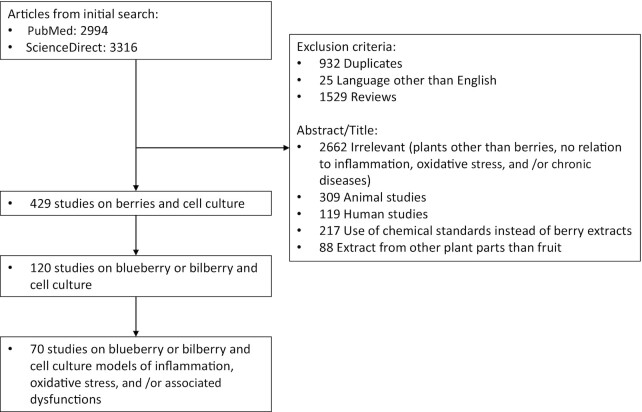
Search queries containing the keywords “inflammation”, “anti-inflammatory”, “oxidative stress”, “cell culture”, “in vitro”, and “berry” (title/abstract/keywords) were conducted in the PubMed and Science Direct databases. The search was conducted for articles through to August 2021. Duplicates, reviews, articles written in languages other than English, and studies using animal models or human participants were excluded (Figure 2) with the caveat that studies focusing on animals and/or humans but containing complementary cell-based experiments were included and an evaluation of the cell model findings used in the current review. From all studies on berries and cell culture-based models retrieved, only studies using blueberries were included. In this article, the term “blueberry” encompasses fruits from the Vaccinium genus described as blueberries, including V. angustifolium, V. corymbosum, V. ashei, V. uliginosum, and the European blueberry also referred to as bilberry, V. myrtillus. In addition, relevant references from earlier reviews were manually entered. A total of 70 articles related to blueberry and cell culture models of inflammation, oxidative stress, and related conditions were included.
FIGURE 2.
Flow diagram of included and excluded studies.
Blueberry phytochemicals: in vitro models of inflammation and oxidative stress
Immune system and inflammation models
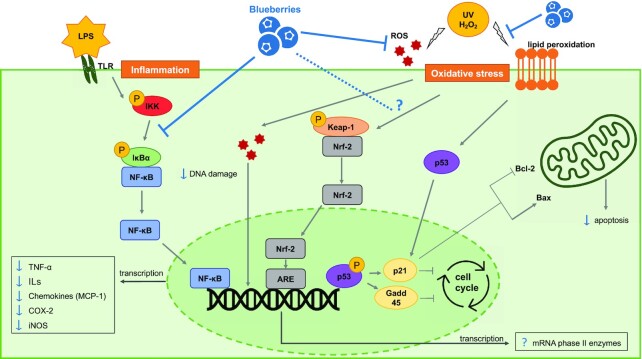
Inflammation is the innate immune system reaction to a stimulus generated by pathogens, damaged cells, carcinogens, toxic compounds, changes in concentrations of reactive oxygen species (ROS), and some foods or metabolites (e.g., certain SFAs and fatty acylcarnitines). General response mechanisms of inflammation have been extensively reviewed elsewhere (30–32). In brief, the NF-κB and mitogen-activated protein kinase [MAPK, subdivided into extracellular-signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38] inflammatory pathways are activated following an external stimulus (e.g., pathogen) and/or by proinflammatory cytokines (e.g., TNF-α). Their activation generates the production of proinflammatory cytokines, including TNF-α, IL-1β, and IL-6, which upon release mobilize immune cells (33). Abnormal activation of inflammation-associated proteins, including NADPH oxidase (NOX) (34), inducible NO synthase (iNOS), and cyclo-oxygenase (COX)-2 (35), and failure to resolve the infection or injury can lead to chronic inflammation linked to diseases and cardiometabolic dysfunction.
Studies on murine cell lines include the extensively used monocyte/macrophage RAW 264.7 cells and primary bone marrow-derived macrophages (BMDMs) that can be readily activated by binding ligand to several toll-like receptors (TLRs). Numerous studies on blueberry treatment of murine cell lines use TLR4-activated models, through the addition of the bacterial cell wall component LPS at doses varying from 0.01 to 10 μg/mL (details in Table 1). This produces a strong inflammatory response through, but not limited to, the expression of IL-6, TNF-α, or NO (36).
TABLE 1.
Cell-culture-based studies on the effect of blueberry phytochemicals on immune system and inflammation models
| Cell line | Treatments (dose, duration) | Challenge (dose, duration) | Controls | N1 | Parameters measured (method) | Effects of treatment compared to challenge | Ref. |
|---|---|---|---|---|---|---|---|
| Whole and/or polyphenolic extracts: | |||||||
| RAW 264.7 | Water and ethanol extracts (5–625 μg/mL, 24 h2) | LPS (10 μg/mL, 24 h) | Vehicle, challenge | 3 | Viability (MTT), NO (Griess) | Dose-dependent ↓ NO production | (52) |
| RAW 264.7 | Polyphenol extract (75 μg/mL, 30 min) | LPS (40 ng/mL, 6 h) | Vehicle, challenge | Gene signals (microarray hybridization), COX-2, IL-1β, IL-6, PTGS2, TNC, TNF, CCL22, IFI1, IFI47 gene expression (qPCR) | ↓ TNF, IL-1β, IL-6, TNC, PTGS2, and COX-2 | (37) | |
| RAW 264.7 | Polyphenol extract (dose study: 10–400 μg/mL, 48 h2 time study: 100 μg/mL, 6–72 h2) | LPS (1 μg/mL, 24 h) | Vehicle, challenge, positive (tea polyphenols, 100 μg/mL) | 3 | IL-1β, IL-6, IL-12 gene expression (qPCR) | Better ↓ IL-1β and IL-6 for doses 200 μg/mL and less. Dose- and time-dependent ↓of IL-12 | (47) |
| RAW 264.7 | Polyphenol extract (100 μM, 1 h + 24 h3) | LPS (5 μg/mL, 24 h) or conditioned media from 3T3-L1 adipocytes (24 h) | Vehicle, challenge | 3 | NO (Griess), iNOS, TNF-a, IL-10, MCP-1 (qPCR) | ↓ of LPS- and conditioned media-induced NO secretion. ↓ of LPS-induced IL-10 gene expression, no effect on LPS-induced iNOS and TNF-α gene expression. ↓ of conditioned media-induced iNOS expression | (53) |
| RAW 264.7 | Polyphenol extract (2.7–8.8 mg fresh blueberry equivalent/mL, 24 h) | LPS (100 ng/mL, 18 h) | Not described | 3 | TNF-α, IL-6 (ELISA) | ↓ of TNF-α and IL-6, with varying inhibitory concentrations depending on the blueberry cultivar. Negative correlation between cytokines IC50 and phenolic acid content of the extracts | (42) |
| RAW 264.7 | Whole and polyphenol extracts (50–150 μg/mL, 1 h + 4 h3) | LPS (1 μg/mL, 4 h) | Vehicle, challenge, positive (DEX, 10 μM) | 3 | Viability (MTT), COX-2, iNOS, IL-6, IL-1β gene expression (qPCR) | ↓ of IL-1β gene expression by polyphenol extract, but not COX-2, iNOS, and IL-6. No effect of whole extract | (45) |
| RAW 264.7 | Freeze-dried pomace extract (10 μg/mL, 1 h) | LPS (10 ng/mL, 4 h) | Vehicle, challenge, positive (DEX, 10 μM) | 3 | Viability (MTT), COX-2, iNOS, IL-6, IL-1β gene expression (qPCR) | ↓ of the 4 gene expressions | (40) |
| RAW 264.7 | Whole extract (250 μg/mL for hybrid cultivars, 50 μg/mL for lowbush, 1 h) | LPS (1 μg/mL, 18 h) | Vehicle, challenge, positive (DEX, 10 μM) | 2 | Viability (MTT), ROS (DCFDA), NO (Griess), IL-1β, COX-2, iNOS, IL-6 gene expression (qPCR) | ↓ proinflammatory cytokines transcription. Better effect for cultivars with higher anthocyanin content | (39) |
| RAW 264.7 | Whole extract (0.2–0.5 mg/mL, 3 h) | LPS (1 μg/mL, 24 h) | Vehicle, challenge | 3–4 | Cell viability (MTT), TNF-α, IL-6, IL-10 (ELISA), COX-2, iNOS (WB) | Slight ↓ IL-6 at 0.2 but not 0.5 mg/mL. Dose-dependent inhibition of TNF-α. ↑ IL-10, no effect on COX-2 and iNOS production | (41) |
| RAW 264.7 | Extractable (EPP) and nonextractable (NEPP) polyphenol extracts (10–400 μg/mL, 6–72 h2) | LPS (1 μg/mL, 24 h) | Vehicle, challenge, positive (tea polyphenols, 100 μg/mL) | 3 | NO (detection kit), iNOS, COX-2 gene expression (qPCR), NF-κB (WB) | ↓ NO production, iNOS gene expression and inhibition of NF-κB activation by both extracts. Higher inhibitory effect of EPP than NEPP on COX-2 gene expression | (50) |
| U-937, differentiated intomacrophages | Phenolic extract (15.6–500 μg/mL, 30 min for luciferase assay, 2 h for ELISA) | F. nucleatum (MOI 100, 6 h for luciferase assay, 48 h for ELISA) | Vehicle, challenge, positive (BAY-11–7082, inhibitor of NF-κB, 25 μM) | 3 | Viability (MTT), NF-κB activity (luciferase activity), TNF-α, IL-1β, IL-6, CXCL8, MMP-8, MMP-9 (ELISA) | Dose-dependent ↓ of NF-κB activation, IL-1β, IL-6, and TNF-α production. ↓ in CXCL8 (doses ≥ 125 μg/mL). ↓ MMP-8 and MMP-9 secretion | (56) |
| THP-1 (monocytes) | Blueberry and bilberry pomace (0.04–1 mg/mL, 1.5 h) | LPS (1 μg/mL, 1 h) | Vehicle, challenge | 3 | Viability (flow cytometry), NF-κB (flow cytometry), IL-8 (ELISA), CCL22, IL-8, IL-10, IL-6, TNF-α, IP10, IL-23, MMP-9 (multiplex assay), IL-6, TGF-β, TNF-α, IL-10, IL-23 (qPCR), COX-2 (fluorometric kit) | ↓ of NF-κB translocation by bilberry extract (1 mg/mL) and blueberry extracts (0.2 and 1 mg/mL). ↓ of proinflammatory cytokines by both berries, except for CCL22 and IL-10. Greater ↓ of COX-2 by blueberry (dose-dependent) than bilberry pomace | (59) |
| THP-1 (monocytes) | Whole extract (10 μg/mL, 20 min + 20 min3 for WB or 24 h3 for other assays) | TNF-α or IFN-γ (100 ng/mL, 30 min or 24 h) | Vehicle, challenge | 4 | STAT1, STAT3, ERK, JNK, p38, NF-κB phosphorylation (WB), TNF-α, MCP-1, IL-6 (ELISA), MCP-1, IL-6, ICAM-1, T-bet, IL-12, IL-1β, IL-10 (qPCR) | IFN-γ challenge: ↓ in STAT1 and STAT3 phosphorylation. No effect on MAPK pathway. Significant ↓ of TNF-α and MCP-1, complete inhibition of IL-6. ↑ in IL-10 gene expression | (61) |
| TNF-α challenge: ↓ MCP-1 and TNF-α secretion. ↑ NF-κB phosphorylation and ↓ ERK. No significant changes for p38 and JNK. ↑ gene expression of IL-6, IL-8, MCP-1, IL-1β, IL10, and ↓ ICAM-1 | |||||||
| THP-1, differentiated intomacrophages | Polyphenol extract (1.53 mg/mL, 2 h) | LPS (5 μg/mL, 48 h) | Vehicle, challenge | 5–6 | Viability (LDH), caspase-3 (fluorescence), NO (Griess), TNF-α, IL-6, and IL-10 gene expression (qPCR), IL-6 secretion (Luminex xMAP) | No effect of blueberry treatment on NO secretion. ↑ TNF-α and ↓ IL-10 gene expression compared to LPS alone. ↓ IL-6 secretion, but no significant effect on IL-6 gene expression | (60) |
| PBMCs | Anthocyanin (10 μg/mL, 30 min3 for WB or 24 h3 for other assays) | IFN-γ (100 ng/mL, 30 min or 24 h) | Vehicle, challenge | 4 | STAT1, STAT3, and p38 phosphorylation (WB), MCP-1, IL-6, ICAM-1, (qPCR) | ↓ STAT1 and STAT3 phosphorylation but ↑ p38 phosphorylation. ↓ ICAM-1, MCP-1, and IL-6 gene expression | (61) |
| Phenolic and polyphenolic fractions: | |||||||
| RAW 264.7 | Whole and polyphenol extracts, anthocyanin, proanthocyanidin phenolic acid fractions (50 μg/mL, 1 h) | LPS (1 μg/mL, 6 h) | Vehicle, challenge, positive (DEX, 10 μM) | 3 | Viability (MTT), COX-2, iNOS, IL-6, IL-1β, MCP-1 gene expression (qPCR) | ↓ COX-2 by all fractions except phenolic acid. Better ↓ iNOS and IL-1β by proanthocyanidin. Better ↓ of MCP-1 expression by anthocyanin | (43) |
| U-937, differentiated intomacrophages | Proanthocyanidin fraction, viability tests (3.9–500 μg/mL, 1 h3), other tests (31.25–125 μg/mL, 2 h for ELISA, 30 min for NF-κB) | Leukotoxin (viability: 1 μg/mL, 1 h), A. actinomycetemcomitans LPS (1 μg/mL, 6–24 h) | Vehicle, challenge, positive (BAY-11–7082, 25 μM) | 3 | Viability (MTT), IL-1β, IL-6, CXCL8, TNF-α, MMP-3, and MMP-9 (ELISA) | ↓ leukotoxin cytotoxic effect on cells by proanthocyanidin extract. Dose-dependent ↓ of proinflammatory cytokine and MMPs production | (58) |
| Anthocyanin fractions: | |||||||
| RAW 264.7 | Anthocyanin (400–1200 μg/mL, 1 h) | LPS (1 μg/mL, 24 h) | Vehicle, challenge, positive (DEX, dose not described) | 3 | Viability (MTT), NO (Griess), PGE2, INF-γ, IL-1β, and IL-6 (ELISA), MCP-1, IL-6, TNF-α, IL-1β, COX-2 gene expression (qPCR), COX-2, NF-κBp65 proteins (WB) | Dose- and time-dependent ↓ of NO production. Dose-dependent ↓ of cytokines. No significant effect on TNF-α gene expression. ↓ COX-2 protein expression and NF-κB p65 | (51) |
| RAW 264.7 and BMDM | Anthocyanin (RAW 264.7: 2.5–20 μg/mL, 12 h + 1 to 3 h3; BMDM: 20 μg/mL, 12 h + 3–12 h3) | LPS (RAW 264.7: 100 ng/mL, 1 h for WB, 3 h for other assays; BMDM: 3–12 h) | Vehicle, challenge | 3–9 | RAW 264.7: TNF-α, IL-1β gene expression (qPCR), TNF-α cytokines (ELISA), NF-κB translocation (WB) | RAW 264.7: ↓ IL-1β and TNF-α gene expressions and TNF-α secretion. ↓ NF-κB translocation | (44) |
| BMDM: ROS (DCFDA), TNF-α, IL-1β, NOX-1 gene expression (qPCR) | BMDM: no alteration in ROS production after 3 h but ↓ in cells expressing Nrf-2 after 12 h. ↓ IL-1β and NOX-1 in both cell phenotypes | ||||||
| U-937, differentiated intomacrophages | Anthocyanin (1–25 μg/mL, 1 h or 3 h2) | LPS (1 μg/mL, 1 h2 or 3 h) | Vehicle, challenge | 3 | TNF-α gene expression (qPCR) | ↓ TNF-α, significant when the treatment was administered before the LPS induction | (57) |
| THP-1 | Anthocyanin (10 μg/mL, 20 min + 30 min3 for WB or 24 h3 for qPCR) | IFN-γ (100 ng/mL, 30 min or 24 h) | Vehicle, challenge | 3 | STAT phosphorylation (WB), IFN-γ R1, IFN-γ R2, SOCS1, SOCS3 (qPCR) | ↓ IFN-γ R1 expression induced by challenge and ↑ in IFN-γ R2 expression decreased by challenge. ↓ SOCS1 and SOCS3 mRNA expression. ↓ STAT phosphorylation, but not through inhibition of protein tyrosine phosphatase activation | (62) |
| Others: | |||||||
| RAW 264.7 | Polyphenol extract (19–78 μg/mL, 1 h), volatile extract (7–28 ng/mL, 1 h) | LPS (100 ng/mL, 24 h) | Vehicle, challenge | 3 | Viability (MTS), NO (Griess), IL-6, TNF-α, PGE2 (ELISA), COX-2, NF-κB, IκBα (WB) | ↓ proinflammatory markers by both extracts. Higher ↓ of IL-6 by volatile extracts | (38) |
N, number of experimental replicates, as stated by the authors. BMDM, bone marrow-derived macrophages; CCL22, CC chemokine ligand 22; COX-2, cyclo-oxygenase-2; CXCL, chemokine (C-X-C motif) ligand; DCFDA, 2′,7′-dichlorofluorescin diacetate; DEX, dexamethasone; ERK, extracellular-signal-regulated kinase; ICAM, intercellular adhesion molecule-1; IFI, interferon-inducible protein; iNOS, inducible nitric oxide synthase; IκBα, NF κ B inhibitor α; JNK, c-Jun N-terminal kinase; LDH, lactate dehydrogenase; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemoattractant protein-1; MMP, matrix metalloproteinase; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium); MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide; Nrf-2, Nuclear factor erythroid 2-related factor 2; NOX-1, NADPH oxidase; PGE2, prostaglandin E2; PTGS2, prostaglandin-endoperoxide synthase; ROS, reactive oxygen species; STAT, signal transducers and activators of transcription; TNC, Tenascin C; SOCS, suppressor of cytokine signaling; WB, Western blot.
