ABSTRACT
Observational studies, randomized controlled trials (RCTs), and Mendelian randomization (MR) studies have yielded inconsistent results on the associations of vitamin D concentrations with multiple health outcomes. In the present umbrella review we aimed to evaluate the effects of low vitamin D concentrations and vitamin D supplementation on multiple health outcomes. We summarized current evidence obtained from meta-analyses of observational studies that examined associations between vitamin D concentrations and multiple health outcomes, meta-analyses of RCTs that investigated the effect of vitamin D supplementation on multiple health outcomes, and MR studies that explored the causal associations of vitamin D concentrations with various diseases (international prospective register of systematic reviews PROSPERO registration number CRD42018091434). A total of 296 meta-analyses of observational studies comprising 111 unique outcomes, 139 meta-analyses of RCTs comprising 46 unique outcomes, and 73 MR studies comprising 43 unique outcomes were included in the present umbrella review. Twenty-eight disease outcomes were identified by both meta-analyses of observational studies and MR studies. Seventeen of these reported disease outcomes had consistent results, demonstrating that lower concentrations of vitamin D were associated with a higher risk for all-cause mortality, Alzheimer's disease, hypertension, schizophrenia, and type 2 diabetes. The combinations of consistent evidence obtained by meta-analyses of observational studies and MR studies together with meta-analyses of RCTs showed that vitamin D supplementation was associated with a decreased risk for all-cause mortality but not associated with the risk for Alzheimer's disease, hypertension, schizophrenia, or type 2 diabetes. The results indicated that vitamin D supplementation is a promising strategy with long-term preventive effects on multiple chronic diseases and thus has the potential to decrease all-cause mortality. However, the current vitamin D supplementation strategy might not be an efficient intervention approach for these diseases, suggesting that new strategies are highly needed to improve the intervention outcomes.
Keywords: umbrella review, vitamin D deficiency, vitamin D supplementation, meta-analysis, multiple health outcomes, observational studies, randomized controlled trials, Mendelian randomization studies
Statement of Significance: No previous systematic effort, to our knowledge, has been made to summarize and appraise evidence obtained in meta-analyses of observational studies, meta-analyses of RCTs, and MR studies on associations of vitamin D concentrations with a range of disease outcomes. This umbrella review takes advantage of the respective strengths of meta-analyses of observational studies, meta-analyses of RCTs, and MR studies by combining and comparing the findings to explore the potential importance of vitamin D in detail and to assess the implications for clinical practice and public health.
Introduction
Vitamin D deficiency is one of the most common health problems worldwide, resulting in poor musculoskeletal health and a range of acute and chronic diseases, such as infectious diseases, pneumonia, cancer, metabolic disorders, cardiovascular disease (CVD), and mortality (1, 2). Additionally, vitamin D supplementation appears to be associated with a decreased risk for several common diseases (i.e., infectious diseases, asthma, cancer, and CVD) and lower all-cause mortality in randomized controlled trials (RCTs) (3–7). Previous studies exploring the associations of vitamin D concentrations with multiple outcomes were biased by many confounding variables, and the observed associations may not be causal (8, 9). Therefore, causality and the direction of associations between vitamin D concentrations and disease outcomes to date remain uncertain.
Genetic data may partially address the limitations of confounding and reverse causality and provide more convincing evidence to explain the underlying causal effects known as Mendelian randomization (MR) (10–12). An MR study exploited natural randomization of genetic variants and prospective design involving exposed genetic variants to provide insight into disease pathogenesis based on observational data (13). Certain genetic variants, which are robustly associated with risk factors but are not associated with confounders, can be used as instrumental variables (IVs) in causal inferences. However, the accumulated results of MR studies have demonstrated that vitamin D deficiency–related genetic risk factors do not predict disease risk (14–19).
RCTs are designed to explore a causal effect of an intervention on a disease; however, the small sample size, limited external validity, short duration of an intervention, and ethical concerns limit the implementation of RCTs. Observational studies are relatively less work than MR studies or RCTs; however, the results provide weak inference of causality due to residual confounding bias, reverse causality, or undetected bias. MR studies, with evidence at the interface between observational studies and RCTs (20), are less susceptible to confounding bias and reverse causality but are restricted by potentially weak IVs and genetic pleiotropy. The 3 types of studies have specific advantages and disadvantages that can complement each other to some extent (21). The present study combined the summary estimates for identical outcomes of observational studies and MR studies. The consistency of these estimates between observational studies and MR studies was tested to provide evidence of statistically significant differences (22, 23). If the results of observational studies and MR studies are consistent, the findings can be combined and compared with the findings of RCTs. The present study made a tradeoff between the effects of vitamin D deficiency and vitamin D supplementation on the diseases to outline the applications and clinical practices related to vitamin D as a causal factor or an intervention factor.
An umbrella review (21, 24, 25) is a popular method for the systematic assessment of evidence from multiple sources and may help in the evaluation of potential biases in associations of exposure with outcome. Therefore, our umbrella review aimed to provide a comprehensive synopsis of associations of low vitamin D concentrations and vitamin D supplementation with multiple outcomes by combining the results of meta-analyses of observational studies, meta-analyses of RCTs, and MR studies.
Methods
This umbrella review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) as CRD42018091434.
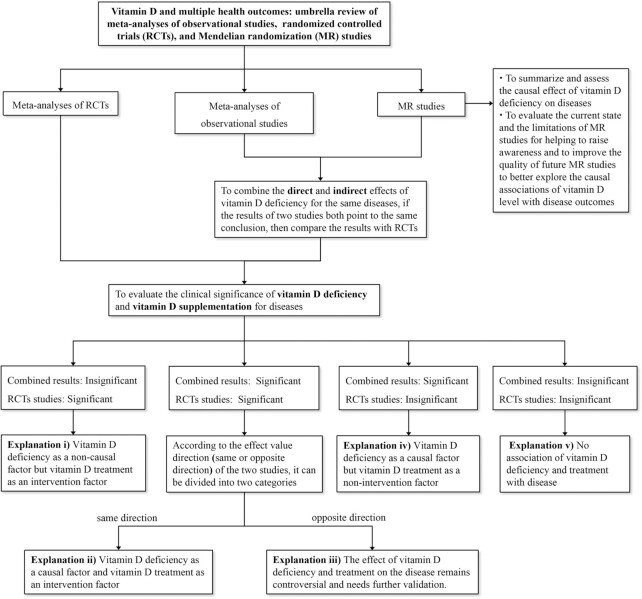
The umbrella review protocol was designed on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (26). The present umbrella review included meta-analyses of observational studies on associations of vitamin D concentration with disease outcomes, meta-analyses of RCTs on associations of vitamin D supplementation with disease outcomes, and MR studies on causal associations of vitamin D concentration with disease outcomes. The results of MR studies and meta-analyses of observational studies or RCTs were combined whenever the data were available for identical disease outcomes. The study design is presented in Figure 1.
FIGURE 1.
Flow diagram of the selection process. MR, Mendelian randomization; RCT, randomized controlled trial.
Search and eligibility criteria
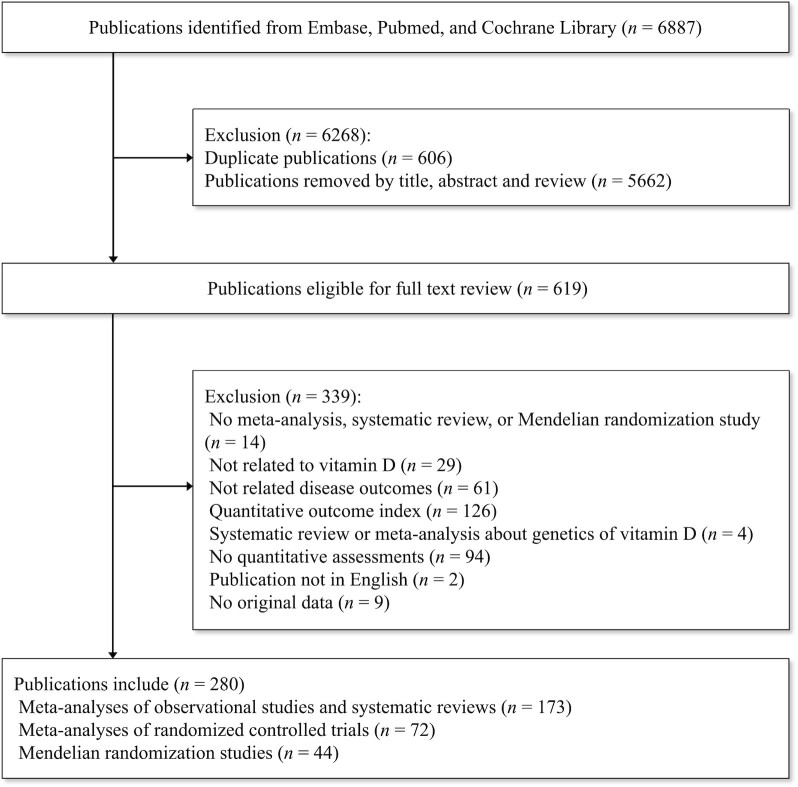
Based on the search strategy, we identified meta-analyses of observational studies, meta-analyses of RCTs, and MR studies by searching the Cochrane, Embase, and PubMed databases from inception to May 2019. The literature search was limited to English language studies involving humans. The search strategy is presented in detail in Supplemental Table 1. Initially, 2 authors of the present study (QT and XM) screened the titles and abstracts of the literature and then reviewed the full text of eligible studies. The detailed process for the present umbrella review is presented in a schematic flowchart in Figure 2. Points of divergence were resolved by discussion among 3 authors of the present study (DL, XM, and QT).
FIGURE 2.
Flow chart of the study design.
We included meta-analyses of observational studies exploring the associations of vitamin D concentration with diseases, meta-analyses of RCTs investigating the effects of vitamin D supplementation on diseases (≥1 intervention of vitamin D compared with either placebo or no vitamin D supplementation), and MR studies investigating causal associations of vitamin D concentration with diseases using vitamin D–related genetic instruments. Only formal quantitative meta-analyses or MR studies were considered. Studies exploring the associations between multiple micronutrient supplements (including vitamin D) and various disease outcomes, systematic reviews or meta-analyses of genetic factors related to vitamin D, studies published in languages other than English, systematic reviews without quantitative assessments, reports containing only an abstract or meeting reports without original data, meta-analyses of observational studies, and RCTs that mainly involved investigations of the effects of a reduction in vitamin D concentration rather than focusing on the effects of vitamin D concentration or vitamin D supplementation on various diseases were further excluded.
Data extraction
Two researchers (QT and XM) were responsible for the extraction of the data, and the third author (DL) verified the data. Included studies were subsequently assessed by 2 authors of the present study (YW and MS) for accuracy and completeness of available information.
We extracted the name of the first author, publication year, study population, study design, vitamin D measurements, number of included studies, number of included participants, number of cases, and investigated diseases from all eligible publications. Depending on 3 specific study types, we further extracted study-specific risk estimates (OR, RR, HR, or regression coefficient β together with their 95% CI) and evaluated the heterogeneity of the studies using the I2 metric. If a meta-analysis presented both overall results and subgroup results, only the overall results were extracted because of the larger sample size. If a publication reported separate meta-analyses for >1 disease, all diseases were assessed separately. If more than a single meta-analysis reported an identical disease in the same population, the results of the largest and most recent study were extracted. In the case of MR studies of identical diseases and populations, we extracted the data corresponding to the largest number of cases and participants. Finally, we assessed the consistency of the findings based on the effects and levels of statistical significance/direction of the reported association for each disease. For MR studies, the data on genetic instruments and the proportion of the variance of a risk factor explained by genetic instruments (percentage explained, R2) were extracted.
Statistical analysis
The 95% prediction interval (PI) was calculated to assess the effect and uncertainty for an expected effect in a new original study (27, 28), which was estimated based on a parametric bootstrap for confidence distribution (R version 4.0.1, R packages: “pimeta”) (29). The I2 metric was used to evaluate the heterogeneity; I2 > 50% was assumed to correspond to high heterogeneity with potential bias. Two measures of publication bias, small study effect and excess significance bias, were calculated (R packages: “meta,” “powerAnalysis” and “pwr”), with P < 0.10 considered to be the result of publication bias (30–32). The small study effect was estimated using Egger's test. The chi-squared test was applied to estimate the excess statistical significance bias of nominally significant results [if observed (O) number > expected (E) number]. For MR studies, the noncentrality parameter–based approach was used to estimate the power of MR studies (33). Statistical power calculations were based on the sample size, a type I error rate (α) of 0.05, proportion of cases in a study, risk estimates (e.g., OR), and R2 and were performed using the online tool mRnd (https://shiny.cnsgenomics.com/mRnd/).
Furthermore, if the data were available for the same disease the findings of meta-analyses of observational studies and MR studies were combined by using tests for the differences and tests for consistency of the estimates (22, 23). The present study used random-effects models based on a normal distribution. Many parameters were not normally distributed; therefore, we transformed these parameters into log-scales that resulted in an approximately normal distribution, and these transformed values were used in subsequent analyses. Rejection of the null hypothesis (at a significance level of 0.05) indicated that risk estimates in meta-analyses of observational studies and MR studies were inconsistent. If obvious inconsistences were not detected, the findings of meta-analyses of observational studies and MR studies were combined, and random-effects models were used to account for heterogeneity. We then combined and compared prior results of meta-analyses of observational studies and MR studies to assess the level of consistency with the data of meta-analyses of RCTs. If the statistical significance levels of the estimates provided by meta-analyses of observational studies and MR studies were inconsistent, we reported the results of the MR studies, which limited the bias due to confounding and reverse causality. Overall, the results of observational studies, MR studies, and RCTs exploring associations of low vitamin D concentrations and vitamin D supplementation with the same disease were divided into 5 categories based on the effects and level of statistical significance/direction, as shown in detail in Figure 1.
