Abstract
Background
Despite benefits of endocrine therapy (ET) for patients with hormone-receptor (HR)-positive breast cancer, many patients do not initiate or discontinue ET against recommendations.
Methods
We identified variables associated with ET initiation and continuation, analyzing pooled data from two longitudinal studies at a National Cancer Institute comprehensive cancer center in St. Louis, Missouri. The sample included 533 women with newly diagnosed, non-metastatic, HR-positive breast cancer who completed interviews at enrollment and 6, 12, and 24 months after definitive surgical treatment. Logistic regression models estimated the adjusted odds ratio and 95% confidence interval (aOR [95% CI]) for each of self-reported ET initiation by the 12-month interview and continuation for ≥12 months by the 24-month interview in association with self-reported diabetes, elevated depressed mood, menopausal-symptom severity and obesity, adjusting for race, age, insurance status, chemotherapy, and radiation therapy.
Results
Overall, 81.4% (434/533) of patients initiated ET, and 86.5% (371/429) continued ET ≥12 months. Patients with diabetes had lower odds of initiating ET (0.50 [0.27-0.91]). Patients reporting greater menopausal-symptom severity had lower odds of continuing ET (0.72 [0.53-0.99]).
Conclusion
Efforts to increase ET initiation among patients with diabetes and better manage severe menopausal symptoms among ET users might promote ET continuation.
Clinical trial information
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-022-09946-x.
Keywords: Breast cancer, Hormone therapy, Diabetes, Menopausal symptoms, Obesity, Depression
Background
About 83% of breast cancer patients in the U.S. have hormone receptor (HR)-positive (i.e., estrogen and/or progesterone receptor-positive) tumors and are eligible for endocrine therapy (ET) [1]. ET significantly reduces risks of breast cancer recurrence and mortality in conjunction with long-term adherence to oral medication [2, 3]. However, many patients do not initiate ET or discontinue use before completing the recommended minimum duration of 5 years [2–6]. In previous studies, 20-30% of HR-positive breast cancer patients did not initiate ET in the first year after diagnosis [2, 4, 6], and up to 51% of users discontinued ET prior to the recommended 5-year treatment duration [5, 6]. Thus, further investigation is needed to identify risk factors for non-initiation and/or discontinuation of ET.
Multiple variables have been associated with breast cancer patients’ ET initiation and/or continuation, such as sociodemographic characteristics [2, 7, 8], comorbidities [2, 7, 9–12], and psychosocial symptoms [6, 13–17]. Being non-Hispanic black [2], having lower socioeconomic status [2, 7], and having public or no insurance [8] are risk factors for ET underutilization. Among patients who received ET, those ≥65 years old were more likely to discontinue ET before completing the 5-year treatment compared to patients 50-64 years old [7].
Greater comorbidity-related burden also was related to higher risk of discontinuation of ET [2, 7, 12] due to comorbidity-related complications [10, 11]. Diabetes is a common comorbidity in breast cancer patients and has been associated with a lower likelihood of receiving chemotherapy and radiation therapy [9]. Obesity is another common comorbidity among patients with breast cancer [18, 19] and reduces the effectiveness of ET [20–22]. Previous studies reported an interaction between obesity and tamoxifen, increasing the risk of developing endometrial cancer [23, 24]. In addition, bipolar depression was inversely associated with ET initiation [6]. Several studies documented that patients with more severe depressive symptoms were less likely to continue ET compared with patients having less severe or no depressive symptoms [13, 14, 17]. Menopausal symptoms also have been associated with discontinuation of ET [15, 16].
However, little is known about the associations of co-existing diabetes and obesity with ET initiation and continuation. Moreover, studies examining the associations of depressed mood and menopausal symptoms with ET were limited by including only older patients (age ≥ 68 years) [6], participants in a randomized controlled trial (RCT) of aromatase inhibitors [14], a lack of racial/ethnic diversity with predominantly white (89-94%) [6, 14, 17], or small (n = 196) samples [16]. To fill these knowledge gaps, we examined associations of diabetes, obesity, depressed mood, and menopausal symptoms with initiation and continuation of ET using pooled data from two prospective studies (described below) to acquire a larger, more sociodemographically and socioeconomically diverse sample of women who had been diagnosed with HR-positive breast cancer. We hypothesized that having diabetes, being obese, and reporting elevated depressed mood and greater menopausal-symptom severity are inversely associated with initiation and continuation of ET, adjusting for demographic and clinical variables.
Methods
Study sample
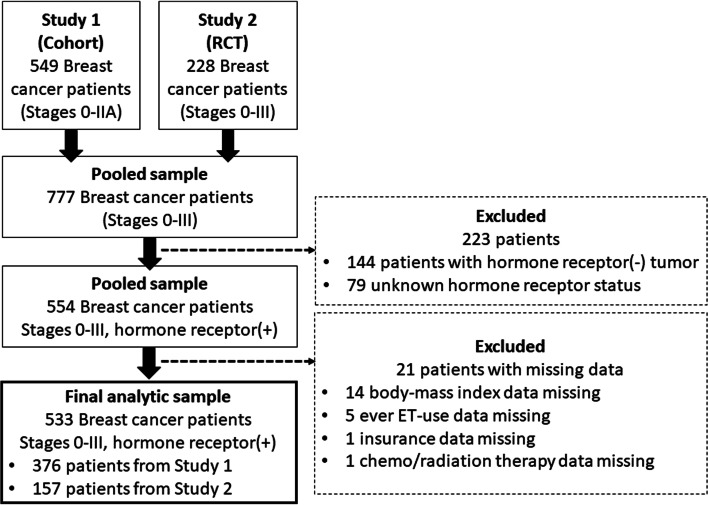
We conducted a secondary analysis of pooled, longitudinal data from two studies (a cohort study and an RCT) examining quality-of-life outcomes among prospectively recruited English-speaking women with newly diagnosed, first-primary, non-metastatic breast cancer, who were receiving treatment at the Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine and at Saint Louis University School of Medicine, both in St. Louis, Missouri, USA. The cohort study included 549 patients with early-stage breast cancer (Stages 0–IIA), who enrolled in the study between October 2003 and June 2007 [25]. The second study included 228 black women with non-metastatic breast cancer (Stages 0–III), who enrolled between December 2009 and 2012 in an RCT testing an interactive, cancer-communication intervention [26]. Both parent studies included multiple interviews over a 2-year follow-up period, with quality of life as the primary outcome [25]. Secondary outcomes of the RCT were adherence to recommended surveillance mammography and endocrine therapy. Patients ≥40 years old were eligible for the cohort study; black patients ≥30 years old were eligible for the second study. In both parent studies, potential participants ≥65 years old had been screened for cognitive impairment, and women with weighted scores > 10 on the Orientation-Memory-Concentration Test [27] were excluded. Thus, 549 patients in the cohort study and 228 patients in the RCT comprised the pool of patients from which this study sample was drawn (Fig. 1).
Fig. 1.
Inclusion/exclusion procedure of study sample
The RCT intervention used videos of black breast cancer survivor stories programmed on a touchscreen tablet-computer (clinicaltrials.gov Trial Number NCT00929084, 06/24/2009) [28]. Further information regarding the RCT, including the full protocol, in compliance with CONSORT guidelines, has been published [29]. Participants received either standard of care for their breast cancer or standard of care plus the video intervention, featuring 207 video clips of black breast cancer survivors telling their stories of living with breast cancer. Stories focused on treatment side effects, coping, social support, healthcare experiences, follow-up care, and quality of life, and could be selected for viewing by story topic or by storyteller [28].
