ABSTRACT
Body composition parameters are not captured by measures of body mass, which may explain inconsistent associations between body weight and prostate cancer (PC) risk. The objective of this systematic review was to characterize the association between fat mass (FM) and fat-free mass (FFM) parameters and PC risk. A search of PubMed, Embase, and Web of Science identified case-control and cohort studies that measured body composition in relation to PC risk. Methodological quality was assessed using the Newcastle-Ottawa Scale (NOS). Thirteen observational studies were included, of which 8 were case-control studies (n = 1572 cases, n = 1937 controls) and 5 were prospective cohort studies (n = 7854 incident cases with PC). The NOS score was 5.9 ± 1.1 for case-control studies and 8.4 ± 1.3 for cohort studies. The most common body composition technique was bioelectrical impedance analysis (n = 9 studies), followed by DXA (n = 2), computed tomography (n = 2), air displacement plethysmography (n = 1), and MRI (n = 1). No case-control studies reported differences in %FM between PC cases and controls and no consistent differences in FM or FFM (in kilograms) were observed. Two out of 5 cohort studies reported that higher %FM was associated with lower PC risk. Conversely, 3 cohort studies reported a greater risk of being diagnosed with advanced/aggressive PC with higher FM (expressed in kilograms, %FM, or fat distribution). Two out of 4 studies (both case-control and cohort) found that higher abdominal adipose tissue was associated with increased PC risk. In conclusion, although results were inconsistent, there is some evidence that FM may be negatively associated with total PC risk but positively associated with the risk of advanced/aggressive PC; modest evidence suggests that abdominal adipose tissue may increase the risk of PC. Future work should elucidate unique patterns of FM distribution and PC risk to triage men at risk for developing PC. This study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database as CRD42019133388.
Keywords: nutrition assessment, carcinoma, cancer, obesity, adiposity, muscle
Statement of Significance: This systematic review found that higher whole-body fat mass was associated with greater advanced prostate cancer risk in some (but not all) studies and abdominal adipose tissue may increase the risk of all stages of prostate cancer. Future research that delineates the specific relationships between body composition compartments with cancer risk may support the development of more targeted approaches to reduce the prevalence of cancer.
Introduction
Prostate cancer (PC) is the second most commonly diagnosed malignancy in males, comprising 13.5% of new cancer diagnoses worldwide (1). Considering the prevalence of PC, elucidating modifiable risk factors for the development of this condition is imperative for creating impactful public health strategies aimed at cancer prevention. Estimates of body size, such as body mass index (BMI), have been investigated as a risk factor for PC; however, substantial inconsistency across previous studies has been reported. Studies have suggested that BMI is negatively (2) or positively (3) associated with PC risk, or that BMI impacts overall and advanced PC risk differentially (4). For example, BMI was inversely associated with localized PC and positively associated with advanced PC in a systematic review (5).
Although BMI is an accessible and inexpensive tool commonly used in clinical settings to predict health risks, the inability to discern body compartments, such as fat mass (FM) and fat-free mass (FFM), is a notable limitation. In fact, body composition varies widely across the BMI spectrum (6). Furthermore, body composition predicts clinical outcomes after cancer diagnosis, independently of BMI (7–10). The impact of body composition on health outcomes might be due, in part, to the disparate metabolic activities of FM and FFM (11, 12). More specifically, FFM is hypothesized to represent “metabolic capacity,” which consists of the organs and tissues that maintain homeostasis, whereas FM is considered a “metabolic load,” which can challenge homeostasis maintenance (11, 13, 14). FM may be of particular importance in predicting cancer risk due to the positive associations of FM with insulin, insulin-like growth factor, and systemic inflammation (15), which may promote tumor growth. These relations between metabolic health and body composition compartments may partially explain the inconsistencies in anthropometric measurements and PC risk observed in previous studies.
Despite the value of body composition in predicting outcomes after cancer diagnosis, relatively fewer studies have described body composition in relation to the risk of developing PC. Because body composition may impact cancer development differently and independently of body mass, the objective of this investigation was to systematically review the association between body composition and the risk of PC.
Methods
Search strategy
This review was planned, conducted, and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations (16). A literature search of online databases (PubMed, Embase, and Web of Science) was performed from inception up to 4 May 2020. As shown in Supplemental Table 1, the search strategy consisted of 2 separate components and each involved keywords and medical subject heading (MeSH) terms related to “prostate cancer” and “body composition” individually. The keywords in each component were linked using “OR” as a Boolean function, and the results of the 2 sections were combined by utilizing the “AND” Boolean in the final search. No search limits were applied regarding date or language of publication. The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42019133388).
Study selection
Records found through the literature search were assessed to determine if they met the following inclusion criteria (17, 18):
Population: Adults (age ≥18 y)
Exposure: Body compartments quantified by bioelectrical impedance analysis (BIA), DXA, computed tomography (CT), air displacement plethysmography (ADP), or MRI
Comparator: Individuals without a diagnosis of PC
Outcome: Individuals diagnosed with PC versus no diagnosis of PC; there was no exclusion criterion relating to cancer stage at diagnosis or medication use
Body composition terminology was described consistently according to the specific compartment being assessed, as described elsewhere (19), irrespective of the terminology used in the selected articles. Due to the nature of the research question, inclusion of randomized controlled trials was not pertinent to the aim of this systematic review. Review articles, case reports, editorials, abstracts, and book chapters were also excluded. Therefore, only cohort and case-control studies that assessed body composition variables associated with PC risk were included. Two of the authors (SAP, MM) independently read titles and abstracts and potentially eligible records were selected for a full-text review. The same authors then reviewed all selected full texts for eligibility. Disagreements were resolved by review and consensus.
Data extraction
From the included studies, relevant data were extracted independently by 3 authors (SAP, MM, CLPO) in Microsoft Excel (version 2102; Microsoft Corporation). Data abstractors were not blinded to information such as authors, institutions of origin, and journal of publication. Information extracted included the following: study characteristics (i.e., first author's last name, article title, year of publication, study design, years of study, and follow-up duration, where applicable), study participants (i.e., sample size, ethnicity, race, and country of origin), body composition variables, outcome (i.e., PC diagnosis, advanced/aggressive PC diagnosis, or incident PC), confounding variables that were controlled for in statistical analyses, results summary (mean differences, ORs, HRs, or risk ratios, where appropriate) and Newcastle-Ottawa Scale (NOS) score. Follow-up time in cohort studies was reported exactly as stated in the study, as conversion from person-years to mean or median was not possible. Where appropriate, results from subanalyses of advanced versus nonadvanced or aggressive versus nonaggressive disease were also extracted. Advanced disease was defined as stage IV (20) or stage III or IV PC (21); aggressive PC was described as Gleason score ≥7 (22, 23), Gleason score ≥8 (20), a combination of Gleason score ≥8 or presence of stage III or IV PC (24, 25), or fatal PC (20). Outcomes of interest included body compartments as measured by BIA, DXA, CT, ADP, or MRI. An exploratory examination of results according to body composition technique was also conducted.
