Abstract
Expression of the Escherichia coli OmpC and OmpF outer membrane proteins is regulated by the osmolarity of the culture media. In contrast, expression of OmpC in Salmonella typhi is not influenced by osmolarity, while OmpF is regulated as in E. coli. To better understand the lack of osmoregulation of OmpC expression in S. typhi, we compared the expression of the ompC gene in S. typhi and E. coli, using ompC-lacZ fusions and outer membrane protein (OMP) electrophoretic profiles. S. typhi ompC expression levels in S. typhi were similar at low and high osmolarity along the growth curve, whereas osmoregulation of E. coli ompC in E. coli was observed during the exponential phase. Both genes were highly expressed at high and low osmolarity when present in S. typhi, while expression of both was regulated by osmolarity in E. coli. Complementation experiments with either the S. typhi or E. coli ompB operon in an S. typhi ΔompB strain carrying the ompC-lacZ fusions showed that both S. typhi and E. coli ompC were not regulated by osmolarity when they were under the control of S. typhi ompB. Interestingly, in the same strain, both genes were osmoregulated under E. coli ompB. Surprisingly, in E. coli ΔompB, they were both osmoregulated under S. typhi or E. coli ompB. Thus, the lack of osmoregulation of OmpC expression in S. typhi is determined in part by the ompB operon, as well as by other unknown trans-acting elements present in S. typhi.
Salmonellae are gram-negative enterobacteria that can be pathogenic for both humans and animals. They cause disease ranging from gastroenteritis to typhoid fever, depending on their serotype and on the infected host. In particular, Salmonella typhi is a human-specific pathogen that causes typhoid fever, a systemic febrile illness acquired by ingesting food or water that has been contaminated by human feces (1).
Escherichia coli, as well as other gram-negative bacteria, exhibits a wide variety of adaptive responses to changes in the environment; these include an increase or decrease in the expression of the major outer membrane porin proteins OmpC and OmpF in response to different demands and stresses (i.e., osmolarity, temperature, pH, oxygen tension, and nutrient starvation) (29). In particular, the influence of osmolarity on the regulation of OmpC and OmpF expression has been extensively studied. OmpF is preferentially expressed in media of low osmolarity, whereas OmpC expression is increased in media of high osmolarity. The E. coli ompB locus, which contains two distinct genes, ompR and envZ, regulates OmpF and OmpC expression at the transcriptional level. EnvZ and OmpR belong to the two-component regulatory systems that respond to environmental stimuli. OmpR, a cytoplasmic protein, is the activator that binds to both the ompF and ompC promoters; EnvZ, an inner membrane protein, is thought to sense an environmental signal in order to modulate OmpR function by phosphorylation and dephosphorylation (for reviews, see references 6, 7, 24, and 29).
The S. typhi ompC gene was isolated and characterized in our laboratory (30, 31). In S. typhi, in contrast to E. coli, OmpC has been observed to be expressed at the same level at both low and high osmolarity, whereas the synthesis of OmpF in both bacteria is regulated in similar manners (32). These findings suggest different mechanisms of osmoregulation of gene expression between E. coli and S. typhi.
We have characterized the S. typhi ompR and envZ genes. Amino acid sequence alignment between the S. typhi OmpR and EnvZ proteins and the corresponding E. coli proteins revealed that S. typhi and E. coli OmpR are identical; in contrast, S. typhi EnvZ shows 95% identity with the E. coli EnvZ protein. Interestingly, most of the differences between the EnvZ proteins lie toward the carboxy terminus, mostly between residues 260 and 450, in a region generally regarded as conserved within the histidine kinase protein family (19).
To determine whether the lack of ompC osmoregulation in S. typhi is mediated by particular features of cis- or trans-acting elements, in this work we analyzed the expression of S. typhi and E. coli ompC-lacZ fusions in both E. coli and S. typhi. The studies were performed with wild-type strains as well as with ΔompB strains complemented with either the E. coli or the S. typhi ompB operon. In the same manner, we analyzed the outer membrane protein (OMP) electrophoretic pattern of the complemented S. typhi and E. coli ΔompB. Our observations support the notion of a functionally polymorphic ompB operon with regard to regulation of OmpC expression in response to changes in osmolarity.
MATERIALS AND METHODS
Bacterial strains, plasmids and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were grown overnight at 37°C in Luria-Bertani (LB) broth plus either ampicillin (250 μg/ml), streptomycin (100 μg/ml), tetracycline (20 μg/ml), kanamycin (20 μg/ml), chloramphenicol (40 μg/ml), or rifampin (150 μg/ml), as required. E. coli SY327λpir was used as the transformation recipient of pKNG101 derivatives. To study OMP expression, medium A (containing, per liter, 7 g of nutrient broth, 1 g of yeast extract, 2 g of glycerol, 3.7 g of K2HPO4, and 1.3 g of KH2PO4) was used (15). To test the influence of osmolarity on OMP expression, medium A was prepared with or without 0.3 M NaCl (high or low osmolarity, respectively). All cultures were grown with vigorous shaking (250 rpm) at 37°C.
TABLE 1.