This study was conducted in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments. Following Institutional Review Board approval at Washington University School of Medicine (WUSM IRB #201102380) and receipt of patients’ informed consent, participants completed a baseline interview 4-6 weeks following definitive surgical treatment (cohort study) or near patients’ surgical post-operative visit or the start of neoadjuvant treatment (RCT). Follow-up interviews in both studies were completed at 6, 12, and 24 months following definitive surgical treatment.
Independent variables: diabetes, obesity, depressed mood, and menopausal symptoms
We used Katz’s validated interview adaptation [30] of the Charlson Comorbidity Index [31] to identify patients at each interview who reported having diabetes that was treated by any of diet modification, oral medication, or insulin. Substantial to near-perfect agreement [32] between self-reported diabetes and the medical-record data has been reported, with kappas ranging from 0.76 to 0.93 across several studies [33–37]. Body mass index (BMI) at each interview was calculated as a ratio of weight to height (kg/m2). We compared obese patients with BMI ≥ 30 kg/m2 to non-obese patients with BMI < 30 kg/m2 [38]. Depressed mood was measured at each interview using the 20-item Center for Epidemiologic Studies Depression (CESD) Scale [39]. Participants rated their experience of depressive symptoms during the past week from “rarely or none of the time” (0) to “most or all of the time” (3). We compared patients with depressed mood scores ≥16, which is indicative of elevated depressed mood, to patients with scores < 16 [39, 40]. Menopausal symptoms were measured using a previously validated 4-item measure [41]. Patients reported the severity hot flashes, cold sweats, night sweats, and vaginal dryness in the past month, ranging from (1) “not at all” to (5) “very much.” An average score of the four menopausal symptoms at each interview was computed. We also calculated the change in menopausal-symptom severity mean scores 12-18 months after patient’s first reported use of ET.
Outcome variables
The timing of ET initiation and continuation were based on patients’ self-reported use at each interview. Near-perfect agreement [32] between the medical record and self-reported receipt of adjuvant ET was previously reported for patients in the parent cohort study (kappa = 0.94) [42]. ET initiation was determined using patients’ affirmative response to the question, “Did you ever take tamoxifen or other forms of endocrine/hormone therapy?” Patients who first reported “yes” to this question at baseline, 6-month, or 12-month interview were considered to have initiated ET. ET continuation was assessed based on patients’ affirmative response to ever using ET and to the question, “Are you still taking tamoxifen or other forms of endocrine/hormone therapy?” Patients were considered to have continued ET for at least 12 months if they 1) first reported ever use at baseline and reported current use at the 12-month interview, or 2) first reported ever use at the 6-month or 12-month interview and reported current use at the 24-month interview. Patients who reported ET use at baseline but not current use at the 12-month interview, and patients who reported ET use at either 6-month or 12-month interview but not current use at the 24-month interview were considered to have discontinued use.
Covariates
Covariates previously shown to be associated with ET use included patients’ race (white or black/Asian/Pacific Islander/unspecified), insurance status (private or other, including public insurance, self-pay, or no insurance), age at enrollment (continuous), and receipt of chemotherapy (yes or no) and radiation therapy (yes or no) during the first year of the study [2, 7, 8]. Since all patients in the RCT and all patients with locally advanced breast cancer were black, and since race was a covariate of interest in this study, we did not include study type or stage in the analysis to avoid overfitting the data.
Statistical analysis
Descriptive statistics are presented by whether or not patients initiated and continued ET. Group differences in continuous variables were tested using t-tests, and differences in categorical variables were tested with Chi-square tests. Logistic regression analysis was used to estimate the adjusted odd ratio (aOR) and 95% of confidence interval (CIs) of ET initiation by the 12-month interview for each of obesity, diabetes, depressed mood, and menopausal-symptom severity, adjusting for covariates. Measures of obesity, diabetes, elevated depressed mood, and menopausal symptoms at the interview prior to patients’ first reported use of ET were included in the analysis of ET initiation; for patients who reported initiating ET at baseline or did not initiate ET by the 12-month interview, these measures at baseline were entered. Among patients who initiated ET by the 12-month interview, logistic regression analysis was used to estimate the aOR of ET continuation for each of obesity, diabetes, depressed mood, and menopausal-symptom severity measured at the interview when ET use was first reported, and the change in menopausal-symptom severity scores 12-18 months after first report of ET initiation, adjusting for the same covariates. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Statistical significance was assessed as two-sided P < 0.05.
Results
Since one of the studies was an RCT, we first determined whether the intervention had an effect on ET initiation and continuation. There were no significant differences between the intervention and control arms in the distributions of the variables (Supplementary Table 1) or in the likelihood of ET initiation and of ET continuation adjusted for all covariates and independent variables of interest (Supplementary Table 2). In addition, there were no significant differences between the two studies in the proportions of black/other (cohort study) or black (RCT) patients with HR-positive tumors eligible for inclusion in the pooled sample (65/110 [59.1%] cohort study vs. 157/228 [68.9%] RCT, P = 0.08), or their proportions in association with each of the outcomes and other variables of interest in this study, except for insurance, radiation therapy, and elevated depressed mood (Table 1). Moreover, the odds of ET initiation and continuation among patients in the cohort study for exposure variables and covariates (Supplementary Table 3) were similar to findings for patients in the RCT (Supplementary Table 2), except that patients in the RCT who reported greater menopausal-symptom severity were more likely to initiate ET. Therefore, pooled data for all eligible, prospectively recruited participants in both studies were included to increase the sample size and diversity.
Table 1.
Patient characteristics of racial minorities by the study samples
| Cohort Black/other | RCT Black | P valued | |
|---|---|---|---|
| Total | 65 | 157 | |
| Age, years | 0.08 | ||
| Mean (SD) | 58.9 (11.4) | 56.2 (10.0) | |
| Insurance status | < 0.01 | ||
| Private | 42 (64.6%) | 67 (42.7%) | |
| Public only or othera | 23 (35.4%) | 90 (57.3%) | |
| Chemotherapy | 0.08 | ||
| No | 47 (72.3%) | 94 (59.9%) | |
| Yes | 18 (27.7%) | 63 (40.1%) | |
| Radiation therapy | < 0.01 | ||
| No | 28 (43.1%) | 35 (22.3%) | |
| Yes | 37 (56.9%) | 122 (77.7%) | |
| Diabetes | 0.05 | ||
| No | 54 (83.1%) | 111 (70.7%) | |
| Yes | 11 (16.9%) | 46 (29.3%) | |
| Obesity | 0.80 | ||
| No (BMI < 30 kg/m2) | 26 (40.0%) | 60 (38.2%) | |
| Yes (BMI ≥ 30 kg/m2) | 39 (60.0%) | 97 (61.8%) | |
| Elevated depressed mood | 0.01 | ||
| No (CESD < 16) | 53 (81.5%) | 101 (64.3%) | |
| Yes (CESD ≥16) | 12 (18.5%) | 56 (35.7%) | |
| Menopausal-symptom severity, mean (SD) | 1.8 (0.9) | 1.9 (0.9) | 0.46 |
| ET initiationb | 0.32 | ||
| No | 11 (16.9%) | 36 (22.9%) | |
| Yes | 54 (83.1%) | 121 (77.1%) | |
| ET continuationc | 0.10 | ||
| No | 5 (9.4%) | 23 (19.3%) | |
| Yes | 48 (90.6%) | 96 (80.7%) | |
RCT randomized controlled trial, ET endocrine therapy, SD standard deviation, BMI body mass index, CESD Center for Epidemiologic Studies Depression scale
a Other insurance status includes no insurance or self-pay
b ET initiated by the 12-month interview
c ET continued for at least 12 months after initiation. Of 175 patients of racial minorities who initiated ET within first 12 months, three were excluded due to missing data for obesity (n = 2, one from the cohort study and the other one from the RCT) and diabetes (n = 1 from the RCT)
d Differences between the cohort study and the RCT in continuous variables were tested with the independent-samples t-test, and differences in categorical variables were tested with Chi-square test
The final sample selection included 533 patients with HR-positive tumors regardless of human epidermal growth factor receptor-2 status (Fig. 1). Only four patients were Hispanic (one white patient and three black patients), so we examined patients’ self-reported race regardless of Hispanic ethnicity. In addition, two patients were Asian Indian/Pakistani, two were Asian/Pacific Islander, and two did not respond to this question. There were no significant differences in the associations observed between each of the independent variables of interest and each outcome, ET initiation and continuation, in models that excluded these six patients (Supplementary Table 4) or combined them with black patients in one category (Tables 2 and 3). Thus, we report findings for the black/other race category that combined these six patients with black patients for analysis to increase statistical power.
