Abstract
Purpose of review:
Given the ongoing rise in prevalence of autism spectrum disorder (ASD) and the challenges in developing and administering interventions to significantly alleviate ASD symptoms, there is an urgent need to identify modifiable risk factors for ASD. The goal of this review is to systematically evaluate the current evidence for an association between conditions related to maternal metabolic syndrome and risk for ASD in offspring focusing on methodically rigorous studies.
Recent findings:
In recent years, multiple studies explored the association between various conditions related to maternal metabolic syndrome (obesity, hypertension, or diabetes prior to, or with onset during pregnancy) and ASD risk in the offspring.
Summary:
Examining large, sufficiently powered, population-based epidemiological studies that explored the association between maternal metabolic syndrome and ASD, we found consistent evidence for an association between maternal preeclampsia and risk for ASD. Other conditions that are part of maternal metabolic syndrome, including maternal obesity, gestational weight gain, diabetes and gestational diabetes, should be studied further with careful attention paid to potential synergistic effects between different metabolic conditions. These findings highlight the need for rigorous, large, population based epidemiological studies of potentially modifiable ASD risk factors that could inform public health interventions.
Keywords: Autism spectrum disorder, maternal metabolic syndrome, preeclampsia, review, epidemiology
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social communication, and restricted and repetitive patterns of behavior [1]. The prevalence of ASD has been increasing in recent decades and current estimates from the Center for Disease Control (CDC) suggest that 1 in 54 children in the US aged 8 years has ASD [2]. ASD is a leading cause of disability and has a significant public health impact with estimated national costs in the United States between $61–66 billion per year for children and $175–196 billion per year for adults [3,4,5].
The etiology of ASD is thought to be multifactorial and includes a combination of genetic and environmental factors, as well as their interaction [6]. Research confirms a strong causal genetic component, however, monozygotic twin studies report concordance rates less than 100%, suggesting a role for environmental factors as well [22–25,84].
Population-based epidemiological studies have been frequently utilized to explore potential environmental risk factors for ASD. For some risk factors, findings were further refined using systematic reviews and meta-analyses [26–50], and several have been consistently associated with ASD, including advanced maternal and paternal age [26–34,40] and birth complications resulting in fetal hypoxia [32,33,50].
Given the challenges in developing and administering interventions to significantly alleviate ASD symptoms, it is critical to continue exploring modifiable risk factors as a means of primary prevention. Among potential risk factors for ASD, maternal metabolic syndrome consists of several conditions with well-studied preventive and treatment approaches [13]. The metabolic syndrome includes a constellation of pathophysiological states including obesity, insulin resistance, hypertension, and hypercholesterolemia, and predisposes individuals to develop numerous medical conditions including diabetes mellitus (DM) [13,14,17]. According to the CDC, the prevalence of obesity continues to rise and is estimated to be 40% among women aged 20–39 [15]. Further, the incidence of metabolic syndrome has paralleled the incidence of obesity [13]. Human and animal studies have shown that maternal prenatal metabolic syndrome includes increased adiposity and insulin resistance and results in an inflammatory state [18,19] as well as altered leptin signaling [68–72]. These changes have significant impact on fetal neurodevelopment [20] secondary to neuroinflammation and can affect synaptic plasticity, oxidative stress, as well neurotrophic and neuroprotective signaling [14,16,20,21,68–71,73,74–79].
Multiple studies have explored the association of ASD with the various conditions related to maternal metabolic syndrome, including maternal obesity and gestational weight gain (GWG)[56,67, 81,86,89], maternal DM [47,55,58,59,60,61,65,66], gestational DM [55,58,59,60,61,65,66], maternal pregnancy induced hypertension, and preeclampsia [37,53,55,57,61,62,63,64]. However, most studies had methodological limitations including small sample size, inconsistent or unreliable reporting of outcomes (i.e., ASD) or maternal exposure, as well as the use of clinical samples that are prone to ascertainment bias [12]. Several reviews have also discussed the association between specific factors of the metabolic syndrome and its association with ASD [14,16,27,30,35–37,67,94,95].
The goal of this review is to systematically evaluate the evidence for the association of factors related to maternal metabolic syndrome and the risk for ASD. In order to minimize bias of results due to methodological limitations, this review will focus on rigorous studies using large, sufficiently powered, population-based epidemiological samples.
Methods
We conducted a systematic literature search in PubMed from inception until June 2020. Medical Subject Headings (MeSH) and relevant key words were used to conduct the search. Key words included Autism/ASD [AND] maternal metabolic syndrome, Autism/ASD [AND] body mass index/BMI, Autism/ASD [AND] weight gain, Autism/ASD [AND] weight, Autism/ASD [AND] obesity, Autism/ASD [AND] gestational weight gain, Autism/ASD [AND] gestational diabetes, Autism/ASD [AND] diabetes, Autism/ASD [AND] hypertension, Autism/ASD [AND] gestational hypertension, Autism/ASD [AND] preeclampsia. In addition, we reviewed references from original papers and review articles to identify additional relevant studies.
In order to meet criteria for inclusion in this review, studies had to have: (1) a well-defined sample of cases drawn from population-based registers or birth cohorts; (2) a population of interest (i.e., individuals with ASD) sample size greater than 1000 ASD cases; (3) use of standardized diagnostic criteria including either the Diagnostic and Statistical Manual for Mental Disorders (DSM-IV [91] or DSM-5 [1]) or the International Classification of Diseases (ICD-9 [93] or ICD-10 [92]); (4) similarly ascertained exposure and outcomes.
All abstracts were reviewed for relevance and excluded if our inclusion criteria were not met. For example, studies were excluded due to lack of population-based samples, too few cases of ASD (<1000), overlapping cohorts with other included studies, or a focus on exposures outside the scope of this review, such as use of medications or complications during labor and delivery (see Figure 1). Full texts of relevant studies were then reviewed by the authors (JK, AR, and AK) to ensure all eligibility criteria were met and 100 percent consensus was reached. Studies used different measures of relative risk to report on the association between maternal conditions and ASD risk, including Odds-Ratio (OR), Relative-Risk (RR) and Hazard-Ratio (HR). When the outcome is rare (as is ASD), then OR and RR are approximately equal and can readily be interchanged. HR is not always interchangeable because risk factors may operate differently to produce the initial manifestation of a disease and its subsequent presentation. For clarity, we summarized study results in terms of the proportion increase in risk. The specific measures of association used in individual studies are listed in the associated Tables.
Figure 1.