Study quality assessment
Methodological quality was assessed using the NOS (26). This assessment tool was chosen because it was specifically designed for nonrandomized trials and includes separate items for case-control and cohort studies. The NOS utilizes a scoring system to assess 3 domains of methodological rigor: 1) selection of study groups (0–5 points), 2) comparability/adjustment for confounding variables (0–3 points), and 3) ascertainment of exposures or outcomes (0–3 points). Two authors (SAP and MM) independently reviewed included articles and assigned scores for each category; scores that differed were discussed until a consensus was met. Higher scores are indicative of higher quality studies.
Results
Description of studies
The literature search retrieved 5015 potentially eligible records. After duplicates were removed, a total of 3399 articles remained. After the initial screening of titles and abstracts, 36 articles were retrieved for full-text review (20–25, 27–56) and 13 met the inclusion criteria. In total, there were 8 case-control studies (22, 23, 44–48, 51) and 5 cohort studies (20, 21, 24, 25, 49). The detailed process of the literature search is shown in Figure 1.
FIGURE 1.
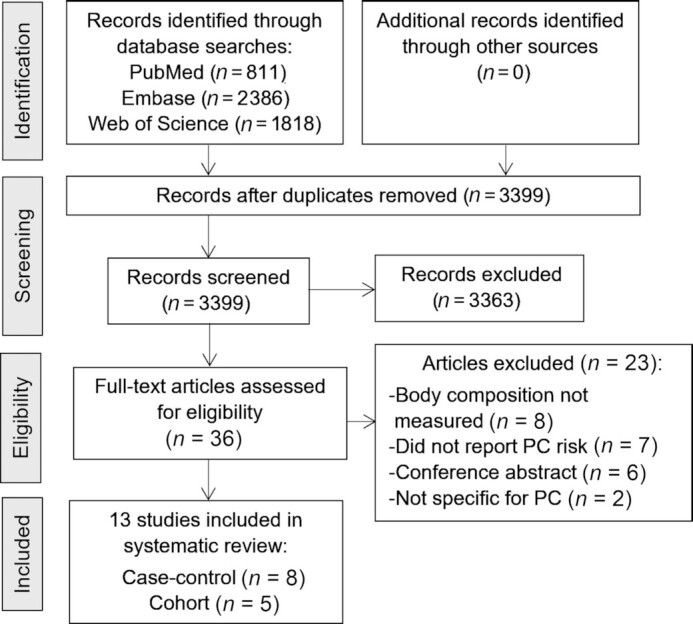
Flow chart to select eligible articles that assessed body composition in relation to PC risk. PC, prostate cancer.
Characteristics of the included case-control studies are presented in Table 1. These studies were conducted between 1993 and 2017 and included 1572 cases and 1937 controls. All cases had newly diagnosed PC, therefore negating the potential effects of cancer treatment on body composition. Cohort studies were conducted between 1990 and 2011 and included 285,865 participants, 7854 of whom were diagnosed with PC (Table 2). Among cohort studies, the minimum follow-up was a mean ± SD of 5.6 ± 1.0 y (49) and the maximum follow-up was a median of 15.5 y (21); follow-up was also reported as 113,535 person-years in 1 study (24).
TABLE 1.
Characteristics of case-control studies included in the systematic review assessing body composition in relation to PC risk1
| Reference | Cases | Controls | Race/ethnicity | Body composition method; variables | Confounding variables controlled for | Results: All PC and advanced or aggressive PC |
|---|---|---|---|---|---|---|
| Cimino et al. 201 (44) | Total: n = 45, in which: T1 stage: n = 21; T2a stage: n = 10; T2b stage: n = 5; T2c stage: n = 4 | Total: n = 105 (benign prostatic hyperplasia) | Not reported | ADP; FM (%) | None | %FM:↔Controls: 32.0 ± 5.1 Cases: 29 ± 8.9P = 0.90 |
| Demark-Wahnefried et al. 1997 (45) | Total: n = 36 | Total: n = 38 | Black or White men (recruitment aim was 80% White, 20% Black) | DXA; FM (kg and %), fat distribution and FFM (kg) | Age, race, age × race interaction | %FM:↔Controls: 24.9 ± 5.9Cases: 24.0 ± 5.6OR (95% CI): 0.82 (0.40–1.68)FM (kg): ↔ (data not reported)FFM (kg): ↔Controls: 64.5 ± 8.6 Cases: 65.1 ± 8.4 OR (95% CI): 1.03 (0.50–2.13)Fat distribution: ↔ (data not reported) |
| Fowke et al. 2012 (46) | Total: n = 809, in which: Gleason 6: n = 402; Gleason 7: n = 272 Gleason 8: n = 135 | Total: n = 1057 | Mostly White | BIA; FM (kg and %) and FFM (kg) | Age, race/ethnicity, family history of PC, PSA level, prostate volume, and current treatment for diabetes, cardiovascular disease, benign prostatic hyperplasia, or hyperlipidemia | %FM: ↔ across all Gleason scores Adjusted mean (95% CI):Controls: 29.2 (28.5, 29.9)Gleason 6: 28.6 (27.7, 29.4)Gleason 7: 29.4 (28.5, 30.4)Gleason 8–10: 30.1 (28.8, 31.4)FM (kg): ↑ in Gleason 7 and 8–10FM as a continuous variable associated with Gleason 8-10 cancer (OR: 1.02; 95% CI: 1.00–1.04)Highest quartile of FM was associated with Gleason 7 cancer (OR: 1.52; 95% CI: 1.00, 2.30)Adjusted mean (95% CI):Controls: 27.1 (25.9, 28.4)Gleason 6: 26.3 (24.7, 27.8)Gleason 7: 28.0 (26.3, 29.7) Gleason 8–10: 29.9 (27.6, 32.2)FFM (kg): ↑ in Gleason 7 & 8-10Highest quartile of FFM was associated with Gleason 7 (OR: 1.72; 95% CI: 1.14–2.61) and Gleason 8–10 (OR: 2.91; 95% CI: 1.56–5.44)FFM above median associated with Gleason 6 (OR: 1.31; 95% CI: 1.02–1.67)Adjusted mean (95% CI):Controls: 62.9 (62.0, 63.8)Gleason 6: 63.1 (62.0, 64.2)Gleason 7: 64.4 (63.1, 65.6)Gleason 8–10: 66.2 (64.5, 67.9) |
| Guerrios-Rivera et al. 2017 (22) | Total: n = 483, in which:Gleason 6: n = 246; Gleason 7-10 (high-grade disease): n = 237 | Total: n = 496 | Cases: 64% Black and 36% Non-BlackControls: 51% Black and 49% Non-Black | BIA; FM (%) | Age, race, previous biopsy, family history of PC, PSA, digital rectal exam findings, prostate volume | All PC: %FM: ↔ Controls: 28.6 ± 6.0 Cases: 28.3 ± 6.7OR: 1.02 (95% CI: 0.99–1.04), P = 0.16 Aggressive PC (Gleason ≥7):%FM: ↑Gleason 6: 27.4 ± 6.9 Gleason 7–10: 29.3 ± 6.4OR: 1.06 (95% CI: 1.03–1.09), P<0.001 |
| Kim et al. 2019 (23) | Total: n = 100 | Total: n = 100 | Not reported | MRI; total abdominal AT and abdominal AT ratio (not specified) | Age, educational level, and family history | All PC: Total abdominal AT (mm2): ↔Total abdominal AT above the median (21,500 mm2): OR: 1.30 (95% CI: 0.37–4.60), P < 0.39Abdominal AT ratio: ↑Abdominal AT ratio above the median (51.5%):OR: 2.23 (95% CI: 1.05–3.93), P < 0.05Controls: 49.4 ± 8.1All PC: 51.6 ± 7.9p<0.04Aggressive PC (Gleason ≥7):Total abdominal AT (mm2): ↔ OR: 1.30 (95% CI: 0.37–4.60), P < 0.35Abdominal AT ratio: ↔ OR: 2.81 (95% CI: 1.16–9.76), P < 0.10 |