Table 2.
Characteristics of the pooled sample and prevalence of ET initiation by the 12-month interview and ET continuation for at least 12 months after initiation among hormone receptor-positive breast cancer patients
| ET initiationa | ET continuationb | |||||||
|---|---|---|---|---|---|---|---|---|
| Independent variables | Total N |
Yes n (%) |
No n (%) |
Pc | Total n |
Yes n (%) |
No n (%) |
Pc |
| Total | 533 | 434 (81.4) | 99 (18.6) | 410 | 370 (90.2) | 40 (9.8) | ||
| Race | ||||||||
| White | 311 | 259 (83.3) | 52 (16.7) | 0.19 | 248 | 226 (91.1) | 22 (8.9) | 0.45 |
| Black/Otherd | 222 | 175 (78.8) | 47 (21.2) | 162 | 144 (88.9) | 18 (11.1) | ||
| Age, years | ||||||||
| Mean (SD) | 58.1 (10.6) | 57.8 (10.2) | 59.5 (11.8) | 0.15 | 57.8 (10.2) | 57.7 (10.3) | 59.3 (9.3) | 0.32 |
| Insurance status | ||||||||
| Private | 389 | 320 (82.3) | 69 (17.7) | 0.41 | 307 | 279 (90.9) | 28 (9.1) | 0.45 |
| Public only or othere | 144 | 114 (79.2) | 30 (20.8) | 103 | 91 (88.4) | 12 (11.6) | ||
| Chemotherapy | ||||||||
| No | 380 | 293 (77.1) | 87 (22.9) | <.01 | 275 | 246 (89.4) | 29 (10.6) | 0.44 |
| Yes | 153 | 141 (92.2) | 12 (7.8) | 135 | 124 (91.8) | 11 (8.2) | ||
| Radiation Therapy | ||||||||
| No | 165 | 108 (65.4) | 57 (34.6) | <.01 | 101 | 89 (88.1) | 12 (11.9) | 0.41 |
| Yes | 368 | 326 (88.6) | 42 (11.4) | 309 | 281 (90.9) | 28 (9.1) | ||
| Diabetes | ||||||||
| No | 447 | 373 (83.4) | 74 (16.6) | <.01 | 350 | 317 (90.6) | 33 (9.4) | 0.59 |
| Yes | 86 | 61 (70.9) | 25 (29.1) | 60 | 53 (88.3) | 7 (11.7) | ||
| Obesity | ||||||||
| No (BMI < 30 kg/m2) | 307 | 249 (81.1) | 58 (18.9) | 0.83 | 238 | 215 (90.3) | 23 (9.7) | 0.94 |
| Yes (BMI ≥ 30 kg/m2) | 226 | 185 (81.9) | 41 (18.1) | 172 | 155 (90.1) | 17 (9.9) | ||
| Elevated depressed mood | ||||||||
| No (CESD < 16) | 422 | 343 (81.3) | 79 (18.7) | 0.87 | 339 | 311 (91.7) | 28 (8.3) | 0.03 |
| Yes (CESD ≥16) | 111 | 91 (82.0) | 20 (18.0) | 71 | 59 (83.1) | 12 (16.9) | ||
| Menopausal-symptom severity, mean (SD) | 1.8 (0.9) | 1.8 (0.9) | 1.6 (0.8) | 0.04 | 2.1 (0.9) | 2.0 (0.9) | 2.4 (1.1) | 0.02 |
| Change in menopausal-symptom severityf, mean (SD) | – | – | – | – | 0.05 (0.75) | 0.07 (0.72) | −0.08 (0.95) | 0.37 |
ET endocrine therapy, SD standard deviation, BMI body mass index, CESD Center for Epidemiologic Studies Depression scale
a Using diabetes, obesity, elevated depressed mood, and menopausal symptoms from the interview prior to first report of ET use. For non-initiators and patients who first reported ever taking ET at baseline, the baseline measures of these four variables were used
b Using diabetes, obesity, elevated depressed mood, and menopausal symptom data from the interview when ET initiation was first reported. Of 434 patients who initiated ET within first 12 months of the study, five were excluded due to missing data for obesity and diabetes, and 19 were excluded due to missing data for menopausal symptom severity at follow-up interviews
c Group differences were tested using independent t-tests for continuous variables and chi-square tests for categorical variables
d This category includes 216 black, two Asian Indian/Pakistani, two Asian/Pacific Islander, and two unspecified race in the ET initiation model, with one fewer Asian/Pacific Islander in the ET continuation model
e Other insurance status includes no insurance or self-pay
f Change in menopausal-symptom severity mean scores from patient’s first reported use of ET to 12-18 month follow-up of ET continuation among patients who had used ET within 12 months of definitive surgery
Table 3.