Bacterial strains and plasmids used
| Strains or plasmids | Description | Reference |
|---|---|---|
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flb5301 deoC1 ptsF25 | 2 |
| SG480Δ900 | MC4100 Δ(envZ-malP)900 malP::neo | 8 |
| MH760 | MC4100 ompR472 malQ+ recA | 9 |
| MH1461 | MC4100 envZ11 malQ+ tpo | 9 |
| SY327λpir | F−araD Δ(lac pro) argE(Am) recA56 nalA; contains the prophage λpir, Rifr | 22 |
| S. typhi strains | ||
| IMSS-1 | 9, 12, d, Vi serotype; reference clinical strain | 30 |
| STY81 | IMSS-1; ΔompB Kmr | This study |
| STYC171 | IMSS-1; ΔompC Kmr | This study |
| STYF302 | IMSS-1; ΔompF Kmr | This study |
| Plasmids | ||
| pIM25 and pIM26 | Vector pUC19, carrying the S. typhi ompB operon in both orientations; Ampr | 19 |
| pIM260 | Vector pACYC184, carrying S. typhi ompB; Cmr | This study |
| pIM262 | Vector pBR322, carrying S. typhi ompB; Ampr | This study |
| pAT224 | Vector pBR322, carrying the E. coli ompB operon; Ampr | 25 |
| pIM40 | Vector pACYC184, carrying the E. coli ompB operon; Cmr | This study |
| pVF27 | Vector pBR322, carrying the S. typhi ompC gene; Ampr | 30 |
| pMY111 | Vector pBR322, carrying the E. coli ompC gene; Ampr | 23 |
| pRCV3 | Vector pACYC184, carrying the S. typhi ompF gene; Cmr | This study |
| pBSL46 | Ampr Kmr; used to provide the Kmr cassette | 20 |
| pMC1871 | Vector pBR322, carrying a promotorless E. coli lacZ gene; Tcr | 35 |
| pSCZ10 | S. typhi ompC-lacZ translational fusion in pMC1871 | This study |
| pECZ20 | E. coli ompC-lacZ translational fusion in pMC1871 | This study |
| pKNG101 | oriR6K StprmobRK2 sacB | 14 |
| pKB8 | Vector pKNG101, carrying a Kmr gene flanked by up- and downstream sequences of the ompB operon | This study |
| pKC17 | Vector pKNG101, carrying a Kmr gene flanked by up- and downstream sequences of the ompC gene | This study |
| pKF30 | Vector pKNG101, carrying a Kmr gene flanked by up- and downstream sequences of the ompF gene | This study |
Construction of S. typhi ompB, ompC, and ompF mutants.
The mutagenesis strategy used to replace the omp loci for a kanamycin resistance (Kmr) gene was based on the sucrose counterselection technique, using suicide clones derived from vector pKNG101 (14). To construct suicide clone pKB8 (Table 1), a fragment carrying both an S. typhi ompB upstream fragment (containing 37 nucleotides [nt] of the structural gene and about 350 nt of the 5′ regulatory region) and a downstream fragment (containing 24 nt of the structural gene and about 200 nt of the 3′ downstream region), as well as the pUC19 vector sequence, was amplified by the inverse PCR method (26), using as template pIM26 DNA (19) and synthetic oligonucleotides Ra and Zb, which were designed to generate BamHI restriction sites where the Kmr gene from pBSL46 (20) was cloned. A SacI restriction fragment encompassing the recombination cassette (the Kmr gene flanked by the upstream and downstream sequences to S. typhi ompB) was gel purified; the ends were blunted and ligated into the SmaI site of pKNG101.
Construction of pKC17 (Table 1) was basically as described for pKB8. Inverse PCR was carried out with pVF27 DNA (30) as the template plus synthetic oligonucleotides C1 and C2, which were also designed to generate a BamHI site to clone the Kmr gene. The upstream fragment contained 40 nt of the structural gene and about 1,300 nt of upstream region, whereas the downstream fragment contained 88 nt of the structural gene and about 500 nt of downstream region. An EcoRV fragment containing this recombination cassette was ligated into the SmaI site of plasmid pKNG101.
Construction of pKF30 was also as described for pKB8. Plasmid pRCV3 (Table 1) and synthetic oligonucleotides S32 and S33, which generate BamHI sites, were used to amplify, by inverse PCR, a fragment carrying regions that flanked the ompF gene (the upstream portion carrying 59 nt of the structural gene and 397 nt of 5′ upstream sequence, and the downstream segment carrying 173 nt of the structural gene and 191 nt of the 3′ downstream region). The Kmr gene was subsequently cloned into the BamHI site; from the resultant plasmid, a SalI/XbaI fragment containing the recombination cassette was cloned into pKNG101.
Construction of plasmids carrying the S. typhi or E. coli ompB operon.
Plasmids pIM25 and pIM26 are derivatives of the high-copy-number vector pUC19, carrying the S. typhi ompB operon in both orientations (19). The ompB operon from pIM25 was subcloned, in both orientations, in a SacI site previously introduced between the EcoRI and NruI sites of pBR322 (a medium-copy-number plasmid), thus generating pIM262 and pIM263. The EcoRV/BamHI fragment of pIM26, containing the ompB operon, was subcloned into pACYC184 (a low-copy-number plasmid) between the EcoRV-BamHI and NruI-BamHI sites to obtain both orientations, thus generating pIM260 and pIM261, respectively. To subclone the E. coli ompB operon, the BamHI/SalI fragment of pAT224 (25) was cloned into pACYC184, generating pIM40.
Construction of S. typhi and E. coli ompC-lacZ fusions.
A fragment containing 1,450 bp of the 5′ upstream regulatory region and the first codon of S. typhi ompC was obtained by PCR from plasmid pVF27 (30), using synthetic oligonucleotides SCSm1 and SCSc1, which generated SmaI and ScaI sites, respectively. This DNA fragment was cloned into the unique SmaI site of the pMC1871 translational fusion vector, which contains a promoterless lacZ gene (Pharmacia Biotech Inc., Uppsala, Sweden) (35), generating plasmid pSCZ10 (S. typhi ompC-lacZ) (Table 1). Similarly, a fragment containing 1,150 bp of the E. coli 5′ ompC upstream regulatory region and the first codon was obtained by PCR from plasmid pMY111 (23), using synthetic oligonucleotides ECSm2 and ECSc2, which generated SmaI and ScaI sites, respectively. This DNA fragment was cloned into the SmaI site of pMC181, generating plasmid pECZ20 (E. coli ompC-lacZ).