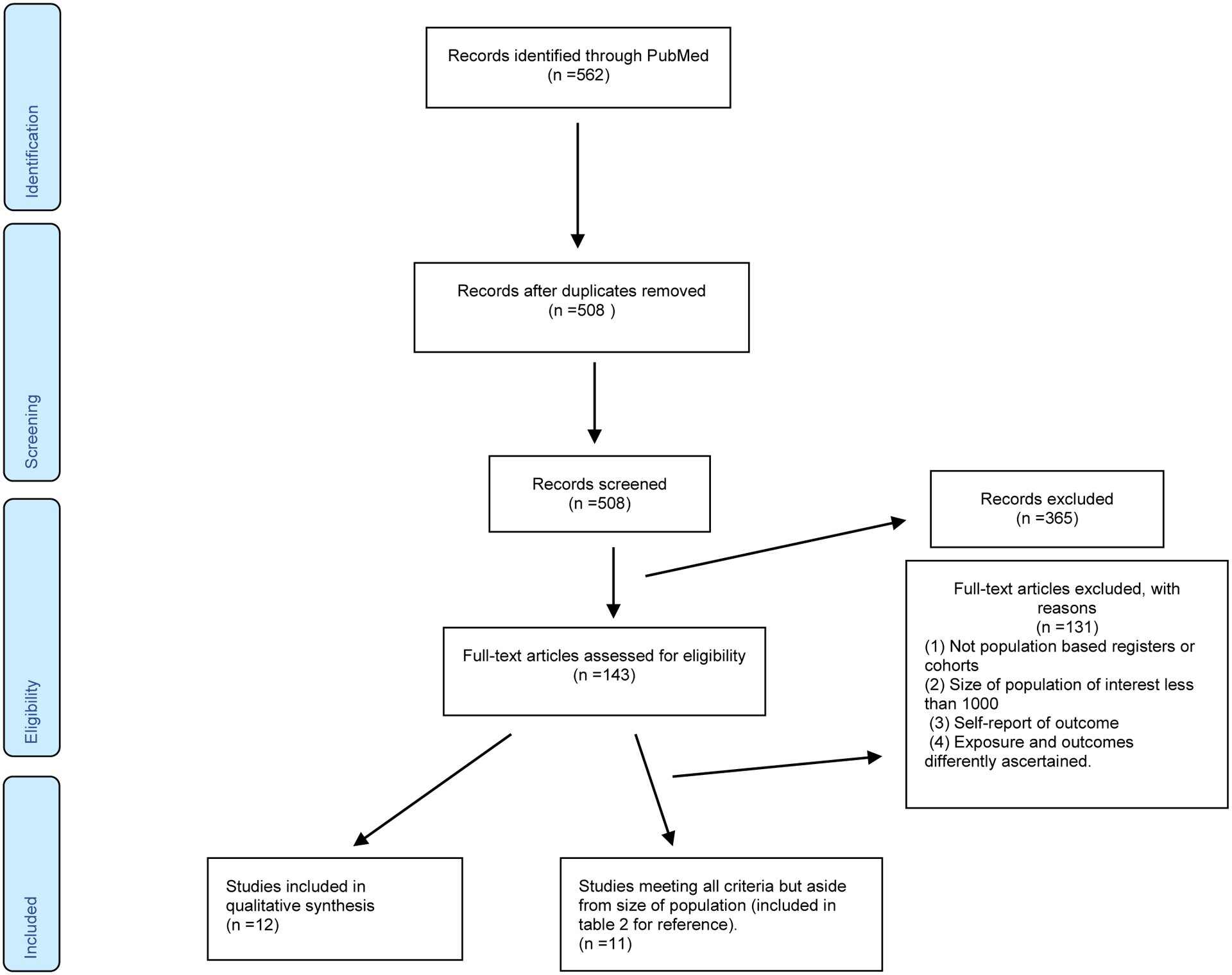
PRISMA flow diagram
Results
A total of 562 publications were found in the initial search. After excluding 54 duplicates and 365 with irrelevant topics, we examined 155 relevant studies. 143 were eliminated after not meeting our inclusion criteria. However, in order to be as thorough as possible and for reference and context, we also summarize results from 11 studies [31,80–83,85–90] that met all of our inclusion criteria except that of sample size (see Table 2). Finally, we identified 12 studies [54–59,61–66] examining the association between aspects of maternal metabolic syndrome and offspring development of ASD (see Figure 1). Study characteristics and summaries of the main findings of individual studies with crude and fully adjusted risk estimates are presented in Table 1 and summarized below. There were three studies from California [58,65,66] with partially overlapping samples (Jo et al., 2019; Xiang et al., 2015 and 2018). All studies were included because they provided additional information relevant to the goals of this review.
Table 2.
Population based studies included in the review
| Author | Publication Date | Study Location | Cohort Size | Study Design | Diagnostic Criteria | Relevant Exposure | Findings |
|---|---|---|---|---|---|---|---|
| Connolly et al. [31] | Aug-16 | Cincinnati Children’s Hospital Medical Center’s 2009–2014 | 503 | case control | ICD9 | pre pregnancy weight/BMI, GDM | Maternal obesity (BMI≥ 30) and GDM were associated with approximately 1.5-fold increased odds of having a child with an ASD. For mothers with both GDM and obesity, the association was twofold. |
| Cordero et al. [80] | Jun-19 | The Study to Explore Early Development (SEED) is a U.S. multisite. California, Colorado, Georgia, Maryland, North Carolina, and Pennsylvania | 698 | case control | ADOS and ADI-R | pre pregnancy DM, GDM and HTN prior and during pregnancy | Hypertension (pre pregnancy and PIH/preeclampsia) was associated with ASD (aOR = 1.69 [95% CI 1.26, 2.26]). Diabetes (pre gestational an GDM clustered) during pregnancy was not associated with ASD (aOR = 1.10 [95% CI 0.77, 1.56]). |
| Dodds et al. [81] | Jul-11 | 1990 and 2002 in Nova Scotia | 924 | cohort | ICD-9 or ICD-10 | pregnancy wt gain, pre pregnancy weight, pre pregnancy HTN, HTN during pregnancy | Univariate: Pregnancy weight gain ≥18 kg RR 1.26 (1.08–1.47), Pregnancy induced hypertension (PIH) RR 1.24 (1.02–1.52), pre pregnancy weight >90kg RR 1.72 (1.39–2.13), pre gestational DM RR1.98 (0.94–4.16), materal weight at delivery >120kg RR 2.18 (1.1–3.16), GDM RR 1.29 (0.9–1.83). Multivariate: Pre-pregnancy weight ≥90 kg RR 1.58 (1.26–1.98), Pregnancy weight gain ≥18 kg RR 1.19 (1.02–1.39), no other data. |
| Getz et al. [82] | Sep-16 | General Practice Research Database from 1993 through 2008 (“about 6% of the population of England, Nortern Ireland, Scotland and Wales” | 889 | case control | E140.00 Infantile autism, *diagnostic source was not reported | maternal pre-pregnancy BMI | ORs for maternal obesity 1.54 [95% CI: 1.26, 1.89] |
| Krakowiak et al. [83] | May-12 | CHARGE (Childhood Autism Risks from Genetics and the Environment) study in California, January 2003 and June 2010 | 517 | case control | ADOS and ADI-R | maternal T2D or GDM in the index pregnancy. Other conditions of interest were hypertension and obesity, defined as BMI >30 with onset before index pregnancy | The association between diabetes (T2DM or GDM) and ASD did not reach statistical significance, the association between hypertension (during pregnancy and before) and ASD was not signinficant. The risk of having a child with ASD to TD, was significantly increased among obese women (ASD, OR: 1.67 [95% CI: 1.10–2.56], |
| Mann et al. [85] | May-10 | South Carolina, 1996 through 2002 | 472 | case control | ICD-9 | Pre-eclampsia/eclampsia | OR 1.69 (1.26–2.28) |
| Shen et al. [86] | Jan-18 | Han Chinese population. | 705 | case control | DSM-IV-TR | maternal pre-pregnancy BMI, GWG | Excessive GWG was associated with autism risk (OR = 1.327, 95% CI: 1.021–1.725) Excessive GWG increased the risk of autism in overweight/obese mothers (OR = 2.468, 95% CI: 1.102–5.526) |
| Surén et al. [87] | May-14 | Norwegian Mother and Child Cohort Study | 419 | case control | DSM-IV-TR | Maternal and paternal height and weight were recorded in a questionnaire completed by the mothers during week 18 of pregnancy | Maternal BMI <18.5: OR 1.39 (0.79–2.46) BMI 25.0–29.9 : 1.29 (0.99–1.69) BMI >30.0: 1.17 (0.81–1.7) |
| Walker et al. [88] | Feb-15 | California January 29, 2003, through April 7, 2011, (CHARGE) study | 517 | case control | ADOS and ADI-R | Preeclampsia | OR 2.36; 95% CI, 1.18–4.68);” |
| Windham et al. [89] | Feb-19 | Study to Explore Early Development (SEED), a multi-site case-control study of children born in 2003–2006. California, Colorado, Georgia, Maryland, North Carolina, and Pennsylvania | 540 | case control | ADOS and ADI-R | Maternal height, weight, and GWG were self-reported | BMI ≥25 & < 30 1.25 (0.94–1.68), BMI>30 OR 1.37 (0.98–1.92). GWG 35–44lb OR 1.52 (1.05–2.22) GWG >44lb OR = 1.58 (1.08–2.31) |
| Xiang et al. [90] | Jun-19 | Kaiser Permanente Southern California (KPSC) hospitals between January 1, 2012, and December 31, 2013 | 707 | cohort | ICD-9 | HbA1c screening in the early prenatal period | HbA1c > 6.5% (n-15) HR 1.79 (1.06–3.00) |
Table 1.