Variables associated with ET initiation by the 12-month interview and ET continuation for at least 12 months after initiation in the pooled sample
| ET initiationa | ET continuationb | |
|---|---|---|
| Independent variables |
aOR (95% CI) (n = 533) |
aOR (95% CI) (n = 410) |
| Race | ||
| White | Ref. | Ref |
| Black/Otherc | 0.66 (0.38-1.13) | 0.85 (0.39-1.85) |
| Age, years | 1.01 (0.98-1.03) | 0.97 (0.93-1.00) |
| Insurance status | ||
| Private | Ref. | Ref |
| Public only or otherd | 0.94 (0.52-1.71) | 1.12 (0.47-2.69) |
| Chemotherapy | ||
| No | Ref. | Ref |
| Yes | 3.47 (1.77-6.78)* | 1.23 (0.57-2.66) |
| Radiation Therapy | ||
| No | Ref. | Ref |
| Yes | 3.94 (2.47-6.31)* | 1.42 (0.70-2.89) |
| Diabetes | ||
| No | Ref. | Ref |
| Yes | 0.50 (0.27-0.91)* | 0.84 (0.34-2.04) |
| Obesity | ||
| No (BMI < 30 kg/m2) | Ref. | Ref |
| Yes (BMI ≥ 30 kg/m2) | 1.12 (0.67-1.88) | 1.03 (0.52-2.03) |
| Elevated depressed mood | ||
| No (CESD < 16) | Ref. | Ref |
| Yes (CESD ≥16) | 0.79 (0.41-1.50) | 0.46 (0.19-1.11) |
| Menopausal-symptom severity | 1.35 (0.99-1.85) | 0.67 (0.45-0.98)* |
| Change in menopausal-symptom severity mean scorese | – | 1.04 (0.66-1.62) |
ET endocrine therapy, BMI body mass index, aOR adjusted odds ratio, CI confidence interval, CESD Center for Epidemiologic Studies Depression scale
a Using diabetes, obesity, elevated depressed mood, and menopausal symptom data from the interview prior to first report of ET initiation. For non-initiators and patients who first reported ever taking ET at baseline, the baseline measures of these four variables were used
b Using diabetes, obesity, elevated depressed mood, and menopausal symptom data from the interview when ET initiation was first reported
c This category includes 216 black, two Asian Indian/Pakistani, two Asian/Pacific Islander, and two unspecified race in the ET initiation model, with one fewer Asian/Pacific Islander in the ET continuation model
d Other insurance status includes no insurance or self-pay
e Change in menopausal-symptom severity mean scores from patient’s first reported use of ET to 12-18 month follow-up of ET continuation among patients who had used ET within 12 months of definitive surgery
* P < 0.05
All participants had non-metastatic breast cancer (24.6% ductal carcinoma in situ, 67.9% early-invasive breast cancer, and 7.5% locally advanced breast cancer). Table 2 presents characteristics of 533 participants in the pooled sample by ET initiation and continuation. Average age was 58.1 years; 41.6% self-identified as black/Asian/unspecified race (40.5% black and 1.1% Asian or unspecified), 42.4% were obese, 16.1% had diabetes, and 20.8% had elevated depressed mood. Additionally, 362 (67.9%) received breast-conserving surgery (BCS), 28.7% received chemotherapy, and 69.0% received radiation therapy. Overall, 81.4% reported initiating ET. A lower proportion of patients with (vs. without) diabetes initiated ET (70.9% vs. 83.4%). Among the 410 patients who initiated ET by the 12-month interview, 90.2% continued ET for at least 12 months.
As shown in Table 3, patients who received chemotherapy (aOR 3.47; 95% CI 1.77-6.78) and radiation therapy (aOR 3.94; 95% CI 2.47-6.31) were each more likely to have initiated ET, but patients with diabetes were less likely to have initiated ET (aOR 0.50; 95% CI 0.27-0.91). Among patients who initiated ET, those who reported more severe menopausal symptoms were less likely to continue ET for at least 12 months (aOR 0.67; 95% CI 0.45-0.98). None of the other variables, including diabetes, depressed mood, obesity, and change in menopausal-symptom severity, was independently associated with ET continuation. However, in sensitivity analyses stratified by race comparing results for only white, only black, and only black/other patients, we observed a significant inverse association between elevated depressed mood and ET continuation among white patients (aOR 0.24; 95% CI 0.07-0.78), but not in either model that included black patients (Table 4).
Table 4.
Variables associated with endocrine therapy (ET) continuation for at least 12 months after initiation, stratified by race
| aOR (95% CI)a | |||
|---|---|---|---|
| Independent variables | White (n = 248) |
Blackb (n = 157) |
Black/Other (n = 162) |
| Age, years | 0.96 (0.92-1.01) | 0.98 (0.92-1.04) | 0.98 (0.92-1.04) |
| Insurance status | |||
| Private | Ref | Ref | Ref |
| Public only or otherc | 5.87 (0.36-94.67) | 0.54 (0.18-1.57) | 0.54 (0.19-1.58) |
| Chemotherapy | |||
| No | Ref | Ref | Ref |
| Yes | 1.16 (0.39-3.46) | 1.58 (0.54-4.58) | 1.58 (0.54-4.57) |
| Radiation Therapy | |||
| No | Ref | Ref | Ref |
| Yes | 1.62 (0.66-3.99) | 1.12 (0.35-3.55) | 1.16 (0.37-3.68) |
| Diabetes | |||
| No | Ref | Ref | Ref |
| Yes | 0.49 (0.13-1.87) | 1.23 (0.39-3.87) | 1.19 (0.38-3.74) |
| Obesity | |||
| No (BMI < 30 kg/m2) | Ref | Ref | Ref |
| Yes (BMI ≥ 30 kg/m2) | 1.20 (0.45-3.19) | 0.85 (0.32-2.26) | 0.83 (0.31-2.19) |
| Elevated depressed mood | |||
| No (CESD < 16) | Ref | Ref | Ref |
| Yes (CESD ≥16) | 0.24 (0.07-0.78)* | 0.96 (0.28-3.27) | 0.94 (0.28-3.23) |
| Menopausal-symptom severity | 0.66 (0.41-1.07) | 0.72 (0.39-1.32) | 0.70 (0.38-1.30) |
| Change in menopausal-symptom severity mean scoresd | 0.77 (0.42-1.43) | 1.42 (0.74-2.73) | 1.40 (0.73-2.69) |
BMI body mass index, aOR adjusted odds ratio, CI confidence interval, CESD Center for Epidemiologic Studies Depression scale
a Using diabetes, obesity, elevated depressed mood, and menopausal symptom data from the interview when ET initiation was first reported
b Five patients (two Asian Indian/Pakistani, one Asian/Pacific Islander, and two unspecified because they did not respond to the question about race) were excluded from this sensitivity analysis stratified by race to compare the associations between ET continuation and variables of interest within each racial group
c Other insurance status includes no insurance or self-pay
d Change in menopausal-symptom severity mean scores from patient’s first reported use of ET to 12-18 month follow-up of ET continuation among patients who had used ET within 12 months of definitive surgery
* P < 0.05
We conducted another sensitivity analysis to explore if we over or underestimated continuation based on the way patients with missing data were classified as continuing or discontinuing ET. Of the 410 patients who had initiated ET (99 initiated ET at baseline, 234 at 6 months, 77 at 12 months), 15 patients with missing data (six between the baseline and 12-month interviews, six between the 6-month and 24-month interviews, and three between the 12-month and 24-month interviews) were excluded, as we could not confirm that they continued ET for at least 12 months; moreover, eight patients who temporarily stopped ET were reassigned to the discontinued group. There were no significant changes in aORs of ET continuation for the independent variables of interest (Supplementary Table 5).
Discussion
We examined the associations of diabetes, obesity, depressed mood, and menopausal-symptom severity with ET initiation and continuation in women with HR-positive non-metastatic breast cancer using longitudinal data. We observed that patients with diabetes were significantly less likely to initiate ET within 12 months of enrollment, and patients reporting more severe menopausal symptoms at the time of ET initiation were significantly less likely to have continued use for at least 12 months.