Evaluation of the quality of evidence
We next assessed the epidemiologic credibility of the 3 types of studies. Meta-analyses of observational studies were divided into 4 classes based on the level of evidence: convincing (class Ⅰ), highly suggestive (class II), suggestive (class III), and weak (class IV) (21). The level of evidence was rated as convincing for studies that were characterized by the following features: a statistical significance level of P < 10–6, >1000 cases, a significant result at P < 0.05 reported for the study with the largest sample size in the meta-analysis, a 95% PI that excluded the null, a low heterogeneity (I2 < 50%), no evidence of small-study effects (P > 0.10), and no evidence of excess significance bias (P > 0.10). Highly suggestive evidence for studies was characterized by the following features: a statistical significance level of P < 10–6, >1000 cases, and a significant result at P < 0.05 reported for the study with the largest sample size in the meta-analysis. Suggestive evidence for studies was characterized by the following features: a statistical significance level of P < 10–3 and >1000 cases. The level of evidence was rated as weak for studies that were characterized by the following feature: a statistical significance level of P < 0.05. The level of evidence for meta-analyses of RCTs was evaluated according to the level of statistical significance, 95% PI, I2, small-study effect bias, and excess significance bias. Additionally, the level of evidence of MR studies was evaluated based on statistical significance and statistical power (34).
Risk of bias assessment
We further assessed the methodological quality of the each included meta-analysis using the Risk of Bias in Systematic Reviews (ROBIS) tool (35). In brief, 4 domains of ROBIS were evaluated separately: 1) study eligibility criteria, 2) identification and selection of studies, 3) data collection and study appraisal, and 4) synthesis and findings, and then concerns about risk of bias of the domains were judged (35). Finally, an overall judgment of risk of bias was made for each meta-analysis, rated as low, high, or unclear risk of bias. Three authors (DL, XM, and QT) assessed the risk of bias of each meta-analysis independently. Any discrepancies were identified and resolved by discussion.
Results
Literature review
A total of 6887 publications were identified across the 3 databases (Embase, PubMed, and Cochrane Database). After removing 606 duplicate publications and 5662 publications based on the title and abstract, we investigated the full text of 619 publications for eligibility. Finally, 339 publications were excluded according to the exclusion criteria, leaving 280 unique publications that met the eligibility criteria (236 publications were meta-analyses, and the remaining 44 publications were MR studies). Overall, 296 meta-analyses of observational studies including 111 unique outcomes were reported in 173 articles (36–208); 139 meta-analyses of RCTs including 46 unique outcomes were reported in 72 articles (3–7, 50, 65, 81, 120, 130, 160, 165, 167, 190, 209–266), and 73 MR studies including 43 unique outcomes were reported in 44 articles (15, 17, 18, 267–307) (Supplemental Tables 2–5). As shown in Figure 3, 13 outcomes overlapped in all 3 study types. Moreover, 28 unique outcomes overlapped in the MR studies and meta-analyses of observational studies, and 24 unique outcomes overlapped in the meta-analyses of RCTs and meta-analyses of observational studies.
FIGURE 3.
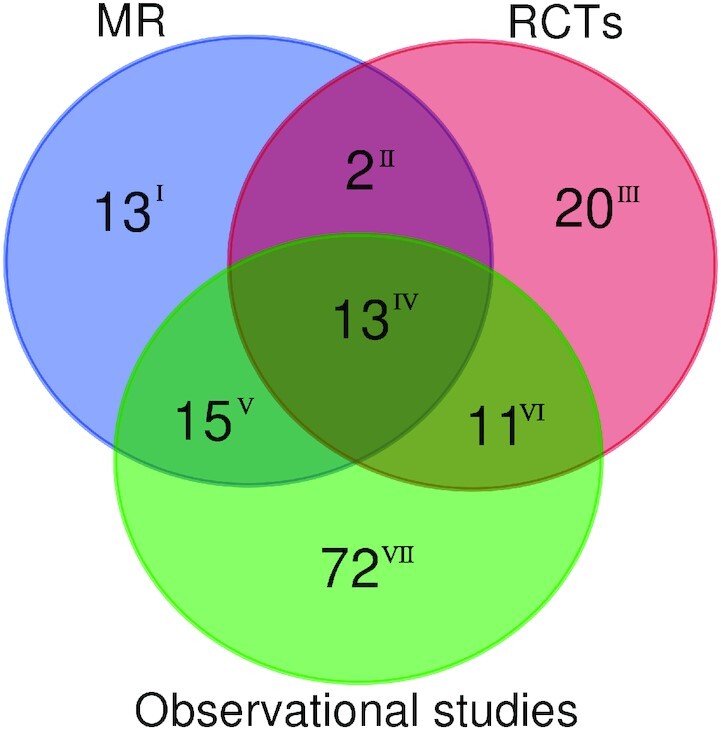
Venn diagram of meta-analyses of observational studies, meta-analyses of RCTs, and MR studies. The plot was performed by R package “VennDiagram.” The number of disease outcomes for overlapping and distinct study types is shown. Ⅰ, only reported in MR studies; ⅠI, reported in MR studies and meta-analyses of RCTs; ⅠII, only reported in meta-analyses of RCTs; ⅠV, reported in all 3 study types; V, reported in MR studies and meta-analyses of observational studies; VI, reported in meta-analyses of RCTs and meta-analyses of observational studies; VII, only reported in meta-analyses of observational studies. MR, Mendelian randomization; RCT, randomized controlled trial.
Risk of bias assessments for the included meta-analyses
Risk of bias assessment of the included meta-analyses is presented in Supplemental Table 4. Most meta-analyses of observational studies and meta-analyses of RCTs (n = 235) were evaluated as high risk of bias, except for 1 study at low risk of bias. After removal of overlapping meta-analyses, all the included meta-analyses of observational studies (n = 104) were at high risk of bias, with 28 (26.9%), 75 (72.1%), 73 (70.2%), and 103 (99.0%) meta-analyses at high risk in the first, second, third, and fourth domain of ROBIS, respectively (Supplemental Figure 1, Supplemental Table 6). Similarly, all of the included meta-analyses of RCTs (n = 50) were rated high risk of bias, with 11 (22.0%), 33 (66.0%), 36 (72.0%), and 47 (94.0%) of studies at high risk in the first, second, third, and fourth domain of ROBIS, respectively (Supplemental Figure 2, Supplemental Table 7).
Characteristics of meta-analyses of observational studies
A total of 136 unique meta-analyses remained after the removal of overlapping meta-analyses (conducted in the same population and evaluating the same outcome), and these studies reported a series of outcomes (Supplemental Table 6). Among these meta-analyses, 97 (71.32%) unique meta-analyses presented statistically significant summary results (P < 0.05). These studies included 3 (37.5%) meta-analyses for skeletal outcomes, 4 (50%) analyses for respiratory illness, 13 (43.33%) analyses for cancer diseases, 13 (100%) analyses for CVD, 5 (83.33%) analyses for diabetes-related diseases, 24 (92.31%) analyses for all-cause or cause-specific mortality, 4 (100%) analyses for neurocognitive disorders, 2 (100%) analyses for skin diseases, 3 (50%) analyses for neonatal, infant, child, or pregnancy-related diseases, 4 (100%) analyses for mental diseases, 3 (75%) analyses for infectious diseases, and 19 (76%) analyses for other outcomes with summary estimates with P < 0.05, which indicated that low concentrations of vitamin D are associated with an increased risk of a disease. Additionally, 2 cancer outcomes (i.e., basal cell cancer and nonmelanoma skin cancer) had summary estimates with P < 0.05 and were associated with high vitamin D concentrations.
Then, we applied the evidence classification criteria described above (Supplemental Table 6). Eighteen (13.14%) meta-analyses had P < 10–6, 130 (94.89%) analyses had a 95% PI that excluded the null, 72 (52.55%) analyses had >1000 cases, 64 (46.72%) analyses did not have high heterogeneity (I2 < 50%), and 34 (24.82%) analyses lacked small-study effects or excess significance bias. Based on these metrics, 3 (2.19%) outcomes presented convincing evidence (class I: hip fracture, sepsis, and sepsis in critically ill patients), 12 (8.76%) outcomes presented highly suggestive evidence, and 18 (13.14%) outcomes presented suggestive evidence. The remaining 66 (48.18%) statistically significant outcomes were assessed as weak evidence (class IV).
Characteristics of meta-analyses of RCTs
Eighty-two nonoverlapping meta-analyses (Supplemental Table 7) were identified for the outcomes related to all-cause or cause-specific mortality (n = 16); cancer diseases (n = 7); skeletal diseases (n = 13); CVD (n = 8); neonatal, infant, child, or pregnancy-related diseases (n = 6); respiratory illness (n = 17); and other outcomes (n = 15). Among 82 nonoverlapping meta-analyses, 26 meta-analyses (32.10%) reported summary results that were statistically significant at P < 0.05. These results included 4 (25%) meta-analyses for all-cause or cause-specific mortality; 1 (14.29%) analysis for cancer diseases; 1 (12.50%) analysis for CVD; 3 (50%) analyses for neonatal, infant, child, or pregnancy-related diseases; 12 (70.59%) analyses for respiratory illness; and 5 (33.33%) analyses for other outcomes that reported summary estimates with P < 0.05, suggesting that vitamin D supplementation is associated with decreased disease risk. Additionally, meta-analyses for 2 outcomes [i.e., hypercalcemia in patients with chronic kidney disease (CKD) and all-cause mortality] had summary estimates with P < 0.05 and suggested that vitamin D supplementation may be associated with increased disease risk.
The results of quality of evidence for meta-analyses of RCTs are showed in Supplemental Table 7. Overall, 28 (34.57%) meta-analyses had P < 0.05 (8 analyses had P < 0.001), 62 (76.54%) analyses had a 95% PI that excluded the null, 56 (69.14%) analyses did not have high levels of heterogeneity (I2 < 50%), and 34 (41.98%) analyses lacked small-study effects or excess significance bias. Only 4 outcomes (CVD events in predialysis CKD patients, low birth weight, pediatric asthma, and hypercalcemia in CKD patients) were characterized by P < 0.001, had a 95% PI excluding the null, and lacked evidence of high heterogeneity or bias.
Characteristics of the MR studies
A total of 47 unique MR studies (Supplemental Table 8) were identified for the following outcomes: cardiovascular outcomes (n = 5), all-cause or cause-specific mortality (n = 5), cancer outcomes (n = 13), and other outcomes (n = 24). In total, statistically significant summary results at P < 0.05 were reported for 8 (17.02%) outcomes (i.e., type 2 diabetes, schizophrenia, Alzheimer's disease, multiple sclerosis, multiple sclerosis in children, hypertension, all-cause mortality, and other mortality). Notably, P < 0.01 was reported for 5 outcomes (hypertension, type 2 diabetes, multiple sclerosis, Alzheimer's disease, and schizophrenia), and only 4 outcomes (myocardial infarction, esophageal adenocarcinoma, fatigue/frailty, and atopic dermatitis) were characterized by power >80%.
Combination of the findings of meta-analyses of observational studies and MR studies
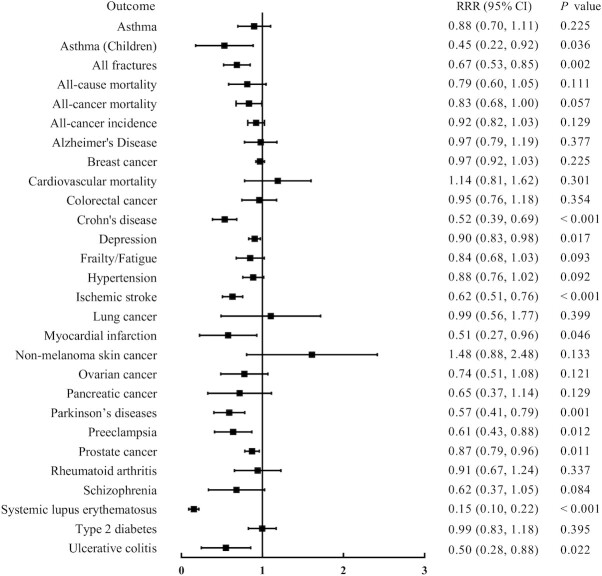
In contrast to the results of meta-analyses of observational studies, which demonstrated significant associations for most disease outcomes (78.57%), the results for most (82.14%) disease outcomes were not significant in the MR studies. A total of 126 outcomes were reported across these 2 study types (i.e., meta-analyses of observational studies and MR studies); 111 outcomes were reported in meta-analyses of observational studies, 43 outcomes were reported in MR studies (some outcomes contained different subgroups, as shown in Supplemental Table 9), and 28 (17.72%) outcomes were assessed by both meta-analyses of observational studies and MR studies. Seventeen outcomes were not significantly different according to the results of interaction analyses testing for differences in the estimates between parallel studies (P ≥ 0.05) (Figure 4). Hence, these outcomes were consistent between meta-analyses of observational studies and MR studies. The results of the present study indicated that lower concentrations of vitamin D were associated with a higher risk of all-cause mortality, Alzheimer's disease, hypertension, schizophrenia, and type 2 diabetes based on comparison of the results of meta-analyses of observational studies and MR studies, and low vitamin D concentration was not a causal risk factor for other disease outcomes (Supplemental Table 9).
FIGURE 4.
Consistency between meta-analyses of observational studies, and MR studies for the same disease outcome. P is the P value in the test for interaction. MR, Mendelian randomization; RRR, ratio of relative risks.
Eleven outcomes were significantly different based on the results of the interaction analysis testing performed in the present study (P < 0.05), indicating that the estimates of parallel studies were different. Low vitamin D concentration was associated with an increased risk for Crohn's disease, depression, ischemic stroke, Parkinson's disease, preeclampsia, systemic lupus erythematosus, ulcerative colitis, all fractures, and asthma (children) only according to meta-analyses of observational studies, and these results were inconsistent with the data of MR studies. Furthermore, vitamin D deficiency was not associated with the risk for lung cancer and pancreatic cancer according to meta-analyses of observational studies, and these findings were inconsistent with those in MR studies (Supplemental Table 9).
Comparison of the results for vitamin D deficiency and vitamin D supplementation
We compared the consistent results of meta-analyses of observational studies and MR studies with those of meta-analyses of RCTs; the data indicated that 7 outcomes overlapped (Table 1). Only all-cause mortality was characterized by identical conclusions (effect and level of statistical significance/direction) across the 3 study types. For the remaining outcomes, nominally significant results at P < 0.05 were reported for colorectal cancer, all-cancer mortality, and asthma by meta-analyses of RCTs, indicating that a low vitamin D concentration was not a causal factor for these disease outcomes; however, vitamin D supplementation was an efficient intervention factor for these disease outcomes. Additionally, only a single outcome (all-cause mortality) was reported by meta-analyses of observational studies with suggestive evidence, by meta-analyses of RCTs with a 95% PI excluding the null, and by MR studies with P < 0.05 (Table 1).