Blueberry treatment administered after challenge.
Blueberry and challenge in coadministration. If no symbol is indicated, the cells were treated with the blueberry extracts first before (Table 2) challenge.
The capacity of blueberry components to reduce proinflammatory marker gene expression and secretion in LPS-induced murine cell models (RAW 264.7 and BMDMs) has been shown in several studies, although the anti-inflammatory effect seems to be carried out by an array of compounds rather than an individual blueberry phenolic fraction. Diverse blueberry products, including blueberry pomace, whole blueberry extracts, polyphenol-rich extracts, or specific phenolic fractions lowered the production of cytokines by the cells in a dose-dependent manner. Further details on the treatments and doses used in the studies are reported in Table 1. For instance, blueberry phytochemicals reduced the gene expression and secretion of proinflammatory cytokines induced by LPS, particularly IL-6 (37–43), IL-1β (37, 39, 40, 43–45), and TNF-α (37, 38, 41, 42, 44) in RAW 264.7 cells and BMDMs compared with the LPS-induced control. However, it is not clear which fraction or specific phenolic compound exerts a more potent effect, especially since their activities seem to be cytokine specific. For example, Esposito et al. (43) reported a better ability of a proanthocyanidin fraction to reduce IL-1β compared with the polyphenol extract, anthocyanin, or phenolic acid fractions. However, IL-6 gene expression was not suppressed by that same proanthocyandin fraction, and monocyte chemoattractant protein (MCP)-1 expression (43), a chemokine that regulates monocyte infiltration (46), was inhibited by the blueberry anthocyanins but not by the other phenolic fractions. Although the large variation between the blueberry treatment doses used across different reports hinders direct comparisons between the studies, most have shown a dose-dependent regulation of cytokines (39, 41, 44). Cheng et al. (47), however, reported 40–60% inhibition of IL-1β gene expression with ≤200 μg/mL blueberry extract, but no effect when the treatment dose was increased to 400 μg/mL (47). This observation was tentatively explained by the increased phagocytic activity of the macrophages in the presence of a high polyphenol concentration.
iNOS is an enzyme responsible for the synthesis of NO, a mediator secreted by neutrophils and macrophages to induce vasodilation, mediate the immune response, or regulate apoptosis (48, 49). After stimulation of RAW 264.7 cells by LPS, blueberry extracts inhibited iNOS gene expression (40, 50) and NO production (38, 39, 50–53), with extracts from anthocyanin-rich cultivars (39) and blueberry proanthocyanidin fractions (43) being particularly effective (Table 1). COX-2 also plays a central role in the induction of inflammation. It is involved in the formation of prostaglandins, including prostaglandin E2 (PGE-2) responsible for the induction of pain (54). Blueberry extracts have consistently reduced COX-2 gene expression in RAW 264.7 cells (37–40, 43, 50, 51) (Table 1), although Mueller et al. (41) and Grace et al. (45) did not report a significant reduction of iNOS and COX-2 gene expression by whole blueberry or polyphenol-rich extracts. Although most studies have focused on blueberry polyphenols, Gu et al. (38) reported the anti-inflammatory effect of the volatile extracts of several berries compared with their phenolic counterpart. Volatile and phenolic blueberry extracts showed similar inhibitory effects on inflammatory cytokines, NO, and COX-2 production, even though the volatile fraction was tested at a lower concentration than the phenolic fraction. This provides initial evidence that the volatile fraction of blueberries also contains molecules with anti-inflammation properties, but this remains to be validated.
The anti-inflammatory activities of blueberry phytochemicals have also been demonstrated in cell lines derived from humans (23), including the U-937 and THP-1 monocyte-like cells, that can be differentiated into macrophages after stimulation with phorbol-12-myristate-13-acetate (PMA) (55), and human primary peripheral blood mononuclear cells (PBMCs). In the context of blueberry studies, a variety of compounds have been used to induce inflammation, including Fusobacterium nucleatum bacteria (56), LPS (57–60), or cytokines (61, 62) (Table 1). Blueberry extracts exerted an inhibitory effect toward cytokine secretion (56, 58–60) and matrix metalloproteinase (MMP)-8 and 9 production (56, 58), in cells triggered by bacteria or LPS. Blueberry extracts decreased TNF-α gene expression induced by LPS in THP-1 monocytes (59) and U937 macrophages (57), but on the contrary, increased its expression in THP-1 differentiated macrophages (60). The regulatory effect reported in most studies was associated with a decrease in NF-κB translocation in THP-1 cells (59). In PBMCs and THP-1 cells alternatively induced with either IFN-γ or TNF-α, the effects of a blueberry treatment were less robust (Table 1). Cytokine secretion and adhesion molecule gene expression were inhibited by a blueberry extract in IFN-γ-induced PBMCs, but the same blueberry extract further increased the proinflammatory marker secretion when the cells were induced with TNF-α (61). These observations were tentatively explained by looking at the pathways activated by the different cytokines (IFN-γ or TNF-α): the signal transducers and activators of transcription (STAT) pathway activation was inhibited by coincubation of IFN-γ with the berry treatment, but NF-κB was enhanced by the addition of TNF-α combined with a blueberry extract (61). A follow-up study demonstrated that the IFN-γ receptor 2, responsible for transducing the signal conveyed by the proinflammatory cytokines, was inhibited by the blueberry anthocyanins (62). These observations on cell models induced with non-LPS ligands suggest that the immunomodulatory effects of blueberry compounds are context and pathway specific.
In summary, blueberry phenolic and polyphenolic extracts have been shown to dampen inflammation in RAW 264.7, U-937, BMDMs, and PBMCs challenged with inflammation inducers, through the reduction of proinflammatory cytokine gene expression and secretion, and inhibition of NF-κB translocation to the nucleus. No specific fraction emerges as being more potent, suggesting a general effect of multiple phytomolecules rather than a single compound. More studies are warranted to better define molecular targets of blueberry-derived molecules and to assess the involvement of TLR-dependent and -independent pathways.
Oxidative stress models
ROS and free radicals are natural by-products of enzymatic reactions produced during metabolism (63, 64). When controlled, ROS production is used for signaling in metabolic processes (65). Environmental factors, lifestyle, and pathologies contribute to an unbalanced state, where ROS production overwhelms the defense capacity of the cells and induces oxidative stress (66). This state leads to protein and nucleic oxidation, and lipid peroxidation, which can impair enzymatic processes, induce breakage of DNA strands, and may lead to cell death (67–69). Endogenous antioxidant defense mechanisms exist in the body to limit the production and deleterious effects of ROS. Superoxide dismutase (SOD), found in the membrane or cytosolic fractions of cells, converts superoxide radicals (O2−) to H2O2 and O2 (70). Glutathione peroxidase (GSH-PX), via the oxidation of glutathione S-transferase, reduces lipid peroxide and converts H2O2 to H2O (71). Enzymes, including DNA glycosylases, repair damaged DNA (72). Oxidation and inflammation are intricately related, as cytokines and chemokines secreted by inflammatory cells can trigger ROS production. In turn, ROS activate proinflammatory pathways, including NF-κB, and sustain the cycle of oxidative and inflammatory stress (10, 65). These conditions favor the development of chronic pathologies such as cancer (73), cardiovascular (74), inflammatory (75), and neurodegenerative diseases (76).
The effect of blueberry phytochemicals on oxidative stress has been evaluated using several cell models, including neurons (77), fibroblasts (78, 79), hepatocytes (80, 81), enterocytes (82–86), and epithelial cells (82, 87). Despite the diversity in models used, studies overlap in terms of the endpoints measured, which focus on evaluation of the modulation in ROS production and lipid peroxidation, increases in antioxidant enzyme activities, and protection of DNA against oxidative damage (Table 2).
TABLE 2.
Cell-culture-based studies on the effect of blueberry phytochemicals on oxidative stress models
| Cell line | Treatments (dose, duration) | Challenge (dose, duration) | Controls | N1 | Parameters measured (method) | Effects of treatment compared to challenge | Ref. |
|---|---|---|---|---|---|---|---|
| Whole and/or polyphenolic extracts: | |||||||
| SH-SY5Y | Lyophilized juice (25–100 μg/mL, 24 h) | H2O2 (100 μM, 30 min) | Vehicle, challenge | 3 | Viability (MTT), ROS (DCFDA), lipid peroxidation (TBARS), antioxidant enzymes activity (spectrophotometric assay) | ↓ intracellular ROS formation and lipid oxidation. ↑ catalase and SOD activity | (77) |
| L-929 | Lyophilized juice (0.05–12.5 mg/mL, 1–1.5 h2) | t-BuOOH (200 μM, 1–1.5 h) | Vehicle, challenge | 3 | ROS (DCFDA) | Dose-dependent ↓ ROS production (dose ≥6.25 mg/mL) | (78) |
| CHO and HT-29 | Pomace (50–200 μg/mL, 20 h) | n/a | Vehicle, positive (CDDO-imidazolide) | 4 | CHO: Nrf-2 luciferase expression (reporter gene assay) | No effect of extract on Nrf-2 luciferase activity nor relative transcription level of phase II enzymes | (82) |
| HT-29: phase II enzyme-transcription (qPCR) | |||||||
| Caco-2 | Whole extract (1.8–7 mg/mL, 2–24 h) | t-BuOOH (100–150 μM, 0.5–1 h) | Vehicle challenge | 3 | Viability (trypan blue exclusion), DNA damage (Comet), free radical generation (electron spin resonance spectroscopy measurements), gene expression (qPCR) | Dose-dependent ↓ DNA damage. ↓ free radical formation at higher concentrations | (83) |
| Phenolic and polyphenolic fractions: | |||||||
| V79-4 | Phenolic extracts, by enzymatic hydrolysates (25–100 μg/mL, 0.5–2 h) | H2O2 (1 mM, 5 min–24 h) | Vehicle challenge | 3 | Viability (MTT), ROS (DCFDA), lipid peroxidation (malondialdehyde measurement), DNA damage (Comet), nuclear morphology (Hoechst staining) | Dose-dependent ↑ ROS scavenging activity. ↓ lipid peroxidation, DNA damage ,and nuclear fragmentation | (79) |
| Caco-2 and HT-29 | Whole extract, anthocyanin, phenolcarbonic acid, polymeric fractions (5–500 μg/mL extract, or corresponding concentration of each fraction in the extract, 1 h) | t-BuOOH (250 μM, 40 min) or Menadione (Caco-2: 6 μM, HT29: 20 μM, 1 h) | Vehicle, challenge | 3–6 | Cytotoxicity (Alamar blue assay), ROS (DCFDA), tGSH concentrations (kinetic assay), oxidative DNA damage (Comet) | Caco-2: ↓ ROS production and DNA damage and ↑ tGSH after 24 h. Fractions: ↓ DNA damage by phenolcarbonic acid fraction only. No modulatory effects of the anthocyanin fraction on Caco-2 | (84) |
| HT-29: Slight ↓ ROS by berry extract after 24 h. No modulation of oxidative DNA damage or tGSH concentrations | |||||||
| Anthocyanin fractions: | |||||||
| Caco-2 and HT-29 | Anthocyanin (0.01–500 μg/mL, 1–24 h) | t-BuOOH (250 μM, 40 min), Menadione (Caco-2: 6 μM, HT29: 20 μM, 1 h) | Vehicle, challenge, positive (quercetin, 30 μM) | 3–6 | Cytotoxicity (Alamar blue assay), ROS (DCFDA), glutathione concentrations (kinetic assay), oxidative DNA damage (Comet) | Caco-2: ↓ ROS production at dose 50 μg/mL. Slight ↓ DNA damage, nonsignificant modulation of tGHS | (85) |
| HT-29: ↓ ROS production (dose 250 μg/mL) | |||||||
| IPEC-1 | Anthocyanin (1.6–200 mg/L, 3–24 h) | AAPH (1–15 mmol/L, 24 h) | Vehicle, challenge | 2 | Viability (MTT), cell cycle (flow cytometer), proliferation (BrdU), ROS (DCFDA) | Dose-dependent ↓ peroxyl radical formation. No protective effect on stress-induced cytotoxicity and cell cycle perturbation | (86) |
| HepG2 | Anthocyanin: water, ethanol, and methanol extracts (75 μg/mL, 12 h) | UV irradiation (4 mJ/cm2) | Vehicle, challenge | 3 | Viability (MTT), γH2AX (immunofluorescence staining), DNA damage (Comet), ROS (DCFDA), p53 and p21 gene expression (qPCR) and protein expression (WB) | Protective effect against DNA damage by methanol extract through ↓ γH2AX phosphorylation and ROS production. ↓ p53 and p21 gene and protein expression by all extracts | (80) |
| HepG2 | Anthocyanin (25–75 μg/mL, overnight) | UV irradiation (30 mJ/cm2) | Vehicle, challenge | 3 | Viability (MTT), morphology (SEM), nuclear morphology (Hoechst staining), cell cycle analysis (flow cytometry), mitochondrial potential (laser confocal scanning microscopy), DNA damage (Comet), Gadd45 and MDM2 gene expression (qPCR), Gadd45, MDM2, p21, and p53 proteins (WB) | ↓ UV damage on cell and nuclear morphology. Restoration of cell cycle and mitochondrial membrane potential. Dose-dependent protection against DNA damage. ↓ Gadd45, MDM2, p21, and p53 expression | (81) |
| HPAEpiC | Anthocyanin (75 μg/mL, 72 h) | Radiation (2 Gy/min, 7.5 min) | Vehicle challenge | — | Caspase-3, Bax, Bcl-2, PKR (qPCR and WB), viability (MTT) | Protective effect against cell death and modulation of Bcl-2, Bax, and caspase 3 gene expression in cells transfected with control small interfering RNA but not PKR-siRNA | (87) |
N, number of experimental replicates, as stated by the authors. AAPH, 2,2-azobis(2-amidinopropane)dihydrochloride; Bax, B-cell lymphoma 2 (Bcl-2) associated X-protein; Bcl-2, B-cell lymphoma 2; BrdU, bromodeoxyuridine; CHO, Chinese hamster ovary; CDDO-imidazolide, 1[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole; DCFDA, 2′,7′-dichlorofluorescin diacetate; Gadd45, growth arrest and DNA damage-45; MDM2, mouse double minute 2; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide; Nrf-2, nuclear factor erythroid 2-related factor-2; PKR, protein kinase R; ROS, reactive oxygen species; SEM, scanning electron microscope; t-BuOOH, tert-butylhydroperoxide; tGSH, total glutathione; SOD, superoxide dismutase; WB, Western blot.
Blueberry and challenge in coadministration. If no symbol is indicated, the cells were treated with the blueberry extracts first before challenge.
The induction of oxidative stress in a variety of cells was attenuated by treatment with blueberry extracts, principally through the decreased formation of ROS (77, 78, 80, 84, 85), but also increased scavenging activity (79), and/or reduction of lipid peroxidation (77, 79). This effect, however, was only partly explained by the regulatory effect of the blueberry compounds on antioxidant enzymes, which were upregulated in neuronal cells treated with blueberry juice (77) and Caco-2 cells incubated with a polyphenol-rich blueberry extract (84). Glutathione concentrations, however, remained unchanged in Caco-2 cells treated with an anthocyanin fraction (85). In addition, a blueberry pomace extract tested on Chinese hamster ovary (CHO) epithelial cells and a human colon cancer cell line (HT-29) failed to stimulate the transcription of detoxification enzymes such as heme oxygenase (HO-1) and NADPH quinone oxidoreductase-1 (NQO-1) (88), both involved in the antioxidant/oxidant balance (89, 90). In contrast with those observations made on blueberry parent compounds, phloroglucinol aldehyde (an anthocyanin degradation colonic product) increased the transcription activity of nuclear factor erythroid 2-related factor 2 (Nrf-2), which when induced by oxidative stress stimulates the transcription of HO-1 and NQO-1 (88). This suggests that blueberry metabolite derivatives may contribute to the antioxidant activity of the berries.
