Abstract
Introduction:
Giardiasis is one of the greatest public parasitic infections causing diarrheal and also known to be associated with high morbidity and mortality, among the children's particularly in developing countries with less cleanliness practices. Thus, studying genomic variety of Giardia intestinalis aids to improve our perspective related to the variability in the genome of the parasite.
Materials and Methods:
In this cross-sectional study, 1006 stool samples were collected from the rural (n = 500) and urban settings (n = 506) from the children (<15 years) with and without symptoms and were screened for the presence of G. intestinalis by polymerase chain reaction (PCR) targeting triosephosphate isomerase gene. Further, all PCR-positive amplicons were subjected to restriction fragment length polymorphism using RsaI restriction enzyme.
Results:
Of the total 1006 stool samples, 500 samples from rural screened by PCR 108 (21%) were found to be positive for assemblage A, 116 (23.2%) belong to assemblage B, and 5 (1%) were mixed assemblages (A + B). Whereas in urban, of the 506 samples screened by PCR, 92 (18.1%) were found to be positive for assemblage A, 93 (18.3%) assemblage B, and 10 (1.9%) were mixed assemblages (A + B). No significant difference was found between the G. intestinalis assemblages with clinical details of symptomatic and asymptomatic in children.
Conclusions:
This signifies the first study inspection in our location to shed lights and delivers some preliminary data on assemblages and subassemblages. The results suggest that anthroponotic transmission could be a foremost transmission path for giardiasis among the study population.
Keywords: Assemblages, diarrhea, genotyping, Giardia intestinalis, polymerase chain reaction and restriction fragment length polymorphism, triosephosphate isomerase geness
INTRODUCTION
Giardia intestinalis (synonyms: Giardia lamblia and Giardia duodenalis) is an intestinal flagellate protozoan considered as the most common cause of parasitic diarrhea. It is also known as one of the major sources of traveler's diarrhea and affects children worldwide.[1,2,3] Giardiasis cases around the world were about 2.5 million cases of diarrhea and nutritional deficits in children every year, especially in developing countries.[4] Since 2004, it has been counted as a part of the World Health Organization's neglected tropical diseases. It is a known fact that children belonging to 1–5 years of age and mainly those from developing countries and under malnourished individuals are most affected.[5,6] It is projected that the occurrence rates are 2%–5% in developed countries and 20%–30% in developing countries.[7,8]
G. intestinalis has been defined as a species complex embracing seven assemblages.[9] The assemblages A and B are most commonly seen as zoonotic infections and C, D, E, F, and G seem to found only in specific hosts.[10] Giardia isolates from the humans and animals characterized by molecular methods at several loci, such as triose phosphate isomerase (tpi) gene, glutamate dehydrogenase, small subunit ribosomal RNA, and β-giardin. These permit identification of different assemblages of Giardia and help in differentiating subassemblages also as well.[11] Assemblage A has been distinguished clearly and affects mostly cattle, canines, felines, with assemblage A sub assemblage Group AI seen to affect a very wide host range. Assemblage A sub assemblage Group AII has been seen to be limited to humans only. It was observed that assemblage B has been detected from dogs, beavers, rats, slow lorises.[12] The current study aimed to elucidate the prevalence and genetic diversity of G. intestinalis among the rural and urban population-based upon polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). It also tried to make an effort to check and relate the association between the clinical features and the infection by various assemblages of G. intestinalis.