Population based studies not included in the review
| Author | Publication date | study location | ASD cases / Non cases | study design | diagnostic criteria | Relevant exposure | Crude data | Fully adjusted |
|---|---|---|---|---|---|---|---|---|
| Andersen et al. [54] | Feb-18 | Denmark (Danish national birth cohort (DNBC) | 1,116 / 81,892 | population based cohort | ICD-10 | maternal pre-pregnancy BMI self report | ASD+ADHD HR: 30 ≤ BMI < 35: 1.43 (1.06;1.92), BMI ≥ 35: 2.11 (1.43;3.13) |
ASD+ADHD HR: BMI<18.5: 1.09 [0.75–1.58] 30 ≤ BMI < 35: 1.42 [1.05–1.92] BMI ≥ 35: 2.08 [1.40–3.08] |
| Burstyn et al. [55] | Sep-10 | Alberta, Canada,between January 1, 1998, and December 31, 2004. | 1,138/218,890 | population based cohort | ICD-9 | pre pregnancy DM, GDM, preeclampsia | RR Prepregnancy DM 1.65 (1.01–2.71) GDM RR 1.24 (0.94–1.65) preeclampsia RR 1.49 (1–2.23) |
|
| Gardner et al. [56] | Jun-15 | sweden (Stockholm Youth Cohort (SYC), a prospective register-based cohort consisting of all individuals born 1984–2007 and resident in Stockholm County) | 6,420/333,057 | population based cohort | ICD-9, ICD-10, DSM-IV | (BMI) at first antenatal visit, GWG, pre gestatonal HTN, pre gestational DM, GDM and preeclampsia | OR: BMI ≥25 & < 30: 1.34 (1.24 – 1.45) BMI ≥ 30: 2.07 (1.85 – 2.32) |
OR: Pre gestational HTN 1.16 (0.85–1.58) Preeclapsia 1.25 (1.07–1.47) Pre gestationl DM 1.53 (1.07–2.2) Gestational DM 1.49 (1.09–2.07)BMI ≥25 & < 30 1.31 (1.21–1.41) BMI ≥ 30 1.94 (1.72–2.17) ALL mother/child pairs: Insufficient GWG-> 1.17 (1.04 – 1.31) Excessive GWG-> 1.12 (1.01 – 1.25) mothers w/normal baseline BMI: Insufficient GWG-> 1.22 (1.07 – 1.40) excessive GWG-> 1.23 (1.08 – 1.40) |
| Getahun et al. [57] | Feb-17 | Kaiser Permanente Southern California hospitals between 1991 and 2009 | 6,255/594,638 | retrospective cohort study | DSM-IV | preeclampsia (ICD-9-CM codes 642.x) | HR 1.38 (1.23, 1.54) | HR 1.26 (1.13, 1.41) |
| Jo et al. [58] | Dec-19 | 246,420 singleton children born in Kaiser Permanente Southern California hospitals in 1999–2009 | 2,471/246,420 | retrospective cohort study | ICD-9 | maternal diabetes during index pregnancy. GDM exposure was further categorized as diagnosis before or after 24 weeks’ gestation. | GDM <24 weeks’ gestation HR 1.40 (1.08–1.81), Pre-existing type 2 diabetes HR 1.65 (1.26–2.16) |
GDM <24 weeks’ gestation HR 1.24 (0.95–1.62) Pre-existing type 2 diabetes HR 1.45 (1.11–1.91) |
| Kong et al. [59] | Feb-20 | nationwide registries in Finland 2004–2014 | 2,346/649,043 | population-based cohort study | ICD-10 | Maternal pre pregnancy body mass index, insulin-treated pregestational diabetes, pre gestational type 2 diabetes and gestational diabetes without insulin treatment. all date obtained from database (no self report) | HR: No DM: BMI ≥25 & < 30 1.14 (1.02–1.28), BMI ≥30 & < 35 1.29 (1.07–1.56), BMI ≥35 1.21 (0.88–1.66). IDDM: BMI ≥25 & < 30 0.43 (0.11–1.72), BMI ≥30 & < 35 3.61 (1.61–8.07), BMI ≥35 5.93 (2.81–12.52). T2DM: BMI ≥25 & < 30 1.68 (0.75–3.75), BMI ≥30 & < 35 1.14 (0.43–3.05), BMI ≥35 2.28 (1.18–4.41). GDM: BMI ≥25 & < 30 1.28 (1.07–1.53), BMI ≥30 & < 35 1.57 (1.26–1.95), BMI ≥35 1.29 (0.95–1.74) |
|
| Lagridge et al. [61] | Jan-13 | Western Australia (WA) between January 1984 and December 1999 | 1,179/383,153 | case contol? | DSM-IIIR, DSM-IV, DSM-IV-TR | Maternal diabetes during pregnancy (gestational diabetes or pre-existing diabetes), Pregnancy hypertension (preeclampsia or essential hypertension). | univarate*: Maternal pre pregnancy DM and GDM: ASD+ID 1.38 (0.86–2.2), ASD−ID 1.35 (0.74–2.46) pregnancy HTN (preeclampsia or essential) ASD+ID 1.34 (1.07–1.67), ASD−ID 0.81 (0.58–1.74) |
Multivariate*: Maternal pre pregnancy DM and GDM: ASD+ID 1.03 (0.62–1.69), ASD−ID 1.3 (0.69–2.45) pregnancy HTN (preeclampsia or essential) ASD+ID 1.34 (1.05, 1.71), ASD−ID 0.73 (0.5–1.07) |
| Maher et al. [62] | Feb-20 | Sweden from 1982 to 2010 | 54,071/2,842,230 | population-based cohort study | ICD-9 and ICD-10 | preeclampsia | Preeclampsia HR 1.36 (1.31–1.43) | HR Preeclampsia 1.25 (1.19, 1.30) Preeclampsia and SgA 1.66 (1.49, 1.85) Preeclampsia without SGA 1.20 (1.14, 1.26) SGA only 1.60 (1.53, 1.67) |
| Polo-Kantola et al. [63] | Feb-14 | Finland from 1990–2005 | 4,713 /total amount of cases not available | Registry-based case-control |
ICD-9 and ICD-10 | maternal high blood pressure included both pre-eclampsia and pregnancy induced hypertension | OR: childhood autism 1.56 (1.–2.1) PDD 1.36 (1.04–1.8) Asperger syndrome 1.3 (1–1.7) |
OR: childhood autism 1.6 (1.1.–2.2) PDD 1.3 (1–1.7) Asperger syndrome 1.03 (0.8–1.4) |
| Sun et al. [64] | Apr-20 | Norway’s Medical Birth Registry January 1, 1991, through December 31, 2009, and followed up through December 31, 2014 | 3,548/980,560 | prospective, population-based cohort study | ICD-9 and ICD-10 | Preeclampsia | term birth: OR 1.37 (1.15–1.62) pre-term+term: OR 1.37 (1.19–1.59) |
term birth: OR 1.29 (1.08–1.54) pre-term+term: OR 1.31 (1.12–1.52) |
| Xiang et al. [65] | Apr-15 | 1995–2009 at Kaiser Permanente Southern California (KPSC) hospitals. | 3,388/322,323 | Retrospective longitudinal cohort study | ICD-9 | Maternal preexisting type 2 diabetes and GDM | Bivariable: T2DM HR 1.59 (1.29–1.95) GDM HR 1.18 (1.04–1.33) GDM ≤ 26wks HR 1.63 (1.35–1.97) GDM >26 &<30 HR 0.94 (0.76–1.15) GDM >30 HR 1.04 (0.81–1.32) |
Multivariable adjused: T2DM HR 1.3 (1.04–1.62) GDM HR 1.03 (0.9–1.17) GDM ≤ 26wks HR 1.4 (1.14–1.72) GDM>26wks HR 0.86 (0.73–1.02) |