A consequence of oxidation is DNA damage (69) and potentially increases in cell death. Blueberry extracts demonstrated a protection against DNA damage induced by hydroxide peroxide (79) and tert-butylhydroperoxide (t-BuOOH) (83, 85), although in Caco-2 cells, only the blueberry phenolcarbonic acid fraction reduced DNA damage compared with whole blueberry extract, anthocyanin, and polymeric fractions (84). Protective effects in terms of DNA damage and cell death were attributed to blueberry anthocyanin fractions in liver cells (HepG2) and pulmonary epithelial cells (HPAEpiC) exposed to light or ionizing radiation, through modulation of apoptosis and cell cycle regulatory gene expressions (80, 81, 87). However, no improvement of cell cycle perturbation induced by 2,2-azobis(2-amidinopropane)dihydrochloride (AAPH) was reported in intestinal epithelial cells IPEC-1 treated with blueberry anthocyanins (86).
To summarize, blueberry extracts and phenolic fractions demonstrated protective effects against oxidative stress, which were mainly explained through reduction of ROS production and protection against DNA damage induced during oxidation. Additional studies comparing cell models and/or blueberry fractions using similar experimental parameters are necessary to demonstrate the effects of the treatment on antioxidant enzymes, and fully understand the antioxidant contribution of blueberry phytochemicals versus their metabolic by-products. There is also a need to evaluate the potential effects of blueberry volatiles in this regard.
Blueberry phytochemicals, inflammation, and oxidative stress: perspectives on physiological functions
Endothelial and vascular inflammation models
Atherosclerosis, a chronic inflammatory disease of the arterial wall (91), is characterized by the buildup of plaques in arteries and is the most frequent underlying condition for the development of cardiovascular diseases (92). Human umbilical vein endothelial cells (HUVECs) and human microvascular vein endothelial cells (HMVECs) provide a model to study normal as well as oxidation and inflammation-related dysfunctions. Studies on the effect of blueberry compounds in endothelial cell models are detailed in Table 3.
TABLE 3.
Cell culture-based studies on the effect of blueberry phytochemicals on endothelial and cardiovascular models
| Cell line (P#1) | Treatments (dose, duration) | Challenge (dose, duration) | Controls | N2 | Parameters measured (method) | Effects of treatment compared to challenge | Ref. |
|---|---|---|---|---|---|---|---|
| Whole and/or polyphenolic extracts: | |||||||
| HUVEC | Whole extract (1000 μg/mL, 3–24 h) | P2X7 agonist bzATP (300 μM) and antagonist AZ11645373 (100 nM, 24 h) | Vehicle, challenge | 3–4 | Viability and proliferation (Trypan blue), vesiculation (calcein staining), Akt, p38 (WB), P2X7 (qPCR) | ↓ HUVEC vesiculation. ↓ Akt phosphorylation, but not p38. ↓ P2X7 transcription. ↓ or prevention of increase of genes involved in extracellular vesicle release | (104) |
| HUVEC (P#2) | Polyphenol extract (0.1–1 mg/mL, 24 h) | Vehicle, negative (wortmannin, 30 nmol/L, 30 min) | 3 | Viability (Trypan blue), apoptosis (colorimetric assay), Akt (Alpha Screen SureFire Assay), migration (scratch assay), angiogenesis (microscopy) | Dose-dependent ↑ Akt activation. ↑ cell migration and capillary-like tube formation | (108) | |
| HUVEC | Polyphenol extract (0.3–30 μg/mL, 4 h to 11 d3 for tube formation assay) | VEGF-A (10 ng/mL) | Vehicle, challenge | 3–6 | Tube formation assay (coculture with fibroblast), proliferation (WST-8), migration (microscopy), ERK, PLC-γ, Akt (WB) | Dose-dependent ↓ tube formation. ↓ cell proliferation and cell migration. ↓ ERK and Akt phosphorylation but not PLC-γ | (109) |
| HUVEC | Polyphenol extract (40 μg/mL, 3–24 h3) | LPS (100 ng/mL, 3–24 h) | Vehicle, challenge | 3 | Viability (MTT), PLC enzyme gene expression (qPCR), PLC enzyme distribution (immunofluorescence) | Time-dependent ↑ cell viability with blueberry treatment. No significant modulation of PLC expression | (110) |
| RAW 264.7 | Polyphenol extract (2.7–8.8 mg fresh blueberry equivalent/mL, 24 h) | LPS (100 ng/mL, 18 h) | Not described | 3 | miRNA expression (qPCR) | ↓ miRNA expression, generally upregulated during vascular inflammation | (42) |
| Polyphenolic fractions: | |||||||
| HMVEC | Anthocyanin and hydroxycinnamic acids fractions (0.01–0.1 mg/mL, 30 min–2 h) | TNF-α (10–20 ng/mL, 6 h) or H2O2 (100 μM, 2 h) | Vehicle, challenge | — | ROS (DCFDA), lipid peroxidation (fluorescent membrane fatty acid probe), ICAM-1, MCP-1, IL-8 (ELISA), viability (MTT) | ↓ ROS production induced by H2O2. Time-dependent but not dose-dependent protection of membrane fatty acid. ↓ production of IL-8, MCP-1, ICAM-1 induced by TNF-α | (95) |
| HUVEC (+ THP-1) | Anthocyanin and phenolic acid fractions (0.01–10 μg/mL, 24 h) | TNF-α (100 ng/mL, 24 h) | Vehicle, challenge | 3 | Viability (MTT), THP-1 cell adhesion (fluorescence of labeled cells) | ↓ THP-1 adhesion by both fractions | (102) |
| Anthocyanin fractions: | |||||||
| HUVEC (+ THP-1) | Anthocyanin (0.01–10 μg/mL, 24 h4) | TNF-α (100 ng/mL, 24 h) | Vehicle, challenge | 3 | THP-1 cell adhesion (fluorescence of labeled cells) | Dose-dependent ↓of THP-1 adhesion | (103) |
| Others: | |||||||
| EA.hy926 | Blueberry exosome-like nanoparticles (20–40 μg/mL, 3 h) | TNF-α (20 ng/mL, 2 h) | Vehicle, challenge, negative (NAC, 200 μM), positive (H2O2, 50 μM, 1 h) | 3 | Viability (SDB), ROS (fluorescent probe dihydroethidium), inflammatory genes (qPCR) | ↓ ROS production. ↓ endothelial activation and inflammation-related gene expression. ↑ antioxidant gene expression | (97) |
P#, passage number used for the experiments (when specified). bzATP, 2′(3′)-O-(4-benzoylbenzoyl)adenosine-5′-triphosphate tri(triethylammonium); CAT, catalase; DCFDA, 2ʹ,7ʹ-dichlorofluorescin diacetate; ERK, extracellular-signal-regulated kinase; HMVEC, human microvascular vein endothelial cell; HUVEC, human umbilical vein endothelial cell; ICAM, intercellular adhesion molecule; MCP-1, monocyte chemoattractant protein-1; miRNA, microRNA; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide; NAC, N-acetyl-L-cysteine; PLC, phospholipase C; ROS, reactive oxygen species; SDB, Sulforhodamine B; THP-1, Tamm-Horsfall Protein 1; VEGF-A, vascular endothelial growth factor A; WB, Western blot; WST, water-soluble tetrazolium.
N, number of experimental replicates, as stated by the authors.
Blueberry and challenge in coadministration. If no symbol is indicated, the cells were treated with the blueberry extracts first before challenge.
Blueberry treatment administered after challenge.
Risk factors including smoking, aging, hypercholesterolemia, and hyperglycemia (93) promote the retention of lipids, particularly LDL prone to oxidation in the vascular wall, causing the activation of inflammatory processes (94). The treatment of endothelial cells exposed to oxidative stress triggers with blueberry anthocyanins demonstrated protective effects toward ROS secretion and lipid peroxidation (95). A similar observation was reported with cells treated with blueberry exosome-like nanoparticles (ELN), an extracellular messenger vesicle presents in plants that contains proteins, lipids, mRNA, and microRNA (miRNA) (96). In addition to reducing ROS production, blueberry ELN also regulated gene expression involved in endothelial activation and leukocyte recruitment [MAPK1 and intercellular adhesion molecule (ICAM)] and inflammation (IL-6, TLR8, and TNF) (97). The antioxidant effect of blueberry extract on endothelial cells is likely due to the activity of several phytochemicals, but the extent to which other compounds contribute to the effect remains unclear, particularly due to the low number of studies focusing on nonphenolic compounds.
The secretion of chemokines and adhesion molecules by the endothelium is primarily regulated by TNF-α and C-reactive protein (CRP) (98), and leads to monocyte recruitment (99). After infiltration, monocytes differentiate into macrophages and phagocytose LDL (100). Macrophages that accumulate lipids eventually turn into foam cells, becoming surrounded by smooth-muscle cells and a collagen matrix, ultimately resulting in plaque formation (101). Blueberry anthocyanins reduced the adhesion of THP-1 monocytes to HUVEC endothelial cells with a better efficacy than the phenolic acid fraction derived from the same extract (102). The action of blueberry anthocyanins was further investigated and the individual compounds malvidin and cyanidin 3-glucoside, protocatechuic, and gallic acid reduced THP-1 adhesion (103). Blueberry extracts also decreased platelet- and endothelial-derived microvesicles through the inhibition of P2X7 transcription and Akt phosphorylation, both contributing to the release of extracellular vesicles (104) associated with monocyte interaction with endothelial cells (105). Su et al. (42) observed that blueberry extracts led to downregulation of noncoding miR-21, miR-146a, and miR125b, miRs typically increased in macrophages involved in plaque formation (Table 3).
In cardiovascular disease, endothelial cell migration and angiogenesis are reduced, leading to structural and functional alterations of the endothelium (106). Akt is a major signaling pathway in angiogenesis, regulating cell survival, cell cycle, and migration (107). Treatment with blueberry polyphenols increased angiogenesis in endothelial cells through the upregulation of the Akt pathway (108). However, abnormal angiogenesis promoted by vascular endothelial growth factor (VEGF) was counterbalanced by blueberry extract treatment through the inhibition of ERK and Akt phosphorylation (104, 109). In addition, blueberry polyphenol extract did not modulate polyphospholipase C (PLC) expression and phosphorylation, involved in angiogenesis, in HUVEC cells following induction with VEGF or LPS (109, 110). These results suggest that blueberry components may support angiogenesis under normal physiological conditions, but attenuate abnormal angiogenesis induced by overactive growth factors.
Blueberries exert protective effects on endothelial models by reducing oxidative stress and monocyte adhesion to the endothelium and modulating angiogenesis. The direct impact of blueberry compounds on endothelial dysfunction in vivo remains to be elucidated, with few studies focusing on the molecular pathways involved in atherosclerosis.
Brain and neuronal inflammation models
Inflammation in the brain can be generated by the dysregulation of inflammatory pathways, molecular signals released by injured neurons, or the accumulation of protein aggregates (111). Brain inflammation has been associated with an impairment of neuron regeneration (112) and an increase in the incidence of neurodegenerative diseases such as multiple sclerosis (113), and Alzheimer's (114) and Parkinson's diseases (115). Mechanisms involved in neurodegeneration have been reviewed in detail by Jellinger (116).
The sensitivity of brain muscarinic receptors to oxidative stress increases with aging (117), and abnormal signaling is implicated in neurodegenerative diseases. Blueberry extracts alleviate oxidative stress in neuronal models through a variety of mechanisms (Table 4), including scavenging of ROS (118) and downregulation of iNOS gene expression (119–121). In COS-7 cells transfected with muscarinic receptors, treatment with a whole blueberry extract reduced activation of the cAMP response element-binding (CREB) pathway, which can be activated by protein aggregates and is involved in oxidative stress in neurons (122). Several blueberry fractions exerted antioxidant effects, although via different mechanisms: a blueberry extract polar fraction (containing high concentrations of polyphenols) and a nonpolar fraction (low in polyphenols) both reduced oxidative stress in TNF-α- and PMA-induced human neuroblastoma (118). The polyphenolic-rich fraction exerted scavenging activity of ROS, whereas the nonpolar fraction disrupted NOX assembly, a mechanism that generates the production of ROS. Blueberry treatment also reduced proinflammatory cytokines in LPS-induced BV-2 cells, through modulation of COX-2 gene expression (119, 120) and the inhibition of NF-κB translocation into the nucleus (123). The phenolic extracts increased concentrations of Arg-1 (124), a marker of the M2 phenotype of macrophages, which promotes a return to homeostasis (125) necessary to avoid chronic inflammation. In a model of microglial cells subjected to inflammation in different glycemic conditions, a blueberry extract was effective at inhibiting inflammatory markers, with a comparable effect to the insulin treatment used in the model (121).
TABLE 4.
Cell-culture-based studies on the effect of blueberry phytochemicals on microglia and neuronal inflammation models
| Cell line | Treatments (dose, duration) | Challenge (dose, duration) | Controls | N1 | Parameters measured (method) | Effects of treatment compared to challenge | Ref. |
|---|---|---|---|---|---|---|---|
| Whole and/or polyphenolic extracts: | |||||||
| Microglia from C57BL/6mice | Polyphenol extract (1 μL, equivalent to about 10 μg/mL, 24 h2) | Glutamate (100 μM, 24 h) or α-synuclein (100 ng/mL, 24 h) | Vehicle, challenge | 3 | Morphology (light microscopy), viability (DAPI staining) | Moderate effect on cell morphology (presence of both healthy and “fried egg” shaped cells). No neuroprotective effects by the treatment against glutamate-induced cell death, but protection against α-synuclein damage | (132) |
| Hippocampal neuronsfrom F344 rats | Whole extract (0.125 mg/mL, 10–30 min2) | Aβ species (10 μM, 10–30 min) | Vehicle, challenge | 4 | Viability (staining exclusion), CREB, ERK, PKC (immunofluorescence), ROS (DCFDA), glutathione (immunofluorescence) | Protection against Aβ-induced cytotoxicity. No ↓ PKCα and PKCγ in aging neurons. ↓ phosphorylation of CREB but no effect on ERK activation. ↓ ROS production and ↑ glutathione concentrations | (131) |
| COS-7 transfected withmuscarinic receptors(M1 and M3) | Whole extract (2 mg/mL, 45 min + 4 h2) | Dopamine (1 mM, 4 h) | Vehicle, challenge, murine receptor cell control | — | PKC, CREB, MAPK (WB) | Further ↑ MAPK phosphorylation induced by dopamine in M1 cells but not M3. ↓ PKCγ and CREB phosphorylation induced by dopamine in both cell types | (122) |
| BV-2 | Whole extract (50–500 μg/mL, 45 min + 4–16 h2) | LPS (100 ng/mL, 4–16 h) | Vehicle, challenge | 3 | NO (Griess), iNOS, and COX-2 gene expression (qPCR), iNOS and COX-2 proteins (WB), IL-1β and TNF-α (ELISA), ROS (DCFDA) | ↓ NO production. Dose-dependent ↓ iNOS and COX-2 gene and protein expression. ↓cytokines and ROS production for treatment dose 100 μg/mL and higher | (119) |
| BV-2 | Whole extract (0.25–2 mg/mL, 1 h) | LPS (100 ng/mL, overnight) | Vehicle, challenge | — | NO (Griess), iNOS, COX-2 protein expression (WB), TNF-α (ELISA) | ↓NO, TNF-α production and iNOS gene expression (doses ≥1 mg/mL). ↓COX-2 gene expression (dose 0.5 mg/mL) | (120) |
| BV-2 | Polyphenol extract (10 ng/mL, 1–6 h2 for qPCR or 24–48 h2 for other assays) | LPS (100 ng/mL, 1–6 h or 24–48 h) | Vehicle, challenge | 3 | Viability (MTT), morphology, iNOS and Arg-1 (immunofluorescence), migration (transwell chambers and scratch assay), Rho GTPases (WB), IL-1β, TNF-α, IL-6 (qPCR) | ↓ migration and prevention of morphology changes. ↓ RAC-1-GTP. Modulation of M1 phenotype acquired after LPS challenge, towards M2 phenotype. ↓ cytokine production | (124) |
| BV-2 | Polyphenolic extract (25–100 μg/mL, 45 min + 16 h2) | LPS (100 ng/mL, 16 h) | Vehicle, challenge | 3–4 | Viability (MTS), NO (Griess), iNOS, and COX-2 (WB, transient transfection/luciferase assay), NF-κB (EMSA) | Dose-dependent ↓ NO production. ↓ iNOS and COX-2 protein and promoter activity. ↓ translocation of NF-κB | (123) |
| HAPI | Whole extract (2 mg/mL, 24 h, alone or with 50 mM insulin) | LPS (100 mg/mL, overnight, in presence of 5, 25, or 50 mM glucose) | Vehicle, challenge | 3 | NO (Griess), TNF-α (ELISA), iNOS (WB), GLUT1, and NOX4 expression (DAPI staining) | Comparison with LPS control: ↓ all marker production or expression by blueberry treatment of all concentrations of glucose (higher inhibition in low glucose concentration). The inhibition effect was more pronounced when blueberry was used with insulin for NO and iNOS | (121) |
| Anthocyanin fractions: | |||||||
| Neuro2a | Anthocyanin (0.5–8-fold molar ratio to Aβ, 15 min–3 h2) | Aβ species (10 μM, 15 min–3 h) | Vehicle, challenge | 3 | Viability (MTS), amyloid formation (fluorescence assay), morphology Aβ samples (microscopy), fibril formation (WB) | Dose-dependent ↓ amyloid formation and ↑ formation of low-molecular weight Aβ species. ↓ cell cytotoxicity induced by Aβ aggregates | (130) |
| Others: | |||||||
| SH-SY5Y | Whole extract, polar (polyphenol-rich) and nonpolar (not polyphenol-rich) fractions (5 μg/mL, 1 h2) | TNF-α (200 ng/mL, 1 h) or PMA (400 ng/mL, 1 h) | Vehicle, challenge, negative: DPI (10 μM), NAC (1 mM), AEBSF (1 mM), GW4896 (13.8 μM), 1 h | 4 | ROS (DCFDA), scavenging capacity (choline oxidation assay), cytotoxicity (MTT), superoxide production (cytochrome C), P67phox and phosphor P40phox (ELISA), P67phox (WB), NOX assembly in lipid raft (confocal microscopy) | ↓ ROS formation by whole extract and nonpolar fraction. ↑ ROS scavenging activity of polar fraction only. ↓ superoxide production and plasma membrane-associated p67phox, ↓ formation of large lipid raft by nonpolar fraction only | (118) |
N, number of experimental replicates, as stated by the authors. AEBSF, 4-(2-aminoethyl) benzenesulfonyl fluoride; Aβ, amyloid β; COX-2, cyclooxygenase-2; CREB, cAMP-response element binding; DAPI, 4′,6-diamidino-2-phenylindole; DCFDA, 2′,7′-dichlorofluorescin diacetate; DPI, diphenyl iodonium; EMSA, electrophoretic-mobility shift assay; ERK, extracellular-signal-regulated kinase; iNOS, inducible nitric oxide synthase; MAPK, mitogen-activated protein kinase; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium); MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide; NAC, N-acetyl-L-cysteine; NOX, NADPH oxidase; PKC, protein kinase C; PMA, phorbol-12-myristate 13-acetate ester; ROS, reactive oxygen species; WB, Western blot.