Breast cancer patients with diabetes have poorer outcomes than patients without diabetes [9, 43, 44]. A higher risk of cancer-specific mortality and all-cause mortality in breast cancer patients with diabetes might be due to underutilization of chemotherapy [9, 43, 44] and radiotherapy [9, 44]. Little is known about an independent contribution of comorbid diabetes to ET use. Studies reported that patients with more comorbidities were less likely to initiate ET and continue to use it [2, 7, 12]. However, these studies did not specifically examine the association between diabetes and ET use. A population-based Dutch study of 9725 breast cancer patients examined receipt of various cancer treatments in association with diabetes; patients with diabetes received less aggressive treatment than patients without diabetes [11]. In that study, age was an effect modifier of the association between diabetes and cancer treatment. Patients < 65 years old with (vs. without) diabetes were less likely to have received adjuvant chemotherapy and more likely to have received surgery and ET; however, patients ≥65 years old with (vs. without) diabetes were less likely to have received radiation therapy as older patients with diabetes were also less likely to have received BCS [11]. Due to a small sample size, because we limited our sample to patients with HR-positive tumors, we could not stratify by age in this study. Various diabetes-related complications may explain the underutilization of recommended cancer treatments [10, 11]. Studies demonstrated increased risk of complications from chemotherapy in breast cancer patients with diabetes [43, 45], thus, these patients may be similarly concerned about increased risk of complications from ET and may explain the observed lower ET use among patients with (vs. without) diabetes. An increased risk of venous thrombosis after tamoxifen use [46–48] may also influence diabetic patient’s ET decision making. Given the established benefits of ET for HR-positive breast cancer and the high prevalence of diabetes among breast cancer patients [49, 50], early patient-provider discussions about breast cancer treatment decisions in the context of diabetes management [51] and increased risk for poorer outcomes [52, 53] are recommended to promote ET initiation in patients with diabetes. The demands of self-management for multiple chronic conditions may be challenging to patients with diabetes for whom ET is recommended.
We found that patients who reported more severe menopausal symptoms at the interview prior to ET initiation (or at baseline if ET initiation was reported then) were less likely to continue ET. While 70-80% of HR-positive breast cancer patients are reported to receive ET within 12 months of diagnosis [2, 4, 6], a large proportion of ET users discontinue use due to side effects such as menopausal symptoms [16, 54–57]. Patients often experience severe menopausal symptoms shortly after initiating ET [55, 56], which may negatively affect ET continuation, especially among younger women who may experience menopausal symptoms for the first time [16]. Hot flashes newly treated after ET initiation were found to be associated with earlier ET discontinuation, whereas pre-existing hot flashes were not [15]. Notably, we did not observe a significant association between ET continuation and change in menopausal-symptom severity after ET initiation. To our knowledge, the independent effects of a multi-item menopausal-symptom severity scale and of change in menopausal-symptom severity on ET continuation have not been reported. However, an RCT examined bother by prior treatment side effects at baseline as well as change in bother by individual ET side effects in association with ET continuation [58], with similar results as ours. Menopausal-symptom severity is a commonly reported barrier to ET adherence [54, 58–60]. However, since studies demonstrated that patients with severe menopausal symptoms may experience a greater benefit from ET [61, 62], timely management of severe menopausal symptoms after ET initiation is needed to promote completion of ET. Various non-hormonal interventions have been recommended to relieve discomfort from menopausal symptoms, including maintaining a healthy weight, engaging in regular physical activity, yoga, relaxation techniques, and acupuncture, and avoiding tobacco and alcohol consumption [63–69]. However, there is a lack of published research examining the impact of non-hormonal interventions on both alleviating menopausal-symptom severity and promoting adherence to ET as recommended. Such studies would make substantial contributions to the literature.
Obesity is a common health condition related to breast cancer [18, 19]. Aromatase inhibitors are less effective in lowering the blood estrogen levels in obese patients than non-obese patients [20–22]. Among tamoxifen users, obese patients have a higher risk of developing endometrial cancer than non-obese patients [23, 24]. For these reasons, there might be an inverse association between obesity and ET use. However, we did not observe a significant association between obesity and either ET initiation or continuation. Since obesity is highly correlated with other comorbidities, including diabetes, simultaneous adjustment for diabetes and obesity might have attenuated the association between obesity and ET use. A multi-institutional study also did not observe a significant association between obesity and ET use [70]. Further analysis using a larger sample would refine our understanding of ET initiation and continuation in obese patients with breast cancer.
Depressed mood is commonly reported by breast cancer patients. Pooled data of 43 breast cancer-patient cohorts indicated that 20% of patients self-reported depressed mood, and depression was particularly high during cancer treatment and decreased afterward [71]. Depressed mood can interrupt individuals’ daily routines including general medication use [72]. A prospective study observed that elevated depressed mood at cancer diagnosis was related to higher risk of discontinuing ET within 12 months of initiation [14]. A cross-sectional study also showed a positive association between depressive symptoms and non-persistence of ET use in 12 months [17]. However, we did not observe a significant association between elevated depressed mood and either ET initiation or continuation. This discrepancy in findings might be due, in part, to differences in study design and patient characteristics, as racial minorities accounted for only 6-11% in the other studies and almost 42% in our study. In our sensitivity analysis stratified by race, only white patients with elevated depressed mood had a lower likelihood of ET continuation. Thus, the high proportion of black patients in our sample might explain the discrepancy between our findings and those of other studies. Sufficiently powered studies with diverse racial/ethnic groups and longer follow-up are warranted to determine the impact of depressed mood on ET continuation.
This study has limitations. First, most patients were recruited from a National Cancer Institute-designated comprehensive cancer center and another academic-medical center in the same U.S. city, so the results might not be generalizable to patients treated in community or rural hospitals. Second, ET initiation and continuation were assessed based on a fixed schedule of study interviews, thus the exact timing of initiation or discontinuation could not be determined. We were unable to assess adherence to ET (taking tamoxifen or aromatase inhibitors as prescribed) due to the lack of reliable prescription data in the medical record. Third, although it is not surprising that women who experienced more severe menopausal symptoms might discontinue ET, our findings were based on a larger, more diverse sample of patients than earlier studies that were limited by smaller and less diverse samples [4, 14, 16, 17]. Importantly, we found that greater menopausal-symptom severity at the interview when ET was first reported, not the change in menopausal-symptom severity after ET initiation, was associated with discontinuation of treatment. Significant associations between ET continuation and change in specific ET side effects also were not observed in a large RCT of anastrozole and exemestane [58]. Fourth, ET use was measured over a 2-year follow-up. Thus, the results might not be applicable to the recommended 5-year continuation of ET. Fifth, the Katz’s validated interview of the Charlson Comorbidity Index does not distinguish between Type I and Type II diabetes per se, which might be associated with ET continuation; thus, we could not account for the potential influence of diabetes type in this analysis. Sixth, household income and medication coverage might be associated with ET use and continuation, as costs for ET medication can be a barrier [73]; however, 6% of participants did not report their annual household income. Instead, as previously reported [74], we used insurance status as an indicator of socioeconomic status, since income and insurance status are correlated. Moreover, higher out-of-pocket cost for other medications, such as diabetes medications, could exacerbate financial barriers to ET initiation and long-term adherence. Side effects of other medications were not measured but could have influenced ET initiation or continuation as well. Lastly, cancer care providers might influence participants’ decisions to use ET [75], but information about the role that providers played in ET decisions was not available.
Despite these limitations, this study provided evidence for the potential roles of common comorbidities, including diabetes and severe menopausal symptoms, in association with ET initiation and continuation among women with non-metastatic breast cancer. Given a high prevalence of pre-existing diabetes and its associated breast cancer mortality risk, understanding the role of potentially modifiable barriers, including patient-provider communication [51, 52, 75], is essential for improving ET use by breast cancer patients with diabetes and their prognosis [52, 76]. Timely and effective management of severe menopausal symptoms in breast cancer patients receiving ET is necessary to facilitate their completion of the recommended course of ET.