MATERIALS AND METHODS
Study population and specimen collection
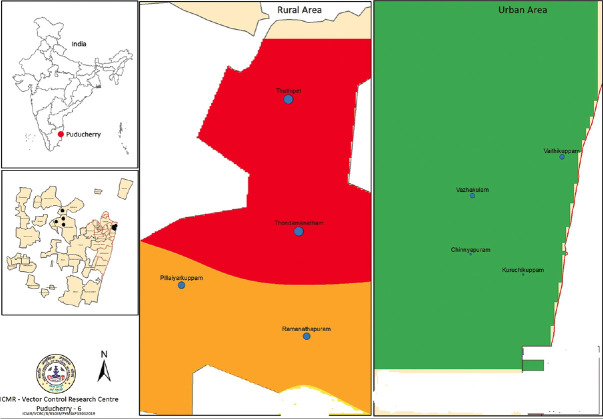
A cross-sectional descriptive study was conducted from September 2016 to December 2018. Sampling was implemented to collect the stool samples among the rural and urban settings of the study population in Pondicherry. [Figure 1] Stool samples were collected from the rural (n = 500) and urban settings (n = 506) in children (<15 years) both the genders with and without symptoms were included in this study. Each children was given a sterile labeled stool container and directed to take a sufficient amount of fresh stool sample. The samples were transported inside an insulated box along with the ice pack to the laboratory within 24 h of collection, and routine examination was done immediately. Samples were aliquoted into two halves in the laboratory for microscopy and PCR.
Figure 1.
A geographic map showing the location of the rural and urban area (blue dots) involved in the study. This map was created using the Esri ArcMap 10.2.1 software. This image has been reproduced with the permission of ICMR, VCRC Pondicherry-06 (Courtesy: Dr. Hari Kishan Raju. K and Dr. P. Jambulingam, Former Director, Vector Control Research Centre (VCRC), ICMR, Pondicherry)
Demographic information collection
Details regarding gender, age, history of the children such as clinical signs and symptoms, source of drinking water supply, food hygiene, availability of toilets at home, number of domestic animals owned by the household, personal hygiene practice, etc., were noted from each children or their parents/guardians using a structured questionnaire. Informed/written consent was obtained from all the children including parents/guardians before data and samples were collected.
Ethical clearance
The study was given clearance by the Institute Ethics Committee (human studies) of Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry (approval No: ECR/342/Inst/PY/2013). To store these samples until the duration of the study period, to adhere to ethical norms for using human subjects for the medical research, all children/guardians/parents were informed about the aim and objectives and goals of the present study. Physicians and laboratory personnel were also explained about the results of the tests, and in case of positive results, the children received an appropriate free of cost treatment from the institute.
Methods used in the detection of parasites
Stool samples were observed both macroscopically and by microscope too. The macroscopic examination was carried out for all the samples instantly without delaying within 24 h after the collection, macroscopic was done to identify consistency, color, and bloodstain of the stool samples. Before and after the formal–ether concentration technique was done, saline and iodine wet mount preparations from each sample were performed and then were examined under low and high power (×10, ×40) magnification of the light microscope to detect intestinal parasites including protozoal trophozoites or cysts.[13]
Deoxyribonucleic acid extraction and polymerase chain reaction assay
All stool samples collected were subjected to DNA extraction using QIAmp DNA Stool Mini Kit (QIAGEN, Valencia, CA, USA) based on the palm plate manufacturer's instructions given from the kit extraction was followed. The purity and yield of the DNA after extraction were checked by ultraviolet absorbance using NanoDrop 2000 C (Thermo Fisher, USA). Then, DNA samples were stored at −20°C for further molecular study.
PCR primers and protocol were followed as per Amar et al. where they target tpi locus for the detection of an assemblage of Giardia species, particularly assemblage A and B.[14] A semi-nested PCR amplification assay was used, which involve two phases: Phase I comprising of a single duplex reaction for genus level and Phase II comprising of two individual reactions particularly for assemblage A and B. Positive DNA controls for both assemblage A and B of G. intestinalis were attained from CMC Vellore, India (Courtesy: Dr. Sitara Swarna Rao). Nuclease-free water was used as negative control.
Restriction fragment length polymorphism analysis
RFLP was performed for distinguishing genotype assemblage A into the sub assemblages A-I or A-II based on the equivalent matched restriction digestion products band size of 437 and 15 bp for assemblage A Group I and 235, 202 and 15 bp for assemblage A Group II and for products band size of 130 and 300 bp for assemblage B Group III and 430 bp for assemblage B Group IV.[14] RFLP examination was achieved by digesting 8 μl of the tpi B-PCR or tpi A-PCR product with master mix consisting of RsaI restriction enzyme (10 U/μl) 0.5 μl and 3 μl of × 1 enzyme buffer solution and 18.5 μl nuclease-free water (New England Biolabs, Massachusetts, United States) bringing to the final volume of 30 μl then cover and kept at 37°C water bath incubation for at least 4 h.