Preparation of OMPs.
OMPs were prepared essentially as described previously (18). Briefly, 50 ml of medium A, with or without 0.3 M NaCl (high or low osmolarity, respectively), was inoculated with 200 μl of a bacterial cell suspension from an overnight LB culture, prepared in phosphate-buffered saline (pH 7.4), and adjusted to an optical density at 600 nm of 1.8. Cultures were incubated at 37°C in a shaking water bath at 200 rpm, and samples were taken each hour, over a 12-h period for assays of the kinetics of OMP expression. For osmoregulation studies, samples were taken at the fifth hour, where the best osmoregulation profiles were obtained.
SDS-PAGE.
Two different gel systems were used to obtain the best separation of the major OMPs. S. typhi OMP preparations were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), at 30 mA for 6 h, in gels containing 11% acrylamide, 0.12% bisacrylamide, and 0.1% SDS. E. coli OMP preparations were analyzed by urea-SDS-PAGE, at 20 mA for 8 h, in gels prepared with 11% acrylamide, 0.3% bisacrylamide, 8 M urea, and 0.1% SDS. For both systems, the discontinuous buffer system of Laemmli (16) was used. The gels were stained with Coomassie brilliant blue. Under these conditions, S. typhi OmpC migrated ahead of OmpF, a feature that has been previously attributed to the ammonium persulfate concentration or to an excess of salt added to the gel (18). The positions of OmpC and OmpF on the OMP profiles were ascertained by comparing the profiles of OMP preparations from the S. typhi mutant strains STYC171 (ΔompC) and STYF302 (ΔompF) or the E. coli mutant strains MH760 (ompR472 OmpC− OmpF+) and MH1461 (envZ11 OmpC+ OmpF−).
Microplate β-galactosidase assays.
β-Galactosidase activity was measured by the method described by Miller (21), adapted as a microtiter plate assay. Briefly, cell samples were washed twice and resuspended in 1× Z buffer (0.06 M Na2HPO4, 0.04 M NaH2PO4, 0.01 M KCl, 0.001 M MgSO4 [pH 7]). Then 20 μl of cell suspension was lysed by addition of 100 μl of lysis mixture (0.22 mg of lysozyme per ml, 0.22% Triton X-100, 1.6× Z buffer, 0.016 M β-mercaptoethanol) for 10 min with shaking at 37°C. Upon addition of 100 μl of substrate solution (1 mg of o-nitrophenyl-β-d-galactoside per ml), the rate of each reaction was obtained by recording the change in absorbance at 415 nm, every 5 s for 3 min, with a Ceres 900 C scanning autoreader and microplate workstation and KC Jr software (Bio-Tek Instruments, Inc.) set in the kinetics mode.
Microplate protein determinations.
Protein concentrations in cell extracts were determined by the method of Lowry et al. (18a), also adapted as a microtiter plate assay as follows. Twenty microliter of cell suspension was treated with 100 μl of reaction mixture containing 98 μl of a carbonate (3.4% Na2CO3)–hydroxide (0.17 N NaOH) solution and 2 μl of a copper (0.85% CuSO4 · 5H2O)–tartrate (1.7% sodium-potassium tartrate) solution for 10 min at room temperature, with the subsequent addition of 100 μl of 16.9% (vol/vol) Folin-Ciocalteu solution for 15 min at room temperature. Absorbance at 620 nm was obtained with a Ceres 900 C scanning autoreader and microplate workstation and KC Jr software set in the endpoint mode.
Recombinant DNA techniques.
All DNA manipulations were performed according to standard protocols (34). Oligonucleotides used for amplification by PCR were provided by the Oligonucleotide Synthesis Facility at our institute. PCRs were performed by using AmpliTaq (Perkin-Elmer) according to the manufacturer’s instructions. Restriction and modification enzymes were used as instructed by the manufacturer (Boehringer Mannheim, New England Biolabs, or Gibco BRL).
RESULTS
Levels of ompC expression in S. typhi are similar at low and high osmolarity along the growth curve.
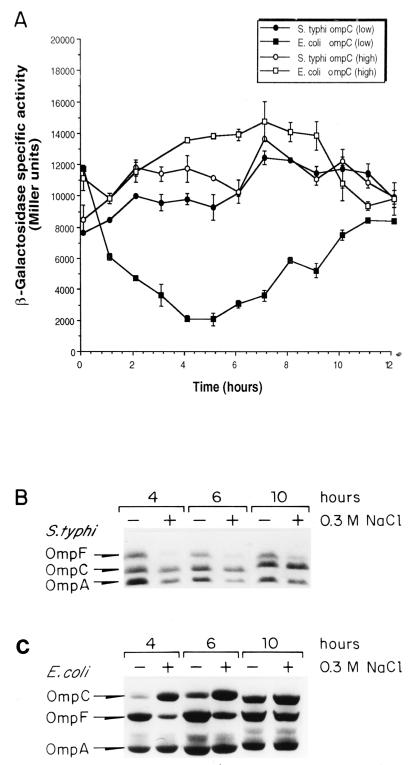
Previous analysis of OMP electrophoretic patterns showed that while OmpF is osmoregulated in S. typhi as it is in E. coli, S. typhi OmpC is highly expressed at both low and high osmolarity (32). However, it had not been tested whether S. typhi ompC expression varied according to the growth phase and how it compared with the kinetics of ompC expression in E. coli. Thus, we compared the expression levels of S. typhi ompC-lacZ and E. coli ompC-lacZ in S. typhi and E. coli wild-type strains, respectively. β-Galactosidase activity was measured from samples obtained throughout the growth curve (exponential to stationary phase) of E. coli MC4100/pECZ20 (plasmid carrying the E. coli ompC-lacZ fusion) and S. typhi IMSS-1/pSCZ10 (plasmid carrying the S. typhi ompC-lacZ fusion) cultures grown in low and high osmolarity (Fig. 1A). S. typhi ompC expression levels were similar at low and high osmolarity throughout the growth curve, whereas E. coli ompC expression was reduced at low osmolarity during the exponential phase, mainly at 4 to 6 h of growth.