Supplementary Information
Acknowledgments
We thank our patient participants, the interviewers, and Ms. Lori Grove in Oncology Data Services at Washington University in St. Louis for assistance with data collection from the medical record. We also thank the physicians in addition to Dr. Margenthaler, who helped us recruit their patients for this study, including Drs. Rebecca Aft, Amy Cyr, Jill Dietz, Timothy Eberlein, Matthew Ellis, William Gillanders, Virginia Herrmann, Donald Lombardi, Cynthia Ma, Loren Michel, Michael Naughton, Antonella Rastelli, Rama Suresh, Marie Taylor, and Imran Zoberi at Washington University in St. Louis School of Medicine, and Drs. Eddie Hsueh and Theresa Schwartz and Ms. Pam Hunborg, RN, at Saint Louis University School of Medicine.
Abbreviations
- aOR
Adjusted odds ratio
- BCS
Breast-conserving surgery
- BMI
Body mass index
- CESD
Center for Epidemiologic Studies Depression
- CI
Confidence interval
- ET
Endocrine Therapy
- HR
Hormone-receptor
- RCT
Randomized controlled trial
Authors’ contributions
B.C. conducted the data analysis and drafted manuscript with support from M.P., D.J., and Y.L. Y.L. and D.J. conceived the original idea and supervised the project. All authors directly participated in interpretation of the results, provided critical comments to the manuscript, revised the text, and have read and approved the final version of the manuscript submitted and agree to be accountable for all aspects of the work.
Funding
This study was funded by a grant from the National Cancer Institute (NCI) and Breast Cancer Stamp Fund (R01 CA102777; PI: DB Jeffe), an NCI Center of Excellence in Cancer Communication Research grant (2P50 CA095815-06; PI: MW Kreuter), and the NCI Cancer Center Support Grant (P30 CA091842; PI: T Eberlein) to the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, MO, for services provided by the Health Behavior, Communication and Outreach Core. The study was also supported by the NCI (R01CA215418; PIs: Y Liu/M Lian) and the American Cancer Society (Denim Days Research Scholar Grant RSG-18-116-01-CPHPS; PI: Y Liu).
Availability of data and materials
The datasets generated and analyzed for the current study are not publicly available as data for the trial aims are still being analyzed. The pooled data may be made available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All study procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments. Following Institutional Review Board approval at Washington University School of Medicine (WUSM IRB #201102380), informed consent was obtained from each participant prior to enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Cancer Society . Breast Cancer Facts & Figures 2019-2020. Atlanta: American Cancer Society, Inc.; 2019. [Google Scholar]
- 2.Farias AJ, Du XL. Racial differences in adjuvant endocrine therapy use and discontinuation in association with mortality among Medicare breast Cancer patients by receptor status. Cancer Epidemiol Biomark Prev. 2017;26(8):1266–1275. doi: 10.1158/1055-9965.EPI-17-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheeler SB, Kohler RE, Reeder-Hayes KE, Goyal RK, Lich KH, Moore A, Smith TW, Melvin CL, Muss HB. Endocrine therapy initiation among Medicaid-insured breast cancer survivors with hormone receptor-positive tumors. J Cancer Surviv. 2014;8(4):603–610. doi: 10.1007/s11764-014-0365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livaudais JC, Hershman DL, Habel L, Kushi L, Gomez SL, Li CI, Neugut AI, Fehrenbacher L, Thompson B, Coronado GD. Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2012;131(2):607–617. doi: 10.1007/s10549-011-1762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, Fehrenbacher L, Gomez SL, Miles S, Neugut AI. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haskins CB, McDowell BD, Carnahan RM, Fiedorowicz JG, Wallace RB, Smith BJ, Chrischilles EA. Impact of preexisting mental illness on breast cancer endocrine therapy adherence. Breast Cancer Res Treat. 2019;174(1):197–208. doi: 10.1007/s10549-018-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman RA, Virgo KS, He Y, Pavluck AL, Winer EP, Ward EM, Keating NL. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117(1):180–189. doi: 10.1002/cncr.25542. [DOI] [PubMed] [Google Scholar]
- 9.Lega IC, Austin PC, Fischer HD, Fung K, Krzyzanowska MK, Amir E, Lipscombe LL. The impact of diabetes on breast Cancer treatments and outcomes: a population-based study. Diabetes Care. 2018;41(4):755–761. doi: 10.2337/dc17-2012. [DOI] [PubMed] [Google Scholar]
- 10.Richardson LC, Pollack LA. Therapy insight: influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol. 2005;2(1):48–53. doi: 10.1038/ncponc0062. [DOI] [PubMed] [Google Scholar]
- 11.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. 2007;120(9):1986–1992. doi: 10.1002/ijc.22532. [DOI] [PubMed] [Google Scholar]
- 12.He W, Fang F, Varnum C, Eriksson M, Hall P, Czene K. Predictors of discontinuation of adjuvant hormone therapy in patients with breast Cancer. J Clin Oncol. 2015;33(20):2262–2269. doi: 10.1200/JCO.2014.59.3673. [DOI] [PubMed] [Google Scholar]
- 13.Mausbach BT, Schwab RB, Irwin SA. Depression as a predictor of adherence to adjuvant endocrine therapy (AET) in women with breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2015;152(2):239–246. doi: 10.1007/s10549-015-3471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidwell KM, Harte SE, Hayes DF, Storniolo AM, Carpenter J, Flockhart DA, Stearns V, Clauw DJ, Williams DA, Henry NL. Patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer. 2014;120(16):2403–2411. doi: 10.1002/cncr.28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemp A, Preen DB, Saunders C, Boyle F, Bulsara M, Malacova E, Roughead EE. Early discontinuation of endocrine therapy for breast Cancer: who is at risk in clinical practice? SpringerPlus. 2014;3(1):282–291. doi: 10.1186/2193-1801-3-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cluze C, Rey D, Huiart L, BenDiane MK, Bouhnik AD, Berenger C, Carrieri MP, Giorgi R. Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann Oncol. 2012;23(4):882–890. doi: 10.1093/annonc/mdr330. [DOI] [PubMed] [Google Scholar]
- 17.Stanton AL, Petrie KJ, Partridge AH. Contributors to nonadherence and nonpersistence with endocrine therapy in breast cancer survivors recruited from an online research registry. Breast Cancer Res Treat. 2014;145(2):525–534. doi: 10.1007/s10549-014-2961-3. [DOI] [PubMed] [Google Scholar]
- 18.Fu MR, Axelrod D, Guth AA, Cleland CM, Ryan CE, Weaver KR, Qiu JM, Kleinman R, Scagliola J, Palamar JJ, et al. Comorbidities and quality of life among breast Cancer survivors: a prospective study. J Pers Med. 2015;5(3):229–242. doi: 10.3390/jpm5030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edgington A, Morgan MA. Looking beyond recurrence: comorbidities in cancer survivors. Clin J Oncol Nurs. 2011;15(1):E3–12. doi: 10.1188/11.CJON.E3-E12. [DOI] [PubMed] [Google Scholar]