| Maturo et al. 2003 (47) | Total: n = 11 | Total: n = 11 | Italian | DXA; FM (kg) and LST (kg)BIA; body cell mass, intra- and extracellular water, and total body water (L) | None | FM (kg): ↔Controls 23.6 ± 7.0Cases: 22.8 ± 5.3P ≥ 0.05LST (kg): ↓Controls: 53.6 ± 5.6Cases: 50.2 ± 5.0P < 0.05Body cell mass (kg): ↓Controls: 28.3 ± 2.6Cases: 26.9 ± 3.0P < 0.05Intracellular water (L): ↔Controls: 23.4 ± 2.4Cases: 22.5 ± 2.9P > 0.05Extracellular water (L): ↔Controls: 23.7 ± 5.Cases: 21.7 ± 5.5 P > 0.05 |
| Total body water (L): ↓ Controls: 47.0 ± 6.9 Cases: 43.8 ± 5.4 P> 0.05 | ||||||
| Moran et al. 2017 (48) | Total: n = 25, in which: Gleason ≥7: n = 7 | Total: n = 67 | Not reported | BIA; FM (kg), visceral AT (%)2 | None | Presented as mean (95% CI): FM (kg): ↔Controls: 20.4 (17.7–23.3) Cases: 19.5 (14.2–17.7)P = 0.09Visceral AT2(%): ↔Controls: 32.2 (30.1–34.3) Cases: 31.6 (27.7–35.4)P = 0.12 |
| von Hafe et al. 2004 (51) | Total: n = 63, in which: localized disease: n = 30; locally advanced disease: n = 12; metastasized disease: n = 21 | Total: n = 63 | White | CT; AT area (abdominal subcutaneous, total abdominal, and visceral) and visceral: subcutaneous AT ratio | None | All PC: AT area (cm2): ↑Controls: 334.3 ± 132.9 Cases: 509.2 ± 226.1OR (95% CI): 3.2 (1.9–5.3)P < 0.001Visceral AT area (cm2): ↑Controls: 177.4 ± 88.4 Cases: 324.7 ± 145P < 0.001OR (95% CI): 4.6 (2.6–8.2)Subcutaneous area (cm2): ↑Controls: 156.9 ± 57.6 Cases: 184.5 ± 85.6P = 0.037OR (95% CI): 1.5 (1.0–2.2)Visceral:subcutaneous AT: ↑Controls: 1.2 ± 0.4 Cases: 1.8 ± 0.4 P < 0.001OR (95% CI): 6.0 (2.3–11.0)Advanced PC: All body composition parameters: ↔ local, locally advanced, metastatic PC |
Values are means ± SDs unless otherwise stated. Where appropriate, only values from adjusted models were included in the table. ADP, air displacement plethysmography; ADT, androgen deprivation therapy; AT, adipose tissue; BIA, bioelectrical impedance analysis; CT, computed tomography; FFM, fat-free mass; FM, fat mass; LST, lean soft tissue; PC, prostate cancer; ↑, significantly associated with higher risk of prostate cancer (P < 0.05); ↔, not associated with risk of prostate cancer (P > 0.05); ↓, significantly associated with lower risk of prostate cancer (P < 0.05).
Visceral AT was estimated by BIA rather than measured directly.
TABLE 2.
Characteristics of cohort studies included in the systematic review assessing body composition in relation to PC risk1
| Reference | Cases | Cohort | Race/ethnicity | Body composition methods; variables | Follow-up duration | Confounding variables controlled for | Results: All PC and advanced or aggressive PC |
|---|---|---|---|---|---|---|---|
| Dickerman et al. 2019 (20) | Total: n = 172, in which: Gleason grade ≥8 (high grade): n = 43; advanced (≥cT3b/N1/M1 at diagnosis or fatal prostate cancer over follow-up): n = 41; fatal: n = 31 | Total: n = 1832 | Not reported | CT; visceral and subcutaneous abdominal AT; intermuscular AT and subcutaneous thigh AT BIA; FM (kg and %) | Median 10.1 y (range: 0.1–13.3 y) until diagnosis and 10.4 y (range: 0.1–13.3 y) until PC death | Age, height, family history of PC, smoking status, education, frequency of moderate/vigorous physical activity during youth and midlife, and presence of a physician visit over the past year | Presented as HR per 1-SD increase in FM (6.8 kg), %FM (5.3%), visceral abdominal AT (85.7 cm2), subcutaneous abdominal AT (85.6 cm2), intermuscular thigh AT (16.0 cm2), and subcutaneous thigh AT (39.2 cm2)All PC : FM: ↔; 0.98 (0.83–1.18)% FM: ↔; 0.99 (0.83–1.18)Visceral abdominal AT ↔; 0.97 (0.83–1.13)Subcutaneous abdominal AT: ↔; 1.02 (0.88–1.19)Intermuscular thigh AT: ↔; 0.91 (0.78–1.08)Subcutaneous thigh AT: ↔; 1.02 (0.88–1.19)Advanced PC:FM: ↔; 1.17 (0.83–1.67)% FM: ↔; 1.19 (0.83–1.69)Visceral abdominal AT: ↑; 1.31 (1.00–1.72)Subcutaneous abdominal AT: ↔; 1.22 (0.91–1.63)Intermuscular thigh AT: ↔; 1.02 (0.75–1.40)Subcutaneous thigh AT: ↔; 1.25 (0.95–1.64)Fatal PC:FM: ↔; 1.17 (0.78–1.75)%FM: ↔; 1.20 (0.80–1.81)Visceral abdominal AT: ↔; 1.24 (0.89–1.73)Subcutaneous abdominal AT: ↔; 1.26 (0.89–1.78)Intermuscular thigh AT: ↔; 1.27 (0.91–1.78)Subcutaneous thigh AT: ↑; 1.37 (1.00–1.88) |
| MacInnis et al. 2003 (24) | Total: n = 477, in which:Gleason 1–4: n = 66; Gleason 5–7: n = 332; Gleason 8–10: n = 79 | Total: n = 16,336 | Italian: n = 2419 Greek: n = 2073Remainder not reported | BIA; FM (kg and %) and FFM (kg) | 113,535 person-years | Age, country of birth, and highest level of education | Presented as RR (95% CI)All PC: FM: ↔; Per 10 kg: 1.08 (0.96–1.22)FM %: ↔; Per 10%: 1.16 (0.99–1.35)FFM: ↔; Per 10 kg: 0.96 (0.82–1.13)Aggressive PC:FM: ↑; Per 10 kg: 1.25 (1.03–1.77)FM %: ↔; Per 10%: 1.39 (0.95–2.06)FFM: ↔; Per 10 kg: 1.25 (0.85–1.83) |
| Moller et al. 2015 (21) | Total: n = 1813, in which: stage 3 or 4: n = 626 | Total: n = 29,944 | Not reported | BIA; FM (%) | Median 15.5 y | Age; stage, where appropriate | Presented as HR (95% CI), 4th vs. 1st quartileAll PC: % FM: ↓; 0.84 (0.74–0.96)Advanced PC:% FM: ↑; 1.31 (1.04–1.64)Quartile 1: 1.3–22.8Quartile 2: 22.9–26.4Quartile 3: 26.5–29.9Quartile 4: 30.0–48.4 |
| Perez-Cornago et al. 2017 (49) | Total: n = 4575 | Total: n = 219,335 | White: n = 205,839; mixed background: n = 1077; Asian: n = 5765; Black: n = 3279; other: n = 1926 | BIA; FM (%) | Mean ± SD: 5.6 ± 1.0 y | Townsend deprivation index, ethnicity, co-habitation status, BMI, cigarette smoking, physical activity, diabetes, enlarged prostate, and family history of PC | Presented as HR (95% CI), 5th vs. 1st quintile FM %: ↓; 0.81 (0.73–0.89),P < 0.001 across quintilesQuintile 1: <20.5Quintile 2: 20.5 - <24Quintile 3: 24 to <26.8Quintile 4: 26.8 to <30.1Quintile 5: ≥30.1 |