FIG. 1.
Kinetics of E. coli or S. typhi ompC and ompF expression throughout the growth curve. (A) S. typhi IMSS-1 carrying plasmid pSCZ10 (S. typhi ompC-lacZ fusion; circles) and E. coli MC4100 carrying plasmid pECZ20 (E. coli ompC-lacZ fusion; squares) were grown in medium A with (open symbols) or without (closed symbols) 0.3 M NaCl (high or low osmolarity, respectively). β-Galactosidase activity was measured from samples taken hourly from duplicate cultures. The data represent the average of three different experiments. (B) S. typhi IMSS-1 and E. coli MC4100 were grown at 37°C in medium A with (+) or without (−) 0.3 M NaCl (high or low osmolarity, respectively); OMPs were purified from samples taken hourly and subjected to SDS-PAGE for S. typhi or to urea-SDS-PAGE for E. coli. Strains carrying deletions in either ompC or ompF were used to ascertain the positions of both porins (see Materials and Methods and Fig. 3). Data for OMPs at 4, 6, and 10 h are shown.
This behavior was also observed when we compared the OMP electrophoretic patterns of S. typhi IMSS-1 and E. coli MC4100 grown in low and high osmolarity (Fig. 1B and C). In S. typhi, OmpC expression was not affected by osmolarity along the different stages of growth (Fig. 1B), as described previously (32). As observed above, E. coli OmpC osmoregulation was most evident during the logarithmic phase (Fig. 1C).
Osmoregulation of S. typhi and E. coli ompC is determined by strain background.
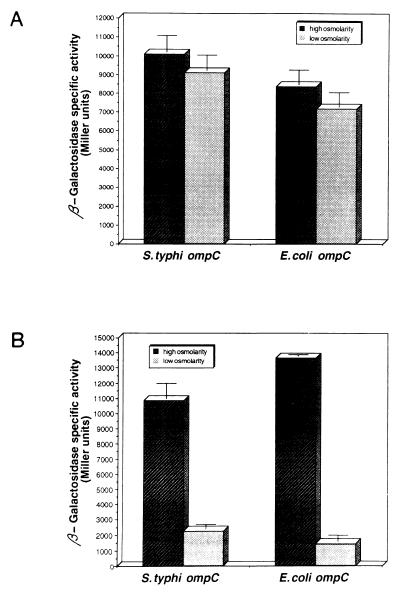
The 5′ upstream regulatory regions of the S. typhi and E. coli ompC genes are slightly different (31). To investigate if these differences in cis-acting elements allow S. typhi to express ompC regardless of medium osmolarity, plasmids pSCZ10 (S. typhi ompC-lacZ) and pECZ20 (E. coli ompC-lacZ) (Table 1) were transformed into either S. typhi IMSS-1 or E. coli MC4100 and grown at either low or high osmolarity. β-Galactosidase activities of transformed cells were measured from samples collected along the growth curve. As shown in Fig. 2, the expression of S. typhi ompC was regulated by osmolarity in E. coli, whereas the expression of E. coli ompC was independent of medium osmolarity in S. typhi. These results indicated that the differences in the regulatory region did not alter the response to osmolarity in E. coli, nor did they mediate the osmolarity-independent expression in S. typhi. Instead, the expression of ompC in response to osmolarity was determined by the strain background.
FIG. 2.
Expression of S. typhi and E. coli ompC-lacZ fusions in a heterologous background. S. typhi IMSS-1 (A) and E. coli MC4100 (B) strains carrying either the S. typhi or the E. coli ompC-lacZ fusion were grown in medium A with (high osmolarity) or without (low osmolarity) 0.3 M NaCl. β-Galactosidase activity was measured from samples taken hourly from duplicate cultures. The data show the activity attained after 5 h of growth, a point where osmoregulation was most apparent for both the E. coli OmpC and OmpF porins, and for S. typhi OmpF, as shown in Fig. 1, and represent the average of three different experiments.
Characterization of S. typhi ompB, ompC, and ompF mutants.
To corroborate the role of the ompB operon in OMP synthesis in S. typhi, a specific mutation was constructed in this locus by replacing this operon with a Kmr cassette, using plasmid pKB8 (Table 1; see Materials and Methods). Mutations in either the ompC or ompF gene were also constructed by using plasmids pKC17 and pKF30 (Table 1; see Materials and Methods). The resultant S. typhi mutant strains were named STY81 (ΔompB), STYC171 (ΔompC), and STYF302 (ΔompF) (Table 1). The disruption of each gene was confirmed by Southern blot hybridization with the appropriate omp probe and the Kmr gene (data not shown).