Polymerase chain reaction amplified product and restriction fragment detection
Two percent agarose gel containing ethidium bromide was used to separate the corresponding band size of 140 bp for assemblage B and 452 bp for assemblage A from the final secondary PCR products. For the RFLP restriction fragment products, 1.5% agarose gel containing ethidium bromide were used to separate the band size pattern and visualized under ultra-violet transilluminator.
Statistical analysis
The association between the assemblages and clinical symptomatology and also the prevalence of G. intestinalis assemblages among the rural and urban of the study population was analyzed by the Chi-square test using the SPSS software version 22 (SPSS Inc., Chicago, Illinois, USA). P ≤ 0.05 is considered statistically significant.
RESULTS
Of the 1006 stool samples collected among the rural (n = 500) and urban (n = 506) study population, 505 were male and 501 female, majority were asymptomatic and very few were symptomatic.
Polymerase chain reaction and assemblage's identification
Initially, all stool samples were screened for the presence of G. intestinalis by microscopy and then later followed by conventional PCR. Using microscopic methods, 159/1006 (15.8%) samples were determined as G. intestinalis positive. However, PCR-based methods enabled us to pick up the additional number of positivity and also help to differentiate G. intestinalis into assemblage A and B. Of the 500 rural samples screened by PCR, 108 (21%) were found to be positive for assemblage A, 116 (23.2%) assemblage B, and 5 (1%) were mixed assemblages (A + B). Among the 506 urban samples screened by PCR, 92 (18.1%) were positive for assemblage A, 93 (18.3%) belong to assemblage B, and 10 (1.9%) were mixed assemblages (A + B) [Table 1]. No significant difference was found between G. intestinalis assemblages’ infections among the rural and urban study population.
Table 1.
Prevalence of Giardia intestinalis assemblages among the study population
| Assemblages | Rural (n=500), n (%) | Urban (n=506), n (%) | P |
|---|---|---|---|
| A | 108 (21) | 92 (18.1) | 0.2 |
| B | 116 (23.2) | 93 (18.3) | |
| Mixed types (A+B) | 5 (1) | 10 (1.9) |
Sub-assemblage identification
The final secondary PCR product of tpi gene-positive for assemblages A & B digested by RsaI restriction endonuclease enzyme (RFLP) assay which helps us to classify into sub assemblages like AI, AII, BIII, BIV, and mixed types, respectively. Of the 500 samples from the rural setting 14 (2.8%) were symptomatic and 215 (43%) were asymptomatic; however, from urban setting, out of the 506 samples 29 (5.7%) were symptomatic and 116 (22.9%) were asymptomatic. All these were confirmed cases of G. intestinalis using PCR. Hence, the distribution of G. intestinalis sub-assemblages among the symptomatic and asymptomatic participants in both rural and urban settings is shown in Tables 2 and 3. No significant difference was found between the G. intestinalis assemblages of the symptomatic and asymptomatic children, as shown in Table 4. Most of the cases of giardiasis were noticed in children belong less than 10 years of age.
Table 2.
Prevalence of Giardia intestinalis assemblages among the cases confirmed by polymerase chain reaction (n=424)
| Assemblages | Symptomatic (n=43; 10%), n (%) | Asymptomatic (n=381; 90%), n (%) | P |
|---|---|---|---|
| A | 16 (37.2) | 184 (48.3) | 0.3 |
| B | 25 (58.1) | 184 (48.3) | |
| Mixed types (A+B) | 2 (4.6) | 13 (3.4) |
Table 3.