| Xiang et al. [66] | Jul-18 | Kaiser Permanente Southern California (KPSC) hospitals from January 1, 1995, through December 31, 2012 | 5,827/419,425 | Retrospective cohort study | ICD-9 | T1DM, T2DM, GDM | T1DM HR 2.36 (1.36–4.12) T2DM HR 1.45 (1.24–1.7) GDM ≤ 26wks HR 1.3 (1.12–1.51) GDM >26wks HR 0.99 (0.88–1.12) |
T1DM HR 2.33 (1.29–4.21) T2DM HR 1.39 (1.18–1.62) GDM ≤ 26wks HR 1.26 (1.08–1.47) GDM >26wks HR 0.98 (0.87–1.1) |
All studies were published between 2010–2020. Ten studies were population-based birth cohort studies [54–59,62,64,65,66] and two were case-control studies [61,63]. Seven different geographic locations were represented, including Denmark, Canada, Sweden, California (US), Finland, Western Australia, and Norway.
Pre-Pregnancy Weight
Among the studies that met criteria for our review, both Andersen and colleagues (2018) and Gardner and colleagues (2015) found a significant association between obese mothers and the risk for ASD. Body mass index (BMI) ≥ 30 was associated with a 1.42 to 1.94 fold greater risk of developing ASD (Table 1). Andersen and colleagues (2018) further observed a possible dose-response relationship with the highest risk in severely obese mothers (BMI ≥ 35). In matched sibling analyses, however, Gardner and colleagues (2018) no longer observed a statistically significant association between maternal BMI and ASD risk.
Multiple metabolic conditions often co-occur. Kong and colleagues (2020) examined the association of BMI with ASD risk based on both absence of DM and presence of subtypes of DM. They found an increased risk of ASD among overweight (BMI ≥ 25), and obese (BMI≥ 30) mothers without DM, but no association among severely obese (BMI ≥ 35) mothers without DM (Table 1). BMI was associated with a higher risk of ASD irrespective of other insulin resistance conditions [i.e., insulin dependent DM (IDDM), Type 2 DM (T2DM) and gestational DM].
Gestational Weight Gain
Only Gardner and colleagues (2015) applied methods that met our inclusion criteria and found that excessive GWG independent of pre pregnancy (baseline) BMI was associated with a 1.12 fold greater risk for developing ASD (Table 1). A similar risk was observed for ASD with and without intellectual disability (ID). A matched sibling analysis showed similar results. In addition, a dose response effect was observed where risk increased with each 5 pound increment of weight gained among mothers with normal baseline BMI. Interestingly, Gardner and colleagues (2015) also showed a significant association of insufficient GWG with the risk of ASD.
Pre-gestational diabetes
Among the five studies [55,58,59,65,66] that examined the association between pre- gestational DM and ASD that met our inclusion criteria, the overall increase in risk varied from 1.39 to 1.65 (Table 1). Of note however, 3 of the studies [58,65,66] had overlapping samples. Similarly to their previous work described above, Kong and colleagues (2020) examined the risk for ASD and pre-gestational T2DM as well as pre-gestational IDDM, stratified according to BMI. A significant association was found between severely obese mothers (BMI ≥ 35) with T2DM or IDDM and the risk for ASD (Table 1). Langridge and colleagues (2013) did not find significant associations between DM and the risk for ASD, however, the type of DM and stage of development (gestational or pre-gestational) were clustered together in this study, making it difficult to compare study results.
Gestational Diabetes
Three studies [55,56,65] evaluated gestational DM as a binary exposure and only one study [56] showed a significant increased risk for ASD among affected mothers. Other studies stratified gestational DM based on gestational age of either 24 weeks [58] (Jo et al., 2019) or 26 weeks [65,66] (Xiang et al., 2015; Xiang et al., 2018) and showed increased risk ranging from 1.24 to 1.63 fold. Kong and colleagues (2018) examined the association of gestational DM with risk for ASD stratified by BMI. A significant association was found among overweight (BMI ≥ 25) and obese (BMI ≥ 30) mothers with DM (Table 1), however, no association was found between severely obese mothers (BMI ≥ 35) with DM and risk for ASD.
Pre-Pregnancy Hypertension
Few studies have reliably examined the association between pre-pregnancy hypertension and offspring development of ASD. Pre-pregnancy hypertension exposure documentation is often lacking or based on maternal self-report and therefore typically clustered with gestational hypertension/preeclampsia in analyses [35]. Among the studies that met our inclusion criteria for review, only Gardner and colleagues (2015) examined the direct association of pre-gestational hypertension with ASD and did not find a significant increase in risk (Table 1). Langridge and colleagues (2013) examined the risk for ASD using clustering of both preeclampsia or essential hypertension and found that risk increased for cases with ASD and ID (Table 1).
When pre-existing hypertension was clustered with pregnancy induced hypertension or pre-eclampsia in other studies, results were inconsistent [80,83]. Maher and colleagues (2020) examined both the effect of pre-gestational hypertension clustered with preeclampsia and preeclampsia alone. They showed decreased effect size resulting in an association that was not statistically significant when preeclampsia was clustered with chronic hypertension (as compared to preeclampsia alone).