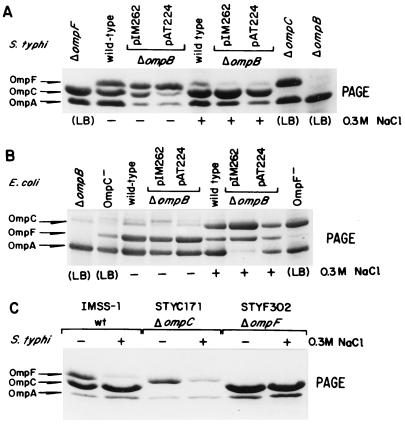
Deletion of the ompB operon abrogated the expression of OmpC and OmpF (Fig. 3A, last lane). Deletion of ompC or ompF generated phenotypes OmpC− OmpF+ and OmpC+ OmpF−, respectively, where each porin was still expressed as in the wild type (Fig. 3A and C). The electrophoretic patterns of these strains were used for confirming the identity of each porin.
FIG. 3.
OMP electrophoretic patterns from S. typhi and E. coli ΔompB strains complemented with either the S. typhi or E. coli ompB operon. Cells were grown in medium A with (+) or without (−) 0.3 M NaCl (high or low osmolarity, respectively) or in LB. OMPs were purified from culture samples obtained after 5 h of growth at 37°C and then subjected to SDS-PAGE (S. typhi) or urea-SDS-PAGE (E. coli) as described in Materials and Methods. Positions of the S. typhi and E. coli OMPs are shown on the left and correspond to the following strains: (A) S. typhi STYF302 (ΔompF), IMSS-1 (wild type), STY81 (ΔompB)/pIM262 (pBR322 carrying S. typhi ompB), STY81 (ΔompB)/pAT224 (pBR322 carrying E. coli ompB), STYC171 (ΔompC), and STY81 (ΔompB); (B) E. coli SG480Δ900 (ΔompB), MH760 (ompR472 OmpC− OmpF+), MC4100 (wild type), SG480Δ900 (ΔompB)/pIM262, SG480Δ900 (ΔompB)/pAT224 and MH1461 (envZ11 OmpC+ OmpF−). (C) OMP profile of control strains used to ascertain the electrophoretic mobilities of OmpC and OmpF: S. typhi IMSS-1 (wild type [wt]), STYC171 (ΔompC), and STYF302 (ΔompF).
The lack of S. typhi ompC osmoregulation was determined both by the S. typhi ompB operon and by another unknown factor(s) present in S. typhi.
To explore the possibility that the sequence differences found between the S. typhi and E. coli ompB operons determined the differences in behavior of OmpC expression in S. typhi (19), the S. typhi STY81 and E. coli SG480Δ900 ompB deletion mutant strains, which fail to produce both OmpC and OmpF, were complemented with either the S. typhi or E. coli ompB operon. Figures 3A and B show the OMP electrophoretic profiles of these strains complemented with one of plasmids pIM262 and pAT224 (pBR322 derivatives carrying the S. typhi and E. coli ompB operons, respectively [Table 1]).
The OMP profile of S. typhi STY81 complemented with S. typhi ompB (pIM262) showed that the proportion of OmpC and OmpF was similar to that of the wild type in both low and high osmolarity (Fig. 3A). Interestingly, when STY81 was transformed with E. coli ompB (pAT224), S. typhi OmpC expression was osmoregulated; that is, its expression with respect to OmpF was reduced in low osmolarity and increased at high osmolarity, as has been described for E. coli (Fig. 3A).
The reciprocal experiments with E. coli SG480Δ900 (ΔompB) were carried out. When complemented with pAT224 (E. coli ompB), this strain showed a wild-type OMP profile in both low and high osmolarity (Fig. 3B). Surprisingly, when complemented with pIM262 (S. typhi ompB), the OMP pattern showed that OmpC expression was still repressed in low osmolarity and induced in high osmolarity (Fig. 3B). Thus, E. coli ompC was osmoregulated even when under the control of the S. typhi ompB operon, as long as it was in E. coli. A quantitative densitometric analysis of the gel bands present in Fig. 3A and B further supported the observations described above (data not shown). Furthermore, the same effects were seen with other constructs, regardless of plasmid copy number (data not shown).
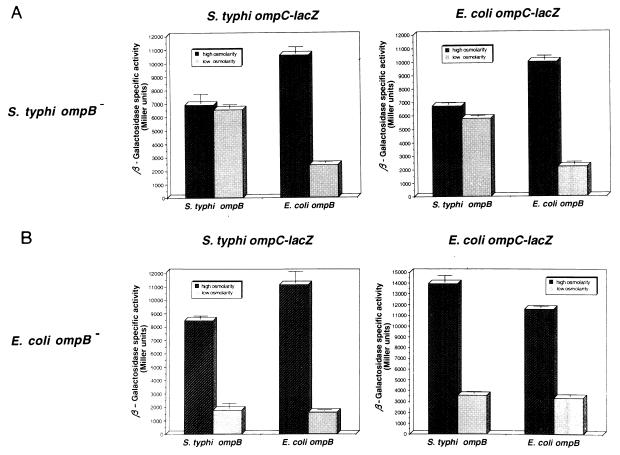
These observations were further confirmed and quantified by measuring the activity of S. typhi ompC-lacZ and E. coli ompC-lacZ fusions (plasmids pSCZ10 and pECZ20, respectively) carried by S. typhi and E. coli ΔompB strains, complemented either with pIM261 (pACYC184 carrying the S. typhi ompB [Table 1]) or with pIM40 (pACYC184 carrying E. coli ompB [Table 1]). Complemented strains were grown in medium A with or without 0.3 M NaCl (high or low osmolarity, respectively), and β-galactosidase activity was measured throughout the growth curve.