Distribution of Giardia intestinalis assemblage (s) and sub-assemblage (s) among symptomatic and asymptomatic in Rural population (n=500)
| Giardia intestinalis genotype assemblage (s) | Sub-assemblage (s) PCR-RFLP | Symptomatic (n=14), n (%) | Asymptomatic (n=215), n (%) |
|---|---|---|---|
| A | A (unidentified) | 2 (14.2) | 14 (6.5) |
| AI | 1 (7.1) | 64 (30) | |
| AII | 2 (14.2) | 22 (10.2) | |
| AI+AII | 1 (7.1) | 2 (0.9) | |
| B | B (unidentified) | 6 (43) | 60 (28) |
| BIII | 1 (7.1) | 18 (8.3)) | |
| BIV | 0 | 19 (9) | |
| BIII+BIV | 0 | 12 (5.5) | |
| Mixed types (A+B) | A+B (unidentified) | 1 (7.1) | 0 (0.4) |
| AI+BIII | 0 | 1 (0.4) | |
| AII+BIV | 0 | 1 (0.4) | |
| BIII+AII | 0 | 1 (0.4) | |
| BIV+AI | 0 | 1 (0.4) |
PCR-RFLP: Polymerase chain reaction-restriction fragment length polymorphism
Table 4.
Distribution of Giardia intestinalis assemblage (s) and sub-assemblage (s) among symptomatic and asymptomatic in urban population (n=506)
| Giardia intestinalis genotype assemblage (s) | Sub-assemblage (s) PCR-RFLP | Symptomatic (n=29), n (%) | Asymptomatic (n=166), n (%) |
|---|---|---|---|
| A | A (unidentified) | 1 (3.4) | 18 (11) |
| AI | 3 (10.3) | 50 (30.1) | |
| AII | 5 (17.2) | 13 (8) | |
| AI+AII | 1 (3.4) | 1 (0.6) | |
| B | B (unidentified) | 11 (38) | 45 (27.1) |
| BIII | 2 (7) | 18 (11) | |
| BIV | 3 (10.3) | 4 (2.4) | |
| BIII+BIV | 2 (7) | 8 (5) | |
| Mixed types (A+B) | A+B (unidentified) | 1 (3.4) | 4 (2.4) |
| AI+BIII | 0 (0) | 2 (1.2) | |
| AII+BIV | 0 (0) | 1 (0.6) | |
| BIII+AII | 0 (0) | 1 (0.6) | |
| BIV+AI | 0 (0) | 1 (0.6) |
PCR-RFLP: Polymerase chain reaction-restriction fragment length polymorphism
DISCUSSION
It is a known fact that Giardia infection occurs in human directly through fecal-oral and ingestion of parasite cysts from the contaminated water or food and also the mode of transmission could be a zoonotic origin.[15,16] The severity of the parasite infection depends on the number or load of cysts ingested (10 cysts or more is infectious).[17] However, studies say that to determine the prevalence of giardiasis in a community, it depends on water sanitation and personal hygiene status.[18] In developing counties, the most important social health problem commonly encountered among children is intestinal parasitic infection. In the rural population, our results showed that 108 (21%), 116 (23.2%), and 5 (1%), whereas in the urban population, 92 (18.1%), 93 (18.3%), and 10 (1.9%) positive isolates of G. intestinalis belonged to assemblages A, B, and mixed types, respectively. Our findings results are in agreement with the increased overall prevalence of assemblage B in the world when compared with assemblage A and mixed assemblage A + B. Our results were found to be alike to some studies carried out in Bangladesh,[19] Philippines,[20] England,[21] Netherlands,[22] and Brazil,[23] but different results were reported from Korea[24] and Mexico[25] where assemblage A was the majority. Our study results showed that the prevalence of giardiasis in Pondicherry was much higher than previous studies conducted by other states in India and other countries. This may be due to sanitary conditions improvement, health-care facilities, and health education in the others states of India and other countries during recent years.