| Wallstrom et al. 2009 (25) | Total: n = 817, in which: T stage ≥3 or N stage ≥1 or M1 or Gleason ≥8 or PSA ≥ 50 ng/mL (aggressive disease): n = 281Nonaggressive disease: n = 530 | Total: n = 10,564 | Not reported | BIA; FM (%) | Mean follow-up time for cases was 11.0 y; mean follow-up for non-cases was 7.7 y | Age, co-habitation status, socioeconomic index, alcohol habits, smoking history, country of birth, and dietary intake of total calcium EPA/DHA, fruits, vegetables, and red meat | Presented as HR (95% CI), 5th vs. 1st quintileAll PC:FM %: ↔; 1.08 (0.86–1.35), P = 0.94 across quintilesAggressive PC:FM % ↔; 1.01 (0.69-1.48), P = 0.35 across quintilesQuintile 1: <18.3 (median: 15.7)Quintile 2: 18.3–21.3 (median: 19.9)Quintile 3: 21.3–23.8 (median: 22.6)Quintile 4: 23.8–26.6 (median: 25.1)Quintile 5: ≥26.6 (median: 28.8) |
AT, adipose tissue; BIA, bioelectrical impedance analysis; BMI, body mass index; CT, computed tomography; EPA/DHA, eicosapentaenoic acid/docosahexaenoic acid; FFM, fat-free mass; FM, fat mass; PC, prostate cancer; PSA, prostate specific antigen; RR, risk ratio; ↑, significantly associated with higher risk of prostate cancer (P < 0.05); ↔, not associated with risk of prostate cancer (P > 0.05); ↓, significantly associated with lower risk of prostate cancer (P < 0.05).
Among all studies, the most common body composition technique used to measure participants’ body compartments was BIA (n = 9 studies) (20–22, 24, 25, 46–49), followed by DXA (n = 2 studies) (45, 47), CT (n = 2 studies) (20, 51), ADP (n = 1 study) (44), and MRI (n = 1 study) (23). Most studies reported body composition as percentage FM of total body weight (%FM) (20–22, 24, 25, 44–46, 49) and FM in kilograms (20, 24, 46–48). Other compartments included FFM (kilograms) (24, 45, 46), lean soft tissue (LST; kilograms) (47), and fat distribution (i.e., abdominal adipose tissue area, visceral adipose tissue, %FM) (20, 23, 45, 48, 51). Eight studies reported body composition in relation to advanced stage or tumor aggressiveness (20–25, 46, 51), of which 4 were case-control and 4 were cohort studies. Case-control NOS scores were 5.9 ± 1.1 and cohort NOS scores were 8.4 ± 1.3 (Supplemental Tables 2 and 3).
Due to differences in body composition measurement techniques, body compartments reported, and population characteristics, a meta-analysis of case-control or cohort studies was not conducted. Therefore, a narrative analysis of the studies uncovered in the systematic literature search is presented below.
Results from case-control studies
%FM
Four case-control studies reported %FM. None of these studies reported differences between PC cases and controls using BIA, DXA, or ADP (22, 44–46).
Absolute FM, FFM, or LST
Of the 4 case-control studies that reported body composition in absolute values, 4 reported FM (45–48) and 3 reported FFM or LST in absolute kilograms (45–47). Moran et al. (48) reported no differences in absolute FM as assessed by BIA between patients with and without PC. Conversely, Fowke et al. (46) reported Gleason 8–10 PC cases had significantly higher levels of FM and FFM measured with BIA compared with controls. Cases with Gleason 7 PC had higher FFM, but no differences were observed between controls and patients with Gleason 6 PC (46). Similarly, healthy control subjects had higher LST but similar FM as measured by DXA compared with those with PC (47). In an analysis controlling for age, race, and race-by-age interaction, 1 study using DXA reported no differences in FFM between PC cases and healthy controls (45).
Fat distribution
Four case-control studies described body fat distribution in the methods (23, 45, 48, 51), but 1 study (45) did not report values from this analysis. One study reported no relation between visceral adipose tissue and PC risk; however, visceral adipose tissue was estimated with BIA (with no equation provided) rather than measured directly (48). von Hafe et al. (51) used CT imaging to assess body composition and found that PC patients had higher mean total adipose tissue area, visceral adipose tissue area, and visceral-to-subcutaneous ratio than controls. Similarly, Kim and Joung (23) attained measures of abdominal adipose tissue ratio via MRI, although the methods did not describe what the ratio consisted of. Nevertheless, PC patients had higher abdominal adipose tissue ratio compared with controls; higher odds of PC were observed among individuals with an abdominal adipose tissue ratio above the median.
Advanced or aggressive disease
Four case-control studies reported body composition in relation to aggressiveness or stage of disease (22, 23, 46, 51). Patients with high-grade PC had higher %FM compared with controls in 1 study (22) and another found that higher FM and FFM was associated with higher Gleason scores (46)(both via BIA). Another investigation (23) reported that higher abdominal adipose tissue ratio (by MRI) was associated with higher odds of PC with a Gleason score ≥7, but this relation was no longer significant in a model adjusted for age, educational level, and family history of PC. von Hafe et al. (51) reported no differences in CT-assessed body composition parameters among patients with local, locally advanced, and metastatic disease.