Expression of S. typhi ompC-lacZ or E. coli ompC-lacZ in S. typhi ΔompB showed that expression of S. typhi or E. coli ompC was regulated by osmolarity when the strain was complemented with the E. coli ompB operon but was independent of osmolarity when the strain was complemented with the S. typhi ompB operon (Fig. 4A). In contrast, analysis of the expression of S. typhi ompC-lacZ or E. coli ompC-lacZ in E. coli ΔompB showed that expression of S. typhi and E. coli ompC was regulated by osmolarity when the strain was complemented with E. coli ompB or even with the S. typhi ompB operon (Fig. 4B).
FIG. 4.
Expression of S. typhi and E. coli ompC-lacZ fusions in ΔompB mutant strains complemented with a homologous or heterologous ompB operon. Expression of S. typhi ompC-lacZ and E. coli ompC-lacZ fusions in S. typhi ΔompB and E. coli ΔompB strains complemented with the S. typhi ompB (pIM261) or E. coli ompB (pIM40) operon was measured after growth in medium A with or without 0.3 M NaCl (high or low osmolarity, respectively). β-Galactosidase activity was assayed from samples taken hourly from duplicate cultures. The data show the activity after 5 h of growth and represent the average of two different experiments.
All of these results lead to the notion that the EnvZ and OmpR regulators coded by the S. typhi ompB operon, in conjunction with one or more components present in S. typhi, determine the high levels of ompC expression at high and low osmolarity.
DISCUSSION
The amount of the OmpC and OmpF proteins in the outer membrane of E. coli varies depending on the osmolarity of the culture media; however, it is considered that the total amount of these two proteins remains constant, since their relative levels fluctuate in a reciprocal manner. An increase in osmolarity results in a decrease in the amount of the OmpF protein, with a concomitant increase in the amount of the OmpC protein (6, 7). Interestingly, in S. typhi, OmpC is highly expressed independent of medium osmolarity, whereas OmpF is osmoregulated as it is in E. coli (32). In this work, we have shown that S. typhi OmpC is synthesized similarly at low and high osmolarity throughout the growth curve (Fig. 1), while the osmoregulation of OmpC expression in E. coli is observed mainly during the logarithmic phase.
We have also shown that the S. typhi and E. coli ompC genes are highly expressed at both low and high osmolarity in S. typhi but are osmoregulated in E. coli. We have observed in S. typhi a slight decrease in the activity of ompC-lacZ fusions under low osmolarity (Fig. 1A and 2), but this does not account for a noticeable reduction of protein incorporated in the outer membrane, as clearly happens in E. coli (Fig. 1B). In other words, in S. typhi, OmpC is always more abundant than OmpF, regardless of the growth conditions. This has also been observed by others, even though ompC expression in S. typhi was found to be slightly influenced by medium osmolarity and oxygen availability (3).
We have also demonstrated that expression of OmpC and OmpF porins is abolished in our S. typhi ΔompB strain (Fig. 3A), as has been also shown for an ΔompR derivative of S. typhi Ty2 (28). Therefore, S. typhi OmpC and OmpF are regulated by the OmpR and EnvZ proteins, as the E. coli major porins (9, 10). On the other hand, deletion of either ompC or ompF had no effect on expression of the gene coding for the other major porin: osmoregulation of OmpF synthesis was independent of OmpC expression; likewise, OmpC was still highly expressed in a ΔompF background (Fig. 3C).
Moreover, our analysis of the expression of S. typhi and E. coli ompC-lacZ fusions (Fig. 4), in cross-complementation experiments with either the S. typhi or E. coli ompB operons in either the S. typhi or E. coli ΔompB background showed that both S. typhi and E. coli ompC are not regulated by osmolarity when they are under the control of S. typhi ompB in an S. typhi background. Interestingly, in this background, both genes are osmoregulated under E. coli ompB. In contrast, in E. coli they are both osmoregulated under E. coli ompB and, surprisingly, are also osmoregulated by S. typhi ompB (Fig. 4). Furthermore, similar results were observed with OMP electrophoretic patterns from S. typhi and E. coli ΔompB strains complemented with either the S. typhi or E. coli ompB operon (Fig. 3A and B).
Thus, there appear to be unknown factors in S. typhi that, together with the EnvZ and OmpR regulatory proteins, determine the particular behavior of OmpC expression. The alternative of having a factor present in E. coli, but absent in S. typhi, that allows osmoregulation of ompC is not supported by our observations, since both S. typhi ompC and E. coli ompC were osmoregulated in S. typhi under E. coli ompB (Fig. 4). However, we cannot discount more complex models, such as one in which the unknown factor(s) is present both in S. typhi and E. coli but acts somewhat differently in the two species.
In particular, it is tempting to speculate that differences between sequences in the EnvZ proteins of S. typhi and E. coli may account for the different levels of ompC expression, by mediating different molecular interactions. As mentioned above, comparison of OmpR and EnvZ protein sequences from E. coli and S. typhi has shown that the OmpR regulatory proteins are identical in S. typhi and in E. coli, while the EnvZ sensor protein differs in 21 of 450 amino acid residues between the two bacteria (19). The EnvZ protein belongs to the family of histidine kinase proteins, which is defined by regions of conserved sequences generally located near the C terminus (Fig. 5) (37). The whole C terminus of the sensor protein is the transmitter module, acting as a kinase/phosphatase upon the N-terminal receiver module of the OmpR regulator protein (12). It is thus remarkable that 18 of 21 differences between the E. coli and S. typhi EnvZ proteins lie toward the carboxy terminus, between residues 260 and 450 (19) (Fig. 5). In this context, analyses of chimeric proteins and site-directed mutants will be required in order to characterize the molecular features in EnvZ that confer its particular behavior in S. typhi.
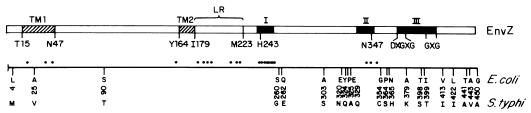
FIG. 5.