A further classification of the assemblages A and B into subassemblages based on RFLP of the tpi gene segments of the final secondary PCR product showed that cysts belonged to sub-assemblage AI, AII, and AI + AII, some samples belonged to the BIII, BIV, and BIII + BIV and some showed mixed types infection such as AI + BIII, AII + BIV, BIII + AII, and BIV + AI, respectively. Previous reports specified that the AI sub-assemblage and B assemblage have a very wide host range infections, which included domestic animals and cattle while the AII sub-assemblage is more limited to a precise host of human infections.[15,26] Thus, it is probable that the AI and B infections types found in this study could be through zoonotic route while the AII infection type could be through anthroponotic route.
In some of the previous studies, Giardia infection by assemblage B has been correlated with severe symptomatic diarrheal cases,[27,28] while in the other study, the results showed a significant association between assemblage A with severe symptoms presented and some studies have shown does not have significant between assemblages with clinical symptomology.[29,30] However, in our study, all positive cases majority were found to be assemblage B, the correlation of different assemblages with symptomatic and asymptomatic giardiasis cases does not have any significance.
In our study, we found that a maximum number of Giardia cases were noticed in children which is <10 years of age, and majority of the giardiasis cases belong to asymptomatic cases which may act like carrier individual, and very less Giardia positive cases belong to the symptomatic individual. Similar result has also been stated from different parts of the country that the prevalence rate seen higher in 0–4 years. Two to eight years, among the children <10 years of age and most of the Giardia-positive cases belong to asymptomatic when compared with the symptomatic case.[31,32,33]
In our study we found that the overall prevalence rate of Giardia in rural populations is comparatively slightly higher than the urban population, This could be due to some reason that those peoples leaving in the rural area still taking bath and wash their dishes, clothes using canals or unclean water, this could be easily contaminated and the parasite may survive and grow. Furthermore, there are more poultry, pets, livestock, and other domestic animals moving around freely in rural communities, this may serve as the potential sources of human zoonotic infections.[15] There are limited reports conducted earlier in various settings such as tertiary health care hospital and schools’ children in Pondicherry to give the microscopic prevalence data of parasitic infections/infestations, but our study focused on the prevalence rate and genetic diversity of parasitic infections among the rural and urban study population in the communities with special consideration to G. intestinalis. To the best of our information, this study could be taken as the first study ever conducted in Pondicherry communities, which will enlighten the primary healthcare professionals in the various community an idea on the distribution of the genotypes and sub genotypes of G. intestinalis isolates by PCR amplification tpi gene as the target gene from patients with giardiasis in Pondicherry.
Limitations of the study
The current study was examined only a single stool collected at one point of time from each children. If triple feces samples were collected consecutively at a different time interval from each children and tested, it could have improved the occurrence of the parasitic infection rate to some extent compared to that of a single sample. This would support and gave a stronger presentation of the intestinal parasitic infections.
CONCLUSIONS
It is a known fact that rural areas face many problems when it comes to parasitic infection compared with urban areas. The burden of this kind of infection in the rural areas may be due to open defecation, no proper sanitation facilities, lack of knowledge about the infection regarding mode of transmission, fecal contamination of food and water, personal hygiene practices, awareness creation programs all these factors raise the prevalence rate in the rural areas when compared with urban areas. The present study reflects the high prevalence rate, and genetic diversity was found in the rural areas when compared with the urban areas. Some of the assemblages were found to be those of the animals and this may point to as a source of infections from animals. Hence, the way how animals are reared and kept also may be taken into account. Hence, to control raise in the prevalence rate of the parasitic infection, necessary action must be taken such as interventions, improving the sanitary facilities, de-worming programs, educated regarding fecal contamination of food and water, and the necessity of drinking with boiling water. With all these precaution measures, it may help to lower down the occurrence rate of intestinal parasitic infection, which could improve the health status and sound being of both the children and community as well.
Financial support and sponsorship
This study was financially supported by Intramural Research Grant from the Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to prompt our sincere gratefulness to all enrolled children who provided samples and details for questionnaire, staff nurses, Anganwadi workers both rural and urban for their enormous helped in data collection as well in samples collection and special thanks to Dr. Sitara Swarna Rao of CMC Vellore for her kind and support who spared us with the DNA positive controls of both assemblage A and B of G. intestinalis.