Blueberry and challenge in coadministration. If no symbol is indicated, the cells were treated with the blueberry extracts first before challenge.
Protein aggregations in the brain result from the abnormal deposition of misfolded proteins, leading to the accumulation of fibrils, including α-synuclein (αSyn) or amyloid-β (Aβ) into plaques (126). Accumulation of Aβ aggregates is associated with impairment of normal physiological activity of the neurons, increased production of ROS (127), and activation of microglia and astrocytes (128). Immune cells also generate Aβ (129), therefore upregulating the inflammatory cascade. A blueberry extract and an anthocyanin fraction conferred protection against protein aggregate-induced cytotoxicity in mouse neuroblastoma and rat hippocampal neurons challenged with Aβ species (130, 131), most likely through the inhibition of the CREB pathway but with no effect on ERK activation (131). αSyn-challenged mouse microglia were also protected by polyphenol extracts, but not the cells challenged with glutamate, which is sometimes found in excess in pathological conditions (132).
Based on results in cell models, it is possible that the active compounds of blueberries could dampen the development of neurodegenerative diseases by reducing oxidative stress and inflammation, which limits the formation of protein aggregates and associated cell damage. It is important to remember that although cell culture studies of neurons and glial cells provide useful information to understand inflammation mechanisms in the brain, these models may be oversimplified due to the lack of a blood-brain barrier, which is critical in maintaining homeostasis (133).
Dermal inflammation models
The skin is subjected to constant challenge, including pollution, physical damage (wounds), and light irradiation, that play a role in the initiation of inflammation (134). Cell models include keratinocytes, found in the epidermis (135), and fibroblasts that synthesize the extracellular matrix, used alone or in coculture (136). Recently, 3D skin models and a human skin equivalent have been developed to provide more complex models for testing toxicity, absorption, and metabolism with the presence of several epidermal layers and stratum corneum (137, 138).
Blueberry extracts were used to treat human keratinocytes (HaCaT) and a human skin model (“EpiDerm”) (Table 5) challenged with O3 exposure, a toxic pollutant that can alter the redox status of the skin and induce inflammation (138). O3 impaired wound healing of the cells, but function was recovered with a blueberry polyphenol treatment by activating cellular antioxidant defense systems, reducing H2O2 production (138). A blueberry polyphenol extract was compared with 2 anthocyanin and proanthocyanidin-rich extracts to investigate wound healing in human fibroblasts (HDFa), and anti-inflammatory and antioxidant effects on murine macrophages. The fractions were both effective at reducing ROS production and alleviated inflammation by reducing the expression of COX-2 and iNOS in the macrophages, but the protective effects of proanthocyanidins were more pronounced than for the other blueberry polyphenols (139). Blueberry treatments, obtained through several extraction methods, were also effective at reducing NO production of human foreskin fibroblast (HFF-1) induced by IL-2β (140).
TABLE 5.
Cell-culture-based studies on the effect of blueberry phytochemicals on dermal inflammation models
| Cell line | Treatments (dose, duration) | Challenge (dose, duration) | Controls | N1 | Parameters measured (method) | Effects of treatment compared to challenge | Ref. |
|---|---|---|---|---|---|---|---|
| Whole and/or polyphenolic extracts: | |||||||
| HaCaT and 3D skin model“EpiDerm” | Polyphenol extract (HaCat: 10 μg/mL, 24 h; 3D model: 100 μg/mL, 24 h) | O3 (HaCat: 0.5 ppm, 1 h; 3D model: 0.5 ppm, 5 h) | Vehicle, challenge | 3 | Cytotoxicity (LDH), wound closure (scratch wound healing), migration (transwell), proliferation (BrdU), H2O2 (DCFDA), inflammasome activation (ASC oligomerization), ASC and NLRP1 (immunocytochemistry) caspase 1 (WB), ASC, caspase 1, and IL-18 gene expression (qPCR) | ↑ keratinocyte wound closure by increased migration and proliferation. ↓ inflammasome activation by limited induction of ASC, transcription of caspase 1 and IL-18, and ↓ oligomerization of ASC | (138) |
| HaCaT | Polyphenol extract (5–100 mg/L, 1 h + 4 h2 or 4 h2 only) | UV-A (10–40 J/cm2), H2O2 (0.3 mM, 4 h3) | Vehicle, challenge | 3–4 | Viability (LDH and neutral red retention), ROS (DCFDA), lipid peroxidation (TBARS), glutathione (DTNB) | Dose-dependent ↓ ROS production. ↓ membrane lipid peroxidation. Protection against glutathione depletion | (149) |
| HaCaT | Phenolic extract (5–50 mg/L, 1 h + 4–8 h2 or 4/8 h2 only) | UV-B (50–1000 mJ/cm2) | Vehicle, challenge | 3 | Cytotoxicity (LDH), proliferation (BrdU), DNA damage (gel electrophoresis), caspase-3 and -9 activity (fluorescent assay), caspase-3 activation (WB), RONS (dihydrorhodamine 123), IL-6 (ELISA) | ↓ LDH release and ↑ proliferation at higher treatment doses. ↓ DNA damage and apoptosis. ↓ RONS generation, better for cells treated after irradiation only. ↓ IL-6 production | (144) |
| HaCaT and HFF (aloneand in coculture) | Phenolic extract (6–10 mg/mL, 2 h + 12 h) | UV-C (8 mJ, cm2, 5 s, 270–290 nm) | Vehicle, challenge | 3 | Viability (methylene blue), DNA damage (DNA ladder assay), SOD and MDA content (kits), MMP-1 (ELISA), p38MAPK, c-fos, c-Jun, MMP-1, IL-1β, IL-8, TNF-a, and IL-6 (qPCR), 38MAPK, c-fos, c-Jun, MMP-1 (WB) | Protection against UV-C-induced cytotoxicity and ↓ DNA damage. ↓ MMP-1 only in coculture system. ↓ accumulation of MDA and ↑ activity of SOD. ↓ proinflammatory gene expression. ↓ MAPK pathway through ↓ MMP-1, c-fos, and c-Jun expressions in HFF and coculture but not HaCat | (150) |
| Phenolic and polyphenolic fractions: | |||||||
| RAW 264.7 and HDFa | Polyphenol extract, anthocyanin and proanthocyanidin fractions (50 μg/mL, 24 h3) | RAW 264.7: LPS (10 μL, 24) | Vehicle, challenge | 3 | Cytotoxicity (MTT) RAW 264.7: ROS (DCFDA), NO (Griess), COX-2, iNOS (qPCR) | RAW 264.7: No effect of polyphenol extract on ROS production. ↓ ROS by anthocyanin and proanthocyanidin fractions. Highest NO suppression with proanthocyanidin fraction. ↓ COX-2 and iNOS by all treatments | (139) |
| HDFa: cell migration (fluorescence) | HDFa: ↑ wound repair by proanthocyanidin B2 | ||||||
| Anthocyanin fractions: | |||||||
| Human dermal fibroblast | Anthocyanin (1–10 mg/L, 48 h2) | UV-B (100 mJ/cm2, 312 nm) | Vehicle, challenge | 3 | Viability (MTT), ROS (DCFDA), ASK-1, JNK, p38, c-Jun, p53, STAT-1, MMP-1, 8 and 13, IκB, NF-κB (WB), procollagen, collagen (immunocytochemistry), procollagen, MMP-1 (qPCR), TNF-α, IL-8, IL-6, IL-1β (ELISA) | Dose-dependent ↑ cell viability. Inhibition of reduction of procollagen and collagen induced by UV-B. ↓ MMP production and gene expression increased by the UV-B challenge. ↓ proinflammatory cytokine secretion, ↓ translocation of NF-κB and ↓ activation of ASK-1-MAPK pathway | (145) |
| Others: | |||||||
| HFF1 | Blueberry extracts, comparison of extraction methods (12.5 μg/mL, 90 min) | IL-2β (10 μg/mL, 30 min) | Vehicle, challenge, positive (L-NIL, 1 μg/mL) | 4 | Viability (MTT), NO (Griess) | ↓NO production by all fruit extracts. Stronger inhibitory effect for extract obtained by decoction method | (140) |
N, number of experimental replicates, as stated by the authors. ASC, apoptosis-associated speck-like; ASK, apoptosis signal-regulating kinase-1; BrdU, bromodeoxyuridine; COX-2, cyclo-oxygenase-2; DCFDA, 2ʹ,7ʹ-dichlorofluorescin diacetate; DTNB, 5,5′-dithiobis-(2-nitrobenzoic acid); HFF, human foreskin fibroblast; iNOS, inducible nitric oxide synthase; IκB, NF κ B inhibitor; JNK, c-Jun N-terminal kinase; LDH, lactate dehydrogenase; L-NIL, L-N6-(1-iminoethyl)lysine; MAPK, mitogen-activated protein kinase; MDA, malondialdehyde; MMP-1, matrix metalloproteinase-1; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide; NLRP, nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing; RONS, reactive oxygen and nitrogen species; ROS, reactive oxygen species; SOD, superoxide dismutase; STAT-1, signal transducers and activators of transcription-1; WB, Western blot.
Blueberry treatment administered after challenge.
Blueberry and challenge in coadministration. If no symbol is indicated, the cells were treated with the blueberry extracts first before challenge.
The major risk factor for developing skin cancer is sun exposure. DNA absorbs UV-B, which can induce the formation of dimeric pyrimidine bases (141). These mutations are usually repaired by nucleotide excision, although certain mutations, notably in the p53 gene, result in the loss of this repair function (142), possibly leading to the development of squamous or basal cell carcinoma (143). In cells exposed to UV-B, responsible for DNA damage and initiation of proinflammatory pathways, a blueberry extract protected DNA against strand break formation (144). Blueberry extracts also demonstrated anti-inflammatory effects in UV-B-challenged cells and reduced cytokine gene expression through inhibition of NF-κB and MAPK activations (145). UV-A, in contrast, is less absorbed by DNA, but can interact with exogenous chromophores and induce lipid peroxidation, which generates ROS (146). These reactive species can lead to the oxidation of nucleic acid, DNA strand breaks (147), and increased MMP-1 activity (148), potentially increasing the risk of aggressive cancer. The increase of oxidative stress by UV-A on keratinocytes was partially reduced by a blueberry phenolic extract through decreased ROS production and lipid peroxidation (149). The third type of sunlight irradiation, UV-C, has the weakest penetration rate in the atmosphere, but has very high energy, possibly contributing to oxidative stress, inflammation, and DNA damage in the skin cells. A blueberry extract applied to HaCaT keratinocytes and HFF fibroblasts irradiated with UV-C showed protective effects against oxidative stress and DNA damage. The anti-inflammatory effects of the treatment were, however, only characterized in the keratinocyte and fibroblast coculture and the activation of MMP-1 expression by fibroblasts after irradiation was only observed in the presence of keratinocytes, suggesting interactions between the 2 types of cells (150). These observations support the need to use more complex models (coculture or 3D skin model) to investigate mechanisms mediated by the interaction between cell types in the skin. Limiting the studies to one type of cell may leave out crucial information regarding the physiological effects of the treatments on the skin. The results to date in cell models suggest that blueberry derivatives could have a protective effect on skin damage in response to sun and other stressors; however, the extent to which these molecules are stored and present in skin cells or associated tissue layers in vivo is not clear.
Ocular inflammation models
Artificial lights, environment, diet deficiencies, pathologies, and aging can impact oxidative stress and inflammation in the eyes, and may contribute to diabetic retinopathy (151) or age-related macular degeneration (152). The eye tissues and fluids contain little extracellular SOD, hence they have less protection against superoxide radical damage (153). Despite having higher SOD activity, the retina is at more risk of oxidative stress due to its high content of unsaturated fatty acids and high exposure to light (154) leading to lipid peroxidation, which can, in turn, react with DNA, proteins, and lipids, inducing cell damage (155).
Studies on blueberry compounds applied to cell-based models relevant to eye function have typically used light irradiation (UV, blue light, visible light) (156–159), a chemical challenge (e.g., H2O2, DHA) (160–162), or a combination of both (155, 163, 164) to induce inflammation (Table 6). The protective effect of blueberry compounds on oxidative stress induced by light in ocular models has been tentatively explained through a decrease in ROS production and lipid peroxidation. In murine photoreceptors (661 W line) induced with UV and blue light, blueberry extracts exerted protective effects through regulation of ROS production, limiting cell death induced by the light through inhibition of p38-MAPK, JNK, and NF-κB pathways (156, 157).
TABLE 6.