Schematic representation of the EnvZ protein. The sequences corresponding to conserved regions in histidine kinase proteins (I, II, and III) (37) are indicated by solid boxes. Region I contains the conserved histidine residue (H243) (11, 36), region II contains the conserved asparagine (N347) (5), and region III has a conserved glycine-rich segment. This last region is represented by five residues that are especially conserved (DXGXG…GXG). Hydrophobic putative membrane-spanning sequences (TM1 and TM2) are indicated by hatched boxes. The LR (putative linker) region has been suggested to modulate the activity of EnvZ (27). Mutants in the envZ gene which produced an amino acid substitution in the EnvZ protein that provided insight into its function as an osmolarity sensor are indicated (black circles) (12, 13). Other residues that have been implicated in the overall mechanism of osmoregulation by EnvZ are also shown. Spontaneous mutants have been found clustered in regions LR, I, and III (references 7 and 11 and references therein). Below the schematic representation of EnvZ domains, amino acid differences between E. coli and S. typhi EnvZ proteins are shown; the numbers represent amino acid positions.
Moreover, it is thought that the concentration of OmpR-phosphate modulates the reciprocal regulation of porin gene expression in E. coli in response to osmolarity (24, 29, 33); however, it is uncertain if different OmpR-phosphate levels play a role in the osmolarity-independent expression of ompC in S. typhi. In particular, since S. typhi OmpF expression is osmoregulated as in E. coli, one could envision that the OmpR-phosphate levels indeed change according to osmolarity. Furthermore, the observation that the S. typhi ompB operon is able to correctly osmoregulate porin synthesis in an E. coli background also suggests that S. typhi EnvZ can modulate the phosphorylation of OmpR in response to osmolarity.
These observations have evidenced differences between S. typhi and E. coli that could play a role in bacterial physiology, possibly by having an effect on how the bacteria survive in the environment or during pathogenesis. However, we do not know whether the dissimilar levels of ompC expression can affect bacterial virulence or any specific physiological function. It is interesting that the ompB operon has been involved in bacterial virulence, which reflects the pleiotropic role of this regulatory system in the physiology of Salmonella (4, 17, 28). Another functional polymorphism has been found in Salmonella. The spvR genes of S. dublin and S. typhimurium determine the different regulation patterns of SpvA; moreover, the two spvR genes have relatively few differences in their nucleotide sequences (38). These findings raise the interesting question of whether the allelic differences in genes, and subtle differences in the regulatory mechanisms, play a role in the host spectrum or the pathogenesis of salmonellosis. Our interest in resolving this question has led us to probe structure-function relationships in EnvZ.
ACKNOWLEDGMENTS
We thank Eugenio López-Bustos for help with the densitometric analysis.
This research was supported by grants from the Universidad Nacional Autónoma de México (DGAPA IN206594 to E.C. and J.L.P.; PADEP 30382, 30503, and 30530 to I.M.-F.), by grants from the Consejo Nacional de Ciencia y Tecnología, México (CONACyT 3466-N to E.C.), and by an International Research Scholar Award (75191-527102 to E.C.) from the Howard Hughes Medical Institute, Chevy Chase Md. I.M.-F. was supported by a Ph.D. fellowship (90278) from the CONACyT.
REFERENCES
- 1.Calva E, Puente J L, Calva J J. Research opportunities in typhoid fever: epidemiology and molecular biology. BioEssays. 1988;9:173–177. doi: 10.1002/bies.950090509. [DOI] [PubMed] [Google Scholar]
- 2.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 3.Contreras I, Muñoz L, Toro C S, Mora G C. Heterologous expression of Escherichia coli porin genes in Salmonella typhi Ty2: regulation by medium osmolarity, temperature and oxygen availability. FEMS Microbiol Lett. 1995;133:105–111. doi: 10.1111/j.1574-6968.1995.tb07869.x. [DOI] [PubMed] [Google Scholar]
- 4.Dorman C J, Chatfield S, Higgins C F, Hayward C, Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect Immun. 1989;57:2136–2140. doi: 10.1128/iai.57.7.2136-2140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutta R, Inouye M. Reverse phosphotransfer from OmpR to EnvZ in a kinase−/phosphatase+ mutant of EnvZ (EnvZ-N347D), a bifunctional signal transducer of Escherichia coli. J Biol Chem. 1996;271:1424–1429. doi: 10.1074/jbc.271.3.1424. [DOI] [PubMed] [Google Scholar]
- 6.Forst S, Inouye M. Environmentally regulated gene expression for membrane proteins in Escherichia coli. Annu Rev Cell Biol. 1988;4:21–42. doi: 10.1146/annurev.cb.04.110188.000321. [DOI] [PubMed] [Google Scholar]
- 7.Forst S A, Roberts D L. Signal transduction by the EnvZ-OmpR phosphotransfer system in bacteria. Res Microbiol. 1994;145:363–373. doi: 10.1016/0923-2508(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 8.Garret S, Taylor R K, Silhavy T J, Berman M L. Isolation and characterization of ΔompB strains of Escherichia coli by a general method based on gene fusions. J Bacteriol. 1985;162:840–844. doi: 10.1128/jb.162.2.840-844.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall M N, Silhavy T J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981;151:1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- 10.Hall M N, Silhavy T J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K-12. J Mol Biol. 1981;146:23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- 11.Hsing W, Silhavy T J. Function of conserved His-243 in phosphatase activity of EnvZ, the sensor for porin osmoregulation in Escherichia coli. J Bacteriol. 1997;179:3729–3735. doi: 10.1128/jb.179.11.3729-3735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igo M M, Silhavy T J. EnvZ, a transmembrane environmental sensor of Escherichia coli K-12 is phosphorylated in vitro. J Bacteriol. 1988;170:5971–5973. doi: 10.1128/jb.170.12.5971-5973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanamaru K, Aiba H, Mizushima S, Mizuno T. Signal transduction and osmoregulation in Escherichia coli. A single amino acid change in the protein kinase, EnvZ, results in loss of its phosphorylation and dephosphorylation abilities with respect to the activator protein, OmpR. J Biol Chem. 1989;264:21633–21637. [PubMed] [Google Scholar]