Gestational Hypertension/Preeclampsia
Gestational hypertension and preeclampsia are typically clustered together in analyses and consistently found to be associated with increased risk of ASD in offspring. Both large scale studies included in this review (see Table 1) [55,56,57,61,62,63,64] and smaller scale studies (see Table 2) [80,81,83,85,88] support this association. Of the five studies [55,56,57,62,64] to measure gestational hypertension and/or preeclampsia as an exposure that met our inclusion criteria, all five found increased risk of ASD, ranging between 1.25 to 1.49 fold. Langridge and colleagues (2013) found that risk increase was restricted to ASD with ID, a result in part supported by Polo-Kantola and colleagues (2014) who found risk increasing as a function of severity related to ASD diagnostic subtype (see Table 1). Maher and colleagues also showed that the association between preeclampsia and ASD was present in a sibling matched analysis. However, further stratification of data to examine ASD with and without ID showed the highest risk for ASD with ID and the lowest (but still statistically significant) risk for ASD without ID.
Discussion
This study systematically evaluated the evidence for the association between maternal metabolic syndrome around pregnancy and risk for ASD in offspring. By focusing on large population based studies, we endeavor to limit potential biases and draw evidence-based conclusions.
The most consistent association across studies was observed for preeclampsia and ASD. This is further supported by a sibling analysis [62], suggesting limited familial confounding factors. Additional sub-analyses [61,62] showed even greater effects for preeclampsia when restricting analysis to offspring with ASD and ID compared to ASD without ID. Sun and colleagues [64] examined whether pre-term birth contributes to the observed risk, as delivery is considered a treatment for preeclampsia. They found only minimal difference in risk (OR of 1.29 for term only vs 1.31 for all births including pre-term) suggesting that pre-term birth is not a strong factor in the relationship between preeclampsia and ASD.
While the etiology of how preeclampsia contributes to the risk of ASD is not fully elucidated, several mechanisms have been repeatedly proposed. Preeclampsia, a condition associated with chronic immune activation, contributes to an increase in the circulation of pro-inflammatory cytokines such as IL-6 and CRP, which can impact the development of the hypothalamic-pituitary-adrenal (HPA) axis as well as neurotransmitter pathways in the developing fetus [16,97]. In addition, the placental dysfunction seen with preeclampsia can result in reduced placental perfusion, causing fetal hypoxia and oxidative stress [37,57,62,74,94].
Inflammation has been suggested as a primary contributor to the adverse effects of the metabolic syndrome, and one potential mechanism through which pre-eclampsia as well as the other metabolic conditions contribute to the development of ASD. A chronic low grade inflammatory state is thought to be present in obese mothers, which accompanies the fetus during its intrauterine development [18]. Animal studies suggest that low grade inflammation and release of pro-inflammatory cytokines from adipose tissue play a role in the development of insulin resistance [16,96]. This in turn can impact the developing fetus by inducing a chronic state of inflammation via circulating cytokines that are able to cross the placenta and impact the inflammatory profile of the fetus, as well as its brain development [19,20]. In addition, the fetus’ intrauterine exposure to a modified nutrient environment with high concentrations of glucose and leptin, can also contribute to alteration in its brain development. Leptin signaling, similarly to pro-inflammatory cytokines, was found to have effects on the HPA axis, synaptic plasticity, and BDNF signaling [14,16,59,60,98]. Furthermore, these intrauterine effects can be perpetuated by alteration in brain development as well as epigenetic mechanisms [14].
Given that other conditions comprising the maternal metabolic syndrome are significant factors contributing to maternal gestational inflammation and the potential mechanisms associated with the risk of developing ASD, it is important to continue investigating the association between those factors and ASD risk. In this review, we include at least one study supporting an association with ASD risk for each of the conditions comprising the maternal metabolic syndrome (pre-gestational DM, GDM, pre-pregnancy weight and GWG). However, this is not sufficient to draw definitive conclusions.
In summary, current evidence from large scale population-based epidemiological studies support an association between preeclampsia and ASD. Evidence for other maternal conditions which are part of the metabolic syndrome was limited due to a paucity of studies. Given the frequent co-occurrence of the conditions comprising the metabolic syndrome, potential synergistic effects should be systematically studied. The use of multiple study designs, especially those that control for potential familial confounding (e.g., sibling control studies), and examining the effects of medical interventions (e.g., pharmaco-epidemiological studies) are imperative for evaluating potential mechanisms and preventive approaches in the future.
Key points:
Multiple studies explored the association between various conditions related to maternal metabolic syndrome and ASD risk with mixed results
Current evidence from large scale population based epidemiological studies support an association between preeclampsia and ASD
Given the frequent co-occurrence of the conditions comprising the metabolic syndrome, potential synergistic effects should be systematically studied
Acknowledgements:
Financial support:
This study was supported by grant HD098883 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (AR and AK) and by the Beatrice and Samuel A. Seaver Foundation.
Conflicts of interest:
AK receives research support from AMO Pharma and consults to Ovid Therapeutics and Acadia.
References
- 1.Association, A.P. Diagnostic and statistical manual of mental disorders (DSM- 5®). 2013: American Psychiatric Pub. [DOI] [PubMed] [Google Scholar]
- 2.Maenner MJ. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill Summ. 2020;69. doi: 10.15585/mmwr.ss6904a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buescher AVS, Cidav Z, Knapp M, Mandell DS. Costs of Autism Spectrum Disorders in the United Kingdom and the United States. JAMA Pediatr. 2014;168(8):721–728. doi: 10.1001/jamapediatrics.2014.210 [DOI] [PubMed] [Google Scholar]
- 4.Lyall K, Croen L, Daniels J, et al. The Changing Epidemiology of Autism Spectrum Disorders. Annu Rev Public Health. 2017;38:81–102. doi: 10.1146/annurev-publhealth-031816-044318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45(3):601–613. doi: 10.1017/S003329171400172X [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Leventhal BL. Genetic Epidemiology and Insights into Interactive Genetic and Environmental Effects in Autism Spectrum Disorders. Biol Psychiatry. 2015;77(1):66–74. doi: 10.1016/j.biopsych.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei X-Y, Li Y-J, Ou J-J, Li Y-M. Association between parental body mass index and autism spectrum disorder: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2019;28(7):933–947. doi: 10.1007/s00787-018-1259-0 [DOI] [PubMed] [Google Scholar]; ■ This is a review examining the association of parental BMI and ASD risk. Found that maternal obesity and overweight were associated with increased risk of ASD.