REFERENCES
- 1.Molina N, Polverino D, Minvielle M, Basualdo J. PCR amplification of triosephosphate isomerase gene of Giardia lamblia in formalinfixed feces. Rev Latinoam Microbiol. 2007;49:6–11. [PubMed] [Google Scholar]
- 2.Ekdahl K, Andersson Y. Imported giardiasis: Impact of international travel, immigration, and adoption. Am J Trop Med Hyg. 2005;72:825–30. [PubMed] [Google Scholar]
- 3.Adam RD. The Giardia lamblia genome. Int J Parasitol. 2000;30:475–84. doi: 10.1016/s0020-7519(99)00191-5. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Intestinal parasites control: Burden and trends. WHO Division of Control of Tropical Diseases. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 5.Miotti PG, Gilman RH, Santosham M, Ryder RW, Yolken RH. Age-related rate of seropositivity of antibody to Giardia lamblia in four diverse populations. J Clin Microbiol. 1986;24:972–5. doi: 10.1128/jcm.24.6.972-975.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nygård K, Schimmer B, Søbstad Ø, Walde A, Tveit I, Langeland N, et al. A large community outbreak of waterborne giardiasis-delayed detection in a non-endemic urban area. BMC Public Health. 2006;6:141. doi: 10.1186/1471-2458-6-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Control of Tropical Diseases. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 8.Thompson RC. Giardiasis as a reemerging infectious disease and its zoonotic potential. Int J Parasitol. 2000;30:1259–67. doi: 10.1016/s0020-7519(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 9.Gelanew T, Lalle M, Hailu A, Pozio E, Cacciò SM. Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Trop. 2007;102:92–9. doi: 10.1016/j.actatropica.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 11.Read CM, Monis PT, Thompson RC. Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCRRFLP. Infect Genet Evol. 2004;4:125–30. doi: 10.1016/j.meegid.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Homan WL, Mank TG. Human giardiasis: Genotype linked differences in clinical symptomatology. Int J Parasitol. 2001;31:822–6. doi: 10.1016/s0020-7519(01)00183-7. [DOI] [PubMed] [Google Scholar]
- 13.Allen AV, Ridley DS. Further observations on the formol-ether concentration technique for faecal parasites. J Clin Pathol. 1970;23:545–6. doi: 10.1136/jcp.23.6.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amar CF, Dear PH, Pedraza-Díaz S, Looker N, Linnane E, McLauchlin J. Sensitive PCR-restriction fragment length polymorphism assay for detection and genotyping of Giardia duodenalis in human feces. J Clin Microbiol. 2002;40:446–52. doi: 10.1128/JCM.40.2.446-452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14:447–75. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flanagan PA. Giardia--diagnosis, clinical course and epidemiology. A review. Epidemiol Infect. 1992;109:1–22. [PMC free article] [PubMed] [Google Scholar]
- 17.Rendtorff RC. The experimental transmission of human intestinal protozoan parasites. II. Giardia lamblia cysts given in capsules. Am J Hyg. 1954;59:209–20. doi: 10.1093/oxfordjournals.aje.a119634. [DOI] [PubMed] [Google Scholar]
- 18.Stuart JM, Orr HJ, Warburton FG, Jeyakanth S, Pugh C, Morris I, et al. Risk factors for sporadic giardiasis: A case-control study in southwestern England. Emerg Infect Dis. 2003;9:229–33. doi: 10.3201/eid0902.010488. [DOI] [PubMed] [Google Scholar]
- 19.Haque R, Mondal D, Kirkpatrick BD, Akther S, Farr BM, Sack RB, et al. Epidemiologic and clinical characteristics of acute diarrhea with emphasis on Entamoeba histolytica infections in preschool children in an urban slum of Dhaka, Bangladesh. Am J Trop Med Hyg. 2003;69:398–405. [PubMed] [Google Scholar]