TABLE 1.
Comparison of the evidence for disease outcomes examined by 3 types of studies1
| Meta-analysis of observational studies | Meta-analysis of RCTs | MR study | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | Risk estimates (95% CI) | Evidence class2 | Risk of bias level3 | Risk estimates (95% CI) | Evidence | Risk of bias level3 | Risk estimate (95% CI) | Evidence |
| All-cause mortality | 0.61 (0.52, 0.76) | Ⅲ | High | 0.93 (0.88, 0.98) | P < 0.05, 95%PI excluded the null | High | 0.77 (0.62, 0.95) | n = 95,766 |
| Breast cancer | 0.99 (0.98, 1.00) | NS | High | 0.97 (0.95, 1.00) | P > 0.05, 95%PI excluded the null | High | 1.02 (0.97, 1.08) | n = 228,95; P = 0.47; power = 0.13 |
| CVD mortality | 0.88 (0.80, 0.96) | Ⅳ | High | 0.96 (0.93, 1.00) | P > 0.05, 95%PI excluded the null | High | 0.77 (0.55, 1.08) | n = 95,766 |
| Colorectal cancer | 0.87 (0.77, 0.99) | Ⅳ | High | 0.94 (0.91, 0.97) | NA | High | 0.92 (0.76, 1.10) | n = 95,906; P = 0.36; power = 0.29 |
| Asthma | 0.92 (0.77, 1.10) | NS | High | 0.73 (0.58, 0.92) | P < 0.05, 95%PI excluded the null | High | 1.03 (0.90, 1.19) | n = 146,761; P = 0.63; power = 0.25 |
| All-cancer incidence | 0.89 (0.81, 0.97) | Ⅳ | High | 0.98 (0.93, 1.03) | P > 0.05, 95%PI excluded the null | High | 0.97 (0.91, 1.04) | n = 438,870 |
| All-cancer mortality | 0.80 (0.70, 0.91) | Ⅲ | High | 0.87 (0.79, 0.96) | P < 0.05, 95%PI excluded the null | High | 0.97 (0.84, 1.11) | n = 438,870 |
Data are consistent results of meta-analyses of observational studies and MR studies with the results of meta-analyses of RCTs. CVD, cardiovascular disease; MR, Mendelian randomization; NA, not available; NS, not significant (P ≥ 0.05); PI, prediction interval; RCT, randomized controlled trial; ROBIS, Risk of Bias in Systematic Reviews.
Evidence class criteria: class I (convincing), a statistical significance level of P < 10–6, >1000 cases, a significant result at P < 0.05 reported by the study with the largest sample size in the meta-analysis, a 95% PI that excluded the null, a low heterogeneity (I2 < 50%), no evidence of small-study effects (P > 0.10) and of excess significance bias (P > 0.10); class II (highly suggestive), a statistical significance level of P < 10–6, >1000 cases, and a significant result at P < 0.05 reported by the study with the largest sample size in the meta-analysis; class III (suggestive), a statistical significance level of P < 10–3 and >1000 cases; class IV (weak), a statistical significance level of P < 0.05.
The risk of bias was assessed by ROBIS: low risk of bias (Low), the findings of the meta-analysis are likely to be reliable, domain 1–4 (study eligibility criteria, identification and selection of studies, data collection and study appraisal, and synthesis and findings) did not raise any concerns with the review process or concerns were appropriately considered in the review conclusions, the conclusions were supported by the evidence and included consideration of the relevance of included studies; high risk of bias (High), ≥1 of the concerns raised during the domain 1–4 assessment was not addressed in the review conclusions, the review conclusions were not supported by the evidence, or the conclusions did not consider the relevance of the included studies to the review question; unclear risk of bias (Unclear), there is insufficient information reported to make a judgement on risk of bias
In addition, 14 outcomes were examined in meta-analyses of observational studies, excluding MR studies, and meta-analyses of RCTs (Table 2). According to meta-analyses of observational studies, 10 (71.43%) outcomes presented nominally significant results at P < 0.05. However, 3 (30%) outcomes related to all-cause mortality (prostate cancer patients, small for gestational age, and sustained virologic response to hepatitis C virus) corresponded to nominally significant results according to meta-analyses of both RCTs and observational studies. Two outcomes had consistent effects and levels of statistical significance/direction in both meta-analyses of observational studies and meta-analyses of RCTs. Only a single outcome (all-cause mortality in prostate cancer patients) was inconsistent based on the direction of the association/effect in meta-analyses of observational studies and meta-analyses of RCTs. Comparison with the most significant outcomes (71.43%) according to meta-analyses of observational studies indicated that the results for the majority (70%) of the outcomes investigated by meta-analyses of RCTs were not statistically significant.
TABLE 2.
Comparison of the results from the meta-analysis of observational studies and the meta-analysis of RCTs1
| Meta-analysis | ||
|---|---|---|
| Outcomes2 | Observational studies | RCTs |
| All-cause mortality | ||
| Chronic kidney disease | 0.73 (0.65, 0.82) | 0.84 (0.46, 1.52) |
| Critically ill | 0.72 (0.50, 1.04) | 0.70 (0.50, 0.98) |
| Elderly | 0.61 (0.52, 0.71) | 1.04 (0.91, 1.17) |
| Prostate cancer | 0.91 (0.84, 0.98) | 1.19 (1.03, 1.38) |
| Bladder cancer | 0.75 (0.65, 0.85) | 0.92 (0.66, 1.28) |
| CVD | 0.66 (0.56, 0.77) | 0.90 (0.77, 1.05) |
| Hip fracture | 0.68 (0.60, 0.78) | 1.11 (0.97, 1.27) |
| Preterm birth | 0.86 (0.62, 1.20) | 0.57 (0.36, 0.91) |
| Small for gestational age | 0.65 (0.48, 0.86) | 0.72 (0.52, 0.99) |
| Stillbirth | 0.98 (0.92, 1.04) | 0.35 (0.06, 1.99) |
| Stroke | 0.60 (0.48, 0.72) | 1.09 (0.92, 1.30) |
| Sustained virological response in hepatitis C virus | 0.63 (0.45, 0.89) | 0.22 (0.08, 0.60) |
| Total CVD events | 0.90 (0.86, 0.94) | 1.20 (0.48, 2.99) |
| Tuberculosis | 0.86 (0.53, 1.41) | 0.61 (0.24, 1.56) |
Values are risk estimates (95% CI). CVD, cardiovascular disease; MR, Mendelian randomization; RCT, randomized controlled trial.
Presented disease outcomes examined with both meta-analyses of observational studies without MR studies and the meta-analyses of RCTs.
Discussion
The present umbrella review integrated the results of MR studies and RCTs to avoid the inevitable bias or reverse causality of observational studies. The results indicated that low vitamin D concentrations were a causal risk factor for all-cause mortality, Alzheimer's disease, hypertension, schizophrenia, and type 2 diabetes. These findings were combined with the results of meta-analyses of RCTs, and the data indicated that vitamin D supplementation decreased the risk for all-cause mortality. The present study is the first attempt to evaluate associations of low vitamin D concentration and vitamin D supplementation with health outcomes by combining the findings of meta-analyses of observational studies, meta-analyses of RCTs, and MR studies. Unlike a previous umbrella review of systematic reviews and meta-analyses of observational studies and RCTs that assessed associations of vitamin D with multiple health outcomes (25), the present study also combined and compared the findings reported by MR studies.
Comparison of the findings of both MR studies and meta-analyses of observational studies with the data reported by meta-analyses of RCTs indicated significant associations of low vitamin D concentration and vitamin D supplementation with all-cause mortality (136, 214, 278). Associations of low vitamin D concentration with Alzheimer's disease, hypertension, schizophrenia, and type 2 diabetes were significant only in meta-analyses of observational studies and in MR studies, and meta-analyses of RCTs reported little or no effect of vitamin D supplementation on the prevention or treatment of these diseases. Overall, assessments of associations of vitamin D deficiency and vitamin D supplementation with the same diseases yielded conflicting results, and the effects of vitamin D deficiency and vitamin D supplementation on all-cause mortality were consistent. These findings suggested that vitamin D supplementation has a long-term effect on the prevention of overall mortality. In addition, future studies should focus on the impact of low vitamin D concentration and vitamin D supplementation on the incidence of the diseases and on healthy life expectancy. Importantly, genetic variants related to vitamin D deficiency, especially variants of the vitamin D receptor, binding protein, and metabolizing enzyme 1‐α‐hydroxylase, may contribute to these divergent results. Additionally, increasing evidence suggests that genetic variation may impact variable results reported in the case of vitamin D supplementation in various trials (308, 309), indicating that additional studies are required to assess the roles of genetic variations as potential determinants of beneficial and negative effects of over-the-counter supplements used for health promotion. Future studies need to focus on the combined effects of genetic variations and vitamin D supplementation on Alzheimer's disease, hypertension, schizophrenia, and type 2 diabetes. Notably, some subjects without vitamin D deficiency but with genetic variations suffered from these diseases, and in some subjects with genetic variations, vitamin D intervention had no effect. Future RCTs should consider interindividual differences and identify whether certain subgroups of individuals may benefit from vitamin D supplementation in the context of disease outcomes. The purpose of MR studies should not be limited to simple exploration of whether low vitamin D concentration is a causative factor or a biomarker of a disease and should also aim to suggest effective interventions that consider both genetic variations and low vitamin D concentration.
Moreover, associations of low vitamin D concentration and vitamin D supplementation with 14 overlapping health outcomes were supported by evidence that was not fully consistent. These outcomes included all-cause mortality in prostate cancer patients, small for gestational age, and sustained virologic response to hepatitis C virus, which showed a significant association in both meta-analyses of observational studies and meta-analyses of RCTs. Inconsistent evidence was presented for other outcomes. Inconsistencies between observational and randomized evidence may be due to the low frequency of these outcomes, which can limit the conclusions of randomized trials, thus necessitating additional validation by MR studies.
MR is rapidly becoming a powerful method for inferring causality based on routinely conducted observational studies. The present study aimed to investigate the current status and limitations of MR studies for exploring the causal associations of vitamin D concentrations with disease outcomes. Most of the findings of the present umbrella review indicated that genetic risk factors related to vitamin D concentration did not predict disease risk. These null findings may be explained by 4 reasons. First, the findings could have been influenced by a bias of weak IVs because variability of vitamin D concentration explained by single nucleotide polymorphisms was 1.61–2.84%. Therefore, additional genome-wide association studies are needed to systematically explore genetic variants related to vitamin D concentration. Moreover, it is important to investigate network relationships between genetic variants and other molecular intermediates (e.g., DNA methylation, gene expression, metabolites, and metagenomic information) in vitamin D deficiency to understand the molecular mechanism related to vitamin D concentration, including the application of systems biology and pharmacogenomics. Second, these null findings suggested that associations between vitamin D concentration and diseases can be attributed to a reverse causation bias. Additional bidirectional MR studies are needed to prove this hypothesis and are expected to identify interventions aiming to reduce the prevalence of low vitamin D concentrations. Third, MR studies consider lifelong effects of genetic variations on diseases; however, the association of vitamin D concentrations with disease outcomes may vary over time and are not constant. Thus, evaluations are limited due to the cross-sectional observational nature of current MR studies. Therefore, MR studies should incorporate some follow-up data to evaluate the effects of vitamin D concentrations on various diseases and to investigate the changes in the effects of genetic variants over time on these diseases. Finally, a previous study demonstrated that vitamin D supplementation was effective only in subjects with baseline 25(OH)D concentrations of no more than 30 nmol/L, suggesting that associations between the 25(OH)D concentration and disease outcomes may be nonlinear (310–312). The MR analysis included an assumption of linearity (313), suggesting that nonlinear relationships could not be tested and could have supported the null hypothesis of the lack of an effect of 25(OH)D concentration on the diseases. Therefore, the null findings indicated a possible lack of linear causal associations of 25(OH)D concentrations with the diseases. Nonlinear MR studies are needed to prove this hypothesis and are expected to explore the real effects of the vitamin D deficiency on disease risk. Thus, we hope to contribute to more reliable evaluations of MR findings.
The present study has several limitations. First, the risk of type I errors may be increased due to testing multiple outcomes; however, this risk is generally acceptable considering the exploratory nature of umbrella reviews. Second, selected studies could have been heterogeneous due to the variable methodological quality of meta-analyses. The diagnostic criteria used for low vitamin D concentration and outcomes could have influenced estimated effects and increased between-study heterogeneity. In addition, most included meta-analyses were at high risk of bias, which might decrease the robustness of statistical analyses. The studies did not control for confounding factors that could have mediated associations between vitamin D concentration and outcomes because this information was often unavailable in published meta-analyses. Additionally, some potential outcomes for vitamin D concentration and vitamin D supplementation have not been subjected to meta-analyses at present. Notably, comparisons of the results included evidence from MR studies that assessed the effects of vitamin D deficiency on outcomes, which was largely negative or inconclusive due to a bias of weak IVs. Comparison of the results has to account for differences in the duration and timing of exposure to vitamin D. For example, vitamin D concentrations can be influenced by various factors, such as exposure, sunlight, altitude, and race, potentially leading to unidentified biases. Considering these caveats, the main strengths of this umbrella review include a topically comprehensive literature search, inclusion of a large body of evidence, and systematic quantitative and qualitative approaches used to assess the quality of available evidence.
Conclusions
Low vitamin D concentrations are a causal factor for multiple noncommunicable chronic diseases. Accordingly, vitamin D supplementation is a promising strategy with long-term preventive effects on these diseases and thus decreases all-cause mortality. However, the current vitamin D supplementation strategy might not be an efficient intervention factor for these diseases, suggesting that new strategies are highly needed to improve the intervention outcomes. Considering the existence of high risk bias in original meta-analyses, the finding might not robust enough, and needs confirming in future studies. Future studies should focus on personalized interventions for diseases involving considerations of genetic variations in combination with low vitamin D concentrations.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—YW, QM: contributed to the concept and design; DL, XM, QT, WC, XF, LW, MS, QM, WW: contributed to the acquisition and analysis of data; DL, XM, QT: drafted the manuscript; WW, QM, YW: guarantee this work and have full access to all of the data and take responsibility for the integrity of the data; all authors: made important contributions to editing and critically revising the manuscript for important intellectual content; and all authors: read and approved the final manuscript.