- 9.Godfrey KM, Reynolds RM, Prescott SL, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5(1):53–64. doi: 10.1016/S2213-8587(16)30107-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poston L, Caleyachetty R, Cnattingius S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. The Lancet Diabetes & Endocrinology. 2016;4(12):1025–1036. doi: 10.1016/S2213-8587(16)30217-0 [DOI] [PubMed] [Google Scholar]
- 11.Lyall K, Pauls DL, Santangelo SL, Santangelo S, Spiegelman D, Ascherio A. Maternal early life factors associated with hormone levels and the risk of having a child with an autism spectrum disorder in the nurses health study II. J Autism Dev Disord. 2011;41(5):618–627. doi: 10.1007/s10803-010-1079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch Pediatr Adolesc Med. 2007;161(4):326–333. doi: 10.1001/archpedi.161.4.326 [DOI] [PubMed] [Google Scholar]
- 13.Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20(2). doi: 10.1007/s11906-018-0812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivell A, Mattson MP. Intergenerational Metabolic Syndrome and Neuronal Network Hyperexcitability in Autism. Trends Neurosci. 2019;42(10):709–726.doi: 10.1016/j.tins.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Reviewed potential mechanisms in which excessive consumption of energy dense food could be associated with increased risk of ASD.
- 15.Hales CM. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. 2020;(360):8. [PubMed] [Google Scholar]
- 16.Kong L, Chen X, Gissler M, Lavebratt C. Relationship of prenatal maternal obesity and diabetes to offspring neurodevelopmental and psychiatric disorders: a narrative review. Int J Obes (Lond). Published online June 3, 2020. doi: 10.1038/s41366-020-0609-4 [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ This is a review examining the association between maternal diabetes and obesity with increased risk of neurodevelopmental and psychiatric disorders in the offspring. They found that maternal obesity and diabetes was associated with development of ASD and ADHD.
- 17.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482 [DOI] [PubMed] [Google Scholar]
- 18.Pantham P, Aye ILMH, Powell TL. Inflammation in Maternal Obesity and Gestational Diabetes Mellitus. Placenta. 2015;36(7):709–715. doi: 10.1016/j.placenta.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sureshchandra S, Marshall NE, Wilson RM, et al. Inflammatory Determinants of Pregravid Obesity in Placenta and Peripheral Blood. Front Physiol. 2018;9. doi: 10.3389/fphys.2018.01089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang NM, Cowan M, Moonah SN, Petri WA. The Impact of Systemic Inflammation on Neurodevelopment. Trends Mol Med. 2018;24(9):794–804. doi: 10.1016/j.molmed.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpita B, Muti D, Dell’Osso L. Oxidative Stress, Maternal Diabetes, and Autism Spectrum Disorders. Oxid Med Cell Longev. 2018;2018. doi: 10.1155/2018/3717215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey A, Le Couteur A, Gottesman I, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25(1):63–77. doi: 10.1017/S0033291700028099 [DOI] [PubMed] [Google Scholar]
- 23.Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: A decade of new twin studies. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2011;156(3):255–274. doi: 10.1002/ajmg.b.31159 [DOI] [PubMed] [Google Scholar]
- 24.Deng W, Zou X, Deng H, et al. The Relationship Among Genetic Heritability, Environmental Effects, and Autism Spectrum Disorders: 37 Pairs of Ascertained Twin Study. J Child Neurol. 2015;30(13):1794–1799. doi: 10.1177/0883073815580645 [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg RE, Law JK, Yenokyan G, McGready J, Kaufmann WE, Law PA. Characteristics and Concordance of Autism Spectrum Disorders Among 277 Twin Pairs. Arch Pediatr Adolesc Med. 2009;163(10):907–914. doi: 10.1001/archpediatrics.2009.98 [DOI] [PubMed] [Google Scholar]
- 26.Hisle-Gorman E, Susi A, Stokes T, Gorman G, Erdie-Lalena C, Nylund CM. Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatr Res. 2018;84(2):190–198. doi: 10.1038/pr.2018.23 [DOI] [PubMed] [Google Scholar]
- 27.Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism. 2017;8. doi: 10.1186/s13229-017-0121-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Geng H, Liu W, Zhang G. Prenatal, perinatal, and postnatal factors associated with autism: A meta-analysis. Medicine (Baltimore). 2017;96(18):e6696. doi: 10.1097/MD.0000000000006696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guinchat V, Thorsen P, Laurent C, Cans C, Bodeau N, Cohen D. Pre-, peri- and neonatal risk factors for autism. Acta Obstet Gynecol Scand. 2012;91(3):287–300. doi: 10.1111/j.1600-0412.2011.01325.x [DOI] [PubMed] [Google Scholar]
- 30.Kim JY, Son MJ, Son CY, et al. Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. Lancet Psychiatry. 2019;6(7):590–600. doi: 10.1016/S2215-0366(19)30181-6 [DOI] [PubMed] [Google Scholar]; ■ This is a review examining environmental risk factors for ASD. They found increased risk with increased maternal age and aspects of the maternal metabolic syndrome.
- 31.Connolly N, Anixt J, Manning P, Ping-I Lin D, Marsolo KA, Bowers K. Maternal metabolic risk factors for autism spectrum disorder-An analysis of electronic medical records and linked birth data. Autism Res. 2016;9(8):829–837. doi: 10.1002/aur.1586 [DOI] [PubMed] [Google Scholar]
- 32.Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry. 2009;195(1):7–14. doi: 10.1192/bjp.bp.108.051672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128(2):344–355. doi: 10.1542/peds.2010-1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandin S, Hultman CM, Kolevzon A, Gross R, MacCabe JH, Reichenberg A. Advancing maternal age is associated with increasing risk for autism: a review and meta-analysis. J Am Acad Child Adolesc Psychiatry 2012; 51: 477–86. [DOI] [PubMed] [Google Scholar]
- 35.Xu R-T, Chang Q-X, Wang Q-Q, et al. Association between hypertensive disorders of pregnancy and risk of autism in offspring: a systematic review and meta-analysis of observational studies. Oncotarget. 2018;9(1):1291–1301. doi: 10.18632/oncotarget.23030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Tang S, Xu S, Weng S, Liu Z. Maternal Body Mass Index and Risk of Autism Spectrum Disorders in Offspring: A Meta-analysis. Sci Rep. 2016;6:34248. doi: 10.1038/srep34248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dachew BA, Mamun A, Maravilla JC, Alati R. Pre-eclampsia and the risk of autism-spectrum disorder in offspring: meta-analysis. Br J Psychiatry 2018; 212: 142–47. [DOI] [PubMed] [Google Scholar]
- 38.Andalib S, Emamhadi MR, Yousefzadeh-Chabok S, et al. Maternal SSRI exposure increases the risk of autistic offspring: A meta-analysis and systematic review. Eur Psychiatry 2017; 45: 161–63. [DOI] [PubMed] [Google Scholar]
- 39.Morales DR, Slattery J, Evans S, Kurz X. Antidepressant use during pregnancy and risk of autism spectrum disorder and attention deficit hyperactivity disorder: systematic review of observational studies and methodological considerations. BMC Med 2018; 16: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Advanced parental age and autism risk in children: a systematic review and meta-analysis. Acta Psychiatr Scand 2017; 135: 29–41. [DOI] [PubMed] [Google Scholar]