Cell-culture-based studies on the effect of blueberry phytochemicals on ocular inflammation and oxidative stress models
| Cell line (P#1) | Treatments (dose, duration) | Challenge (dose, duration) | Controls | N2 | Parameters measured (method) | Effects of treatment compared to challenge | Ref. |
|---|---|---|---|---|---|---|---|
| Whole and/or polyphenolic extracts: | |||||||
| 661 W | Polyphenol extract (1−30 μg/mL, 1 h + 20 min3 + 24 h4) | UV-A light (4 J/cm2, 20 min) | Vehicle, challenge, positive (NAC, 0.3–1 mM) | 6 | Cytotoxicity (fluorescent microscopy), ROS (DCFDA), MAPK, Akt, JNK (WB) | ↓ morphological defects induced by UV-A. Inhibition of reduction of metabolic activity. ↓ ROS production and ↓ phosphorylation of p38 MAPK and JNK. No downregulation of Akt | (156) |
| 661 W | Polyphenol extract (1–10 μg/mL, 1 h + 6 h3) | Blue light (460–470 nm, 2500 lx, 6 h) | Vehicle, challenge, extract, positive (NAC, 0.3–1 mM) | 6 | Viability (WST-8), cytotoxicity (fluorescent microscopy), ROS (DCFDA), MAPK, NF-κB, LC-3 (WB), caspase activity | Dose-dependent ↓ cell death and ROS production. ↓ p38 MAPK and NF-κB, and inhibition of upregulation of LC-3. ↓ the activation of caspase 3/7 | (157) |
| ARPE-19 | Polyphenol extract (0.01–100 μg/mL, 4 h) | H2O2 (500 μM, 2 h) | Vehicle, challenge, positive (BHT, 300–1000 μM) | 3 | Viability (MTT), ROS (DCFDA), HO-1, GST-pi (WB) | ↓ ROS production. ↑ HO-1 and GST-pi proteins and mRNA | (160) |
| Phenolic and polyphenolic fractions: | |||||||
| ARPE-19 | Polyphenol extract, anthocyanin, phenolic acid, and flavonoid fractions (10–50 μg/mL, 24 h) | DHA (0.05 mmol/L, 24 h) + light irradiation (3500 lx, 12 h) | DHA + light challenge | 3 | Viability (MTT), ROS (DCFDA), lipid peroxidation (MDA) | No effect on ROS and lipid peroxidation for low doses of any fractions. 25 μg/mL: ↓ oxidative stress for polyphenol extract and flavonoid fraction. ↓ oxidative stress by all fractions (dose 50 μg/mL), better efficiency of polyphenol and flavonoid fractions | (155) |
| ARPE-19 | Polyphenol extract, anthocyanin, phenolic acid, and flavonoid fractions (5–50 μg/mL, 24 h) | DHA (0.05 mmol/L, 24 h) + light irradiation (3500 lx, 12 h) | DHA + light challenge | 3 | Viability (MTT), cytotoxicity (LDH), VEGF (ELISA), phagocytic index (fluorescent microspheres), senescence (β-galactosidase activity), lipid peroxidation (MDA), ROS (DCFDA) | ↑ cell viability and ↓ VEGF production. Dose-dependent amelioration of phagocytic function. Amelioration of senescence by all fractions, but better performance of polyphenol extract than fractions on viability and senescence. ↓ lipid peroxidation by all fractions, best effect for flavonoid fraction | (163) |
| Anthocyanin fractions: | |||||||
| ARPE-19 | Purified anthocyanin fractions (100 μM, 14–15 h3) | Bisretinoid fluorophore A2E (100 μM) + endoperoxide (A2E oxidant, 10 mM), 14–15 h or light (0.36 mW/mm2, 10 min, 430 nm) | Vehicle, challenge | 3 | Viability (dye), A2E concentrations (HPLC), membrane permeability (DAPI) | ↓ photo-oxidation consumption and oxidation of A2E by anthocyanins. ↓ death of cells illuminated with blue light and pretreated with anthocyanins. ↓ number of permeabilized cells | (164) |
| ARPE-19 | Malvidin and delphinidin 3-glucoside extracted from blueberry (10–40 μg/mL3) | Visible light (2500 lx, 12 h, 420–800 nm) | Vehicle, challenge | 3 | Viability (MTT), ROS (DCFDA), VEGF (ELISA), β-galactosidase activity (staining) | Dose-dependent ↓ ROS production. ↓ VEGF by malvidin 3-glucoside but not delphinidin 3-glucoside. No effect on senescence | (158) |
| ARPE-19 | Anthocyanin (0.1–10 μg/mL, 24 h) | Light irradiation (2500 lx, 12 h) | Vehicle, challenge | 3 | Viability (MTT), cytotoxicity (LDH), senescence (β-galactosidase activity), VEGF (ELISA), ROS (DCFDA) | ↓ cell senescence after light exposure. ↓ ROS production. ↑ cell viability (doses 1 and 10 μg/mL) and ↓ VEGF expression for lower doses of blueberry extract | (159) |
| ARPE-19 | Anthocyanin (5 μg/mL, 6 h) | H2O2 (800 μM, 2 h) | Vehicle, challenge | 3–4 | Viability (MTT), apoptosis (annexin V detection), ROS (DCFDA), CAT, GSH-PX, MDA, SOD, VEGF (ELISA), pro-caspase, Bcl-2, Bax, Akt, ERK, p38, VEGF (WB) | ↓ apoptosis induced by challenge. ↓ caspase-3 and Bax, ↑ Bcl-2. ↓ ROS production. No effect on lipid peroxidation and antioxidant enzymes (CAT and SOD) production but ↑ GSH-PX. ↓ VEGF content but not protein expression. ↑Akt and ↓ ERK activations | (161) |
| HRCEC (P3-5) | Anthocyanin (10 μg/mL, 24 h) | Glucose (30 mmol/L, 24–48 h) | Vehicle, challenge | 3 | Viability (MTT), ROS (DCFDA), CAT, SOD, NO, eNOS, ACE, VEGF, ICAM-1, NF-κB (ELISA), NOX-4, Akt, VEGF (WB) | ↓ ROS secretion. ↑ antioxidant enzyme activity. ↓ NOX-4 protein expression after 48 h. No significant reduction in NO production but ↓ eNOS activity at 24 h. ↓ VEGF production. No effect on ICAM-1 and NF-κB | (162) |
P#, passage number used for the experiments (when specified). ACE, angiotensin-converting enzyme; BHT, butylated hydroxytoluene; CAT, catalase; DAPI, 4′,6-diamidino-2-phenylindole; DCFDA, 2ʹ,7ʹ-dichlorofluorescin diacetate; eNOS, endothelial nitric oxide synthase; ERK, extracellular-signal-regulated kinase; GSH-PX, glutathione-peroxidase; GST-pi, glutathione S-transferase-pi; HO-1, heme-oxygenase; ICAM-1, intercellular adhesion molecule-1; JNK, c-Jun N-terminal kinase; LDH, lactate dehydrogenase; MAPK, mitogen-activated protein kinase; MDA, malondialdehyde; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide; NAC, N-acetyl-L-cysteine; NO, nitric oxide; NOX, NADPH oxidase; ROS, reactive oxygen species; SOD, superoxide dismutase; VEGF, vascular endothelial growth factor; WB, Western blot; WST, water-soluble tetrazolium.
N: number of experimental replicates, as stated by the authors.
Blueberry and challenge in coadministration. If no symbol is indicated, the cells were treated with the blueberry extracts first before challenge.
Blueberry treatment administered after challenge.
The effects of blueberry extracts on alternatively challenged cells (H2O2 and glucose) were also reported (Table 6), but more research is needed to confirm the mechanisms behind their antioxidant and anti-inflammatory effects. Oxidative stress was alleviated by blueberry compounds in chemically challenged (H2O2) retinal pigment epithelial cells through an increased production of antioxidant enzymes (160). Blueberry anthocyanins modulated oxidative stress through a decrease in ROS production and increase in glutathione peroxidase activity in ARPE-19 cells induced with H2O2 (161). In 661 W cells challenged with high glucose, anthocyanins reduced ROS secretion and NOX protein expression, but limited anti-inflammatory effects were reported with no clear modulation of NF-κB (162).
Several studies have reported the effects of an array of phenolic fractions (anthocyanins, flavonoids, and phenolic acid). Blueberry anthocyanins tested on light-induced ARPE-19 cells helped mitigate lipid peroxidation (159), whereas malvidin 3-glucoside, purified from a blueberry extract, decreased ROS and VEGF production in irradiated cells (158). However, a blueberry flavonoid fraction and whole polyphenol mixture were more effective than the anthocyanin and phenolic acid fractions at reducing lipid peroxidation (155, 163). These observations support the evidence for a general effect to reduce cell stress markers in ocular cell models when using multiple blueberry fractions and phytochemicals, with a potentially stronger activity of the total polyphenol extracts as opposed to individual compounds. Thus, testing the effects of dietary blueberries or their derivatives on eye function is an interesting avenue for future research.
Intestinal inflammation models
Inflammatory bowel diseases (IBDs) encompass chronic inflammation-related disorders in the gastrointestinal tract. Chronic inflammation, associated with oxidative stress, increases the risk of developing colorectal cancers (165). Even though genetic predispositions have been documented (166), environmental factors such as the gut microbiota composition, influenced by lifestyle and diet, and its interaction with the intestinal mucosa, are key factors in increasing the prevalence of IBD (167, 168).
The effects of blueberry compounds on intestinal models are reported in Table 7, along with experimental conditions. IBDs are characterized by a loss of intestinal barrier integrity through the alteration of tight junction proteins (169). Although it is not clear if this loss of permeability is a cause or consequence of IBD, it promotes the chance of pathogens to enter the mucosa, triggering inflammation (170). Blueberry extracts were tested on Caco-2 cell permeability using a transepithelial electrical resistance (TEER) measurement. The anthocyanin and total polyphenolic fractions decreased permeability of the cell monolayer induced by Escherichia coli challenge, but the proanthocyanidin fraction did not restore TEER values. The authors suggested an interaction between proanthocyanidins, cell surface proteins, and the ability of E. coli to adhere to the cells (171).
TABLE 7.
Cell-culture-based studies on the effect of blueberry phytochemicals on intestinal inflammation models
| Cell line | Treatments (dose, duration) | Challenge (dose, duration) | Controls | N1 | Parameters measured (method) | Effects of treatment compared to challenge | Ref. |
|---|---|---|---|---|---|---|---|
| Whole and/or polyphenolic extracts: | |||||||
| T84 | Whole extract (2.5–25 μg/mL, 1 h + 4 or 16 h2) | Cytokine mixture: TNF-α (10 ng/mL), IL-1β (5 ng/mL), and IFN-γ (10 ng/mL, 4 or 16 h) | Vehicle, challenge | 3 | Viability (reazurin reduction assay), IP-10, IL-8, TNF-α (qPCR), cytokines (proteome profiler human cytokine antibody array) | Dose-dependent ↓ TNF-α and IP10 gene expression. Complete inhibition of synthesis of IP-10, I-TAC, and sICAM, and slight ↓ GRO-α and IL-8, all increased by challenge | (174) |
| NCM 356 and CCD 841CoN | Whole extract (25 mg/mL, 48 h3) | Proinflammatory cocktail TNF-α (100 ng/mL), IL-1β (100 ng, mL), LPS (10 μg/mL), IFN-γ (5 μg/mL), 24 h | Vehicle, challenge, negative (PBS, 1:20 dilution) | 5 | ROS (fluorescence microscopy and flow cytometry), cell death (flow cytometry) | ↓ ROS production in nuclear and cytoplasmic compartment of both cell lines. ↓ cell death | (175) |
| Phenolic and polyphenolic fractions: | |||||||
| Caco-2 | Polyphenol extract, anthocyanin and proanthocyanidin fractions (10–100 μg/mL, 24–72 h2) | E. coli (5 × 104 CFU/mL, 24–72 h) | Vehicle, challenge | 3 | Membrane permeability–surrogate marker (TEER) | ↑ TEER value after 48 h treatment with polyphenol and anthocyanin fractions. Further ↓ TEER after 24 h by proanthocyanidin | (171) |
| Caco-2 (transfected withplasmid pNiFty2-Luc) | Anthocyanin, phenolic (5–100 μg/mL, 4 h2) and water-soluble extracts (25–100 μg/mL, 4 h2) | IL-1β (2 ng/mL, 4 h) | Vehicle | 8 | NF-κB (luciferase assay), viability (MTS) | No effect of phenolic and water-soluble fractions on NF-κB induction, dose-dependent ↓ by anthocyanin fraction | (176) |
| Anthocyanin fractions: | |||||||
| T84 | Anthocyanin (10 μg/mL, 20 min + 20 min2 for WB or 24 h2 for other assays) | TNF-α or IFN-γ, 100 ng/mL, 30 min2 or 24 h2 | Vehicle, challenge | 4 | STAT1, STAT3, ERK, JNK, p38, NF-κB phosphorylation (WB) | ↓ phosphorylation of STAT1 and STAT3. No effect on MAPK pathway. ↑ NF-κB phosphorylation | (61) |
N, number of experimental replicates, as stated by the authors. ERK, extracellular-signal-regulated kinase; GRO-α, growth-related oncogene α; ICAM-1, intercellular adhesion molecule-1; IP-10, IFN-γ-inducible protein-10; I-TAC, interferon-inducible T-cell α chemoattractant; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemoattractant protein-1; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; ROS, reactive oxygen species; sICAM, soluble intercellular adhesion molecule-1; STAT, signal transducers and activators of transcription; TEER, transepithelial/endothelial electrical resistance; WB, Western blot.
Blueberry and challenge in coadministration. If no symbol is indicated, the cells were treated with the blueberry extracts first before challenge.
Blueberry treatment administered after challenge.
Regarding oxidative and inflammatory markers, a number of cytokine gene expressions have been found to increase in colonic mucosa of ulcerative colitis patients due to excessive immune response and immune cell reactivity (172, 173). In T84 cells, the increase in TNF-α and IP-10 gene expressions by a cytokine cocktail were counteracted by a blueberry extract and the production of IL-8 protein was slightly, but not significantly inhibited (174). ROS production in a similar cell model was also decreased by blueberry compounds (175). However, the effects of blueberry compounds on the NF-κB pathway remain unclear, with contradictory effects reported in intestinal models of inflammation. The response induced by cytokines was dose-dependently alleviated by a blueberry anthocyanin fraction in Caco-2 cells through the inactivation of NF-κB, but a water extract from the same fruits did not modulate the inflammatory response (176). An anthocyanin extract used on T84 human colon cells decreased the activation of the STAT pathway, upregulated during IBD, but also upregulated the NF-κB pathway (62).
A strong advantage of using an intestinal model is the possibility to study both the effect of polyphenol compounds and their colonic metabolites in a more physiologically relevant situation than for other systems in the body, as both parent molecules and metabolites are present in the intestinal epithelial cell environment despite the low bioavailability of polyphenolic compounds. The limited number of studies on blueberry phytochemicals and cell culture models of intestinal inflammation, the diversity of cell lines used, and parameters measured speak to the need for more studies to determine how blueberries modulate gut function and health.
Inflammation and oxidative stress: summary of the bioactivity of blueberry phytochemicals
Because both inflammation and oxidative stress are frequently associated with the development of chronic diseases (10), it is important to understand how dietary factors impact these outcomes. Here, we have reviewed studies reporting the effects of blueberry phytochemicals on cell culture models of inflammation and/or oxidative stress (Figure 3). Blueberry phenolic compounds and more broadly, phytochemicals, exert regulatory effects including a decrease in proinflammatory gene expression/production in part through the modulation of the NF-κB pathway. A modulation of the MAPK pathway by blueberry phytochemicals is less evident with contradictory observations reported but may also play a role. Blueberry phytochemicals decreased DNA damage in cells in vitro, via the reduction of ROS production, lipid peroxidation, and an increase in antioxidant enzyme activities.
FIGURE 3.
Summary of proposed cellular regulatory mechanisms of blueberry extracts on inflammatory or oxidative stress pathways investigated through cell-based models. Blue lines and arrows show the modulatory effects of blueberry phytochemicals. ↓, downregulation or reduction; ⊢, inhibition; ?, contradictory results. ARE, antioxidant response element; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; COX-2, cyclooxygenase-2; IKK, inhibitory kappa B kinase; iNOS, inducible nitric oxide synthase; IκBα, inhibitor of nuclear factor kappa B-alpha; Keap-1, Kelch Like erythroid-derived CNC homology Associated Protein 1; Nrf-2, nuclear erythroid 2-related factor 2; P, phosphorylated; TLR, toll-like receptor.
Despite many in vitro studies on blueberry extracts, no specific compounds have emerged as singly responsible for the regulatory effects on inflammation and oxidative stress. Virtually all studies have focused on blueberry (poly)phenolic extracts or fractions, with a large emphasis on anthocyanins. Health effects of dietary anthocyanins have been extensively reported and discussed (177–179), and berries provide an excellent vector for anthocyanin consumption. Blueberries have a complex anthocyanin profile and both major anthocyanidin derivatives, malvidin and delphinidin, have demonstrated a reduction of inflammatory markers in different in vitro models of intestinal inflammation (174) and endothelial dysfunction (103, 180). Although it is highly likely that anthocyanins largely contribute to the health benefits provided by blueberries, as supported by the number of studies focusing on those compounds, it is doubtful that they are entirely responsible for the bioactivities.
Several in vitro studies compared different fractions of blueberry phytochemicals, with reports of similar or better effects by other phenolic fractions and/or whole blueberry extract compared with anthocyanins (43, 84, 139, 158, 163). These different studies highlight that mechanisms of action of individual blueberry compounds and fractions are context and/or model specific. More studies comparing the effect on individual compounds and well-defined combinations of molecules in different systems are needed to investigate the impact of a system's environment or system-specific regulation on the bioactivity of blueberries. Although the amplitude of the effect of individual compounds appears to be widely specific to the model studied, the use of whole fractions of the fruits seems to alleviate inflammation and/or oxidative stress more consistently across models, despite not always demonstrating the strongest effects compared with specific blueberry fractions.
As the health effects of polyphenols have been extensively described, more data on other phytochemicals should be gathered as they may also exert health benefits. Other notable phytochemicals in blueberries include ascorbic acid (181), polysaccharides (182), and volatile compounds (183) and could contribute to inflammatory or oxidative responses of cells to stimuli. A blueberry volatile extract, high in monoterpenes (linalool, linalool oxide, and α-terpineol), modulated the inflammatory response in LPS-induced RAW 264.7 cells through inhibition of the NF-κB pathway (38). Phenolic compounds, although carrying anti-inflammatory and antioxidant modulatory effects, may not be solely responsible for the health benefits of blueberries. Whether the phytochemicals act in synergy or target different molecular pathways remains to be elucidated.