Results from cohort studies
%FM
All 5 cohort studies measured %FM using BIA. Higher %FM was associated with lower risk of PC when individuals were grouped according to %FM quartiles (21) or quintiles (49). Three other studies using BIA (20, 24, 25) reported that %FM was not associated with PC risk.
Absolute FM, FFM, or LST
Two cohort studies (20, 24) presented FM in absolute kilograms, estimated using BIA; one of these also reported FFM in this manner (24). There were no associations between body composition and total or advanced PC risk in either study.
Fat distribution
Only 1 cohort study assessed fat distribution, which was measured using CT (20). No measure of visceral or subcutaneous adipose tissue was associated with PC risk.
Advanced or aggressive disease
Four cohort studies stratified results by PC stage or aggressiveness (20, 21, 24, 25). Moller et al. (21) reported a higher risk of developing advanced PC with higher %FM (by BIA). Another study using BIA (24) reported that FM in kilograms was associated with higher risk of aggressive PC (Gleason score 8–10 vs. Gleason score 1–4). Similarly, Dickerman et al. (20) reported that visceral adipose tissue measured by CT was associated with increased risk of advanced PC and thigh subcutaneous adipose tissue was associated with increased risk of fatal PC. However, these results were not apparent when BMI was included in the models. Two studies reported no relation between %FM measured with BIA (20, 25) or body fat distribution measured with CT (20) and aggressive PC.
PC risk according to body composition technique
Due to the inherent differences in body composition techniques, PC risk among both case-control and cohort studies was assessed according to technique (Table 3). There was no apparent bias or pattern of PC risk that could be explained by PC methodology.
TABLE 3.
Association between body compartments and risk of total PC in cohort and case-control studies by body composition technique1
| Fat mass, kg | % Fat mass | FFM, LST, or body cell mass | Total and intra- or extracellular water | Total abdominal adiposity | Abdominal AT ratio2 | Intermuscular or subcutaneous thigh AT | Visceral abdominal AT | Subcutaneous abdominal AT | |
|---|---|---|---|---|---|---|---|---|---|
| ADP | ↔ | ||||||||
| DXA | ↔↔ | ↔ | ↔↓ | ||||||
| BIA | ↑ ↔↔↔ | ↓↓↔↔↔↔↔ | ↑ ↓↔ | ↓↔↔ | ↔3 | ||||
| MRI | ↔ | ↑ | |||||||
| CT | ↑ | ↑ | ↔ | ↑↔ | ↑↔ |
Each arrow represents one result from studies that were included in this systematic review, fully described in Table 1 (i.e., case-control studies) and Table 2 (i.e., cohort studies). ADP, air displacement plethysmography; AT, adipose tissue; BIA, bioelectrical impedance analysis; CT, computed tomography; FFM, fat-free mass; LST, lean soft tissue; PC, prostate cancer; ↑, significantly associated with higher risk of prostate cancer (P < 0.05); ↔, not associated with risk of prostate cancer (P > 0.05); ↓, significantly associated with lower risk of prostate cancer (P<0.05).
Visceral adipose tissue was estimated by BIA rather than measured directly.
Discussion
The present systematic review is the first to assess objectively-measured body composition in relation to PC risk. Percentage FM did not differ between cases and controls, although higher %FM was associated with lower risk of PC in 2 out of 5 cohort studies. Some results also suggest that abdominal FM may increase the risk of advanced or aggressive PC. Although FM might relate to PC risk, our results were inconsistent and discrepancies in PC staging, population, and body composition techniques are apparent; therefore, these results should be interpreted with caution.
Previous data on anthropometric measures of obesity and PC risk are conflicting. One systematic review concluded that obesity determined by BMI ≥30 kg/m2, but not waist circumference, was weakly associated with higher PC risk (3). In contrast, another systematic review reported that BMI was inversely associated with localized PC and positively associated with advanced PC (5), similar to recent Mendelian randomization studies that found genetically predicted BMI or FM was associated with lower PC risk (57, 58). Although anthropometric variables such as weight and height used to derive BMI are accessible and low-burden, body composition varies greatly across age, sex, and BMI categories (6), which may partly explain the discrepancies in PC risk according to anthropometric measurements.
Our findings suggest that higher %FM might be protective against the development of PC. Of note, 3 studies included in this literature review (21, 23, 47) did not report associations between BMI and PC but found associations between body composition parameters and PC risk. More specifically, %FM (21, 47) and abdominal adipose tissue ratio (23) may be more sensitive than anthropometric measurements in predicting PC risk. Conversely, Dickerman et al. (20) conducted a sensitivity analysis in which inclusion of BMI in the models attenuated the relation between body composition parameters and PC risk. While a superficial interpretation may support the notion that BMI predicts PC or negates the effect of body composition on PC risk, null findings in the supplementary models are likely a result of high collinearity among variables in the same model. However, inclusion of such measurements is not currently routinely obtained in clinical practice or large prospective research studies, which hinders cancer risk estimates based on body composition.
Higher %FM may relate to greater PC risk due to underlying metabolic, hormonal, and inflammatory mechanisms underpinning the association between FM and cancer development. Men with obesity have lower concentrations of androgens and adiponectin and higher concentrations of insulin and insulin-like growth factor (59, 60), the latter of which is associated with increased PC risk (61). Obesity is also related to lower concentrations of free testosterone and higher systemic inflammation, which may also promote tumor growth (62, 63). However, we found that %FM often did not relate to PC risk, and in fact, 2 studies (out of 5) suggested that higher %FM was related to lower PC risk (21, 49). Notably, studies that reported an inverse association between PC risk and %FM were prospective cohort studies, which are more appropriate for establishing prognosis and risk compared with case-control designs and—in this review—had higher ratings of study quality according to NOS scores. One factor that may contribute to the unexpected association between %FM and PC risk is fat distribution. Higher abdominal adiposity is associated with altered concentrations of adipokines, insulin resistance, impaired glucose and fat metabolism, and endothelial dysfunction (63, 64). Pischon et al. (65) observed that waist circumference—an anthropometric-based estimate of abdominal FM—was positively associated with PC risk, although this has not been corroborated by other investigations (3, 66, 67). Objective measurements of body composition provide more specific information of fat distribution that may impact cancer development. We observed modest positive associations between abdominal obesity and PC risk in the present review (20, 23, 51) and contradictory evidence that higher %FM may relate to lower PC risk; therefore, further investigation of whole-body FM parameters and specific adipose tissue depots (e.g., visceral, subcutaneous) in relation to PC risk is warranted.
Where appropriate, we extracted data on the occurrence of aggressive PC, since this may develop from physiological processes that are different from nonaggressive PC. Interestingly, of the 8 studies that reported results of aggressive PC separately from all PC stages combined (20–24, 46, 51), 5 reported modest positive associations between high FM and advanced PC risk (20–22, 24, 46). Notably, one of these studies determined FM distribution via CT imaging and reported that visceral and thigh subcutaneous adiposity was associated with greater risk of advanced and fatal PC, respectively (20). Previous findings from the European Prospective Investigation into Cancer and Nutrition have found that higher waist circumference was associated with greater risk of developing advanced and high-grade PC, especially among men with a normal BMI (65). Therefore, FM and fat distribution may promote aggressive disease directly through altered metabolic pathways or may be a marker for lifestyle patterns (i.e., physical activity, dietary intake) that influence both FM accumulation and aggressive disease in a manner somewhat independent of body weight.