- 41.Lam J, et al. A systematic review and meta-analysis of multiple airborne pollutants and autism spectrum disorder. PLoS One. 2016;11(9):e0161851. ->pollution [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang T, et al. Serum concentration of 25-hydroxyvitamin D in autism spectrum disorder: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2016;25(4):341–50. [DOI] [PubMed] [Google Scholar]
- 43.Rosen BN, Lee BK, Lee NL, Yang Y, Burstyn I. Maternal Smoking and Autism Spectrum Disorder: A Meta-analysis. J Autism Dev Disord. 2015;45(6):1689–1698. doi: 10.1007/s10803-014-2327-z [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi T, et al. Autism spectrum disorder and prenatal exposure to selective serotonin reuptake inhibitors: a systematic review and meta- analysis. Reprod Toxicol. 2016;65:170–8. [DOI] [PubMed] [Google Scholar]
- 45.Wu S, et al. Family history of autoimmune diseases is associated with an increased risk of autism in children: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2015;55:322–32. [DOI] [PubMed] [Google Scholar]
- 46.Jiang HY, et al. Maternal infection during pregnancy and risk of autism spectrum disorders: a systematic review and meta-analysis. Brain Behav Immun. 2016;58:165–72. [DOI] [PubMed] [Google Scholar]
- 47.Xu G, et al. Maternal diabetes and the risk of autism spectrum disorders in the offspring: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conde-Agudelo A, Rosas-Bermudez A, Norton MH. Birth spacing and risk of autism and other neurodevelopmental disabilities: a systematic review. Pediatrics. 2016;137(5). [DOI] [PubMed] [Google Scholar]
- 49.Curran EA, et al. Research review: birth by caesarean section and development of autism spectrum disorder and attention-deficit/ hyperactivity disorder: a systematic review and meta-analysis. J Child Psychol Psychiatry. 2015;56(5):500–8. [DOI] [PubMed] [Google Scholar]
- 50.Modabbernia A, et al. Impaired gas exchange at birth and risk of intellectual disability and autism: a meta-analysis. J Autism Dev Disord. 2016;46(5):1847–59. [DOI] [PubMed] [Google Scholar]
- 51.El Marroun H, White T, Verhulst FC, Tiemeier H. Maternal use of antidepressant or anxiolytic medication during pregnancy and childhood neurodevelopmental outcomes: a systematic review. Eur Child Adolesc Psychiatry. 2014;23(10):973–992. doi: 10.1007/s00787-014-0558-3 [DOI] [PubMed] [Google Scholar]
- 52.Rais TB, Rais A. Association Between Antidepressants Use During Pregnancy and Autistic Spectrum Disorders: A Meta-analysis. Innov Clin Neurosci. 2014;11(5–6):18–22. [PMC free article] [PubMed] [Google Scholar]
- 53.Xu R-T, Chang Q-X, Wang Q-Q, et al. Association between hypertensive disorders of pregnancy and risk of autism in offspring: a systematic review and meta-analysis of observational studies. Oncotarget. 2018;9(1):1291–1301. doi: 10.18632/oncotarget.23030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andersen CH, Thomsen PH, Nohr EA, Lemcke S. Maternal body mass index before pregnancy as a risk factor for ADHD and autism in children. Eur Child Adolesc Psychiatry. 2018;27(2):139–148. doi: 10.1007/s00787-017-1027-6 [DOI] [PubMed] [Google Scholar]
- 55.Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can. 2010;30(4):125–134. [PubMed] [Google Scholar]
- 56.Gardner RM, Lee BK, Magnusson C, et al. Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: Results from a Swedish total population and discordant sibling study. Int J Epidemiol. 2015;44(3):870–883. doi: 10.1093/ije/dyv081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Getahun D, Fassett MJ, Peltier MR, et al. Association of Perinatal Risk Factors with Autism Spectrum Disorder. Am J Perinatol. 2017;34(3):295–304. doi: 10.1055/s-0036-1597624 [DOI] [PubMed] [Google Scholar]
- 58.Jo H, Eckel SP, Chen J-C, et al. Gestational diabetes mellitus, prenatal air pollution exposure, and autism spectrum disorder. Environ Int. 2019;133(Pt A):105110. doi: 10.1016/j.envint.2019.105110 [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ This study evaluated the potential combined impact of air pollution and maternal diabetes on risk of developing ASD. They found that early onset gestational diabetes could increase the fetus susceptibility to O3 and thereby increasing risk of developing ASD.
- 59.Kong L, Nilsson IAK, Brismar K, Gissler M, Lavebratt C. Associations of Different Types of Maternal Diabetes and Body Mass Index With Offspring Psychiatric Disorders. JAMA Netw Open. 2020;3(2):e1920787. doi: 10.1001/jamanetworkopen.2019.20787 [DOI] [PubMed] [Google Scholar]; ■ This study examined the potential synergistic effect between maternal diabetes and obesity on the risk of developing psychiatric disorders in the offspring.
- 60.Kong L, Norstedt G, Schalling M, Gissler M, Lavebratt C. The Risk of Offspring Psychiatric Disorders in the Setting of Maternal Obesity and Diabetes. Pediatrics. 2018;142(3). doi: 10.1542/peds.2018-0776 [DOI] [PubMed] [Google Scholar]
- 61.Langridge AT, Glasson EJ, Nassar N, et al. Maternal conditions and perinatal characteristics associated with autism spectrum disorder and intellectual disability. PLoS ONE. 2013;8(1):e50963. doi: 10.1371/journal.pone.0050963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maher GM, O’Keeffe GW, Dalman C, et al. Association between preeclampsia and autism spectrum disorder: a population-based study. J Child Psychol Psychiatry. 2020;61(2):131–139. doi: 10.1111/jcpp.13127 [DOI] [PubMed] [Google Scholar]; ■ This study examined the associated between maternal preeclampsia and ASD development. They found that preeclampsia is a risk factor for ASD development.
- 63.Polo-Kantola P, Lampi KM, Hinkka-Yli-Salomäki S, Gissler M, Brown AS, Sourander A. Obstetric risk factors and autism spectrum disorders in Finland. J Pediatr. 2014;164(2):358–365. doi: 10.1016/j.jpeds.2013.09.044 [DOI] [PubMed] [Google Scholar]
- 64.Sun BZ, Moster D, Harmon QE, Wilcox AJ. Association of Preeclampsia in Term Births With Neurodevelopmental Disorders in Offspring. JAMA Psychiatry. Published online April 1, 2020. doi: 10.1001/jamapsychiatry.2020.0306 [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ This study examined the association of preeclampsia in term births with risk of neurodevelopmental disorders in the offspring. They found potential effect of preeclampsia on the offspring neurodevelopment.
- 65.Xiang AH, Wang X, Martinez MP, et al. Association of maternal diabetes with autism in offspring. JAMA. 2015;313(14):1425–1434. doi: 10.1001/jama.2015.2707 [DOI] [PubMed] [Google Scholar]
- 66.Xiang AH, Wang X, Martinez MP, Page K, Buchanan TA, Feldman RK. Maternal Type 1 Diabetes and Risk of Autism in Offspring. JAMA. 2018;320(1):89–91. doi: 10.1001/jama.2018.7614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tian Z-X, Wan M, Gao Y-L, et al. Gestational weight gain and risk of autism spectrum disorders in offspring: a systematic review and meta-analysis. J Obstet Gynaecol. Published online December 2, 2019:1–8. doi: 10.1080/01443615.2019.1676211 [DOI] [PubMed] [Google Scholar]; ■ This is a review and meta-analysis examining gestational weight gain as a potential risk for ASD development. They found that excessive gestational weight gain is associated with ASD development.