Notes
Supported by grants from the National Natural Science Foundation of China (NSFC 81872682 and NSFC 81773527) and the China–Australian Collaborative Grant, China Scholarship Council (NSFC 81561128020-NHMRC APP1112767). DL was supported by the China Scholarship Council (CSC 201908110339). The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–2 and Supplemental Tables 1–9 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
DL, XM, and QT contributed equally to this work.
Abbreviations used: 25(OH)D, 25-hydroxyvitamin D; CKD, chronic kidney disease; CVD, cardiovascular disease; IVS, instrumental variables; MR, Mendelian randomization; PI, prediction interval; PRISMA, Preferred Reporting Items of Systematic Reviews and Meta-analyses; PROSPERO, International Prospective Register of Systematic Reviews; RCT, randomized controlled trial; ROBIS, Risk of Bias in Systematic Reviews.
Contributor Information
Di Liu, Beijing Key Laboratory of Clinical Epidemiology, School of Public Health, Capital Medical University, Beijing, China; Centre for Biomedical Information Technology, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, Guangdong, China.
Xiaoni Meng, Beijing Key Laboratory of Clinical Epidemiology, School of Public Health, Capital Medical University, Beijing, China.
Qiuyue Tian, Beijing Key Laboratory of Clinical Epidemiology, School of Public Health, Capital Medical University, Beijing, China.
Weijie Cao, Beijing Key Laboratory of Clinical Epidemiology, School of Public Health, Capital Medical University, Beijing, China.
Xin Fan, School of Basic Medical Sciences, Capital Medical University, Beijing, China.
Lijuan Wu, Beijing Key Laboratory of Clinical Epidemiology, School of Public Health, Capital Medical University, Beijing, China.
Manshu Song, Centre for Precision Health, School of Medical and Health Sciences, Edith Cowan University, Perth, WA, Australia.
Qun Meng, Beijing Key Laboratory of Clinical Epidemiology, School of Public Health, Capital Medical University, Beijing, China.
Wei Wang, Beijing Key Laboratory of Clinical Epidemiology, School of Public Health, Capital Medical University, Beijing, China; Centre for Precision Health, School of Medical and Health Sciences, Edith Cowan University, Perth, WA, Australia; School of Public Health, Shandong First Medical University and Shandong Academy of Medical Science, Tai'an, Shandong, China.
Youxin Wang, Beijing Key Laboratory of Clinical Epidemiology, School of Public Health, Capital Medical University, Beijing, China; Centre for Precision Health, School of Medical and Health Sciences, Edith Cowan University, Perth, WA, Australia.
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080s–6s. [DOI] [PubMed] [Google Scholar]
- 2. Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88(7):720–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(12):827–38. [DOI] [PubMed] [Google Scholar]
- 4. Pojsupap S, Iliriani K, Sampaio TZ, O'Hearn K, Kovesi T, Menon K, McNally JD. Efficacy of high-dose vitamin D in pediatric asthma: a systematic review and meta-analysis. J Asthma. 2015;52(4):382–90. [DOI] [PubMed] [Google Scholar]
- 5. Shahvazi S, Soltani S, Ahmadi SM, de Souza RJ, Salehi-Abargouei A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51(1):11–21. [DOI] [PubMed] [Google Scholar]
- 6. Li XH, Feng L, Yang ZH, Liao YH. Effect of active vitamin D on cardiovascular outcomes in predialysis chronic kidney diseases: a systematic review and meta-analysis. Nephrology. 2015;20(10):706–14. [DOI] [PubMed] [Google Scholar]
- 7. Martineau AR, Jolliffe DA, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall ECet al. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess. 2019;23(2):1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saraff V, Shaw N. Sunshine and vitamin D. Arch Dis Child. 2016;101(2):190–2. [DOI] [PubMed] [Google Scholar]
- 9. Maxwell SM, Salah SM, Bunn JE. Dietary habits of the Somali population in Liverpool, with respect to foods containing calcium and vitamin D: a cause for concern?. J Hum Nutr Diet. 2006;19(2):125–7. [DOI] [PubMed] [Google Scholar]
- 10. Burgess S, Timpson NJ, Ebrahim S, Davey Smith G. Mendelian randomization: where are we now and where are we going?. Int J Epidemiol. 2015;44(2):379–88. [DOI] [PubMed] [Google Scholar]
- 11. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318(19):1925–6. [DOI] [PubMed] [Google Scholar]
- 12. Roberts R. Mendelian randomization studies promise to shorten the journey to FDA approval. JACC Basic Transl Sci. 2018;3(5):690–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thanassoulis G, O'Donnell CJ. Mendelian randomization: nature's randomized trial in the post-genome era. JAMA. 2009;301(22):2386–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meng X, Li X, Timofeeva MN, He Y, Spiliopoulou A, Wei WQ, Gifford A, Wu H, Varley T, Joshi Pet al. Phenome-wide Mendelian-randomization study of genetically determined vitamin D on multiple health outcomes using the UK Biobank study. Int J Epidemiol. 2019;48(5):1425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manousaki D, Mokry LE, Ross S, Goltzman D, Richards JB. Mendelian randomization studies do not support a role for vitamin D in coronary artery disease. Circ Cardiovasc Genet. 2016;9(4):349–56. [DOI] [PubMed] [Google Scholar]
- 16. Noordam R, Hamer MA, Pardo LM, van der Nat T, Kayser M, Slagboom PE, Uitterlinden A, Zillikens MC, Beekman Met al. No causal association between 25-hydroxyvitamin D and features of skin aging: evidence from a bidirectional Mendelian randomization study. J Invest Dermatol. 2017;137(11):2291–7. [DOI] [PubMed] [Google Scholar]
- 17. Larsson SC, Singleton AB, Nalls MA, Richards JB. No clear support for a role for vitamin D in Parkinson's disease: a Mendelian randomization study. Mov Disord. 2017;32(8):1249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hysinger EB, Roizen JD, Mentch FD, Vazquez L, Connolly JJ, Bradfield JP, Almoguera B, Sleiman PM, Allen JL, Levine MAet al. Mendelian randomization analysis demonstrates that low vitamin D is unlikely causative for pediatric asthma. J Allergy Clin Immunol. 2016;138(6):1747–9. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li SS, Gao LH, Zhang XY, He JW, Fu WZ, Liu YJ, Hu YQ, Zhang ZL. Genetically low vitamin D levels bone mineral density, and bone metabolism markers: a Mendelian randomisation study. Sci Rep. 2016;6:33202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X, Meng X, Timofeeva M, Tzoulaki I, Tsilidis KK, Ioannidis JP, Campbell H, Theodoratou E. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ. 2017;357:j2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lawlor DA, Tilling K, Davey Smith G.Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45(6):1866–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Köhler CA, Evangelou E, Stubbs B, Solmi M, Veronese N, Belbasis L, Bortolato B, Melo MCA, Coelho CA, Fernandes BSet al. Mapping risk factors for depression across the lifespan: an umbrella review of evidence from meta-analyses and Mendelian randomization studies. J Psychiatr Res. 2018;103:189–207. [DOI] [PubMed] [Google Scholar]
- 25. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19(22):3127–31. [DOI] [PubMed] [Google Scholar]
- 29. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 30. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. 2007;4(3):245–53. [DOI] [PubMed] [Google Scholar]
- 32. Ioannidis JP. Excess significance bias in the literature on brain volume abnormalities. Arch Gen Psychiatry. 2011;68(8):773–80. [DOI] [PubMed] [Google Scholar]
- 33. Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Whiting P, Savović J, Higgins JP, Caldwell DM, Reeves BC, Shea B, Davies P, Kleijnen J, Churchill R; ROBIS group . ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feng Y, Cheng G, Wang H, Chen B. The associations between serum 25-hydroxyvitamin D level and the risk of total fracture and hip fracture. Osteoporos Int. 2017;28(5):1641–52. [DOI] [PubMed] [Google Scholar]
- 37. Bergink AP, Zillikens MC, Van Leeuwen JP, Hofman A, Uitterlinden AG, van Meurs JB. 25-Hydroxyvitamin D and osteoarthritis: a meta-analysis including new data. Semin Arthritis Rheum. 2016;45(5):539–46. [DOI] [PubMed] [Google Scholar]
- 38. Song GG, Bae SC, Lee YH. Association between vitamin D intake and the risk of rheumatoid arthritis: a meta-analysis. Clin Rheumatol. 2012;31(12):1733–9. [DOI] [PubMed] [Google Scholar]
- 39. Lin J, Liu J, Davies ML, Chen W. Serum vitamin D level and rheumatoid arthritis disease activity: review and meta-analysis. PLoS One. 2016;11(1):e0146351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yin L, Ordonez-Mena JM, Chen T, Schottker B, Arndt V, Brenner H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: a systematic review and meta-analysis. Prev Med. 2013;57(6):753–64. [DOI] [PubMed] [Google Scholar]
- 41. Wei MY, Garland CF, Gorham ED, Mohr SB, Giovannucci E. Vitamin D and prevention of colorectal adenoma: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2958–69. [DOI] [PubMed] [Google Scholar]
- 42. Fedirko V, Bostick RM, Goodman M, Flanders WD, Gross MD. Blood 25-hydroxyvitamin D3 concentrations and incident sporadic colorectal adenoma risk: a pooled case-control study. Am J Epidemiol. 2010;172(5):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Choi YJ, Kim YH, Cho CH, Kim SH, Lee JE. Circulating levels of vitamin D and colorectal adenoma: a case-control study and a meta-analysis. World J Gastroenterol. 2015;21(29):8868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30(2):113–25. [DOI] [PubMed] [Google Scholar]
- 45. Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128(6):1414–24. [DOI] [PubMed] [Google Scholar]
- 46. Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29(28):3775–82. [DOI] [PubMed] [Google Scholar]
- 47. Lee JE, Li H, Chan AT, Hollis BW, Lee IM, Stampfer MJ, Wu K, Giovannucci E, Ma J. Circulating levels of vitamin D and colon and rectal cancer: the Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev Res (Phila). 2011;4(5):735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li M, Chen P, Li J, Chu R, Xie D, Wang H. Review: the impacts of circulating 25-hydroxyvitamin D levels on cancer patient outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99(7):2327–36. [DOI] [PubMed] [Google Scholar]
- 49. Liu Y, Yu Q, Zhu Z, Zhang J, Chen M, Tang P, Li K. Vitamin and multiple-vitamin supplement intake and incidence of colorectal cancer: a meta-analysis of cohort studies. Med Oncol. 2015;32(1):434. [DOI] [PubMed] [Google Scholar]
- 50. Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: circulating vitamin D and ovarian cancer risk. Gynecol Oncol. 2011;121(2):369–75. [DOI] [PubMed] [Google Scholar]
- 51. Touvier M, Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, Riboli E, Hercberg S, Norat T. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20(5):1003–16. [DOI] [PubMed] [Google Scholar]
- 52. Yin L, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis of longitudinal studies: serum vitamin D and prostate cancer risk. Cancer Epidemiol. 2009;33(6):435–45. [DOI] [PubMed] [Google Scholar]
- 53. Gilbert R, Martin RM, Beynon R, Harris R, Savovic J, Zuccolo L, Bekkering GE, Fraser WD, Sterne JA, Metcalfe C. Associations of circulating and dietary vitamin D with prostate cancer risk: a systematic review and dose-response meta-analysis. Cancer Causes Control. 2011;22(3):319–40. [DOI] [PubMed] [Google Scholar]
- 54. Xu Y, Shao X, Yao Y, Xu L, Chang L, Jiang Z, Lin Z. Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: new findings from an updated meta-analysis. J Cancer Res Clin Oncol. 2014;140(9):1465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu J, Huang W, Zhou R, Jia S, Tang W, Luo Y, Zhang J. Serum/plasma 25-hydroxyvitamin D and risk of lung, breast and prostate cancer: A meta-analysis. Int J Clin Exp Med. 2016;9(2):2728–37. [Google Scholar]
- 56. Gao J, Wei W, Wang G, Zhou H, Fu Y, Liu N. Circulating vitamin D concentration and risk of prostate cancer: a dose-response meta-analysis of prospective studies. Ther Clin Risk Manage. 2018;14:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121(2):469–77. [DOI] [PubMed] [Google Scholar]
- 58. Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer. 2010;46(12):2196–205. [DOI] [PubMed] [Google Scholar]
- 59. Mohr SB, Gorham ED, Alcaraz JE, Kane CJ, Macera CA, Parsons JK, Wingard DL, Garland CF. Serum 25-hydroxyvitamin D and prevention of breast cancer: pooled analysis. Anticancer Res. 2011;31(9):2939–48. [PubMed] [Google Scholar]
- 60. Bauer SR, Hankinson SE, Bertone-Johnson ER, Ding EL. Plasma vitamin D levels, menopause, and risk of breast cancer: dose-response meta-analysis of prospective studies. Medicine (Baltimore). 2013;92(3):123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen P, Li M, Gu X, Liu Y, Li X, Li C, Wang Y, Xie D, Wang F, Yu Cet al. Higher blood 25(OH)D level may reduce the breast cancer risk: evidence from a Chinese population based case-control study and meta-analysis of the observational studies. PLoS One. 2013;8(1):e49312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang D, Velez de-la-Paz OI, Zhai JX, Liu DW. Serum 25-hydroxyvitamin D and breast cancer risk: a meta-analysis of prospective studies. Tumor Biol. 2013;34(6):3509–17. [DOI] [PubMed] [Google Scholar]
- 63. Kim Y, Je Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: a meta-analysis. Br J Cancer. 2014;110(11):2772–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Estebanez N, Gomez-Acebo I, Palazuelos C, Llorca J, Dierssen-Sotos T. Vitamin D exposure and risk of breast cancer: a meta-analysis. Sci Rep. 2018;8(1):9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hossain S, Beydoun MA, Beydoun HA, Chen X, Zonderman AB, Wood RJ. Vitamin D and breast cancer: a systematic review and meta-analysis of observational studies. Clin Nutr ESPEN. 2019;30:170–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rose AA, Elser C, Ennis M, Goodwin PJ. Blood levels of vitamin D and early stage breast cancer prognosis: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;141(3):331–9. [DOI] [PubMed] [Google Scholar]