Although the scope of this review is limited to blueberries, the anti-inflammatory and antioxidant effects and mechanisms are likely applicable to other commonly consumed berries. Berries are generally rich in polyphenols, particularly anthocyanins, flavonols, and proanthocyanidins, but the profile of each berry species, and even within varieties, harbors differences in terms of the individual compounds present and their respective concentration (184). Gasparrini et al. (15) reviewed in detail the anti-inflammatory effects of several berries in cellular models using LPS-induced inflammation, and consistently report alleviation of inflammation by berry phytochemicals through inhibition of NF-κB and MAPK pathways. Other reviews also discuss and compare the anti-inflammatory properties of berries, in preclinical and human models (10, 16, 185). Moore et al. (186) and Gu et al. (38) have reported similar anti-inflammatory effects of berry volatiles compared with phenolic extracts for cranberries, blackberries, blueberries, red and black raspberries, and strawberries. Notably, the bioactivities of berry polyphenol extracts do not always explain the overall anti-inflammatory effects observed with whole berries (84, 187, 188), highlighting that potential health effects of berries as a group derived from highly diverse phytomolecules.
After consumption, blueberries and their phytochemicals undergo metabolism through phase II enzymatic reactions in the enterocytes and hepatocytes (189) or microbial metabolism in the gut (190). Metabolites are more likely to reach target sites inside the body and exert health benefits than their parent compounds (191). Evidence of the role of blueberry metabolites (e.g., protocatechuic acid and gallic acid, the catabolites for cyanidin and delphinidin 3-glucoside, respectively) in the modulation of inflammation and/or oxidative stress has also been established (103). Metabolites of elderberry were tested in RAW 264.7 and dendritic cells, and p-coumaric, homovanillic, 4-hydroxybenzoic, ferulic, protocatechuic, caffeic, and vanillic acids [also reported to be blueberry metabolites (25, 192)], exerted a dose-response inhibitory effect on NO (193). Studies regarding berry catabolites are less abundant than studies on berry parent phytochemicals but have gained interest in more recent literature. These studies of microbe- and host-modified phytochemicals are extremely important to fully understand the potential anti-inflammatory effects of blueberry consumption. Although most of the evidence focuses on the effect of individual compounds, it is essential to consider the potency of these metabolites in profiles similar to what occurs physiologically. To take the compound profile and physiologically available doses into account, Rutledge et al. (194) treated LPS-induced rat microglial cells with serum from subjects having regularly consumed blueberry, strawberry, or a placebo powder blends over 90 d. The blueberry consumption decreased NO production, TNF-α secretion, iNOS expression, and moderately modulated COX-2 protein expression in the cells (194). This type of design allows the integration of a more realistic profile of parent compounds and metabolites from blueberry consumption, at physiological doses, within a cell-culture-based model.
The current review summarizes the extensive amount of literature available on blueberry phytochemicals and inflammation using cell-based models. This choice comes with limitations, since it can be challenging to interpret results using specific concentrations of berry-derived molecules on cells when concentrations of these metabolites at the site of the target organs may not be established. There have been major differences in concentrations used to treat the cells, ranging anywhere from tens of μg/mL to mg/mL for total polyphenols and from tens of ng/mL to ≤1.2 mg/mL for anthocyanin fractions. Some of these concentrations are much higher than the blood concentrations that would be present in the body after consumption, as bioavailability of anthocyanins in the body is estimated to be lower than 2%, and peaking at 100 nmol/L after consumption of grape/blueberry juice (195). The relevance of the findings of cell-culture-based studies in complex human systems needs further investigation. These studies should comprise of well-controlled clinical trials, with the relevant choice of placebo controls and inclusion criteria depending on the specific blueberry phytochemical and physiological condition investigated. Future studies should also quantify the entire suite of berry-derived molecules and derivatives in key pools such as the blood, concurrently with physiologic indices of inflammation and oxidative stress.
Conclusions
General anti-inflammatory and antioxidant outcomes are consistently reported for blueberry extracts or derivatives across many studies. However, results observed in diverse cell culture studies from different investigators are challenging to interpret due to the differences in protocol, treatment, cell line, and analyzed markers. More studies investigating the effects of blueberry extracts on different systems and using comparable conditions would be valuable. Cell-culture-based models are not suitable to draw definitive conclusions on the effects of blueberry compounds on complex physiological processes occurring in the human body. Limitations include the compartmentalization of the observations in space and time: the compounds are only available in the form they are distributed and to the type of cells tested, outside of any regulatory processes by surrounding local tissues or on the whole-body scale, and tested on a one-time, acute, and usually high-dose treatment. Thus, precautions should be taken when drawing conclusions from simplified models, especially when using pharmacological doses of compounds. Despite the limitations, cell-culture-based studies have yielded critical information regarding mechanisms of action of blueberry phytochemicals, and have provided consistent evidence that components of blueberries have anti-inflammation and antioxidant properties, which likely contribute to health and functional benefits attributed to blueberries.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—LF-L: wrote the manuscript; JIB: developed the concept for the manuscript; JIB, SHA, and LH: contributed to writing the manuscript and editing the manuscript; and all authors: contributed to, read, and approved the final manuscript.
Notes
This work was supported in part by USDA, National Institute of Food and Agriculture, and Hatch Act Funding.
Author disclosures: SHA is a member of the Advances in Nutrition Editorial Board. The authors report no conflicts of interest.
Abbreviations used: AAPH, 2,2-azobis(2-amidinopropane)dihydrochloride; Aβ, amyloid-β; αSyn, α-synuclein; BMDM, bone marrow-derived macrophage; COX-2, cyclo-oxygenase-2; CREB, cAMP response element-binding; ELN, exosome-like nanoparticles; ERK, extracellular-signal-regulated kinase; HFF, human foreskin fibroblast; HO-1, heme oxygenase; HUVEC, human umbilical vein endothelial cell; IBD, inflammatory bowel disease; iNOS, inducible NO synthase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; NOX, NADPH oxidase; NQO-1, NADPH quinone oxidoreductase-1; PBMC, primary peripheral blood mononuclear cell; PMA, phorbol-12-myristate-13-acetate; ROS, reactive oxygen species; SOD, superoxide dismutase; STAT, signal transducers and activators of transcription; TEER, transepithelial electrical resistance; TLR, toll-like receptor.
Contributor Information
Laura Felgus-Lavefve, Department of Food Science, University of Arkansas, Fayetteville, AR, USA.
Luke Howard, Department of Food Science, University of Arkansas, Fayetteville, AR, USA.
Sean H Adams, Department of Surgery, School of Medicine, University of California Davis, Sacramento, CA, USA; Center for Alimentary and Metabolic Science, School of Medicine, University of California Davis, Sacramento, CA, USA.
Jamie I Baum, Department of Food Science, University of Arkansas, Fayetteville, AR, USA; Center for Human Nutrition, University of Arkansas System Division of Agriculture, Fayetteville, AR, USA.
References
- 1. Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006;54(11):4069–75. [DOI] [PubMed] [Google Scholar]
- 2. Cho MJ, Howard LR, Prior RL, Clark JR. Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry and red grape genotypes determined by high-performance liquid chromatography/mass spectrometry. J Sci Food Agric. 2004;84(13):1771–82. [Google Scholar]
- 3. Prior RL, Lazarus SA, Cao G, Muccitelli H, Hammerstone JF. Identification of procyanidins and anthocyanins in blueberries and cranberries (Vaccinium spp.) using high-performance liquid chromatography/mass spectrometry. J Agric Food Chem. 2001;49(3):1270–6. [DOI] [PubMed] [Google Scholar]
- 4. Kalt W, McDonald JE, Ricker RD, Lu X. Anthocyanin content and profile within and among blueberry species. Can J Plant Sci. 1999;79(4):617–23. [Google Scholar]
- 5. Cho MJ, Howard LR, Prior RL, Clark JR. Flavonol glycosides and antioxidant capacity of various blackberry and blueberry genotypes determined by high-performance liquid chromatography/mass spectrometry. J Sci Food Agric. 2005;85(13):2149–58. [Google Scholar]
- 6. Vrhovsek U, Masuero D, Palmieri L, Mattivi F. Identification and quantification of flavonol glycosides in cultivated blueberry cultivars. J Food Compos Anal. 2012;25(1):9–16. [Google Scholar]
- 7. Rodriguez-Mateos A, Cifuentes-Gomez T, Tabatabaee S, Lecras C, Spencer JPE. Procyanidin, anthocyanin, and chlorogenic acid contents of highbush and lowbush blueberries. J Agric Food Chem. 2012;60(23):5772–8. [DOI] [PubMed] [Google Scholar]
- 8. Kalt W, Cassidy A, Howard LR, Krikorian R, Stull AJ, Tremblay F, Zamora-Ros R. Recent research on the health benefits of blueberries and their anthocyanins. Adv Nutr. 2020;11(2):224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodriguez-Mateos A, Heiss C, Borges G, Crozier A. Berry (poly)phenols and cardiovascular health. J Agric Food Chem. 2014;62(18):3842–51. [DOI] [PubMed] [Google Scholar]
- 10. Joseph SV, Edirisinghe I, Burton-Freeman BM. Berries: anti-inflammatory effects in humans. J Agric Food Chem. 2014;62(18):3886–903. [DOI] [PubMed] [Google Scholar]
- 11. Bertoia ML, Mukamal KJ, Cahill LE, Hou T, Ludwig DS, Mozaffarian D, Willett WC, Hu FB, Rimm EB. Changes in intake of fruits and vegetables and weight change in United States men and women followed for up to 24 years: analysis from three prospective cohort studies. PLoS Med. 2015;12(9):e1001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muraki I, Imamura F, Manson JE, Hu FB, Willett WC, Dam RM, Sun Q. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347(1):f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cutler BR, Petersen C, Anandh Babu PV. Mechanistic insights into the vascular effects of blueberries: evidence from recent studies. Mol Nutr Food Res. 2017;61(6):1600271. [DOI] [PubMed] [Google Scholar]
- 14. Tran PHL, Tran TTD. Blueberry supplementation in neuronal health and protective technologies for efficient delivery of blueberry anthocyanins. Biomolecules. 2021;11(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gasparrini M, Forbes-Hernandez TY, Cianciosi D, Quiles JL, Mezzetti B, Xiao J, Giampieri F, Battino M. The efficacy of berries against lipopolysaccharide-induced inflammation: a review. Trends Food Sci Technol. 2021; in press. [Google Scholar]
- 16. Land Lail H, Feresin RG, Hicks D, Stone B, Price E, Wanders D. Berries as a treatment for obesity-induced inflammation: evidence from preclinical models. Nutrients. 2021;13(2):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, Aston CE, Lyons TJ. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. 2010;140(9):1582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Du C, Smith A, Avalos M, South S, Crabtree K, Wang W, Kwon Y-H, Vijayagopal P, Juma S. Blueberries improve pain, gait performance, and inflammation in individuals with symptomatic knee osteoarthritis. Nutrients. 2019;11(2):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr. 2010;140(10):1764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nair AR, Mariappan N, Stull AJ, Francis J. Blueberry supplementation attenuates oxidative stress within monocytes and modulates immune cell levels in adults with metabolic syndrome: a randomized, double-blind, placebo-controlled trial. Food Funct. 2017;8(11):4118–28. [DOI] [PubMed] [Google Scholar]
- 21. Johnson SA, Feresin RG, Navaei N, Figueroa A, Elam ML, Akhavan NS, Hooshmand S, Pourafshar S, Payton ME, Arjmandi BH. Effects of daily blueberry consumption on circulating biomarkers of oxidative stress, inflammation, and antioxidant defense in postmenopausal women with pre- and stage 1-hypertension: a randomized controlled trial. Food Funct. 2017;8(1):372–80. [DOI] [PubMed] [Google Scholar]
- 22. Elisia I, Pae HB, Lam V, Cederberg R, Hofs E, Krystal G. Comparison of RAW264.7, human whole blood and PBMC assays to screen for immunomodulators. J Immunol Methods. 2018;452:26–31. [DOI] [PubMed] [Google Scholar]
- 23. Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol. 2014;23(1):37–45. [DOI] [PubMed] [Google Scholar]
- 24. Kapałczyńska M, Kolenda T, Przybyła W, Zajączkowska M, Teresiak A, Filas V, Ibbs M, Bliźniak R, Łuczewski Ł, Lamperska K. 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14(4):910–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhong S, Sandhu A, Edirisinghe I, Burton-Freeman B. Characterization of wild blueberry polyphenols bioavailability and kinetic profile in plasma over 24-h period in human subjects. Mol Nutr Food Res. 2017;61(12):1700405. [DOI] [PubMed] [Google Scholar]
- 26. Crozier A, Del Rio D, Clifford MN. Bioavailability of dietary flavonoids and phenolic compounds. Mol Aspects Med. 2010;31(6):446–67. [DOI] [PubMed] [Google Scholar]
- 27. Czank C, Cassidy A, Zhang Q, Morrison DJ, Preston T, Kroon PA, Botting NP, Kay CD. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13)C-tracer study. Am J Clin Nutr. 2013;97(5):995–1003. [DOI] [PubMed] [Google Scholar]
- 28. González-Barrio R, Borges G, Mullen W, Crozier A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J Agric Food Chem. 2010;58(7):3933–9. [DOI] [PubMed] [Google Scholar]
- 29. Kaur G, Dufour JM. Cell lines: valuable tools or useless artifacts. Spermatogenesis. 2012;2(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dorrington MG, Fraser IDC. NF-κB signaling in macrophages: dynamics, crosstalk, and signal integration. Front Immunol. 2019;10:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92(2):689–737. [DOI] [PubMed] [Google Scholar]
- 33. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. [DOI] [PubMed] [Google Scholar]
- 34. Vaziri ND, Dicus M, Ho ND, Boroujerdi-Rad L, Sindhu RK. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int. 2003;63(1):179–85. [DOI] [PubMed] [Google Scholar]
- 35. LaPointe Margot C, Isenović E. Interleukin-1β regulation of inducible nitric oxide synthase and cyclooxygenase-2 involves the p42/44 and p38 MAPK signaling pathways in cardiac myocytes. Hypertension. 1999;33(1 Pt 2):276–82. [DOI] [PubMed] [Google Scholar]
- 36. Berghaus LJ, Moore JN, Hurley DJ, Vandenplas ML, Fortes BP, Wolfert MA, Boons G-J. Innate immune responses of primary murine macrophage-lineage cells and RAW 264.7 cells to ligands of toll-like receptors 2, 3, and 4. Comp Immunol Microbiol Infect Dis. 2010;33(5):443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen J, Uto T, Tanigawa S, Kumamoto T, Fujii M, Hou D-X. Expression profiling of genes targeted by bilberry (Vaccinium myrtillus) in macrophages through DNA microarray. Nutr Cancer. 2008;60(sup1):43–50. [DOI] [PubMed] [Google Scholar]
- 38. Gu I, Brownmiller C, Stebbins NB, Mauromoustakos A, Howard L, Lee S-O. Berry phenolic and volatile extracts inhibit pro-inflammatory cytokine secretion in LPS-stimulated RAW264.7 cells through suppression of NF-κB signaling pathway. Antioxidants. 2020;9(9):871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grace MH, Xiong J, Esposito D, Ehlenfeldt M, Lila MA. Simultaneous LC-MS quantification of anthocyanins and non-anthocyanin phenolics from blueberries with widely divergent profiles and biological activities. Food Chem. 2019;277:336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiong J, Chan YH, Rathinasabapathy T, Grace MH, Komarnytsky S, Lila MA. Enhanced stability of berry pomace polyphenols delivered in protein-polyphenol aggregate particles to an in vitro gastrointestinal digestion model. Food Chem. 2020;331:127279. [DOI] [PubMed] [Google Scholar]