- 68.Carpita B, Muti D, Dell’Osso L. Oxidative Stress, Maternal Diabetes, and Autism Spectrum Disorders. Oxid Med Cell Longev. 2018;2018:3717215. doi: 10.1155/2018/3717215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sullivan EL, Nousen L, Chamlou K. Maternal High Fat Diet Consumption during the Perinatal Period Programs Offspring Behavior. Physiol Behav. 2014;123. doi: 10.1016/j.physbeh.2012.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Angelidou A, Asadi S, Alysandratos K-D, Karagkouni A, Kourembanas S, Theoharides TC. Perinatal stress, brain inflammation and risk of autism-Review and proposal. BMC Pediatr. 2012;12:89. doi: 10.1186/1471-2431-12-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Contu L, Hawkes CA. A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. Int J Mol Sci. 2017;18(5). doi: 10.3390/ijms18051093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edlow AG. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn. 2017;37(1):95–110. doi: 10.1002/pd.4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gumusoglu SB, Chilukuri ASS, Santillan DA, Santillan MK, Stevens HE. Neurodevelopmental Outcomes of Prenatal Preeclampsia Exposure. Trends Neurosci. 2020;43(4):253–268. doi: 10.1016/j.tins.2020.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Review of epidemiological and neuroscience studies discussing the association of preeclampsia with impact on offspring neurodevelopment.
- 74.Maher GM, McCarthy FP, McCarthy CM, et al. A perspective on pre-eclampsia and neurodevelopmental outcomes in the offspring: Does maternal inflammation play a role? Int J Dev Neurosci. 2019;77:69–76. doi: 10.1016/j.ijdevneu.2018.10.004 [DOI] [PubMed] [Google Scholar]; ■ Review of potential mechanisms implicated in the association of preeclampsia with impact on neurodevelopment.
- 75.Maldonado-Ruiz R, Garza-Ocañas L, Camacho A. Inflammatory domains modulate autism spectrum disorder susceptibility during maternal nutritional programming. Neurochem Int. 2019;126:109–117. doi: 10.1016/j.neuint.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 76.Rivera HM, Christiansen KJ, Sullivan EL. The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci. 2015;9:194. doi: 10.3389/fnins.2015.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valleau JC, Sullivan EL. The impact of leptin on perinatal development and psychopathology. J Chem Neuroanat. 2014;61–62:221–232. doi: 10.1016/j.jchemneu.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Burg JW, Sen S, Chomitz VR, Seidell JC, Leviton A, Dammann O. The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatr Res. 2016;79(1–1):3–12. doi: 10.1038/pr.2015.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X, Lu J, Xie W, et al. Maternal diabetes induces autism-like behavior by hyperglycemia-mediated persistent oxidative stress and suppression of superoxide dismutase 2. Proc Natl Acad Sci USA. 2019;116(47):23743–23752. doi: 10.1073/pnas.1912625116 [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ This study discusses potential mechanism in which maternal diabetes increases risk for ASD.
- 80.Cordero C, Windham GC, Schieve LA, et al. Maternal diabetes and hypertensive disorders in association with autism spectrum disorder. Autism Res. 2019;12(6):967–975. doi: 10.1002/aur.2105 [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ This study examined the association of pregnancy complications with ASD development. They found an association between gestational high blood pressure and ASD and other neurodevelopmental disorders.
- 81.Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord. 2011;41(7):891–902. doi: 10.1007/s10803-010-1114-8 [DOI] [PubMed] [Google Scholar]
- 82.Getz KD, Anderka MT, Werler MM, Jick SS. Maternal prepregnancy body mass index and autism spectrum disorders among offspring: a population-based case-control study. Paediatr Perinat Epidemiol. 2016;30(5):479–487. doi: 10.1111/ppe.12306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krakowiak P, Walker CK, Bremer AA, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129(5):e1121–1128. doi: 10.1542/peds.2011-2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hallmayer J, Cleveland S, Torres A, et al. Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Arch Gen Psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mann JR, McDermott S, Bao H, Hardin J, Gregg A. Pre-eclampsia, birth weight, and autism spectrum disorders. J Autism Dev Disord. 2010;40(5):548–554. doi: 10.1007/s10803-009-0903-4 [DOI] [PubMed] [Google Scholar]
- 86.Shen Y, Dong H, Lu X, et al. Associations among maternal pre-pregnancy body mass index, gestational weight gain and risk of autism in the Han Chinese population. BMC Psychiatry. 2018;18(1):11. doi: 10.1186/s12888-018-1593-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Surén P, Gunnes N, Roth C, et al. Parental obesity and risk of autism spectrum disorder. Pediatrics. 2014;133(5):e1128–1138. doi: 10.1542/peds.2013-3664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, Hertz-Picciotto I. Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr. 2015;169(2):154–162. doi: 10.1001/jamapediatrics.2014.2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Windham GC, Anderson M, Lyall K, et al. Maternal Pre-pregnancy Body Mass Index and Gestational Weight Gain in Relation to Autism Spectrum Disorder and other Developmental Disorders in Offspring. Autism Res. 2019;12(2):316–327. doi: 10.1002/aur.2057 [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ This study examined the association between maternal weight and gestational weight gain and risk of offspring development of neurodevelopmental disorders. They found potential association of gestational weight gain and ASD and pre-pregnancy overweight/obesity and other neurodevelopmental disorders.
- 90.Xiang AH, Chow T, Martinez MP, et al. Hemoglobin A1c Levels During Pregnancy and Risk of Autism Spectrum Disorders in Offspring. JAMA. Published online June 9, 2019. doi: 10.1001/jama.2019.8584 [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Examined the association of hemoglobin A1c levels and risk of ASD. They found an association with values above 6.5 however findings were limited due to sample size.
- 91.Association, A.P. Diagnostic and statistical manual of mental disorders (4th ed., Text Revision). 2000: American Psychiatric Pub. [Google Scholar]
- 92.World Health Organization. (2004). ICD-10 : international statistical classification of diseases and related health problems : tenth revision, 2nd ed. World Health Organization. [Google Scholar]
- 93.World Health Organization. (1978). International classification of diseases : [9th] ninth revision, basic tabulation list with alphabetic index. World Health Organization. [Google Scholar]
- 94.Maher GM, O’Keeffe GW, Kearney PM, et al. Association of Hypertensive Disorders of Pregnancy With Risk of Neurodevelopmental Disorders in Offspring: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2018;75(8):809–819. doi: 10.1001/jamapsychiatry.2018.0854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanchez CE, Barry C, Sabhlok A, et al. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. Obes Rev. 2018;19(4):464–484. doi: 10.1111/obr.12643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Richardson AC, Carpenter MW. Inflammatory Mediators in Gestational Diabetes Mellitus. Obstetrics and Gynecology Clinics of North America. 2007;34(2):213–224. doi: 10.1016/j.ogc.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 97.Piazza FV, Segabinazi E, de Meireles ALF, et al. Severe Uncontrolled Maternal Hyperglycemia Induces Microsomia and Neurodevelopment Delay Accompanied by Apoptosis, Cellular Survival, and Neuroinflammatory Deregulation in Rat Offspring Hippocampus. Cell Mol Neurobiol. 2019;39(3):401–414. doi: 10.1007/s10571-019-00658-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Valleau JC, Sullivan EL. The Impact of Leptin on Perinatal Development and Psychopathology. J Chem Neuroanat. 2014;0:221–232. doi: 10.1016/j.jchemneu.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]