- 67. Chen GC, Zhang ZL, Wan Z, Wang L, Weber P, Eggersdorfer M, Qin LQ, Zhang W. Circulating 25-hydroxyvitamin D and risk of lung cancer: a dose–response meta-analysis. Cancer Causes Control. 2015;26(12):1719–28. [DOI] [PubMed] [Google Scholar]
- 68. Zhang L, Wang S, Che X, Li X. Vitamin D and lung cancer risk: a comprehensive review and meta-analysis. Cell Physiol Biochem. 2015;36(1):299–305. [DOI] [PubMed] [Google Scholar]
- 69. Liu J, Dong Y, Lu C, Wang Y, Peng L, Jiang M, Tang Y, Zhao Q. Meta-analysis of the correlation between vitamin D and lung cancer risk and outcomes. Oncotarget. 2017;8(46):81040–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wei H, Jing H, Wei Q, Wei G, Heng Z. Associations of the risk of lung cancer with serum 25-hydroxyvitamin D level and dietary vitamin D intake: a dose-response PRISMA meta-analysis. Medicine (Baltimore). 2018;97(37):e12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Purdue MP, Freedman DM, Gapstur SM, Helzlsouer KJ, Laden F, Lim U, Maskarinec G, Rothman N, Shu XO, Stevens VLet al. Circulating 25-hydroxyvitamin D and risk of non-hodgkin lymphoma: cohort consortium vitamin D pooling project of rarer cancers. Am J Epidemiol. 2010;172(1):58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lu D, Chen J, Jin J. Vitamin D status and risk of non-Hodgkin lymphoma: a meta-analysis. Cancer Causes Control. 2014;25(11):1553–63. [DOI] [PubMed] [Google Scholar]
- 73. Park HY, Hong YC, Lee K, Koh J. Vitamin D status and risk of non-Hodgkin lymphoma: an updated meta-analysis. PLoS One. 2019;14(4):e0216284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, Qi D, Patel AV, Helzlsouer KJ, Weinstein SJ, McCullough ML, Purdue MP, Shu XOet al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: cohort consortium vitamin D pooling project of rarer cancers. Am J Epidemiol. 2010;172(1):81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu SL, Zhao YP, Dai MH, You L, Wen Z, Xu JW. Vitamin D status and the risk of pancreatic cancer: a meta-analysis. Chin Med J (Engl). 2013;126(17):3356–9. [PubMed] [Google Scholar]
- 76. Zhang X, Huang XZ, Chen WJ, Wu J, Chen Y, Wu CC, Wang ZN. Plasma 25-hydroxyvitamin D levels, vitamin D intake, and pancreatic cancer risk or mortality: a meta-analysis. Oncotarget. 2017;8(38):64395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Caini S, Boniol M, Tosti G, Magi S, Medri M, Stanganelli I, Palli D, Assedi M, Marmol VD, Gandini S. Vitamin D and melanoma and non-melanoma skin cancer risk and prognosis: a comprehensive review and meta-analysis. Eur J Cancer. 2014;50(15):2649–58. [DOI] [PubMed] [Google Scholar]
- 78. Zgaga L, O'Sullivan F, Cantwell MM, Murray LJ, Thota PN, Coleman HG. Markers of vitamin D exposure and esophageal cancer risk: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2016;25(6):877–86. [DOI] [PubMed] [Google Scholar]
- 79. Zhao J, Wang H, Zhang Z, Zhou X, Yao J, Zhang R, Liao L, Dong J. Vitamin D deficiency as a risk factor for thyroid cancer: a meta-analysis of case-control studies. Nutrition. 2019;57:5–11. [DOI] [PubMed] [Google Scholar]
- 80. Hu MJ, Zhang Q, Liang L, Wang SY, Zheng XC, Zhou MM, Yang YW, Zhong Q, Huang F. Association between vitamin D deficiency and risk of thyroid cancer: a case–control study and a meta-analysis. J Endocrinol Invest. 2018;41(10):1199–210. [DOI] [PubMed] [Google Scholar]
- 81. Chen F, Li Q, Yu Y, Yang W, Shi F, Qu Y. Association of vitamin C, vitamin D, vitamin E and risk of bladder cancer: a dose-response meta-analysis. Sci Rep. 2015;5:9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang H, Wen X, Zhang Y, Wei X, Liu T. Vitamin D deficiency and increased risk of bladder carcinoma: a meta-analysis. Cell Physiol Biochem. 2015;37(5):1686–92. [DOI] [PubMed] [Google Scholar]
- 83. Khayatzadeh S, Feizi A, Saneei P, Esmaillzadeh A. Vitamin D intake, serum vitamin D levels, and risk of gastric cancer: a systematic review and meta-analysis. J Res Med Sci. 2015;20(8):790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lin G, Ning L, Gu D, Li S, Yu Z, Long Q, Hou LN, Tan WL. Examining the association of circulating 25-hydroxyvitamin D with kidney cancer risk: a meta-analysis. Int J Clin Exp Med. 2015;8(11):20499–507. [PMC free article] [PubMed] [Google Scholar]
- 85. Yan L, Gu Y, Luan T, Miao M, Jiang L, Liu Y, Li P, Zeng X. Associations between serum vitamin D and the risk of female reproductive tumors. Medicine (Baltimore). 2018;97(15):e0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang R, Li B, Gao X, Tian R, Pan Y, Jiang Y, Gu H, Wang Y, Wang Y, Liu G. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am J Clin Nutr. 2017;105(4):810–19. [DOI] [PubMed] [Google Scholar]
- 87. Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, Kandala NB, Clarke A, Franco OH. Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas. 2010;65(3):225–36. [DOI] [PubMed] [Google Scholar]
- 88. Sokol SI, Tsang P, Aggarwal V, Melamed ML, Srinivas VS. Vitamin D status and risk of cardiovascular events: lessons learned via systematic review and meta-analysis. Cardiol Rev. 2011;19(4):192–201. [DOI] [PubMed] [Google Scholar]
- 89. Wang L, Song Y, Manson JE, Pilz S, Marz W, Michaelsson K, Lundqvist A, Jassal SK, Barrett-Connor E, Zhang Cet al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5(6):819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Huang J, Wang Z, Hu Z, Jiang W, Li B. Association between blood vitamin D and myocardial infarction: a meta-analysis including observational studies. Clin Chim Acta. 2017;471:270–5. [DOI] [PubMed] [Google Scholar]
- 91. Brondum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG. 25-Hydroxyvitamin D levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol. 2012;32(11):2794–802. [DOI] [PubMed] [Google Scholar]
- 92. Sun Q, Pan A, Hu FB, Manson JE, Rexrode KM. 25-Hydroxyvitamin D levels and the risk of stroke: a prospective study and meta-analysis. Stroke. 2012;43(6):1470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhou R, Wang M, Huang H, Li W, Hu Y, Wu T. Lower vitamin D status is associated with an increased risk of ischemic stroke: a systematic review and meta-analysis. Nutrients. 2018;10(3):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chowdhury R, Stevens S, Ward H, Chowdhury S, Sajjad A, Franco OH. Circulating vitamin D, calcium and risk of cerebrovascular disease: a systematic review and meta-analysis. Eur J Epidemiol. 2012;27(8):581–91. [DOI] [PubMed] [Google Scholar]
- 95. Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152(5):307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens. 2011;29(4):636–45. [DOI] [PubMed] [Google Scholar]
- 97. Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol. 2013;28(3):205–21. [DOI] [PubMed] [Google Scholar]
- 98. Qi D, Nie XL, Wu S, Cai J. Vitamin D and hypertension: prospective study and meta-analysis. PLoS One. 2017;12(3):e0174298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ometto F, Stubbs B, Annweiler C, Duval GT, Jang W, Kim HT, McCarroll K, Cunningham C, Soysal P, Isik ATet al. Hypovitaminosis D and orthostatic hypotension: a systematic review and meta-analysis. J Hypertens. 2016;34(6):1036–43. [DOI] [PubMed] [Google Scholar]
- 100. Zhang Z, Yang Y, Ng CY, Wang D, Wang J, Li G, Liu T. Meta-analysis of vitamin D deficiency and risk of atrial fibrillation. Clin Cardiol. 2016;39(9):537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lupoli R, Vaccaro A, Ambrosino P, Poggio P, Amato M, Di Minno MND. Impact of vitamin D deficiency on subclinical carotid atherosclerosis: a pooled analysis of cohort studies. J Clin Endocrinol Metab. 2017;102(7):2146–53. [DOI] [PubMed] [Google Scholar]
- 102. Chen FH, Liu T, Xu L, Zhang L, Zhou XB. Association of serum vitamin D level and carotid atherosclerosis: a systematic review and meta-analysis. J Ultrasound Med. 2018;37(6):1293–303. [DOI] [PubMed] [Google Scholar]
- 103. Iannuzzo G, Forte F, Lupoli R, Di Minno MND. Association of vitamin D deficiency with peripheral arterial disease: a meta-analysis of literature studies. J Clin Endocrinol Metab. 2018;103(6):2107–15. [DOI] [PubMed] [Google Scholar]
- 104. Dong JY, Zhang WG, Chen JJ, Zhang ZL, Han SF, Qin LQ. Vitamin D intake and risk of type 1 diabetes: a meta-analysis of observational studies. Nutrients. 2013;5(9):3551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65(9):1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Forouhi NG, Ye Z, Rickard AP, Khaw KT, Luben R, Langenberg C, Wareham NJ. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: results from the European Prospective Investigation into Cancer (EPIC)–Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia. 2012;55(8):2173–82. [DOI] [PubMed] [Google Scholar]
- 107. Afzal S, Bojesen SE, Nordestgaard BG. Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin Chem. 2013;59(2):381–91. [DOI] [PubMed] [Google Scholar]
- 108. Khan H, Kunutsor S, Franco OH, Chowdhury R. Vitamin D, type 2 diabetes and other metabolic outcomes: a systematic review and meta-analysis of prospective studies. Proc Nutr Soc. 2013;72(1):89–97. [DOI] [PubMed] [Google Scholar]
- 109. Zhao LM, Tian XQ, Ge JP, Xu YC. Vitamin D intake and type 2 diabetes risk: a meta-analysis of prospective cohort studies. Afr Health Sci. 2013;13(4):1130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, Hu FB. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36(5):1422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lucato P, Solmi M, Maggi S, Bertocco A, Bano G, Trevisan C, Manzato E, Sergi G, Schofield P, Kouidrat Yet al. Low vitamin D levels increase the risk of type 2 diabetes in older adults: a systematic review and meta-analysis. Maturitas. 2017;100:8–15. [DOI] [PubMed] [Google Scholar]
- 112. Poel YH, Hummel P, Lips P, Stam F, van der Ploeg T, Simsek S. Vitamin D and gestational diabetes: a systematic review and meta-analysis. Eur J Intern Med. 2012;23(5):465–9. [DOI] [PubMed] [Google Scholar]
- 113. Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O'Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. [DOI] [PubMed] [Google Scholar]
- 114. Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2013;26(9):889–99. [DOI] [PubMed] [Google Scholar]
- 115. Zhang MX, Pan GT, Guo JF, Li BY, Qin LQ, Zhang ZL. Vitamin D deficiency increases the risk of gestational diabetes mellitus: a meta-analysis of observational studies. Nutrients. 2015;7(10):8366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lu M, Xu Y, Lv L, Zhang M. Association between vitamin D status and the risk of gestational diabetes mellitus: a meta-analysis. Arch Gynecol Obstet. 2016;293(5):959–66. [DOI] [PubMed] [Google Scholar]
- 117. Amraei M, Mohamadpour S, Sayehmiri K, Mousavi SF, Shirzadpour E, Moayeri A. Effects of vitamin D deficiency on incidence risk of gestational diabetes mellitus: a systematic review and meta-analysis. Front Endocrinol. 2018;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hu L, Zhang Y, Wang X, You L, Xu P, Cui X, Zhu L, Ji C, Guo X, Wen J. Maternal vitamin D status and risk of gestational diabetes: a meta-analysis. Cell Physiol Biochem. 2018;45(1):291–300. [DOI] [PubMed] [Google Scholar]
- 119. Nargesi S, Ghorbani A, Shirzadpour E, Mohamadpour M, Mousavi SF, Amraei M. A systematic review and meta-analysis of the association between vitamin D deficiency and gestational diabetes mellitus. Biomed Res Therapy. 2018;5(3):2078–95. [Google Scholar]
- 120. Zhang Y, Gong Y, Xue H, Xiong J, Cheng G. Vitamin D and gestational diabetes mellitus: a systematic review based on data free of Hawthorne effect. BJOG. 2018;125(7):784–93. [DOI] [PubMed] [Google Scholar]
- 121. Derakhshanian H, Shab-Bidar S, Speakman JR, Nadimi H, Djafarian K. Vitamin D and diabetic nephropathy: a systematic review and meta-analysis. Nutrition. 2015;31(10):1189–94. [DOI] [PubMed] [Google Scholar]
- 122. Luo BA, Gao F, Qin LL. The association between vitamin D deficiency and diabetic retinopathy in type 2 diabetes: a meta-analysis of observational studies. Nutrients. 2017;9(3):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Dai J, Jiang C, Chen H, Chai Y. Vitamin D and diabetic foot ulcer: a systematic review and meta-analysis. Nutr Diabetes. 2019;9(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis. 2011;58(3):374–82. [DOI] [PubMed] [Google Scholar]
- 125. Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daurès JP, Argilés A. Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am J Nephrol. 2013;37(3):239–48. [DOI] [PubMed] [Google Scholar]
- 126. Patel S, Patel S, Makki H. Vitamin D deficiency is not associated with increased mortality in critically ill: a meta-analysis. Crit Care Med. 2013;41(12):A132. [Google Scholar]
- 127. Rush L, McCartney G, Walsh D, MacKay D. Vitamin D and subsequent all-age and premature mortality: a systematic review. BMC Public Health. 2013;13:1, 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Schottker B, Ball D, Gellert C, Brenner H. Serum 25-hydroxyvitamin D levels and overall mortality. A systematic review and meta-analysis of prospective cohort studies. Ageing Res Rev. 2013;12(2):708–18. [DOI] [PubMed] [Google Scholar]
- 129. Zheng Z, Shi H, Jia J, Li D, Lin S. Vitamin D supplementation and mortality risk in chronic kidney disease: a meta-analysis of 20 observational studies. BMC Nephrology. 2013;14:1, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, Khan H, Baena CP, Prabhakaran D, Hoshen MBet al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Fan H, Yu W, Cao H, Li J, Liu B, Wang J, Shao Y, Fan Y, Yang J, Zhang Qet al. Meta-analysis of circulating 25-hydroxyvitamin D levels and risk of cardiovascular and all-cause mortality in elderly population. Int J Cardiol. 2014;176(3):1025–9. [DOI] [PubMed] [Google Scholar]