- 41. Mueller M, Hobiger S, Jungbauer A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010;122(4):987–96. [Google Scholar]
- 42. Su X, Zhang J, Wang H, Xu J, He J, Liu L, Zhang T, Chen R, Kang J. Phenolic acid profiling, antioxidant, and anti-inflammatory activities, and miRNA regulation in the polyphenols of 16 blueberry samples from China. Molecules. 2017;22(2):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Esposito D, Chen A, Grace MH, Komarnytsky S, Lila MA. Inhibitory effects of wild blueberry anthocyanins and other flavonoids on biomarkers of acute and chronic inflammation in vitro. J Agric Food Chem. 2014;62(29):7022–8. [DOI] [PubMed] [Google Scholar]
- 44. Lee SG, Kim B, Yang Y, Pham TX, Park Y-K, Manatou J, Koo SI, Chun OK, Lee J-Y. Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J Nutr Biochem. 2014;25(4):404–11. [DOI] [PubMed] [Google Scholar]
- 45. Grace MH, Esposito D, Dunlap KL, Lila MA. Comparative analysis of phenolic content and profile, antioxidant capacity, and anti-inflammatory bioactivity in wild Alaskan and commercial Vaccinium berries. J Agric Food Chem. 2014;62(18):4007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cheng A, Yan H, Han C, Wang W, Tian Y, Chen X. Polyphenols from blueberries modulate inflammation cytokines in LPS-induced RAW264.7 macrophages. Int J Biol Macromol. 2014;69:382–7. [DOI] [PubMed] [Google Scholar]
- 48. Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15(6):252–9. [DOI] [PubMed] [Google Scholar]
- 49. Lowenstein CJ, Padalko E. iNOS (NOS2) at a glance. J Cell Sci. 2004;117(14):2865–7. [DOI] [PubMed] [Google Scholar]
- 50. Cheng A, Han C, Fang X, Sun J, Chen X, Wan F. Extractable and non-extractable polyphenols from blueberries modulate LPS-induced expression of iNOS and COX-2 in RAW264.7 macrophages via the NF-κB signalling pathway. J Sci Food Agric. 2016;96(10):3393–400. [DOI] [PubMed] [Google Scholar]
- 51. Xu W, Zhou Q, Yao Y, Li X, Zhang J-L, Su G-H, Deng A-P. Inhibitory effect of gardenblue blueberry (Vaccinium ashei reade) anthocyanin extracts on lipopolysaccharide-stimulated inflammatory response in RAW 264.7 cells. Journal of Zhejiang University-SCIENCE B. 2016;17(6):425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Samad NB, Debnath T, Ye M, Hasnat MdA, Lim BO. In vitro antioxidant and anti-inflammatory activities of Korean blueberry (Vaccinium corymbosum L.) extracts. Asian Pac J Trop Biomed. 2014;4(10):807–15. [Google Scholar]
- 53. Reyes-Farias M, Vasquez K, Ovalle-Marin A, Fuentes F, Parra C, Quitral V, Jimenez P, Garcia-Diaz DF. Chilean native fruit extracts inhibit inflammation linked to the pathogenic interaction between adipocytes and macrophages. J Med Food. 2015;18(5):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Simon LS. Role and regulation of cyclooxygenase-2 during inflammation. Am J Med. 1999;106(5):37S–42S. [DOI] [PubMed] [Google Scholar]
- 55. Zeng C, Wang W, Yu X, Yang L, Chen S, Li Y. Pathways related to PMA-differentiated THP1 human monocytic leukemia cells revealed by RNA-Seq. Science China Life Sciences. 2015;58(12):1282–7. [DOI] [PubMed] [Google Scholar]
- 56. Ben Lagha A, Dudonné S, Desjardins Y, Grenier D. Wild blueberry (Vaccinium angustifolium ait.) polyphenols target Fusobacterium nucleatum and the host inflammatory response: potential innovative molecules for treating periodontal diseases. J Agric Food Chem. 2015;63(31):6999–7008. [DOI] [PubMed] [Google Scholar]
- 57. Taverniti V, Dalla Via A, Minuzzo M, Del Bo’ C, Riso P, Frøkiær H, Guglielmetti S. In vitro assessment of the ability of probiotics, blueberry and food carbohydrates to prevent S. pyogenes adhesion on pharyngeal epithelium and modulate immune responses. Food Funct. 2017;8(10):3601–9. [DOI] [PubMed] [Google Scholar]
- 58. Ben Lagha A, LeBel G, Grenier D. Dual action of highbush blueberry proanthocyanidins on Aggregatibacter actinomycetemcomitans and the host inflammatory response. BMC Complement Alternat Med. 2018;18(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kunrade L, Rembergs R, Jēkabsons K, Kļaviņš L, Kļaviņš M, Muceniece R, Riekstiņa U. Inhibition of NF-κB pathway in LPS-stimulated THP-1 monocytes and COX-2 activity in vitro by berry pomace extracts from five Vaccinium species. J Berry Res. 2020;10(3):381–96. [Google Scholar]
- 60. Ovalle-Marin A, Reyes-Farias M, Vasquez K, Parra-Ruiz C, Quitral V, Jimenez P, Garcia L, Ramirez LA, Quezada J, Gonzalez-Muniesa Pet al. Maqui, calafate, and blueberry fruits extracts treatments suppress the pathogenic interaction amongst human adipocytes and macrophages. J Berry Res. 2020;10(3):531–45. [Google Scholar]
- 61. Roth S, Spalinger MR, Müller I, Lang S, Rogler G, Scharl M. Bilberry-derived anthocyanins prevent IFN-γ-induced pro-inflammatory signalling and cytokine secretion in human THP-1 monocytic cells. Digestion. 2014;90(3):179–89. [DOI] [PubMed] [Google Scholar]
- 62. Roth S, Spalinger MR, Gottier C, Biedermann L, Zeitz J, Lang S, Weber A, Rogler G, Scharl M. Bilberry-derived anthocyanins modulate cytokine expression in the intestine of patients with ulcerative colitis. PLoS One. 2016;11(5):e0154817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. [DOI] [PubMed] [Google Scholar]
- 64. Schrader M, Fahimi HD. Mammalian peroxisomes and reactive oxygen species. Histochem Cell Biol. 2004;122(4):383–93. [DOI] [PubMed] [Google Scholar]
- 65. Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122(6):877–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Carraro E, Schilirò T, Biorci F, Romanazzi V, Degan R, Buonocore D, Verri M, Dossena M, Bonetta S, Gilli G. Physical activity, lifestyle factors and oxidative stress in middle age healthy subjects. Int J Environ Res Public Health. 2018;15(6):1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Loidl A, Sevcsik E, Riesenhuber G, Deigner H-P, Hermetter A. Oxidized phospholipids in minimally modified low density lipoprotein induce apoptotic signaling via activation of acid sphingomyelinase in arterial smooth muscle cells. J Biol Chem. 2003;278(35):32921–8. [DOI] [PubMed] [Google Scholar]
- 68. Starke-Reed PE, Oliver CN. Protein oxidation and proteolysis during aging and oxidative stress. Arch Biochem Biophys. 1989;275(2):559–67. [DOI] [PubMed] [Google Scholar]
- 69. Eiberger W, Volkmer B, Amouroux R, Dhérin C, Radicella JP, Epe B. Oxidative stress impairs the repair of oxidative DNA base modifications in human skin fibroblasts and melanoma cells. DNA Repair (Amst). 2008;7(6):912–21. [DOI] [PubMed] [Google Scholar]
- 70. Marín-García J. Chapter 14 - Oxidative stress and cell death in cardiovascular disease: a post-genomic appraisal. In: Marín-García J, editor. Post-Genomic Cardiology. (Second Edition). Boston: Academic Press; 2014. p. 471–98. [Google Scholar]
- 71. Tabet F, Touyz RM. Chapter 30 - Reactive oxygen species, oxidative stress, and vascular biology in hypertension. In: Lip GYH, Hall JE, editors. Comprehensive Hypertension. Philadelphia: Mosby; 2007. p. 337–47. [Google Scholar]
- 72. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci. 1993;90(17):7915–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431(7007):461–6. [DOI] [PubMed] [Google Scholar]
- 74. Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, Channon KM. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res. 2000;86(9):E85–90. [DOI] [PubMed] [Google Scholar]
- 75. Genser D, Kang MH, Vogelsang H, Elmadfa I. Status of lipid soluble antioxidants and TRAP in patients with Crohn's disease and healthy controls. Eur J Clin Nutr. 1999;53(9):675–9. [DOI] [PubMed] [Google Scholar]
- 76. Gabbita SP, Lovell MA, Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer's disease. J Neurochem. 1998;71(5):2034–40. [DOI] [PubMed] [Google Scholar]
- 77. Cásedas G, González-Burgos E, Smith C, López V, Gómez-Serranillos MP. Regulation of redox status in neuronal SH-SY5Y cells by blueberry (Vaccinium myrtillus L.) juice, cranberry (Vaccinium macrocarpon A.) juice and cyanidin. Food Chem Toxicol. 2018;118:572–80. [DOI] [PubMed] [Google Scholar]
- 78. Girard-Lalancette K, Pichette A, Legault J. Sensitive cell-based assay using DCFH oxidation for the determination of pro- and antioxidant properties of compounds and mixtures: analysis of fruit and vegetable juices. Food Chem. 2009;115(2):720–6. [Google Scholar]
- 79. Senevirathne M, Kim S-H, Jeon Y-J. Protective effect of enzymatic hydrolysates from highbush blueberry (Vaccinium corymbosum L.) against hydrogen peroxide-induced oxidative damage in Chinese hamster lung fibroblast cell line. Nutr Res Pract. 2010;4(3):183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu W, Lu X, He G, Gao X, Li M, Wu J, Li Z, Wu J, Wang J, Luo C. Cytosolic protection against ultraviolet induced DNA damage by blueberry anthocyanins and anthocyanidins in hepatocarcinoma Hepg2 cells. Biotechnol Lett. 2013;35(4):491–8. [DOI] [PubMed] [Google Scholar]
- 81. Liu W, Lu X, He G, Gao X, Xu M, Zhang J, Li M, Wang L, Li Z, Wang Let al. Protective roles of Gadd45 and MDM2 in blueberry anthocyanins mediated DNA repair of fragmented and non-fragmented DNA damage in UV-irradiated Hepg2 cells. Int J Mol Sci. 2013;14(11):21447–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kropat C, Mueller D, Boettler U, Zimmermann K, Heiss EH, Dirsch VM, Rogoll D, Melcher R, Richling E, Marko D. Modulation of Nrf2-dependent gene transcription by bilberry anthocyanins in vivo. Mol Nutr Food Res. 2013;57(3):545–50. [DOI] [PubMed] [Google Scholar]
- 83. van Breda SGJ, Briedé JJ, de Kok T. Improved preventive effects of combined bioactive compounds present in different blueberry varieties as compared to single phytochemicals. Nutrients. 2018;11(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Juadjur A, Mohn C, Schantz M, Baum M, Winterhalter P, Richling E. Fractionation of an anthocyanin-rich bilberry extract and in vitro antioxidative activity testing. Food Chem. 2015;167:418–24. [DOI] [PubMed] [Google Scholar]
- 85. Schantz M, Mohn C, Baum M, Richling E. Antioxidative efficiency of an anthocyanin rich bilberry extract in the human colon tumor cell lines Caco-2 and HT-29. J Berry Res. 2010;1(1):25–33. [Google Scholar]
- 86. Kšonžeková P, Mariychuk R, Eliašová A, Mudroňová D, Csank T, Király J, Marcinčáková D, Pistl J, Tkáčiková L. In vitro study of biological activities of anthocyanin-rich berry extracts on porcine intestinal epithelial cells. J Sci Food Agric. 2016;96(4):1093–100. [DOI] [PubMed] [Google Scholar]
- 87. Liu Y, Tan D, Tong C, Zhang Y, Xu Y, Liu X, Gao Y, Hou M. Blueberry anthocyanins ameliorate radiation-induced lung injury through the protein kinase RNA-activated pathway. Chem Biol Interact. 2015;242:363–71. [DOI] [PubMed] [Google Scholar]
- 88. Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Araujo J, Zhang M, Yin F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol. 2012;3:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ross D, Siegel D. Functions of NQO1 in cellular protection and COQ10 metabolism and its potential role as a redox sensitive molecular switch. Front Physiol. 2017;8:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Park K-H, Park WJ. Endothelial dysfunction: clinical implications in cardiovascular disease and therapeutic approaches. J Korean Med Sci. 2015;30(9):1213–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ross R. The pathogenesis of atherosclerosis – an update. N Engl J Med. 1986;314(8):488–500. [DOI] [PubMed] [Google Scholar]
- 93. Hadi HAR, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1(3):183–98. [PMC free article] [PubMed] [Google Scholar]
- 94. Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita Tet al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386(6620):73–7. [DOI] [PubMed] [Google Scholar]
- 95. Youdim KA, McDonald J, Kalt W, Joseph JA. Potential role of dietary flavonoids in reducing microvascular endothelium vulnerability to oxidative and inflammatory insults. J Nutr Biochem. 2002;13(5):282–8. [DOI] [PubMed] [Google Scholar]
- 96. Xiao J, Feng S, Wang X, Long K, Luo Y, Wang Y, Ma J, Tang Q, Jin L, Li Xet al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ. 2018;6:e5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. De Robertis M, Sarra A, D'Oria V, Mura F, Bordi F, Postorino P, Fratantonio D. Blueberry-derived exosome-like nanoparticles counter the response to TNF-α-induced change on gene expression in EA.hy926 cells. Biomolecules. 2020;10(5):742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bruunsgaard H, Skinhøj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121(2):255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci. 2006;103(27):10340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis: An Official Journal of the American Heart Association, Inc. 1986;6(2):131–8. [DOI] [PubMed] [Google Scholar]
- 102. Del Bo’ C, Roursgaard M, Porrini M, Loft S, Møller P, Riso P. Different effects of anthocyanins and phenolic acids from wild blueberry (Vaccinium angustifolium) on monocytes adhesion to endothelial cells in a TNF-α stimulated proinflammatory environment. Mol Nutr Food Res. 2016;60(11):2355–66. [DOI] [PubMed] [Google Scholar]
- 103. Del Bo’ C, Marino M, Riso P, Møller P, Porrini M. Anthocyanins and metabolites resolve TNF-α-mediated production of E-selectin and adhesion of monocytes to endothelial cells. Chem Biol Interact. 2019;300:49–55. [DOI] [PubMed] [Google Scholar]
- 104. Bryl-Górecka P, Sathanoori R, Arevström L, Landberg R, Bergh C, Evander M, Olde B, Laurell T, Fröbert O, Erlinge D. Bilberry supplementation after myocardial infarction decreases microvesicles in blood and affects endothelial vesiculation. Mol Nutr Food Res. 2020;64(20):2000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Curtis AM, Edelberg J, Jonas R, Rogers WT, Moore JS, Syed W, Mohler ER. Endothelial microparticles: sophisticated vesicles modulating vascular function. Vasc Med. 2013;18(4):204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Lee Murfee W, Pacher P, Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15(9):555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90(12):1243–50. [DOI] [PubMed] [Google Scholar]
- 108. Tulio AZ, Chang C, Edirisinghe I, White KD, Jablonski JE, Banaszewski K, Kangath A, Tadapaneni RK, Burton-Freeman B, Jackson LS. Berry fruits modulated endothelial cell migration and angiogenesis via phosphoinositide-3 kinase/protein kinase B pathway in vitro in endothelial cells. J Agric Food Chem. 2012;60(23):5803–12. [DOI] [PubMed] [Google Scholar]
- 109. Matsunaga N, Chikaraishi Y, Shimazawa M, Yokota S, Hara H. Vaccinium myrtillus (bilberry) extracts reduce angiogenesis in vitro and in vivo. Evid Based Complement Alternat Med. 2010;7(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lo Vasco VR, Leopizzi M, Di Maio V, Di Raimo T, Cesa S, Masci A, Rocca CD. LPS, oleuropein and blueberry extracts affect the survival, morphology and phosphoinositide signalling in stimulated human endothelial cells. J Cell Commun Signal. 2017;11(4):317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease – a double-edged sword. Neuron. 2002;35(3):419–32. [DOI] [PubMed] [Google Scholar]
- 112. Ekdahl CT, Claasen J-H, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci. 2003;100(23):13632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Lassmann H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(5):1175–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer's disease. Brain Res. 1998;780(2):294–303. [DOI] [PubMed] [Google Scholar]
- 115. Chen H, O'Reilly EJ, Schwarzschild MA, Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson's disease. Am J Epidemiol. 2008;167(1):90–5. [DOI] [PubMed] [Google Scholar]
- 116. Jellinger KA. Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med. 2010;14(3):457–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Joseph JA, Villalobos-Molina R, Denisova N, Erat S, Cutler R, Strain J. Age differences in sensitivity to H2O2- or no-induced reductions in K+-evoked dopamine release from superfused striatal slices: reversals by PBN or trolox. Free Radic Biol Med. 1996;20(6):821–30. [DOI] [PubMed] [Google Scholar]