- 14.Kaniga K, Detor I, Cornelis G R. A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaZ gene of Yersinia enterocolitica. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 15.Kawaji H, Mizuno T, Mizushima S. Influence of molecular size and osmolarity of sugars and dextrans on the synthesis of outer membrane proteins O-8 and O-9 of Escherichia coli K-12. J Bacteriol. 1979;140:843–847. doi: 10.1128/jb.140.3.843-847.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Lindgren S W, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobos S R, Mora G C. Alteration in the electrophoretic mobility of OmpC due to variations in the ammonium persulfate concentration in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Electrophoresis. 1991;12:448–450. doi: 10.1002/elps.1150120615. [DOI] [PubMed] [Google Scholar]
- 18a.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Martínez-Flores I, Bustamante V H, Puente J L, Calva E. Cloning and characterization of the Salmonella typhi ompR and envZ genes. Asia Pac J Mol Biol Biotechnol. 1995;3:135–144. [Google Scholar]
- 20.Mikhail F A, Shokolenko I N, Croughan T P. Improved antibiotic-resistance gene cassettes and omega elements for Escherichia coli vector construction and in vitro deletion/insertion mutagenesis. Gene. 1995;160:63–67. doi: 10.1016/0378-1119(95)00108-i. [DOI] [PubMed] [Google Scholar]
- 21.Miller J H. Experiments in molecular genetics. 1972. pp. 352–355. and 403–404. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]
- 22.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizuno T, Chou M Y, Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. J Biol Chem. 1983;258:6932–6940. [PubMed] [Google Scholar]
- 24.Mizuno T, Mizushima S. Signal transduction and gene regulation through the phosphorylation of two regulatory components: the molecular basis for the osmotic regulation of the porin genes. Mol Microbiol. 1990;4:1077–1082. doi: 10.1111/j.1365-2958.1990.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 25.Mizuno T, Wurtzel E T, Inouye M. Cloning of the regulatory genes (ompR and envZ) for the matrix proteins of the Escherichia coli outer membrane. J Bacteriol. 1982;150:1462–1466. doi: 10.1128/jb.150.3.1462-1466.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochman H, Gerber A S, Hartl D L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park H, Inouye M. Mutational analysis of the linker region of EnvZ, an osmosensor in Escherichia coli. J Bacteriol. 1997;179:4382–4390. doi: 10.1128/jb.179.13.4382-4390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickard D, Li J, Roberts M, Maskell D, Hone D, Levine M, Dougan G, Chatfield S. Characterization of defined ompR mutants of Salmonella typhi: ompR is involved in the regulation of Vi polysaccharide expression. Infect Immun. 1994;62:3984–3993. doi: 10.1128/iai.62.9.3984-3993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pratt L A, Hsing W, Gibson K E, Silhavy T J. From acids to osmZ: multiple factors influence synthesis of the OmpF and OmpC porins in Escherichia coli. Mol Microbiol. 1996;20:911–917. doi: 10.1111/j.1365-2958.1996.tb02532.x. [DOI] [PubMed] [Google Scholar]
- 30.Puente J L, Alvarez-Scherer V, Gosset G, Calva E. Comparative analysis of the Salmonella typhi and Escherichia coli ompC genes. Gene. 1989;83:197–206. doi: 10.1016/0378-1119(89)90105-4. [DOI] [PubMed] [Google Scholar]
- 31.Puente J L, Flores V, Fernández M, Fuchs Y, Calva E. Isolation of an ompC-like outer membrane protein gene from Salmonella typhi. Gene. 1987;61:75–83. doi: 10.1016/0378-1119(87)90366-0. [DOI] [PubMed] [Google Scholar]
- 32.Puente J L, Verdugo-Rodriguez A, Calva E. Expression of Salmonella typhi and Escherichia coli OmpC is influenced differently by medium osmolarity; dependence on Escherichia coli OmpR. Mol Microbiol. 1991;5:1205–1210. doi: 10.1111/j.1365-2958.1991.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 33.Russo F D, Silhavy T J. EnvZ controls the concentration of phosphorylated OmpR to mediate osmoregulation of the porin genes. J Mol Biol. 1991;222:567–580. doi: 10.1016/0022-2836(91)90497-t. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Shapira S K, Chou J, Richaud F V, Casadaban M J. New versatile plasmid vectors of hybrid proteins code by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of β-galactosidase. Gene. 1983;25:71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- 36.Skarphol K, Waukan J, Forst S A. Role of H-243 in the phosphatase activity of EnvZ in Escherichia coli. J Bacteriol. 1997;179:1413–1416. doi: 10.1128/jb.179.4.1413-1416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stock J B, Ninfa A J, Stock A M. Protein phosphorylation and regulation of adaptative responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taira S, Heiskanen P, Hurme R, Heikkila H, Riikonen P, Rhen M. Evidence for functional polymorphism of the spvR gene regulating virulence gene expression in Salmonella. Mol Gen Genet. 1995;246:437–444. doi: 10.1007/BF00290447. [DOI] [PubMed] [Google Scholar]