- 132. Garland CF, Kim JJ, Mohr SB, Gorham ED, Grant WB, Giovannucci EL, Baggerly L, Hofflich H, Ramsdell JW, Zeng Ket al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am J Public Health. 2014;104(8):e43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Maalmi H, Ordonez-Mena JM, Schottker B, Brenner H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: systematic review and meta-analysis of prospective cohort studies. Eur J Cancer. 2014;50(8):1510–21. [DOI] [PubMed] [Google Scholar]
- 134. Tomson J, Emberson J, Hill M, Gordon A, Armitage J, Shipley M, Collins R, Clarke R. Vitamin D and risk of death from vascular and non-vascular causes in the Whitehall study and meta-analyses of 12,000 deaths. Eur Heart J. 2013;34(18):1365–74. [DOI] [PubMed] [Google Scholar]
- 135. Wang W, Li G, He X, Gao J, Wang R, Wang Y, Zhao W. Serum 25-hydroxyvitamin D levels and prognosis in hematological malignancies: a systematic review and meta-analysis. Cell Physiol Biochem. 2015;35(5):1999–2005. [DOI] [PubMed] [Google Scholar]
- 136. Jayedi A, Soltani S, Shab-Bidar S. Vitamin D status and all-cause mortality in patients with chronic kidney disease: a systematic review and dose-response meta-analysis. J Clin Endocrinol Metab. 2017;102(7):2136–45. [DOI] [PubMed] [Google Scholar]
- 137. Wang YM, Tang CL, Che M, Wang XQ, Li AC. Prognostic value of vitamin D in patients with pneumonia: a systematic review and meta-analysis. Trop J Pharm Res. 2017;16(9):2267–73. [Google Scholar]
- 138. Song ZY, Yao Q, Zhuo Z, Ma Z, Chen G. Circulating vitamin D level and mortality in prostate cancer patients: a dose-response meta-analysis. Endocr Connect. 2018;7(12):R294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Xu J, Yuan X, Tao J, Yu N, Wu R, Zhang Y. Association of circulating 25-Hydroxyvitamin D levels with colorectal cancer: an updated meta-analysis. J Nutr Sci Vitaminol(Tokyo) 2018;64(6):432–44. [DOI] [PubMed] [Google Scholar]
- 140. Zhang Y, Darssan D, Pascoe EM, Johnson DW, Pi H, Dong J. Vitamin D status and mortality risk among patients on dialysis: a systematic review and meta-analysis of observational studies. Nephrol Dial Transplant. 2018;33(10):1742–51. [DOI] [PubMed] [Google Scholar]
- 141. Yang F, Ren H, Gao Y, Zhu Y, Huang W. The value of severe vitamin D deficiency in predicting the mortality risk of patients with liver cirrhosis: a meta-analysis. Clin Res Hepatol Gastroenterol. 2019;43(6):722–9. [DOI] [PubMed] [Google Scholar]
- 142. Hu K, Callen DF, Li J, Zheng H. Circulating vitamin D and overall survival in breast cancer patients: a dose-response meta-analysis of cohort studies. Integr Cancer Ther. 2018;17(2):217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Mohr SB, Gorham ED, Kim J, Hofflich H, Cuomo RE, Garland CF. Could vitamin D sufficiency improve the survival of colorectal cancer patients?. J Steroid Biochem Mol Biol. 2015;148:239–44. [DOI] [PubMed] [Google Scholar]
- 144. de Haan K, Groeneveld AB, de Geus HR, Egal M, Struijs A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Critical Care. 2014;18(6):660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zhang YP, Wan YD, Sun TW, Kan QC, Wang LX. Association between vitamin D deficiency and mortality in critically ill adult patients: a meta-analysis of cohort studies. Critical Care. 2014;18(6):684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Shen L, Ji HF. Vitamin D deficiency is associated with increased risk of Alzheimer's disease and dementia: evidence from meta-analysis. Nutr J. 2015;14:1, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Jayedi A, Rashidy-Pour A, Shab-Bidar S. Vitamin D status and risk of dementia and Alzheimer's disease: a meta-analysis of dose-response. Nutr Neurosci. 2019;22(11):750–9. [DOI] [PubMed] [Google Scholar]
- 148. Lv Z, Qi H, Wang L, Fan X, Han F, Wang H, Bi S. Vitamin D status and Parkinson's disease: a systematic review and meta-analysis. Neurol Sci. 2014;35(11):1723–30. [DOI] [PubMed] [Google Scholar]
- 149. Shen L, Ji HF. Associations between vitamin D status, supplementation, outdoor work and risk of Parkinson's disease: a meta-analysis assessment. Nutrients. 2015;7(6):4817–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Luo X, Ou R, Dutta R, Tian Y, Xiong H, Shang H. Association between serum vitamin D levels and Parkinson's disease: a systematic review and meta-analysis. Front Neurol. 2018;9:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhou Z, Zhou R, Zhang Z, Li K. The association between vitamin D status, vitamin D supplementation, sunlight exposure, and Parkinson's disease: a systematic review and meta-analysis. Med Sci Monit. 2019;25:666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Etgen T, Sander D, Bickel H, Sander K, Forstl H. Vitamin D deficiency, cognitive impairment and dementia: a systematic review and meta-analysis. Dement Geriatr Cogn Disord. 2012;33(5):297–305. [DOI] [PubMed] [Google Scholar]
- 153. Goodwill AM, Szoeke C. A systematic review and meta-analysis of the effect of low vitamin D on cognition. J Am Geriatr Soc. 2017;65(10):2161–8. [DOI] [PubMed] [Google Scholar]
- 154. Amraei M, Mohamadpour R, Moayeri A, Abbasi N, Shirzadpour E, Mohamadpour M. Vitamin D and its association with memory and learning: a systematic review and meta-analysis. Biomed Res. 2017;28(17):7427–33. [Google Scholar]
- 155. Wang XR, Xiao JP, Zhang JJ, Wu YG. Decreased serum/plasma vitamin D levels in SLE patients: a meta-analysis. Curr Pharm Des. 2018;24(37):4466–73. [DOI] [PubMed] [Google Scholar]
- 156. Bae SC, Lee YH. Association between vitamin D level and/or deficiency, and systemic lupus erythematosus: a meta-analysis. Cell Mol Biol. 2018;64(1):7–13. [DOI] [PubMed] [Google Scholar]
- 157. Wei Z, Zhang J, Yu X. Maternal vitamin D status and childhood asthma, wheeze, and eczema: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2016;27(6):612–19. [DOI] [PubMed] [Google Scholar]
- 158. Qin LL, Lu FG, Yang SH, Xu HL, Luo BA. Does maternal vitamin D deficiency increase the risk of preterm birth: a meta-analysis of observational studies. Nutrients. 2016;8(5):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Amegah AK, Klevor MK, Wagner CL. Maternal vitamin D insufficiency and risk of adverse pregnancy and birth outcomes: a systematic review and meta-analysis of longitudinal studies. PLoS One. 2017;12(3):e0173605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zhou SS, Tao YH, Huang K, Zhu BB, Tao FB. Vitamin D and risk of preterm birth: up-to-date meta-analysis of randomized controlled trials and observational studies. J Obstet Gynaecol Res. 2017;43(2):247–56. [DOI] [PubMed] [Google Scholar]
- 161. Tous M, Villalobos M, Iglesias L, Fernandez-Barres S, Arija V. Vitamin D status during pregnancy and offspring outcomes: a systematic review and meta-analysis of observational studies. Eur J Clin Nutr. 2020;74(1):36–53. [DOI] [PubMed] [Google Scholar]
- 162. Chen Y, Zhu B, Wu X, Li S, Tao F. Association between maternal vitamin D deficiency and small for gestational age: evidence from a meta-analysis of prospective cohort studies. BMJ Open. 2017;7(8):e016404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hu Z, Tang L, Xu HL. Maternal vitamin D deficiency and the risk of small for gestational age: a meta-analysis. Iran J Public Health. 2018;47(12):1785–95. [PMC free article] [PubMed] [Google Scholar]
- 164. Santamaria C, Bi WG, Leduc L, Tabatabaei N, Jantchou P, Luo ZC, Audibert F, Nuyt AM, Wei SQ. Prenatal vitamin D status and offspring's growth, adiposity and metabolic health: a systematic review and meta-analysis. Br J Nutr. 2018;119(3):310–19. [DOI] [PubMed] [Google Scholar]
- 165. Hyppönen E, Cavadino A, Williams D, Fraser A, Vereczkey A, Fraser WD, Bánhidy F, Lawlor D, Czeizel AE. Vitamin D and pre-eclampsia: original data, systematic review and meta-analysis. Ann Nutr Metab. 2013;63(4):331–40. [DOI] [PubMed] [Google Scholar]
- 166. Tabesh M, Salehi-Abargouei A, Tabesh M, Esmaillzadeh A. Maternal vitamin D status and risk of pre-eclampsia: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98(8):3165–73. [DOI] [PubMed] [Google Scholar]
- 167. Fu ZM, Ma ZZ, Liu GJ, Wang LL, Guo Y. Vitamins supplementation affects the onset of preeclampsia. J Formos Med Assoc. 2018;117(1):6–13. [DOI] [PubMed] [Google Scholar]
- 168. Chu J, Gallos I, Tobias A, Tan B, Eapen A, Coomarasamy A. Vitamin D and assisted reproductive treatment outcome: a systematic review and meta-analysis. Hum Reprod. 2018;33(1):65–80. [DOI] [PubMed] [Google Scholar]
- 169. Lv SS, Wang JY, Wang XQ, Wang Y, Xu Y. Serum vitamin D status and in vitro fertilization outcomes: a systematic review and meta-analysis. Arch Gynecol Obstet. 2016;293(6):1339–45. [DOI] [PubMed] [Google Scholar]
- 170. Su G, Liu Z, Qin X, Hong X, Liu X, Wen Z, Lindholm B, Carrero JJ, Johnson DW, Brusselaers Net al. Vitamin D deficiency and treatment versus risk of infection in end-stage renal disease patients under dialysis: a systematic review and meta-analysis. Nephrol Dial Transplant. 2019;34(1):146–56. [DOI] [PubMed] [Google Scholar]
- 171. Feng H, Xun P, Pike K, Wills AK, Chawes BL, Bisgaard H, Cai W, Wan Y, He K. In utero exposure to 25-hydroxyvitamin D and risk of childhood asthma, wheeze, and respiratory tract infections: a meta-analysis of birth cohort studies. J Allergy Clin Immunol. 2017;139(5):1508–17. [DOI] [PubMed] [Google Scholar]
- 172. Deng QF, Chu H, Wen Z, Cao YS. Vitamin D and urinary tract infection: a systematic review and meta-analysis. Ann Clin Lab Sci. 2019;49(1):134–42. [PubMed] [Google Scholar]
- 173. Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:2, 100–7. [DOI] [PubMed] [Google Scholar]
- 174. Ju SY, Lee YJ, Jeong SN. Serum 25-hydroxyvitamin D levels and the risk of depression: a systematic review and meta-analysis. J Nutr Health Aging. 2013;17(5):447–55. [DOI] [PubMed] [Google Scholar]
- 175. Valipour G, Saneei P, Esmaillzadeh A. Serum vitamin D levels in relation to schizophrenia: a systematic review and meta-analysis of observational studies. J Clin Endocrinol Metab. 2014;99(10):3863–72. [DOI] [PubMed] [Google Scholar]
- 176. Lv WS, Zhao WJ, Gong SL, Fang DD, Wang B, Fu ZJ, Yan SL, Wang YG. Serum 25-hydroxyvitamin D levels and peripheral neuropathy in patients with type 2 diabetes: a systematic review and meta-analysis. J Endocrinol Invest. 2015;38(5):513–18. [DOI] [PubMed] [Google Scholar]
- 177. García-Serna AM, Morales E. Neurodevelopmental effects of prenatal vitamin D in humans: systematic review and meta-analysis. Mol Psychiatry. 2020;25(10):2468–81. [DOI] [PubMed] [Google Scholar]
- 178. Gou X, Pan L, Tang F, Gao H, Xiao D. The association between vitamin D status and tuberculosis in children: a meta-analysis. Medicine (Baltimore). 2018;97(35):e12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Zeng J, Wu G, Yang W, Gu X, Liang W, Yao Y, Song Y. A serum vitamin D level <25nmol/l pose high tuberculosis risk: a meta-analysis. PLoS One. 2015;10(5):e0126014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Zhang LL, Liu CT, Gong J, Liu CT. Vitamin D with asthma and COPD: not a false hope? A systematic review and meta-analysis. Genetics Mol Res. 2014;13(3):7607–16. [DOI] [PubMed] [Google Scholar]
- 181. Man L, Zhang Z, Zhang M, Zhang Y, Li J, Zheng N, Cao Y, Chi M, Chao Y, Huang Qet al. Association between vitamin D deficiency and insufficiency and the risk of childhood asthma: evidence from a meta-analysis. Int J Clin Exp Med. 2015;8(4):5699–706. [PMC free article] [PubMed] [Google Scholar]
- 182. Jat KR, Khairwa A. Vitamin D and asthma in children: a systematic review and meta-analysis of observational studies. Lung India. 2017;34(4):355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Zhu B, Zhu B, Xiao C, Zheng Z. Vitamin D deficiency is associated with the severity of COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:1907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Zhu M, Wang T, Wang C, Ji Y. The association between vitamin D and COPD risk, severity, and exacerbation: an updated systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:2597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Ju SY, Jeong HS, Kim DH. Blood vitamin D status and metabolic syndrome in the general adult population: a dose-response meta-analysis. J Clin Endocrinol Metab. 2014;99(3):1053–63. [DOI] [PubMed] [Google Scholar]
- 186. Zhou J, Huang P, Liu P, Hao Q, Chen S, Dong B, Wang J. Association of vitamin D deficiency and frailty: a systematic review and meta-analysis. Maturitas. 2016;94:70–6. [DOI] [PubMed] [Google Scholar]
- 187. Ju SY, Lee JY, Kim DH. Low 25-hydroxyvitamin D levels and the risk of frailty syndrome: a systematic review and dose-response meta-analysis. BMC Geriatrics. 2018;18(1):206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Luo YQ, Wu XX, Ling ZX, Cheng YW, Yuan L, Xiang C. Association between serum vitamin D and severity of liver fibrosis in chronic hepatitis C patients: a systematic meta-analysis. J Zhejiang Univ Sci B. 2014;15(10):900–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Garcia-Alvarez M, Pineda-Tenor D, Jimenez-Sousa MA, Fernandez-Rodriguez A, Guzman-Fulgencio M, Resino S. Relationship of vitamin D status with advanced liver fibrosis and response to hepatitis C virus therapy: a meta-analysis. Hepatology. 2014;60(5):1541–50. [DOI] [PubMed] [Google Scholar]