- 118. Gustafson SJ, Dunlap KL, McGill CM, Kuhn TB. A nonpolar blueberry fraction blunts NADPH oxidase activation in neuronal cells exposed to tumor necrosis factor-α. Ox Med Cell Longev. 2012;2012:768101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lau FC, Bielinski DF, Joseph JA. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J Neurosci Res. 2007;85(5):1010–17. [DOI] [PubMed] [Google Scholar]
- 120. Carey AN, Fisher DR, Rimando AM, Gomes SM, Bielinski DF, Shukitt-Hale B. Stilbenes and anthocyanins reduce stress signaling in BV-2 mouse microglia. J Agric Food Chem. 2013;61(25):5979–86. [DOI] [PubMed] [Google Scholar]
- 121. Hininger-Favier I, Thangthaeng N, Bielinski DF, Fisher DR, Poulose SM, Shukitt-Hale B. Blueberries and insulin protect microglial cells against high glucose-induced inflammation and restore GLUT-1. J Berry Res. 2021;11(2):201–16. [Google Scholar]
- 122. Joseph JA, Fisher DR, Bielinski D. Blueberry extract alters oxidative stress-mediated signaling in COS-7 cells transfected with selectively vulnerable muscarinic receptor subtypes. J Alzheimers Dis. 2006;9(1):35–42. [DOI] [PubMed] [Google Scholar]
- 123. Lau FC, Joseph JA, McDonald JE, Kalt W. Attenuation of iNOS and COX2 by blueberry polyphenols is mediated through the suppression of NF-kB activation. J Funct Foods. 2009;1(3):274–83. [Google Scholar]
- 124. De Caris MG, Grieco M, Maggi E, Francioso A, Armeli F, Mosca L, Pinto A, D'Erme M, Mancini P, Businaro R. Blueberry counteracts BV-2 microglia morphological and functional switch after LPS challenge. Nutrients. 2020;12(6):1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Cherry JD, Olschowka JA, O'Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chen G, Xu T, Yan Y, Zhou Y, Jiang Y, Melcher K, Xu HE. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin. 2017;38(9):1205–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282(15):11590–601. [DOI] [PubMed] [Google Scholar]
- 128. Dudal S, Krzywkowski P, Paquette J, Morissette C, Lacombe D, Tremblay P, Gervais F. Inflammation occurs early during the abeta deposition process in tgcrnd8 mice. Neurobiol Aging. 2004;25(7):861–71. [DOI] [PubMed] [Google Scholar]
- 129. Oberstein TJ, Spitzer P, Klafki H-W, Linning P, Neff F, Knölker H-J, Lewczuk P, Wiltfang J, Kornhuber J, Maler JM. Astrocytes and microglia but not neurons preferentially generate N-terminally truncated aβ peptides. Neurobiol Dis. 2015;73:24–35. [DOI] [PubMed] [Google Scholar]
- 130. Yamakawa MY, Uchino K, Watanabe Y, Adachi T, Nakanishi M, Ichino H, Hongo K, Mizobata T, Kobayashi S, Nakashima Ket al. Anthocyanin suppresses the toxicity of aβ deposits through diversion of molecular forms in in vitro and in vivo models of Alzheimer's disease. Nutr Neurosci. 2016;19(1):32–42. [DOI] [PubMed] [Google Scholar]
- 131. Brewer GJ, Torricelli JR, Lindsey AL, Kunz EZ, Neuman A, Fisher DR, Joseph JA. Age-related toxicity of amyloid-beta associated with increased pERK and pCREB in primary hippocampal neurons: reversal by blueberry extract. J Nutr Biochem. 2010;21(10):991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Debnath-Canning M, Unruh S, Vyas P, Daneshtalab N, Igamberdiev AU, Weber JT. Fruits and leaves from wild blueberry plants contain diverse polyphenols and decrease neuroinflammatory responses in microglia. J Funct Foods. 2020;68:103906. [Google Scholar]
- 133. Slanzi A, Iannoto G, Rossi B, Zenaro E, Constantin G. In vitro models of neurodegenerative diseases. Front Cell Dev Biol. 2020;8:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kim I, He Y-Y. Ultraviolet radiation-induced non-melanoma skin cancer: regulation of DNA damage repair and inflammation. Genes Dis. 2014;1(2):188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Teimouri A, Yeung P, Agu R. 2D vs. 3D cell culture models for in vitro topical (dermatological) medication testing. Cell Culture IntechOpen. 2018. doi: 10.5772/intechopen.79868. [DOI] [Google Scholar]
- 136. Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998–1008. [DOI] [PubMed] [Google Scholar]
- 137. Monteiro-Riviere NA, Inman AO, Snider TH, Blank JA, Hobson DW. Comparison of an in vitro skin model to normal human skin for dermatological research. Microsc Res Tech. 1997;37(3):172–9. [DOI] [PubMed] [Google Scholar]
- 138. Pambianchi E, Ferrara F, Pecorelli A, Woodby B, Grace M, Therrien J-P, Lila MA, Valacchi G. Blueberry extracts as a novel approach to prevent ozone-induced cutaneous inflammasome activation. Ox Med Cell Longev. 2020;2020:9571490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Esposito D, Overall J, Grace MH, Komarnytsky S, Lila MA. Alaskan berry extracts promote dermal wound repair through modulation of bioenergetics and integrin signaling. Front Pharmacol. 2019;10:1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tenuta MC, Malfa GA, Bonesi M, Acquaviva R, Loizzo MR, Dugay A, Bouzidi C, Tomasello B, Tundis R, Deguin B. LC-ESI-QTOF-MS profiling, protective effects on oxidative damage, and inhibitory activity of enzymes linked to type 2 diabetes and nitric oxide production of Vaccinium corymbosum L (Ericaceae) extracts. J Berry Res. 2020;10(4):603–22. [Google Scholar]
- 141. Young AR, Potten CS, Nikaido O, Parsons PG, Boenders J, Ramsden JM, Chadwick CA. Human melanocytes and keratinocytes exposed to UVB or UVA in vivo show comparable levels of thymine dimers. J Invest Dermatol. 1998;111(6):936–40. [DOI] [PubMed] [Google Scholar]
- 142. Goukassian D, Gad F, Yaar M, Eller MS, Nehal US, Gilchrest BA. Mechanisms and implications of the age-associated decrease in DNA repair capacity. FASEB J. 2000;14(10):1325–34. [DOI] [PubMed] [Google Scholar]
- 143. Pinnell SR. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J Am Acad Dermatol. 2003;48(1):1–22. [DOI] [PubMed] [Google Scholar]
- 144. Svobodová A, Zdarilová A, Vostálová J. Lonicera caerulea and Vaccinium myrtillus fruit polyphenols protect HaCaT keratinocytes against UVB-induced phototoxic stress and DNA damage. J Dermatol Sci. 2009;56(3):196–204. [DOI] [PubMed] [Google Scholar]
- 145. Bae J-Y, Lim SS, Kim SJ, Choi J-S, Park J, Ju SM, Han SJ, Kang I-J, Kang Y-H. Bog blueberry anthocyanins alleviate photoaging in ultraviolet-B irradiation-induced human dermal fibroblasts. Mol Nutr Food Res. 2009;53(6):726–38. [DOI] [PubMed] [Google Scholar]
- 146. Morliere P, Moysan A, Tirache I. Action spectrum for UV-induced lipid peroxidation in cultured human skin fibroblasts. Free Radic Biol Med. 1995;19(3):365–71. [DOI] [PubMed] [Google Scholar]
- 147. de Gruijl FR. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000;319:359–66. [DOI] [PubMed] [Google Scholar]
- 148. Fisher GJ, Choi HC, Bata-Csorgo Z, Shao Y, Datta S, Wang ZQ, Kang S, Voorhees JJ. Ultraviolet irradiation increases matrix metalloproteinase-8 protein in human skin in vivo. J Invest Dermatol. 2001;117(2):219–26. [DOI] [PubMed] [Google Scholar]
- 149. Svobodová A, Rambousková J, Walterová D, Vostalová J. Bilberry extract reduces UVA-induced oxidative stress in HaCaT keratinocytes: a pilot study. Biofactors. 2008;33(4):249–66. [DOI] [PubMed] [Google Scholar]
- 150. Wang H, Liu J, Pang D, Li T, Liu RH. Mechanisms underlying the protective effects of blueberry extract against ultraviolet radiation in a skin cell co-culture system. J Funct Foods. 2019;52:603–10. [Google Scholar]
- 151. Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof Bet al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18(12):1450–52. [DOI] [PubMed] [Google Scholar]
- 152. Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122(4):598–614. [DOI] [PubMed] [Google Scholar]
- 153. Behndig A, Svensson B, Marklund SL, Karlsson K. Superoxide dismutase isoenzymes in the human eye. Invest Ophthalmol Vis Sci. 1998;39(3):471–5. [PubMed] [Google Scholar]
- 154. Richards MJ, Nagel BA, Fliesler SJ. Lipid hydroperoxide formation in the retina: correlation with retinal degeneration and light damage in a rat model of Smith-Lemli-Opitz syndrome. Exp Eye Res. 2006;82(3):538–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Liu Y, Zhang D, Hu J, Liu G, Chen J, Sun L, Jiang Z, Zhang X, Chen Q, Ji B. Visible light-induced lipid peroxidation of unsaturated fatty acids in the retina and the inhibitory effects of blueberry polyphenols. J Agric Food Chem. 2015;63(42):9295–305. [DOI] [PubMed] [Google Scholar]
- 156. Ogawa K, Tsuruma K, Tanaka J, Kakino M, Kobayashi S, Shimazawa M, Hara H. The protective effects of bilberry and lingonberry extracts against UV light-induced retinal photoreceptor cell damage in vitro. J Agric Food Chem. 2013;61(43):10345–53. [DOI] [PubMed] [Google Scholar]
- 157. Ogawa K, Kuse Y, Tsuruma K, Kobayashi S, Shimazawa M, Hara H. Protective effects of bilberry and lingonberry extracts against blue light-emitting diode light-induced retinal photoreceptor cell damage in vitro. BMC Complement Alternat Med. 2014;14(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wang Y, Zhang D, Liu Y, Wang D, Liu J, Ji B. The protective effects of berry-derived anthocyanins against visible light-induced damage in human retinal pigment epithelial cells. J Sci Food Agric. 2015;95(5):936–44. [DOI] [PubMed] [Google Scholar]
- 159. Liu Y, Song X, Zhang D, Zhou F, Wang D, Wei Y, Gao F, Xie L, Jia G, Wu Wet al. Blueberry anthocyanins: protection against ageing and light-induced damage in retinal pigment epithelial cells. Br J Nutr. 2012;108(1):16–27. [DOI] [PubMed] [Google Scholar]
- 160. Milbury PE, Graf B, Curran-Celentano JM, Blumberg JB. Bilberry (Vaccinium myrtillus) anthocyanins modulate heme oxygenase-1 and glutathione S-transferase-pi expression in ARPE-19 cells. Investig Opthalmol Vis Sci. 2007;48(5):2343–9. [DOI] [PubMed] [Google Scholar]
- 161. Huang W-Y, Wu H, Li D-J, Song J-F, Xiao Y-D, Liu C-Q, Zhou J-Z, Sui Z-Q. Protective effects of blueberry anthocyanins against H2O2-induced oxidative injuries in human retinal pigment epithelial cells. J Agric Food Chem. 2018;66(7):1638–48. [DOI] [PubMed] [Google Scholar]
- 162. Huang W, Yan Z, Li D, Ma Y, Zhou J, Sui Z. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Ox Med Cell Longev. 2018;2018:1862462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Liu Y, Liu M, Chen Q, Liu G-M, Cao M-J, Sun L, Lu Z, Guo C. Blueberry polyphenols ameliorate visible light and lipid-induced injury of retinal pigment epithelial cells. J Agric Food Chem. 2018;66(48):12730–40. [DOI] [PubMed] [Google Scholar]
- 164. Jang YP, Zhou J, Nakanishi K, Sparrow JR. Anthocyanins protect against A2E photooxidation and membrane permeabilization in retinal pigment epithelial cells. Photochem Photobiol. 2005;81(3):529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140(6):1807–1816.e1. [DOI] [PubMed] [Google Scholar]
- 166. Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Philip Schumm L, Sharma Y, Anderson CAet al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel JF. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998;115(6):1405–13. [DOI] [PubMed] [Google Scholar]
- 168. Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci. 2007;104(34):13780–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Michielan A, D'Incà R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015;2015:628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50(8):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Polewski MA, Esquivel-Alvarado D, Wedde NS, Kruger CG, Reed JD. Isolation and characterization of blueberry polyphenolic components and their effects on gut barrier dysfunction. J Agric Food Chem. 2020;68(10):2940–7. [DOI] [PubMed] [Google Scholar]
- 172. Funakoshi K, Sugimura K, Anezaki K, Bannai H, Ishizuka K, Asakura H. Spectrum of cytokine gene expression in intestinal mucosal lesions of Crohn's disease and ulcerative colitis. Digestion. 1998;59(1):73–8. [DOI] [PubMed] [Google Scholar]
- 173. Peloquin JM, Goel G, Villablanca EJ, Xavier RJ. Mechanisms of pediatric inflammatory bowel disease. Annu Rev Immunol. 2016;34(1):31–64. [DOI] [PubMed] [Google Scholar]
- 174. Triebel S, Trieu H-L, Richling E. Modulation of inflammatory gene expression by a bilberry (Vaccinium myrtillus L.) extract and single anthocyanins considering their limited stability under cell culture conditions. J Agric Food Chem. 2012;60(36):8902–10. [DOI] [PubMed] [Google Scholar]
- 175. Driscoll K, Deshpande A, Datta R, Ramakrishna W. Anti-inflammatory effects of northern highbush blueberry extract on an in vitro inflammatory bowel disease model. Nutr Cancer. 2020;72(7):1178–90. [DOI] [PubMed] [Google Scholar]
- 176. Taverniti V, Fracassetti D, Del Bo’ C, Lanti C, Minuzzo M, Klimis-Zacas D, Riso P, Guglielmetti S. Immunomodulatory effect of a wild blueberry anthocyanin-rich extract in human Caco-2 intestinal cells. J Agric Food Chem. 2014;62(33):8346–51. [DOI] [PubMed] [Google Scholar]
- 177. Pojer E, Mattivi F, Johnson D, Stockley CS. The case for anthocyanin consumption to promote human health: a review. Compr Rev Food Sci Food Saf. 2013;12(5):483–508. [DOI] [PubMed] [Google Scholar]
- 178. Tsuda T. Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Mol Nutr Food Res. 2012;56(1):159–70. [DOI] [PubMed] [Google Scholar]
- 179. Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51(6):675–83. [DOI] [PubMed] [Google Scholar]
- 180. Huang W-Y, Liu Y-M, Wang J, Wang X-N, Li C-Y. Anti-inflammatory effect of the blueberry anthocyanins malvidin-3-glucoside and malvidin-3-galactoside in endothelial cells. Molecules. 2014;19(8):12827–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. de Souza VR, Pereira PAP, da Silva TLT, de Oliveira Lima LC, Pio R, Queiroz F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014;156:362–8. [DOI] [PubMed] [Google Scholar]
- 182. Deng J, Shi Z-J, Li X-Z, Liu H-M. Soluble polysaccharides isolation and characterization from rabbiteye blueberry (Vaccinium ashei) fruits. BioResources. 2012;8(1):405–19. [Google Scholar]
- 183. Farneti B, Khomenko I, Grisenti M, Ajelli M, Betta E, Algarra AA, Cappellin L, Aprea E, Gasperi F, Biasioli Fet al. Exploring blueberry aroma complexity by chromatographic and direct-injection spectrometric techniques. Front Plant Sc. 2017;8:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Howard LR, Hager T. Berry Fruit Phytochemicals. Berry Fruit: Value-Added Products for Health Promotion. Boca Raton (FL): CRC Press; 2007. p. 444. [Google Scholar]
- 185. Nile SH, Park SW. Edible berries: bioactive components and their effect on human health. Nutrition. 2014;30(2):134–44. [DOI] [PubMed] [Google Scholar]
- 186. Moore K, Howard L, Brownmiller C, Gu I, Lee S-O, Mauromoustakos A. Inhibitory effects of cranberry polyphenol and volatile extracts on nitric oxide production in LPS activated RAW 264.7 macrophages. Food Funct. 2019;10(11):7091–102. [DOI] [PubMed] [Google Scholar]
- 187. Suzuki R, Tanaka M, Takanashi M, Hussain A, Yuan B, Toyoda H, Kuroda M. Anthocyanidins-enriched bilberry extracts inhibit 3T3-L1 adipocyte differentiation via the insulin pathway. Nutr Metab. 2011;8(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Szymanowska U, Baraniak B, Bogucka-Kocka A. Molecules. 2018;23(7):1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Chen Z, Zheng S, Li L, Jiang H. Metabolism of flavonoids in human: a comprehensive review. Curr Drug Metab. 2014;15(1):48–61. [DOI] [PubMed] [Google Scholar]
- 190. Selma MV, Espín JC, Tomás-Barberán FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem. 2009;57(15):6485–501. [DOI] [PubMed] [Google Scholar]
- 191. Lavefve L, Howard LR, Carbonero F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020;11(1):45–65. [DOI] [PubMed] [Google Scholar]
- 192. Feliciano RP, Istas G, Heiss C, Rodriguez-Mateos A. Plasma and urinary phenolic profiles after acute and repetitive intake of wild blueberry. Molecules. 2016;21(9):1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Ho GTT, Wangensteen H, Barsett H. Elderberry and elderflower extracts, phenolic compounds, and metabolites and their effect on complement, RAW 264.7 macrophages and dendritic cells. Int J Mol Sci. 2017;18(3):584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Rutledge GA, Fisher DR, Miller MG, Kelly ME, Bielinski DF, Shukitt-Hale B. The effects of blueberry and strawberry serum metabolites on age-related oxidative and inflammatory signaling in vitro. Food Funct. 2019;10(12):7707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Kuntz S, Rudloff S, Asseburg H, Borsch C, Fröhling B, Unger F, Dold S, Spengler B, Römpp A, Kunz C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br J Nutr. 2015;113(7):1044–55. [DOI] [PubMed] [Google Scholar]