Although this review is the first to investigate body composition and PC risk in a systematic manner, some limitations should be noted when interpreting the results. In particular, a wide array of body composition techniques were utilized among studies, which could negatively influence interpretation of the impact of body composition on PC risk. For example, BIA may produce values of FM that are lower and LST that are higher than those arising from DXA (68). Heterogeneity also occurs across different manufacturers and formulae used to predict body composition from resistance measured by BIA (69, 70). Several studies also reported absolute values of FM or FFM; however, body composition expressed in this manner does not account for total body weight or height. Furthermore, 1 study (44) used controls who were diagnosed with benign prostatic hyperplasia, which might have obscured potential differences in body composition, due to the association of this condition with obesity, diabetes, and higher plasma glucose (71). By design, case-control studies may also introduce additional biases such as those associated with medical access and referral, data ascertainment (e.g., chart review), self-selection in cancer-related research, or collection asynchronous exposures and outcomes (72, 73). Notably, many studies in this review included socioeconomic status and/or race as confounding variables in statistical models or excluded individuals with major comorbidities. Another limitation is the small sample sizes observed in many case-control studies. Investigations of body composition and disease risk may also be prone to bias from pre-diagnosis unintentional weight change directly before diagnosis (74); therefore, weight (or body composition) at diagnosis may not reflect long-term energy balance. This review also uncovered a small number of studies available for hypothesis testing. Because of the differences among study procedures and small number of similar studies, we did not perform a meta-analysis for all PC combined or subgroups according to PC aggressiveness/stage or body composition modality. Nevertheless, we have presented results describing risk of advanced/aggressive PC, where appropriate, and an additional table summarizing body composition techniques to help identify potential biases. Notably, a recent systematic review of body composition in relation to breast cancer risk also reported high heterogeneity among studies (75), suggesting that this issue is common among studies investigating body composition. Variation among study results may also relate to molecular heterogeneity of cancer subtypes that may be differentially affected by body composition.
In conclusion, this systematic review found that body composition may relate to PC risk in a manner different from anthropometric measures (i.e., BMI) alone. Specifically, %FM may associate with lower total PC risk but may increase the risk of developing advanced PC; modest evidence suggests that excess abdominal adipose tissue may also confer increased PC risk. However, these results should be interpreted with caution due to differences in body composition methods and populations among studies. Future research with more equivalent and accurate body composition tools and prospective study designs may further clarify the role of body composition on the development and aggressiveness of PC.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Jessica Thorlakson for her insight on the initial development of our literature search. The authors’ responsibilities were as follows—SAP, MM, CMP, PR, and JL: formulated the research question; SAP and MM: applied the literature search and undertook the screening title and abstract screening; SAP and CLPO: extracted the data and tabulated results; SAP: wrote the initial version of the manuscript; and all authors: contributed to its final version and read and approved the final manuscript.
Notes
This investigation was part of a larger project funded by Prostate Cancer Canada Targeted RFP in Prostate Cancer Prevention. CMP is supported by a Canadian Institutes of Health Research New Investigator Salary Award, a Campus Alberta Innovates Program, and a Canadian Foundation for Innovation John R. Evans Leaders Fund (project no. 34115).
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: ADP, air displacement plethysmography; ADT, androgen deprivation therapy; AT, adipose tissue; BIA, bioelectrical impedance analysis; CT, computed tomography; FFM, fat-free mass; FM, fat mass; LST, lean soft tissue; NOS, Newcastle Ottawa Scale; PC, prostate cancer.
Contributor Information
Sarah A Purcell, Agricultural, Food, and Nutritional Science, University of Alberta, Edmonton, Alberta, Canada; Division of Endocrinology, Metabolism, and Diabetes, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Camila L P Oliveira, Agricultural, Food, and Nutritional Science, University of Alberta, Edmonton, Alberta, Canada.
Michelle Mackenzie, Agricultural, Food, and Nutritional Science, University of Alberta, Edmonton, Alberta, Canada.
Paula Robson, Cancer Care Alberta and the Cancer Strategic Clinical Network, Alberta Health Services, Edmonton, Alberta, Canada.
John D Lewis, Department of Experimental Oncology, University of Alberta, Edmonton, Alberta, Canada.
Carla M Prado, Agricultural, Food, and Nutritional Science, University of Alberta, Edmonton, Alberta, Canada.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet North Am Ed. 2014;384(9945):755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17(8):989–1003. [DOI] [PubMed] [Google Scholar]
- 4. Xie B, Zhang G, Wang X, Xu X. Body mass index and incidence of nonaggressive and aggressive prostate cancer: a dose-response meta-analysis of cohort studies. Oncotarget. 2017;8(57):97584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer—a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23(7):1665–71. [DOI] [PubMed] [Google Scholar]
- 6. Prado CMM, Siervo M, Mire E, Heymsfield SB, Stephan BCM, Broyles S, Smith SR, Wells JCK, Katzmarzyk PT. A population-based approach to define body-composition phenotypes. Am J Clin Nutr. 2014;99(6):1369–77. [DOI] [PubMed] [Google Scholar]
- 7. Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, Strasser F, Thoresen L, Jagoe RT, Chasen Met al. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol. 2015;33(1):90–9. [DOI] [PubMed] [Google Scholar]
- 8. Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016;75(2):188–98. [DOI] [PubMed] [Google Scholar]
- 9. Prado CMM, Mourtzakis M, Baracos V, Reiman T, Sawyer MB, McCargar LJ. Overweight and obese patients with solid tumors may have sarcopenia, poor prognosis and early features of cachexia. Int J Body Compos Res. 2010;8:7–15. [Google Scholar]
- 10. Brown JC, Caan BJ, Meyerhardt JA, Weltzien E, Xiao J, Cespedes Feliciano EM, Kroenke CH, Castillo A, Kwan ML, Prado CM. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I-III colorectal cancer: a population-based cohort study (C-SCANS). J Cachexia Sarcopenia Muscle. 2018;9(4):664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prado CMM, Wells JCK, Smith SR, Stephan BCM, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012;31(5):583–601. [DOI] [PubMed] [Google Scholar]