- 190. Villar LM, Del Campo JA, Ranchal I, Lampe E, Romero-Gomez M. Association between vitamin D and hepatitis C virus infection: a meta-analysis. World J Gastroenterol. 2013;19(35):5917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Upala S, Sanguankeo A, Permpalung N. Significant association between vitamin D deficiency and sepsis: a systematic review and meta-analysis. BMC Anesthesiology. 2015;15:1, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Shanmugalingam T, Crawley D, Bosco C, Melvin J, Rohrmann S, Chowdhury S, Holmberg L, Van Hemelrijck M. Obesity and cancer: the role of vitamin D. BMC Cancer. 2014;14:1, 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Yao Y, Zhu L, He L, Duan Y, Liang W, Nie Z, Jin Y, Wu X, Fang Y. A meta-analysis of the relationship between vitamin D deficiency and obesity. Int J Clin Exp Med. 2015;8(9):14977–84. [PMC free article] [PubMed] [Google Scholar]
- 194. Liu T, Zhong S, Liu L, Liu S, Li X, Zhou T, Zhang J. Vitamin D deficiency and the risk of anemia: a meta-analysis of observational studies. Ren Fail. 2015;37(6):929–34. [DOI] [PubMed] [Google Scholar]
- 195. Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F. Association between inflammatory bowel disease and vitamin D deficiency: a systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21(11):2708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lu C, Yang J, Yu W, Li D, Xiang Z, Lin Y, Yu C. Association between 25(OH)D level, ultraviolet exposure, geographical location, and inflammatory bowel disease activity: a systematic review and meta-analysis. PLoS One. 2015;10(7):e0132036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Wang J, Lv S, Chen G, Gao C, He J, Zhong H, Xu Y. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015;7(4):2485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Stefanic M, Tokic S. Serum 25-hydoxyvitamin D concentrations in relation to Hashimoto's thyroiditis: a systematic review, meta-analysis and meta-regression of observational studies. Eur J Nutr. 2020;59(3):859–72. [DOI] [PubMed] [Google Scholar]
- 199. Xu MY, Cao B, Yin J, Wang DF, Chen KL, Lu QB. Vitamin D and Graves' disease: a meta-analysis update. Nutrients. 2015;7(5):3813–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Annweiler C, Drouet M, Duval GT, Pare PY, Leruez S, Dinomais M, Milea D. Circulating vitamin D concentration and age-related macular degeneration: systematic review and meta-analysis. Maturitas. 2016;88:101–12. [DOI] [PubMed] [Google Scholar]
- 201. Wu W, Weng Y, Guo X, Feng L, Xia H, Jiang Z, Lou J. The association between serum vitamin D levels and age-related macular degeneration: a systematic meta-analytic review. Invest Ophthalmol Vis Sci. 2016;57(4):2168–77. [DOI] [PubMed] [Google Scholar]
- 202. Kim YH, Kim KW, Kim MJ, Sol IS, Yoon SH, Ahn HS, Kim HJ, Sohn MH, Kim KE. Vitamin D levels in allergic rhinitis: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2016;27(6):580–90. [DOI] [PubMed] [Google Scholar]
- 203. Aryan Z, Rezaei N, Camargo CA Jr.. Vitamin D status, aeroallergen sensitization, and allergic rhinitis: a systematic review and meta-analysis. Int Rev Immunol. 2017;36:1, 41–53. [DOI] [PubMed] [Google Scholar]
- 204. Li HB, Tai XH, Sang YH, Jia JP, Xu ZM, Cui XF, Dai S. Association between vitamin D and development of otitis media: a PRISMA-compliant meta-analysis and systematic review. Medicine (Baltimore). 2016;95(40):e4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Tsai TY, Huang YC. Vitamin D deficiency in patients with alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;78(1):207–9. [DOI] [PubMed] [Google Scholar]
- 206. Lee S, Kim BJ, Lee CH, Lee WS. Increased prevalence of vitamin D deficiency in patients with alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2018;32(7):1214–21. [DOI] [PubMed] [Google Scholar]
- 207. Khoshbakht Y, Bidaki R, Salehi-Abargouei A. Vitamin D status and attention deficit hyperactivity disorder: a systematic review and meta-analysis of observational studies. Adv Nutr. 2018;9(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Gao Q, Kou T, Zhuang B, Ren Y, Dong X, Wang Q. The association between vitamin D deficiency and sleep disorders: a systematic review and meta-analysis. Nutrients. 2018;10(10):1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Xu L, Wan X, Huang Z, Zeng F, Wei G, Fang D, Deng W, Li Y. Impact of vitamin D on chronic kidney diseases in non-dialysis patients: a meta-analysis of randomized controlled trials. PLoS One. 2013;8(4):e61387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(16):1730–7. [DOI] [PubMed] [Google Scholar]
- 211. Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF. Vitamin D compounds for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev. 2009;(4):CD005633. [DOI] [PubMed] [Google Scholar]
- 212. Zheng Y, Zhu J, Zhou M, Cui L, Yao W, Liu Y. Meta-analysis of long-term vitamin D supplementation on overall mortality. PLoS One. 2013;8(12):e82109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Avenell A, Mak J, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;2014(4):CD000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Bjelakovic G, Gluud L, Nikolova D, Whitfield K, Krstic G, Wetterslev J, Gluud C. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2(4):307–20. [DOI] [PubMed] [Google Scholar]
- 216. Mann MC, Hobbs AJ, Hemmelgarn BR, Roberts DJ, Ahmed SB, Rabi DM. Effect of oral vitamin D analogs on mortality and cardiovascular outcomes among adults with chronic kidney disease: a meta-analysis. Clin Kidney J. 2015;8(1):41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Xiao L, Xing C, Yang Z, Xu S, Wang M, Du H, Liu K, Huang Z. Vitamin D supplementation for the prevention of childhood acute respiratory infections: a systematic review of randomised controlled trials. Br J Nutr. 2015;114(7):1026–34. [DOI] [PubMed] [Google Scholar]
- 218. Zheng YT, Cui QQ, Hong YM, Yao WG. A meta-analysis of high dose, intermittent vitamin D supplementation among older adults. PLoS One. 2015;10(1):e0115850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Bjelakovic G, Nikolova D, Bjelakovic M, Gluud C. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Putzu A, Belletti A, Cassina T, Clivio S, Monti G, Zangrillo A, Landoni G. Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials. J Crit Care. 2017;38:109–14. [DOI] [PubMed] [Google Scholar]
- 221. Wu HX, Xiong XF, Zhu M, Wei J, Zhuo KQ, Cheng DY. Effects of vitamin D supplementation on the outcomes of patients with pulmonary tuberculosis: a systematic review and meta-analysis. BMC Pulmonary Medicine. 2018;18(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E. Vitamin D supplements and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol. 2019;30(5):733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Wang T, Liu Z, Fu J, Min Z. Meta-analysis of vitamin D supplementation in the treatment of chronic heart failure. Scand Cardiovasc J. 2019;53(3):110–16. [DOI] [PubMed] [Google Scholar]
- 224. Elamin MB, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, Liu H, Lane MA, Mullan RJ, Hazem Aet al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(7):1931–42. [DOI] [PubMed] [Google Scholar]
- 225. Keum N, Giovannucci E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br J Cancer. 2014;111(5):976–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Goulão B, Stewart F, Ford JA, Maclennan G, Avenell A. Cancer and vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2018;107(4):652–63. [DOI] [PubMed] [Google Scholar]
- 227. Buttigliero C, Monagheddu C, Petroni P, Saini A, Dogliotti L, Ciccone G, Berruti A. Prognostic role of vitamin D status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16(9):1215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. De-Regil L, Palacios C, Lombardo L, Peña-Rosas J. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2016;(1):CD008873. [DOI] [PubMed] [Google Scholar]
- 229. Bi WG, Nuyt AM, Weiler H, Leduc L, Santamaria C, Wei SQ. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatrics. 2018;172(7):635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, Newmark HL, Giovannucci E, Wei M, Holick MF. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32(3):210–16. [DOI] [PubMed] [Google Scholar]
- 231. Sperati F, Vici P, Maugeri-Sacca M, Stranges S, Santesso N, Mariani L, Giordano A, Sergi D, Pizzuti L, Di Lauro Let al. Vitamin D supplementation and breast cancer prevention: a systematic review and meta-analysis of randomized clinical trials. PLoS One. 2013;8(7):e69269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Avenell A, Gillespie WJ, Gillespie LD, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev. 2009;(2):CD000227. [DOI] [PubMed] [Google Scholar]
- 233. DIPART (Vitamin D Individual Patient Analysis of Randomized Trials) Group . Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ. 2010;340:b5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, Thoma A, Kiel DP, Henschkowski J. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169(6):551–61. [DOI] [PubMed] [Google Scholar]
- 235. Lai JK, Lucas RM, Clements MS, Roddam AW, Banks E. Hip fracture risk in relation to vitamin D supplementation and serum 25-hydroxyvitamin D levels: a systematic review and meta-analysis of randomised controlled trials and observational studies. BMC Public Health. 2010;10:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Wu Z, Malihi Z, Stewart AW, Lawes CM, Scragg R. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19(7):415–27. [PubMed] [Google Scholar]
- 237. Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152(5):315–23. [DOI] [PubMed] [Google Scholar]
- 238. Mao PJ, Zhang C, Tang L, Xian YQ, Li YS, Wang WD, Zhu XH, Qiu HL, He J, Zhou YH. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol. 2013;169(2):106–11. [DOI] [PubMed] [Google Scholar]
- 239. Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100(3):746–55. [DOI] [PubMed] [Google Scholar]
- 240. Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, Park BJ. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;346:f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. De-Regil LM, Palacios C, Ansary A, Kulier R, Pena-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2012;(2):CD008873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012;26 Suppl 1:(0 1):75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Perez-Lopez FR, Pasupuleti V, Mezones-Holguin E, Benites-Zapata VA, Thota P, Deshpande A, Hernandez AV. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2015;103(5):1278–88. e4. [DOI] [PubMed] [Google Scholar]
- 244. Palacios C, De-Regil LM, Lombardo LK, Pena-Rosas JP. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Khaing W, Vallibhakara SA, Tantrakul V, Vallibhakara O, Rattanasiri S, McEvoy M, Attia J, Thakkinstian A. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9(10):1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Riverin BD, Maguire JL, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLoS One. 2015;10(8):e0136841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Bergman P, Lindh Å U, Björkhem-Bergman L, Lindh JD. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8(6):e65835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Mao S, Huang S. Vitamin D supplementation and risk of respiratory tract infections: a meta-analysis of randomized controlled trials. Scand J Infect Dis. 2013;45(9):696–702. [DOI] [PubMed] [Google Scholar]
- 249. Ahn JG, Lee D, Kim KH. Vitamin D and risk of respiratory tract infections in children: a systematic review and meta-analysis of randomized controlled trials. Pediatr Infect Vaccine. 2016;23(2):109–16. [Google Scholar]
- 250. Gysin DV, Dao D, Gysin CM, Lytvyn L, Loeb M. Effect of vitamin D3 supplementation on respiratory tract infections in healthy individuals: a systematic review and meta-Analysis of randomized controlled trials. PLoS One. 2016;11(9):e0162996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Christensen N, Sondergaard J, Fisker N, Christesen HT. Infant respiratory tract infections or wheeze and maternal vitamin D in pregnancy: a systematic review. Pediatr Infect Dis J. 2017;36(4):384–91. [DOI] [PubMed] [Google Scholar]
- 252. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AAet al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Xia JY, Shi LY, Zhao L, Xu F. Impact of vitamin D supplementation on the outcome of tuberculosis treatment: a systematic review and meta-analysis of randomized controlled trials. Chin Med J. 2014;127(17):3127–34. [PubMed] [Google Scholar]
- 254. Luo J, Liu D, Liu CT. Can vitamin D supplementation in addition to asthma controllers improve clinical outcomes in patients with asthma?: a meta-analysis. Medicine (Baltimore). 2015;94(50):e2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Wang M, Liu M, Wang C, Xiao Y, An T, Zou M, Cheng G. Association between vitamin D status and asthma control: a meta-analysis of randomized trials. Respir Med. 2019;150:85–94. [DOI] [PubMed] [Google Scholar]
- 256. Vahdaninia M, Mackenzie H, Helps S, Dean T. Prenatal intake of vitamins and allergic outcomes in the offspring: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2017;5(3):771–8. e5. [DOI] [PubMed] [Google Scholar]
- 257. Wolsk H, Chawes B, Litonjua A, Hollis B, Waage J, Stokholm J, Bonnelykke K, Bisgaard H, Weiss S. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: a combined analysis of two randomized controlled trials. PLoS One. 2017;12(10):e0186657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. de Borst MH, Hajhosseiny R, Tamez H, Wenger J, Thadhani R, Goldsmith DJ. Active vitamin D treatment for reduction of residual proteinuria: a systematic review. J Am Soc Nephrol. 2013;24(11):1863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Hujoel PP. Vitamin D and dental caries in controlled clinical trials: systematic review and meta-analysis. Nutr Rev. 2013;71(2):88–97. [DOI] [PubMed] [Google Scholar]