- 20.Lee K, Kruper L, Dieli-Conwright CM, Mortimer JE. The impact of obesity on breast Cancer diagnosis and treatment. Curr Oncol Rep. 2019;21(5):41. doi: 10.1007/s11912-019-0787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodwin PJ. Obesity and endocrine therapy: host factors and breast cancer outcome. Breast. 2013;22(Suppl 2):S44–S47. doi: 10.1016/j.breast.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, Stenbygaard LE, Tange UB, Cold S. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29(1):25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein L, Deapen D, Cerhan JR, Schwartz SM, Liff J, McGann-Maloney E, Perlman JA, Ford L. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999;91(19):1654–1662. doi: 10.1093/jnci/91.19.1654. [DOI] [PubMed] [Google Scholar]
- 24.Chlebowski RT, Schottinger JE, Shi J, Chung J, Haque R. Aromatase inhibitors, tamoxifen, and endometrial cancer in breast cancer survivors. Cancer. 2015;121(13):2147–2155. doi: 10.1002/cncr.29332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeffe D, Pérez M, Liu Y, Collins K, Aft R, Schootman M. Quality of life over time in women diagnosed with ductal carcinoma in situ, early-stage invasive breast cancer, and age-matched controls. Breast Cancer Res Treat. 2012;134(1):379–391. doi: 10.1007/s10549-012-2048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez M, Kreuter MW, Yan Y, Thompson T, Sefko J, Golla B, Margenthaler JA, Colditz G, Jeffe DB. Feasibility and acceptability of an interactive Cancer-communication video program using African American breast Cancer survivor stories. J Health Commun. 2020;25(7):566–575. doi: 10.1080/10810730.2020.1821132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983. [DOI] [PubMed]
- 28.Pérez M, Sefko JA, Ksiazek D, Golla B, Casey C, Margenthaler JA, Colditz G, Kreuter MW, Jeffe DB. A novel intervention using interactive technology and personal narratives to reduce cancer disparities: African American breast cancer survivor stories. J Cancer Surviv. 2014;8(1):21–30. doi: 10.1007/s11764-013-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson T, Pérez M, Yan Y, Kreuter MW, Margenthaler JA, Colditz GA, Jeffe DB. Randomized controlled trial of a breast cancer survivor stories intervention for African American women. Soc Sci Med. 2021;270:113663. doi: 10.1016/j.socscimed.2020.113663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996:73–84. [DOI] [PubMed]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Clin Epidemiol. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 33.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57(10):1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Tisnado DM, Adams JL, Liu H, Damberg CL, Chen WP, Hu FA, Carlisle DM, Mangione CM, Kahn KL. What is the concordance between the medical record and patient self-report as data sources for ambulatory care? Med Care. 2006;44(2):132–140. doi: 10.1097/01.mlr.0000196952.15921.bf. [DOI] [PubMed] [Google Scholar]
- 35.Kilbourne AM, Schumacher K, Frayne SM, Cypel Y, Barbaresso MM, Nord KM, Perzhinsky J, Lai Z, Prenovost K, Spiro A, et al. Physical health conditions among a population-based cohort of Vietnam-era women veterans: agreement between self-report and medical records. J Women's Health (Larchmt) 2017;26(11):1244–1251. doi: 10.1089/jwh.2016.6069. [DOI] [PubMed] [Google Scholar]
- 36.Simpson CF, Boyd CM, Carlson MC, Griswold ME, Guralnik JM, Fried LP. Agreement between self-report of disease diagnoses and medical record validation in disabled older women: factors that modify agreement. J Am Geriatr Soc. 2004;52(1):123–127. doi: 10.1111/j.1532-5415.2004.52021.x. [DOI] [PubMed] [Google Scholar]
- 37.Bush TL, Miller SR, Golden AL, Hale WE. Self-report and medical record report agreement of selected medical conditions in the elderly. Am J Public Health. 1989;79(11):1554–1556. doi: 10.2105/AJPH.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control Prevention . Overweight & obesity: defining adult overweight and obesity. In: United States; 2016. [Google Scholar]
- 39.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 40.Sawyer Radloff L, Teri L. Use of the center for epidemiological studies-depression scale with older adults. Clin Gerontol. 1986;5(1-2):119–136. doi: 10.1300/J018v05n01_06. [DOI] [Google Scholar]
- 41.Pérez M, Liu Y, Schootman M, Aft RL, Schechtman KB, Gillanders WE, Jeffe DB. Changes in sexual problems over time in women with and without early-stage breast cancer. Menopause. 2010;17(5):924. doi: 10.1097/gme.0b013e3181d5dd26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez M, Schootman M, Hall L, Jeffe D. Accelerated partial breast irradiation compared with whole breast radiation therapy: a breast cancer cohort study measuring change in radiation side-effects severity and quality of life. Breast Cancer Res Treat. 2017;162(2):329–342. doi: 10.1007/s10549-017-4121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srokowski TP, Fang S, Hortobagyi GN, Giordano SH. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27(13):2170–2176. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao S, Gill AA, Zahm SH, Jatoi I, Shriver CD, McGlynn KA, Zhu K. Diabetes and overall survival among breast Cancer patients in the U.S. military health system. Cancer Epidemiol Biomark Prev. 2018;27(1):50–57. doi: 10.1158/1055-9965.EPI-17-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Decker MR, Greenblatt DY, Havlena J, Wilke LG, Greenberg CC, Neuman HB. Impact of neoadjuvant chemotherapy on wound complications after breast surgery. Surgery. 2012;152(3):382–388. doi: 10.1016/j.surg.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onitilo AA, Doi SA, Engel JM, Glurich I, Johnson J, Berg R. Clustering of venous thrombosis events at the start of tamoxifen therapy in breast cancer: a population-based experience. Thromb Res. 2012;130(1):27–31. doi: 10.1016/j.thromres.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernandez RK, Sørensen HT, Pedersen L, Jacobsen J, Lash TL. Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2009;115(19):4442–4449. doi: 10.1002/cncr.24508. [DOI] [PubMed] [Google Scholar]
- 48.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and bowel project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 49.Bronsveld HK, Jensen V, Vahl P, De Bruin ML, Cornelissen S, Sanders J, Auvinen A, Haukka J, Andersen M, Vestergaard P, et al. Diabetes and breast Cancer subtypes. PLoS One. 2017;12(1):e0170084. doi: 10.1371/journal.pone.0170084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL, Brancati FL, Wolff AC. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 2011;29(1):40–46. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jarvandi S, Pérez M, Margenthaler J, Colditz GA, Kreuter MW, Jeffe DB. Improving lifestyle behaviors after breast Cancer treatment among African American women with and without diabetes: role of health care professionals. Ann Behav Med. 2020. [DOI] [PMC free article] [PubMed]