- 12. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–82. [DOI] [PubMed] [Google Scholar]
- 13. Wells JCK. Historical cohort studies and the early origins of disease hypothesis: making sense of the evidence. Proc Nutr Soc. 2009;68(2):179–88. [DOI] [PubMed] [Google Scholar]
- 14. Siervo M, Prado CM, Mire E, Broyles S, Wells JCK, Heymsfield S, Katzmarzyk PT. Body composition indices of a load-capacity model: gender- and BMI-specific reference curves. Public Health Nutr. 2015;18(7):1245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity?. Curr Opin Endocrinol Diabetes Obesity. 2012;19(2):81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dekkers OM, Vandenbroucke JP, Cevallos M, Renehan AG, Altman DG, Egger M. COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019;16(2):e1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121:1027–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. J Parenter Enteral Nutr. 2014;38(8):940–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dickerman BA, Torfadottir JE, Valdimarsdottir UA, Giovannucci E, Wilson KM, Aspelund T, Tryggvadottir L, Sigurdardottir LG, Harris TB, Launer LJet al. Body fat distribution on computed tomography imaging and prostate cancer risk and mortality in the AGES-Reykjavik study. Cancer. 2019;125:2877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moller H, Roswall N, Van Hemelrijck M, Larsen SB, Cuzick J, Holmberg L, Overvad K, Tjonneland A. Prostate cancer incidence, clinical stage and survival in relation to obesity: a prospective cohort study in Denmark. Int J Cancer. 2015;136(8):1940–7. [DOI] [PubMed] [Google Scholar]
- 22. Guerrios-Rivera L, Howard L, Frank J, De Hoedt A, Beverly D, Grant DJ, Hoyo C, Freedland SJ. Is body mass index the best adiposity measure for prostate cancer risk? Results from a Veterans Affairs biopsy cohort. Urology. 2017;105:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim MS, Joung JY. Visceral obesity assessment by MRI and prostate cancer risk. J Magn. 2019;24(4):698–703. [Google Scholar]
- 24. MacInnis RJ, English DR, Gertig DM, Hopper JL, Giles GG. Body size and composition and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12:1417–21. [PubMed] [Google Scholar]
- 25. Wallstrom P, Bjartell A, Gullberg B, Olsson H, Wirfalt E. A prospective Swedish study on body size, body composition, diabetes, and prostate cancer risk. Br J Cancer. 2009;100(11):1799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tigwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. 2011. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 27. Cimino S, Favilla V, Castelli T, Galvano F, Li Volti G, D'Orazio N, Sortino G, Madonia M, Russo G, Morgia G. Fat mass and waist circumference are positviely associated with glutathione levels in prostate cancer patients. Urology. 2013;82:S1–334. [Google Scholar]
- 28. Fowke JH, Motley SS, Barocas DA. Abstract 944: total body composition and the association with high-grade prostate cancer. Cancer Res. 2011;71:944 LP. [Google Scholar]
- 29. Giles G, MacInnis R, English D, Gertig D, Hooper J. A prospective study of the risk of prostate cancer associated with fat-free mass measured by bioelectric impedance. Epidemiol Biomarkers Prev. 2002;11:1205S. [Google Scholar]
- 30. Gupta A, Pandey A, Ayers C, Beg MS, Lakoski SG, Vega GL, Grundy SM, Johnson DH, Neeland IJ. An analysis of individual body fat depots and risk of developing cancer: insights from the Dallas Heart Study. Mayo Clin Proc. 2017;92(4):536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu X, Rybicki BA, Casey G, Witte JS. Relationship between body size and prostate cancer in a sibling based case-control study. J Urol. 2005;174(6):2169–73. [DOI] [PubMed] [Google Scholar]
- 32. Liu Y, Zheng W. Abstract 5251: prospective investigation of general and visceral obesity, body fat distribution and risk of common cancers using data from the UK Biobank cohort study. Cancer Res. 2018;78:5251 LP. [Google Scholar]
- 33. Lopes I, Pina F, Lunet N, Botelho F, Cruz R, Resende A, Cruz F, Barros H. UP-02.125 BMI and bio-impedance factors among candidates to prostate cancer screening: implication of obesity on actual cancer diagnosis. Urology. 2011;78(3):S302–3. [Google Scholar]
- 34. Nilsen TI, Vatten LJ. Anthropometry and prostate cancer risk: a prospective study of 22,248 Norwegian men. Cancer Causes Control. 1999;10(4):269–75. [DOI] [PubMed] [Google Scholar]
- 35. Moran Pascual E, Martinez Sarmiento M, Budia Alba A, Broseta Rico E, Camara Gomez R, Boronat Tormo F. Central body fat mass measured by bioelectrical impedanciometry but not body mass index is a high-grade prostate cancer risk factor. Urol Int. 2017;98(1):28–31. [DOI] [PubMed] [Google Scholar]
- 36. Qu Y-Y, Dai B, Kong Y-Y, Chang K, Ye D-W, Yao X-D, Zhang S-L, Zhang H-L, Yang W-Y. Influence of obesity on localized prostate cancer patients treated with radical prostatectomy. Asian J Androl. 2013;15(6):747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA. Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol. 2000;151(6):541–9. [DOI] [PubMed] [Google Scholar]
- 38. Song M, Willett WC, Hu FB, Spiegelman D, Must A, Wu K, Chan AT, Giovannucci EL. Trajectory of body shape across the lifespan and cancer risk. Int J Cancer. 2016;138(10):2383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Roermund JGH, Bol GH, Witjes JA, Ruud Bosch JLH, Kiemeney LA, van Vulpen M. Periprostatic fat measured on computed tomography as a marker for prostate cancer aggressiveness. World J Urol. 2010;28:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wosnitzer M, Truesdale M, Sartori S, Ko W, Sandri M, Haramis G, Landman J, Badani K. Evaluation of preoperative bioimpedance spectroscopy quantification of body composition in patients prior to Robotic-assisted Radical Prostatectomy (RARP). J Endourol. 2010;1:A85–6. [PubMed] [Google Scholar]
- 41. Xu WP, Cao DX, Lin ZM, Wu GH, Chen L, Zhang JP, Zhang B, Yang ZA, Jiang Y, Han YSet al. Analysis of energy utilization and body composition in kidney, bladder, and adrenal cancer patients. Urologic Oncol Semin Orig Investig. 2012;30(5):711–8. [DOI] [PubMed] [Google Scholar]
- 42. Zhang Q, Sun L-J, Qi J, Yang Z-G, Huang T. Influence of adipocytokines and periprostatic adiposity measurement parameters on prostate cancer aggressiveness. Asian Pac J Cancer Prev. 2014;15(4):1879–83. [DOI] [PubMed] [Google Scholar]
- 43. Zilli T, Nguyen T V, Bahary J-P, Chagnon M, Dufresne A, Taussky D. Prognostic impact of abdominal adiposity, waist circumference and body mass index in patients with intermediate-risk prostate cancer treated with radiotherapy. Int J Obes. 2011;35(11):1421–6. [DOI] [PubMed] [Google Scholar]
- 44. Cimino S, Favilla V, Russo GI, Galvano F, Li Volti G, Barbagallo I, Giofre SV, D'Orazio N, DI Rosa A, Madonia Met al. Oxidative stress and body composition in prostate cancer and benign prostatic hyperplasia patients. Anticancer Res Greece. 2014;34:5051–6. [PubMed] [Google Scholar]