- 260. James E, Dobson R, Kuhle J, Baker D, Giovannoni G, Ramagopalan SV. The effect of vitamin D-related interventions on multiple sclerosis relapses: a meta-analysis. Multiple Sclerosis Journal. 2013;19(12):1571–9. [DOI] [PubMed] [Google Scholar]
- 261. Li J, Chen N, Wang D, Zhang J, Gong X. Efficacy of vitamin D in treatment of inflammatory bowel disease: a meta-analysis. Medicine (Baltimore). 2018;97(46):e12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Wang J, Feng M, Ying S, Zhou J, Li X. Efficacy and safety of vitamin D supplementation for pulmonary tuberculosis: a systematic review and meta-analysis. Iran J Public Health. 2018;47(4):466–72. [PMC free article] [PubMed] [Google Scholar]
- 263. Li G, Mbuagbaw L, Samaan Z, Falavigna M, Zhang S, Adachi JD, Cheng J, Papaioannou A, Thabane L. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99(3):757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and colorectal adenoma risk. Prev Med. 2011;53(1-2):10–16. [DOI] [PubMed] [Google Scholar]
- 265. Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2011;(7):CD007470. [DOI] [PubMed] [Google Scholar]
- 266. Rejnmark L, Avenell A, Masud T, Anderson F, Meyer HE, Sanders KM, Salovaara K, Cooper C, Smith HE, Jacobs ETet al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J Clin Endocrinol Metab. 2012;97(8):2670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Vitamin D concentration, obesity, and risk of diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(4):298–306. [DOI] [PubMed] [Google Scholar]
- 268. Ye Z, Sharp SJ, Burgess S, Scott RA, Imamura F, Langenberg C, Wareham NJ, Forouhi NG. Association between circulating 25-hydroxyvitamin D and incident type 2 diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 2015;3(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Lu L, Bennett DA, Millwood IY, Parish S, McCarthy MI, Mahajan A, Lin X, Bragg F, Guo Y, Holmes MVet al. Association of vitamin D with risk of type 2 diabetes: a Mendelian randomisation study in European and Chinese adults. PLoS Med. 2018;15(5):e1002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Vimaleswaran KS, Cavadino A, Berry DJ, Jorde R, Dieffenbach AK, Lu C, Alves AC, Heerspink HJ, Tikkanen E, Eriksson Jet al. Association of vitamin D status with arterial blood pressure and hypertension risk: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Mokry LE, Ross S, Ahmad OS, Forgetta V, Smith GD, Goltzman D, Leong A, Greenwood CM, Thanassoulis G, Richards JB. Vitamin D and risk of multiple sclerosis: a Mendelian randomization study. PLoS Med. 2015;12(8):e1001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Rhead B, Baarnhielm M, Gianfrancesco M, Mok A, Shao X, Quach H, Shen L, Schaefer C, Link J, Gyllenberg Aet al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurology Genetics. 2016;2(5):e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Gianfrancesco MA, Stridh P, Rhead B, Shao X, Xu E, Graves JS, Chitnis T, Waldman A, Lotze T, Schreiner Tet al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset MS. Neurology. 2017;88(17):1623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Mokry LE, Ross S, Morris JA, Manousaki D, Forgetta V, Richards JB. Genetically decreased vitamin D and risk of Alzheimer disease. Neurology. 2016;87(24):2567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Larsson SC, Traylor M, Malik R, Dichgans M, Burgess S, Markus HS. Modifiable pathways in Alzheimer's disease: Mendelian randomisation analysis. BMJ. 2017;359:j5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Larsson SC, Traylor M, Markus HS, Michaëlsson K. Serum parathyroid hormone, 25-hydroxyvitamin D, and risk of Alzheimer's disease: a Mendelian randomization study. Nutrients. 2018;10(9):1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Trummer O, Pilz S, Hoffmann MM, Winkelmann BR, Boehm BO, März W, Pieber TR, Obermayer-Pietsch B, Renner W. Vitamin D and mortality: a Mendelian randomization study. Clin Chem. 2013;59(5):793–7. [DOI] [PubMed] [Google Scholar]
- 278. Afzal S, Brøndum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ. 2014;349:g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Aspelund T, Grübler MR, Smith AV, Gudmundsson EF, Keppel M, Cotch MF, Harris TB, Jorde R, Grimnes G, Joakimsen Ret al. Effect of genetically low 25-hydroxyvitamin D on mortality risk: Mendelian randomization analysis in 3 large European cohorts. Nutrients. 2019;11(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Chandler PD, Tobias DK, Wang L, Smith-Warner SA, Chasman DI, Rose L, Giovannucci EL, Buring JE, Ridker PM, Cook NRet al. Association between vitamin D genetic risk score and cancer risk in a large cohort of US. Nutrients. 2018;10(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Ong JS, Gharahkhani P, An J, Law MH, Whiteman DC, Neale RE, MacGregor S. Vitamin D and overall cancer risk and cancer mortality: a Mendelian randomization study. Hum Mol Genet. 2018;27(24):4315–22. [DOI] [PubMed] [Google Scholar]
- 282. Trummer O, Langsenlehner U, Krenn-Pilko S, Pieber TR, Obermayer-Pietsch B, Gerger A, Renner W, Langsenlehner T. Vitamin D and prostate cancer prognosis: a Mendelian randomization study. World J Urol. 2016;34(4):607–11. [DOI] [PubMed] [Google Scholar]
- 283. Ong JS, Cuellar-Partida G, Lu Y, Fasching PA, Hein A, Burghaus S, Beckmann MW, Lambrechts D, Van Nieuwenhuysen E, Vergote Iet al. Association of vitamin D levels and risk of ovarian cancer: a Mendelian randomization study. Int J Epidemiol. 2016;45(5):1619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Dimitrakopoulou VI, Tsilidis KK, Haycock PC, Dimou NL, Al-Dabhani K, Martin RM, Lewis SJ, Gunter MJ, Mondul A, Shui IMet al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ. 2017;359:j4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Theodoratou E, Palmer T, Zgaga L, Farrington SM, McKeigue P, Din FV, Tenesa A, Davey-Smith G, Dunlop MG, Campbell H. Instrumental variable estimation of the causal effect of plasma 25-hydroxy-vitamin D on colorectal cancer risk: a Mendelian randomization analysis. PLoS One. 2012;7(6):e37662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. He Y, Timofeeva M, Farrington SM, Vaughan-Shaw P, Svinti V, Walker M, Zgaga L, Meng X, Li X, Spiliopoulou Aet al. Exploring causality in the association between circulating 25-hydroxyvitamin D and colorectal cancer risk: a large Mendelian randomisation study. BMC Medicine. 2018;16(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Dong J, Gharahkhani P, Chow WH, Gammon MD, Liu G, Caldas C, Wu AH, Ye W, Onstad L, Anderson LAet al. No association between vitamin D status and risk of barrett's esophagus or esophageal adenocarcinoma: a Mendelian randomization study. Clin Gastroenterol Hepatol. 2019;17(11):2227–35. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Dudding TA-OX, Johansson M, Thomas SJ, Brennan P, Martin RM, Timpson NJ. Assessing the causal association between 25-hydroxyvitamin D and the risk of oral and oropharyngeal cancer using Mendelian randomization. Int J Cancer. 2018;143(5):1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Wang S, Huo D, Kupfer S, Alleyne D, Ogundiran TO, Ojengbede O, Zheng WA-OX, Nathanson KL, Nemesure B, Ambs Set al. Genetic variation in the vitamin D related pathway and breast cancer risk in women of African ancestry in the root consortium. Int J Cancer. 2018;142(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Jiang X, Dimou NL, Al-Dabhani K, Lewis SJ, Martin RM, Haycock PC, Gunter MJ, Key TJ, Eeles RA, Muir Ket al. Circulating vitamin D concentrations and risk of breast and prostate cancer: a Mendelian randomization study. Int J Epidemiol. 2019;48(5):1416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Sun YQ, Brumpton BM, Bonilla C, Lewis SJ, Burgess S, Skorpen F, Chen Y, Nilsen TIL, Romundstad PR, Mai XM. Serum 25-hydroxyvitamin D levels and risk of lung cancer and histologic types: a Mendelian randomisation analysis of the HUNT study. Eur Respir J. 2018;51(6):1800329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Winsløw UC, Nordestgaard BG, Afzal S. High plasma 25-hydroxyvitamin D and high risk of nonmelanoma skin cancer: a Mendelian randomization study of 97 849 individuals. Br J Dermatol. 2018;178(6):1388–95. [DOI] [PubMed] [Google Scholar]
- 293. Takahashi H, AJA-Ohoo C, AA-Ohoo S, PJA-Ohoo L, BA-Ohoo K, Ostrom QT, KA-Ohoo L, Eckel-Passow JE, Armstrong GN, Claus EBet al. Mendelian randomisation study of the relationship between vitamin D and risk of glioma. Sci Rep. 2018;8(1):2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Dudding T, Thomas SJ, Duncan K, Lawlor DA, Timpson NJ. Re-examining the association between vitamin D and childhood caries. PLoS One. 2015;10(12):e0143769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Manousaki D, Paternoster LA-O, Standl MA-O, Moffatt MA-O, Farrall MA-O, Bouzigon EA-O, Strachan DA-O, Demenais F, Lathrop M, Cookson Wet al. Vitamin D levels and susceptibility to asthma, elevated immunoglobulin e levels, and atopic dermatitis: a Mendelian randomization study. PLoS Med. 2017;14(5):e1002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Cuellar-Partida G, Williams KM, Yazar S, Guggenheim JA, Hewitt AW, Williams C, Wang JJ, Kho PF, Saw SM, Cheng CYet al. Genetically low vitamin D concentrations and myopic refractive error: a Mendelian randomization study. Int J Epidemiol. 2017;46(6):1882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Havdahl A, Mitchell R, Paternoster L, Davey Smith G. Investigating causality in the association between vitamin D status and self-reported tiredness. Sci Rep. 2019;9(1):2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Bae SC, Lee YH. Vitamin D level and risk of systemic lupus erythematosus and rheumatoid arthritis: a Mendelian randomization. Clin Rheumatol. 2018;37(9):2415–21. [DOI] [PubMed] [Google Scholar]
- 299. Magnus MC, Miliku K, Bauer A, Engel SM, Felix JF, Jaddoe VWV, Lawlor DA, London SJ, Magnus P, McGinnis Ret al. Vitamin D and risk of pregnancy related hypertensive disorders: Mendelian randomisation study. BMJ. 2018;361:k2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Taylor AE, Burgess S, Ware JJ, Gage SH, Richards JB, Davey Smith G, Munafo MR. Investigating causality in the association between 25(OH)D and schizophrenia. Sci Rep. 2016;6:1, 26496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Bowman K, Jones L, Pilling LC, Delgado J, Kuchel GA, Ferrucci L, Fortinsky RH, Melzer D. Vitamin D levels and risk of delirium: a Mendelian randomization study in the UK Biobank. Neurology. 2019;92(12):e1387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Wang N, Chen C, Zhao L, Chen Y, Han B, Xia F, Cheng J, Li Q, Lu Y. Vitamin D and nonalcoholic fatty liver disease: bi-directional Mendelian randomization analysis. EBioMedicine. 2018;28:187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Lund-Nielsen J, Vedel-Krogh S, Kobylecki CJ, Brynskov J, Afzal S, Nordestgaard BG. Vitamin D and inflammatory bowel disease: Mendelian randomization analyses in the Copenhagen studies and UK Biobank. J Clin Endocrinol Metab. 2018;103(9):3267–77. [DOI] [PubMed] [Google Scholar]
- 304. Michaëlsson K, Melhus H, Larsson SC. Serum 25-hydroxyvitamin D concentrations and major depression: a Mendelian randomization study. Nutrients. 2018;10(12):1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Brondum-Jacobsen P, Benn M, Afzal S, Nordestgaard BG. No evidence that genetically reduced 25-hydroxyvitamin D is associated with increased risk of ischaemic heart disease or myocardial infarction: a Mendelian randomization study. Int J Epidemiol. 2015;44(2):651–61. [DOI] [PubMed] [Google Scholar]
- 306. Larsson SC, Traylor M, Mishra A, Howson JMM, Michaëlsson K, Markus HS. Serum 25-hydroxyvitamin D concentrations and ischemic stroke and its subtypes. Stroke. 2018;49(10):2508–11. [DOI] [PubMed] [Google Scholar]
- 307. Trajanoska K, Morris JA, Oei L, Zheng HF, Evans DM, Kiel DP, Ohlsson C, Richards JB, Rivadeneira F. Assessment of the genetic and clinical determinants of fracture risk: genome wide association and Mendelian randomisation study. BMJ. 2018;362:k3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Hall KT, Buring JE, Mukamal KJ, Vinayaga Moorthy M, Wayne PM, Kaptchuk TJ, Battinelli EM, Ridker PM, Sesso HD, Weinstein SJet al. COMT and alpha-tocopherol effects in cancer prevention: gene-supplement interactions in two randomized clinical trials. J Natl Cancer Inst. 2019;111(7):684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Yang W, Liu L, Masugi Y, Qian ZR, Nishihara R, Keum N, Wu K, Smith-Warner S, Ma Y, Nowak JAet al. Calcium intake and risk of colorectal cancer according to expression status of calcium-sensing receptor (CASR). Gut. 2018;67(8):1475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Crowe FL, Thayakaran R, Gittoes N, Hewison M, Thomas GN, Scragg R, Nirantharakumar K. Non-linear associations of 25-hydroxyvitamin D concentrations with risk of cardiovascular disease and all-cause mortality: results from the health improvement network (THIN) database. J Steroid Biochem Mol Biol. 2019;195:105480. [DOI] [PubMed] [Google Scholar]
- 311. Esteghamati A, Fotouhi A, Faghihi-Kashani S, Hafezi-Nejad N, Heidari B, Sheikhbahaei S, Zandieh A, Nakhjavani M. Non-linear contribution of serum vitamin D to symptomatic diabetic neuropathy: a case-control study. Diabetes Res Clin Pract. 2016;111:44–50. [DOI] [PubMed] [Google Scholar]
- 312. Zittermann A. Vitamin D status, supplementation and cardiovascular disease. Anticancer Res. 2018;38(2):1179–86. [DOI] [PubMed] [Google Scholar]
- 313. Revez JA, Lin T, Qiao Z, Xue A, Holtz Y, Zhu Z, Zeng J, Wang H, Sidorenko J, Kemper KEet al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat Commun. 2020;11(1):1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.