- 20.Yason JA, Rivera WL. Genotyping of Giardia duodenalis isolates among residents of slum area in Manila, Philippines. Parasitol Res. 2007;101:681–7. doi: 10.1007/s00436-007-0533-8. [DOI] [PubMed] [Google Scholar]
- 21.Amar CF, Dear PH, McLauchlin J. Detection and genotyping by real-time PCR/RFLP analyses of Giardia duodenalis from human faeces. J Med Microbiol. 2003;52:681–3. doi: 10.1099/jmm.0.05193-0. [DOI] [PubMed] [Google Scholar]
- 22.van der Giessen JW, de Vries A, Roos M, Wielinga P, Kortbeek LM, Mank TG. Genotyping of Giardia in Dutch patients and animals: A phylogenetic analysis of human and animal isolates. Int J Parasitol. 2006;36:849–58. doi: 10.1016/j.ijpara.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Kohli A, Bushen OY, Pinkerton RC, Houpt E, Newman RD, Sears CL, et al. Giardia duodenalis assemblage, clinical presentation and markers of intestinal inflammation in Brazilian children. Trans R Soc Trop Med Hyg. 2008;102:718–25. doi: 10.1016/j.trstmh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yong T, Han K, Yang H, Park S. PCR-RFLP analysis of Giardia intestinalis using a Giardia-specific gene, GLORF-C4. Parasite. 2002;9:65–70. doi: 10.1051/parasite/200209165. [DOI] [PubMed] [Google Scholar]
- 25.Ponce-Macotela M, Martínez-Gordillo MN, Bermúdez-Cruz RM, Salazar-Schettino PM, Ortega-Pierres G, Ey PL. Unusual prevalence of the Giardia intestinalis A-II subtype amongst isolates from humans and domestic animals in Mexico. Int J Parasitol. 2002;32:1201–2. doi: 10.1016/s0020-7519(02)00086-3. [DOI] [PubMed] [Google Scholar]
- 26.Thompson RC. Giardia infection. In: Palmer SR, Soulsby EJ, Simpson DI, editors. Zoonosis, Biology, Clinical Practice and Public Health Control. Oxford: Oxford University Press; 1998. pp. 545–61. [Google Scholar]
- 27.Homan WL, Mank TG. Human giardiasis: genotype linked differences in clinical symptomatology. Int J Parasitol. 2001;31:822–6. doi: 10.1016/s0020-7519(01)00183-7. [DOI] [PubMed] [Google Scholar]
- 28.Gelanew T, Lalle M, Hailu A, Pozio E, Cacciò SM. Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Trop. 2007;102:92–9. doi: 10.1016/j.actatropica.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Read C, Walters J, Robertson ID, Thompson RC. Correlation between genotype of Giardia duodenalis and diarrhoea. Int J Parasitol. 2002;32:229–31. doi: 10.1016/s0020-7519(01)00340-x. [DOI] [PubMed] [Google Scholar]
- 30.Haque R, Roy S, Kabir M, Stroup SE, Mondal D, Houpt ER. Giardia assemblage A infection and diarrhea in Bangladesh. J Infect Dis. 2005;192:2171–3. doi: 10.1086/498169. [DOI] [PubMed] [Google Scholar]
- 31.Tamer GS, Kasap M, Er DK. Genotyping and phylogenetic analysis of Giardia duodenalis isolates from Turkish children. Med Sci Monit. 2015;21:526–32. doi: 10.12659/MSM.892318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Basha NR, Zaki MM, Hassanin OM, Rehan MK, Omran D. Giardia Assemblages A and B in diarrheic patients: A comparative study IN Egyptian Children and Adults. J Parasitol. 2016;102:69–74. doi: 10.1645/14-676. [DOI] [PubMed] [Google Scholar]
- 33.de Lucio A, Martínez-Ruiz R, Merino FJ, Bailo B, Aguilera M, Fuentes I, et al. Molecular Genotyping of Giardia duodenalis Isolates from Symptomatic Individuals Attending Two Major Public Hospitals in Madrid, Spain. PLoS One. 2015;10:e0143981. doi: 10.1371/journal.pone.0143981. [DOI] [PMC free article] [PubMed] [Google Scholar]