- 45. Demark-Wahnefried W, Conaway MR, Robertson CN, Mathias BJ, Anderson EE, Paulson DF. Anthropometric risk factors for prostate cancer. Nutr Cancer. 1997;28(3):302–7. [DOI] [PubMed] [Google Scholar]
- 46. Fowke JH, Motley SS, Concepcion RS, Penson DF, Barocas DA. Obesity, body composition, and prostate cancer. BMC Cancer. 2012;12(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maturo G, Vespasiani G, Mohamed EI, Maiolo C, Finazzi Agro E, Forte F, De Lorenzo A. Evaluating body composition of Italian prostate cancer patients without metastases. Acta Diabetol. 2003;40(Suppl 1):s168–70. [DOI] [PubMed] [Google Scholar]
- 48. Moran E, Martinez M, Budia A, Broseta E, Camara R, Boronat F. The role of IGF-1 and the distribution of body fat in decreasing the number of prostate rebiopsies. Actas Urol Esp. 2017;41(2):82–7. [DOI] [PubMed] [Google Scholar]
- 49. Perez-Cornago A, Key TJ, Allen NE, Fensom GK, Bradbury KE, Martin RM, Travis RC. Prospective investigation of risk factors for prostate cancer in the UK Biobank cohort study. Br J Cancer. 2017;117(10):1562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Londen GJ, Levy ME, Perera S, Nelson JB, Greenspan SL. Body composition changes during androgen deprivation therapy for prostate cancer: a 2-year prospective study. Crit Rev Oncol Hematol. 2008;68:172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. von Hafe P, Pina F, Perez A, Tavares M, Barros H. Visceral fat accumulation as a risk factor for prostate cancer. Obes Res. 2004;12:1930–5. [DOI] [PubMed] [Google Scholar]
- 52. Dal Maso L, Zucchetto A, La Vecchia C, Montella M, Conti E, Canzonieri V, Talamini R, Tavani A, Negri E, Garbeglio Aet al. Prostate cancer and body size at different ages: an Italian multicentre case-control study. Br J Cancer. 2004;90:2176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Allott EH, Howard LE, Song H-J, Sourbeer KN, Koontz BF, Salama JK, Freedland SJ. Racial differences in adipose tissue distribution and risk of aggressive prostate cancer among men undergoing radiotherapy. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Andersson SO, Wolk A, Bergstrom R, Adami HO, Engholm G, Englund A, Nyren O. Body size and prostate cancer: a 20-year follow-up study among 135006 Swedish construction workers. J Natl Cancer Inst. 1997;89(5):385–9. [DOI] [PubMed] [Google Scholar]
- 55. Beebe-Dimmer JL, Dunn RL, Sarma A V, Montie JE, Cooney KA. Features of the metabolic syndrome and prostate cancer in African-American men. Cancer. 2007;109(5):875–81. [DOI] [PubMed] [Google Scholar]
- 56. Beebe-Dimmer JL, Faerber GJ, Morgenstern H, Werny D, Wojno K, Halstead-Nussloch B, Cooney KA. Body composition and serum prostate-specific antigen: review and findings from Flint Men's Health Study. Urology. 2008;71(4):554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Davies NM, Gaunt TR, Lewis SJ, Holly J, Donovan JL, Hamdy FC, Kemp JP, Eeles R, Easton D, Kote-Jarai Zet al. The effects of height and BMI on prostate cancer incidence and mortality: a Mendelian randomization study in 20,848 cases and 20,214 controls from the PRACTICAL Consortium. Cancer Causes Control. 2015;26(11):1603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vithayathil M, Carter P, Kar S, Mason AM, Burgess S, Larsson SC. Body size and composition and risk of site-specific cancers in the UK Biobank and large international consortia: a Mendelian randomisation study. PLoS Med. 2021;18(7):e1003706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–25. [DOI] [PubMed] [Google Scholar]
- 60. World Cancer Research Fund International/American Institute for Cancer Research Continuous Update Project Report . Diet, nutrition, physical activity, and prostate cancer. [Internet]2014; [accessed 28 December 21]. Available from: https://www.wcrf.org/wp-content/uploads/2021/02/prostate-cancer-report.pdf. [Google Scholar]
- 61. Roddam AW, Allen NE, Appleby P, Key TJ, Ferrucci L, Carter HB, Metter EJ, Chen C, Weiss NS, Fitzpatrick Aet al. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med. 2008;149:461–71, W83-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kelly DM, Jones TH. Testosterone and obesity. Obes Rev. 2015;16(7):581–606. [DOI] [PubMed] [Google Scholar]
- 63. Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85(1009):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ritchie SA, Connell JMC. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17(4):319326. [DOI] [PubMed] [Google Scholar]
- 65. Pischon T, Boeing H, Weikert S, Allen N, Key T, Johnsen NF, Tjonneland A, Severinsen MT, Overvad K, Rohrmann Set al. Body size and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3252–61. [DOI] [PubMed] [Google Scholar]
- 66. Pichardo MS, Smith CJ, Dorsey TH, Loffredo CA, Ambs S. Association of anthropometric measures with prostate cancer among African American men in the NCI-Maryland Prostate Cancer Case-Control Study. Cancer Epidemiol Biomarkers Prev. 2018;27(8):936LP–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lavalette C, Trétarre B, Rebillard X, Lamy P-J, Cénée S, Menegaux F. Abdominal obesity and prostate cancer risk: epidemiological evidence from the EPICAP study. Oncotarget. 2018;9(77):34485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Moore ML, Benavides ML, Dellinger JR, Adamson BT, Tinsley GM. Segmental body composition evaluation by bioelectrical impedance analysis and dual-energy X-ray absorptiometry: quantifying agreement between methods. Clin Nutr. 2020;39(9):2802–10. [DOI] [PubMed] [Google Scholar]
- 69. Shepherd JA, Fan B, Lu Y, Wu XP, Wacker WK, Ergun DL, Levine MA. A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J Bone Miner Res. 2012;27(10):2208–16. [DOI] [PubMed] [Google Scholar]
- 70. Franco-Villoria M, Wright CM, McColl JH, Sherriff A, Pearce MS. Assessment of adult body composition using bioelectrical impedance: comparison of researcher calculated to machine outputted values. BMJ Open. 2016;6(1):e008922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Parsons JK, Carter HB, Partin AW, Windham BG, Metter EJ, Ferrucci L, Landis P, Platz EA. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006;91(7):2562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yuan W, Beaulieu-Jones BK, Yu K-H, Lipnick SL, Palmer N, Loscalzo J, Cai T, Kohane IS. Temporal bias in case-control design: preventing reliable predictions of the future. Nat Commun. 2021;12(1):1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sutton-Tyrrell K. Assessing bias in case-control studies. Proper selection of cases and controls. Stroke. 1991;22(7):938–42. [DOI] [PubMed] [Google Scholar]
- 74. Nicholson BD, Hamilton W, Koshiaris C, Oke JL, Hobbs FDR, Aveyard P. The association between unexpected weight loss and cancer diagnosis in primary care: a matched cohort analysis of 65,000 presentations. Br J Cancer. 2020;122(12):1848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Namazi N, Irandoost P, Heshmati J, Larijani B, Azadbakht L. The association between fat mass and the risk of breast cancer: a systematic review and meta-analysis. Clin Nutr. 2019;38(4):1496–503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