- 52.Luo J, Hendryx M, Virnig B, Wen S, Chlebowski R, Chen C, Rohan T, Tinker L, Wactawski-Wende J, Lessin L. Pre-existing diabetes and breast cancer prognosis among elderly women. Br J Cancer. 2015;113(5):827–832. doi: 10.1038/bjc.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jarvandi S, Pérez M, Schootman M, Jeffe DB. Pre-existing diabetes in early stage breast cancer patients is associated with lack of improvement in quality of life 2 years after diagnosis. Int J Behav Med. 2016;23(6):722–729. doi: 10.1007/s12529-016-9577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bluethmann SM, Murphy CC, Tiro JA, Mollica MA, Vernon SW, Bartholomew LK. Deconstructing decisions to initiate, maintain, or discontinue adjuvant endocrine therapy in breast Cancer survivors: a mixed-methods study. Oncol Nurs Forum. 2017;44(3):E101–e110. doi: 10.1188/17.ONF.E101-E110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y, Dorjgochoo T, Bao PP, Zheng Y, Cai H, Lu W, Shu XO. Menopausal symptoms among breast cancer patients: a potential indicator of favorable prognosis. PLoS One. 2013;8(9):e75926. doi: 10.1371/journal.pone.0075926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glaus A, Boehme C, Thurlimann B, Ruhstaller T, Hsu Schmitz SF, Morant R, Senn HJ, von Moos R. Fatigue and menopausal symptoms in women with breast cancer undergoing hormonal cancer treatment. Ann Oncol. 2006;17(5):801–806. doi: 10.1093/annonc/mdl030. [DOI] [PubMed] [Google Scholar]
- 57.Harris PF, Remington PL, Trentham-Dietz A, Allen CI, Newcomb PA. Prevalence and treatment of menopausal symptoms among breast cancer survivors. J Pain Symptom Manag. 2002;23(6):501–509. doi: 10.1016/S0885-3924(02)00395-0. [DOI] [PubMed] [Google Scholar]
- 58.Wagner LI, Zhao F, Goss PE, Chapman J-AW, Shepherd LE, Whelan TJ, Mattar BI, Bufill JA, Schultz WC, LaFrancis IE, et al. Patient-reported predictors of early treatment discontinuation: treatment-related symptoms and health-related quality of life among postmenopausal women with primary breast cancer randomized to anastrozole or exemestane on NCIC Clinical Trials Group (CCTG) MA.27 (E1Z03) Breast Cancer Res Treat. 2018;169(3):537–548. doi: 10.1007/s10549-018-4713-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wheeler SB, Roberts MC, Bloom D, Reeder-Hayes KE, Espada M, Peppercorn J, Golin CE, Earp JA. Oncology providers' perspectives on endocrine therapy prescribing and management. Patient preference and adherence. 2016;10:2007–2019. doi: 10.2147/PPA.S95594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wells KJ, Pan TM, Vázquez-Otero C, Ung D, Ustjanauskas AE, Muñoz D, Laronga C, Roetzheim RG, Goldenstein M, Carrizosa C, et al. Barriers and facilitators to endocrine therapy adherence among underserved hormone-receptor-positive breast cancer survivors: a qualitative study. Support Care Cancer. 2016;24(10):4123–4130. doi: 10.1007/s00520-016-3229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fontein D, Seynaeve C, Hadji P, Hille E, van de Water W, Putter H, Kranenbarg E, Hasenburg A, Paridaens RJ, Vannetzel J-M. Specific adverse events predict survival benefit in patients treated with tamoxifen or aromatase inhibitors: an international tamoxifen exemestane adjuvant multinational trial analysis. J Clin Oncol. 2013;31(18):2257–2264. doi: 10.1200/JCO.2012.45.3068. [DOI] [PubMed] [Google Scholar]
- 62.Fontein D, Houtsma D, Hille E, Seynaeve C, Putter H, Meershoek-Klein Kranenbarg E, Guchelaar HJ, Gelderblom H, Dirix L, Paridaens R. Relationship between specific adverse events and efficacy of exemestane therapy in early postmenopausal breast cancer patients. Ann Oncol. 2012;23(12):3091–3097. doi: 10.1093/annonc/mds204. [DOI] [PubMed] [Google Scholar]
- 63.Cusack L, Brennan M, Baber R, Boyle F. Menopausal symptoms in breast cancer survivors: management update. Br J Gen Pract. 2013;63(606):51–52. doi: 10.3399/bjgp13X660977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deng G, Vickers A, Yeung S, D'Andrea GM, Xiao H, Heerdt AS, Sugarman S, Troso-Sandoval T, Seidman AD, Hudis CA, et al. Randomized, controlled trial of acupuncture for the treatment of hot flashes in breast cancer patients. J Clin Oncol. 2007;25(35):5584–5590. doi: 10.1200/JCO.2007.12.0774. [DOI] [PubMed] [Google Scholar]
- 65.Bokmand S, Flyger H. Acupuncture relieves menopausal discomfort in breast cancer patients: a prospective, double blinded, randomized study. Breast. 2013;22(3):320–323. doi: 10.1016/j.breast.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 66.Lesi G, Razzini G, Musti MA, Stivanello E, Petrucci C, Benedetti B, Rondini E, Ligabue MB, Scaltriti L, Botti A, et al. Acupuncture as an integrative approach for the treatment of hot flashes in women with breast Cancer: a prospective multicenter randomized controlled trial (AcCliMaT) J Clin Oncol. 2016;34(15):1795–1802. doi: 10.1200/JCO.2015.63.2893. [DOI] [PubMed] [Google Scholar]
- 67.Hickey M, Szabo RA, Hunter MS. Non-hormonal treatments for menopausal symptoms. Bmj. 2017;359:j5101. doi: 10.1136/bmj.j5101. [DOI] [PubMed] [Google Scholar]
- 68.Chan CW, Tai D, Kwong S, Chow KM, Chan DN, Law BM. The effects of pharmacological and non-pharmacological interventions on symptom management and quality of life among breast cancer survivors undergoing adjuvant endocrine therapy: a systematic review. Int J Environ Res Public Health. 2020;17(8):2950. doi: 10.3390/ijerph17082950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Franzoi MA, Agostinetto E, Perachino M, Del Mastro L, de Azambuja E, Vaz-Luis I, Partridge AH, Lambertini M. Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. The Lancet Oncology. 2021;22(7):e303–e313. doi: 10.1016/S1470-2045(20)30666-5. [DOI] [PubMed] [Google Scholar]
- 70.Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, Hayden J, Tarpinian K, Yakim E, Flockhart DA, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30(9):936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krebber AM, Buffart LM, Kleijn G, Riepma IC, de Bree R, Leemans CR, Becker A, Brug J, van Straten A, Cuijpers P, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121–130. doi: 10.1002/pon.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lingam R, Scott J. Treatment non-adherence in affective disorders. Acta Psychiatr Scand. 2002;105(3):164–172. doi: 10.1034/j.1600-0447.2002.1r084.x. [DOI] [PubMed] [Google Scholar]
- 73.Riley GF, Warren JL, Harlan LC, Blackwell SA. Endocrine therapy use among elderly hormone receptor-positive breast cancer patients enrolled in Medicare part D. Medicare Medicaid Res Rev. 2011;1(4). [DOI] [PMC free article] [PubMed]
- 74.Thompson T, Rodebaugh TL, Pérez M, Struthers J, Sefko JA, Lian M, Schootman M, Jeffe DB. Influence of neighborhood-level factors on social support in early-stage breast cancer patients and controls. Soc Sci Med. 2016;156:55–63. doi: 10.1016/j.socscimed.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Friese CR, Pini TM, Li Y, Abrahamse PH, Graff JJ, Hamilton AS, Jagsi R, Janz NK, Hawley ST, Katz SJ, et al. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat. 2013;138(3):931–939. doi: 10.1007/s10549-013-2499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Erickson K, Patterson RE, Flatt SW, Natarajan L, Parker BA, Heath DD, Laughlin GA, Saquib N, Rock CL, Pierce JP. Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol. 2011;29(1):54. doi: 10.1200/JCO.2010.29.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed for the current study are not publicly available as data for the trial aims are still being analyzed. The pooled data may be made available from the corresponding author on reasonable request.